Abstract
Background
China has a high incidence of esophageal cancer (EC), mainly squamous cell carcinoma, which is a serious threat to human life. Previous studies have shown that artemisinin can inhibit the proliferation and metastasis of cancer cells, thus inhibiting the progression of cancer. Aerobic glycolysis plays an important role in the uncontrolled growth of tumor cells. However, there are still different opinions on the anti-cancer mechanism, and there have been few studies involving EC. Our pre-experiment found that artemisinin can inhibit the progression of EC by directly regulating aerobic glycolysis.
Methods
The EC cell lines KYSE-150 and KYSE-170 were used to detect the effects of artemisinin on cell viability, proliferation, metastasis, and aerobic glycolysis. Network pharmacology technology was used to explore the potential molecular mechanism of artemisinin inhibiting the development of EC through aerobic glycolysis and the findings were verified by molecular docking.
Results
Artemisinin could inhibit the proliferation, metastasis, and glycolysis of esophageal squamous cell carcinoma (ESCC), and this was verified by the expression of key metastatic proteins (N-cadherin) and key enzymes of glycolysis [hypoxia-inducible factor-1α (HIF-1α), pyruvate kinase M2 (PKM2)]. Through network pharmacology, we found the potential therapeutic target of artemisinin, HIF-1α. The results of molecular docking showed that artemisinin could directly target HIF-1α and promote its degradation.
Conclusions
Artemisinin can target HIF-1α to reduce the level of glycolysis and inhibit the development of EC, which may become a targeted drug for the treatment of EC.
Keywords: Esophageal squamous cell carcinoma (ESCC), artemisinin, aerobic glycolysis, hypoxia-inducible factor-1α (HIF-1α)
Introduction
The latest global cancer statistics show that esophageal cancer (EC) is one of the most common malignant tumors worldwide, with the 8th and 6th highest morbidity and mortality, respectively. There are significant regional differences in the incidence of EC. China comprises a large proportion of EC cases worldwide, with the number of new cases annually accounting for about half of the total number of EC patients worldwide (1). The incidence and mortality of EC rank 6th and 4th, respectively, among all malignant tumors in China (2). Since there are no obvious symptoms in the early stage of EC, it is often diagnosed at a late stage, seriously endangering people’s health. In view of the high mortality and short survival time of patients with locally advanced and advanced EC, how to optimize treatment to improve the prognosis is still an urgent health problem to be addressed in China and worldwide.
The traditional methods of cancer treatment include surgery, radiotherapy, and chemotherapy. Although they can play a role in improving the survival time of patients, the therapeutic effect has remained unsatisfactory (3). Additional therapeutic challenges include that some tumors are not suitable for surgery, patient intolerance to radiotherapy and chemotherapy, and the development of drug resistance during chemotherapy. Serious adverse reactions have also been common in tumor immunotherapy in recent years (4,5). Therefore, the development of new safe and effective antineoplastic drugs is currently a research hotspot.
Traditional Chinese medicine (TCM) has accumulated experience in tumor treatment since ancient times. With the continuous progress and development of modern medicine, the anti-tumor efficacy and mechanism of a large number of active substances in TCM have been elucidated (6). Whether used alone or as adjuvant drugs for radiotherapy, chemotherapy, or other drugs, TCM shows great clinical anti-tumor application value. Therefore, strengthening the development and application of TCM and its extracts is an effective way to enhance the treatment of cancer.
Artemisinin is the most effective drug in the treatment of malaria, and combination therapy based on artemisinin is the most effective and important means for the treatment of malaria (7). Through in-depth research of artemisinin, we found that it can inhibit a variety of cancers through selective cytotoxicity (8-10). As there is no cross-resistance of traditional therapeutic drugs, their use can circumvent the multiple drug resistance of tumor cells (11). Previous studies have found that a certain dose of artemisinin can promote cancerous cells of the lung, liver, cervix, breast, and other cancer types to stop growing or even undergo apoptosis. When used in combination with traditional chemotherapeutic drugs, it can exert a synergistic anti-tumor effect (12-14). However, controversy remains regarding the anti-cancer mechanism, and there have been few studies involving EC.
Our pre-experiment found that artemisinin can inhibit the progression of EC by directly regulating aerobic glycolysis. This study aimed to clarify this mechanism. We present the following article in accordance with the MDAR reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-877/rc).
Methods
Cell culture
Human EC cell lines KYSE-150 (Shanghai Zhong Qiao Xin Zhou Biotechnology Co., Ltd., Shanghai, China) and KYSE-170 (Procell Life Science and Technology Co., Ltd., Wuhan, China) were cultured in Roswell Park Memorial Institute (RMPI)-1640 (HyClone, Logan, UT, USA) medium containing 10% fetal bovine serum (FBS; PAN, Adenbach, Germany), containing penicillin and streptomycin. The cells were cultured in a cell incubator with 5% volume fraction of CO2 at 37 ℃, and the state of cell proliferation was observed. Subsequent experiments used log-phase cells without special instructions. Artemisinin (Lot. No.113E022; Solarbio, Beijing, China) was added to the medium at different concentrations and diluted in 0.01% dimethyl sulfoxide (DMSO) in the medium.
Cell viability and proliferation assays
The effects of artemisinin on KYSE-150 and KYSE-170 cells were detected by CellTiter 96® AQueous cell proliferation assay kit (Lot 0000499780; Promega, Madison, WI, USA). The cells were inoculated in a 96-well plate at the density of 1,000 cells per well and incubated with 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS tetrazolium compound) for 4 hours, then the absorbance was measured at 490 nm with a miniature plate reader.
Transwell assays
The digested cells were suspended again in serum-free medium; 1×105 cells and matrix gel were added to the upper chamber, and the complete culture medium containing serum was added to the lower chamber. After incubation at 37 ℃ in 5% CO2 incubator for 36 hours, the cells were fixed with 4% paraformaldehyde and stained with 0.1% crystal violet. The cells were counted under a microscope [Eclipse E200, Nikon Instruments (Shanghai) Co., Ltd.].
Seahorse assays
After 48 hours of treatment with artemisinin, KYSE-150 and KYSE-170 cells were inoculated on XF96 cell culture plate with a cell density of 1×104 cells per well. The extracellular acidification rate (ECAR) of glycolysis level was detected by Seahorse XFe96 Analyzer (US411225; Agilent, Santa Clara, CA, USA) and Agilent Seahorse XFe96 Extracellular Flux Assay kits (Lot. W13221). The specific experimental procedure was carried out according to the manufacturer’s instructions.
Western blotting analysis
Precooled radioimmunoprecipitation assay (RIPA) cleavage buffer was used to cleave cells for 45 minutes on ice, which were then centrifuged at 4 ℃ at a rate of 12,000 rpm/min for 10 minutes. The concentration of extracted protein was determined by bicinchoninic acid (BCA) protein concentration determination kit. A total of 50 µg protein was loaded on the gel, separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), and transferred to a polyvinylidene fluoride (PVDF) membrane. The membrane was sealed with 5% skimmed milk powder at room temperature for 1 hour, and incubated with antibodies [anti-GAPDH, 1:10,000; anti-HIF-1α, 1:500; anti-pyruvate kinase M2 (PKM2), 1:500; anti-E-cadherin, 1:1,500; anti-N-cadherin, 1:1,500] overnight at 4 ℃, washed with 0.1% Tween 3 times, and incubated with horseradish peroxide-coupled secondary antibody (1:2,000) for 1.5 hours at room temperature. The membrane was washed with Tris buffered saline with Tween 20 (TBST) containing 0.1% Tween 3 times and the immunoreactive bands were detected by scanner.
Construction of a core target network of artemisinin-aerobic glycolysis-EC
We input the related information of artemisinin, aerobic glycolysis, and EC into the GeneCards database (https://www.genecards.org/), and select the correlation score ≥10.00. We filtered the related genes and retrieved genes that target common to all three. The data were mapped by Venny mapping software (https://bioinfogp.cnb.csic.es/tools/venny/) to obtain the common potential targets and the target protein interaction network was constructed by the Search Tool for the Retrieval of Interacting Genes/Proteins (STRING) database (https://string-db.org/). The common potential targets were analyzed by Metascape database (https://metascape.org/) for Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis.
Molecular docking
We downloaded the protein structure from the Research Collaboratory for Structural Bioinformatics Protein Data Bank (RCSB’s PDB) database (https://www.rcsb.org/), removed ligands with PYMOL (https://pymol.org/2/), removed water molecules with AutoDock 4.2 (https://autodock.scripps.edu/), and added total hydrogen. The ingredient of artemisinin was downloaded through Traditional Chinese Medicine Systems Platform (TCMSP; https://tcmsp-e.com/tcmsp.php), introduced into AutoDock4.2, and total hydrogen was added as ligand. Finally, PYMOL and LIGPLOT (https://www.ebi.ac.uk/thornton-srv/software/LIGPLOT/) are used for visualization.
Cycloheximide inhibition experiment
The cells growing in exponential phase were inoculated in a 24-well plate according to the amount of about 5×104 cells/well, and cultured overnight in a 5% CO2 incubator at 37 ℃ until the cells grew to 50–80% density; the required plasmids were transfected with Lipofectamine 2000 (Thermo Fisher, Waltham, MA, USA); after transfection for 24 hours, 25 µg/mL of actinoketone was added for a certain period of time, and then the cells were collected, lysed and subjected to Western blotting.
Statistical analysis
The software SPSS 21.0 (IBM Corp., Armonk, NY, USA) and Excel were used for statistical analysis. Experimental data were the results of three biological replications and were expressed as mean ± standard deviation (SD). T-test was used for statistical analysis between the two groups. A P value <0.05 was considered statistically significant.
Results
Screening for artemisinin validity
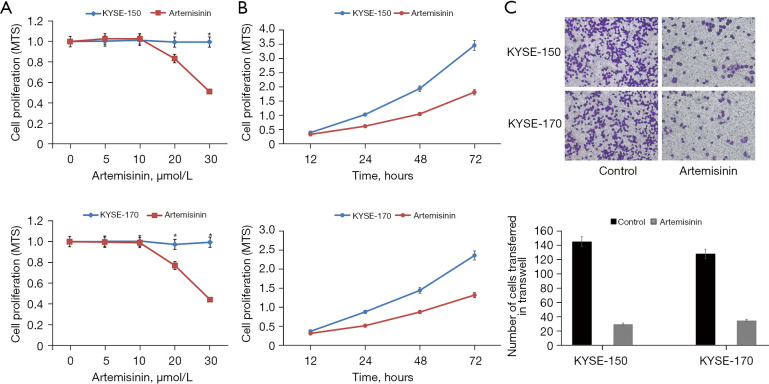
According to a previous study, the semi-inhibitory concentration of artemisinin on all kinds of tumor cell lines ranges from a few micromoles to dozens of micromoles (15). Although this concentration is higher than the nanomole concentration of its antimalarial treatment, it has less dosage and low toxicity compared with traditional chemotherapeutic drugs. In order to evaluate the effect of artemisinin on the EC cell line, we tested the cell activity at different concentrations. The results showed that the cell activity of KYSE-150 and KYSE-170 cells decreased significantly at the dose of 20 µmol/L. We chose 10 µmol/L as a non-toxic dose of artemisinin for follow-up experiments (Figure 1A).
Figure 1.
The role of artemisinin in EC cells. (A) The effect of different artemisinin concentrations on the viability of EC cells. (B) The effect of artemisinin on the proliferation of EC cells. (C) The effect of artemisinin on the metastasis of EC cells (crystal violet, ×400). *, P<0.05. EC, esophageal cancer.
Artemisinin inhibits cell proliferation and metastasis of EC cells
We added 10 µmol/L artemisinin to KYSE-150 and KYSE-170 EC cells for culture, and observed the value-added of EC cells at 12, 24, 48, and 72 hours of artemisinin exposure, respectively. The results showed that artemisinin significantly inhibited the growth of EC cells (Figure 1B). Through the transwell experiment, we found that the transfer of KYSE-150 decreased by 80%, and the transfer of KYSE-170 decreased by 73% (Figure 1C).
Artesunate inhibits aerobic glycolysis in EC cells
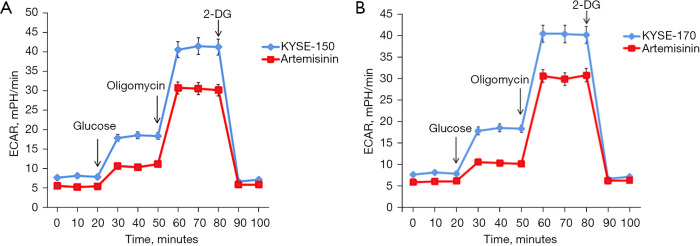
We detected the effect of artemisinin on aerobic glycolysis of EC cells by ECAR. The results showed that artemisinin significantly inhibited the level of aerobic glycolysis in KYSE-150 and KYSE-170 cells (Figure 2).
Figure 2.
Artemisinin inhibits glycolysis in EC cells. (A) The effect of artemisinin on the glycolysis rate was tested by ECAR in KYSE-150 cells. (B) The effect of artemisinin on the glycolysis rate was tested by ECAR in KYSE-170 cells. EC, esophageal cancer; ECAR, extracellular acidification rate; 2-DG, 2-deoxy-D-glucose.
Artemisinin inhibits the expression of transfer key proteins and glycolysis of key enzymes
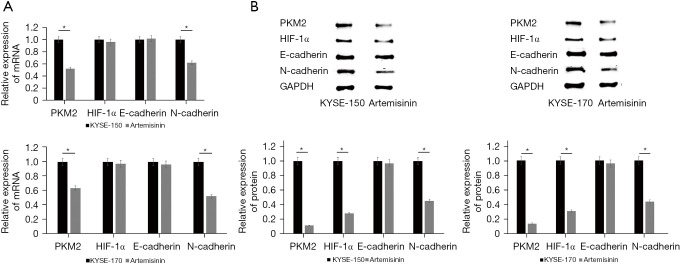
Because artemisinin inhibits the metastasis and glycolysis of EC cells, we verified the expression of key metastatic proteins (E-cadherin, N-cadherin) and glycolysis key rate-limiting enzymes (HIF-1α, PKM2). The results of quantitative polymerase chain reaction (q-PCR) test showed that the messenger RNA (mRNA) expression of N-cadherin and PKM2 decreased significantly after the application of artemisinin (Figure 3A). The results of Western blot showed that the protein expression levels of N-cadherin, HIF-1α, and PKM2 decreased significantly after artemisinin treatment (Figure 3B).
Figure 3.
Artemisinin inhibits the transfer of key proteins and glycolysis of key enzymes. (A) The levels of the transfer of key proteins and glycolysis of key enzymes were tested by q-PCR. (B) The levels of the transfer of key proteins and glycolysis of key enzymes were tested by Western blotting. *, P<0.05. q-PCR, quantitative polymerase chain reaction; PKM2, pyruvate kinase M2; HIF-1α, hypoxia-inducible factor-1α.
Cyberpharmacology predicts potential targets for the treatment of EC by aerobic glycolysis of artemisinin
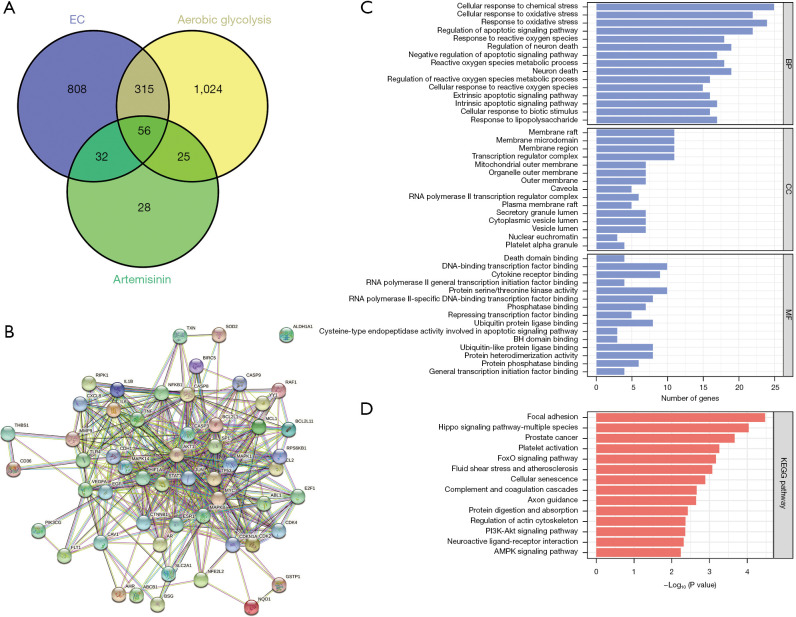
We input EC related information into the GeneCards database, selected correlation score ≥10.00, filtered the related genes, and retrieved 1,211 potential targets of EC. Similarly, 141 targets related to artemisinin and 1,420 targets related to aerobic glycolysis were found. The data of the 3 potential targets were mapped by Venny drawing software, and 56 common potential targets were obtained (Figure 4A). The target protein interaction network was constructed with 56 common potential targets through the STRING database (Figure 4B). The 56 common potential targets were analyzed by Metascape database for GO and KEGG enrichment analysis (Figure 4C,4D). Among them, we found the classic PI3K/AKT signal pathway, which is related to proliferation, differentiation, and apoptosis. The downstream target is mTOR, and the downstream transcription factors of mTOR include HIF-1α, c-Myc, FOXO, and so on. In the above experiments, we found that the protein level of HIF-1α in EC cells decreased significantly after the use of artemisinin.
Figure 4.
Construction of an artemisinin-glycolysis-EC core target network. (A) Artemisinin-glycolysis-EC common target intersection map. (B) Target protein interaction network. (C) Artemisinin-glycolysis-EC common target GO analysis. (D) Artemisinin-glycolysis-EC common target KEGG analysis. EC, esophageal cancer; BP, biological processes; CC, cellular components; MF, molecular function; GO, Gene Ontology; KEGG, Kyoto Encyclopedia of Genes and Genomes.
Artemisinin targets and degrades HIF-1α
We simulated the interaction between artemisinin and HIF-1α by molecular docking. The results showed that artemisinin was linked to the Asp571 (H) site of HIF-1α (Figure 5A). In order to determine the changes of HIF-1α protein after artemisinin treatment, cells were treated with cyclohexylamine. The results showed that artemisinin treatment of KYSE-150 and KYSE-170 promoted the degradation rate of HIF-1α protein (Figure 5B,5C).
Figure 5.
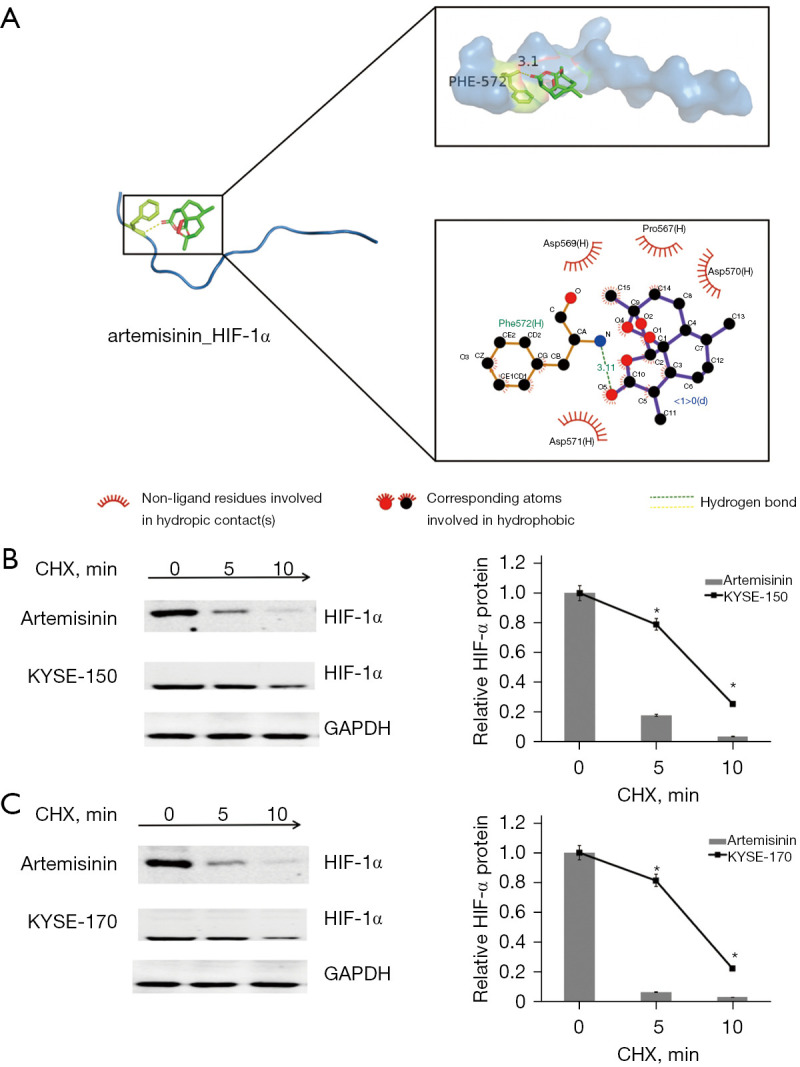
Artemisinin targets and degrades HIF-1α. (A) The binding site of artemisinin and HIF-1α. (B) The half-life of HIF-1α protein was assessed in KYSE-150 cells. Cells were treated with artemisinin. (C) The half-life of HIF-1α protein was assessed in KYSE-170 cells. Cells were treated with artemisinin. *, P<0.05. HIF-1α, hypoxia-inducible factor-1α; CHX, cycloheximide.
Discussion
Uncontrolled cell growth is the most important feature of cancer. After the tumor volume increases, the blood supply cannot keep up with the growth rate of fluid tissue, resulting in the formation of an anoxic microenvironment in the tumor (16). In the process of rapid growth of tumor cells, oxygen supply cannot meet the energy needs of mitochondria, so it can make up for the lack of energy of tumor cells by up-regulating the level of glycolysis, so as to escape the death caused by hypoxia (17). Aerobic glycolysis is also known as the Warburg effect. As a marker of tumor energy metabolism, the Warburg effect has been confirmed in many types of cancer cells (18,19). The glycolysis ability of tumor cells is 20–30 times higher than that of normal cells. The enhancement of glycolysis is proportional to the growth rate of cancer and is closely related to the invasive growth of the tumor (20). Relevant research shows, the risk characteristics of cancer prognosis were screened by glycolysis gene expression profiling, and the results showed that the glycolysis-related gene sets differed significantly between adjacent cancer samples and paracancerous samples, and had good diagnostic significance for the survival prediction of patients (21). In turn, we can construct a prognostic model of glycolysis mRNA for EC, aiming to discover a genetic marker associated with glycolysis that can predict the survival of patients with EC.
Hypoxia-inducible factor-1 (HIF-1) is a transcriptional nuclear protein with a wide range of target genes, including a variety of target genes related to hypoxia adaptation, inflammatory development, and tumor growth. The ability of tumor cells to resist oxygen depletion is mainly attributed to the accumulation of HIF-1. The activation of HIF-1 promotes the Warburg effect through the transition from oxidative phosphorylation to glycolysis. HIF-1 is an isodimer consisting of 2 basic helix-cyclic-helical Per-Arnt-Sim (PAS) proteins, HIF-1α and HIF-1β. HIF-1α is expressed cumulatively under hypoxic conditions, and HIF-1β is constitutive expression. It can be seen from the KEGG database that HIF-1α target genes, including genes encoding glycolysis receptors and enzymes (GLUT1, HKII, LDHA, PDK1), are adapted to the hypoxic state of tumors. Therefore, we believe that HIF-1α is one of the important reasons for the enhancement of aerobic glycolysis in tumor cells, and targeted regulation of HIF-1α has become an effective way to inhibit glycolysis for cancer treatment (22).
With the deepening of the research on the modernization of TCM, we understand that TCM can improve the immune activity of the body, regulate specific signal pathways, inhibit tumor angiogenesis, and inhibit tumor cell invasion and metastasis. As a result, TCM has garnered increasing attention (23,24). The botanical agents and their derivatives, including flavonoid, isoflavone, icariin and curcumin, have been proved to be effective in many types of cancer therapy (25,26). For example, Luteolin, Apigenin, Quercetin, Artemisinin, Sinomenine, and many more.
Artemisinin is a sesquiterpene lactone compound with a unique chemical structure derived from artemisia arbutus of the comfrey genus. It has been shown to have anti-tumor, anti-viral, anti-fungal, anti-tuberculosis, and immunomodulatory effects. Artemisinin and its derivatives directly kill tumor cells through cytotoxic effects, induce cell cycle arrest, regulate tumor microenvironment, and thus inhibit tumor cell invasion and metastasis (27). We can think of artemisinin’s anti-tumor effects as all-encompassing. However, its mechanism had remained unclear. Therefore, this study explored the role and mechanism of artemisinin in EC.
We chose 10 µmol/L artemisinin as a non-toxic dose and found that artemisinin could inhibit the proliferation and metastasis of EC cells. In our previous studies, we found that aerobic glycolysis is a prominent feature of glucose metabolism in cancer progression, which can continuously support cancer cell proliferation and metastasis. Therefore, we examined the effect of artemisinin on aerobic glycolysis of EC and found that artemisinin could significantly inhibit glycolysis and the expression of key glycolytic proteins HIF-1α and PKM2.
Nowadays, with the development of the economy and the improvement of living standards, human beings are more and more eager for health and longevity, which is inseparable from the research and development of new drugs, but today’s new drug research and development has many difficulties, low bioavailability, low activity, high research and development costs, long research and development time, etc. The emergence of network pharmacology can bring new ideas and methods to drug research and development, it uses the principles of system biology, network biology, gene redundancy and other principles to explain the process of disease development, using the overall view of network balance to understand the interaction between drugs and the body, and its “multi-gene, multi-target” characteristics coincide with the treatment concept of complex diseases, so its application value is extremely high .We found 56 potential common targets of ES-artemisinin-aerobic glycolysis by using network pharmacology technology. Through GO and KEGG enrichment analyses, we obtained the related biological processes and signal pathways of artemisinin regulating ES through aerobic glycolysis pathway, in which we found PI3K/AKT signal pathway. The downstream target of the PI3K/AKT signal pathway is HIF-1α, the key protein of aerobic glycolysis which can be significantly inhibited by artemisinin.
We believe that HIF-1α can be used as a direct target for artemisinin to inhibit glycolysis of EC cells. We simulated the interaction between artemisinin and HIF-1α by molecular docking. It was found that artemisinin could connect with the Asp571 (H) site of HIF-1α and promote the degradation of HIF-1α. As shown earlier, HIF-1α plays an important role in aerobic glycolysis, and artemisinin inhibits glycolysis by inhibiting HIF-1α. This also explains the mechanism by which artemisinin inhibits the proliferation and metastasis of EC cells. The rapid proliferation of tumor cells benefits from glycolysis to obtain the energy for growth and diffusion. Glycolysis is inhibited by artemisinin, resulting in insufficient supply of energy and materials, and effective control of cancer cell proliferation and metastasis.
PKM2 is a specific enzyme in cancer tissue, which is highly expressed in many cancer cells. It can promote aerobic glycolysis by regulating transcriptional regulation. It is the final rate-limiting step of catalytic glycolysis. At the same time, it is closely related to the occurrence, development, and metastasis of malignant tumors (28). Studies have shown that PKM2 and HIF-1α compensate each other and cooperate with each other. HIF-1α can activate PKM2 gene transcription, and PKM2 in turn interacts with HIF-1α subunits and promotes the transactivation of HIF-1α target genes, thus enhancing the cell response to hypoxia or oncogene activation (29). It is worth noting that artemisinin significantly decreased the expression of PKM2 in both the mRNA and protein levels of EC cells. However, there was no significant difference in mRNA expression level of HIF-1α, but its protein expression level decreased significantly. It is well known that protein levels in a steady state are largely determined by the number of transcripts, yet in dynamic stages, such as cell differentiation or stress, the post-transcriptional process may lead to more changes. Of course, there is also post-translation regulation or modification. Or the drug itself is a protein directly acting on HIF-1α, which seems to be consistent with the results of our molecular docking experiments. However, it is clear that artemisinin directly regulates the protein level of HIF-1α directly beyond the transcriptional stage, at least for now.
The transformation of glucose utilization by tumor cells from oxidative phosphorylation to glycolysis is now considered to be a major feature of tumors. This change in energy metabolism is regulated by complex factors, including the pressure of tumor microenvironment and genetic changes. Some studies have shown that inhibition of the tumor glycolysis pathway can effectively inhibit tumor cell proliferation and metastasis and even kill tumor cells (30,31). Therefore, we believe that artemisinin can inhibit the proliferation and metastasis of ES by regulating the glycolysis level. Unfortunately, for some special reason we were unable to complete animal experiments. It is undeniable that animal experiments are a key step towards clinical application of artemisinin, so we look forward to the next opportunity to complete animal experiments for in vivo validation.
Compared with the energy metabolism of normal cells, tumor cells are a complex process of variation; especially in the process of glucose metabolism, there are still many questions to be solved. Glycolysis of tumor cells is controlled by the expression of key enzymes. Therefore, people are exploring the targeted treatment of tumors by inhibiting the activities of key enzymes in the tumor glycolysis pathway. However, the activity of glycolytic enzymes may change due to the influence of tumor microenvironment, and the inhibition of a single target may not be enough to inhibit tumor proliferation, or even cause drug resistance. The combined therapy of multiple glycolytic enzyme targets is worthy of our further study.
In summary, we demonstrated that artemisinin can target HIF-1α targets to reduce glycolysis levels and thus inhibit the development of EC.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-877/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-877/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-877/coif). The authors have no conflicts of interest to declare.
(English Language Editor: J. Jones)
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2.Zhang S, Sun K, Zhen R, et al. Cancer incidence and mortality in China, 2015. Journal of the National Cancer Center 2021;1:2-11. 10.1016/j.jncc.2020.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zeng H, Zheng R, Guo Y, et al. Cancer survival in China, 2003-2005: a population-based study. Int J Cancer 2015;136:1921-30. 10.1002/ijc.29227 [DOI] [PubMed] [Google Scholar]
- 4.Shahbar A, Alshamrani M, Aseeri M, et al. Safety and discontinuation rate of immune checkpoint inhibitors used in patients with solid and hematological malignancies. A population based retrospective analysis. J Oncol Pharm Pract 2022;28:771-6. 10.1177/10781552211017634 [DOI] [PubMed] [Google Scholar]
- 5.Sun G, Liu H, Shi X, et al. Treatment of patients with cancer using PD-1/PD-L1 antibodies: Adverse effects and management strategies (Review). Int J Oncol 2022;60:74. 10.3892/ijo.2022.5364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Niu H, Liu J. Advances in TCM treatment for metastasis of tumors. J Tradit Chin Med 2003;23:151-7. [PubMed] [Google Scholar]
- 7.Eastman RT, Fidock DA. Artemisinin-based combination therapies: a vital tool in efforts to eliminate malaria. Nat Rev Microbiol 2009;7:864-74. 10.1038/nrmicro2239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun X, Yan P, Zou C, et al. Targeting autophagy enhances the anticancer effect of artemisinin and its derivatives. Med Res Rev 2019;39:2172-93. 10.1002/med.21580 [DOI] [PubMed] [Google Scholar]
- 9.Woerdenbag HJ, Moskal TA, Pras N, et al. Cytotoxicity of artemisinin-related endoperoxides to Ehrlich ascites tumor cells. J Nat Prod 1993;56:849-56. 10.1021/np50096a007 [DOI] [PubMed] [Google Scholar]
- 10.Lai HC, Singh NP, Sasaki T. Development of artemisinin compounds for cancer treatment. Invest New Drugs 2013;31:230-46. 10.1007/s10637-012-9873-z [DOI] [PubMed] [Google Scholar]
- 11.Reiter C, Herrmann A, Çapci A, et al. New artesunic acid homodimers: potent reversal agents of multidrug resistance in leukemia cells. Bioorg Med Chem 2012;20:5637-41. 10.1016/j.bmc.2012.07.015 [DOI] [PubMed] [Google Scholar]
- 12.Cao Y, Feng YH, Gao LW, et al. Artemisinin enhances the anti-tumor immune response in 4T1 breast cancer cells in vitro and in vivo. Int Immunopharmacol 2019;70:110-6. 10.1016/j.intimp.2019.01.041 [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y, Wang Y, Li Y, et al. Dihydroartemisinin and artesunate inhibit aerobic glycolysis via suppressing c-Myc signaling in non-small cell lung cancer. Biochem Pharmacol 2022;198:114941. 10.1016/j.bcp.2022.114941 [DOI] [PubMed] [Google Scholar]
- 14.Hu CJ, Zhou L, Cai Y. Dihydroartemisinin induces apoptosis of cervical cancer cells via upregulation of RKIP and downregulation of bcl-2. Cancer Biol Ther 2014;15:279-88. 10.4161/cbt.27223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Efferth T, Dunstan H, Sauerbrey A, et al. The anti-malarial artesunate is also active against cancer. Int J Oncol 2001;18:767-73. 10.3892/ijo.18.4.767 [DOI] [PubMed] [Google Scholar]
- 16.Qiu GZ, Jin MZ, Dai JX, et al. Reprogramming of the Tumor in the Hypoxic Niche: The Emerging Concept and Associated Therapeutic Strategies. Trends Pharmacol Sci 2017;38:669-86. 10.1016/j.tips.2017.05.002 [DOI] [PubMed] [Google Scholar]
- 17.Moreno-Sánchez R, Rodríguez-Enríquez S, Marín-Hernández A, et al. Energy metabolism in tumor cells. FEBS J 2007;274:1393-418. 10.1111/j.1742-4658.2007.05686.x [DOI] [PubMed] [Google Scholar]
- 18.Updegraff BL, Zhou X, Guo Y, et al. Transmembrane Protease TMPRSS11B Promotes Lung Cancer Growth by Enhancing Lactate Export and Glycolytic Metabolism. Cell Rep 2018;25:2223-2233.e6. 10.1016/j.celrep.2018.10.100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiao L, Zhang HL, Li DD, et al. Regulation of glycolytic metabolism by autophagy in liver cancer involves selective autophagic degradation of HK2 (hexokinase 2). Autophagy 2018;14:671-84. 10.1080/15548627.2017.1381804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu L, Chen X, Wang L, et al. Oncogenic virus-induced aerobic glycolysis and tumorigenesis. J Cancer 2018;9:3699-706. 10.7150/jca.27279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang L, Zhao L, Bi J, et al. Glycolysis gene expression profilings screen for prognostic risk signature of hepatocellular carcinoma. Aging (Albany NY) 2019;11:10861-82. 10.18632/aging.102489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zheng F, Chen J, Zhang X, et al. The HIF-1α antisense long non-coding RNA drives a positive feedback loop of HIF-1α mediated transactivation and glycolysis. Nat Commun 2021;12:1341. 10.1038/s41467-021-21535-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu TJ, Hu S, Qiu ZD, et al. Anti-Tumor Mechanisms Associated With Regulation of Non-Coding RNA by Active Ingredients of Chinese Medicine: A Review. Front Oncol 2020;10:634936. 10.3389/fonc.2020.634936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu D, Li A, Lv Y, et al. Traditional Chinese Medicine: A Class of Potentially Reliable Epigenetic Drugs. Front Pharmacol 2022;13:907031. 10.3389/fphar.2022.907031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Horie S. Chemoprevention of prostate cancer: soy isoflavones and curcumin. Korean J Urol 2012;53:665-72. 10.4111/kju.2012.53.10.665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sarkar FH, Li Y, Wang Z, et al. Lesson learned from nature for the development of novel anti-cancer agents: implication of isoflavone, curcumin, and their synthetic analogs. Curr Pharm Des 2010;16:1801-12. 10.2174/138161210791208956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li D, Zhang J, Zhao X. Mechanisms and Molecular Targets of Artemisinin in Cancer Treatment. Cancer Invest 2021;39:675-84. 10.1080/07357907.2021.1954190 [DOI] [PubMed] [Google Scholar]
- 28.Wong N, Ojo D, Yan J, et al. PKM2 contributes to cancer metabolism. Cancer Lett 2015;356:184-91. 10.1016/j.canlet.2014.01.031 [DOI] [PubMed] [Google Scholar]
- 29.Demaria M, Poli V. PKM2, STAT3 and HIF-1α: The Warburg's vicious circle. JAKSTAT 2012;1:194-6. 10.4161/jkst.20662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang RA, Lu YY, Fan DM. Reasons for cancer metastasis: A holistic perspective. Mol Clin Oncol 2015;3:1199-202. 10.3892/mco.2015.623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johar D, Elmehrath AO, Khalil RM, et al. Protein networks linking Warburg and reverse Warburg effects to cancer cell metabolism. Biofactors 2021;47:713-28. 10.1002/biof.1768 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as