Abstract
Background
About 10–20% of patients with anal squamous cell carcinoma (SCCa) present with metastatic disease and are usually treated with systemic chemotherapy. However, primary tumor control is crucial as local failure is associated with significant morbidity. Using the largest cohort to date, we report the impact of local therapy on survival among patients with metastatic anal SCCa.
Methods
Data were collected from US hospitals that contributed to the National Cancer Database (NCDB) between 2004 and 2015. Patients who did not receive palliative systemic chemotherapy were excluded from analysis. Univariate (UVA) and multivariable analyses (MVA) were performed to identify factors associated with patient outcome. Kaplan-Meier analysis and Cox proportional hazards models were used to evaluate the association between tumor/patient characteristics and overall survival (OS).
Results
A total of 1,160 patients were identified over the 12 years of study. Median age was 57 years. Majority were female (64.9%), non-Hispanic Whites (79.1%) and had Charlson-Deyo Score of 0 (83.6%). Most common metastatic sites were liver (25.9%), lung (11.6%) and bone (8.5%). More than 79% of the patients had received radiation to the primary site, and 10.4% underwent surgical resection for local control. Use of local therapy correlated closely with OS on MVA (HR 0.66; 0.55–0.79; P<0.001), with a 12-month and 5-year OS rates of 72.8% and 25.7% respectively, compared with 61.1% and 14.6% for patients treated with chemotherapy only. Poor prognostic factors included male gender (HR 1.44; 1.24–1.67; P<0.001), age >70 years (HR 1.28; 1.02–1.62; P=0.034), lack of health insurance (HR 1.32; 1.02–1.71; P=0.034), and cloacogenic zone location (HR 4.02; 1.43–11.30; P=0.008). There was no benefit from abdominoperineal resection (mOS =19.7 months; HR 1.05; 0.48–2.29; P=0.909), but both local resection of the primary (mOS =24.8 months, HR 0.48; 0.29–0.80; P=0.005) and palliative radiation (mOS =22.6 months; HR 0.66; 0.55–0.79; P<0.001) were associated with improved OS.
Conclusions
In addition to systemic therapy, resection of the primary tumor or palliative radiation improved OS in patients with anal SCCa. Patients unlikely to benefit from local control were those >70 years of age, male, lack of health insurance and cloacogenic carcinoma.
Keywords: Metastatic anal cancer, local therapy, surgical resection, palliative radiation, overall survival
Introduction
Anal cancer incidence has been steadily increasing in the US over the past 4 decades, with reported incidence of 9,090 cases in 2021—an estimated 3,020 men and 6,070 women (1,2). The strongest causal relationship for anal cancer is infection with HPV, mainly HPV-16, as more than 90% of anal cancers are associated with HPV infection (3). The HPV vaccine impact on the primary prevention of SCCA in the US may not be obvious for another two decades, since decreased incidence lags behind infection prevention. In addition, patients infected with HIV have >40 times increased incidence of anal cancer compared with the general population (4). Interestingly, unlike other HIV-associated cancers, anal cancer incidence in patients infected with HIV has not declined since the introduction of highly active antiretroviral therapy (HAART) (5,6): one possible explanation is that HAART does not cause regression of anal intraepithelial neoplasia (AIN), the presumed precursor of invasive anal cancer (7,8). In addition, the duration of immune dysfunction from HIV infection to anal cancer diagnosis is longer in patients who have developed anal cancer in the era of HAART (7). Hence, the speculation is that the duration rather than the severity of immune suppression determines the risk of invasive anal cancer (6).
About 10–20% of patients with anal squamous cell carcinoma (SCCa) present with metastatic disease and are usually treated with palliative systemic chemotherapy. Results from the phase II InterAACT trial, the first international prospective randomized trial in advanced anal cancer, provide strong evidence for the use of carboplatin and paclitaxel as the first-line treatment of patients with metastatic anal cancer (9). The study enrolled 91 patients between December 2013 and November 2017 in Australia, Germany, Norway, the United Kingdom, and the United States. The overall response rate (ORR) for cisplatin plus 5-fluorouracil (5FU) was 57% vs. 59% for carboplatin plus paclitaxel. However, cisplatin-5FU was associated with significantly more adverse events compared to carboplatin-paclitaxel (62% vs. 36%, P=0.016) and lower overall survival (12.3 vs. 20 months, P=0.014). Due to biologic implications, the role of immunotherapy has been actively studied of the recent years. PD-L1 checkpoint inhibitors nivolumab and pembrolizumab have demonstrated encouraging activity in heavily pre-treated advanced anal SCC. Phase Ib KEYNOTE-028 trial had 20 different cohorts of previously treated patients with PD-L1-positive advanced tumors. In the anal cancer cohort, 74% (32 pts) of patients had PD-L1 expression ≥1% in tumor cells. ORR was 17%, and 10 patients showed stable disease. Median PFS and OS were 3.0 and 9.3 months, respectively. Pembrolizumab in SCCA patients was deemed safe, without unexpected toxicities. Integration of immunotherapy with anti-EGFR (CARACAS phase II study) or antiangiogenics (as recently proven in hepatocellular carcinoma by IMBRAVE 150 trial) have also been evaluated in clinical trials which may bring practice changing developments in the systemic treatment of anal SCC. Lastly, vaccines and adoptive T-cell therapies may also be useful in the near future (10).
Local control of the primary tumor is important since local failure is associated with significant morbidity such as pain, severe discomfort and bleeding (11). However, there are limited guidelines and published data on the impact of local therapy on survival in metastatic anal cancer. Using the largest cohort to date, we report the impact of local therapy on survival among patients with metastatic anal SCCa. We present the following article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-125/rc).
Methods
Data
We conducted a retrospective analysis of patients with metastatic anal SCCa using the National Cancer Database (NCDB). The NCDB is hospital-based cancer registry that collects and reports prospective data on over 70% of cancer incidents diagnosed at more than 1500 Commission on Cancer (CoC)-accredited centers in the United States. The NCDB was established in 1989 as a nationwide, facility-based, comprehensive clinical surveillance resource oncology data in the US. The staging system used was in accordance with the AJCC Staging Manual 6th edition for data between 2004 and 2009, and the 7th edition from 2010 and 2015. Since NCDB contains fully de-identified data, this study was conducted under waiver of consent guidelines of Emory University institutional review board (IRB). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Study patients
We identified all patients with anal SCCa reported between 2004 and 2015. Primary inclusion criteria included squamous cell histology and clinical stage IV metastatic disease. We excluded patients with carcinoma in situ, patients who had an unknown or no metastatic status at diagnosis, lack of pathologic confirmation or patients who did not receive palliative systemic chemotherapy.
Variables
Variables of interest included demographic, clinical, and treatment data. Charlson-Deyo index provided measure of comorbid conditions (index range, 0 to ≥2; higher values indicate greater burden of comorbid conditions). Additional clinical data captured and analyzed included age at diagnosis, sex, race, insurance status, primary tumor site, year at diagnosis, age at diagnosis, histology, grade, surgical margins, tumor size, regional lymph nodes examined, metastatic sites, radiation treatment and type of surgery.
Statistical analysis
Patient demographic, disease, and treatment characteristics were compared between patient who received local therapy and those who did not. ANOVA tests and Chi-square tests were used for numeric and categorical variables. Univariate (UVA) and multivariate analyses (MVA) with Cox proportional hazards models were used to assess the associations between tumor/patient characteristics and overall survival (OS). Kaplan-Meier analysis with log-rank tests were generated to depict and compare OS between interested patient characteristic categories.
Results
Demographic characteristics
Between 2004 and 2015, a total of 1,160 patients with primary diagnosis of metastatic SCCa were identified from the database, included and analyzed in this study. Patients who have recurrent metastatic disease were not included in this study. Patient and treatment characteristics are summarized in Table 1. Median age at diagnosis was 57 years. Majority of the patients were female (65%), younger than 60 years (59%) and had Charlson-Deyo Score of 0 (83.6%). Non-Hispanic Whites were 79% of the patients, followed by non-Hispanic Blacks at approximately 12%. Nine hundred forty-eight (82%) patients received local therapy: 827 (71%) received radiation only, 95 (8%) patients underwent local resection, and 26 (2%) patients underwent abdominoperineal (radical) resection (APR). Most patients received local therapy concurrently with chemotherapy (35%), while others were before (18%) or after chemotherapy (23%). Only 212 (18%) patients did not receive any local therapy. Almost all the patients, including those who did (96%) or did not (98%) receive local therapy, had a documented clinical cM1 disease at diagnosis and received systemic therapy for their metastatic disease. The most common metastatic sites were liver (25.9%), lung (11.6%) and bone (8.5%). Only a minority of patients (5%) had multiple metastatic disease sites at diagnosis.
Table 1. Demographics of study population.
| Covariate | Statistics | Level | If received local therapy | Parametric P value* |
|
|---|---|---|---|---|---|
| No (N=212) | Yes (N=948) | ||||
| Sex | N (Col %) | Male | 59 (27.83) | 348 (36.71) | 0.014 |
| N (Col %) | Female | 153 (72.17) | 600 (63.29) | ||
| Age at diagnosis, years | N (Col %) | <60 | 124 (58.49) | 562 (59.28) | 0.033 |
| N (Col %) | 60–70 | 71 (33.49) | 257 (27.11) | ||
| N (Col %) | >70 | 17 (8.02) | 129 (13.61) | ||
| Race | N (Col %) | Non-Hispanic White | 169 (79.72) | 748 (78.9) | 0.155 |
| N (Col %) | Non-Hispanic Black | 17 (8.02) | 120 (12.66) | ||
| N (Col %) | Hispanic | 18 (8.49) | 57 (6.01) | ||
| N (Col %) | Other | 6 (2.83) | 14 (1.48) | ||
| N (Col %) | Unknown | 2 (0.94) | 9 (0.95) | ||
| Year of diagnosis | N (Col %) | ≥2004, ≤2008 | 53 (25) | 264 (27.85) | 0.358 |
| N (Col %) | >2008, ≤2011 | 46 (21.7) | 240 (25.32) | ||
| N (Col %) | >2011, ≤2014 | 80 (37.74) | 325 (34.28) | ||
| N (Col %) | >2014, ≤2015 | 33 (15.57) | 119 (12.55) | ||
| Primary payor | N (Col %) | Not insured | 17 (8.02) | 92 (9.7) | 0.163 |
| N (Col %) | Private | 108 (50.94) | 421 (44.41) | ||
| N (Col %) | Medicaid/Medicare/Other Government | 81 (38.21) | 420 (44.3) | ||
| N (Col %) | Unknown | 6 (2.83) | 15 (1.58) | ||
| Median income quartiles 2000 | N (Col %) | < $30,000 | 33 (15.57) | 152 (16.03) | 0.509 |
| N (Col %) | $30,000–$35,999 | 37 (17.45) | 195 (20.57) | ||
| N (Col %) | $36,000–$45,999 | 54 (25.47) | 267 (28.16) | ||
| N (Col %) | ≥ $46,000 | 80 (37.74) | 300 (31.65) | ||
| N (Col %) | Not Available | 8 (3.77) | 34 (3.59) | ||
| Urban/rural 2003 | N (Col %) | Metro | 169 (79.72) | 792 (83.54) | 0.543 |
| N (Col %) | Urban | 32 (15.09) | 121 (12.76) | ||
| N (Col %) | Rural | 6 (2.83) | 17 (1.79) | ||
| N (Col %) | Unknown | 5 (2.36) | 18 (1.9) | ||
| Histology | N (Col %) | Squamous cell carcinoma | 197 (92.92) | 872 (91.98) | 0.645 |
| N (Col %) | Squamous cell carcinoma keratinizing | 15 (7.08) | 76 (8.02) | ||
| Primary site | N (Col %) | C210-Anus, NOS | 101 (47.64) | 397 (41.88) | 0.372 |
| N (Col %) | C211-Anal canal | 84 (39.62) | 418 (44.09) | ||
| N (Col %) | C212-Cloacogenic zone | 0 (0) | 4 (0.42) | ||
| N (Col %) | C218-Overlapping lesion of rectum/anal canal | 27 (12.74) | 129 (13.61) | ||
| Tumor size | N (Col %) | No mass or tumor found | 1 (0.47) | 2 (0.21) | 0.004 |
| N (Col %) | <0.5 cm | 51 (24.06) | 268 (28.27) | ||
| N (Col %) | ≥0.5 cm | 66 (31.13) | 379 (39.98) | ||
| N (Col %) | Unknown, size not stated, microscopic focus | 94 (44.34) | 299 (31.54) | ||
| Surgical margins | N (Col %) | Yes | 0 (0) | 56 (5.91) | <0.001 |
| N (Col %) | No | 0 (0) | 36 (3.8) | ||
| N (Col %) | Not available | 212 (100) | 856 (90.3) | ||
| Grade | N (Col %) | Well differentiated, differentiated, NOS | 3 (1.42) | 32 (3.38) | 0.277 |
| N (Col %) | Moderately differentiated | 67 (31.6) | 327 (34.49) | ||
| N (Col %) | Poorly differentiated/anaplastic | 81 (38.21) | 356 (37.55) | ||
| N (Col %) | Cell type not determined/stated | 61 (28.77) | 233 (24.58) | ||
| Charlson-Deyo Score | N (Col %) | 0 | 174 (82.08) | 796 (83.97) | 0.090 |
| N (Col %) | 1 | 29 (13.68) | 89 (9.39) | ||
| N (Col %) | 2+ | 9 (4.25) | 63 (6.65) | ||
| Surgical resection | N (Col %) | No | 212 (100) | 827 (87.24) | <0.001 |
| N (Col %) | Yes | 0 (0) | 121 (12.76) | ||
| Radiation therapy | N (Col %) | No | 212 (100) | 23 (2.43) | <0.001 |
| N (Col %) | Yes | 0 (0) | 925 (97.57) | ||
| Type of surgery | N (Col %) | No | 212 (100) | 827 (87.24) | <0.001 |
| N (Col %) | Local | 0 (0) | 95 (10.02) | ||
| N (Col %) | Radical | 0 (0) | 26 (2.74) | ||
| Overall survival (months) | N | 212 | 948 | 0.004 | |
| Mean | 22.01 | 27.81 | |||
| Median | 15.02 | 18.4 | |||
| Min | 0.33 | 0.66 | |||
| Max | 146.66 | 160.3 | |||
| Std. Dev. | 23.11 | 26.78 | |||
| Age at diagnosis | N | 212 | 948 | 0.365 | |
| Mean | 57.2 | 57.95 | |||
| Median | 57.5 | 57 | |||
| Min | 25 | 25 | |||
| Max | 80 | 90 | |||
| Std. Dev. | 9.69 | 11.12 | |||
*, The parametric P value is calculated by ANOVA for numerical covariates and chi-square test for categorical covariates. Col %, percentages by column; NOS, not otherwise specified; ANOVA, analysis of variance.
Impact of local therapy
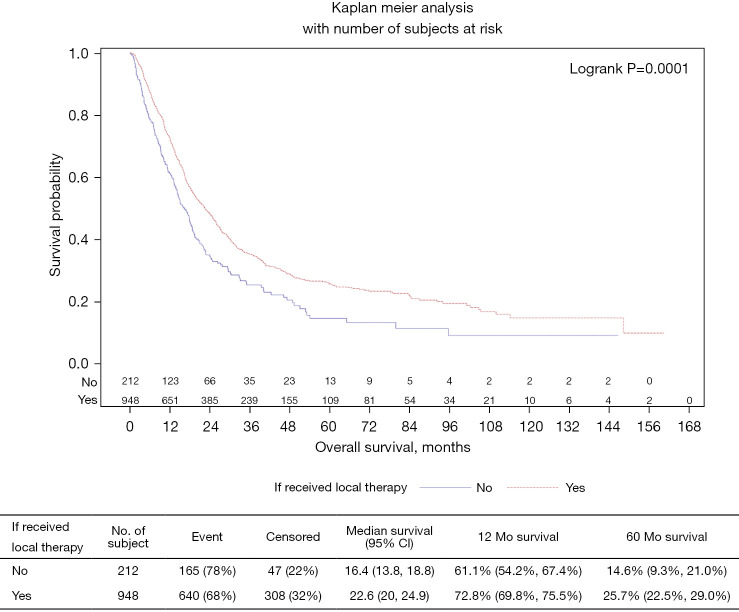
On univariate analysis, patients who received local therapy (N=948; 82%) were more likely to be alive (32%) compared to patients who did not receive local therapy (N=212; 18%; P=0.003). The majority of patients who received local therapy had clinical staging of cT2 or higher (77%) and cN2 or higher (57%). Only 13% of patients who received local therapy underwent surgical resection. Among those who received surgical resection, the majority of patients underwent local resection (78%) as opposed to radical or abdominoperineal resection (22%). Median overall survival (mOS) of patients who received local therapy was 22.6 months (95% CI: 20–24.9) vs. 16.4 months (95% CI: 13.8–18.8) for patients who did not receive local therapy (P=0.001) (Figure 1).
Figure 1.
Local therapy vs. no local therapy/systemic chemotherapy only.
Outcomes related to modality of local therapy
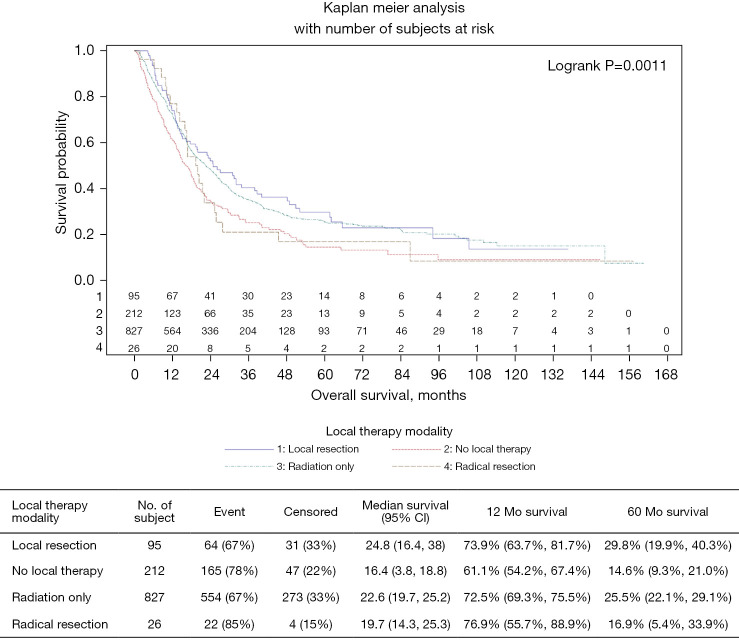
The median OS of patients who received local surgical resection was 24.8 months (95% CI: 16.4–38), 22.6 months (95% CI: 19.7–25.2) for patients treated with radiation only, 19.7 months (95% CI: 14.3–25.3) for radical surgical resection and 16.4 months (95% CI: 13.8–18.8) for patients who did not receive any local therapy in addition to systemic chemotherapy (P=0.0011; Figure 2). The 5-year overall survival (OS) of patients who underwent local resection or radiation therapy (RT) only were 29.8% and 25.5% respectively, compared to patients who underwent radical resection or no local therapy (16.9% and 14.6% respectively). Patients treated with local resection had a 1-year OS of 73.9% (95% CI: 63.7–81.7%), while those who received radiation only or no local therapy were 72.5% (95% CI: 69.3–75.5%) and 61.1% (95% CI: 54.2–67.4%) respectively. Interestingly, the one-year OS for patients who underwent radical resection/APR was 76.9% (95% CI: 55.7–88.9%). The vast majority of patients who had local therapy, regardless of modality of treatment, were younger than 60 years (Table 2). Overall Survival was the lowest among patients with multiple metastatic sites at 11.1 months compared to patients with single metastatic site involvement: mOS was 14.9 months for Lung metastasis followed by 17.3 months for liver metastasis and 17.5 months for bone metastasis.
Figure 2.
Modality of local therapy vs. systemic chemotherapy only.
Table 2. Univariate association with local therapy modality.
| Covariate | Statistics | Level | Local therapy modality | Parametric P value* | |||
|---|---|---|---|---|---|---|---|
| No local therapy, N=212 | Radiation only, N=827 | Local resection, N=95 | Radical resection, N=26 | ||||
| Age at diagnosis, years | N (Col %) | <60 | 124 (58.49) | 479 (57.92) | 69 (72.63) | 14 (53.85) | 0.022 |
| N (Col %) | 60–70 | 71 (33.49) | 232 (28.05) | 17 (17.89) | 8 (30.77) | ||
| N (Col %) | >70 | 17 (8.02) | 116 (14.03) | 9 (9.47) | 4 (15.38) | ||
| Sex | N (Col %) | Male | 59 (27.83) | 286 (34.58) | 49 (51.58) | 13 (50.00) | <0.001 |
| N (Col %) | Female | 153 (72.17) | 541 (65.42) | 46 (48.42) | 13 (50.00) | ||
| Race | N (Col %) | Non-Hispanic White | 169 (79.72) | 656 (79.32) | 71 (74.74) | 21 (80.77) | 0.032 |
| N (Col %) | Non-Hispanic Black | 17 (8.02) | 98 (11.85) | 20 (21.05) | 2 (7.69) | ||
| N (Col %) | Hispanic | 18 (8.49) | 53 (6.41) | 3 (3.16) | 1 (3.85) | ||
| N (Col %) | Other | 6 (2.83) | 11 (1.33) | 1 (1.05) | 2 (7.69) | ||
| N (Col %) | Unknown | 2 (0.94) | 9 (1.09) | 0 (0) | 0 (0) | ||
| Primary payor | N (Col %) | Not insured | 17 (8.02) | 81 (9.79) | 9 (9.47) | 2 (7.69) | 0.255 |
| N (Col %) | Private | 108 (50.94) | 356 (43.05) | 52 (54.74) | 13 (50) | ||
| N (Col %) | Medicaid/Medicare/Other Government | 81 (38.21) | 377 (45.59) | 32 (33.68) | 11 (42.31) | ||
| N (Col %) | Unknown | 6 (2.83) | 13 (1.57) | 2 (2.11) | 0 (0) | ||
| Median income quartiles 2000 | N (Col %) | < $30,000 | 33 (15.57) | 134 (16.20) | 16 (16.84) | 2 (7.69) | 0.085 |
| N (Col %) | $30,000–$35,999 | 37 (17.45) | 172 (20.80) | 23 (24.21) | 0 (0) | ||
| N (Col %) | $36,000–$45,999 | 54 (25.47) | 239 (28.90) | 17 (17.89) | 11 (42.31) | ||
| N (Col %) | $46,000+ | 80 (37.74) | 253 (30.59) | 35 (36.84) | 12 (46.15) | ||
| N (Col %) | Not Available | 8 (3.77) | 29 (3.51) | 4 (4.21) | 1 (3.85) | ||
| Urban/Rural 2003 |
N (Col %) | Metro | 169 (79.72) | 685 (82.83) | 82 (86.32) | 25 (96.15) | 0.370 |
| N (Col %) | Urban | 32 (15.09) | 113 (13.66) | 8 (8.42) | 0 (0) | ||
| N (Col %) | Rural | 6 (2.83) | 14 (1.69) | 3 (3.16) | 0 (0) | ||
| N (Col %) | Unknown | 5 (2.36) | 15 (1.81) | 2 (2.11) | 1 (3.85) | ||
| Year of diagnosis | N (Col %) | ≥2004, ≤2008 | 53 (25) | 229 (27.69) | 25 (26.32) | 10 (38.46) | 0.249 |
| N (Col %) | >2008, ≤2011 | 46 (21.7) | 200 (24.18) | 33 (34.74) | 7 (26.92) | ||
| N (Col %) | >2011, ≤2014 | 80 (37.74) | 290 (35.07) | 27 (28.42) | 8 (30.77) | ||
| N (Col %) | >2014, ≤2015 | 33 (15.57) | 108 (13.06) | 10 (10.53) | 1 (3.85) | ||
| Histology | N (Col %) | Squamous cell carcinoma | 197 (92.92) | 763 (92.26) | 86 (90.53) | 23 (88.46) | 0.795 |
| N (Col %) | Squamous cell carcinoma keratinizing | 15 (7.08) | 64 (7.74) | 9 (9.47) | 3 (11.54) | ||
| Primary site | N (Col %) | C210-Anus, NOS | 101 (47.64) | 347 (41.96) | 42 (44.21) | 8 (30.77) | 0.024 |
| N (Col %) | C211-Anal canal | 84 (39.62) | 366 (44.26) | 44 (46.32) | 8 (30.77) | ||
| N (Col %) | C212-Cloacogenic zone | 0 (0) | 4 (0.48) | 0 (0) | 0 (0) | ||
| N (Col %) | C218-Overlapping lesion of rectum/anal canal | 27 (12.74) | 110 (13.3) | 9 (9.47) | 10 (38.46) | ||
| Tumor size, cm |
N (Col %) | No mass or tumor found | 1 (0.47) | 2 (0.24) | 0 (0) | 0 (0) | 0.002 |
| N (Col %) | <0.5 | 51 (24.06) | 222 (26.84) | 39 (41.05) | 7 (26.92) | ||
| N (Col %) | ≥0.5 | 66 (31.13) | 328 (39.66) | 38 (40.0) | 13 (50.0) | ||
| N (Col %) | Unknown, size not stated, microscopic focus | 94 (44.34) | 275 (33.25) | 18 (18.95) | 6 (23.08) | ||
| Surgical margins | N (Col %) | Yes | 0 (0) | 0 (0) | 50 (52.63) | 6 (23.08) | <0.001 |
| N (Col %) | No | 0 (0) | 0 (0) | 20 (21.05) | 16 (61.54) | ||
| N (Col %) | Not Available | 212 (100.00) | 827 (100.00) | 25 (26.32) | 4 (15.38) | ||
| Grade | N (Col %) | Well differentiated, differentiated, NOS | 3 (1.42) | 28 (3.39) | 4 (4.21) | 0 (0) | 0.244 |
| N (Col %) | Moderately differentiated | 67 (31.6) | 281 (33.98) | 35 (36.84) | 11 (42.31) | ||
| N (Col %) | Poorly differentiated/anaplastic | 81 (38.21) | 304 (36.76) | 42 (44.21) | 10 (38.46) | ||
| N (Col %) | Cell type not determined/stated | 61 (28.77) | 214 (25.88) | 14 (14.74) | 5 (19.23) | ||
| Charlson-Deyo Score | N (Col %) | 0 | 174 (82.08) | 693 (83.8) | 79 (83.16) | 24 (92.31) | 0.059 |
| N (Col %) | 1 | 29 (13.68) | 83 (10.04) | 5 (5.26) | 1 (3.85) | ||
| N (Col %) | 2+ | 9 (4.25) | 51 (6.17) | 11 (11.58) | 1 (3.85) | ||
| Surgical resection | N (Col %) | No | 212 (100.0) | 827 (100.0) | 0 (0) | 0 (0) | <0.001 |
| N (Col %) | Yes | 0 (0) | 0 (0) | 95 (100.0) | 26 (100.0) | ||
| Radiation therapy | N (Col %) | No | 212 (100.00) | 0 (0) | 16 (16.84) | 7 (26.92) | <0.001 |
| N (Col %) | Yes | 0 (0) | 827 (100.00) | 79 (83.16) | 19 (73.08) | ||
| Type of surgery | N (Col %) | No | 212 (100.00) | 827 (100.00) | 0 (0) | 0 (0) | <0.001 |
| N (Col %) | Local | 0 (0) | 0 (0) | 95 (100.00) | 0 (0) | ||
| N (Col %) | Radical | 0 (0) | 0 (0) | 0 (0) | 26 (100.00) | ||
| Overall survival (months) | N | 212 | 827 | 95 | 26 | 0.019 | |
| Mean | 22.01 | 27.44 | 30.89 | 28.23 | |||
| Median | 15.02 | 18.3 | 19.75 | 19.7 | |||
| Min | 0.33 | 0.66 | 4.04 | 1.81 | |||
| Max | 146.66 | 160.3 | 136.71 | 157.11 | |||
| Std. Dev. | 23.11 | 26.43 | 28.46 | 31.74 | |||
| Age at diagnosis | N | 212 | 827 | 95 | 26 | 0.005 | |
| Mean | 57.2 | 58.33 | 54.28 | 59.04 | |||
| Median | 57.5 | 58 | 54 | 58 | |||
| Min | 25 | 25 | 32 | 42 | |||
| Max | 80 | 90 | 76 | 88 | |||
| Std. Dev. | 9.69 | 11.15 | 10.17 | 11.3 | |||
*, The parametric P value is calculated by ANOVA for numerical covariates and chi-square test for categorical covariates. Col %, percentages by column; NOS, not otherwise specified; ANOVA, analysis of variance.
Factors associated with survival
Univariate association showed inferior OS in patients who did not receive any local therapy (HR 1.40; 95% CI: 1.18–1.66; P<0.001) (Table 3) while there was a statistically significant survival benefit for patients who underwent local resection (HR 0.66; 95% CI: 0.49–0.88; P=0.004) including radiation treatment (HR 0.72; 0.60–0.86; P<0.001). On multivariate analysis, the survival benefit for patients who received local therapy remained statistically significant (HR 0.66; 0.55–0.79; P<0.001). Poor prognostic factors included male gender (HR 1.44; 95% CI: 1.24–1.67; P<0.001), age >70 years (HR 1.28; 95% CI: 1.02–1.62; P=0.034), lack of health insurance (HR 1.32; 95% CI: 1.02–1.71; P=0.034), and Cloacogenic zone primary location (HR 4.02; 95% CI: 1.43–11.30; P=0.008).
Table 3. Univariate association with overall survival (from diagnosis).
| Covariate | Level | N | Overall survival (months) | ||
|---|---|---|---|---|---|
| Hazard ratio (95% CI) | HR P value | Log-rank P value | |||
| Received local therapy |
No | 212 | 1.40 (1.18–1.66) | <0.001 | <0.001 |
| Yes | 948 | Reference | – | ||
| Local therapy modality |
Radical resection | 26 | 0.88 (0.57–1.38) | 0.587 | 0.001 |
| Local resection | 95 | 0.66 (0.49–0.88) | 0.004 | ||
| Radiation only | 827 | 0.72 (0.60–0.86) | <0.001 | ||
| No local therapy | 212 | Reference | – | ||
| Metastatic involvement site | Others | 716 | 0.80 (0.67–0.96) | 0.018 | <0.001 |
| Bone | 62 | 0.91 (0.64–1.29) | 0.588 | ||
| Lung | 72 | 1.08 (0.79–1.49) | 0.619 | ||
| Multiple sites | 85 | 1.43 (1.07–1.91) | 0.015 | ||
| Liver | 225 | Reference | – | ||
| Sequence of chemo and local therapy |
During chemo | 416 | 0.75 (0.61–0.91) | 0.004 | <0.001 |
| After chemo | 274 | 0.77 (0.62–0.95) | 0.015 | ||
| Unknown | 252 | 1.09 (0.88–1.34) | 0.436 | ||
| Before chemo | 218 | Reference | – | ||
| Age at diagnosis, years | >70 | 146 | 1.44 (1.18–1.77) | <0.001 | 0.002 |
| 60–70 | 328 | 1.11 (0.95–1.30) | 0.203 | ||
| <60 | 686 | Reference | – | ||
| Sex | Male | 407 | 1.38 (1.20–1.59) | <0.001 | <0.001 |
| Female | 753 | Reference | – | ||
| Race | Unknown | 11 | 0.89 (0.40–1.99) | 0.782 | 0.870 |
| Other | 20 | 1.20 (0.71–2.05) | 0.491 | ||
| Hispanic | 75 | 0.89 (0.66–1.20) | 0.442 | ||
| Non-Hispanic Black | 137 | 0.97 (0.78–1.20) | 0.759 | ||
| Non-Hispanic White | 917 | Reference | – | ||
| Primary payor | Not Insured | 109 | 1.30 (1.01–1.67) | 0.039 | <0.001 |
| Medicaid/Medicare/Other Government | 501 | 1.37 (1.18–1.58) | <0.001 | ||
| Unknown | 21 | 1.00 (0.58–1.70) | 0.987 | ||
| Private | 529 | Reference | – | ||
| Median income quartiles 2000 | < $30,000 | 185 | 1.23 (1.00–1.52) | 0.051 | 0.043 |
| $30,000–$35,999 | 232 | 1.05 (0.87–1.28) | 0.600 | ||
| $36,000–$45,999 | 321 | 1.10 (0.92–1.31) | 0.296 | ||
| Not Available | 42 | 0.65 (0.42–1.00) | 0.048 | ||
| $46,000+ | 380 | Reference | – | ||
| Urban/rural 2003 | Urban | 153 | 1.10 (0.89–1.35) | 0.376 | 0.701 |
| Rural | 23 | 1.22 (0.76–1.94) | 0.411 | ||
| Unknown | 23 | 1.07 (0.66–1.73) | 0.790 | ||
| Metro | 961 | Reference | – | ||
| Year of diagnosis |
≥ 2004, ≤2008 | 317 | 1.12 (0.86–1.45) | 0.405 | 0.021 |
| >2008, ≤2011 | 286 | 0.93 (0.71–1.21) | 0.568 | ||
| >2011, ≤2014 | 405 | 0.85 (0.66–1.10) | 0.221 | ||
| >2014, ≤2015 | 152 | Reference | – | ||
| Histology | Squamous cell carcinoma keratinizing | 91 | 0.97 (0.75–1.26) | 0.830 | 0.828 |
| Squamous cell carcinoma | 1069 | Reference | – | ||
| Primary site | C218-Overlapping lesion of rectum/anal canal | 156 | 1.04 (0.84–1.29) | 0.719 | 0.073 |
| C212-Cloacogenic zone | 4 | 2.91 (1.08–7.80) | 0.034 | ||
| C211-Anal canal | 502 | 0.92 (0.79–1.07) | 0.289 | ||
| C210-Anus, NOS | 498 | Reference | – | ||
| Tumor size, cm | No mass or tumor found | 3 | 1.31 (0.33–5.27) | 0.702 | 0.008 |
| <0.5 | 319 | 0.82 (0.69–0.98) | 0.025 | ||
| Unknown, size not stated, microscopic focus | 393 | 1.11 (0.95–1.31) | 0.194 | ||
| ≥0.5 | 445 | Reference | – | ||
| Surgical margins | Yes | 56 | 1.26 (0.77–2.04) | 0.357 | 0.651 |
| Not available | 1068 | 1.13 (0.77–1.65) | 0.526 | ||
| No | 36 | Reference | – | ||
| Grade | Well differentiated, differentiated, NOS | 35 | 1.05 (0.70–1.57) | 0.830 | 0.148 |
| Moderately differentiated | 394 | 0.84 (0.72–0.99) | 0.043 | ||
| Cell type not determined, not stated | 294 | 0.86 (0.72–1.03) | 0.102 | ||
| Poorly differentiated/anaplastic | 437 | Reference | – | ||
| Charlson-Deyo score | 2+ | 72 | 1.28 (0.96–1.70) | 0.090 | 0.161 |
| 1 | 118 | 1.13 (0.90–1.41) | 0.306 | ||
| 0 | 970 | Reference | – | ||
| Received surgical resection | Yes | 121 | 0.92 (0.73–1.15) | 0.439 | 0.439 |
| No | 1039 | Reference | – | ||
| Received radiation | Yes | 925 | 0.72 (0.61–0.85) | <0.001 | <0.001 |
| No | 235 | Reference | – | ||
| Type of surgery | Local | 95 | 0.86 (0.66–1.10) | 0.232 | 0.380 |
| Radical | 26 | 1.15 (0.75–1.76) | 0.517 | ||
| No | 1039 | Reference | – | ||
| Age at diagnosis | 1,160 | 1.01 (1.00–1.02) | 0.002 | – | |
NOS, not otherwise specified.
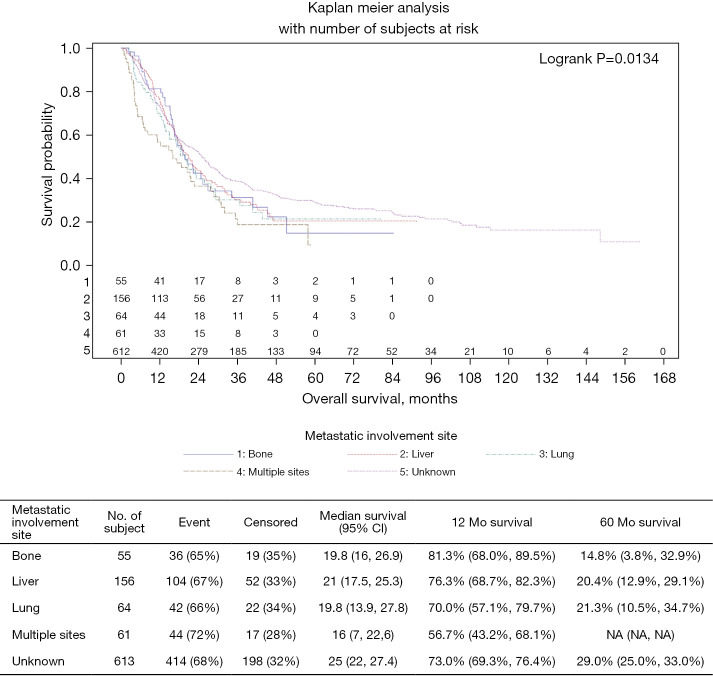
There was no benefit associated with APR (mOS =19.7 months; HR 1.05; 95% CI: 0.48–2.29; P=0.909), but both local resection of the primary (mOS =24.8 months, HR 0.48; 95% CI: 0.29–0.80; P=0.005) and palliative radiation (mOS =22.6 months; HR 0.66; 95% CI: 0.55–0.79; P<0.001) were associated with improved OS (Table 4). Median OS remained lowest among patients with multiple metastatic sites (16 months) compared to patients with liver metastasis (21 months), lung metastasis (19.8 months) and bone metastasis (19.8 months) (P=0.0134; Figure 3).
Table 4. Multivariable survival analysis of OS main effect.
| Covariate | Level | N | Overall survival (months) | ||
|---|---|---|---|---|---|
| Hazard ratio (95% CI) | HR P value | Type 3 P value | |||
| Received local therapy | Yes | 948 | 0.66 (0.55–0.79) | <0.001 | <0.001 |
| No | 212 | Reference | – | ||
| Sequence of chemo and local therapy |
During chemo | 416 | 0.77 (0.63–0.94) | 0.011 | <0.001 |
| After chemo | 274 | 0.72 (0.58–0.89) | 0.003 | ||
| Unknown | 252 | 1.15 (0.93–1.43) | 0.199 | ||
| Before chemo | 218 | Reference | – | ||
| Age at diagnosis, years | >70 | 146 | 1.28 (1.02–1.61) | 0.036 | 0.111 |
| 60–70 | 328 | 1.08 (0.91–1.28) | 0.404 | ||
| <60 | 686 | Reference | – | ||
| Sex | Male | 407 | 1.46 (1.26–1.70) | <0.001 | <0.001 |
| Female | 753 | Reference | – | ||
| Primary payor | Not insured | 109 | 1.33 (1.03–1.72) | 0.030 | 0.007 |
| Medicaid/Medicare/Other Government | 501 | 1.29 (1.09–1.53) | 0.002 | ||
| Unknown | 21 | 0.85 (0.49–1.48) | 0.569 | ||
| Private | 529 | Reference | – | ||
| Year of diagnosis | ≥2004, ≤2008 | 317 | 1.09 (0.83–1.42) | 0.543 | 0.007 |
| >2008, ≤2011 | 286 | 0.86 (0.66–1.13) | 0.292 | ||
| >2011, ≤2014 | 405 | 0.80 (0.61–1.04) | 0.099 | ||
| >2014, ≤2015 | 152 | Reference | – | ||
| Primary site | C218-Overlapping lesion of rectum/anal canal | 156 | 0.93 (0.75–1.16) | 0.525 | 0.030 |
| C212-Cloacogenic zone | 4 | 4.47 (1.58–12.62) | 0.005 | ||
| C211-Anal canal | 502 | 0.95 (0.82–1.11) | 0.525 | ||
| C210-Anus, NOS | 498 | Reference | – | ||
| Grade | Well differentiated, differentiated, NOS | 35 | 1.06 (0.69–1.61) | 0.799 | 0.075 |
| Moderately differentiated | 394 | 0.83 (0.70–0.98) | 0.031 | ||
| Cell type not determined, not stated | 294 | 0.82 (0.69–0.99) | 0.040 | ||
| Poorly differentiated/anaplastic | 437 | Reference | – | ||
| Charlson-Deyo Score | 2+ | 72 | 1.10 (0.82–1.48) | 0.536 | 0.181 |
| 1 | 118 | 1.24 (0.98–1.57) | 0.075 | ||
| 0 | 970 | Reference | – | ||
Number of observations in the original data set =1,160. Number of observations used =1,160. Backward selection with an alpha level of removal of .20 was used. The following variables were removed from the model: Histology, Median Income Quartiles 2000, TUMOR_SIZE_AllYear, Urban/Rural 2003, Race, and Surgical Margins.
Figure 3.
Metastatic sites treated by local therapy.
Local therapy before, during or after chemotherapy
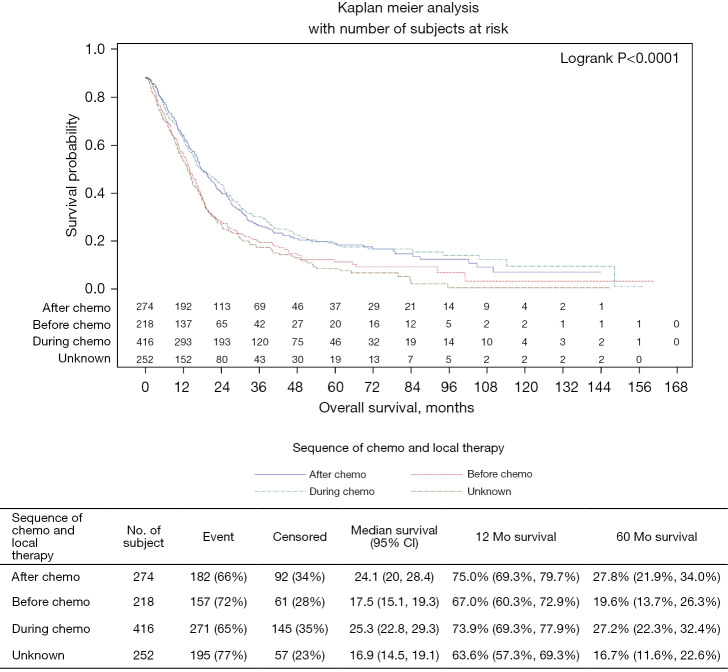
The majority of patients (59%) underwent local therapy during or after chemotherapy. Patients who underwent local therapy during or after chemotherapy had a mOS of 25.3 months (95% CI: 22.8–29.3) and 24.1 months (95% CI: 20–28.4) respectively. Local treatment before systemic chemotherapy initiation was associated with an inferior mOS of 17.5 months (95% CI: 15.1–19.3) (Figure 4). Moreover, the five-year OS for patients who underwent local therapy during or after chemotherapy were 27.2% and 27.8% respectively compared to 19.6% 5-year OS for patients who underwent local therapy before systemic chemotherapy. The survival benefits were statistically significant for local therapy during (HR 0.77; 95% CI: 0.63–0.94; P=0.011) or after chemotherapy (HR 0.72; 95% CI: 0.58–0.89; P=0.03) (Table 4).
Figure 4.
Sequence of chemotherapy and local therapy.
Discussion
Anal cancer is relatively rare, representing less than 2.5% of all gastrointestinal (GI) malignancies. However, incidence has been rising, with 9,090 cases in 2021 (6,070 in women and 3,020 in men) (1,2). The risk of being diagnosed with anal cancer during one’s lifetime is about 1 in 500 which is mostly HPV-related cancer. Patients with metastatic disease have a poor five-year overall survival (18%) compared to patients with localized disease (78%) (5). Median survival for patients with metastatic anal cancer is approximately 12 months (11). The presentation with an initial distant metastatic disease occurs in about 5–8% of patients diagnosed with anal cancer, and metastatic progression following initial treatment is seen in about 10–20% of cases (12). In a retrospective study of 92 patients with anal cancer, 17% of patients had local recurrence and 9% had distant metastasis (13). Most anal cancers are squamous cell cancers or cloacogenic zone cancers, with a few adenocarcinomas that are treated using the treatment paradigms for rectal adenocarcinoma.
Treatment for localized disease is well established with concurrent chemoradiation as the standard of care (Nigro protocol); however, the optimal treatment for metastatic anal cancer remains a therapeutic challenge due to the relatively small number of cases and limited published data. Until recently, the combination of Cisplatin and 5-Fluorouracil (5FU) was the widely acceptable initial systemic therapy for advanced or metastatic anal SCCa. The phase II InterAACT trial showed the favorable adverse effect profile of carboplatin and paclitaxel compared to 5FU and cisplatin as the first-line treatment, despite comparable ORR of 59% vs. 57% respectively (9,14). The trial recruited 91 patients at multiple centers between 2013 and 2017. Secondary survival end points were PFS (8.1 vs. 5.7 months, P=0.375) and overall survival (OS) (20 vs. 12.3 months, P=0.014), favoring carboplatin and paclitaxel combination. The toxicity profile showed 36% serious adverse event (SAE) rate with carboplatin and paclitaxel vs. 62% with Cisplatin/5-FU (P=0.0016).
The role of immunotherapy is emerging: NCT04444921 is a randomized phase 3 trial comparing chemotherapy alone (carboplatin + paclitaxel) to chemotherapy plus nivolumab for treatment-naïve metastatic anal cancer (15). A recent randomized phase II study CARACAS addressed the safety and efficacy of dual EGFR and PD-L1 blockade in who had progressed after at least one line of treatment (16). Sixty patients were randomized to avelumab alone or in combination with cetuximab: The primary endpoint ORR was 17% in the combination arm vs. 10% in the avelumab alone arm. With a median follow-up of 11 months, the PFS was 3.88 vs. 2.05 months, respectively. A favorable safety profile was observed in both arms.
An important consideration in metastatic anal cancer is the control of the primary tumor as local failure is associated with significant morbidity. However, optimal management of the primary symptomatic or bulky tumor in metastatic anal cancer is unknown. Current NCCN guidelines recommend palliative RT to be administered with chemotherapy for local control of symptomatic bulky primary (17). The panel identified benefits of systemic chemotherapy first since most patients (especially those naïve to chemotherapy) have rapid relief of symptoms as the primary tumor responds to systemic treatment. Control of distant disease is also achieved with frontline systemic therapy. However, chemo-radiation therapy is a viable frontline option for symptomatic patients in an attempt to provide relief of anal discomfort as quickly as possible (18). Notwithstanding, the optimal approach to patients who present with metastatic anal carcinoma and a symptomatic primary tumor remains unknown.
This is the largest series of metastatic anal SCCa patients and the first to report potential benefits of local therapy on survival. This study specifically addressed the impact of local therapy on survival, the benefit of palliative RT compared to local or radical surgical resection, as well as the sequence of therapies. More than 79% of the patients received radiation to the primary site, and 10.4% underwent surgical resection for local control. Use of local therapy correlated closely with OS on MVA (HR 0.66; 0.55–0.79; P<0.001), with a 12-month and 5-year OS rates of 72.8% and 25.7% respectively, compared with 61.1% and 14.6% for patients treated with chemotherapy only. This supports the use of local control of the primary symptomatic/bulky tumor.
When comparing local therapy modality, local resection had the best 5-year OS survival outcome (29.8%) followed by palliative radiation (25.5%) and radical resection/APR (16.9%) as opposed to no local therapy (14.6%) (P=0.0011). Interestingly, patients who underwent radical surgical resection/APR derived a short-term benefit and had the best short-term outcome with 1-year OS 76.9%. However, these patients quickly lost the benefit thereafter (Figure 2) possibly due to long term complications of radical resections/APR surgery in addition to permanent colostomy complications, infections and fistulas. Moreover, there was no survival benefit on multivariate analysis for patients who underwent APR (mOS =19.7 months; HR 1.05; 0.48–2.29; P=0.909).
A previous study showed a survival benefit for a subset of patients (n=33) who underwent multidisciplinary management of their metastatic disease with potentially curative intent (19). The median PFS was significantly longer at 16 months (95% CI: 9.2–22.8; P<0.001) compared to those patients treated with only palliative systemic chemotherapy (5 months; 95% CI: 3.5–6.5; P<0.001). The difference in OS was also longer with a median OS of 53 months (95% CI: 28.3–77.6) compared to only palliative systemic chemotherapy (17 months; 95% CI: 13.9–20.1; P<0.001). Furthermore, the role of palliative surgical intervention or RT in patients with metastatic disease who previously received radiation treatment is unclear.
There are several limitations to our study, including the retrospective nature and possible treatment bias. Palliative radiation modality was more commonly used overall, but the database does not allow direct comparison between radiation therapy, local surgical intervention or radical resection/APR. The database does not include HIV status, and positive patients generally tend to have inferior outcomes (20). Unfortunately, the NCDB does not contain details regarding radiation or chemotherapy (e.g., dosage, length of treatment, etc.). Nonetheless, there are several strengths to our analysis. Our large number of patients bolsters outcomes evaluation compared to previously reported small case series. Most importantly, the statistically significant OS benefit for patients who underwent local resection of the primary should prompt a review of current treatment strategies for patients with metastatic anal SCCa.
In conclusion, the addition of local control to systemic therapy (resection of the primary tumor or palliative radiation) improved OS in patients with metastatic anal SCCa. Patients older than 70 years of age, male, lack of health insurance and cloacogenic carcinoma should be carefully assessed before undergoing local therapies in the metastatic due to less demonstrable benefits.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Ethical approval was based on Emory University IRB waiver policy, since patient information in the database is completely de-identified and the database is legally accessible to the public. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-125/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-125/coif). WLS reports that he receives consulting fees from Bristol Myers Squibb, Mylan, Ipsen Pharmaceuticals, Lexicon, Blueprints and Eisai. MD reports that she receives consulting fees from Novartis and Guardant Health. OBA report that he receives consulting fees from Ipsen Pharmaceuticals, Natera, Taiho, Pfizer, QED Therapeutics as well as research support from Taiho Oncology, Ipsen Pharmaceuticals, GSK, Bristol Myers Squibb, PCI Biotech AS, ASCO, Calithera Biosciences, Inc., SynCore Biotechnology Co., Ltd., Mabspace Biosciences, Corcept Therapeutics. The other authors have no conflicts of interest to declare.
References
- 1.Siegel RL, Miller KD, Fuchs HE, et al. Cancer Statistics, 2021. CA Cancer J Clin 2021;71:7-33. 10.3322/caac.21654 [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013;63:11-30. 10.3322/caac.21166 [DOI] [PubMed] [Google Scholar]
- 3.Daling JR, Madeleine MM, Johnson LG, et al. Human papillomavirus, smoking, and sexual practices in the etiology of anal cancer. Cancer 2004;101:270-80. 10.1002/cncr.20365 [DOI] [PubMed] [Google Scholar]
- 4.Sischy B, Doggett RL, Krall JM, et al. Definitive irradiation and chemotherapy for radiosensitization in management of anal carcinoma: interim report on Radiation Therapy Oncology Group study no. 8314. J Natl Cancer Inst 1989;81:850-6. 10.1093/jnci/81.11.850 [DOI] [PubMed] [Google Scholar]
- 5.Johnson LG, Madeleine MM, Newcomer LM, et al. Anal cancer incidence and survival: the surveillance, epidemiology, and end results experience, 1973-2000. Cancer 2004;101:281-8. 10.1002/cncr.20364 [DOI] [PubMed] [Google Scholar]
- 6.Bower M, Powles T, Newsom-Davis T, et al. HIV-associated anal cancer: has highly active antiretroviral therapy reduced the incidence or improved the outcome? J Acquir Immune Defic Syndr 2004;37:1563-5. 10.1097/00126334-200412150-00004 [DOI] [PubMed] [Google Scholar]
- 7.Fox P, Stebbing J, Portsmouth S, et al. Lack of response of anal intra-epithelial neoplasia to highly active antiretroviral therapy. AIDS 2003;17:279-80. 10.1097/00002030-200301240-00028 [DOI] [PubMed] [Google Scholar]
- 8.Palefsky JM, Holly EA, Ralston ML, et al. Prevalence and risk factors for human papillomavirus infection of the anal canal in human immunodeficiency virus (HIV)-positive and HIV-negative homosexual men. J Infect Dis 1998;177:361-7. 10.1086/514194 [DOI] [PubMed] [Google Scholar]
- 9.Rao S, Sclafani F, Eng C, et al. International Rare Cancers Initiative Multicenter Randomized Phase II Trial of Cisplatin and Fluorouracil Versus Carboplatin and Paclitaxel in Advanced Anal Cancer: InterAAct. J Clin Oncol 2020;38:2510-8. 10.1200/JCO.19.03266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farias JPF, Rangel da Silva MHC, Jácome AA. Emerging and Experimental Agents for Anal Cancer: What is New? J Exp Pharmacol 2021;13:433-40. 10.2147/JEP.S262342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim R, Byer J, Fulp WJ, et al. Carboplatin and paclitaxel treatment is effective in advanced anal cancer. Oncology 2014;87:125-32. 10.1159/000361051 [DOI] [PubMed] [Google Scholar]
- 12.Ghosn M, Kourie HR, Abdayem P, et al. Anal cancer treatment: current status and future perspectives. World J Gastroenterol 2015;21:2294-302. 10.3748/wjg.v21.i8.2294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Glynne-Jones R, Nilsson PJ, Aschele C, et al. Anal cancer: ESMO-ESSO-ESTRO clinical practice guidelines for diagnosis, treatment and follow-up. Eur J Surg Oncol 2014;40:1165-76. 10.1016/j.ejso.2014.07.030 [DOI] [PubMed] [Google Scholar]
- 14.Hung A, Crane C, Delclos M, et al. Cisplatin-based combined modality therapy for anal carcinoma: a wider therapeutic index. Cancer 2003;97:1195-202. 10.1002/cncr.11161 [DOI] [PubMed] [Google Scholar]
- 15.Roth MT, Catalano PJ, Ciombor KK, et al. A randomized phase III study of immune checkpoint inhibition with chemotherapy in treatment-naive metastatic anal cancer patients: A trial of the ECOG-ACRIN cancer research group (EA2176). Paper presented at: 2021 ASCO Annual Meeting; May 28 2021, 2021. [Google Scholar]
- 16.Lonardi S, Prete AA, Morano F, et al. Randomized phase II trial of avelumab alone or in combination with cetuximab for patients with previously treated, locally advanced, or metastatic squamous cell anal carcinoma: the CARACAS study. J Immunother Cancer 2021;9:e002996. 10.1136/jitc-2021-002996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shridhar R, Shibata D, Chan E, et al. Anal cancer: current standards in care and recent changes in practice. CA Cancer J Clin 2015;65:139-62. 10.3322/caac.21259 [DOI] [PubMed] [Google Scholar]
- 18.Martini G, Arrichiello G, Borrelli C, et al. How I treat anal squamous cell carcinoma. ESMO Open 2020;4:e000711. 10.1136/esmoopen-2020-000711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eng C, Chang GJ, You YN, et al. The role of systemic chemotherapy and multidisciplinary management in improving the overall survival of patients with metastatic squamous cell carcinoma of the anal canal. Oncotarget 2014;5:11133-42. 10.18632/oncotarget.2563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim JH, Sarani B, Orkin BA, et al. HIV-positive patients with anal carcinoma have poorer treatment tolerance and outcome than HIV-negative patients. Dis Colon Rectum 2001;44:1496-502. 10.1007/BF02234605 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as