Abstract
Since more than 3 decades, schizophrenia (SZ) has been regarded as a neurodevelopmental disorder. The neurodevelopmental hypothesis proposes that SZ is associated with genetic and environmental risk factors, which influence connectivity in neuronal circuits during vulnerable developmental periods. We carried out a non-systematic review of genetic/environmental factors that increase SZ risk in light of its neurodevelopmental hypothesis. We also reviewed the potential impact of SZ-related environmental and genetic risk factors on grey and white matter pathology and brain function based on magnetic resonance imaging and post-mortem studies. Finally, we reviewed studies that have used patient-derived neuronal models to gain knowledge of the role of genetic and environmental factors in early developmental stages. Taken together, these studies indicate that a variety of environmental factors may interact with genetic risk factors during the pre- or postnatal period and/or during adolescence to induce symptoms of SZ in early adulthood. These risk factors induce disturbances of macro- and microconnectivity in brain regions involving the prefrontal, temporal and parietal cortices and the hippocampus. On the molecular and cellular level, a disturbed synaptic plasticity, loss of oligodendrocytes and impaired myelination have been shown in brain regions of SZ patients. These cellular/histological phenotypes are related to environmental risk factors such as obstetric complications, maternal infections and childhood trauma and genetic risk factors identified in recent genome-wide association studies. SZ-related genetic risk may contribute to active processes interfering with synaptic plasticity in the adult brain. Advances in stem cell technologies are providing promising mechanistic insights into how SZ risk factors impact the developing brain. Further research is needed to understand the timing of the different complex biological processes taking place as a result of the interplay between genetic and environmental factors.
Keywords: Schizophrenia, Neurodevelopment, Risk genes, Environmental factors, Connectivity, Synaptic plasticity, Neuron, Oligodendrocyte
Introduction
Mental disorders, including schizophrenia (SZ), are the leading medical cause of years lived with disability worldwide (GBD 2019 Diseases and Injuries Collaborators 2020). SZ is a severe neuropsychiatric disease that typically emerges in late adolescence, persists throughout adult life, and affects about 1% of the population (Jablensky 1995). Together, the direct and indirect costs of SZ-related psychotic disorders amount to €93.9 billion (Gustavsson et al. 2011). The high hospitalization rates and high levels of disease-related incapacity to work and early retirement lead to a high disease burden (GBD 2019 Diseases and Injuries Collaborators 2020). Furthermore, a substantial proportion of patients (30–50%) experience an unfavourable disease course and residual symptoms, i.e., cognitive impairment and negative symptoms, that remain after acute treatment (Falkai and Schmitt 2022). These symptoms are generally very difficult to treat with psychotherapy or antipsychotics and cause multifaceted disability, including functional impairments in everyday life that prevent successful social and professional reintegration (Nielsen et al. 2015). In SZ, twin studies have reported a heritability estimate of this disorder of about 60–80% (Sullivan et al. 2003). In addition, environmental factors such as obstetric complications, virus infections of the mother and childhood trauma also contribute to an increased risk of the disease (Schmitt et al. 2014).
The neurodevelopmental hypothesis
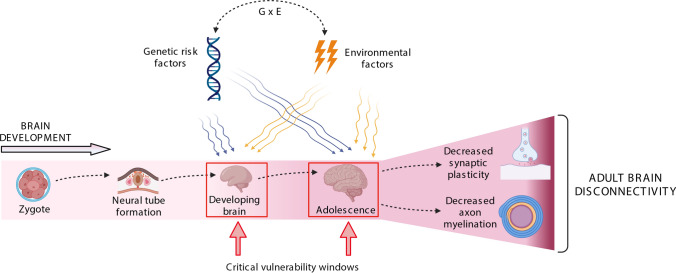
It has been proposed that a variety of environmental factors may interact with genetic risk factors during the pre- or postnatal period to induce symptoms of SZ in early adulthood (Schmitt et al. 2014). This neurodevelopmental hypothesis was first introduced in 1986 (Weinberger 1986) and proposes that SZ is related to genetic and environmental adverse conditions leading to abnormal brain development during the perinatal period, whereas symptoms of the disease appear in early adulthood (Weinberger 1996). In SZ, a pre-existing disturbed neuronal network may be triggered towards disease during a vulnerable period in adolescence. Specifically, the second trimester and perinatal period has been implicated in the pathophysiology of SZ (Fatemi and Folsom 2009). Synaptogenesis is established during the second and third trimester of pregnancy and continues during childhood (Hall and Bray 2022). In a proposed "two-hit" model, early perinatal insults (genetic background and/or environmental factors) may lead to dysfunction of neuronal networks and a vulnerable status, while a second "hit" during a critical brain development period in adolescence may induce the onset of the disease (Fig. 1) (Keshavan and Hogarty 1999). During this critical period in adolescence, a synaptic pruning process with excessive elimination of synapses and loss of synaptic plasticity may lead to a disturbed microconnectivity and exacerbation of symptoms in the predisposed brain (Keshavan and Hogarty 1999; Schmitt et al. 2014). Additionally, myelination of the heteromodal association cortex like the prefrontal cortex occurs during this period (Peters et al. 2012) and a deficit in myelination and oligodendrocyte number may contribute to disturbed macroconnectivity in SZ (Hof et al. 2003; Schmitt et al. 2009; Falkai et al. 2016). According to the neurodevelopmental hypothesis, prodromal and symptoms of SZ occur for the first time in adolescence (Häfner 2007). Along the same lines, those subjects who will eventually suffer SZ already show unspecific signs of a slight brain dysfunction before the onset of the disease, manifested as a mild cognitive impairment or subtle motor abnormalities (Cuesta et al. 2018; Kahn 2020).
Fig. 1.
Impact of genetic and environmental factors during neurodevelopment in schizophrenia: two vulnerable periods in brain development are the prenatal period and adolescence. During these critical periods, genetic and environmental risk factors of schizophrenia act together to induce deficits in synaptic plasticity and myelination. As consequence, impaired micro- and macroconnectivity is the basis of cognitive deficits and symptoms of the disease, which arise in young adulthood
SZ-related genetic factors with impact on neurodevelopment
Family, twin, and adoption studies have provided compelling evidence of the contribution of genetic factors to SZ risk, with an estimated heritability (h2) around 60 to 80% (Sullivan et al. 2003). These estimates have been confirmed by nation-wide register-based studies (Lichtenstein et al. 2009). Recent large-scale genomics approaches have finally improved our understanding of how specific genetic factors contribute to SZ risk. Genome-wide association studies (GWASs) have provided convincing evidence of the remarkable role that common genetic variants play in the definition of the individual vulnerability background to suffer SZ (Dennison et al. 2020).
The largest GWAS in SZ to date has reported 287 independent genetic risk loci for SZ (Trubetskoy et al. 2022). The results of the study provided biological insight into the biological underpinnings of SZ: the associations were found to be enriched in cortical inhibitory interneurons and excitatory neurons in the cerebral cortex and hippocampus (pyramidal and granule cells), reinforcing the notion that SZ is primarily a neuronal disorder. The cellular components or molecular functions more enriched for these associations were related to neuronal excitability, and synaptic (especially the post-synapse) structure and function. In addition, the enrichment of these associations in biological processes such as nervous system development, regulation of neuron differentiation, or neurogenesis, were of special interest within the framework of the neurodevelopmental hypothesis of SZ. Moreover, the C4A locus in the Major Histocompatibility Complex (MHC) in chromosome 6, which is the locus with the top association with SZ, has been shown to participate in synaptic pruning, determination of synapse density, and microglial engulfment of synapses (Sekar et al. 2016; Yilmaz et al. 2021).
Moreover, large-scale genomic studies analysing structural variants (copy number variation, CNV) or rare (low frequency) genetic variation have also provided convergent evidence regarding the impact of SZ genetic risk burden on the synapse (Marshall et al. 2017; Singh et al. 2022). The case of structural variants is especially interesting since several of the CNV loci identified in this study overlap with genomic regions previously implicated with developmental syndromes like autism spectrum disorder (2p16.3 [NRXN1 gene, Neurexin-1 protein]) (Tromp et al. 2021) or 22q11.2 deletion syndrome (Francisco 2022). However, an important limitation of genetic association studies is that they are not designed to discriminate the developmental stage at which each genetic risk factor contributes to the individual vulnerability background.
Despite this hurdle, a growing body of evidence indicates that some of the genes involved in SZ risk act, at least, during prenatal development (Hall and Bray 2022). NRXN1, overlapping with a SZ risk CNV locus (2p16.3), plays a remarkable role in synapse formation at early stages of development (Tromp et al. 2021). Exome sequencing has identified several genes also important during early stages of development for neurite outgrowth and axon/dendrite branching: SP4 (Transcription Factor Sp4), SETD1A (Histone-lysine N-methyltransferase SETD1A), or TRIO (Triple functional domain protein) (Singh et al. 2022). Finally, GWAS have identified common risk variants in several genes that also participate in neurite outgrowth and synapse formation: ZNF804A (Zinc finger protein 804A), CNTN4 (Contactin-4), LRRC4B (Leucine-rich repeat-containing protein 4B), or DCC (Netrin receptor DCC) (Trubetskoy et al. 2022). Several of these genes (NRXN1, TRIO) play a prominent role in synaptic plasticity throughout development and adult life. In these cases, identifying the developmental timing of their aetiological mechanism using only genetic association studies remains a challenge. A better knowledge of the effects of genetic variation on gene regulation during pre- and postnatal development could help to clarify these questions. In this vein, some studies have integrated the results of GWAS with information on quantitative trait loci (QTLs) known to be active in the foetal brain. These approaches have shown that mechanistic effects of CNTN4, PCDHA7, PCDHA8 (Protocadherins alpha-7 and alpha-8), and other genes on synaptic SZ risk are very likely to take place in utero, although posterior postnatal influences cannot be ruled out (Walker et al. 2019; Hall et al. 2021). This is the case of NRNX1 and TRIO genes, which also have clear functions in glutamatergic transmission in the adult hippocampus (Hall and Bray 2022). Such a dual role also can be observed in CACNA1C gene, one of the most robust transdiagnostic genetic markers for SZ and bipolar disorder (Mullins et al. 2021; Trubetskoy et al. 2022). CACNA1C codes for a voltage-dependent calcium channel, voltage-dependent L-type calcium channel subunit alpha-1C (also known as Cav1.2), with an important role both in synaptic plasticity in adult brain (Nanou and Catterall 2018) and regulation of Ca2+ activity and formation of neuronal networks during neurodevelopment (Smedler et al. 2022). Interestingly, a recent post-mortem study in the temporal cortex (BA21) of SZ patients has reported a downregulation of Cav1.2 (CACNA1C) and Cav1.3 (CACNA1D) mRNAs (Schmitt et al. 2022).
Induced pluripotent stem cell (IPSC) modelling
Recent advances in stem cell technology, now allowing to obtain iPSCs from blood, skin, or other somatic tissues and their reprogramming into cell types of the central nervous system, holds promise for the understanding of the developmental functional cellular mechanisms that connect genetic with an increased SZ risk at unprecedented resolution (Howes and Shatalina 2022). For example, several recent studies have shown the deleterious effect of deletions and aberrant expression of NRXN1 gene on neuronal excitability and synaptic function in iPSC-derived neurons (Flaherty et al. 2019; Pak et al. 2021; Avazzadeh et al. 2021). In the case of ZNF804 gene, IPSC-derived neurons were used to uncover a novel subcellular distribution within somatodendritic compartments and the regulatory function of this molecule in neurite formation and dendritic spine structure (Deans et al. 2017). Another study based on IPSC-derived developing cortical interneurons has shown that several SZ GWAS loci converge on the PKC pathway, leading to an abnormal arborization during development (Liu et al. 2022). The same study, interestingly, observed that in IPSC-derived developing glutamatergic neurons SZ GWAS loci converge on the ion transport pathway, and disruption of one of the members of this pathway, CACNA1D, led to alteration in calcium currents in these developing cells (Liu et al. 2022).
Such cellular models have also provided interesting hints to understand the role of classical chromosomal aberrations known to increase risk for schizophrenia. In the case of 22q11.2 deletion, the use of such models has allowed the identification of trans effects of this deletion during the process of neuronal differentiation with a large effect on genes previously identified in SZ GWAS, as for example MEF2C (Myocyte-specific enhancer factor 2C) (Nehme et al. 2022). A similar approach based on the 15q13.3 deletion identified a SZ-associated loss of function genetic variant in the OTUD7A gene (OTU domain-containing protein 7A), mapping to this region, that led to impaired synapse development in IPSC-derived excitatory neurons (Kozlova et al. 2022).
Environmental factors contributing to risk of SZ
In SZ, robust evidence indicates that cannabis use, exposure to stressful events during childhood and adulthood, and a history of obstetric complications are well-replicated risk factors (Belbasis et al. 2018). However, these environmental factors, which play a role during the prenatal period and adolescence have also been related to depression, anxiety, autism spectrum disorder and attention deficit hyperactivity disorder (ADHD) (Markham and Koenig 2011; Class et al. 2014). The second trimester of pregnancy and the adolescence are particularly vulnerable brain development periods very sensible to environmental stressors (Fig. 1). Several meta-analyses have shown an association between birth and obstetric complications and SZ. The pooled odds ratio for the exposure to OCs on subsequent development of SZ was 2.0 (Geddes and Lawrie 1995). Obstetric complications include bleeding, preeclampsia, diabetes, rhesus incompatibility, asphyxia, uterine atony, and with the highest risk connected to emergency caesarean section and placental abruption. The foetal abnormalities with the highest effect on SZ risk are low birth weight (OR ~ 3.2), small head circumference (OR ~ 1.6) and congenital malformations (OR ~ 2–2.5) (Waddington et al. 2008; Harper et al. 2015). SZ has been associated with low gestational age at birth with an odds ratio of 3.2 (Hultman et al. 1999). Maternal bleeding during pregnancy has been found to be associated with SZ with an odds ratio of 3.5 (Hultman et al. 1999). Low birth weight is a general marker of disturbances of the intrauterine environment (Fineberg et al. 2013). Another meta-analysis found associations between SZ and different obstetric complications, use of incubator, prematurity and premature rupture of membranes (Geddes et al. 1999). Studies with individuals at high risk for psychosis who converted into SZ showed that they present more obstetric complications compared to non-converting individuals (Mittal et al. 2009). A common factor of these complications is perinatal hypoxia (Zornberg et al. 2000), which in animal models induced SZ-associated behavioural deficits in early adulthood, such as deficits in prepulse inhibition of acoustic startle response (Fendt et al. 2008). A recent meta-analysis revealed that SZ patients with obstetric complications had a poorer verbal and working memory performance than patients without obstetric complications (Amoretti et al. 2022).
Maternal stress during the prenatal period has been shown to be a risk factor of SZ (Markham and Koenig 2011). These risk factors include maternal psychological stress exposure due to e.g. unwantedness of a pregnancy, war experience or natural disaster (Brown 2002; Spauwen et al. 2004). Children of mothers who experienced serious life events such as war experience developed SZ more frequently than expected (van Os and Selten 1998). Prenatal stress is known to influence function of the hypothalamic–pituitary–adrenal (HPA) axis, which is the major stress neuroendocrine system of the body, and the protective capacity of the placenta (Weinstock 2008). Childhood trauma is a severe form of stress, which influences the HPA axis and renders individuals more vulnerable to develop SZ (Popovic et al. 2019). In a meta-analysis of 18 case–control studies, adverse experiences in childhood significantly increased the risk to develop SZ (Varese et al. 2012). Specifically, a strong association between childhood adversity, including trauma, and SZ has been shown with odds ratio between 2 and 3 (Varese et al. 2012). Epidemiological studies show that early stress in the form of abuse and neglect during childhood plays an important role as a risk factor for SZ (Bonoldi et al. 2013). In SZ patients, although the most frequently reported subtype of trauma was emotional neglect, also rates of physical abuse and physical neglect were increased (Larsson et al. 2013). However, childhood trauma is not only a risk factor for SZ, but also for other mental disorders such as affective disorders and ADHD (Popovic et al. 2019).
Potential stress-related factors for SZ are migration and urbanicity. A meta-analysis reported an association with urban environment and SZ (van Os et al. 2010). Individuals living in a higher degree of urbanization had a higher risk to develop SZ than people living in rural areas (Pedersen and Mortensen 2001). In healthy probands, city living was associated with increased amygdala activity, whereas urban upbringing affected the anterior cingulate cortex, and the stress response (Lederbogen et al. 2011). In first- and second-generation migrants as well as in minority groups across all cultures, psychotic symptoms have been shown to be increased (Rapoport et al. 2012) (37). It has been assumed that social status, e.g. occupying a minority position or experiencing social exclusion, promotes the development of SZ (van Os et al. 2010). In addition, maternal malnutrition has been related to the risk of brain defects and neuropsychiatric disorders including SZ (Cortés-Albornoz et al. 2021). Famine periods during second world war and in China doubled the risk for SZ (Susser et al. 1996; Xu et al. 2009). As consequence of malnutrition, deficits in vitamin D, polyunsaturated fatty acids, folic acid, choline, and iron intake have been regarded to play a role in the pathophysiology of the disease (Martinat et al. 2021; Freedman et al. 2021).
Infections during pregnancy activate the maternal immune system and can trigger neuroinflammation of the foetal brain during neurodevelopment. Evidence from animal studies suggests that SZ-related symptoms can be induced by viral infections with e.g. Influenza A and Cytomegalovirus (Elgueta et al. 2022) or perinatal induction of neuroinflammation with e.g. poly I:C, which mimics anti-viral innate immune responses (Ding et al. 2019). Severe neuroinflammation during pregnancy has been linked to preterm births, abortions, and microcephaly (Ganguli and Chavali 2021). In nonhuman primates, maternal immune activation induces cognitive dysfunction and deficits in brain growth, characterized by grey and white matter prefrontal volume deficits in adulthood (Vlasova et al. 2021). Future studies should investigate the impact of SARS-CoV-2 infection during the prenatal period since the consequences of the cytokine storm on brain development are unknown (Figueiredo et al. 2021). In fact, retrospective studies have shown an association between SZ and timing of birth during infectious epidemics induced by influenza, polio, diphtheria and measles (Eyles 2021).
Impact of neurodevelopmental disturbances on brain connectivity in SZ
One of the first neuroimaging findings in SZ was the enlargement of ventricles (Johnstone et al. 1976). This was followed by magnetic resonance imaging (MRI) studies demonstrating more subtle grey matter volume loss especially in the prefrontal cortex, superior temporal gyrus and cingulate cortex (Qi et al. 2022). A meta-analysis of voxel-based morphometry and diffusion tensor imaging studies revealed widespread white matter alterations including decreased fractional anisotropy in fronto-temporal-limbic pathways (Vitolo et al. 2017). Dysconnectivity in fronto-temporal and limbic regions has been described by resting-state functional MRI studies (Brandl et al. 2019). One of the most replicated structural MRI-based finding in SZ is hippocampal volume reduction with volume loss in all subregions (Haukvik et al. 2018). The neuronal network between the prefrontal cortex and hippocampus is critical for cognitive domains such as working memory and verbal memory (Bähner and Meyer-Lindenberg 2017; Vargas et al. 2018). Such a brain network has been shown to be disturbed in SZ, mainly due to neurodevelopmental disturbances (Bullmore et al. 1997; Peters et al. 2012). Animal models studies provided convergent evidence, since perinatal hippocampal lesions induced dysfunction of the prefrontal cortex in early adulthood of rats, leading to an impaired SZ-related behaviour such as reduced prepulse inhibition of acoustic startle response (Lipska 2004).
In adolescent subjects with clinical high risk for SZ, those who convert to psychosis showed accelerated gray matter reduction in the prefrontal cortex and enlarged ventricles compared with those subjects who did not convert and healthy controls (Cannon et al. 2015). A recent meta-analysis confirmed grey matter volume loss of the right and left superior frontal gyrus in subjects at high risk for psychosis (Ding et al. 2019). Moreover, lower cortical thickness has been observed in a large sample of individuals with clinical high risk for psychosis (ENIGMA Clinical High Risk for Psychosis Working Group et al. 2021). Additionally, patients with adolescent onset of SZ had white matter abnormalities compared to healthy controls, pointing to a neurodevelopmental pathology (Seitz-Holland et al. 2022). During this vulnerable brain period, the prefrontal cortex matures through synaptic pruning and myelination (Huttenlocher 1979; Huttenlocher and Dabholkar 1997; Gogtay et al. 2004). Accordingly, a loss of synaptic elements in the prefrontal cortex has been detected in post-mortem studies in SZ (Berdenis van Berlekom et al. 2020). This loss of microconnectivity can be accompanied by a deficit in myelination, leading to disturbed macroconnectivity. In the prefrontal cortex and hippocampal subregion cornu ammonis 4, a loss of oligodendrocytes, which are the myelinating glia cells of the brain, has been reported in SZ (Hof et al. 2003; Schmitt et al. 2009; Falkai et al. 2016). The loss of hippocampal oligodendrocytes was associated with decreased volumes in the neuronal Papez circuit, pointing to impaired connectivity (Falkai et al. 2020).
Using neuroimaging, environmental factors have been shown to influence connectivity in SZ. For instance, in patients with SZ, childhood trauma was associated with disturbances of white matter integrity and functional connectivity in neuronal networks (Cancel et al. 2019). Animal models have shown that chronic stress results in degeneration of hippocampal neurons and atrophy of dendrites (Sapolsky et al. 1990; Watanabe et al. 1992). In SZ patients and their siblings, foetal hypoxia predicted reduced gray matter volume and increased cerebrospinal fluid, most strongly in the temporal lobe. In SZ patients, prenatal hypoxia correlated also with ventricular enlargement (Cannon et al. 2002). Obstetric complications induce brain abnormalities ranging from decreased grey matter volume and increased ventricles up to reduced hippocampus volume (Costas-Carrera et al. 2020). A reduced hippocampus volume has been reported in SZ patients and controls with obstetric complications (Haukvik et al. 2010). Asphyxia at birth was related to smaller intracranial volume and smaller cortical surface areas in frontal, temporal, insular and parietal regions (Wortinger et al. 2020). In premature infants, perinatal white matter injury is based on hypoxia and is accompanied by neuroinflammation, decreased oligodendrocyte maturation and myelin damage (Motavaf and Piao 2021). Elevated expression of inflammation-related genes and an activation of microglia, the resident immune defenders of the brain, have been detected in post-mortem studies in SZ (van Kesteren et al. 2017). A relationship between premature birth, perinatal hypoxia, white matter deficits with oligodendrocyte damage and an activated immune system has been proposed to underlie the pathophysiology of SZ (Chew et al. 2013; Jenkins 2013). In this context, maternal infection with immune activation during pregnancy has been shown to impair dendritic spine development and to impair synaptic plasticity (Pekala et al. 2021). Reduced synaptic plasticity along with reduced dendritic spines, decreased expression of synaptic genes and abnormal synaptic neurotransmission has been reported in SZ, is related to impaired connectivity (Fig. 1) and results in cognitive deficits (Wu et al. 2022).
Neuroimaging studies have provided interesting evidence of the interplay between SZ genetic risk and brain structure/function leading to behavioural outcomes frequently observed in SZ patients. The analysis of a large cohort of twins analysed the relationship between SZ risk, brain structure and cognitive performance (Toulopoulou et al. 2015). This study showed that at least a fraction of SZ genetic risk is related to an abnormal early development of the brain eventually leading to cognitive deficits. In addition, a recent meta-analysis based on first-degree relatives of SZ patients (therefore carriers of SZ genetic risk) has shown that these individuals present alterations in corticostriatal-thalamic networks, spanning the dorsolateral prefrontal cortex and temporal regions (Cattarinussi et al. 2022b). However, it is not yet clear if brain abnormalities associated with an impaired neurodevelopment in SZ are related just to volume changes or an abnormal connectivity of neuronal networks.
Molecular genetics studies have tried to determine if genetic risk variants in genes with a clear role in neurodevelopment contribute to brain abnormalities observed in SZ patients. Studies using global polygenic risk scores for SZ have shown extensive heterogeneity in the results, with positive or negative correlations with cortical thickness in fronto-temporal areas (Cattarinussi et al. 2022a). Such a lack of specificity might be due to the fact that polygenic risk scores summarize genetic risk irrespective of their possible neurodevelopmental / adult timing. Despite these limitations, some studies based on children cohorts have identified the effect of polygenic risk of SZ with higher global cortical thickness, smaller white matter volumes of the fornix and cingulum, larger medial occipital surface area and smaller surface area of lateral and medial temporal regions (Fernandez-Cabello et al. 2022). Polygenic risk scores based only on genetic variants related to neurodevelopment have so far shown inconclusive results regarding changes in brain structure in non-clinical subjects (Van der Auwera et al. 2017; Spalthoff et al. 2019). However, one of these studies identified an interesting association of TLE1 gene (Transducin-like enhancer protein 1) with increases of cortical thickness in the upper left temporal gyrus (Spalthoff et al. 2019).
Other studies have analysed the effect of specific genetic variants in genes with a clear role in neurodevelopment using a classical candidate gene design (Gurung and Prata 2015). Among all analysed genes, two of them seem to be the ones with a larger effect on brain connectivity: CACNA1C and ZNF804A. The evidence of an effect on brain structure/volume of genetic variants in CACNA1C is weak due to the lack of convincing replication of original findings (Gurung and Prata 2015). However, literature regarding the functional effects of this gene is more consistent (Guardiola-Ripoll et al. 2022). One of the most replicated findings is the influence of CACNA1C genetic variants on the connectivity between the dorsolateral prefrontal cortex and the hippocampus (Paulus et al. 2014). Noteworthy, a very similar effect has been also observed for ZNF804A gene (Esslinger et al. 2009, 2011). CACNA1C has also been associated with decreased functional connectivity between the right dorsolateral prefrontal cortex and right superior occipital gyrus/cuneus and anterior cingulate cortex (Cosgrove et al. 2017), and reduced activation of the left inferior frontal gyrus (Zhang et al. 2019). With regard to ZNF804A, many studies based on resting-state paradigms have shown that genetic variation in this gene has an impact on the positive functional coupling between the left precentral gyrus/inferior frontal gyrus and both the left inferior frontal gyrus, and the left posterior cingulate gyrus (Tecelão et al. 2018). Additionally, the functional connectivity between the hippocampus and the dorsolateral prefrontal cortex is impaired (Zhang et al. 2018). This gene has also been associated with dorsolateral prefrontal cortex coupling with the hippocampus and prefrontal cortex (Rasetti et al. 2011; Zhao et al. 2020; Yang et al. 2021). Finally, a recent study has identified a genetic interaction effect between CACNA1C and ZNF804A modulating the activity ventral caudate medially and within the left hemisphere, the superior and inferior orbitofrontal gyrus, the superior temporal pole and the ventral-anterior insula during a working memory task (Guardiola-Ripoll et al. 2022).
Other genes that harbour genome-wide associated genetic variants have also been analysed in this context: TCF4 (Transcription factor 4), ANK3 (Ankyrin-3), or NCAN (Neurocan core protein), among others (Gurung and Prata 2015). However, the evidence of the effect of these genes on connectivity changes with a neurodevelopmental origin is less convincing. Even for CACNA1C and ZNF804A, although results are more solid, the question remains whether their influence on the aforementioned connectivity parameters has a neurodevelopmental component or involve neural network regulation in the adult brain (or both).
Interplay between genetic and environmental factors in SZ
Despite compelling evidence of the contribution to risk of genetic and environmental factors to SZ risk, their interplay within a neurodevelopmental framework has not yet been understood. Gene x Environment interactions (G x E) have been hypothesised to play a central role in the differential risk of SZ (van Os et al. 2008). Under this model, the individual genetic background modulates the sensitivity to environmental factors. Several studies have shown that G x E processes are important upon exposure to infections, cannabis use, psychosocial stress, or childhood adversity (Wahbeh and Avramopoulos 2021). However, current evidence suggest that G × E might not be the only process relevant for SZ risk. Other models like GE correlation (Warrier et al. 2021), or even pure additive models with no interaction (Pignon et al. 2022) might also drive the effects of childhood trauma or other sources of psychosocial stress on SZ risk.
Conclusion
Taken together, the results from genetic and environmental factors highlight the role of synaptic dysfunction an impaired myelination in the pathophysiology of SZ. Synaptic plasticity is a key biological process not only in the adult brain but also in developmental stages of the central nervous system, during the establishment and consolidation of neural networks (Forsyth and Lewis 2017). Here, we show that at least part of the genetic and environmental risk of SZ contributes to neurodevelopmental abnormalities that may lead to vulnerable synaptic networks and impaired myelination in the adult brain. Subsequently, SZ genetic risk may also contribute to active processes interfering with synaptic plasticity in the adult brain. Evidence supports such a dual role of SZ genetic risk throughout brain development and adolescence. Further research is needed to understand i) the timing of the different complex biological processes taking place, and ii) the interplay between genetic and environmental factors during these processes. Here, recent ground-breaking advances in stem cell methodologies may pave the way for the identification of the specific neurodevelopmental mechanisms that increase SZ risk.
Author contributions
AS had the idea for the article. All authors contributed to manuscript preparation and editing. AS and SP performed literature search and creation of the figure.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Declarations
Conflict of interests
All authors disclose financial or non-financial interests that are directly or indirectly related to the work.
Data availabilitys
Data are not available.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Amoretti S, Rabelo-da-Ponte FD, Garriga M, et al. Obstetric complications and cognition in schizophrenia: a systematic review and meta-analysis. Psychol Med. 2022 doi: 10.1017/S0033291722002409. [DOI] [PubMed] [Google Scholar]
- Avazzadeh S, Quinlan LR, Reilly J, et al. NRXN1α+/- is associated with increased excitability in ASD iPSC-derived neurons. BMC Neurosci. 2021;22:56. doi: 10.1186/s12868-021-00661-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bähner F, Meyer-Lindenberg A. Hippocampal-prefrontal connectivity as a translational phenotype for schizophrenia. Eur Neuropsychopharmacol. 2017;27:93–106. doi: 10.1016/j.euroneuro.2016.12.007. [DOI] [PubMed] [Google Scholar]
- Belbasis L, Köhler CA, Stefanis N, et al. Risk factors and peripheral biomarkers for schizophrenia spectrum disorders: an umbrella review of meta-analyses. Acta Psychiatr Scand. 2018;137:88–97. doi: 10.1111/acps.12847. [DOI] [PubMed] [Google Scholar]
- Berdenis van Berlekom A, Muflihah CH, Snijders GJLJ, et al. Synapse pathology in schizophrenia: a meta-analysis of postsynaptic elements in postmortem brain studies. Schizophr Bull. 2020;46:374–386. doi: 10.1093/schbul/sbz060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonoldi I, Simeone E, Rocchetti M, et al. Prevalence of self-reported childhood abuse in psychosis: a meta-analysis of retrospective studies. Psychiatry Res. 2013;210:8–15. doi: 10.1016/j.psychres.2013.05.003. [DOI] [PubMed] [Google Scholar]
- Brandl F, Avram M, Weise B, et al. Specific substantial dysconnectivity in schizophrenia: a transdiagnostic multimodal meta-analysis of resting-state functional and structural magnetic resonance imaging studies. Biol Psychiatry. 2019;85:573–583. doi: 10.1016/j.biopsych.2018.12.003. [DOI] [PubMed] [Google Scholar]
- Brown AS. Prenatal risk factors and schizophrenia. Expert Rev Neurother. 2002;2:53–60. doi: 10.1586/14737175.2.1.53. [DOI] [PubMed] [Google Scholar]
- Bullmore ET, Frangou S, Murray RM. The dysplastic net hypothesis: an integration of developmental and dysconnectivity theories of schizophrenia. Schizophr Res. 1997;28:143–156. doi: 10.1016/s0920-9964(97)00114-x. [DOI] [PubMed] [Google Scholar]
- Cancel A, Dallel S, Zine A, et al. Understanding the link between childhood trauma and schizophrenia: a systematic review of neuroimaging studies. Neurosci Biobehav Rev. 2019;107:492–504. doi: 10.1016/j.neubiorev.2019.05.024. [DOI] [PubMed] [Google Scholar]
- Cannon M, Caspi A, Moffitt TE, et al. Evidence for early-childhood, pan-developmental impairment specific to schizophreniform disorder: results from a longitudinal birth cohort. Arch Gen Psychiatry. 2002;59:449–456. doi: 10.1001/archpsyc.59.5.449. [DOI] [PubMed] [Google Scholar]
- Cannon TD, Chung Y, He G, et al. Progressive reduction in cortical thickness as psychosis develops: a multisite longitudinal neuroimaging study of youth at elevated clinical risk. Biol Psychiatry. 2015;77:147–157. doi: 10.1016/j.biopsych.2014.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattarinussi G, Delvecchio G, Sambataro F, Brambilla P. The effect of polygenic risk scores for major depressive disorder, bipolar disorder and schizophrenia on morphological brain measures: a systematic review of the evidence. J Affect Disord. 2022;310:213–222. doi: 10.1016/j.jad.2022.05.007. [DOI] [PubMed] [Google Scholar]
- Cattarinussi G, Kubera KM, Hirjak D, et al. Neural correlates of the risk for schizophrenia and bipolar disorder: a meta-analysis of structural and functional neuroimaging studies. Biol Psychiatry. 2022;92:375–384. doi: 10.1016/j.biopsych.2022.02.960. [DOI] [PubMed] [Google Scholar]
- Chew L-J, Fusar-Poli P, Schmitz T. Oligodendroglial alterations and the role of microglia in white matter injury: relevance to schizophrenia. Dev Neurosci. 2013;35:102–129. doi: 10.1159/000346157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Class QA, Abel KM, Khashan AS, et al. Offspring psychopathology following preconception, prenatal and postnatal maternal bereavement stress. Psychol Med. 2014;44:71–84. doi: 10.1017/S0033291713000780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortés-Albornoz MC, García-Guáqueta DP, Velez-van-Meerbeke A, Talero-Gutiérrez C. Maternal nutrition and neurodevelopment: a scoping review. Nutrients. 2021;13:3530. doi: 10.3390/nu13103530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove D, Mothersill O, Kendall K, et al. Cognitive characterization of Schizophrenia risk variants involved in synaptic transmission: evidence of CACNA1C’s role in working memory. Neuropsychopharmacology. 2017;42:2612–2622. doi: 10.1038/npp.2017.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costas-Carrera A, Garcia-Rizo C, Bitanihirwe B, Penadés R. Obstetric complications and brain imaging in Schizophrenia: a systematic review. Biol Psychiatry Cogn Neurosci Neuroimaging. 2020;5:1077–1084. doi: 10.1016/j.bpsc.2020.07.018. [DOI] [PubMed] [Google Scholar]
- Cuesta MJ, Moreno-Izco L, Ribeiro M, et al. Motor abnormalities and cognitive impairment in first-episode psychosis patients, their unaffected siblings and healthy controls. Schizophr Res. 2018;200:50–55. doi: 10.1016/j.schres.2017.10.035. [DOI] [PubMed] [Google Scholar]
- Deans PJM, Raval P, Sellers KJ, et al. Psychosis risk candidate ZNF804A localizes to synapses and regulates neurite formation and dendritic spine structure. Biol Psychiatry. 2017;82:49–61. doi: 10.1016/j.biopsych.2016.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennison CA, Legge SE, Pardiñas AF, Walters JTR. Genome-wide association studies in schizophrenia: Recent advances, challenges and future perspective. Schizophr Res. 2020;217:4–12. doi: 10.1016/j.schres.2019.10.048. [DOI] [PubMed] [Google Scholar]
- Ding S, Hu Y, Luo B, et al. Age-related changes in neuroinflammation and prepulse inhibition in offspring of rats treated with Poly I: C in early gestation. Behav Brain Funct. 2019;15:3. doi: 10.1186/s12993-019-0154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgueta D, Murgas P, Riquelme E, et al. Consequences of viral infection and cytokine production during pregnancy on brain development in offspring. Front Immunol. 2022;13:816619. doi: 10.3389/fimmu.2022.816619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENIGMA Clinical High Risk for Psychosis Working Group. Jalbrzikowski M, Hayes RA, et al. Association of structural magnetic resonance imaging measures with psychosis onset in individuals at clinical high risk for developing psychosis: an ENIGMA working group mega-analysis. JAMA Psychiat. 2021;78:753–766. doi: 10.1001/jamapsychiatry.2021.0638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esslinger C, Walter H, Kirsch P, et al. Neural mechanisms of a genome-wide supported psychosis variant. Science. 2009;324:605. doi: 10.1126/science.1167768. [DOI] [PubMed] [Google Scholar]
- Esslinger C, Kirsch P, Haddad L, et al. Cognitive state and connectivity effects of the genome-wide significant psychosis variant in ZNF804A. Neuroimage. 2011;54:2514–2523. doi: 10.1016/j.neuroimage.2010.10.012. [DOI] [PubMed] [Google Scholar]
- Eyles DW. How do established developmental risk-factors for schizophrenia change the way the brain develops? Transl Psychiatry. 2021;11:158. doi: 10.1038/s41398-021-01273-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkai P, Schmitt A. Failed regeneration and inflammation in schizophrenia: two sides of the same coin? J Neural Transm (vienna) 2022;129:611–615. doi: 10.1007/s00702-022-02496-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkai P, Malchow B, Wetzestein K, et al. Decreased oligodendrocyte and neuron number in anterior hippocampal areas and the entire hippocampus in Schizophrenia: a stereological postmortem study. Schizophr Bull. 2016;42(Suppl 1):S4–S12. doi: 10.1093/schbul/sbv157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkai P, Raabe F, Bogerts B, et al. Association between altered hippocampal oligodendrocyte number and neuronal circuit structures in schizophrenia: a postmortem analysis. Eur Arch Psychiatry Clin Neurosci. 2020;270:413–424. doi: 10.1007/s00406-019-01067-0. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Folsom TD. The neurodevelopmental hypothesis of schizophrenia, revisited. Schizophr Bull. 2009;35:528–548. doi: 10.1093/schbul/sbn187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fendt M, Lex A, Falkai P, et al. Behavioural alterations in rats following neonatal hypoxia and effects of clozapine: implications for schizophrenia. Pharmacopsychiatry. 2008;41:138–145. doi: 10.1055/s-2008-1058107. [DOI] [PubMed] [Google Scholar]
- Fernandez-Cabello S, Alnæs D, van der Meer D, et al. Associations between brain imaging and polygenic scores of mental health and educational attainment in children aged 9–11. Neuroimage. 2022;263:119611. doi: 10.1016/j.neuroimage.2022.119611. [DOI] [PubMed] [Google Scholar]
- Figueiredo DLA, Ximenez JPB, Seiva FRF, et al. COVID-19: the question of genetic diversity and therapeutic intervention approaches. Genet Mol Biol. 2021;44:e20200452. doi: 10.1590/1678-4685-GMB-2020-0452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fineberg AM, Ellman LM, Buka S, et al. Decreased birth weight in psychosis: influence of prenatal exposure to serologically determined influenza and hypoxia. Schizophr Bull. 2013;39:1037–1044. doi: 10.1093/schbul/sbs084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaherty E, Zhu S, Barretto N, et al. Neuronal impact of patient-specific aberrant NRXN1α splicing. Nat Genet. 2019;51:1679–1690. doi: 10.1038/s41588-019-0539-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsyth JK, Lewis DA. Mapping the consequences of impaired synaptic plasticity in schizophrenia through development: an integrative model for diverse clinical features. Trends Cogn Sci. 2017;21:760–778. doi: 10.1016/j.tics.2017.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francisco AA. 22q11.2 Deletion syndrome as a neural model for Schizophrenia. Biol Psychiatry. 2022;92:338–340. doi: 10.1016/j.biopsych.2022.06.014. [DOI] [PubMed] [Google Scholar]
- Freedman R, Hunter SK, Law AJ, et al. Choline, folic acid, Vitamin D, and fetal brain development in the psychosis spectrum. Schizophr Res. 2021;S0920–9964(21):00128–136. doi: 10.1016/j.schres.2021.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguli S, Chavali PL. Intrauterine viral infections: impact of inflammation on fetal neurodevelopment. Front Neurosci. 2021;15:771557. doi: 10.3389/fnins.2021.771557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GBD Diseases and Injuries Collaborators (2020) Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2019;396:1204–1222. doi: 10.1016/S0140-6736(20)30925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geddes JR, Lawrie SM. Obstetric complications and schizophrenia: a meta-analysis. Br J Psychiatry. 1995;167:786–793. doi: 10.1192/bjp.167.6.786. [DOI] [PubMed] [Google Scholar]
- Geddes JR, Verdoux H, Takei N, et al. Schizophrenia and complications of pregnancy and labor: an individual patient data meta-analysis. Schizophr Bull. 1999;25:413–423. doi: 10.1093/oxfordjournals.schbul.a033389. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci USA. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guardiola-Ripoll M, Almodóvar-Payá C, Lubeiro A, et al. A functional neuroimaging association study on the interplay between two schizophrenia genome-wide associated genes (CACNA1C and ZNF804A) Eur Arch Psychiatry Clin Neurosci. 2022 doi: 10.1007/s00406-022-01447-z. [DOI] [PubMed] [Google Scholar]
- Gurung R, Prata DP. What is the impact of genome-wide supported risk variants for schizophrenia and bipolar disorder on brain structure and function? A systematic review. Psychol Med. 2015;45:2461–2480. doi: 10.1017/S0033291715000537. [DOI] [PubMed] [Google Scholar]
- Gustavsson A, Svensson M, Jacobi F, et al. Cost of disorders of the brain in Europe 2010. Eur Neuropsychopharmacol. 2011;21:718–779. doi: 10.1016/j.euroneuro.2011.08.008. [DOI] [PubMed] [Google Scholar]
- Häfner H. On the track of initial symptoms of depression, anxiety disorders and schizophrenia. Timely treatment saves much suffering. MMW Fortschr Med. 2007;149:26–27. [PubMed] [Google Scholar]
- Hall J, Bray NJ. Schizophrenia genomics: convergence on synaptic development, adult synaptic plasticity, or both? Biol Psychiatry. 2022;91:709–717. doi: 10.1016/j.biopsych.2021.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall LS, Pain O, O’Brien HE, et al. Cis-effects on gene expression in the human prenatal brain associated with genetic risk for neuropsychiatric disorders. Mol Psychiatry. 2021;26:2082–2088. doi: 10.1038/s41380-020-0743-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper S, Towers-Evans H, MacCabe J. The aetiology of schizophrenia: what have the Swedish Medical Registers taught us? Soc Psychiatry Psychiatr Epidemiol. 2015;50:1471–1479. doi: 10.1007/s00127-015-1081-7. [DOI] [PubMed] [Google Scholar]
- Haukvik UK, Saetre P, McNeil T, et al. An exploratory model for G × E interaction on hippocampal volume in schizophrenia; obstetric complications and hypoxia-related genes. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:1259–1265. doi: 10.1016/j.pnpbp.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Haukvik UK, Tamnes CK, Söderman E, Agartz I. Neuroimaging hippocampal subfields in schizophrenia and bipolar disorder: a systematic review and meta-analysis. J Psychiatr Res. 2018;104:217–226. doi: 10.1016/j.jpsychires.2018.08.012. [DOI] [PubMed] [Google Scholar]
- Hof PR, Haroutunian V, Friedrich VL, et al. Loss and altered spatial distribution of oligodendrocytes in the superior frontal gyrus in schizophrenia. Biol Psychiatry. 2003;53:1075–1085. doi: 10.1016/s0006-3223(03)00237-3. [DOI] [PubMed] [Google Scholar]
- Howes OD, Shatalina E. Integrating the neurodevelopmental and dopamine hypotheses of Schizophrenia and the role of cortical excitation-inhibition balance. Biol Psychiatry. 2022;92:501–513. doi: 10.1016/j.biopsych.2022.06.017. [DOI] [PubMed] [Google Scholar]
- Hultman CM, Sparén P, Takei N, et al. Prenatal and perinatal risk factors for schizophrenia, affective psychosis, and reactive psychosis of early onset: case-control study. BMJ. 1999;318:421–426. doi: 10.1136/bmj.318.7181.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttenlocher PR. Synaptic density in human frontal cortex - developmental changes and effects of aging. Brain Res. 1979;163:195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol. 1997;387:167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Jablensky A. Schizophrenia: recent epidemiologic issues. Epidemiol Rev. 1995;17:10–20. doi: 10.1093/oxfordjournals.epirev.a036164. [DOI] [PubMed] [Google Scholar]
- Jenkins TA. Perinatal complications and schizophrenia: involvement of the immune system. Front Neurosci. 2013;7:110. doi: 10.3389/fnins.2013.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone EC, Crow TJ, Frith CD, et al. Cerebral ventricular size and cognitive impairment in chronic schizophrenia. Lancet. 1976;2:924–926. doi: 10.1016/s0140-6736(76)90890-4. [DOI] [PubMed] [Google Scholar]
- Kahn RS. On the origins of Schizophrenia. Am J Psychiatry. 2020;177:291–297. doi: 10.1176/appi.ajp.2020.20020147. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Hogarty GE. Brain maturational processes and delayed onset in schizophrenia. Dev Psychopathol. 1999;11:525–543. doi: 10.1017/s0954579499002199. [DOI] [PubMed] [Google Scholar]
- Kozlova A, Zhang S, Kotlar AV, et al. Loss of function of OTUD7A in the schizophrenia- associated 15q13.3 deletion impairs synapse development and function in human neurons. Am J Hum Genet. 2022;109:1500–1519. doi: 10.1016/j.ajhg.2022.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson S, Andreassen OA, Aas M, et al. High prevalence of childhood trauma in patients with schizophrenia spectrum and affective disorder. Compr Psychiatry. 2013;54:123–127. doi: 10.1016/j.comppsych.2012.06.009. [DOI] [PubMed] [Google Scholar]
- Lederbogen F, Kirsch P, Haddad L, et al. City living and urban upbringing affect neural social stress processing in humans. Nature. 2011;474:498–501. doi: 10.1038/nature10190. [DOI] [PubMed] [Google Scholar]
- Lichtenstein P, Yip BH, Björk C, et al. Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: a population-based study. Lancet. 2009;373:234–239. doi: 10.1016/S0140-6736(09)60072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipska BK. Using animal models to test a neurodevelopmental hypothesis of schizophrenia. J Psychiatry Neurosci. 2004;29:282–286. [PMC free article] [PubMed] [Google Scholar]
- Liu D, Zinski A, Mishra A, et al. Impact of schizophrenia GWAS loci converge onto distinct pathways in cortical interneurons vs glutamatergic neurons during development. Mol Psychiatry. 2022 doi: 10.1038/s41380-022-01654-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham JA, Koenig JI. Prenatal stress: role in psychotic and depressive diseases. Psychopharmacology. 2011;214:89–106. doi: 10.1007/s00213-010-2035-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall CR, Howrigan DP, Merico D, et al. Contribution of copy number variants to schizophrenia from a genome-wide study of 41,321 subjects. Nat Genet. 2017;49:27–35. doi: 10.1038/ng.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinat M, Rossitto M, Di Miceli M, Layé S. Perinatal dietary polyunsaturated fatty acids in brain development, role in neurodevelopmental disorders. Nutrients. 2021;13:1185. doi: 10.3390/nu13041185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal VA, Willhite R, Daley M, et al. Obstetric complications and risk for conversion to psychosis among individuals at high clinical risk. Early Interv Psychiatry. 2009;3:226–230. doi: 10.1111/j.1751-7893.2009.00137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motavaf M, Piao X. Oligodendrocyte development and implication in perinatal white matter injury. Front Cell Neurosci. 2021;15:764486. doi: 10.3389/fncel.2021.764486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins N, Forstner AJ, O’Connell KS, et al. Genome-wide association study of more than 40,000 bipolar disorder cases provides new insights into the underlying biology. Nat Genet. 2021;53:817–829. doi: 10.1038/s41588-021-00857-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanou E, Catterall WA. Calcium channels, synaptic plasticity, and neuropsychiatric disease. Neuron. 2018;98:466–481. doi: 10.1016/j.neuron.2018.03.017. [DOI] [PubMed] [Google Scholar]
- Nehme R, Pietiläinen O, Artomov M, et al (2022) The 22q11.2 region regulates presynaptic gene-products linked to schizophrenia. Nat Commun 13:3690. 10.1038/s41467-022-31436-8 [DOI] [PMC free article] [PubMed]
- Nielsen RE, Levander S, Kjaersdam Telléus G, et al. Second-generation antipsychotic effect on cognition in patients with schizophrenia—a meta-analysis of randomized clinical trials. Acta Psychiatr Scand. 2015;131:185–196. doi: 10.1111/acps.12374. [DOI] [PubMed] [Google Scholar]
- Pak C, Danko T, Mirabella VR, et al. Cross-platform validation of neurotransmitter release impairments in schizophrenia patient-derived NRXN1-mutant neurons. Proc Natl Acad Sci USA. 2021;118:e2025598118. doi: 10.1073/pnas.2025598118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus FM, Bedenbender J, Krach S, et al. Association of rs1006737 in CACNA1C with alterations in prefrontal activation and fronto-hippocampal connectivity. Hum Brain Mapp. 2014;35:1190–1200. doi: 10.1002/hbm.22244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen CB, Mortensen PB. Evidence of a dose-response relationship between urbanicity during upbringing and schizophrenia risk. Arch Gen Psychiatry. 2001;58:1039–1046. doi: 10.1001/archpsyc.58.11.1039. [DOI] [PubMed] [Google Scholar]
- Pekala M, Doliwa M, Kalita K. Impact of maternal immune activation on dendritic spine development. Dev Neurobiol. 2021;81:524–545. doi: 10.1002/dneu.22804. [DOI] [PubMed] [Google Scholar]
- Peters BD, Szeszko PR, Radua J, et al. White matter development in adolescence: diffusion tensor imaging and meta-analytic results. Schizophr Bull. 2012;38:1308–1317. doi: 10.1093/schbul/sbs054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignon B, Peyre H, Ayrolles A, et al. Genetic and psychosocial stressors have independent effects on the level of subclinical psychosis: findings from the multinational EU-GEI study. Epidemiol Psychiatr Sci. 2022;31:e68. doi: 10.1017/S2045796022000464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovic D, Schmitt A, Kaurani L, et al. Childhood trauma in Schizophrenia: current findings and research perspectives. Front Neurosci. 2019;13:274. doi: 10.3389/fnins.2019.00274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Z, Wang J, Gong J, et al. Common and specific patterns of functional and structural brain alterations in schizophrenia and bipolar disorder: a multimodal voxel-based meta-analysis. J Psychiatry Neurosci. 2022;47:E32–E47. doi: 10.1503/jpn.210111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport JL, Giedd JN, Gogtay N. Neurodevelopmental model of schizophrenia: update 2012. Mol Psychiatry. 2012;17:1228–1238. doi: 10.1038/mp.2012.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasetti R, Sambataro F, Chen Q, et al. Altered cortical network dynamics: a potential intermediate phenotype for Schizophrenia and association with ZNF804A. Arch Gen Psychiatry. 2011;68:1207–1217. doi: 10.1001/archgenpsychiatry.2011.103. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Uno H, Rebert CS, Finch CE. Hippocampal damage associated with prolonged glucocorticoid exposure in primates. J Neurosci. 1990;10:2897–2902. doi: 10.1523/JNEUROSCI.10-09-02897.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt A, Steyskal C, Bernstein H-G, et al. Stereologic investigation of the posterior part of the hippocampus in schizophrenia. Acta Neuropathol. 2009;117:395–407. doi: 10.1007/s00401-008-0430-y. [DOI] [PubMed] [Google Scholar]
- Schmitt A, Malchow B, Hasan A, Falkai P. The impact of environmental factors in severe psychiatric disorders. Front Neurosci. 2014;8:19. doi: 10.3389/fnins.2014.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt A, Uhrig S, Spanagel R, et al (2022) Post-mortem gene expression of calcium channels Cav1.2 and Cav1.3 in schizophrenia. Eur Arch Psychiatry Clin Neurosci. 10.1007/s00406-022-01482-w [DOI] [PMC free article] [PubMed]
- Seitz-Holland J, Nägele FL, Kubicki M, et al. Shared and distinct white matter abnormalities in adolescent-onset schizophrenia and adolescent-onset psychotic bipolar disorder. Psychol Med. 2022 doi: 10.1017/S003329172200160X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekar A, Bialas AR, de Rivera H, et al. Schizophrenia risk from complex variation of complement component 4. Nature. 2016;530:177–183. doi: 10.1038/nature16549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh T, Poterba T, Curtis D, et al. Rare coding variants in ten genes confer substantial risk for schizophrenia. Nature. 2022;604:509–516. doi: 10.1038/s41586-022-04556-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smedler E, Louhivuori L, Romanov RA, et al. Disrupted Cacna1c gene expression perturbs spontaneous Ca2+ activity causing abnormal brain development and increased anxiety. Proc Natl Acad Sci USA. 2022;119:e2108768119. doi: 10.1073/pnas.2108768119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalthoff R, Degenhardt F, Awasthi S, et al. Effects of a neurodevelopmental genes based polygenic risk score for schizophrenia and single gene variants on brain structure in non-clinical subjects: a preliminary report. Schizophr Res. 2019;212:225–228. doi: 10.1016/j.schres.2019.07.061. [DOI] [PubMed] [Google Scholar]
- Spauwen J, Krabbendam L, Lieb R, et al. Early maternal stress and health behaviours and offspring expression of psychosis in adolescence. Acta Psychiatr Scand. 2004;110:356–364. doi: 10.1111/j.1600-0447.2004.00429.x. [DOI] [PubMed] [Google Scholar]
- Sullivan PF, Kendler KS, Neale MC. Schizophrenia as a complex trait: evidence from a meta-analysis of twin studies. Arch Gen Psychiatry. 2003;60:1187–1192. doi: 10.1001/archpsyc.60.12.1187. [DOI] [PubMed] [Google Scholar]
- Susser E, Neugebauer R, Hoek HW, et al. Schizophrenia after prenatal famine. Further evidence. Arch Gen Psychiatry. 1996;53:25–31. doi: 10.1001/archpsyc.1996.01830010027005. [DOI] [PubMed] [Google Scholar]
- Tecelão D, Mendes A, Martins D, et al. The impact of psychosis genome-wide associated ZNF804A variation on verbal fluency connectivity. J Psychiatr Res. 2018;98:17–21. doi: 10.1016/j.jpsychires.2017.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toulopoulou T, van Haren N, Zhang X, et al. Reciprocal causation models of cognitive vs volumetric cerebral intermediate phenotypes for schizophrenia in a pan-European twin cohort. Mol Psychiatry. 2015;20:1386–1396. doi: 10.1038/mp.2014.152. [DOI] [PubMed] [Google Scholar]
- Tromp A, Mowry B, Giacomotto J. Neurexins in autism and schizophrenia-a review of patient mutations, mouse models and potential future directions. Mol Psychiatry. 2021;26:747–760. doi: 10.1038/s41380-020-00944-8. [DOI] [PubMed] [Google Scholar]
- Trubetskoy V, Pardiñas AF, Qi T, et al. Mapping genomic loci implicates genes and synaptic biology in schizophrenia. Nature. 2022;604:502–508. doi: 10.1038/s41586-022-04434-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Auwera S, Wittfeld K, Shumskaya E, et al. Predicting brain structure in population-based samples with biologically informed genetic scores for schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2017;174:324–332. doi: 10.1002/ajmg.b.32519. [DOI] [PubMed] [Google Scholar]
- van Os J, Selten JP. Prenatal exposure to maternal stress and subsequent schizophrenia. The May 1940 invasion of The Netherlands. Br J Psychiatry. 1998;172:324–326. doi: 10.1192/bjp.172.4.324. [DOI] [PubMed] [Google Scholar]
- van Kesteren CFMG, Gremmels H, de Witte LD, et al. Immune involvement in the pathogenesis of schizophrenia: a meta-analysis on postmortem brain studies. Transl Psychiatry. 2017;7:e1075. doi: 10.1038/tp.2017.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Os J, Rutten BP, Poulton R. Gene-environment interactions in schizophrenia: review of epidemiological findings and future directions. Schizophr Bull. 2008;34:1066–1082. doi: 10.1093/schbul/sbn117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Os J, Kenis G, Rutten BPF. The environment and schizophrenia. Nature. 2010;468:203–212. doi: 10.1038/nature09563. [DOI] [PubMed] [Google Scholar]
- Varese F, Smeets F, Drukker M, et al. Childhood adversities increase the risk of psychosis: a meta-analysis of patient-control, prospective- and cross-sectional cohort studies. Schizophr Bull. 2012;38:661–671. doi: 10.1093/schbul/sbs050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas T, Dean DJ, Osborne KJ, et al. Hippocampal subregions across the psychosis spectrum. Schizophr Bull. 2018;44:1091–1099. doi: 10.1093/schbul/sbx160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitolo E, Tatu MK, Pignolo C, et al. White matter and schizophrenia: a meta-analysis of voxel-based morphometry and diffusion tensor imaging studies. Psychiatry Res Neuroimaging. 2017;270:8–21. doi: 10.1016/j.pscychresns.2017.09.014. [DOI] [PubMed] [Google Scholar]
- Vlasova RM, Iosif A-M, Ryan AM, et al. Maternal immune activation during pregnancy alters postnatal brain growth and cognitive development in nonhuman primate offspring. J Neurosci. 2021;41:9971–9987. doi: 10.1523/JNEUROSCI.0378-21.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddington JL, Brown AS, Lane A, et al. Congenital anomalies and early functional impairments in a prospective birth cohort: risk of schizophrenia-spectrum disorder in adulthood. Br J Psychiatry. 2008;192:264–267. doi: 10.1192/bjp.bp.107.035535. [DOI] [PubMed] [Google Scholar]
- Wahbeh MH, Avramopoulos D. Gene-environment interactions in schizophrenia: a literature review. Genes (basel) 2021;12:1850. doi: 10.3390/genes12121850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker RL, Ramaswami G, Hartl C, et al. Genetic control of expression and splicing in developing human brain informs disease mechanisms. Cell. 2019;179:750–771.e22. doi: 10.1016/j.cell.2019.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrier V, Kwong ASF, Luo M, et al. Gene-environment correlations and causal effects of childhood maltreatment on physical and mental health: a genetically informed approach. Lancet Psychiatry. 2021;8:373–386. doi: 10.1016/S2215-0366(20)30569-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y, Gould E, Cameron HA, et al. Phenytoin prevents stress- and corticosterone-induced atrophy of CA3 pyramidal neurons. Hippocampus. 1992;2:431–435. doi: 10.1002/hipo.450020410. [DOI] [PubMed] [Google Scholar]
- Weinberger DR. On the plausibility of “the neurodevelopmental hypothesis” of schizophrenia. Neuropsychopharmacology. 1996;14:1S–11S. doi: 10.1016/0893-133X(95)00199-N. [DOI] [PubMed] [Google Scholar]
- Weinberger DR (1986) The pathogenesis of schizophrenia: a neurodevelopmental theory. In: Neurology of Schizophrenia. Elsevier, pp 387–405
- Weinstock M. The long-term behavioural consequences of prenatal stress. Neurosci Biobehav Rev. 2008;32:1073–1086. doi: 10.1016/j.neubiorev.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Wortinger LA, Engen K, Barth C, et al. Asphyxia at birth affects brain structure in patients on the schizophrenia-bipolar disorder spectrum and healthy participants. Psychol Med. 2020 doi: 10.1017/S0033291720002779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X-L, Yan Q-J, Zhu F. Abnormal synaptic plasticity and impaired cognition in schizophrenia. World J Psychiatry. 2022;12:541–557. doi: 10.5498/wjp.v12.i4.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M-Q, Sun W-S, Liu B-X, et al. Prenatal malnutrition and adult schizophrenia: further evidence from the 1959–1961 Chinese famine. Schizophr Bull. 2009;35:568–576. doi: 10.1093/schbul/sbn168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Xu F, He Y, et al. Association between ZNF804A gene rs1344706 polymorphism and brain functions in healthy individuals: a systematic review and voxel-based meta-analysis. Neuropsychiatr Dis Treat. 2021;17:2925–2935. doi: 10.2147/NDT.S322114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz M, Yalcin E, Presumey J, et al. Overexpression of schizophrenia susceptibility factor human complement C4A promotes excessive synaptic loss and behavioral changes in mice. Nat Neurosci. 2021;24:214–224. doi: 10.1038/s41593-020-00763-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Yan H, Liao J, et al. ZNF804A variation may affect hippocampal-prefrontal resting-state functional connectivity in schizophrenic and healthy individuals. Neurosci Bull. 2018;34:507–516. doi: 10.1007/s12264-018-0221-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Wang Y, Zhang Q, et al. The effects of CACNA1C gene polymorphism on prefrontal cortex in both schizophrenia patients and healthy controls. Schizophr Res. 2019;204:193–200. doi: 10.1016/j.schres.2018.09.007. [DOI] [PubMed] [Google Scholar]
- Zhao W, Chen X, Zhang Q, et al. Effect of ZNF804A gene polymorphism (rs1344706) on the plasticity of the functional coupling between the right dorsolateral prefrontal cortex and the contralateral hippocampal formation. Neuroimage Clin. 2020;27:102279. doi: 10.1016/j.nicl.2020.102279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zornberg GL, Buka SL, Tsuang MT. Hypoxic-ischemia-related fetal/neonatal complications and risk of schizophrenia and other nonaffective psychoses: a 19-year longitudinal study. Am J Psychiatry. 2000;157:196–202. doi: 10.1176/appi.ajp.157.2.196. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are not available.