Abstract
Introduction
The aim of this work is to evaluate baricitinib safety with respect to venous thromboembolism (VTE), major adverse cardiovascular events (MACE), and serious infection relative to tumor necrosis factor inhibitors (TNFi) in patients with rheumatoid arthritis (RA).
Methods
Patients with RA from 14 real-world data sources (three disease registries, eight commercial and three government health insurance claims databases) in the United States (n = 9), Europe (n = 3), and Japan (n = 2) were analyzed using a new user active comparator design. Propensity score matching (1:1) controlled for potential confounding. Meta-analysis of incidence rate ratios (IRR) and incidence rate differences (IRD) for each outcome, from each data source was executed using modified Poisson regression and Cochran–Mantel–Haenszel analysis.
Results
Of 9013 eligible baricitinib-treated patients, 7606 were propensity score-matched with TNFi-treated patients, contributing 5879 and 6512 person-years of baricitinib and TNFi exposure, respectively. Across data sources, 97 patients (56 baricitinib) experienced VTE during follow-up, 93 experienced MACE (54 baricitinib), and 321 experienced serious infection (176 baricitinib). Overall IRRs comparing baricitinib with TNFi treatment were 1.51 (95% CI 1.10, 2.08) for VTE, 1.54 (95% CI 0.93, 2.54) for MACE, and 1.36 (95% CI 0.86, 2.13) for serious infection. IRDs for VTE, MACE, and serious infection, respectively, were 0.26 (95% CI −0.04, 0.57), 0.22 (95% CI −0.07, 0.52), and 0.57 (95% CI −0.07, 1.21) per 100 person-years greater for baricitinib than TNFi.
Conclusions
Overall results suggest increased risk of VTE with baricitinib versus TNFi, with consistent point estimates from the two largest data sources. A numerically greater risk was observed for MACE and serious infection when comparing baricitinib versus TNFi, with different point estimates from the two largest data sources. Findings from this study and their impact on clinical practice should be considered in context of limitations and other evidence regarding the safety and efficacy of baricitinib and other Janus kinase inhibitors.
Trial registration:
EU PAS Register (http://encepp.eu), identifier #32271.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40744-022-00505-1.
Keywords: Baricitinib, Rheumatoid arthritis, Venous thromboembolism, Major adverse cardiovascular event, Serious infection, JAK inhibitors, Tumor necrosis factor inhibitors
Key Summary Points
| Why carry out this study? |
| This study aimed to provide information about the safety profile of baricitinib relative to tumor necrosis factor inhibitors (TNFi) in real-world patients receiving treatment for rheumatoid arthritis during routine clinical care. |
| During the 24-week placebo-controlled period of the rheumatoid arthritis clinical program, a numerical imbalance of venous thromboembolism between baricitinib 4 mg (6 of 997 patients) and placebo (0 of 1070 patients) suggested the potential for an increased risk of venous thromboembolism in baricitinib-treated patients. Incidence rates remained stable over long-term follow-up in single-arm studies. |
| What was learned from the study? |
| After propensity score matching, patients treated with baricitinib (average treatment length: 9 months; range < 3–17 months) had a 1.5-fold statistically significant increased risk of venous thromboembolism (IRR = 1.51, 95% CI 1.10, 2.08; IR difference = 0.26, 95% CI −0.04, 0.57) compared to patients treated with TNFi. |
| A non-statistically significant increased risk of major adverse cardiovascular events (IRR = 1.54, 95% CI 0.93, 2.54; IR difference = 0.22, 95% CI −0.07, 0.52 per 100 person-years) and serious infection (IRR = 1.36, 95% CI 0.86, 2.13; IR difference = 0.57, 95% CI −0.07, 1.21 per 100 person-years) were also observed compared to patients treated with TNFi. |
Introduction
Rheumatoid arthritis (RA) is a systemic inflammatory disease characterized by synovial inflammation causing pain, swelling, stiffness, and progressive joint damage. Patients with RA also experience an increased risk of significant non-musculoskeletal comorbidities, including malignancy [1], infection, including tuberculosis (TB) [2], venous thromboembolism (VTE) [3–5], cardiovascular disease [6–8], and overall early mortality [9, 10], among others.
Current treatment of RA is typically initiated with a conventional disease-modifying antirheumatic drug (cDMARD) such as methotrexate. Patients with poor control of disease activity can receive additional treatment with biologic DMARDs (bDMARD), such as tumor necrosis factor inhibitors (TNFi) and oral targeted synthetic DMARDs (tsDMARD), such as Janus kinase inhibitors (JAKi). Baricitinib, an oral selective JAK1/JAK2 inhibitor, is approved for the treatment of adults with moderately to severely active RA, moderate-to-severe atopic dermatitis, severe alopecia areata, and hospitalized patients with SARS-CoV-2 (COVID-19).
The safety profile of baricitinib for the treatment of RA is based on clinical trial data from over 14,000 person-years of exposure [11]. During the 24-week placebo-controlled period of the baricitinib RA clinical program, a numerical imbalance of VTE between baricitinib 4 mg (6 of 997 patients) and placebo (0 of 1070 patients) suggested the potential for an increased risk of VTE in baricitinib-treated patients [12]. The duration of the comparative observation period and number of patients in these data, i.e., the total person-time available, limited the evaluation of uncommon events such as MACE and VTE [11]. Differences between patients who volunteer to participate in clinical trials and those who do not and between the clinical care received in trials and real-world settings can also influence the observed safety profile of a medication. Therefore, post-authorization safety studies within real-world populations are typically conducted to better characterize and establish the safety profile of medications.
Study B023 was initiated in January 2020 and aimed to compare the safety of baricitinib for the treatment of patients with RA in routine care, with TNFi for risk of VTE, MACE, or serious infection. A meta-analysis was used to combine results across 14 post-marketing data sources, including bDMARD and disease registries, administrative claims databases and national healthcare systems, in Europe, the United States (US), and Japan.
Methods
Data and Study Design
A new user active comparator design was used to reduce the risk of confounding and selection bias [13]. New users were defined as patients without prior use of the index medications (baricitinib or the specific TNFi or biosimilar) during the baseline period. The baseline period, or covariate assessment window, was defined as the 6-months prior to and including the cohort entry date. A schematic of the study design is available in Supplementary Material, Figure S1.
This study analyzed longitudinal information collected for purposes unrelated to the study objectives from 14 sources. Data came primarily from health insurance claims records and existing RA registries in Europe, the US, and Japan (Table 1; Supplementary Material, Table S1). All patients who were present in the data sources between the start of market availability of Olumiant (baricitinib) and the initiation of analyses were evaluated for eligibility. All data sources provided available longitudinal information on patient demographics, in- and outpatient medical diagnoses and procedures, including RA diagnosis, comorbidities, and prescription dispensing records, including treatments for RA, and healthcare resource utilization.
Table 1.
Contribution of patients and person-time to VTE analyses, by data source
| Data source | Country | Eligible (unmatched) patients | Patients included in analysesa | ||||
|---|---|---|---|---|---|---|---|
| Baricitinib (n) |
TNFi (n) |
Baricitinib (n) |
Baricitinib exposure (PY) |
TNFi (n) |
TNFi exposure (PY) |
||
| US data | |||||||
| Aetna/Healthagen | US | 69 | 289 | 37 | 13 | 37 | 22 |
| Anthem (HIRD) | US | 255 | 1304 | 123 | 69 | 123 | 99 |
| CorEvitas US | US | 118 | 1897 | 112 | 76 | 112 | 85 |
| HealthVerity PS20 | US | 933 | 3953 | 748 | 236 | 748 | 3785 |
| Humana | US | 89 | 154 | 49 | 20 | 49 | 21 |
| Marketscan | US | 257 | 1599 | 185 | 84 | 185 | 78 |
| MDR | US | 188 | 1686 | 114 | 61 | 114 | 70 |
| Optum® Clinformatics® | US | 348 | 1441 | 284 | 118 | 284 | 163 |
| PharMetrics Plus | US | 473 | 6576 | 261 | 141 | 261 | 159 |
| Europe and Japan data | |||||||
| ARTIS | Sweden | 1737 | 6230 | 1685 | 2314 | 1685 | 2608 |
| BKK | Germany | 851 | 3332 | 765 | 539 | 765 | 544 |
| CorEvitas JP | Japan | 210 | 354 | 171 | 200 | 171 | 248 |
| JMDC | Japan | 243 | 1721 | 213 | 154 | 213 | 115 |
| SNDS | France | 3242 | 10,202 | 2859 | 1855 | 2859 | 1923 |
| Total | 9013 | 40,738 | 7606 | 5879 | 7606 | 6512 | |
ARTIS anti-rheumatic therapy in Sweden, BKK Betriebskrankenkasse, HIRD HealthCore Integrated Research Database, JMDC JMDC, Inc.’s claims database, JP Japan, MACE major adverse cardiovascular event, MDR military health system data repository, n number of patients, Optum® Clinformatics® Optum’s de-identified Clinformatics® Data Mart Database, PS20 Private Source 20, PY person-years, SNDS Système National des Données de Santé, TNFi tumor necrosis factor inhibitor, US United States, VTE venous thromboembolism
aAvailable information on sample size (n) and baricitinib exposure (PY) among eligible propensity score-matched patients analyzed to assess risk of VTE
All data were de-identified to ensure patient confidentiality and used in accordance with data license agreements. This study was registered on the European Post-authorization Study (EU PAS) register (#32271; https://www.encepp.eu/encepp/studiesDatabase.jsp), where the protocol and a detailed study report will be available.
Study Population
The study population consisted of adults who were incident users of baricitinib (4 and 2 mg) or a specific TNFi (adalimumab, certolizumab pegol, etanercept, golimumab, or infliximab) or biosimilars. Cohort entry was defined as the date of first dispensing of baricitinib or a specific TNFi during the study period. In addition to the specific medications, patients in claims data were required to have a diagnosis of RA (ICD-10-CM M05–M05.9, M06.0, M06.8, and M06.9, or corresponding regional ICD-10 codes) from a physician encounter during the baseline period. In one US, claims-based study, the positive predictive value (PPV) of these codes for RA was 86% [14]. Similar criteria were used in registries, with RA diagnosis and treatments identified from information contributed by rheumatologists. Patients in claims data were required to have continuous medical and prescription drug coverage for ≥ 6 months prior to cohort entry and throughout their follow-up, with any gaps limited to ≤ 45 days. Patients showing prior use of another JAKi, or with a dispensing of any combination of two or more bDMARDs and/or tsDMARDs on the cohort entry date, were excluded. In US data sources, patients in the TNFi cohort were required to have prior treatment with ≥ 1 TNFi identified during the baseline period to mirror the US indication for baricitinib [15]. This was not required for data collected outside of the US. Patients eligible for either treatment cohort were prioritized for entry to the baricitinib cohort to maximize cohort size. Patients with a baseline history of the outcome under analysis were excluded from analysis of the same outcome. Patients were also excluded from VTE and MACE analyses if there was evidence of anticoagulant use on the cohort entry date.
Exposure and Outcome Definitions
Exposures were based on an as-treated definition, with patients followed for outcomes from the start of treatment until treatment discontinuation or switch (including to another TNFi for patients in the TNFi cohort), initiation of a concomitant bDMARD or tsDMARD, disenrollment from the insurance plan or registry, death (where available), or the end of the study period. Exposure to baricitinib was defined based on aggregate doses (2 and 4 mg).
The primary outcome was VTE, a composite of pulmonary embolism, deep-vein thrombosis, or other venous thrombosis. In claims data, VTE was identified using a validated case definition based on ICD-10 diagnosis codes, health care setting (emergent, inpatient, or outpatient), and, in some cases, dispensing of low molecular weight heparin or oral anticoagulant (PPV = 75.5%; see Supplementary Material, Methods). The definition was updated from a previous algorithm [16] and further adaptations were made to reflect differences in regional coding and healthcare systems. The case definition does not consider the fatality of an event. In French claims data (Système National des Données de Santé [SNDS]), the validated case definition included evidence of imaging procedures to address the absence of outpatient diagnosis codes (PPV ≥ 92%) [17]. In CorEvitas registry data, physician diagnosis and adjudicated endpoints, within the registry procedures, were used to identify VTE. In the Anti-Rheumatic Therapy in Sweden (ARTIS) data source, VTE was defined by a validated algorithm based on ICD-10 from the Swedish National Patient Register (PPV = 87%) [18].
MACE and serious infection were examined as secondary outcomes. MACE was identified in claims data based on ICD-10 diagnosis codes for myocardial infarction (PPV ≥ 93%) [19] or ischemic or hemorrhagic stroke (PPV ≥ 82% for ischemic and ≥ 87% for hemorrhagic stroke) [20], with local adaptation for other ICD-10 coding schemes. In registry data, MACE was defined based on physician diagnosis and adjudicated endpoints per registry procedures [21]. Serious infection was identified in claims data based on primary ICD-10 diagnosis codes from an inpatient stay (PPV = 90.2%) [22]. In registry data, serious infection was based on clinical judgement and adjudicated events when available for a specified infection. MACE and serious infections that met the case definition and subsequently led to death were included although fatal outcomes were not specifically identified. Hospitalized TB was also assessed, as a component of serious infection, and separately as a descriptive outcome.
Covariate Assessment
Patient characteristics were evaluated for potential imbalances in risk factors between groups, i.e., confounding, for each outcome. These included demographics, medical history, and comorbidities, RA treatments, and health care resource utilization evaluated from information available during baseline. Baseline was defined as the 6-month period prior to cohort entry, up to and including the cohort entry date.
Statistical Analysis
Comparative analyses were implemented after 1:1 baricitinib:TNFi nearest-neighbor propensity score matching [23, 24] to create comparison groups with a balanced distribution of baseline risk factors. Propensity score models were generated separately for each outcome, i.e., VTE, MACE, or serious infection. The performance of propensity score matching across baseline variables was assessed using standardized differences, with differences of ≤ 0.10 considered acceptable. The variables considered for inclusion in the propensity score models were risk factors specific to each outcome, including information on patient demographics, medical history including comorbidities, and RA disease treatments; not all data sources had data available on each risk factor (Supplementary Material, Table S2).
Within each data source, patient characteristics, e.g., baseline demographic and clinical conditions, were summarized by treatment group (baricitinib versus TNFi) in unmatched and matched cohorts. Patients were permitted to contribute person-time and events to only a single treatment group, either the TNFi cohort or the baricitinib cohort in an analysis. For all comparative analyses, baricitinib was the treatment of interest and the TNFi cohort was the reference group.
Modified Poisson regression was used to generate an overall incidence rate ratio (IRR) from meta-analysis, as a measure of association comparing events in baricitinib and TNFi treatment cohorts. This allows inclusion of data from all sources, including those with low or no events in either or both cohorts. Both random effects and fixed effect regression models were implemented. Only results from the fixed effect model are reported for the IRR since the data were too sparse and the variance/covariance matrix of the random effects matrix did not converge. Heterogeneity in the treatment effect was assessed using the standard Cochran χ2 test, and the magnitude of heterogeneity was evaluated using the I-squared statistic [25]; however, the sparse data from several sources limited the ability of these tests to detect heterogeneity.
Using Cochran−Mantel–Haenszel analysis, an overall incidence rate difference (IRD) was also estimated for each outcome as a supplemental result. Both the random and fixed effect model results were estimated but the random effects result is reported as the main IRD finding since it allows that the treatment effect may vary in different populations.
A sensitivity analysis was executed to understand the potential impact of bias due to unmeasured confounding by smoking, body mass index (BMI), and disease activity in US data sources and SNDS (details in Supplementary Material). Additional pre-planned sensitivity analyses to understand the impact of geography, disease severity, and length of baseline period are detailed in the final study report available on the EU PAS register.
Compliance with Ethics Guidelines
Study B023 was conducted in accordance with ethical principles of the Helsinki Declaration of 1964 and its later amendments, and Good Clinical Practice guidelines. Ethical approval was provided by Advarra IRB Committee, a centralized IRB in the USA, (Reference Pro00042607), and CNIL for French SNDS data (reference 919392). All data were de-identified to ensure patient confidentiality and used in accordance with data license agreements. The requirement for informed consent was therefore waived.
Results
Study Population
Patients were identified from 14 data sources across Europe, the US, and Japan (Table 1). Of 9013 eligible patients treated with baricitinib, 7606 (84%) were propensity score-matched 1:1 with patients treated with TNFi and included in the comparative analysis of VTE, for a total 5879 and 6512 person-years of baricitinib and TNFi, respectively. A greater proportion of eligible patients were successfully matched in European (ARTIS 97%, Betriebskrankenkasse [BKK] 90%, SNDS 88%) than in US (70% overall) or Japan (85% overall) data. On average, patients were followed for 9 and 10 months of baricitinib and TNFi treatment, respectively. The largest data sources, ARTIS and SNDS, contributed 2314 (1685) and 1855 (2859) person-years of baricitinib exposure (and patients) to the meta-analysis, respectively, with an average follow-up of 16 and 8 months. These data sources contributed 39% (ARTIS) and 32% (SNDS) of the total baricitinib exposure, i.e., person-time, to the meta-analysis for VTE, with the third largest source, BKK in Germany, contributing 9%. Person-time and counts of patients in the MACE and serious infection analysis cohorts did not differ meaningfully from the VTE analysis cohort and are therefore not reported.
Patient characteristics were described from information available prior to cohort entry, i.e., the baseline period 6 months prior to and including the date of initiation of the index medication (claims data, ARTIS) and information collected at enrolment (CorEvitas). Prior to matching, patients treated with baricitinib were more likely to be female and older than those treated with TNFi, with non-US patients treated with the 2-mg dose tending to be more than a decade older. Baricitinib cohort patients were more likely to have received bDMARDs or concomitant cDMARDs during baseline and to take more medications (e.g., antibiotics, antihypertensives, beta-blockers, calcium channel blockers, and statins) compared to patients treated with TNFi (Supplementary Material, Tables S3–S6). After propensity score matching, differences between treatment groups resolved, with little to no difference remaining in the prevalence of measured risk factors between treatment cohorts for each outcome analyzed. Because the 6-month baseline may have limited information available on clinical history, a sensitivity analysis in French data extended the baseline to 2 years. No important differences emerged with this extended period (Supplementary Material, Table S7). Selected characteristics of patients from the largest US and European data sources initiating baricitinib or TNFi treatment and included in comparative analyses are described in Table 2, and the remaining data sources are included in Supplementary Material, Tables S8-S11.
Table 2.
Selected baseline demographics and disease characteristics of patients in propensity score-matched VTE cohorts of the largest US and European data sources
| HealthVerity PS20 | ARTIS | SNDS | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Baricitinib (N = 748) | TNFi (N = 748) | Standardized difference | Baricitinib (N = 1685) | TNFi (N = 1685) | Standardized difference | Baricitinib (N = 2859) | TNFi (N = 2859) | Standardized difference | |
| Exposure | |||||||||
| Total, PY | 236 | 378 | 2314 | 2608 | 1855 | 1923 | |||
| Mean (SD), days | 115 (112) | 184 (164) | 0.49 | 502 (346) | 565 (424) | −0.17 | 237 (195) | 246 (206) | −0.04 |
| Median, days | 69 | 129 | 454 | 454 | 173 | 175 | |||
| Min, max, days | 0, 718 | 0, 808 | 1, 1310 | 9, 1458 | 0, 831 | 0, 851 | |||
| Demographics | |||||||||
| Mean age, years (SD) | 55 (11) | 56 (12) | 0.06 | 59 (14) | 59 (14) | −0.01 | 58 (13) | 58 (13) | 0.00 |
| Sex, female; n (%) | 643 (86) | 635 (85) | 0.03 | 1382 (82) | 1393 (83) | 0.02 | 2268 (79) | 2303 (81) | 0.03 |
| Clinical characteristics | |||||||||
| RA Severity; mean (SD)a | 4 (1) | 4 (1) | 0.06 | 5 (1) | 4 (1) | NA | 7 (1) | 6 (1) | 0.03 |
| DAS28 < 2.6; n (%) | – | – | – | 57 (6) | 97 (12) | −0.11 | – | – | – |
| DAS28 < 3.2; n (%) | – | – | – | 73 (8) | 66 (8) | 0.02 | – | – | – |
| DAS28 < 5.2; n (%) | – | – | – | 454 (49) | 376 (48) | 0.11 | – | – | – |
| DAS28 > 5.2; n (%) | – | – | – | 340 (37) | 248 (32) | 0.14 | – | – | – |
| cDMARDs, baseline; n (%) | 352 (47) | 426 (57) | 0.20 | 935 (55) | 1024 (61) | −0.11 | 1945 (68) | 1882 (66) | 0.05 |
| Methotrexate | 218 (29) | 212 (28) | 0.02 | 724 (43) | 727 (43) | 0.00 | 1523 (53) | 1486 (52) | 0.03 |
| Leflunomide | 89 (12) | 108 (14) | 0.08 | 74 (4) | 103 (6) | −0.09 | 361 (13) | 310 (11) | 0.06 |
| Hydroxychloroquine | 110 (15) | 142 (19) | 0.11 | 92 (5) | 123 (7) | −0.07 | 165 (6) | 138 (5) | 0.04 |
| Sulfasalazine | 38 (5) | 50 (7) | 0.07 | 154 (9) | 241 (14) | −0.16 | 101 (4) | 105 (4) | −0.01 |
| bDMARDs, baseline; n (%) | 271 (36) | 716 (96) | 1.61 | 908 (54) | 1039 (62) | −0.16 | 1599 (56) | 1611 (56) | −0.01 |
| cDMARDs concomitant with bDMARD, baseline; n (%) | 158 (21) | 371 (50) | 0.62 | NA | NA | – | 910 (32) | 847 (30) | 0.05 |
| Glucocorticoids, baseline; n (%) | 349 (47) | 353 (47) | 0.01 | 1073 (64) | 1052 (62) | 0.03 | 2026 (71) | 2002 (70) | 0.02 |
| CVD risk factors; n (%) | |||||||||
| Diabetes | 145 (19) | 140 (19) | 0.02 | 141 (8) | 114 (7) | 0.06 | 283 (10) | 271 (10) | 0.01 |
| Antidiabetic agents | 90 (12) | 111 (15) | 0.08 | 141 (8) | 112 (7) | 0.06 | 276 (10) | 253 (9) | 0.03 |
| Atrial arrhythmia/fibrillation | 23 (3) | 20 (3) | 0.02 | < 5 | 7 (0) | −0.4 | 29 (1) | 27 (1) | 0.01 |
| Coronary artery disease | 42 (6) | 33 (4) | 0.06 | < 5 | < 5 | − 0.03 | 127 (4) | 121 (4) | 0.01 |
| Congestive heart failureb | 7 (1) | 2 (0) | 0.09 | < 5 | < 5 | 0.00 | 12 (0.4) | 13 (0.5) | −0.01 |
| Dyslipidemia | 269 (36) | 375 (37) | 0.02 | < 5 | < 5 | – | NA | NA | – |
| Stroke | NA | NA | – | NA | NA | – | 25 (1) | 22 (1) | 0.01 |
| Hypertension | 321 (43) | 338 (45) | 0.05 | 12 (1) | 5 (0) | 0.06 | NA | NA | – |
| Anti-hypertensive medication | 265 (35) | 378 (51) | 0.31 | 718 (43) | 678 (40) | 0.05 | 973 (34) | 976 (34) | −0.00 |
| Statins | 158 (21) | 191 (26) | 0.10 | 228 (14) | 246 (15) | −0.03 | 394 (14) | 372 (13) | 0.02 |
| Aspirin, prescription | 16 (2) | 20 (3) | 0.04 | 157 (9) | 169 (10) | −0.03 | 37 (1) | 35 (1) | 0.01 |
| Anticoagulant | 20 (3) | 18 (2) | 0.02 | 66 (4) | 51 (3) | 0.05 | 130 (5) | 125 (4) | 0.01 |
| HRT | 47 (6) | 38 (5) | 0.05 | 132 (8) | 138 (8) | −0.01 | 213 (8) | 218 (8) | −0.01 |
| Oral contraceptive | 21 (3) | 20 (3) | 0.01 | 61 (4) | 53 (3) | 0.02 | 147 (5) | 153 (5) | −0.01 |
| Cox-2 inhibitor | 36 (5) | 48 (6) | 0.07 | 121 (7) | 108 (6) | 0.03 | 156 (6) | 175 (6) | −0.03 |
| Obesity | 212 (28) | 210 (28) | 0.01 | NA | NA | – | NA | NA | – |
| Smokingc | 111 (15) | 108 (14) | 0.01 | 803 (48) | 738 (44) | 0.08 | NA | NA | – |
| Other comorbidities | |||||||||
| Cancer | 58 (8) | 56 (8) | 0.01 | < 5 | < 5 | −0.02 | 88 (3) | 94 (3) | −0.01 |
ARTIS anti-rheumatic therapy in Sweden, bDMARDs biologic disease-modifying antirheumatic drugs, cDMARDs conventional synthetic disease-modifying antirheumatic drugs, CVD cardiovascular disease, DAS28 Disease Activity Score 28, HRT hormone replacement therapy, max maximum, min minimum, N number in specific category, n number of patients, NA not available, PS20 private source 20, PY person-years, RA rheumatoid arthritis, SD standard deviation, SNDS Système National des Données de Santé, TNFi tumor necrosis factor inhibitor
aDisease severity is based on the claims-based index of rheumatoid arthritis severity (CIRAS index) in HealthVerity PS20, BKK, and SNDS; and DAS28 for ARTIS data
bCongestive heart failure in PS20 and SNDS was defined as congestive heart failure, hospitalized
cSmoking status is not directly available in claims data sources and is based on ICD-10 codes related to smoking cessation and other measures such as tobacco use disorder, counselling visits for smoking, and antismoking prescription medications. In ARTIS, smoking status is collected directly from patients and defined as current or former smoker
For some data sources, low counts (i.e., < 5) were masked as required to maintain data privacy, as required by local regulations
Primary Outcome—VTE
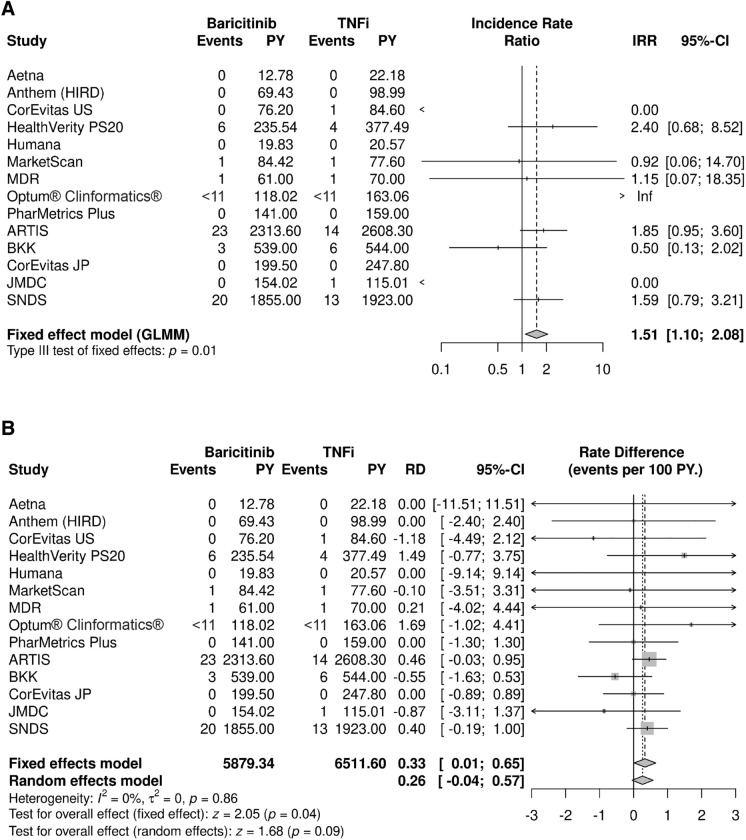
Across all data sources, 97 patients experienced a VTE during a mean overall follow-up of 9 months (baricitinib) and 10 months (TNFi), 56 of whom were treated with baricitinib. In decreasing frequency of cases, the data sources where at least five patients experienced VTE during follow-up were ARTIS (n = 37), SNDS (n = 33), HealthVerity Private Source 20 (PS20) (n = 10), and BKK (n = 9). The overall IRR was statistically significantly elevated for baricitinib vs. TNFi (IRR = 1.51; 95% CI 1.10, 2.08) (Fig. 1A). The IRD between baricitinib and TNFi was 0.26 (95% CI −,0.04, 0.57) per 100 person-years (Fig. 1B) from the random effects model, with the greater rate among patients treated with baricitinib; the IRD was not statistically significant. In other words, assuming a constant rate over time, for every 1000 patients treated with baricitinib instead of a TNFi, an additional three VTE would be expected each year. Both random and fixed effect model results were estimated for IRD (Fig. 1B) but only the random effects result is presented as the main IRD finding since it allows that the treatment effect may vary in different populations and point estimates did not differ. The incidence of VTE in each data source is provided in the Supplementary Material (Table S12). A bias analysis carried out to assess the possible impact of unmeasured confounding by smoking, obesity, and disease activity on the effect of baricitinib on VTE compared to TNFi suggested that results were unlikely to have been meaningfully impacted by not controlling for these factors (Supplementary Material, Table S13).
Fig. 1.
Meta-analysis for VTE comparing baricitinib and TNF inhibitors showing A incidence rate ratios and B incidence rate differences. ARTIS anti-rheumatic therapy in Sweden, BKK Betriebskrankenkasse, CI confidence interval, GLMM generalized linear mixed model, HIRD HealthCore Integrated Research Database, IRR incidence rate ratio, JMDC JMDC, Inc.’s claims database, JP Japan, MDR military health system data repository, Optum® Clinformatics® Optum’s de-identified Clinformatics® Data Mart Database, PS20 private source 20, PY person-years, RD rate difference, SNDS Système National des Données de Santé, TNFi tumor necrosis factor inhibitor, US United States, VTE venous thromboembolism. For some data sources, low counts (i.e., <11) were masked as required to maintain data privacy, as required by local regulations
Clinical characteristics of patients with VTE were similar to those of the overall RA cohorts, except for age and sex (Table 3) but comparisons are limited by the small number of patients with events. The mean age of patients with a VTE appeared higher (mean age in ARTIS 64 years; SNDS mean 68 years) than the mean age of patients included in VTE analyses (mean age in ARTIS 59 years; SNDS mean 58 years), although sample sizes were small and no statistical comparisons were made. In the ARTIS, SNDS, and BKK data sources, almost all patients in the baricitinib cohort with a VTE during follow-up were male, unlike for TNFi cohorts (Table 3). Notably within PS20, all six patients with VTE treated with baricitinib, and one of four patients with VTE treated with TNFi, had a recent hospitalization within the 4 weeks prior to the event. Within data sources with at least five patients with VTE, there were no other differences in selected VTE clinical risk factors, although the number of patients with events limits these qualitative descriptions. The distribution of time to VTE was variable, ranging from 1 to 1458 days. For the baricitinib cohort, the mean (median) time to event was 502 (454) days in ARTIS and 227 (204) days in SNDS, consistent with the mean (median) follow-up time in these cohorts. In the TNFi cohort, the mean (median) time to event was 565 (454) days in ARTIS and 181 (113) days in SNDS, consistent with the mean (median) follow-up time in these cohorts.
Table 3.
Demographic characteristics (sex and age) of patients with events in the VTE analysis cohort in data sources with greater than five events
| Total patients | Patients with VTE | |||||
|---|---|---|---|---|---|---|
| Baricitinib cohort | TNFi cohort | Overall | Baricitinib cohort | TNFi cohort | Overall | |
| ARTIS | N = 1685 | N = 1685 | N = 3370 | N = 23 | N = 14 | N = 37 |
| Age, years; mean (SD) | 59 (14) | 59 (14) | 59 (14) | 63 (10) | 65 (11) | 64 (11) |
| Sex, female; n (%) | 1382 (82) | 1393 (83) | 2775 (82) | 18 (78) | 11 (79) | 29 (78) |
| SNDS | N = 2859 | N = 2859 | N = 5718 | N = 20 | N = 13 | N = 33 |
| Age, years; mean (SD) | 58 (13) | 58 (13) | 58 (11) | 69 (10) | 66 (12) | 68 (11) |
| Sex, female; n (%) | 2268 (79) | 2303 (81) | 4571 (80) | ≤ 10 | ≤ 10 | ≤ 10 |
| PS20 | N = 748 | N = 748 | N = 1496 | N = 6 | N = 4 | N = 10 |
| Age, years; mean (SD) | 55 (11) | 56 (12) | 55 (12) | 60 (9) | 55 (6) | 58 (8) |
| Sex, female; n (%) | 643 (86) | 635 (85) | 1278 (85) | 5 (83) | 3 (75) | 8 (80) |
| BKK | N = 765 | N = 765 | N = 1530 | N = 3 | N = 6 | N = 9 |
| Age, years; mean (SD) | 57 (13) | 56 (14) | 56 (13) | 54 (2) | 59 (16) | 57 (13) |
| Sex, female; n (%) | 572 (75) | 573 (75) | 1145 (75) | 1 (33) | 5 (83) | 6 (67) |
For some data sources, low counts (i.e., ≤ 10) were masked as required to maintain data privacy, as required by local regulations
ARTIS anti-rheumatic therapy in Sweden, BKK Betriebskrankenkasse, N number in specific category, n number of patients, PS20 private source 20, SD standard deviation, SNDS Système National des Données de Santé, VTE venous thromboembolism
Secondary Outcomes
MACE
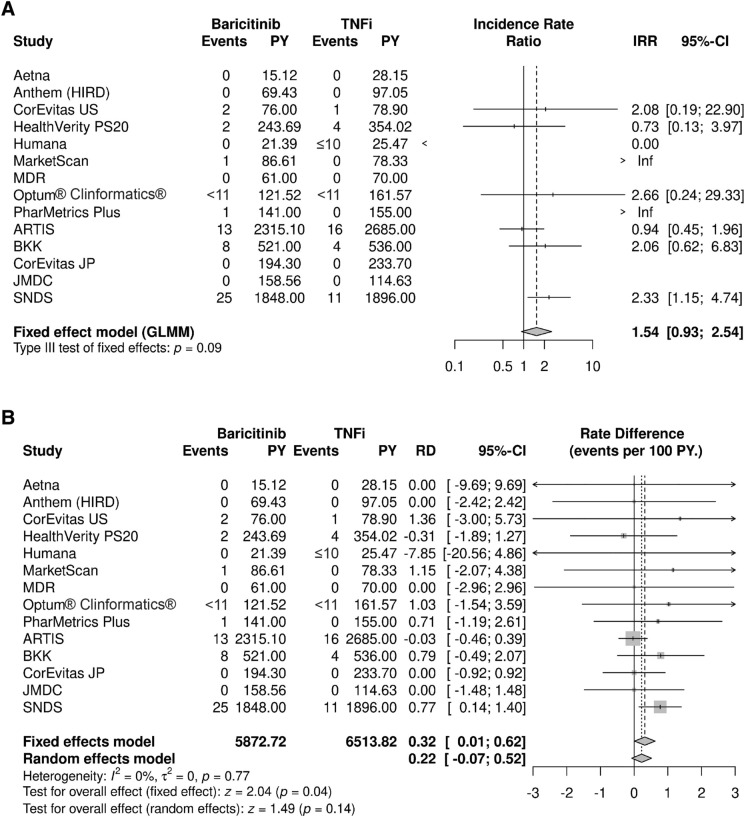
A total of 93 patients experienced MACE during a mean overall follow-up of 8 months (baricitinib) and 10 months (TNFi), 54 of whom were receiving treatment with baricitinib. There were four data sources with more than five patients with a MACE during follow-up: ARTIS (n = 29), SNDS (n = 36), BKK (n = 12), and PS20 (n = 6). A numerically greater, non-statistically significant overall IRR was estimated when baricitinib was compared with TNFi with respect to risk of MACE (IRR = 1.54; 95% CI 0.93, 2.54; Fig. 2A). The difference in incidence rates (IRD) between baricitinib and TNFi was 0.22 (95% CI −0.07, 0.52) per 100 person-years from the random effects model, with a non-significant greater rate observed in patients treated with baricitinib (Fig. 2B). Stated differently, for every 1000 patients treated with baricitinib instead of a TNFi, an additional two MACE would be expected each year. Both random and fixed effect model results were estimated for IRD (Fig. 2B) but only the random effects result is presented as the main IRD finding since it allows that the treatment effect may vary in different populations and point estimates did not differ. The incidence of MACE in each data source is provided in the Supplementary Material (Table S12).
Fig. 2.
Meta-analysis for MACE comparing baricitinib and TNF inhibitors showing A incidence rate ratios and B incidence rate differences. ARTIS anti-rheumatic therapy in Sweden, BKK Betriebskrankenkasse, CI confidence interval, GLMM generalized linear mixed model, HIRD HealthCore Integrated Research Database, IRR incidence rate ratio, JMDC JMDC, Inc.’s claims database, JP Japan, MACE major adverse cardiovascular event, MDR military health system data repository, Optum® Clinformatics® Optum’s de-identified Clinformatics® Data Mart Database, PS20 private source 20, PY person-years, RD rate difference, SNDS Système National des Données de Santé, TNFi tumor necrosis factor inhibitor, US United States. For some data sources, low counts (i.e., ≤ 10) were masked as required to maintain data privacy, as required by local regulations
Clinical characteristics and use of RA medications in patients with MACE were generally consistent with the overall cohort of RA patients, except for age (Supplementary Material, Table S14) although these are qualitative comparisons limited by the small number of events. The mean age of patients treated with baricitinib with a MACE appeared higher (mean age in ARTIS 68 years; SNDS 68 years) than the overall age of the baricitinib cohort included in MACE analyses (mean age in ARTIS 59 years; SNDS 58 years). The majority of French patients in both treatment cohorts who experienced MACE were male (n = 22 of 36 overall; Supplementary Material, Table S14). The distribution of time to MACE was variable, ranging from 1 to 1460 days. For the baricitinib cohort, the mean (median) time to event was 503 (454) days in ARTIS and 216 (171) days in SNDS, consistent with the mean follow-up, i.e., treatment, in these cohorts. In the TNFi cohort, the mean (median) time to event was 583 (484) days in ARTIS and 226 (174) days in SNDS, consistent with the mean (median) follow-up time in these cohorts.
Serious Infection
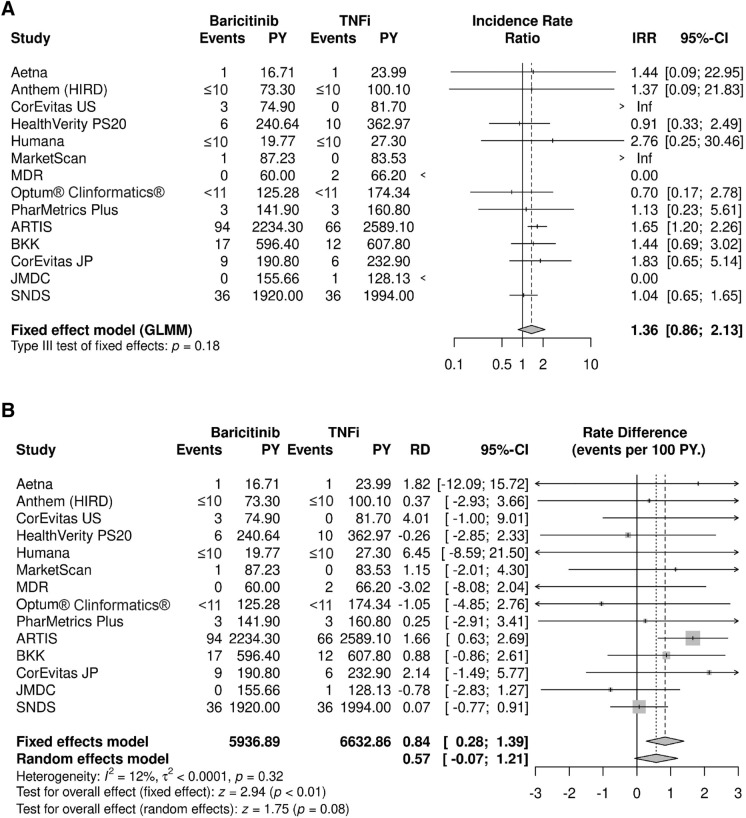
There were 321 patients with serious infections during a mean overall follow-up of 10 months (baricitinib) and 11 months (TNFi), 176 of whom were treated with baricitinib. There were several data sources with more than five patients with serious infection during follow-up: ARTIS (n = 160), SNDS (n = 72), BKK (n = 29), PS20 (n = 16), CorEvitas Japan (n = 15), and PharMetrics Plus (n = 6). A numerically greater, non-statistically significant overall IRR was estimated when comparing risk of serious infection in baricitinib vs. TNFi cohorts (IRR = 1.36; 95% CI 0.86, 2.13) (Fig. 3A). The IRD between baricitinib and TNFi was 0.57 (95% CI −0.07, 1.21) per 100 person-years from the random effects model (Fig. 3B), with a greater incidence rate among patients treated with baricitinib; this difference was not statistically significant. This would mean for every 1000 patients treated with baricitinib instead of a TNFi, six additional serious infections would be expected each year. Both random and fixed effect model results were estimated for IRD (Fig. 3B) but only the random effects result is presented as the main IRD finding since it allows that the treatment effect may vary in different populations and point estimates did not differ. The incidence of serious infection in each data source is provided in the Supplementary Material (Table S12).
Fig. 3.
Meta-analysis for serious infection comparing baricitinib and TNF inhibitors showing A incidence rate ratios and B incidence rate differences. ARTIS anti-rheumatic therapy in Sweden, BKK Betriebskrankenkasse, CI confidence interval, GLMM generalized linear mixed model, HIRD HealthCore Integrated Research Database, IRR incidence rate ratio, JMDC JMDC, Inc.’s claims database, JP Japan, MDR military health system data repository, Optum® Clinformatics® Optum’s de-identified Clinformatics® Data Mart Database, PS20 private source 20, PY person-years, RD rate difference, SNDS Système National des Données de Santé, TNFi tumor necrosis factor inhibitor, US United States. For some data sources, low counts (i.e., ≤ 10) were masked as required to maintain data privacy, as required by local regulations
Clinical characteristics of patients with serious infections were similar to those observed in the overall cohort of RA patients but sample sizes were too small to be informative. The three data sources with the greatest number of serious infection events, consistent with their overall sample sizes, were ARTIS (n = 160), SNDS (n = 72), and BKK (n = 29). Within these sources, results suggest that patients with serious infections may be older and more often male, than patients in the overall cohorts (Supplementary Material, Table S15). However, these are qualitative observations that were not statistically tested.
The distribution of time to serious infection was variable, with minimum of 1 day and maximum of 1460 days. Optum's de-identified Clinformatics® Data Mart Database (Optum® Clinformatics®) and BKK reported the shortest time to serious infection. For the baricitinib cohort, mean (median) time to serious infection was 80 (79) days in Optum® Clinformatics® (n < 11) and 107 (100) days in BKK (n = 17). For the TNFi cohort, time to serious infection was 205 (216) in Optum® Clinformatics® (n < 11) and 188 (96) in BKK (n = 12). ARTIS (n = 94) reported the longest mean (median) time to serious infection, of 485 (428) days in the baricitinib cohort and 562 (453) in the TNFi cohort, consistent with the mean follow-up times of these cohorts.
Among the total 9013 eligible patients treated with baricitinib available prior to matching, there were no cases of hospitalized TB recorded; three cases in total were identified in the TNFi cohort.
Discussion
Study B023 aimed to compare the safety of baricitinib with TNFi for the treatment of patients with RA in routine care for risk of VTE, MACE, or serious infection. A meta-analysis was used to combine results across 14 post-marketing data sources in Europe, the US, and Japan. With a mean overall exposure of 9 months, treatment with baricitinib was associated with a significantly increased risk of VTE versus TNFi (IRR = 1.51, 95% CI 1.10, 2.08). The incidence rate was greater among patients treated with baricitinib than with TNFi, with an IRD of 0.26 (95% CI −0.04, 0.57) per 100 PY. Risk of MACE was also numerically greater with baricitinib versus TNFi, although not statistically significant, during a mean overall exposure of 8 months (IRR = 1.54, 95% 0.93, 2.54; IRD = 0.22 95% CI −0.07, 0.52 per 100 PY). Results for serious infection also estimated a numerically greater, non-statistically significant risk with baricitinib than with TNFi during a mean overall exposure of 10 months (IRR = 1.36, 95% CI 0.86, 2.13; IRD = 0.57, 95% CI −0.07, 1.21 per 100 PY). Overall incidence rates were not estimated in the study and comparative risk should be interpreted in terms of patient cohorts or populations, rather than individual risk.
Patients with RA are at greater risk of a wide range of comorbidities [26], including the conditions evaluated in this study. Risk of VTE in this population is increased by 30–40% compared to the general population [3, 5, 27], and has been associated with disease activity [28]. Among patients receiving treatment for RA, particularly those proceeding through a sequence of advanced therapies [29], risk of VTE was elevated with bDMARDs compared to cDMARDs or methotrexate treatment [5, 30].
Few studies have assessed the comparative risk of VTE associated with JAKi, the most notable of which was the post-marketing study for tofacitinib, the ORAL Surveillance randomized trial in patients with RA enriched for MACE risk factors [31]. A significant imbalance occurred in the incidence of pulmonary embolism and all-cause mortality in the 10-mg twice-daily arm of the trial, which led to a US Food and Drug Administration (FDA) black box warning update in 2021 for all JAKi approved in the US for the treatment of RA and other inflammatory conditions. Using US claims data (2012–2019), Desai et al. [32] compared new users of tofacitinib (5301 person-years) with TNFi (75,824 person-years) and did not detect a meaningful difference in risk of VTE (HR = 1.13; 95% CI 0.77, 1.65). A meta-analysis of data from 29 randomized trials (13,910 patients) found no significant association with risk of VTE for JAKi compared to placebo (odds ratio 0.91; 95% CI 0.57, 1.47), with consistent results for baricitinib (odds ratio 1.12; 95% CI 0.27, 4.69) [33]. Most recently, however, in an observational study conducted within the ARTIS data the risk of VTE in patients with RA treated with baricitinib (3412 person-years) was 1.79-fold (95% CI 1.25, 2.55) greater compared to patients treated with TNFi after adjusting for treatment history, smoking, and RA disease-related variables, i.e., DAS28, CRP, and HAQ [34].
Findings from previous studies examining the association between JAKi and cardiovascular outcomes have not been consistent. Results from the ORAL Surveillance randomized trial identified a 1.33-fold (95% CI 0.91, 1.94) greater risk of MACE with tofacitinib versus TNFi treatment in a cohort of patients enriched for cardiovascular risk factors (50 years or older with ≥ 1 risk factor) [31]. This elevated risk was present for both the 5- and 10-mg doses, although not statistically significant given the low incidence. The observational, non-randomized STAR-RA study, which compared tofacitinib with TNFi, detected a similar 1.24-fold (95% 0.90, 1.69) risk of MACE in a cohort designed to emulate the high-risk ORAL Surveillance population [35]. However, no difference in risk was detected when the comparison was made in the same data, but in an unselected real-world cohort with greater generalizability (HR = 1.01; 95% 0.83, 1.23). The authors hypothesize that the association between tofacitinib and cardiovascular outcomes is modified by baseline cardiovascular risk. An analysis of the ORAL Surveillance population found that increased risk of MACE was mainly observed in patients with older age and previous atherosclerotic cardiovascular disease [36].
The B023 study also calculated a numerically elevated risk of MACE (IRR = 1.54; 95% CI 0.93, 2.54), but point estimates from the two data sources that contributed the most person-time and events were not aligned, with IRRARTIS = 0.94 (95% CI 0.45, 1.96) and IRRSNDS = 2.33 (95% CI 1.15, 4.74). One explanation for this observed difference in point estimates may be the different proportions of more refractory patients in each data source. Patients who are first to initiate newly approved medications may have more refractory disease or differ in other important ways such as more comorbidities. The index period for Swedish ARTIS patients included in B023 (Feb 2017 to Dec 2020) is more than 1 year longer compared to French patients in SNDS (Sept 2017 to Dec 2019). In France, national guidance in 2017–2018 required French patients treated with baricitinib to have had previously failed treatment with bDMARDs [37]. This suggests that a greater proportion of patients in SNDS could be more refractory users compared to the ARTIS data. The observed differences in ARTIS and SNDS point estimates may therefore reflect baseline differences in risk that modify the relative risk of MACE, as proposed by the STAR-RA authors Khosrow-Khavar et al. [35]. While B023 was not designed to test for differences in MACE risk by baseline risk, this explanation is supported by the different incidence rates of MACE in ARTIS vs. SNDS (0.56 vs. 1.4 per 100 person-years, respectively). Alternatively, given the low incidence of MACE in general and in B023, this may also simply reflect variability due to low patient counts.
Patients with RA have an elevated risk of infection due to disease and therapeutic interventions [2]. Findings from interventional studies show that JAKi users have a similar risk of serious infection as TNFi users [38]. Incidence rates from development programs have tended to fall in the range of 3–4 cases per 100 person-years, with increased risk in older patients [38]. ORAL Surveillance detected a non-statistically significant, elevated risk of serious infection for treatment with 5 mg tofacitinib compared to TNFi (HR = 1.17; 95% CI 0.92, 1.50).
Incidence rates of serious infection in the B023 data sources were generally consistent for TNFi [31] and numerically greater for baricitinib compared to clinical trial rates [11]. The increased rates observed in the baricitinib cohorts may reflect a general upward shift in claims data due to differences in the case definitions between trials and claims data, differences in the populations analyzed, or both. Either way, the overall relative risk of serious infection estimated by the B023 meta-analysis was modestly increased with differences once again observed between the individual ARTIS (IRR = 1.65; 95% CI 1.20, 2.26) and SNDS (IRR = 1.04; 95% CI 0.65, 1.65) point estimates. This result does not support the hypothesis that the difference in effect estimates is related to a larger proportion of early adopters in SNDS than in ARTIS. However, rates of infection vary considerably by time since treatment start and there are important differences in mean follow-up between the two sources (ARTIS 1.3 years vs. SNDS 8 months).
Based on extensive longitudinal data from the baricitinib cohort from the clinical development program (> 14,000 person-years, median exposure 1683 days, max exposure 3405 days), the rate of VTE (pulmonary embolism or deep-vein thrombosis), MACE (myocardial infarction, stroke, and cardiovascular deaths), and serious infection during a median 4.6 years (maximum 9.3 years) treatment with baricitinib remained stable over time at 0.49 (95% CI 0.38–0.61), 0.51 (95% CI 0.40, 0.64), and 2.58 (95% CI 2.33, 2.86) per 100 person-years [11]. There did not appear to be differences between the 4 mg (VTE, MACE, and serious infection IRs of 0.51, 0.54, and 2.62, respectively, per 100 person-years) and 2 mg (VTE, MACE, and serious infection IRs of 0.49, 0.42, and 2.13, respectively, per 100 person-years) doses based on the available information. Observed VTE, MACE, and serious infection IRs in patients treated with baricitinib from the clinical development program and other RA populations from various external sources suggests they are numerically similar although no statistical comparison was conducted [39]. However, results from controlled comparative studies, including observational studies such as B023, suggest that incidence rates of these safety outcomes in patients treated with JAKi, including baricitinib, are elevated compared to similar populations treated with TNFi. The rate from the baricitinib cohort in the clinical program (VTE IR = 0.49 per 100 person-years) is not comparable with rates from individual B023 data sources (where VTE IR ranged from 0.60 to 2.55 per 100 person-years for baricitinib cohorts and 0.54 to 1.40 per 100 person-years for TNFi cohorts) as outcome definitions, prevalence of risk factors, and patient populations were different. Similar caution should be applied comparing IR from individual B023 data sources with results from external sources. The characteristics of patients evaluated in the B023 study are those of real-world patients with RA treated with baricitinib, particularly in Europe, where the large majority of eligible patients were included in analyses after propensity score matching to TNFi.
In the future, results from two ongoing post-marketing randomized trials, RA-BRANCH (NCT04086745) and RA-BRIDGE (NCT03915964), will be available to provide a more complete understanding of the risk of VTE, MACE, and serious infection associated with baricitinib compared to TNFi in high-risk patients with ≥ 1 VTE risk factor and inadequate response or intolerance to ≥ 1 prior cDMARD or bDMARD.
Strengths and Limitations
Several strengths and limitations should be considered when interpreting the results of this study. Since this study was not randomized and is based on data collected for other purposes, the potential for bias due to confounding is a concern. Several risk factors known to be associated with the outcomes evaluated in this study are not available or only partially complete. Claims data present limited ability to control for confounding by lifestyle factors such as BMI and smoking, or clinical measures of disease such as severity, activity, duration, or treatment history. A summary of each limitation along with the mitigations taken to address it is presented below for consideration.
First, we give a brief review of the strengths of this study. There was broad geographic representation of patients with RA receiving treatment with baricitinib in routine care. Second, the study used validated case definitions confirming the accuracy of VTE identified in French, Swedish, and US data with PPV of 75.5-92% [16–18]. Third, the study implemented several design and analysis strategies to control for and assess potential confounding, including the use of an active comparator new user study design, propensity score matching, and sensitivity analyses. Finally, the implementation of a common analytic strategy executed across individual data sources may also have reduced a source of heterogeneity.
There are also limitations. RA disease activity is a risk factor for each of the study outcomes (VTE, MACE, and serious infection) [28, 40, 41]. Inclusion of traditional risk factors may not account fully or at all for the effects of RA. In an effort to partially control for disease activity, an RA-specific measure of healthcare resource utilization was included in all propensity score models, but this measure is known to be a poor proxy [42]. A simple bias analysis was conducted to quantify the magnitude of bias that could have been introduced due to unmeasured confounding by disease activity [43]. For all outcomes, the bias analysis result suggested that the final interpretation of the study results is unlikely to have changed if information about disease activity had been fully accounted for. In support of this, a study in US RA registry patients showed that adding disease activity to a model of traditional risk factors for cardiovascular disease contributed limited additional ability to predict risk (change in c-statistic = 0.04) [44]. Further, the recent analysis conducted in ARTIS patients found that additionally adjusting for RA disease measures (treatment history, DAS28, CRP, and HAQ) did not attenuate the association between baricitinib and VTE [34].
Another limitation of the study was the length of follow-up, which was brief. This study was designed to provide rapid insight into the safety of baricitinib with respect to specific outcomes rather than to evaluate long-term safety. The 9-month average follow-up of patients may have limited the ability to fully evaluate risk.
Next, insurance claims data present limited ability to control for confounding by lifestyle factors such as BMI and smoking, both of which are risk factors for the outcomes investigated in B023. In addition to the study design and analytic strategies, such as propensity score matching and active comparator new user design, which were incorporated to minimize the impact of these potentially confounding factors, additional bias analyses were used to assess the robustness of results to missing information on BMI and smoking. As before, quantitative evaluation of the magnitude of potential bias that could have occurred due to BMI or smoking suggests that the study results were unlikely to have been impacted in an important way by not controlling for these factors.
Finally, baseline risk factors were assessed in the 6 months prior to initiation of study drug. This period may be too short to allow for complete assessment of patient comorbidities and relevant risk factors. To evaluate the impact, a sensitivity analysis in the SNDS data extended the baseline to 2 years. As expected, the overall prevalence of comorbidities increased, but no differences appeared between the treatment cohorts that were more extreme than the prevalences evaluated in the bias analyses, suggesting these differences would not meaningfully impact results. This was not evaluated in other data sources.
To date, Study B023 is the largest, real-world observational study evaluating VTE, MACE, and serious infections among patients treated with baricitinib compared to similar patients treated with TNFi. Despite the limitations, this large, multi-database study provides important additional information on the safety of baricitinib into the evolving landscape of safety for JAKi.
Conclusions
In conclusion, this study suggests that patients receiving treatment with baricitinib for RA have an increased risk of VTE compared with TNFi treatment. A numerically greater IRR was estimated for baricitinib compared to TNFi for MACE, but this did not attain statistical significance and point estimates from the largest data sources differed. Similarly, the overall IRR estimating risk of serious infection was numerically greater for baricitinib compared to TNFi and non-statistically significant. Findings from this study and their impact on clinical practice should be considered in context of limitations and other evidence regarding the safety and efficacy of baricitinib and other JAK inhibitors.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank those whose scientific input helped shape the design of the study and sensitivity analyses and generally improved the scientific rigor of the study, including FDA reviewers. We also thank the French Scientific Advisory Board for scientific and medical input (Dr. Vincent Germain, Pr. Christophe Richez), the French Adjudication Committee who supported the case definition for VTE in French data (Dr. Carine Boulon, Dr. Eléonore Brunetti-Casassus, Pr. Joël Constans, Dr. Antoine Diard), the CORPUS Team for providing complementary data for sensitivity analyses (Pr. Francis Guillemin, Marc Soudant), and the ESPOIR Team for providing complementary data for sensitivity analyses (Pr Bernard Combe, Phillipe Dieudé, Nathalie Rincheval), and many others. The authors also wish to thank the clinical experts at the US Department of Defense and all those whose time and expertise allow data to be available for research.
Funding
This study was sponsored by Eli Lilly and Company under license from Incyte. The study sponsor funded the journal’s Rapid Service Fee.
Medical Writing, Editorial, and Other Assistance
Catherine Lynch, Eli Lilly and Company, provided medical writing and editorial assistance.
Author Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Claudia A Salinas, Anthony Louder, Jennifer Polinski, Tancy C. Zhang, Hannah Bower, Syd Phillips, Yufei Song, Emaan Rashidi, Rafia Bosan, Hsiu-Ching Chang, Nicole Foster, Bernice Gershenson, Baojin Zhu, Douglas Faries, Xiaodan Mai, Brett T Doherty, Angela Grelaud, Nicolas H. Thurin, Johan Askling, and Walter Deberdt. The first draft of the manuscript was written by Claudia A. Salinas and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Disclosures
Claudia A Salinas, Peter Fischer, Baojin Zhu, Douglas Faries, and Walter Deberdt are employees and shareholders of Eli Lilly and Company. Anthony Louder was an employee of Aetion, Inc. at the time of study conduct. Jennifer Polinski and Tancy C. Zhang are employees of and hold stock options in Aetion, Inc. Hannah Bower has nothing to disclose. Syd Phillips was a full-time employee of IQVIA at the time of study conduct. Yufei Song, Emaan Rashidi, Rafia Bosan, and Hsiu-Ching Chang are employees of IQVIA. Nicole Foster and Bernice Gershenson are employees of CorEvitas, LLC. Hisashi Yamanaka has received speaker or consultant fees from Teijin Pharma and YL Biologics. Mitsumasa Kishimoto has received consulting fees and/or honoraria from AbbVie, Amgen, Asahi-Kasei Pharma, Astellas, Ayumi Pharma, BMS, Chugai, Daiichi-Sankyo, Eisai, Eli Lilly, Gilead, Janssen, Kyowa Kirin, Novartis, Ono Pharma, Pfizer, Tanabe-Mitsubishi, Teijin Pharma, and UCB Pharma. Yoshiya Tanaka has received speaking fees and/or honoraria from Behringer-Ingelheim, Eli Lilly and Company, AbbVie, Gilead, AstraZeneca, Bristol-Myers, Chugai, Daiichi-Sankyo, Eisai, Pfizer, Mitsubishi-Tanabe, GlaxoSmithKline, received research grants from Asahi-Kasei, AbbVie, Chugai, Eisai, Takeda, Daiichi-Sankyo, Behringer-Ingelheim. Xiaodan Mai was an employee of HealthCore, Inc. at the time of study conduct. Current affiliation: Department of Population Medicine, Harvard Medical School and Harvard Pilgrim Health Care Institute, Boston, MA. Brett T Doherty is an employee of HealthCore, Inc. Angela Grelaud and Nicolas H. Thurin are researchers at Bordeaux PharmacoEpi, a research platform of the University of Bordeaux and its subsidiary the ADERA, which performs financially supported studies for public and private partners, including Eli Lilly and Company, in compliance with the ENCePP Code of Conduct. Johan Askling—Karolinska Institutet has entered into research agreements with AbbVie, Astra-Zeneca, BMS, Eli Lilly, Galapagos, MSD, Pfizer, Roche, Samsung Bioepis, Sanofi, and UCB, with JA as PI, mainly regarding the safety monitoring of rheumatology immunomodulators.
Compliance with Ethics Guidelines
Study B023 was conducted in accordance with ethical principles of the Helsinki Declaration of 1964 and its later amendments, and Good Clinical Practice guidelines. Ethical approval was provided by Advarra IRB Committee, a centralized IRB in the USA, (Reference Pro00042607), and CNIL for French SNDS data (reference 919392). All data were de-identified to ensure patient confidentiality and used in accordance with data license agreements. The requirement for informed consent was therefore waived.
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available as they are commercially owned or limited to analyses approved by the data owner within national borders.
References
- 1.Simon TA, Thompson A, Gandhi KK, Hochberg MC, Suissa S. Incidence of malignancy in adult patients with rheumatoid arthritis: a meta-analysis. Arthritis Res Ther. 2015;17:212. doi: 10.1186/s13075-015-0728-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Doran MF, Crowson CS, Pond GR, O'Fallon WM, Gabriel SE. Frequency of infection in patients with rheumatoid arthritis compared with controls: a population-based study. Arthritis Rheum. 2002;46:2287–2293. doi: 10.1002/art.10524. [DOI] [PubMed] [Google Scholar]
- 3.Kim SC, Schneeweiss S, Liu J, Solomon DH. Risk of venous thromboembolism in patients with rheumatoid arthritis. Arthritis Care Res (Hoboken) 2013;65:1600–1607. doi: 10.1002/acr.22039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee JJ, Pope JE. A meta-analysis of the risk of venous thromboembolism in inflammatory rheumatic diseases. Arthritis Res Ther. 2014;16:435. doi: 10.1186/s13075-014-0435-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ogdie A, Kay McGill N, Shin DB, et al. Risk of venous thromboembolism in patients with psoriatic arthritis, psoriasis and rheumatoid arthritis: a general population-based cohort study. Eur Heart J. 2018;39:3608–3614. doi: 10.1093/eurheartj/ehx145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aviña-Zubieta JA, Choi HK, Sadatsafavi M, Etminan M, Esdaile JM, Lacaille D. Risk of cardiovascular mortality in patients with rheumatoid arthritis: a meta-analysis of observational studies. Arthritis Care Res. 2008;59:1690–1697. doi: 10.1002/art.24092. [DOI] [PubMed] [Google Scholar]
- 7.Avina-Zubieta JA, Thomas J, Sadatsafavi M, Lehman AJ, Lacaille D. Risk of incident cardiovascular events in patients with rheumatoid arthritis: a meta-analysis of observational studies. Ann Rheum Dis. 2012;71:1524–1529. doi: 10.1136/annrheumdis-2011-200726. [DOI] [PubMed] [Google Scholar]
- 8.Picerno V, Ferro F, Adinolfi A, Valentini E, Tani C, Alunno A. One year in review: the pathogenesis of rheumatoid arthritis. Clin Exp Rheumatol. 2015;33:551–558. [PubMed] [Google Scholar]
- 9.Mutru O, Laakso M, Isomäki H, Koota K. Ten year mortality and causes of death in patients with rheumatoid arthritis. Br Med J (Clin Res Ed) 1985;290:1797. doi: 10.1136/bmj.290.6484.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sihvonen S, Korpela M, Laippala P, Mustonen J, Pasternack A. Death rates and causes of death in patients with rheumatoid arthritis: a population-based study. Scand J Rheumatol. 2004;33:221–227. doi: 10.1080/03009740410005845. [DOI] [PubMed] [Google Scholar]
- 11.Taylor PC, Takeuchi T, Burmester GR, et al. Safety of baricitinib for the treatment of rheumatoid arthritis over a median of 4.6 and up to 9.3 years of treatment: Final results from long-term extension study and integrated database. Ann Rheum Dis. 2022;81:335–343. doi: 10.1136/annrheumdis-2021-221276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taylor PC, Weinblatt ME, Burmester GR, et al. Cardiovascular safety during treatment with baricitinib in rheumatoid arthritis. Arthritis Rheumatol. 2019;71:1042–1055. doi: 10.1002/art.40841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lund JL, Richardson DB, Stürmer T. The active comparator, new user study design in pharmacoepidemiology: historical foundations and contemporary application. Curr Epidemiol Rep. 2015;2:221–228. doi: 10.1007/s40471-015-0053-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim SY, Servi A, Polinski JM, et al. Validation of rheumatoid arthritis diagnoses in health care utilization data. Arthritis Res Ther. 2011;13:R32. doi: 10.1186/ar3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olumiant [package insert]. Indianapolis, IN: Eli Lilly and Company; 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/207924s006lbl.pdf. Accessed 16 September 2022.
- 16.Fang MC, Fan D, Sung SH, et al. Validity of using inpatient and outpatient administrative codes to identify acute venous thromboembolism: the CVRN VTE study. Med Care. 2017;55:e137–e143. doi: 10.1097/MLR.0000000000000524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thurin NH, Bosco-Levy P, Blin P, et al. Intra-database validation of case-identifying algorithms using reconstituted electronic health records from healthcare claims data. BMC Med Res Methodol. 2021;21:95. doi: 10.1186/s12874-021-01285-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Molander V, Bower H, Askling J. Validation and characterization of venous thromboembolism diagnoses in the Swedish National Patient Register among patients with rheumatoid arthritis. Scand J Rheumatol. 2022 doi: 10.1080/03009742.2021.2001907:1-7. [DOI] [PubMed] [Google Scholar]
- 19.Fralick M, Kesselheim AS, Avorn J, Schneeweiss S. Use of health care databases to support supplemental indications of approved medications. JAMA Intern Med. 2018;178:55–63. doi: 10.1001/jamainternmed.2017.3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCormick N, Bhole V, Lacaille D, Avina-Zubieta JA. Validity of diagnostic codes for acute stroke in administrative databases: a systematic review. PLoS ONE. 2015;10:e0135834. doi: 10.1371/journal.pone.0135834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kremer JM. The Corrona US registry of rheumatic and autoimmune diseases. Clin Exp Rheumatol. 2016;34:S96–S99. [PubMed] [Google Scholar]
- 22.Schneeweiss S. Developments in post-marketing comparative effectiveness research. Clin Pharmacol Ther. 2007;82:143–156. doi: 10.1038/sj.clpt.6100249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. doi: 10.1093/biomet/70.1.41. [DOI] [Google Scholar]
- 24.Kuss O, Blettner M, Börgermann J. Propensity score: an alternative method of analyzing treatment effects. Deutsch Arzteblatt Int. 2016;113:597–603. doi: 10.3238/arztebl.2016.0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taylor PC, Atzeni F, Balsa A, Gossec L, Müller-Ladner U, Pope J. The key comorbidities in patients with rheumatoid arthritis: a narrative review. J Clin Med. 2021;10:509. doi: 10.3390/jcm10030509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li L, Lu N, Avina-Galindo AM, et al. The risk and trend of pulmonary embolism and deep vein thrombosis in rheumatoid arthritis: a general population-based study. Rheumatology. 2021;60:188–195. doi: 10.1093/rheumatology/keaa262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Molander V, Bower H, Frisell T, Askling J. Risk of venous thromboembolism in rheumatoid arthritis, and its association with disease activity: a nationwide cohort study from Sweden. Ann Rheum Dis. 2021;80:169–175. doi: 10.1136/annrheumdis-2020-218419. [DOI] [PubMed] [Google Scholar]
- 29.Liang H, Danwada R, Guo D, et al. Incidence of inpatient venous thromboembolism in treated patients with rheumatoid arthritis and the association with switching biologic or targeted synthetic disease-modifying antirheumatic drugs (DMARDs) in the real-world setting. RMD Open. 2019;5:e001013. doi: 10.1136/rmdopen-2019-001013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim SC, Solomon DH, Liu J, Franklin JM, Glynn RJ, Schneeweiss S. Risk of venous thromboembolism in patients with rheumatoid arthritis: Initiating disease-modifying antirheumatic drugs. Am J Med. 2015;128(539):e7–17. doi: 10.1016/j.amjmed.2014.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ytterberg SR, Bhatt DL, Mikuls TR, et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med. 2022;386:316–326. doi: 10.1056/NEJMoa2109927. [DOI] [PubMed] [Google Scholar]
- 32.Desai RJ, Pawar A, Khosrow-Khavar F, Weinblatt ME, Kim SC. Risk of venous thromboembolism associated with tofacitinib in patients with rheumatoid arthritis: a population-based cohort study. Rheumatology. 2022;61:121–130. doi: 10.1093/rheumatology/keab294. [DOI] [PubMed] [Google Scholar]
- 33.Bilal J, Riaz IB, Naqvi SAA, et al. Janus kinase inhibitors and risk of venous thromboembolism: a systematic review and meta-analysis. Mayo Clin Proc. 2021;96:1861–1873. doi: 10.1016/j.mayocp.2020.12.035. [DOI] [PubMed] [Google Scholar]
- 34.Molander V, Bower H, Frisell T, Delcoigne B, Di Giuseppe D, Askling J. Venous thromboembolism with JAK inhibitors and other immune-modulatory drugs: a Swedish comparative safety study among patients with rheumatoid arthritis. Ann Rheum Dis. 2022 doi: 10.1136/ard-2022-223050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khosrow-Khavar F, Kim SC, Lee H, Lee SB, Desai RJ. Tofacitinib and risk of cardiovascular outcomes: results from the safety of tofacitinib in routine care patients with rheumatoid arthritis (STAR-RA) study. Ann Rheum Dis. 2022;81:798–804. doi: 10.1136/annrheumdis-2021-221915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Szekanecz Z, Giles JT, Buch MH, et al. POS0110 Incidence of major adverse cardiovascular events stratified by geographic region and baseline cardiovascular risk: a post hoc analysis of oral surveillance. Ann Rheum Dis. 2022;81:278–279. doi: 10.1136/annrheumdis-2022-eular.1180. [DOI] [Google Scholar]
- 37.Olumiant: Avis de la Commission de la Transparence du 21 juin 2017. https://webzine.has-sante.fr/upload/docs/evamed/CT-16116_OLUMIANT_PIC_INS_Avis%202_CT16116.pdf. Accessed September 16, 2022.
- 38.Strand V, Ahadieh S, French J, et al. Systematic review and meta-analysis of serious infections with tofacitinib and biologic disease-modifying antirheumatic drug treatment in rheumatoid arthritis clinical trials. Arthritis Res Ther. 2015;17:362. doi: 10.1186/s13075-015-0880-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bieber T, Feist E, Irvine AD, et al. A review of safety outcomes from clinical trials of baricitinib in rheumatology, dermatology and COVID-19. Adv Ther. 2022;39:4910–4960. doi: 10.1007/s12325-022-02281-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gabriel SE. Cardiovascular morbidity and mortality in rheumatoid arthritis. Am J Med. 2008;121:S9–14. doi: 10.1016/j.amjmed.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mehta B, Pedro S, Ozen G, et al. Serious infection risk in rheumatoid arthritis compared with non-inflammatory rheumatic and musculoskeletal diseases: a US national cohort study. RMD Open. 2019;5:e000935. doi: 10.1136/rmdopen-2019-000935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ting G, Schneeweiss S, Scranton R, et al. Development of a health care utilisation data-based index for rheumatoid arthritis severity: a preliminary study. Arthritis Res Ther. 2008;10:R95. doi: 10.1186/ar2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schneeweiss S. Sensitivity analysis and external adjustment for unmeasured confounders in epidemiologic database studies of therapeutics. Pharmacoepidem Drug Safe. 2006;15:291–303. doi: 10.1002/pds.1200. [DOI] [PubMed] [Google Scholar]
- 44.Solomon DH, Kremer J, Curtis JR, et al. Explaining the cardiovascular risk associated with rheumatoid arthritis: traditional risk factors versus markers of rheumatoid arthritis severity. Ann Rheum Dis. 2010;69:1920–1925. doi: 10.1136/ard.2009.122226. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available as they are commercially owned or limited to analyses approved by the data owner within national borders.