Abstract
Purpose of Review
Recent data identifies increases in young ischemic and hemorrhagic strokes. We provide a contemporary overview of current literature on stroke among young patients or premature stroke along with directions for future investigation.
Recent Findings
Strokes in the young are highly heterogenous and often cryptogenic. Sex distribution and risk factors shift from women among the youngest age groups (< 35) to men over the age of 45, with a coinciding rise in traditional vascular risk factors. Incidence is higher in minority and socioeconomically disadvantaged populations, and the impact of stroke among these communities may be exaggerated by disparities in symptom recognition and access to care. Special diagnostic work-up may be needed, and a lower threshold for diagnosis is warranted as potential misdiagnosis is a concern and may preclude necessary triage and management.
Summary
Although “premature strokes” form a relatively small proportion of total incidence, they vary greatly across subgroups and present an outsized impact on quality of life and productivity.
Keywords: Ischemic stroke, Young stroke, Stroke epidemiology, Stroke etiology, Stroke outcomes, Intracerebral hemorrhage
Introduction
Stroke (including ischemic and hemorrhagic phenotypes) remains the second leading cause of death globally and the third leading causing of death and disability [1]. Over the last two decades (1990–2019), there has been an increase in absolute number of incident strokes by 70%, prevalent strokes by 85%, deaths from stroke by 43%, and disability adjusted life-years (DALYs) by 32% [1]. Although a decline in age-standardized incidence rate has been reported, among people < 70 years both prevalence and incidence rates have increased by 22% and 15%, respectively. In addition to aggregate global data, emerging evidence of the rising burden of strokes among the young is also documented from national and regional analyses [2, 3•, 4••]. These so called “premature strokes” have a disproportionate societal burden, as they curtail productive years of life and predispose younger adults to longer-term sequalae and complications, including recurrent cerebrovascular events. We provide a contemporary review of the epidemiology, risk factors, etiological mechanisms, and outcomes of premature stroke. Though we broadly address all stroke sub-types, the review primarily focuses on ischemic strokes (IS), which constitute 87% and 63% of all stroke sub-types in the USA and globally, respectively.
What Is Premature Stroke?
In the current literature, the terms “premature stroke,” “early-onset stroke,” and “stroke among the young/young stroke” have been used interchangeably. This is perhaps because there is no firm definition for what constitutes a premature stroke. Additionally, there is not a consistent definition of “young” or “juvenile” ages. While the lower age cutoff is fairly uniform at 18 years, upper limits span the decade between 45 and 55 years of age, with 45 and 50 representing the most frequently selected cutoffs. These cutoffs fall below the critical age of 55, after which stroke incidence doubles for each advancing decade of age [5]. Furthermore, they represent the lowest quartile of the overall stroke age distribution, the median of which is 60 to 65 years. To provide a more inclusive assessment, we will approach the topic through the broader age range of 18–55, which covers most relevant research.
Epidemiology of Premature Stroke
As with older populations, IS typically accounts for the greatest proportion of incident strokes (44–65%) among younger patients, followed by intracerebral hemorrhage (ICH; 17–39%) and subarachnoid hemorrhage (SAH; 16–20%) [6, 7]. Rates vary considerably between cohorts, and a meta-analysis reports wide ranges for each subtype [8]. Overall, the incidence of stroke under 44 has increased from 5–17 per 100,000 person-years in the 1990s to 11–28 in more recent estimates [4••, 9, 10].
Age and Sex
Young stroke accounts for 10–15% of total stroke patients. However, heterogeneity within the young is high and may present analytical challenges [11, 12]. Although convenient, treating the 18–55 age group as a monolith may overlook shifts in risk factors and etiologies at different stages of adulthood. Stroke incidence is age-specific even within the young, as occurrence rises exponentially across the 15–50 age range [13]. Therefore, it may be valuable to study epidemiology of stroke in the young in subgroups of 18–34, 35–45, and 46–55 years of age. While stroke within the 18–34 age range is uncommon [13], women have a 26–56% higher likelihood of pre-mature IS than men, depending on stroke subtype [14, 15]. From 35 years of age onwards, overall stroke incidence increases, with males (vs. females) at an increasingly higher stroke risk. This age-associated increase in stroke risk among males is largely attributable to traditional risk factors [12, 16, 17•]. Stroke subtype also seems to change with age. The proportion of lacunar and large artery strokes increases for patients over 40, while cardioembolic, cryptogenic, and “other” stroke types decrease [13, 18, 19]. In ICH populations, recent significant increases in incidence rates have been found for patients in the 18–44 and 45–64 age ranges over the past two decades [3•]. The effect modification of age and stroke risk by sex among the young has largely been reported from high-income countries. It is likely that varying patterns of differential stroke risk between males and females will be observed in global data [4••].
Race and Ethnicity
Black and African American cohorts have 2–5 times higher stroke risk across various sub-types [20–23], with disparities appearing to be highest within the 35–44 age group. Specific increases have been noted for lacunar stroke, mediated by an increased prevalence of hypertension among African Americans [18]. These findings are noticeably linked to geography. Studies from Europe report overall similar trends, albeit to a lesser extent [24]. Studies from the Caribbean offer contrasting evidence, indicating that the increased prevalence found in Black and African American cohorts may stem primarily from socioeconomic and environmental variables [20]. Black patients who experience premature strokes show higher rates of hypertension, type II Diabetes, and congestive heart failure [25]. Similarly available evidence suggests that Hispanic cohorts have higher rates of stroke than non-Hispanic Whites [21, 26]. Overall, higher odds for IS are reported among both young racial minorities and Hispanic populations within the USA [27], although lower odds for transient ischemic attacks (TIAs) and IS have been documented specifically within the 20–24 age range [27].
Geography
Extending outside of the USA, Boot et al. have provided a valuable perspective on global distribution of premature stroke. Generally, rates of young stroke appear to be consistent across North America, Australia, and Asia, at a rate of 20 per 100,000 person-years. This rate is slightly lower in European studies but approximately doubles among African cohorts [4••]. Although stroke prevalence is relatively higher, the overall rates of smoking within African countries have decreased over the past few decades [28, 29], and perspectives on how this may change the incidence of premature stroke are lacking. Compared to trends commonly found within overall US cohorts, trends among the young in Asian countries show increased proportions of large-vessel thrombosis and intra-cranial atherosclerosis [30–32]. Certain uncommon cerebrovascular conditions such as Moyamoya disease have been increasingly reported in Asian populations [33], although it is doubtful that Moyamoya alone accounts for the increasing incidence of stroke among the young. Finally, a recent study within a small Saudi Arabian cohort identified a high rate of dyslipidemia (71.4%) and small vessel occlusion (31.7%). While etiologies appear to have some differences across international cohorts, vascular risk factors do appear to be largely consistent, with multiple risk factors appearing in stroke patients [34]. This similar risk-factor profile provides a shared avenue for intervention and improvement of modifiable vascular risks as a priority for young populations worldwide.
Etiology
Unlike older patients, etiologically most premature IS are classified as cryptogenic (24–53%), followed by those that may be due to cardio-embolism (10–34%), large artery atherosclerosis (4–29%), or small vessel disease (12–26%) [10, 13, 35, 36]. Cervicospinal dissection appears to be particularly prominent as well, causing up to 35% of IS among the young [37]. Among young cryptogenic strokes, patent foramen ovale (PFO) has been found in 40–56% of cases [38, 39] and patients showed high rates of hyperlipidemia, hormonal contraception, and migraine with aura, which may interact with PFO to increase cryptogenic stroke risk [40, 41]. Young ICH is most frequently linked with vascular malformation or hypertensive etiologies, depending on the cohort [42, 43]. Taken together, these findings show noteworthy differences between the causes and presentations of stroke between premature and older cohorts.
Risk Factors
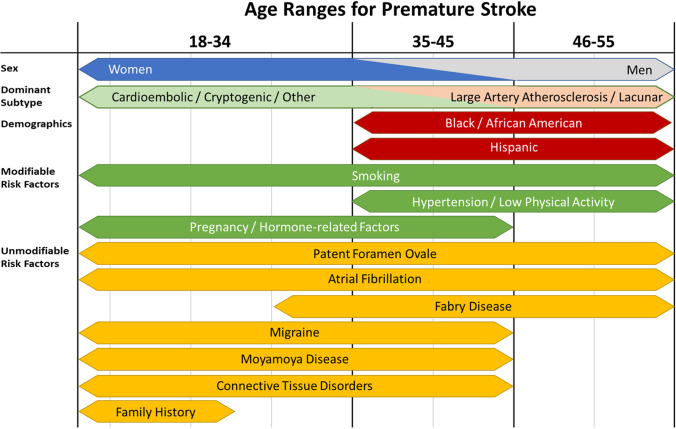
The modifiable risk factors associated with premature IS are similar to those among older populations. Primary risks across the young age range include hypertension, smoking, low physical activity, and hyperlipidemia [44]. Trends additionally demonstrate increased stroke incidence associated with growing cumulative comorbidity burden, even at this younger age. An overview of risk factors and premature stroke characteristics is provided in Fig. 1.
Fig. 1.
Overview of major risk factors and characteristics of premature stroke across the age ranges where they are most prominent
Modifiable Risk Factors
While the overall distribution of young IS etiologies may differ from typical age groups, the vascular risk factors that accumulate over time are more consistent with older cohorts. The first and perhaps most singular vascular risk for young patients is smoking. Current smoking among the premature cohorts is highly common, appearing in up to 44% of young IS patients, while it only appears to be linked with ~ 24% of older strokes [45, 46]. This relationship is dose-dependent—an increase in smoking behaviors yields corresponding increases in the development of atherosclerotic and cardioembolic strokes [46, 47]. Further, the risk posed by smoking is not self-contained and noticeably interacts with other risks, including oral contraception and migraine, to compound total risk of IS [48, 49].
Much like smoking, hypertension and low physical activity present a considerable risk for increased IS risk [50]. These risks appear to be greater in men and increased in the 35–44 and 45 + age ranges, aligning with the shift in stroke risk from women to men [12, 14, 17•, 44]. Although it may be less common in young patients, the risk incurred by hypertension is noticeably higher than in older patients [51], possibly owing to reduced recognition and treatment among the young [52•]. Investigations into obesity have yielded contrasting results. Some recent evidence suggests that obesity portends greater risks of IS within the young adult population with increasing body mass index yielding incremental increases in IS hazard [53–55]. However, other studies have found no significant association between obesity and stroke risk after controlling for other pertinent variables [55]. Dyslipidemia represents another significant vascular risk factor commonly tied to premature IS, with higher incidence in men [12, 56] and even among children [57]. Despite this common association, the overall mechanism linking lipid profiles to stroke remains somewhat uncertain [58].
This apparent cluster of vascular risk factors is highly interconnected, with smoking contributing to hypertension and hyperlipidemia and a marked association between hypertension and hyperlipidemia [59, 60]. Notably, however, each of these factors has shown an increased prevalence among males and generally provides the greatest impact in the 35–55 age cohorts, where stroke is more common among men [12, 56]. Young women may be exposed to a separate set of risks. Pregnancy stands out as a clear gender-specific risk within younger age groups; however, current evidence links it to < 5% of strokes among young women [13, 15]. Oral contraception and hormone replacement therapy also increase stroke risk via thromboembolic mechanisms [11, 13, 61]. Recent meta-analysis has suggested a dose and time-dependent relationship between oral contraception and stroke, with every 10-µg dose increase and each additional 5 years of usage increasing IS risk by 20% [62]. These factors provide notable, age-dependent risks for women that contribute to the increased female stroke prevalence within the youngest age groups.
Genetic and Unmodifiable Risk Factors
From the current discussion, we can see that modifiable risk factors associated with young IS broadly group into hypertension-related and pregnancy/hormone-related groups. While these stand as independent risks, they may augment the risk associated with genetic factors and chronic diseases. Since a considerable number of IS in the young are attributed to cryptogenic or other etiologies, there may be several underlying conditions contributing to IS risk. Particular conditions of interest are PFO, migraine with aura, atrial fibrillation (Afib), Fabry disease, Moyamoya, and connective tissue disorders.
Patent Foramen Ovale is relatively common. Most estimations suggest that it may appear in about 27% of the general population with variably reported sex differences [15, 63, 64]. Incidence in patients with cryptogenic stroke is much higher, and it has been found in up to 62.6% of young strokes of unknown etiology [35, 39]. Furthermore, PFO also interacts with pregnancy and other thrombotic risks [65]. Given the relatively high prevalence of PFO in the general population, its true causality with IS among the young is a matter of current debate [66]. Nevertheless, observational data report PFOs to be related to stroke in patients without other risk factors [63], and surgical closure may reduce recurrent stroke risk among young patients. While both PFO closure and anticoagulation appear to reduce IS, the use of long-term anticoagulation at a young age may increase risk of bleeding disorders [67, 68].
Similar to PFO, Afib presents an increased risk for embolism through the disruption of regular blood flow, likely compounded by other larger systemic abnormalities [69]. There is evidence that PFO contributes to atrial vulnerability, increasing the odds of arrhythmias [70]. Within the context of young stroke, Afib rates are much lower; however, it still presents a noteworthy risk for IS [71]. Similar to older populations, diagnostic challenges of Afib, primarily due to paroxysmal episodes, may result in its underreporting [72]. An association has also been identified between Afib and migraine with aura, compared to migraine without aura [73]. Migraine, in itself, also presents an increased risk for cardioembolic stroke, with vascular dysfunction identified in migraneurs [48]. The overall prevalence of migraine with aura is higher in young women, and the present IS risk is compounded by other factors such as smoking and the use of oral contraception [49, 74].
Fabry disease is a lysosomal storage disorder that leads to thickening within larger vessels [75]. While rare (1 per 100,000) in the general population, 24–48% of patients with Fabry disease experience an incident stroke, particularly at a younger age (28–54 years) [76, 77]. Whereas Fabry disease is a hereditary trait, the cause of Moyamoya disease is uncertain. Moyamoya, which causes progressive narrowing of cranial arteries, appears primarily in people younger than 50 [4••] and is more frequent in low income and urban populations. Risks also appear increased among women, people aged 18–44, and Asian/Pacific Islanders, with high prevalence specifically noted in Japanese cohorts. [78, 79]. While Moyamoya is more strongly associated with risks of IS, it also presents an increased risk of hemorrhage [80].
Just as PFO is linked to cryptogenic stroke, and Fabry Disease and Moyamoya are associated with atherosclerosis, connective tissue disorders may contribute to cervicospinal dissection. A number of conditions may be assessed here, including Ehlers-Danlos syndrome, fibromuscular dysplasia, and Marfan syndrome [35]. These represent genetic disorders that increase the fragility of blood vessels and subsequent stroke risks [35, 81]. This increases the risks associated with trauma and may lead to cervical artery dissection. While options are limited to improve these risks, recent opinion highlights a need to assess possible traumatic triggers in young patients with potential stroke [82].
It should be noted that the risk of premature stroke carries a heritable component even in the absence of recognized genetic conditions. Results from the Framingham Heart Study indicate that the risk of IS was more than doubled among children whose parents had history of premature stroke (< 65 years) [83], whereas genome-based variability only explains around 38% of IS risk [84]. While collective evidence directly assessing the contribution of family history to stroke among the young is limited, results do suggest that premature IS patients are more likely to have a positive family history than older patients [85], and family history carries the strongest association within the youngest age group (15–24 year) compared to 25–34 or 35–49 year subgroups [86]. This heritability also appears constant across IS subtypes [85].
Social Determinants of Health
Coinciding with the geographic and racial disparities, socioeconomic factors and social determinants contribute significantly to both the incidence and outcomes of IS [87]. An important note should be made regarding potential disparities between urban and rural populations among the young. Extending from what we know about older populations and disparities within risk factors [88], it seems likely that young IS rates would be similarly elevated. Considering that social isolation and influence of social determinants increase IS incidence among older populations [89, 90], it may be expected that such social factors would carry similar impact in young cohorts. Direct studies are limited; however, current reports have found that young patients who achieved a favorable post-IS outcome (modified Rankin Scale [mRS] score of 0–1) were more likely to be college educated and practice sports [91]. Studies of premature heart disease have shown elevated burdens of risk factors, including those with important links to premature IS, in patients with low socioeconomic status. Furthermore, patients with higher sociodemographic risks are less likely to be aware of stroke symptoms [52•]. These factors may be further compounded by access and quality of care disparities, as higher socioeconomic status patients are more likely to receive high quality in-hospital and rehabilitative care [87]. This remains a topic with limited evidence and a high potential for future research.
Diagnosis and Treatment
In part due to their rarity, premature strokes carry an increased chance of misdiagnosis, particularly among patients under 35 and those with posterior circulation strokes [92]. Studies report headache and peripheral vertigo as the most common symptoms resulting in misdiagnoses of posterior circulation strokes. However, such misdiagnoses may also be attributable to emergency medical staff as opposed to neurologist-based assessments [92, 93]. Up to 50% of young adults with apparent stroke-like symptoms may not have a stroke mimic [94]. Thus, diagnostic accuracy is exceedingly critical among young patients with stroke symptoms, and expanded utilization of magnetic resonance imaging (MRI) to confirm suspected stroke should be considered [95].
Similar to stroke among older adult populations, management of premature strokes is driven by specific stroke types and etiologies [96]. In the acute IS phase, thrombolysis and reperfusion strategies remain the most effective options [94]. Given the varied etiology, a more thorough diagnostic workup for young IS patients may be warranted. Further management is directed by objectives of avoiding complications and promoting recovery. Secondary stroke prevention strategies would be overlapping with stroke in older adults but are largely driven by specific stroke etiology. For example, following dissection, antithrombotic (antiplatelet or anticoagulant) modalities require consideration [97]. Use of statins after IS is also associated with lower rates of mortality and decreased stroke recurrence [98]. Despite established treatment and secondary prevention strategies remaining effective for younger populations, young patients are more likely to experience delays in contacting emergency services, seeking appropriate care, and receiving accurate diagnosis, which hinders treatment and jeopardizes recovery [92, 94, 99]. The interplay of socioeconomic factors with delay or suboptimal care also requires special consideration. For example, disadvantaged patients tend to have longer reperfusion times [100]. Perhaps the most effective avenue to reduce the burden of premature strokes is through development of targeted primary prevention strategies, implemented across a robust public health infrastructure. These may include cessation of smoking, awareness and appropriate treatment of hypertension and dyslipidemia, and improved diet and lifestyle choices.
Outcomes
Premature strokes are associated with lower mortality as compared to older stroke patients. However, in-hospital death remains a significant risk, occurring in ~ 5–8% of premature ischemic and 12–34% of premature hemorrhage strokes [42, 101–103]. Mortality rates for IS are estimated at 10% within 5 years, increasing to 27% within 20 years [104].
In general, younger patients exhibit better functional recovery and outcomes than older groups [105], with higher rates of younger patients achieving mRS scores of 0–1 or 0–2 at follow-up [11, 106]. This may partly be due to an increase likelihood of receiving rehabilitative care [105]. Additionally, despite early benefits, a prospective study demonstrated that 20-year mortality rates among premature stroke patients are significantly higher than the general populace when matched for age, sex, and calendar-year [104].
Despite lower mortality, even mild impairments have been shown to result in a substantial decrease in quality of life and lost DALYs. Poor functional outcome, defined as mRS scores 3–6, were recorded in 6–20% of young stroke patients [11].
An Australian study reported an average 5-year economic burden of approximately $150,000 per stroke patient, with patients experiencing a loss of 3.05 Quality Adjusted Life years (QALY) over 5 years post-stroke and 14.22 QALYs lost over 30 years [107]. Based on data from 18–64-year-old cohorts, the cost of stroke in terms of lost DALYs is also expected to be higher in developing countries [108]. Other studies have reported that young post-stroke patients also show persistent disabilities such as significantly slower walking speeds, which is strongly associated with the ability to return to work [109]. This, paired with long-term persistent cognitive impairments experienced by up to 50% of young stroke patients [110, 111] and a number of psychosocial dysfunctions (anxiety, depression, etc.), markedly diminishes quality of life [112]. Collectively, these consequences introduce prohibitive costs for young stroke patients.
Gaps in Knowledge and Further Research
Large gaps remain in our understanding of premature stroke. Dyslipidemia is a recognized and significant risk among young adults; however, its causal mechanism is not completely understood. Knowledge regarding the apparent heritability of premature strokes is also lacking. Atrial fibrillation and PFO, while both recognized as risk factors, have open questions regarding their true prevalence in the population.
On a population level, research effort is also needed to investigate potential socioeconomic and treatment disparities and how these may feed into the experience of stroke and recovery. This is true in both domestic and global contexts, as data are lacking for many diverse countries and populations [4••]. Concordantly, awareness of stroke risks and symptoms is lower among minorities, and disadvantaged communities and improvement of these may reduce disparity. Providers, too, may benefit from additional training or the use of MRI to improve diagnostic accuracy. Moreover, while trends and patterns have emerged among young stroke patients, little effectively explains the recent rise in premature stroke incidence.
Finally, respiratory infection has been shown to increase risks of atherothromboembolic and cardioembolic stroke [113, 114]. As we continue to investigate the consequences of the global COVID-19 pandemic, emergent information suggests that COVID-linked IS occurs most often within the presence of other comorbidities [115]. It is plausible that the increasing comorbidity burdens of young adults, paired with reduced activity due to COVID restrictions, may further increase the relative risk of premature stroke.
Conclusion
There is now ample evidence of increasing incidence for ischemic and hemorrhagic stroke in the young. The population experiencing premature stroke is heterogeneous in terms of epidemiology, etiology, and modifiable and unmodifiable risk factors. Generally, the youngest group of patients contains a higher proportion of women and is more likely to be coagulopathic in nature. On the other hand, as age increases, premature stoke patients show a gradual buildup of traditional vascular risk factors and begin to etiologically resemble strokes encountered at older ages. Astute clinical exam and low threshold for diagnosis are necessary. However, the cornerstone of reducing young stroke burden is to be driven by primary prevention strategies implemented across a strong public health infrastructure. Such measures particularly need to address systemic sources of health inequity and disparity.
Declarations
Conflict of Interest
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Evidence-Based Medicine, Clinical Trials and Their Interpretations
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.GBD Stroke Collaborators (2021) Global, regional, and national burden of stroke and its risk factors, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2019;20:795–820. doi: 10.1016/S1474-4422(21)00252-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Béjot Y, Delpont B, Giroud M. Rising stroke incidence in young adults: more epidemiological evidence, more questions to be answered. J Am Heart Assoc. 2016;5:e003661. doi: 10.1161/JAHA.116.003661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bako AT, Pan A, Potter T, Tannous J, Johnson C, Baig E, Meeks J, Woo D, Vahidy FS. Contemporary trends in the nationwide incidence of primary intracerebral hemorrhage. Stroke. 2022;53:e70–e74. doi: 10.1161/STROKEAHA.121.037332. [DOI] [PubMed] [Google Scholar]
- 4.Boot E, Ekker MS, Putaala J, Kittner S, De Leeuw F-E, Tuladhar AM. Ischaemic stroke in young adults: a global perspective. J Neurol Neurosurg Psychiatry. 2020;91:411–417. doi: 10.1136/jnnp-2019-322424. [DOI] [PubMed] [Google Scholar]
- 5.Yousufuddin M, Young N. Aging and ischemic stroke Aging (Albany NY) 2019;11:2542–2544. doi: 10.18632/aging.101931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kissela BM, Khoury JC, Alwell K, et al. Age at stroke: temporal trends in stroke incidence in a large, biracial population. Neurol. 2012;79:1781–1787. doi: 10.1212/WNL.0b013e318270401d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen C-Y, Lin P-T, Wang Y-H, et al. Etiology and risk factors of intracranial hemorrhage and ischemic stroke in young adults. J Chin Med Assoc. 2021;84:930–936. doi: 10.1097/JCMA.0000000000000598. [DOI] [PubMed] [Google Scholar]
- 8.Marini C, Russo T, Felzani G. Incidence of stroke in young adults: a review. Stroke Res Treat. 2010;2011:535672. doi: 10.4061/2011/535672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Madsen TE, Khoury JC, Leppert M, et al. Temporal trends in stroke incidence over time by sex and age in the GCNKSS. Stroke. 2020;51:1070–1076. doi: 10.1161/STROKEAHA.120.028910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smajlović D. Strokes in young adults: epidemiology and prevention. Vasc Health Risk Manag. 2015;11:157–164. doi: 10.2147/VHRM.S53203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maaijwee NAMM, Rutten-Jacobs LCA, Schaapsmeerders P, van Dijk EJ, de Leeuw F-E. Ischaemic stroke in young adults: risk factors and long-term consequences. Nat Rev Neurol. 2014;10:315–325. doi: 10.1038/nrneurol.2014.72. [DOI] [PubMed] [Google Scholar]
- 12.Putaala J, Yesilot N, Waje-Andreassen U, et al. Demographic and geographic vascular risk factor differences in European young adults with ischemic stroke: the 15 cities young stroke study. Stroke. 2012;43:2624–2630. doi: 10.1161/STROKEAHA.112.662866. [DOI] [PubMed] [Google Scholar]
- 13.Putaala J, Metso AJ, Metso TM, Konkola N, Kraemer Y, Haapaniemi E, Kaste M, Tatlisumak T. Analysis of 1008 consecutive patients aged 15 to 49 with first-ever ischemic stroke: the Helsinki young stroke registry. Stroke. 2009;40:1195–1203. doi: 10.1161/STROKEAHA.108.529883. [DOI] [PubMed] [Google Scholar]
- 14.Leppert MH, Ho PM, Burke J, Madsen TE, Kleindorfer D, Sillau S, Daugherty S, Bradley CJ, Poisson SN. Young women had more strokes than young men in a large, United States claims sample. Stroke. 2020;51:3352–3355. doi: 10.1161/STROKEAHA.120.030803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ekker MS, Verhoeven JI, Vaartjes I, van Nieuwenhuizen KM, Klijn CJM, de Leeuw F-E. Stroke incidence in young adults according to age, subtype, sex, and time trends. Neurology. 2019;92:e2444–e2454. doi: 10.1212/WNL.0000000000007533. [DOI] [PubMed] [Google Scholar]
- 16.Tang M, Yao M, Zhu Y, Peng B, Zhou L, Ni J. Sex differences of ischemic stroke in young adults—a single-center Chinese cohort study. J Stroke Cerebrovasc Dis. 2020;29:105087. doi: 10.1016/j.jstrokecerebrovasdis.2020.105087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leppert MH, Burke JF, Lisabeth LD, et al. Systematic review of sex differences in ischemic strokes among young adults: are young women disproportionately at risk? Stroke. 2022;53:319–327. doi: 10.1161/STROKEAHA.121.037117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trivedi MM, Ryan KA, Cole JW. Ethnic differences in ischemic stroke subtypes in young-onset stroke: the Stroke Prevention in Young Adults Study. BMC Neurol. 2015;15:221. doi: 10.1186/s12883-015-0461-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Larrue V, Berhoune N, Massabuau P, Calviere L, Raposo N, Viguier A, Nasr N. Etiologic investigation of ischemic stroke in young adults. Neurol. 2011;76:1983–1988. doi: 10.1212/WNL.0b013e31821e5517. [DOI] [PubMed] [Google Scholar]
- 20.Griffiths D, Sturm J (2011) Epidemiology and etiology of young stroke. Stroke Res Treat10.4061/2011/209370 [DOI] [PMC free article] [PubMed]
- 21.Kissela B, Schneider A, Kleindorfer D, et al. Stroke in a biracial population: the excess burden of stroke among blacks. Stroke. 2004;35:426–431. doi: 10.1161/01.STR.0000110982.74967.39. [DOI] [PubMed] [Google Scholar]
- 22.Chong JY, Sacco RL. Epidemiology of Stroke in young adults: race/ethnic differences. J Thromb Thrombolysis. 2005;20:77–83. doi: 10.1007/s11239-005-3201-9. [DOI] [PubMed] [Google Scholar]
- 23.Aradine EM, Ryan KA, Cronin CA, et al. Black-White differences in ischemic stroke risk factor burden in young adults. Stroke. 2022;53:e66–e69. doi: 10.1161/STROKEAHA.121.034314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gulli G, Rutten-Jacobs LCA, Kalra L, Rudd AG, Wolfe CDA, Markus HS. Differences in the distribution of stroke subtypes in a UK black stroke population—final results from the South London Ethnicity and Stroke Study. BMC Med. 2016;14:77. doi: 10.1186/s12916-016-0618-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones EM, Okpala M, Zhang X, et al. Racial disparities in post-stroke functional outcomes in young patients with ischemic stroke. J Stroke Cerebrovasc Dis. 2020;29:104987. doi: 10.1016/j.jstrokecerebrovasdis.2020.104987. [DOI] [PubMed] [Google Scholar]
- 26.Jacobs BS, Boden-Albala B, Lin I-F, Sacco RL. Stroke in the young in the northern Manhattan stroke study. Stroke. 2002;33:2789–2793. doi: 10.1161/01.STR.0000038988.64376.3A. [DOI] [PubMed] [Google Scholar]
- 27.Trimble B, Morgenstern LB. Stroke in minorities. Neurol Clin. 2008;26:1177–1190. doi: 10.1016/j.ncl.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jafari A, Rajabi A, Gholian-Aval M, Peyman N, Mahdizadeh M, Tehrani H. National, regional, and global prevalence of cigarette smoking among women/females in the general population: a systematic review and meta-analysis. Environ Health Prev Med. 2021;26:5. doi: 10.1186/s12199-020-00924-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sreeramareddy CT, Acharya K. Trends in prevalence of tobacco use by sex and socioeconomic status in 22 sub-Saharan African countries, 2003–2019. JAMA Netw Open. 2021;4:e2137820. doi: 10.1001/jamanetworkopen.2021.37820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wasay M, Kaul S, Menon B, et al. Ischemic stroke in young Asian women: risk factors, subtypes and outcome. Cerebrovasc Dis. 2010;30:418–422. doi: 10.1159/000317075. [DOI] [PubMed] [Google Scholar]
- 31.Li F, Yang L, Yang R, Xu W, Chen F-P, Li N, Zhang J-B. Ischemic stroke in young adults of northern China: characteristics and risk factors for recurrence. Eur Neurol. 2017;77:115–122. doi: 10.1159/000455093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brouwer J, Smaal JA, Emmer BJ, et al. Endovascular thrombectomy in young patients with stroke: a MR CLEAN registry study. Stroke. 2022;53:34–42. doi: 10.1161/STROKEAHA.120.034033. [DOI] [PubMed] [Google Scholar]
- 33.Kim JS. Moyamoya disease: epidemiology, clinical features, and diagnosis. J Stroke. 2016;18:2–11. doi: 10.5853/jos.2015.01627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Niu J-W, Gao S, Cui L-Y, Peng B, Zhu Y-C, Ni J, Zhou L-X, Yao M, Xu W-H. Intracranial atherosclerosis in Chinese young adult stroke patients. J Stroke Cerebrovasc Dis. 2014;23:1519–1523. doi: 10.1016/j.jstrokecerebrovasdis.2013.12.030. [DOI] [PubMed] [Google Scholar]
- 35.Yahya T, Jilani MH, Khan SU, et al. Stroke in young adults: current trends, opportunities for prevention and pathways forward. Am J Prev Cardiol. 2020;3:100085. doi: 10.1016/j.ajpc.2020.100085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Alebeek ME, Arntz RM, Ekker MS, Synhaeve NE, Maaijwee NA, Schoonderwaldt H, van der Vlugt MJ, van Dijk EJ, Rutten-Jacobs LC, de Leeuw F-E. Risk factors and mechanisms of stroke in young adults: the FUTURE study. J Cereb Blood Flow Metab. 2018;38:1631–1641. doi: 10.1177/0271678X17707138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leys D, Bandu L, Hénon H, Lucas C, Mounier-Vehier F, Rondepierre P, Godefroy O. Clinical outcome in 287 consecutive young adults (15 to 45 years) with ischemic stroke. Neurology. 2002;59:26–33. doi: 10.1212/WNL.59.1.26. [DOI] [PubMed] [Google Scholar]
- 38.Alsheikh-Ali AA, Thaler DE, Kent DM. Patent foramen ovale in cryptogenic stroke: incidental or pathogenic? Stroke. 2009;40:2349–2355. doi: 10.1161/STROKEAHA.109.547828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Handke M, Harloff A, Olschewski M, Hetzel A, Geibel A. Patent foramen ovale and cryptogenic stroke in older patients. N Engl J Med. 2007;357:2262–2268. doi: 10.1056/NEJMoa071422. [DOI] [PubMed] [Google Scholar]
- 40.Divišová P, Šaňák D, Král M, et al. Young cryptogenic ischemic stroke: a descriptive analysis of clinical and laboratory characteristics, outcomes and stroke recurrence. J Stroke Cerebrovasc Dis. 2020;29:105046. doi: 10.1016/j.jstrokecerebrovasdis.2020.105046. [DOI] [PubMed] [Google Scholar]
- 41.Martinez-Majander N, Artto V, Ylikotila P, et al. Association between migraine and cryptogenic ischemic stroke in young adults. Ann Neurol. 2021;89:242–253. doi: 10.1002/ana.25937. [DOI] [PubMed] [Google Scholar]
- 42.Tatlisumak T, Cucchiara B, Kuroda S, Kasner SE, Putaala J. Nontraumatic intracerebral haemorrhage in young adults. Nat Rev Neurol. 2018;14:237–250. doi: 10.1038/nrneurol.2018.17. [DOI] [PubMed] [Google Scholar]
- 43.Ruíz-Sandoval JL, Cantu C, Barinagarrementeria F. Intracerebral hemorrhage in young people: analysis of risk factors, location, causes, and prognosis. Stroke. 1999;30:537–541. doi: 10.1161/01.STR.30.3.537. [DOI] [PubMed] [Google Scholar]
- 44.George MG. Risk factors for ischemic stroke in younger adults. Stroke. 2020;51:729–735. doi: 10.1161/STROKEAHA.119.024156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parikh NS, Chatterjee A, Díaz I, Merkler AE, Murthy SB, Iadecola C, Navi BB, Kamel H. Trends in active cigarette smoking among stroke survivors in the United States, 1999 to 2018. Stroke. 2020;51:1656–1661. doi: 10.1161/STROKEAHA.120.029084. [DOI] [PubMed] [Google Scholar]
- 46.Markidan J, Cole JW, Cronin CA, Merino JG, Phipps MS, Wozniak MA, Kittner SJ. Smoking and risk of ischemic stroke in young men. Stroke. 2018;49:1276–1278. doi: 10.1161/STROKEAHA.117.018859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shah RS, Cole JW. Smoking and stroke: the more you smoke the more you stroke. Expert Rev Cardiovasc Ther. 2010;8:917–932. doi: 10.1586/erc.10.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pezzini A, Grassi M, Lodigiani C, et al. Predictors of migraine subtypes in young adults with ischemic stroke: the Italian project on stroke in young adults. Stroke. 2011;42:17–21. doi: 10.1161/STROKEAHA.110.592246. [DOI] [PubMed] [Google Scholar]
- 49.MacClellan LR, Giles W, Cole J, Wozniak M, Stern B, Mitchell BD, Kittner SJ. Probable migraine with visual aura and risk of ischemic stroke: the stroke prevention in young women study. Stroke. 2007;38:2438–2445. doi: 10.1161/STROKEAHA.107.488395. [DOI] [PubMed] [Google Scholar]
- 50.Aigner A, Grittner U, Rolfs A, Norrving B, Siegerink B, Busch MA. Contribution of established stroke risk factors to the burden of stroke in young adults. Stroke. 2017;48:1744–1751. doi: 10.1161/STROKEAHA.117.016599. [DOI] [PubMed] [Google Scholar]
- 51.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mszar R, Mahajan S, Valero-Elizondo J, Yahya T, Sharma R, Grandhi GR, Khera R, Virani SS, Lichtman J, Khan SU. Association between sociodemographic determinants and disparities in stroke symptom awareness among US young adults. Stroke. 2020;51:3552–3561. doi: 10.1161/STROKEAHA.120.031137. [DOI] [PubMed] [Google Scholar]
- 53.Bardugo A, Fishman B, Libruder C, et al. Body mass index in 1.9 million adolescents and stroke in young adulthood. Stroke. 2021;52:2043–2052. doi: 10.1161/STROKEAHA.120.033595. [DOI] [PubMed] [Google Scholar]
- 54.Jaakonmäki N, Zedde M, Sarkanen T, et al. Obesity and the risk of cryptogenic ischemic stroke in young adults. J Stroke Cerebrovasc Dis. 2022;31:106380. doi: 10.1016/j.jstrokecerebrovasdis.2022.106380. [DOI] [PubMed] [Google Scholar]
- 55.Mitchell AB, Cole JW, McArdle PF, Cheng Y-C, Ryan KA, Sparks MJ, Mitchell BD, Kittner SJ. Obesity increases risk of ischemic stroke in young adults. Stroke. 2015;46:1690–1692. doi: 10.1161/STROKEAHA.115.008940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sultan S, Elkind MSV. The growing problem of stroke among young adults. Curr Cardiol Rep. 2013;15:421. doi: 10.1007/s11886-013-0421-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sarecka-Hujar B, Sordyl J, Małecka-Tendera E, Kopyta I. Levels of lipid parameters in children with arterial ischemic stroke and headache: case-control study and meta-analysis. Brain Sci. 2021;11:417. doi: 10.3390/brainsci11040417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Menet R, Bernard M, ElAli A. Hyperlipidemia in stroke pathobiology and therapy: insights and perspectives. Front Physiol. 2018;9:488. doi: 10.3389/fphys.2018.00488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Talpur MTH, Katbar MT, Shabir KU, Shabir KU, Yaqoob U, Jabeen S, Zia D. Prevalence of dyslipidemia in young adults. Prof Med J. 2020;27:987–993. [Google Scholar]
- 60.Ruixing Y, Jinzhen W, Weixiong L, Yuming C, Dezhai Y, Shangling P. The environmental and genetic evidence for the association of hyperlipidemia and hypertension. J Hypertens. 2009;27:251–258. doi: 10.1097/HJH.0b013e32831bc74d. [DOI] [PubMed] [Google Scholar]
- 61.Gialeraki A, Valsami S, Pittaras T, Panayiotakopoulos G, Politou M. Oral contraceptives and HRT risk of thrombosis. Clin Appl Thromb Hemost. 2018;24:217–225. doi: 10.1177/1076029616683802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li F, Zhu L, Zhang J, He H, Qin Y, Cheng Y, Xie Z. Oral contraceptive use and increased risk of stroke: a dose–response meta-analysis of observational studies. Front Neurol. 2019;10:993. doi: 10.3389/fneur.2019.00993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kent DM, Ruthazer R, Weimar C, et al. An index to identify stroke-related vs incidental patent foramen ovale in cryptogenic stroke. Neurol. 2013;81:619–625. doi: 10.1212/WNL.0b013e3182a08d59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nedeltchev K, Wiedmer S, Schwerzmann M, Windecker S, Haefeli T, Meier B, Mattle HP, Arnold M. Sex differences in cryptogenic stroke with patent foramen ovale. Am Heart J. 2008;156:461–465. doi: 10.1016/j.ahj.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 65.Chen L, Deng W, Palacios I, Inglessis-Azuaje I, McMullin D, Zhou D, Lo EH, Buonanno F, Ning M. Patent foramen ovale (PFO), stroke and pregnancy. J Investig Med. 2016;64:992–1000. doi: 10.1136/jim-2016-000103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Putaala J. Ischemic stroke in the young: current perspectives on incidence, risk factors, and cardiovascular prognosis. Eur Stroke J. 2016;1:28–40. doi: 10.1177/2396987316629860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mas J-L, Derumeaux G, Guillon B, et al. Patent foramen ovale closure or anticoagulation vs. antiplatelets after stroke. N Engl J Med. 2017;377:1011–1021. doi: 10.1056/NEJMoa1705915. [DOI] [PubMed] [Google Scholar]
- 68.Kottoor SJ, Arora RR. Cryptogenic stroke: to close a patent foramen ovale or not to close? J Cent Nerv Syst Dis. 2018;10:1179573518819476. doi: 10.1177/1179573518819476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kamel H, Okin PM, Elkind MSV, Iadecola C. Atrial fibrillation and mechanisms of stroke: time for a new model. Stroke. 2016;47:895–900. doi: 10.1161/STROKEAHA.115.012004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Berthet K, Lavergne T, Cohen A, Guize L, Bousser MG, Le Heuzey JY, Amarenco P. Significant association of atrial vulnerability with atrial septal abnormalities in young patients with ischemic stroke of unknown cause. Stroke. 2000;31:398–403. doi: 10.1161/01.STR.31.2.398. [DOI] [PubMed] [Google Scholar]
- 71.Morseth B, Geelhoed B, Linneberg A, et al. Age-specific atrial fibrillation incidence, attributable risk factors and risk of stroke and mortality: results from the MORGAM Consortium. Open Heart. 2021;8:e001624. doi: 10.1136/openhrt-2021-001624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Šaňák D, Hutyra M, Král M, et al. Atrial fibrillation in young ischemic stroke patients: an underestimated cause? Eur Neurol. 2015;73:158–163. doi: 10.1159/000369793. [DOI] [PubMed] [Google Scholar]
- 73.Kuybu O, Amireh A, Davis D, Kelley RE, Javalkar V. Prevalence of ischemic stroke and atrial fibrillation in young patients with migraine national inpatient sample analysis. J Stroke Cerebrovasc Dis. 2020;29:104972. doi: 10.1016/j.jstrokecerebrovasdis.2020.104972. [DOI] [PubMed] [Google Scholar]
- 74.Øie LR, Kurth T, Gulati S, Dodick DW. Migraine and risk of stroke. J Neurol Neurosurg Psychiatry. 2020;91:593–604. doi: 10.1136/jnnp-2018-318254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ortiz A, Germain DP, Desnick RJ, et al. Fabry disease revisited: management and treatment recommendations for adult patients. Mol Genet Metab. 2018;123:416–427. doi: 10.1016/j.ymgme.2018.02.014. [DOI] [PubMed] [Google Scholar]
- 76.Kolodny E, Fellgiebel A, Hilz MJ, Sims K, Caruso P, Phan TG, Politei J, Manara R, Burlina A. Cerebrovascular involvement in Fabry disease: current status of knowledge. Stroke. 2015;46:302–313. doi: 10.1161/STROKEAHA.114.006283. [DOI] [PubMed] [Google Scholar]
- 77.Buechner S, Moretti M, Burlina AP, et al. Central nervous system involvement in Anderson-Fabry disease: a clinical and MRI retrospective study. J Neurol Neurosurg Psychiatry. 2008;79:1249–1254. doi: 10.1136/jnnp.2008.143693. [DOI] [PubMed] [Google Scholar]
- 78.Ghaffari-Rafi A, Ghaffari-Rafi S, Leon-Rojas J. Socioeconomic and demographic disparities of moyamoya disease in the United States. Clin Neurol Neurosurg. 2020;192:105719. doi: 10.1016/j.clineuro.2020.105719. [DOI] [PubMed] [Google Scholar]
- 79.Burke GM, Burke AM, Sherma AK, Hurley MC, Batjer HH, Bendok BR. Moyamoya disease: a summary. Neurosurg Focus. 2009;26:E11. doi: 10.3171/2009.1.FOCUS08310. [DOI] [PubMed] [Google Scholar]
- 80.Yasaka M, Minematsu K. Stroke in young adults in Japan. Rinsho Shinkeigaku. 2005;45:842–845. [PubMed] [Google Scholar]
- 81.Vanakker OM, Hemelsoet D, De Paepe A. Hereditary connective tissue diseases in young adult stroke: a comprehensive synthesis. Stroke Res Treat. 2011;2011:712903. doi: 10.4061/2011/712903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stack CA, Cole JW. The clinical approach to stroke in young adults. In: Dehkharghani S, editor. Stroke. Brisbane, AU: Exon Publications; 2021. p. 53–78. [PubMed]
- 83.Seshadri S, Beiser A, Pikula A, Himali JJ, Kelly-Hayes M, Debette S, DeStefano AL, Romero JR, Kase CS, Wolf PA. Parental occurrence of stroke and risk of stroke in their children: the Framingham study. Circulation. 2010;121:1304–1312. doi: 10.1161/CIRCULATIONAHA.109.854240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bevan S, Traylor M, Adib-Samii P, et al. Genetic heritability of ischemic stroke and the contribution of previously reported candidate gene and genomewide associations. Stroke. 2012;43:3161–3167. doi: 10.1161/STROKEAHA.112.665760. [DOI] [PubMed] [Google Scholar]
- 85.Cheng Y-C, Cole JW, Kittner SJ, Mitchell BD. Genetics of ischemic stroke in young adults. Circ Cardiovasc Genet. 2014;7:383–392. doi: 10.1161/CIRCGENETICS.113.000390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.MacClellan LR, Mitchell BD, Cole JW, Wozniak MA, Stern BJ, Giles WH, Brown DW, Sparks MJ, Kittner SJ. Familial aggregation of ischemic stroke in young women: the Stroke Prevention in Young Women Study. Genet Epidemiol. 2006;30:602–608. doi: 10.1002/gepi.20171. [DOI] [PubMed] [Google Scholar]
- 87.Marshall IJ, Wang Y, Crichton S, McKevitt C, Rudd AG, Wolfe CD. The effects of socioeconomic status on stroke risk and outcomes. The Lancet Neurol. 2015;14:1206–1218. doi: 10.1016/S1474-4422(15)00200-8. [DOI] [PubMed] [Google Scholar]
- 88.Kamin Mukaz D, Dawson E, Howard VJ, Cushman M, Higginbotham JC, Judd SE, Kissela BM, Safford MM, Soliman EZ, Howard G (2021) Rural/urban differences in the prevalence of stroke risk factors: a cross-sectional analysis from the REGARDS study. J Rural Health10.1111/jrh.12608 [DOI] [PMC free article] [PubMed]
- 89.Reshetnyak E, Ntamatungiro M, Pinheiro LC, Howard VJ, Carson AP, Martin KD, Safford MM. Impact of multiple social determinants of health on incident stroke. Stroke. 2020;51:2445–2453. doi: 10.1161/STROKEAHA.120.028530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Salaycik KJ, Kelly-Hayes M, Beiser A, Nguyen A-H, Brady SM, Kase CS, Wolf PA. Depressive symptoms and risk of stroke: the Framingham Study. Stroke. 2007;38:16–21. doi: 10.1161/01.STR.0000251695.39877.ca. [DOI] [PubMed] [Google Scholar]
- 91.Franc D, Šaňák D, Divišová P, et al. Socioeconomic status and lifestyle in young ischaemic stroke patients: a possible relationship to stroke recovery and risk of recurrent event. Cent Eur J Public Health. 2021;29:223–229. doi: 10.21101/cejph.a6697. [DOI] [PubMed] [Google Scholar]
- 92.Kuruvilla A, Bhattacharya P, Rajamani K, Chaturvedi S. Factors associated with misdiagnosis of acute stroke in young adults. J Stroke Cerebrovasc Dis. 2011;20:523–527. doi: 10.1016/j.jstrokecerebrovasdis.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 93.León Cejas L, Mazziotti J, Zinnerman A, Nofal P, Fernández Pardal M, Bonardo P, Reisin R. Misdiagnosis of acute ischemic stroke in young patients. Medicina (B Aires) 2019;79:90–94. [PubMed] [Google Scholar]
- 94.Singhal AB, Biller J, Elkind MS, Fullerton HJ, Jauch EC, Kittner SJ, Levine DA, Levine SR. Recognition and management of stroke in young adults and adolescents. Neurol. 2013;81:1089–1097. doi: 10.1212/WNL.0b013e3182a4a451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bhattacharya P, Nagaraja N, Rajamani K, Madhavan R, Santhakumar S, Chaturvedi S. Early use of MRI improves diagnostic accuracy in young adults with stroke. J Neurol Sci. 2013;324:62–64. doi: 10.1016/j.jns.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 96.Jauch EC, Saver JL, Adams HP, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:870–947. doi: 10.1161/STR.0b013e318284056a. [DOI] [PubMed] [Google Scholar]
- 97.Markus HS, Levi C, King A, Madigan J, Norris J, Cervical Artery Dissection in Stroke Study (CADISS) Investigators Antiplatelet therapy vs anticoagulation therapy in cervical artery dissection: the Cervical Artery Dissection in Stroke Study (CADISS) randomized clinical trial final results. JAMA Neurol. 2019;76:657–664. doi: 10.1001/jamaneurol.2019.0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.van Dongen MME, Aarnio K, Martinez-Majander N, Pirinen J, Sinisalo J, Lehto M, Kaste M, Tatlisumak T, de Leeuw F-E, Putaala J. Use of statins after ischemic stroke in young adults and its association with long-term outcome. Stroke. 2019;50:3385–3392. doi: 10.1161/STROKEAHA.119.026992. [DOI] [PubMed] [Google Scholar]
- 99.Brouwer-Goossensen D, den Hertog HM, Mastenbroek-de Jong MA, van Gemert-Pijnen LJEWC, Taal E. Patient perspectives on health-related behavior change after transient ischemic attack or ischemic stroke. Brain Behav. 2021;11:e01993. doi: 10.1002/brb3.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Biswas S, Andrianopoulos N, Duffy SJ, et al. Impact of socioeconomic status on clinical outcomes in patients with ST-segment-elevation myocardial infarction. Circ Cardiovasc Qual Outcomes. 2019;12:e004979. doi: 10.1161/CIRCOUTCOMES.118.004979. [DOI] [PubMed] [Google Scholar]
- 101.Bernardo F, Rebordão L, Machado S, Salgado V, Pinto AN. In-hospital and long-term prognosis after spontaneous intracerebral hemorrhage among young adults aged 18–65 years. J Stroke Cerebrovasc Dis. 2019;28:104350. doi: 10.1016/j.jstrokecerebrovasdis.2019.104350. [DOI] [PubMed] [Google Scholar]
- 102.Balci K, Utku U, Asil T, Celik Y. Ischemic stroke in young adults: risk factors, subtypes, and prognosis. Neurologist. 2011;17:16–20. doi: 10.1097/NRL.0b013e3181f954a7. [DOI] [PubMed] [Google Scholar]
- 103.Khan SU, Khan MZ, Khan MU, et al. Clinical and economic burden of stroke among young, midlife, and older adults in the United States, 2002–2017. Mayo Clin Proc Innov Qual Outcomes. 2021;5:431–441. doi: 10.1016/j.mayocpiqo.2021.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rutten-Jacobs LCA, Arntz RM, Maaijwee NAM, Schoonderwaldt HC, Dorresteijn LD, van Dijk EJ, de Leeuw F-E. Long-term mortality after stroke among adults aged 18 to 50 years. JAMA. 2013;309:1136–1144. doi: 10.1001/jama.2013.842. [DOI] [PubMed] [Google Scholar]
- 105.Campo M, Toglia J, Jaywant A, O’Dell MW. Young individuals with stroke in rehabilitation: a cohort study. Int J Rehabil Res. 2021;44:314–322. doi: 10.1097/MRR.0000000000000491. [DOI] [PubMed] [Google Scholar]
- 106.Putaala J, Strbian D, Mustanoja S, Haapaniemi E, Kaste M, Tatlisumak T. Functional outcome in young adult ischemic stroke: impact of lipoproteins. Acta Neurol Scand. 2013;127:61–69. doi: 10.1111/j.1600-0404.2012.01683.x. [DOI] [PubMed] [Google Scholar]
- 107.Tan E, Gao L, Collier JM, Ellery F, Dewey HM, Bernhardt J, Moodie M. The economic and health burden of stroke among younger adults in Australia from a societal perspective. BMC Public Health. 2022;22:218. doi: 10.1186/s12889-021-12400-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Krishnamurthi RV, Moran AE, Feigin VL, et al. Stroke prevalence, mortality and disability-adjusted life years in adults aged 20–64 years in 1990–2013: data from the global burden of disease 2013 study. Neuroepidemiol. 2015;45:190–202. doi: 10.1159/000441098. [DOI] [PubMed] [Google Scholar]
- 109.Jarvis HL, Brown SJ, Price M, Butterworth C, Groenevelt R, Jackson K, Walker L, Rees N, Clayton A, Reeves ND. Return to employment after stroke in young adults: how important is the speed and energy cost of walking? Stroke. 2019;50:3198–3204. doi: 10.1161/STROKEAHA.119.025614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pinter D, Enzinger C, Gattringer T, et al. Prevalence and short-term changes of cognitive dysfunction in young ischaemic stroke patients. Eur J Neurol. 2019;26:727–732. doi: 10.1111/ene.13879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Schaapsmeerders P, Maaijwee NAM, van Dijk EJ, Rutten-Jacobs LCA, Arntz RM, Schoonderwaldt HC, Dorresteijn LDA, Kessels RPC, de Leeuw F-E. Long-term cognitive impairment after first-ever ischemic stroke in young adults. Stroke. 2013;44:1621–1628. doi: 10.1161/STROKEAHA.111.000792. [DOI] [PubMed] [Google Scholar]
- 112.Maaijwee Na MM, Tendolkar I, Rutten-Jacobs LCA, Arntz RM, Schaapsmeerders P, Dorresteijn LD, Schoonderwaldt HC, van Dijk EJ, de Leeuw FE. Long-term depressive symptoms and anxiety after transient ischaemic attack or ischaemic stroke in young adults. Eur J Neurol. 2016;23:1262–1268. doi: 10.1111/ene.13009. [DOI] [PubMed] [Google Scholar]
- 113.Sebastian S, Stein LK, Dhamoon MS. Infection as a stroke trigger. Stroke. 2019;50:2216–2218. doi: 10.1161/STROKEAHA.119.025872. [DOI] [PubMed] [Google Scholar]
- 114.Paganini-Hill A, Lozano E, Fischberg G, Perez Barreto M, Rajamani K, Ameriso SF, Heseltine PNR, Fisher M. Infection and risk of ischemic stroke: differences among stroke subtypes. Stroke. 2003;34:452–457. doi: 10.1161/01.STR.0000053451.28410.98. [DOI] [PubMed] [Google Scholar]
- 115.Qureshi AI, Baskett WI, Huang W, et al. Acute ischemic stroke and COVID-19. Stroke. 2021;52:905–912. doi: 10.1161/STROKEAHA.120.031786. [DOI] [PMC free article] [PubMed] [Google Scholar]