Abstract
Background
The postoperative recurrence rate is the main factor affecting the prognosis of hepatocellular carcinoma (HCC) patients, this study sought to investigate the value of contrast-enhanced ultrasound (CEUS) quantitative parameters in predicting the recurrence and the survival of HCC patients after thermal ablation.
Methods
The data of 97 patients with pathologically diagnosed HCC who underwent thermal ablation were retrospectively included in this study. The patients had an average age of 46.6 years (range, 23–79 years), and 79 were male and 18 were female. CEUS follow-up was performed at 1- and 3-month after thermal ablation, then at 6-month intervals thereafter for 5 years. CEUS was performed before thermal ablation, and the results were analyzed quantitatively using CEUS perfusion software (VueBox®, Bracco, Italy). The ratios of the CEUS quantitative parameters between the HCC lesions and reference liver parenchyma were calculated. The parameters included the average contrast signal intensity (MeanLin), peak enhancement (PE), rising time (RT), fall time (FT), time to peak (TTP), mean transit time (mTT), perfusion index (PI), Wash-in Area Under the Curve (WiAUC), Wash-in Rate (WiR), Wash-in Perfusion Index (WiPI), Wash-out Area Under the Curve (WoAUC), Wash-out Rate (WoR), and WiAUC + WoAUC (WiWoAUC). The correlations between the preoperative CEUS quantitative parameter ratios, the blood laboratory indexes, postoperative recurrence, and survival were analyzed using log-rank tests and a Cox regression model.
Results
The average follow-up duration period was 79 months (range, 5–145 months). The average recurrence time after ablation was 1–127 months, and the median disease-free survival time was 21 months. The 1-, 3- and 5-year survival rates were 96.9%, 92.3%, and 80.6%, respectively. The log-rank tests showed that tumor size, prothrombin time, and WiAUC, WoAUC, and WiWoAUC ratios were predictors of survival, and aspartate aminotransferase was a predictor of recurrence. The Cox regression analysis showed that tumor size [odds ratio (OR): 6.421; 95% CI: 1.434–28.761] and alanine transaminase (OR: 0.88; 95% CI: 0.010–0.742) were predictors of a poor prognosis.
Conclusions
CEUS quantitative parameters before thermal ablation and blood laboratory indexes provide potential clinical value for predicting the postoperative recurrence and survival of HCC patients.
Keywords: Contrast-enhanced ultrasound (CEUS), hepatocellular carcinoma (HCC), ablation, prognosis
Introduction
According to the latest annual data from CANCER TODAY, in 2020, liver cancer was the 6th most common cancer worldwide. Due to the large number of hepatitis B patients, hepatocellular carcinoma (HCC) has become one of the most common malignant tumors in China and severely affects the life and health of patients (1-3). In recent years, thermal ablation has been rapidly developed as a minimally invasive interventional therapy, and its principle is that for 1 or more specific tumor lesions in an organ, the biological effect of heat will directly lead to the irreversible damage or coagulative necrosis of tumor cells in the lesion tissue. As a local, minimally invasive, safe, and repeatable treatment, thermal ablation therapy has a satisfactory long-term survival rate in the treatment of HCC (4,5). However, the postoperative recurrence rate is still the main factor affecting the prognosis of HCC patients (6). Thus, it is of great clinical value to identify prognosis predictors for HCC patients after thermal ablation. Previous studies have shown that the prognostic factors after HCC ablation include age, Child-Pugh classification, tumor size, and tumor number (7-9). If patients have similar characteristics above, does it mean they have the same prognosis? As the only cancer that can be diagnosed by enhanced imaging and medical history, does enhanced imaging have some value in its prognosis?
Contrast-enhanced ultrasound (CEUS) has been widely applied in the diagnosis, monitoring, and follow-up of HCC. It makes full use of vascular perfusion information to analyze the nature of lesions and thereby provides a real-time and non-invasive visualization of HCC. CEUS can be used for preoperative diagnosis, postoperative efficacy evaluation and recurrence evaluation of HCC, and can be used as a means of postoperative follow-up (10). As early as 2012, the European Union of Biomedical Ultrasonic Societies noted the advantages and necessity of using CEUS for HCC condition monitoring and recurrence control, and the importance of CEUS has continued to increase each year (11).
Compared to contrast-enhanced computerized tomography, CEUS can be used to observe liver lesions in a dynamic and real-time manner and perform continuous imaging with high temporal resolution during the whole enhancement period, which need not be limited to a specific predetermined time. Conventional CEUS provides contrast wash-in and wash-out information from a real-time dynamic video, but it can not quantify the elaboration and analysis the parameters. A CEUS software analysis can quantify and refine the analysis and parameters, but it has many requirements in terms of image quality and storage time. To date, there is little evidence in China on its use in predicting the prognosis and recurrence of HCC ablation according to the characteristic parameters analyzed by CEUS software.
With the development of quantitative software, dynamic contrast-enhanced ultrasound (DCE-US) enables the quantitative assessment of tumor perfusion through time-intensity curves. The percentage variation of DCE-US has been reported to be predictive of tumor response and correlated with the progression-free survival (PFS) and overall survival (OS) of HCC patients after antiangiogenic therapies. A multicenter study of >500 patients at 19 centers showed that DCE-US was correlated with PFS and OS (12). A 40% decrease in the area under the curve (AUC) at 1 month was found to be correlated to PFS and OS in patients treated with tyrosine kinase inhibitors (13). However, little is known about the prognostic value of DCE-US for HCC patients before ablation.
In this study, we retrospectively collected the pre-ablation CEUS images and follow-up data of HCC patients after thermal ablation and explored the correlations between CEUS quantitative parameters and patient outcomes to investigate the prognostic value of quantitative parameters in predicting the recurrence and survival of HCC patients. We present the following article in accordance with the STARD reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-919/rc).
Methods
Study design
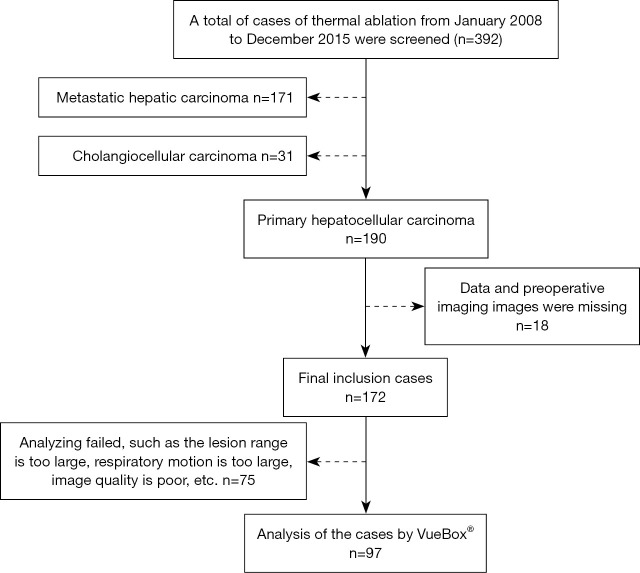
The basic data and information of 392 HCC patients treated at our hospital with thermal ablation from January 2008 to December 2016 were retrospectively collected. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Xijing Hospital (No. KY20222243-C-1) and individual consent for this retrospective analysis was waived. The enrollment process is shown in Figure 1. To be eligible for inclusion in this study, patients had to meet the following inclusion criteria: (I) be a male or female, aged 18–70 years; (II) have been pathologically diagnosed with HCC; (III) have chosen and consented to receive thermal ablation treatment, and meet the diagnosis and treatment standards for thermal ablation; (IV) have complete imaging data available (including data obtained within 2 weeks before ablation); (V) have survival follow-up results for 5 years or more.
Figure 1.
Flow chart for participant inclusion.
Patients were excluded from the study if they met any of the following exclusion criteria: (I) had incomplete data; and/or (II) had angiography results that could not be analyzed by software because, for example, the lesion range was too large to be referenced or analyzed, the patient's respiratory motion was too large, or the image quality was poor.
Imaging studies
Instruments and contrast agents
The following instruments and contrast agents were used in this study: ultrasound (Siemens Acuson Sequoia, Phillip EpiQ7) equipped with C1-5 probe, and mechanical index (MI) under contrast conditions was 0.07; Ultrasound contrast agent SF6 microbubble (SonoVue, Bracco). Before use, the ultrasound contrast agent was mixed with 5 mL of normal saline and shaken well to prepare the fresh suspension.
Basic ultrasound examination
Each patient was first examined by unenhanced ultrasound and confirmed to have no more than 3 focal liver lesions under gray-scale imaging. Each lesion was measured, located, and characterized (e.g., in terms of its boundary, shape, and echo). A Color Doppler ultrasound was used to assess the blood supply. A representative checking sequence was stored digitally.
CEUS examination
Each patient was instructed to breathe quietly. The best cut surface of the lesion was selected, the probe position was fixed, switched the ultrasound instrument to CEUS imaging mode with a MI <0.07, 1.6–2.4 mL of contrast agent was injected into the cubital vein group, and the tube was then flushed with 5–10 mL of normal saline. The perfusion process of the lesion was continuously observed in real time for at least 3 min, and the time was recorded.
CEUS qualitative analysis
The dynamic imaging data were collected and stored in a hard drive. The data were read and analyzed by 2 doctors using a double-blind method. If the 2 doctors disagreed about the analysis results, a 3rd senior doctor made a decision. Based on the echogenicity of the lesion at the arteriography stage, portal vein stage and, delayed stage relative to the liver parenchyma, the enhancement degree was divided into high, equal, and low, respectively. The angiography was divided into the following 3 phases: the arterial phase (8–30 s), the portal vein phase (30–120 s), and the delayed phase (120–360 s).
CEUS quantitative analysis
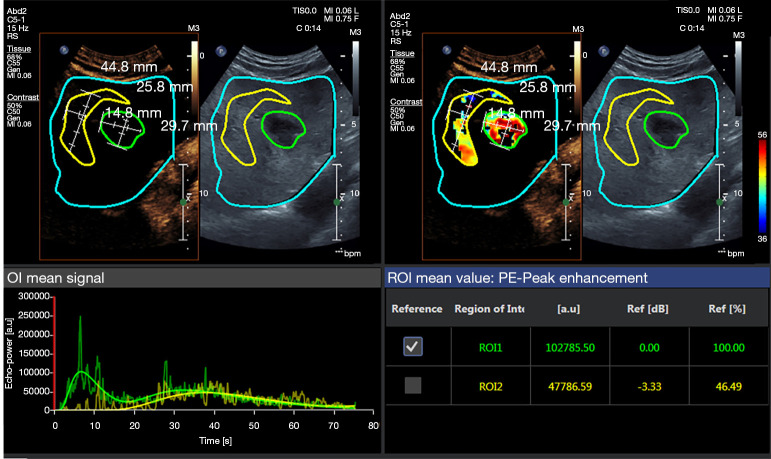
For each patient, 1 lesion was included analyzed. The data were analyzed and read by 2 experienced imaging diagnostic doctors who had worked for at least 5 years. These doctors also operated the CEUS. The digitally stored ultrasound loops were evaluated using perfusion quantification software (VueBox®, Bracco, Italy). Regions of interest (ROIs) were manually selected in a HCC lesion without any necrotic area and the surrounding tissue. The parameters were calculated for each ROI, including the average contrast signal intensity (MeanLin), peak enhancement (PE), rising time (RT), fall time (FT), time to peak (TTP), mean transit time (mTT), perfusion index (PI), Wash-in Area Under the Curve (WiAUC), Wash-in Rate (WiR), Wash-in Perfusion Index (WiPI), Wash-out Area Under the Curve (WoAUC), Wash-out Rate (WoR), and WiAUC + WoAUC (WiWoAUC). The ratio of each parameter between the HCC lesion and surrounding tissue was calculated by the software and exported using a calculation protocol (Figure 2).
Figure 2.
Representative VueBox® analysis results of CEUS images and time-intensity curves for HCC. The ROIs were drawn for the quantitative analysis (Blue: Delimitation ROI; Green: lesion area; Yellow: normal reference area of liver parenchyma). CEUS, contrast-enhanced ultrasound; HCC, hepatocellular carcinoma; ROI, regions of interest.
Data collection
We conducted a retrospective analysis of the data of 392 HCC patients. We collected the patients’ basic data and information before ablation (including liver disease history,age,gender, diabetes mellitus, history of surgery, medication history, smoking, and drinking data), and tumor lesion information (e.g., CEUS quantitative parameters, tumor size, clinical stage, number of tumors, alpha fetoprotein(AFP)value, liver function index, recurrence time, and survival time). VueBox® software analysis the CEUS data. The patients were followed-up 1, 3, and 6 months after thermal ablation, and then every 6 months till 5 years after ablation. Follow-up was carried out by outpatient revisit and telephone. The patients were grouped according to whether the tumor recurred or not. The recurrence time and disease-free survival (DFS) were recorded. Among the data, age, gender, the 5-year survival rate, tumor-free survival, recurrence time, and quantitative parameters obtained by the VueBox® software analysis were the main observation indexes in this study. The basic information of the patients, including tumor size, tumor number, AFP index, and etc, were the secondary observation indexes.
Statistical analysis
SPSS 19.0 statistical package was used to analyze the data. The counting data are expressed as the frequency and percentage, and the measurement data are expressed as the median (quartile). The log-rank test was used for the univariate analysis. Cox regression was used for the multivariate analysis. The COX regression model takes survival as the outcome variable, covariates included each CEUS quantitative parameters, and for indicators with a P value <0.1 of univariable log-rank test analysis. A P value <0.05 indicated a statistically significant difference.
Results
Clinical characteristics of patients
The study comprised 97 patients who were scheduled to receive thermal ablation therapy. These patients had an average age of 46.6 years (range, 23–79 years), and 79 were male and 18 were female. Among the 97 patients, 69 had single tumors and 28 had multiple tumors. The number of lesions totaled 137. Of the lesions, 51% (49/97) had a diameter of <3 cm, 34% (33/97) had a diameter of 3–5 cm, and 15% (15/97) had a diameter of >5 cm. The perfusion area was correctly detected in 87 of the 97 patients via a VueBox® perfusion analysis. Thus, the sensitivity of VueBox® to HCC was 89.7%.
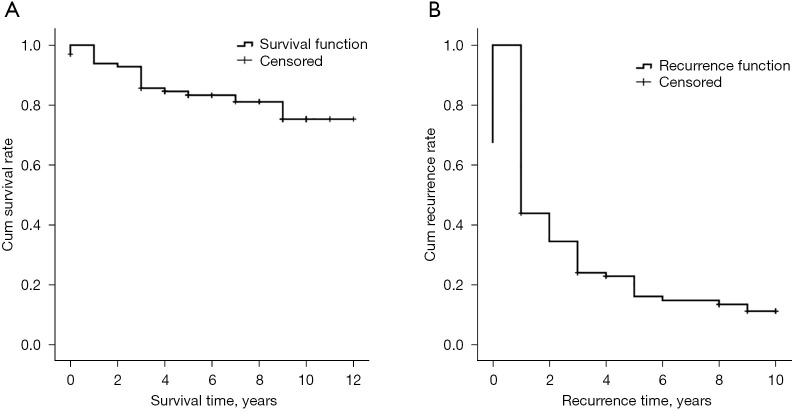
The average follow-up duration was 79 months (range, 5–145 months). The 1-, 3-, and 5-year survival rates were 96.9%, 92.3%, and 80.6%, respectively. The recurrence time was 1–127 months after ablation, and the median DFS time was 21 months (see Figure 3).
Figure 3.
Survival time and recurrence time of the 97 patients.
The quantitative parameters of HCC before thermal ablation
The quantitative parameters obtained by the VueBox® software, including the MeanLin ratio, PE ratio, WiAUC ratio, RT ratio, MTT ratio, TTP ratio, WiR ratio, WiPI ratio, WoAUC ratio, WiWoAUC ratio, FT ratio, and WoR ratio, were included in the analysis. Table 1 shows the quantitative parameters before thermal ablation.
Table 1. The quantitative parameters of the HCC patients before thermal ablation (n=97).
| Parameters | Median | Percentiles | |
|---|---|---|---|
| 25% | 75% | ||
| MeanLin ratio | 286.8779 | 96.3173 | 984.6491 |
| PE ratio | 204.9808 | 76.9554 | 784.7166 |
| WiAUC ratio | 200.5386 | 53.2323 | 1,054.9668 |
| RT ratio | 93.6101 | 49.4687 | 155.9767 |
| mTT ratio | 74.7832 | 35.4109 | 264.5717 |
| TTP ratio | 87.0456 | 50.4155 | 113.2722 |
| WiR ratio | 244.6876 | 44.6620 | 1,403.5424 |
| WiPI ratio | 213.0246 | 76.1688 | 773.7563 |
| WoAUC ratio | 249.3960 | 58.3067 | 850.7691 |
| WiWoAUC ratio | 239.3049 | 62.4973 | 963.8172 |
| FT ratio | 96.1480 | 42.2038 | 276.0448 |
| WoR ratio | 263.7977 | 48.3121 | 1,065.7915 |
MeanLin, the average contrast signal intensity; PE, peak enhancement; WiAUC, Wash-in Area Under the Curve; RT, rising time; mTT, mean transit time; TTP, time to peak; WiR, Wash-in Rate; WiPI, Wash-in Perfusion Index; WoAUC, Wash-out Area Under the Curve; WiWoAUC, WiAUC + WoAUC; FT, fall time; WoR, Wash-out Rate.
Prognosis analysis
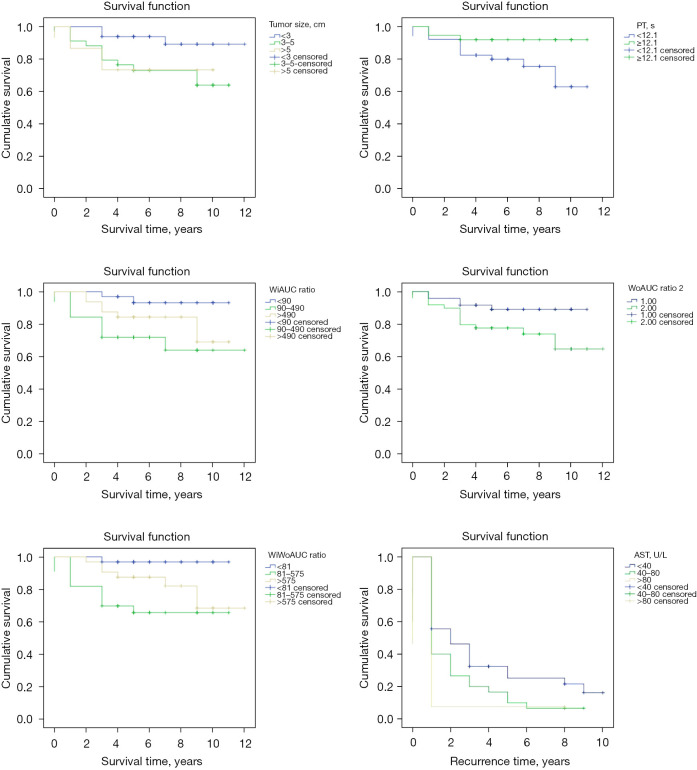
The univariate analysis showed that the WiAUC, WoAUC, and WiWoAUC ratios, tumor size, and prothrombin time (PT) were predictors of survival, and aspartate aminotransferase (AST) was a predictor of recurrence (see Table 2 and Figure 4).
Table 2. Univariate analysis of each survival factor (n=97).
| Factor | n | Overall survival (year) | |||
|---|---|---|---|---|---|
| Estimate | 95% CI | χ2 | P | ||
| Tumor size (cm) | 6.668 | 0.036 | |||
| <3 | 49 | 11.153 | 10.354–11.952 | ||
| 3–5 | 33 | 8.521 | 7.229–9.812 | ||
| >5 | 15 | 7.800 | 5.923–9.677 | ||
| PT (s) | 4.676 | 0.031 | |||
| ≤12.1 | 61 | 8.794 | 7.751–9.837 | ||
| >12.1 | 36 | 10.243 | 9.418–11.069 | ||
| WiAUC ratio | 7.259 | 0.027 | |||
| <90 | 33 | 10.534 | 9.908–11.159 | ||
| 90–490 | 32 | 8.694 | 6.986–10.402 | ||
| >490 | 32 | 9.343 | 8.229–10.470 | ||
| WoAUC ratio | 4.280 | 0.039 | |||
| ≤249.396 | 48 | 10.072 | 9.293–10.851 | ||
| >249.396 | 49 | 9.314 | 8.110–10.518 | ||
| WiWoAUC ratio | 10.317 | 0.006 | |||
| <81 | 32 | 10.750 | 10.268–11.232 | ||
| 81–575 | 33 | 7.875 | 6.347–9.403 | ||
| >575 | 32 | 10.191 | 9.016–11.367 | ||
| PE ratio | 2.305 | 0.316 | |||
| <99 | 33 | 9.303 | 8.059–10.547 | ||
| 99–511 | 32 | 10.794 | 9.676–11.912 | ||
| >511 | 32 | 8.732 | 7.483–9.980 | ||
| RT ratio | 1.730 | 0.421 | |||
| <59 | 32 | 10.417 | 9.137–11.696 | ||
| 59–122 | 33 | 9.731 | 8.692–10.769 | ||
| >122 | 32 | 8.625 | 7.260–9.990 | ||
| FT ratio | 1.836 | 0.399 | |||
| <57 | 32 | 9.906 | 8.891–10.921 | ||
| 57–165 | 33 | 9.487 | 8.381–10.592 | ||
| >165 | 32 | 9.382 | 7.889–10.874 | ||
Estimation is limited to the largest survival time if it is censored. PT, prothrombin time; WiAUC, Wash-in Area Under the Curve; WoAUC, Wash-out Area Under the Curve; WiWoAUC, WiAUC + WoAUC; PE, peak enhancement; RT, rising time; FT, fall time.
Figure 4.
Survival curve for each survival factor (n=97). PT, prothrombin time; WiAUC, Wash-in Area Under the Curve; WoAUC, Wash-out Area Under the Curve; WiWoAUC, WiAUC + WoAUC; AST, aspartate aminotransferase.
The Cox regression analysis showed that tumor size [odds ratio (OR): 6.421; 95% CI: 1.434–28.761] and alanine transaminase (ALT) (OR: 0.88; 95% CI: 0.010–0.742) were predictors of a poor prognosis.
Discussion
CEUS is becoming more and more important in the monitoring and preoperative and postoperative assessment of malignant liver tumors. CEUS can be used as an important detection method for surgical resection, radio frequency ablation (RFA), microwave ablation (MWA), and hepatic artery chemoembolization (TACE) to evaluate the microvascularization of normal liver tissue, ablation/embolization defects, and changes in blood supply at the liver margin. Its advantages are as follows: (I) It enables the real-time imaging and the high-time resolution evaluation of target lesions; (II) the high enhancement and clearance of the arterial phase can be evaluated with high temporal resolution; (III) the diagnostic reliability can be increased by repeatable injections, from different angles, using different parameters to evaluate the enhancement pattern, and it is possible to detect lesions that were missed on computed tomography (CT) or magnetic resonance imaging (MRI); and (IV) it has a high spatial resolution, can find small anatomical or pathological structures that are not shown on CT or MRI, and has certain advantages in identifying and finding lesions (14,15). However, CEUS also has some limitations. Notably, the analysis of CEUS results has a certain relationship with the experience of the operating physicians. Questionable nodules need to be reviewed by senior physicians to ascertain the results. Thus, while CEUS has many advantages, quantitative analysis results cannot be obtained, images cannot be digitized, and it is difficult to provide a theoretical basis for a large number of retrospective analyses.
CEUS and perfusion software VueBox® can be combined to quantify and evaluate tumor microvascularization (16,17). VueBox® is a software tool that helps to calculate and record parameters in lesions and other tissues that show pathological behaviors. It is useful for treatment monitoring, the control of disease changes, and the detection of abnormal perfusion patterns. After the analysis of the GI-Perfusion application package, the perfusion parameters can be calculated, and the perfusion volume can be estimated based on the analysis of the time series of the 2-dimensional contrast-enhanced images. Perfusion parameters include PE, RT, and the WiR and WiAUC ratios. Time parameters can be represented by absolute values (e.g., RT), and amplitude parameters can be represented by relative values (compared to a reference area) (e.g., WiR, PE, and WiAUC).
VueBox® perfusion imaging analysis has been proven to be useful in identifying HCC vascular changes (15,18,19). The software can dynamically evaluate flow and volume parameters, such as TTP, RT, mTT, the perfusion rate, and peak value (16,20). This study evaluated the preoperative ultrasound images of HCC with VueBox® software to obtain quantitative information indicators about the contrast, such as the peak value and TTP, to effectively help to distinguish changes in perfusion patterns. This study showed that the ratios of WiAUC, WoAUC, and WiWoAUC were predictors of survival.
The TTP, RT, and mTT of VueBox® in the CEUS of HCC before thermal ablation were not correlated with the prognosis and recurrence of HCC (P>0.05). This may be because too many influencing factors were included in the analysis, and the sample size (n=97) was not sufficiently large. The quantitative results obtained from the VueBox® analysis and preoperative univariate and multivariate analysis results provide an important reference for the CEUS image analyses and survival information before thermal ablation, and also clarify the research direction on parameter selection for future prospective studies.
This study had several limitations. The purpose of this retrospective study was to investigate the value of quantitative CEUS parameters in predicting the recurrence and survival of HCC patients after thermal ablation. However, this retrospective study had a high rate of follow-up loss and high patient heterogeneity. Consequently, the patients in this study had higher 3- and 5-year survival rates than those previously reported in the literature. To improve the reliability of the data, we implemented strict inclusion and exclusion criteria and established a series of case screening requirements to reduce study bias. In the future, we intend to conduct relevant prospective studies to further explore the value of the quantitative parameters of CEUS in the prognosis of HCC after thermal ablation.
Conclusions
In conclusion, a qualitative analysis of CEUS images combined with a quantitative analysis by VueBox® not only effectively improves the detection of malignant lesions that cannot be detected by the naked eye, but also transforms them into more intuitive and scientific quantitative results. The complementarity of the quantitative and qualitative analysis of preoperative CEUS images brings us a step forward in the accurate diagnosis and treatment for preoperative analysis of HCC patients treated with thermal ablation. In this study, CEUS quantitative parameter images before thermal ablation and blood laboratory indexes provided valuable prediction information about the postoperative recurrence and survival of HCC patients.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: The study was supported by Shaanxi Province Science and Technology Innovation Project Plan (No. 2015KTCL03-11).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Xijing Hospital (No. KY20222243-C-1H) and individual consent for this retrospective analysis was waived.
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-919/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-919/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-919/coif). The authors have no conflicts of interest to declare.
(English Language Editor: L. Huleatt)
References
- 1.Couri T, Pillai A. Goals and targets for personalized therapy for HCC. Hepatol Int 2019;13:125-37. 10.1007/s12072-018-9919-1 [DOI] [PubMed] [Google Scholar]
- 2.Li Z, Yu Q, Lu X, et al. Efficacy of radiofrequency ablation versus laparoscopic liver resection for hepatocellular carcinoma in China: a comprehensive meta-analysis. Wideochir Inne Tech Maloinwazyjne 2021;16:455-71. 10.5114/wiitm.2021.105377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zongyi Y, Xiaowu L. Immunotherapy for hepatocellular carcinoma. Cancer Lett 2020;470:8-17. 10.1016/j.canlet.2019.12.002 [DOI] [PubMed] [Google Scholar]
- 4.Radjenović B, Sabo M, Šoltes L, et al. On Efficacy of Microwave Ablation in the Thermal Treatment of an Early-Stage Hepatocellular Carcinoma. Cancers (Basel) 2021;13:5784. 10.3390/cancers13225784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu F, Rhim H. Thermal ablation for hepatocellular carcinoma: what's new in 2019. Chin Clin Oncol 2019;8:58. 10.21037/cco.2019.11.03 [DOI] [PubMed] [Google Scholar]
- 6.Shiina S, Sato K, Tateishi R, et al. Percutaneous Ablation for Hepatocellular Carcinoma: Comparison of Various Ablation Techniques and Surgery. Can J Gastroenterol Hepatol 2018;2018:4756147. 10.1155/2018/4756147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brunello F, Cantamessa A, Gaia S, et al. Radiofrequency ablation: technical and clinical long-term outcomes for single hepatocellular carcinoma up to 30 mm. Eur J Gastroenterol Hepatol 2013;25:842-9. 10.1097/MEG.0b013e32835ee5f1 [DOI] [PubMed] [Google Scholar]
- 8.Shiina S, Tateishi R, Arano T, et al. Radiofrequency ablation for hepatocellular carcinoma: 10-year outcome and prognostic factors. Am J Gastroenterol 2012;107:569-77; quiz 578. 10.1038/ajg.2011.425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Francica G, Saviano A, De Sio I, et al. Long-term effectiveness of radiofrequency ablation for solitary small hepatocellular carcinoma: a retrospective analysis of 363 patients. Dig Liver Dis 2013;45:336-41. 10.1016/j.dld.2012.10.022 [DOI] [PubMed] [Google Scholar]
- 10.Huang Q, Zeng Q, Long Y, et al. Fusion imaging techniques and contrast-enhanced ultrasound for thermal ablation of hepatocellular carcinoma - A prospective randomized controlled trial. Int J Hyperthermia 2019;36:1207-15. 10.1080/02656736.2019.1687945 [DOI] [PubMed] [Google Scholar]
- 11.Sidhu PS, Cantisani V, Dietrich CF, et al. The EFSUMB Guidelines and Recommendations for the Clinical Practice of Contrast-Enhanced Ultrasound (CEUS) in Non-Hepatic Applications: Update 2017 (Short Version). Ultraschall Med 2018;39:154-80. 10.1055/s-0044-101254 [DOI] [PubMed] [Google Scholar]
- 12.Lassau N, Bonastre J, Kind M, et al. Validation of dynamic contrast-enhanced ultrasound in predicting outcomes of antiangiogenic therapy for solid tumors: the French multicenter support for innovative and expensive techniques study. Invest Radiol 2014;49:794-800. 10.1097/RLI.0000000000000085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O'Connor JP, Aboagye EO, Adams JE, et al. Imaging biomarker roadmap for cancer studies. Nat Rev Clin Oncol 2017;14:169-86. 10.1038/nrclinonc.2016.162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aubé C, Oberti F, Lonjon J, et al. EASL and AASLD recommendations for the diagnosis of HCC to the test of daily practice. Liver Int 2017;37:1515-25. 10.1111/liv.13429 [DOI] [PubMed] [Google Scholar]
- 15.Wilson SR, Lyshchik A, Piscaglia F, et al. CEUS LI-RADS: algorithm, implementation, and key differences from CT/MRI. Abdom Radiol (NY) 2018;43:127-42. 10.1007/s00261-017-1250-0 [DOI] [PubMed] [Google Scholar]
- 16.Maxeiner A, Fischer T, Schwabe J, et al. Contrast-Enhanced Ultrasound (CEUS) and Quantitative Perfusion Analysis in Patients with Suspicion for Prostate Cancer. Ultraschall Med 2019;40:340-8. 10.1055/a-0594-2093 [DOI] [PubMed] [Google Scholar]
- 17.Platz Batista da Silva N, Jung EM, Jung F, et al. VueBox® perfusion analysis of contrast-enhanced ultrasound (CEUS) examinations in patients with primary hyperparathyroidism for preoperative detection of parathyroid gland adenoma. Clin Hemorheol Microcirc 2018;70:423-31. 10.3233/CH-189307 [DOI] [PubMed] [Google Scholar]
- 18.Wood JM, Hussey DJ, Woods CM, et al. Does gene expression in laryngeal subsites differ between patients with laryngopharyngeal reflux and controls? Clin Otolaryngol 2018;43:158-63. 10.1111/coa.12918 [DOI] [PubMed] [Google Scholar]
- 19.Dong Y, Qiu Y, Yang D, et al. Potential application of dynamic contrast enhanced ultrasound in predicting microvascular invasion of hepatocellular carcinoma. Clin Hemorheol Microcirc 2021;77:461-9. 10.3233/CH-201085 [DOI] [PubMed] [Google Scholar]
- 20.Rennert J, Wiesinger I, Schicho A, et al. Color coded perfusion imaging with contrast enhanced ultrasound (CEUS) for post-interventional success control following trans-arterial chemoembolization (TACE) of hepatocellular carcinoma. PLoS One 2019;14:e0217599. 10.1371/journal.pone.0217599 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as