Abstract
Purpose
Lung cancer is one of the most common malignant tumors. Most patients develop spinal metastases during the course of cancer and suffer skeletal-related events. Currently, no consensus has been reached on the prognostic factors in patients undergoing surgeries. This study aimed to answer two questions: (1) what are the effects of surgical intervention, and (2) what are the factors associated with postoperative survival.
Methods
Searches were performed on electronic databases including PubMed, Ovid/MEDLINE, Cochrane, and Scopus for articles published before February of 2022, involving the survival factors of patients with spinal metastasis. Multiple data items were considered, such as baseline demographics, surgical details, clinical outcome, and prognostic factors. The analysis was performed in Review Manager (RevMan) 5.5. The prognostic factors of survival were analyzed with univariate and multivariate cox regression analysis.
Results
Finally, 14 studies with 813 patients were identified. Their 6, 12, and 24 months survival rates ranged from 18 to 58%, 18 to 22.4%, and 0 to 58.5%, respectively. The pooled hazard ratio of preoperative ambulatory status and the number of involved vertebrae demonstrated statistical significance, while no significant prognostic effect on the overall survival was found for targeted therapy, visceral metastases, chemotherapy, radiotherapy, or postoperative ambulatory status.
Conclusion
Overall, surgical intervention could achieve significant pain relief and neurological function improvements. For patients receiving surgery for spinal metastasis from lung cancer, preoperative ambulatory status and the number of involved vertebrae were significant prognostic factors associated with their survival.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00586-022-07444-z.
Keywords: Prognostic factors, Spinal metastases, Lung cancer, Surgical intervention, Meta-analysis
Introduction
As one of the most common malignant tumors, approximately 1.8 million new lung cancer cases are diagnosed worldwide annually [1]. Around 40% of the patients with advanced lung cancer develop bone metastases, where the spinal column is the most common site involved [2, 3]. Skeletal-related events, including intractable pain, pathological vertebral fractures, and spinal cord compression, might accompany spinal metastasis. For such patients, surgical intervention could achieve better pain relief, improved ambulatory function, and extended survival, as reported in several studies of the last decades [4–8]. Surgical treatments include radical and palliative resections with anterior, posterior, or anteroposterior approaches. Other than open surgery, percutaneous stabilization and cement augmentation are less invasive surgical techniques.
Surgery with adjuvant radiotherapy could achieve superior neurological function improvements compared with radiotherapy alone, which has significant implications for spinal oncology surgery. However, such surgeries often have high perioperative mortality and perioperative morbidity risk [9]. As technically demanding surgeries, experienced surgical teams are indispensable. In the meantime, several prospective studies indicated that surgical interventions did not appear to prolong patient survival [10–13]. Thus, the prevailing view was that surgical treatments are only appropriate for patients expected to survive for a sufficient period post-surgery. Some scholars considered the predicted survival above 3 months as the cut-off for surgery, whereas others advocate the cut-off of at least 6 months [14, 15].
Effective management of spinal metastasis requires multidisciplinary collaboration between surgery, radiotherapy, chemotherapy, and corticosteroid therapy. The advent of bisphosphonates and denosumab has improved the therapies for cancer patients with bone metastasis. In any case, survival prognostication plays a key role in developing individualized treatment options, especially when surgical interventions are involved. As a result, the prognostic factors of patients with spinal metastasis have been a topic of great interest for several decades. Several classical prognostic score systems, such as the modified Tokuhashi and the Tomita scoring systems, consider visceral metastases and the number of metastasis foci as prognostic factors associated with patient survival. Whereas the New England Spinal Metastasis Score (NESMS) regards serum albumin and ambulatory function as prognostic factors [16–18].
A wide variety of prognostic factors of spinal metastasis has been identified in previous studies. However, no consensus has been reached on the most accurate prognostic factors in patients receiving surgery for spinal metastasis from primary lung cancer. Through this systematic review and meta-analysis of the literature concerning surgical management of spinal metastasis secondary to lung cancer, we sought to answer two questions: (1) what are the postoperative outcomes (pain relief and neurological function improvement), and (2) what are the factors associated with survival. To the best of our knowledge, this is the first study combining a systematic review of the literature and a quantitative meta-analysis to investigate the surgical intervention efficacy to spinal metastasis secondary to lung cancer and identify significant prognostic factors during surgical treatments.
Materials and methods
Data source and search strategy
The systematic review and meta-analysis were conducted according to the preferred reporting items for systematic reviews and meta-analyses protocol [19]. The PRISMA checklist was presented in Additional file 1. A certified, experienced librarian carried out an extensive search of electronic databases in PubMed, Embase and Cochrane Library for published articles between 2000 and 2022 (over the last two decades) involving survival factors for patients with spinal metastasis. Literature retrieving was carried out through a combined searching of subject terms (“MeSH” on PubMed and “Emtree” on Embase) and free terms on PubMed and Embase, and through keywords searching on Cochrane Library. Searching strategies used on PubMed and Embase was presented in Additional file 2. And the searching on Cochrane Library was conducted with the following keywords: “spinal metastasis; lung cancer; prognostic factor.” Additionally, some else reference studies of relative articles and reviews were screened and hand-searched for possible inclusion. The search limits were English language, studies conducted in humans, and full text available.
Selection criteria
We reviewed the references of all retrieved articles to manually identify additional relevant articles. All identified articles were systematically assessed according to the inclusion and exclusion criteria. The inclusion criteria were defined as prospective and retrospective cohort series reporting the survival factors in adult patients with spinal metastases. Exclusion criteria were defined as concerning no survival factor, mixed report with other bone metastases, duplicated reports, no multivariate analysis, and other publication types including case report, review article, or technical report. Patients who received vertebral augmentation were also excluded. Additional articles were identified from the references of the retrieved articles on survival in spinal metastases.
Data extraction
After removing duplicated publications, two reviewers independently screened the abstracts and titles. Then, full-paper readings were performed to determine the final inclusion. Disagreements were resolved by consensus reached via discussion. The following data items of the included studies were considered: study design (author, year, prospective or retrospective), study population (diagnosis, patient number, age), cancer histology, surgical details (intraoperative time and intraoperative blood loss), use of adjuvant therapy (chemotherapy, radiotherapy, and targeted therapy), type of surgery, survival period, neurologic parameters and so on. A third reviewer participated in the discussion to resolve discrepancies between the first two reviewers.
Methodological quality and risk of bias
The Newcastle–Ottawa scale (NOS) was adopted to assess the methodological quality of the included articles, which contained three quality item categories, i.e., group selection, group comparability, and outcome of interest ascertainment. Higher NOS scores indicate better quality. The risks of bias in the included studies were assessed using the Cochrane handbook for systematic reviews based on six different domains, which were graded as “low” or “high” risk of bias, or “unclear” [20, 21].
Statistical analysis
All meta-analyses were performed in Review Manager 5.5. As the prognostic effects of the factors were represented with hazard ratio (HR) and 95% confidence interval (95% CI) in the primary studies, the meta-analysis was performed with HR as the effect size. The I2 statistic was adopted to assess the heterogeneity of all included studies. If I2 ≤ 50%, the assumption of homogeneity was accepted, and a fixed-effects model was used. Otherwise, low homogeneity was assumed, and a random-effects model was adopted. Pooled estimates and effect sizes were represented with forest plots. Prognostic factors of survival were also analyzed with univariate and multivariate cox regression analysis. P-values less than 0.05 were considered statistically significant.
Result
Study selection
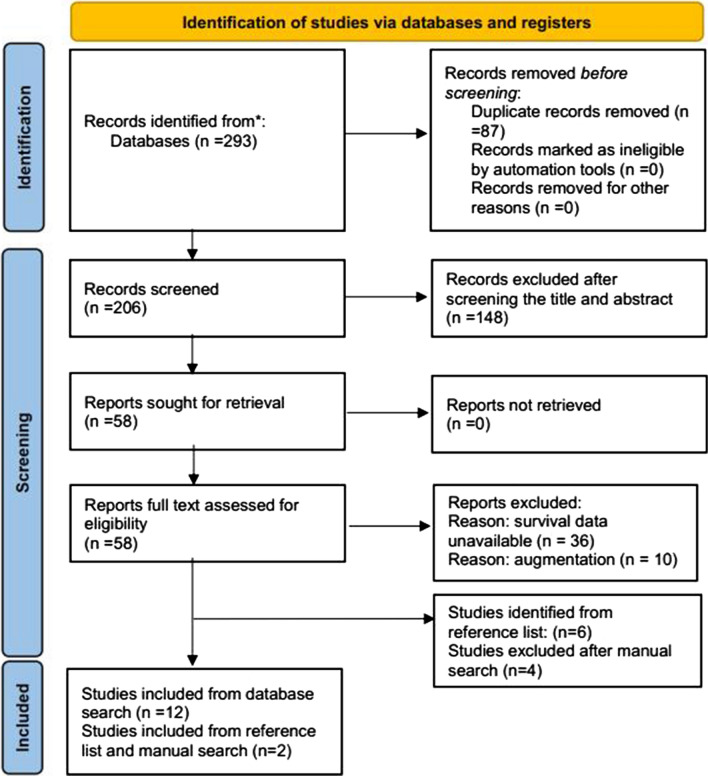
The search yielded 58 relevant studies, from which 22 duplicate records were excluded. A total of 36 articles were reviewed, 18 of which were rejected for not including any survival analysis, and 4 were excluded as the reported patients received vertebral augmentation. Thus, 14 studies published between 2009 and 2021 based on 813 patients were included according to the inclusion and exclusion criteria [22–35]. Figure 1 shows the searching and selecting procedures.
Fig. 1.
Study flow chart
Patient characteristics
The specific characteristics of each included study are listed in Table 1. In terms of study design, 13 of the included studies were retrospective, and the remaining one was prospective. In addition, the primary site of spinal metastasis was mixed in two of the included articles, and the available data of the patients with primary lung cancer were extracted. Lung cancer histology was reported in 12 articles, 6 of which only focused on patients with non-small cell lung cancer (NSCLC), while the other half included both NSCLC patients and small cell lung cancer (SCLC) patients. Patient age and gender distributions were provided in 13 articles. The study population was largely elderly males in all included studies.
Table 1.
Characteristics of patients included in the review
| No | Author | Year | Design | Country | Period | Histology | Sex, (M/F) | Age | NOS |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Chaichana KL | 2009 | R | USA | 1996–2006 | NSLC: 85%; SLC: 15% | 17/10 | Mean ± SD: 62.0 ± 11.0 | 8 |
| 2 | Lei M | 2016 | R | China | 2005–2015 | NSLC: 100% | 48/25 | Median: 57 | 7 |
| 3 | Chen YJ | 2015 | R | China | 2000–2010 | NSLC: 100% | 34/16 | Median (range): 62 (20–87) | 7 |
| 4 | Park SJ | 2016 | P | Korea | 2010–2014 | NSLC: 100% | 27/23 | Mean ± SD: 58.0 ± 11.3 | 8 |
| 5 | Zairi F | 2016 | R | France | 2005–2011 | NSLC: 84.9%; SLC: 15.1% | 45/8 | Median (range): 59 (36–78) | 9 |
| 6 | Tang Y | 2015 | R | China | 2002–2013 | NSLC: 100% | 75/41 | Median (range): 55.0 (26–79) | 7 |
| 7 | Lin HH | 2019 | R | China | 2001–2011 | NSLC: 100% | 39/26 | Mean ± SD: 60.7 ± 11.3 | 8 |
| 8 | Jung JM | 2021 | R | Korea | 2004–2019 | NSLC: 100% | 39/19 | Mean ± SD: 62.0 ± 9.6 | 7 |
| 9 | Chen Q | 2021 | R | China | 2009–2021 | NSLC: 94%; SLC: 6% | 60/23 | Median (range): 60 (42–81) | 7 |
| 10 | Zhang C | 2020 | R | China | 2009–2015 | NA | 9/6 | Median (range): 50 (40–68) | 7 |
| 11 | Fukuhara A | 2010 | R | Japan | 1999–2007 | NSLC: 76.9%; SLC: 23.1% | 9/5 | Median (range): 63 (54–78) | 8 |
| 12 | Hessler C | 2011 | R | German | 1999–2004 | NSLC: 71.4%; SLC: 28.6% | 49/27 | Median (range): 62 (37–89) | 8 |
| 13 | Truong VT | 2021 | R | France | 2008–2018 | NSLC: 92%; SLC: 8% | 45/42 | Mean ± SD: 61.3 ± 1.9 | 7 |
| 14 | Tancioni F | 2012 | R | Italy | 2004–2007 | NA | NA | NA | 8 |
R retrospective, P prospective, NSLC non-small cell lung cancer, SLC small cell lung cancer
Table 2 presents the treatment details of the included studies. Adjuvant therapies of surgical intervention, including chemotherapy, radiotherapy, targeted therapy, and bisphosphonate therapy, were reported in 13 of the included studies. Specifically, 290 (48.5%) of 598 patients reported in 11 included studies received chemotherapy [22–25, 28–34]; 367 (64.2%) of 572 patients reported in 10 included studies underwent radiotherapy [22, 24, 27, 29–35]; 137 patients (38.9%) reported in 5 studies received targeted therapy [23, 27, 28, 30, 31]; and 79 patients (60.3%) reported in 2 studies received bisphosphonate therapy [27, 31]. As for surgical technique, the relevant data of specific surgery and corresponding patients’ number were provided in 12 studies [23–34], involving 740 patients. Among them, a total of 413 patients (55.8%) received decompression with or without fusion, which is the most common surgery type in all included studies, followed by corpectomy (16,121.8%) and spondylectomy (12,917.4%). Almost all of the decompression surgery used posterior approach (99.8%), which was also mostly used in the corpectomy (66.5%). The significant surgical complications mentioned in 12 included articles are listed in Table 3 [22–29, 31–34], with wound infections reported in 6 articles and dehiscence reported in 7 articles being the most common. One-month postoperative mortalities were reported in 6 articles as 3.8–19.7%. Notably, one study reported a significantly higher one-month postoperative mortality (19.7%) than others (3.8–5.5%) [33].
Table 2.
Surgical technique and adjuvant therapy
| Author | Number | Chemotherapy (%) | Radiotherapy (%) | Targeted therapy (%) | Bisphosphonate (%) | Surgical |
|---|---|---|---|---|---|---|
| Chaichana KL | 27 | 19 | 19 | – | – | A (25.9%), P (33.3%), A-P (40.7%); fusion (81.5%); gross total resection (29.6%) |
| Lei M | 73 | 39.7 | – | 35.6 | – | P decompression + fusion (100%) |
| Chen YJ | 50 | 100 | 100 | – | – | A corpectomy + reconstruction + P fusion (6%), A decompression + fusion (4%), P decompression + fusion (90%) |
| Park SJ | 50 | 48 | – | – | – | P laminectomy + fusion (32%), P corpectomy + fusion (54%), A corpectomy + fusion (14%) |
| Zairi F | 53 | – | – | – | – | Decompression (47.2%), decompression + stabilization (52.8%) |
| Tang Y | 116 | – | 62.9 | 28.4 | 59.5 | P En-bloc spondylectomy (14.7%), subtotal spondylectomy P (59.5%), A (25.9%) |
| Lin HH | 65 | 46.2 | – | 53.8 | – | P (100%) fusion with or without decompression |
| Jung JM | 58 | 55.2 | 25.9 | – | – | P laminectomy (36.2%), A corpectomy (41.4%), A-P spondylectomy (22.4%) |
| Chen Q | 83 | 59 | 50.6 | 43.4 | – |
P En-bloc or intralesional excision (32.5%), P decompressive laminectomy (67.5%) |
| Zhang C | 15 | 53.3 | 93.3 | 46.7 | 66.7 | P decompression + radiofrequency ablation (100%) |
| Fukuhara A | 14 | 92.9 | 92.9 | – | – |
P decompression + fusion (85.7%) A decompression + fusion (14.3%) |
| Hessler C | 76 | 22.4 | 65.8 | – | – |
P decompression + fusion (54%), P spondylodesis (13.2%), corpectomy + fusion A (22.4%), P (10.5%) |
| Truong VT | 87 | 37.9 | 67.8 | – | – | P laminectomy + fusion (13.8%), P corpectomy + fusion (82.8%), A corpectomy + fusion (3.4%) |
| Tancioni F | 46 | – | 100 | – | – | Minimal resection with or without instrumentation, subtotal tumorectomy, spondylectomy and vertebrectomy |
Surgical techniques and adjuvant therapies in all included studies were summarized in this table
P prospective, A anterior, P posterior
Table 3.
Surgical complications
| Author | Wound infection | Wound dehiscence | CSF leak | Neurological deterioration | Epidural haematoma | Mortality (< 30 days) |
|---|---|---|---|---|---|---|
| Chaichana KL | – | 1 | 3 | – | – | – |
| Lei M | – | – | – | 3 | – | 4 (5.5%) |
| Chen YJ | 3 | 1 | 2 | 1 | – | 3 (6%) |
| Park SJ | 1 | 1 | – | 1 | – | – |
| Zairi F | – | – | – | – | 3 | 2 (3.8%) |
| Tang Y | – | – | – | – | – | 6 (5.2%) |
| Lin HH | 5 | 1 | – | – | – | – |
| Jung JM | – | 1 | – | – | – | – |
| Chen Q | – | – | – | – | – | – |
| Zhang C | 1 | – | – | – | – | – |
| Fukuhara A | 1 | – | – | – | – | – |
| Hessler C | – | 6 | – | 7 | – | 15 (19.7%) |
| Truong VT | 2 | 1 | – | – | 1 | 4 (4.6%) |
| Tancioni F | – | – | – | – | – | – |
The major surgical complications mentioned in included studies were summarized in this table
CSF cerebrospinal fluid
Study quality and risk of bias
NOS was adopted to assess the methodological quality of identified articles. The studies with the score of 7 was considered good quality. According to this method, the NOS scale evidenced an overall high quality of the included studies. There are 7 included studies with a NOS score of 7, 6 studies with a NOS score of 8, one study with a NOS score of 9. All included studies were assessed at high risk of performance bias consequently.
Evaluation of surgery outcome
The surgical intervention outcomes reported by the included studies involved pain relief and neurological function improvements. As detailed in Tables 4, 5 studies evaluated the patients’ preoperative and postoperative pain status [27–31]. The preoperative VAS scores of the included patients were calculated as 7.2 ± 1.8, which fell to 2.8 ± 1.6 after surgical intervention. Regarding the other surgical outcome, 11 studies evaluated the neurological function improvements achieved by surgeries [22–28, 30–32, 34] using the Frankel scale (8 studies) [23, 24, 26–28, 30–32], the ASIA score (1 study) [34], the Nurick grade (1 study) [25], and the criterion whether each patient could walk independently or not. The specific numbers of preoperatively and postoperatively ambulatory patients were reported in 10 studies, where 146 of 550 patients regained ambulation [22–28, 31, 32, 34]. Changes in patients’ preoperative and postoperative Frankel scale grades specified in 4 studies are summarized in Table 5 [26, 28, 31, 32].
Table 4.
Evaluation of surgery outcome
| Author | Pre-VAS | Pos-VAS | Regained ambulation (n) | Pos-ambulatory (n) | Median Survival (m) |
|---|---|---|---|---|---|
| Chaichana KL | – | – | 4 | 24 | 4.3 |
| Lei M | – | – | 17 | 50 | 6 |
| Chen YJ | – | – | 22 | 32 | 7.5 |
| Park SJ | – | – | 23 | 34 | 5.2 |
| Zairi F | – | – | 10 | 36 | 2.75 |
| Tang Y | 7.76 ± 1.06 | 1.97 ± 1.04 | 45 | – | 15 |
| Lin HH | 6.8 ± 0.5 | 3.8 ± 1.3 | 10 | 59 | 11 |
| Jung JM | 8.7 ± 1.1 | 4.3 ± 1.3 | – | – | 33.6 |
| Chen Q | 5.6 ± 2.4 | 2.1 ± 1.3 | – | – | 12 |
| Zhang C | 7.86 ± 0.86 | 3.51 ± 1.32 | 6 | 15 | 11 |
| Fukuhara A | – | – | 5 | 10 | 5 |
| Hessler C | – | – | – | – | 3.6 |
| Truong VT | – | – | 4 | 83 | 4.1 |
| Tancioni F | – | – | – | – | 9 |
The surgical outcomes included the median VAS scale, the number of patients who regained ambulation after surgery, the number of ambulatory postoperatively and the median survival
Pre preoperative, Pos postoperative, n number, m month
Table 5.
Evaluation of Frankel grade
| Preoperative | Postoperative | Total | ||||
|---|---|---|---|---|---|---|
| A | B | C | D | E | ||
| A | 1 | 1 | 2 | 1 | 0 | 5 |
| B | 0 | 7 | 3 | 6 | 0 | 16 |
| C | 0 | 1 | 8 | 26 | 5 | 40 |
| D | 1 | 0 | 1 | 13 | 23 | 38 |
| E | 0 | 2 | 0 | 0 | 46 | 48 |
| Total | 2 | 11 | 14 | 46 | 74 | 147 |
Evaluation of survival outcome
The median survival was between 3.6 and 33.6 months in all identified studies. The 6, 12, and 24 months survival rates range from 18 to 58%, 18 to 22.4%, and 0 to 58.5%, respectively, as listed in Table 6. Jung JM [29] reported the longest median survival among all the included studies (33.6 months) in patients who received laminectomy, corpectomy, and spondylectomy. Prognostic factors for overall survival time identified by 9 studies are summarized in Table 7 [23–27, 29, 30, 34, 35]. Multivariate analysis revealed preoperative ambulatory status, chemotherapy, radiotherapy, targeted therapy, the number of involved vertebrae, visceral metastases, time developing, neurologic deficits, preoperative performance status, postoperative performance status, postoperative ambulatory status, age, extraspinal bone metastasis foci, and tobacco use as variables associated with survival.
Table 6.
Evaluation of survival analysis
| Author | Median Survival (m) | 6-month survival (%) | 12-month survival (%) | 24-month survival (%) |
|---|---|---|---|---|
| Chaichana KL | 4.3 | 18 | 18 | 0 |
| Lei M | 6 | 49 | 22 | – |
| Chen YJ | 7.5 | 58 | – | – |
| Park SJ | 5.2 | 49.4 | 22.4 | – |
| Tang Y | 15 | – | – | 58.5 |
| Zhang C | 11 | – | – | 53.3 |
| Fukuhara A | 5 | 35.7 | – | – |
The median survival and survival rate in 6, 12 and 24 months were summarized in this table
Table 7.
Prognostic factors of survival in the univariate and multivariate analysis
| Author | Univariate analysis | Multivariate analysis |
|---|---|---|
| Lei M | Preoperative ambulatory status, targeted therapy, preoperative ECOG PS, number of involved vertebrae, visceral metastases, time developing neurologic deficits | Preoperative ambulatory status, targeted therapy, number of involved vertebrae, visceral metastasis, time developing neurologicr deficits |
| Chen YJ | Histology of primary tumor, preoperative Karnofsky PS, postoperative Frankel grade | Preoperative Karnofsky PS |
| Park SJ | Time developing neurologic deficits, responsiveness to preoperative chemotherapy, postoperative chemotherapy, postoperative ECOG PS, postoperative ambulatory status | Time developing neurologic deficits, postoperative chemotherapy, postoperative ECOG PS |
| Zairi F | Preoperative Karnofsky PS, preoperative Frankel grade, the delay between the cancer diagnosis and the occurrence of spine metastases | – |
| Tang Y | Bisphosphonate treatment, radiotherapy, targted therapy, postoperative Frankel grade | Radiotherapy, targted therapy, postoperative Frankel grade |
| Chen Q | Age, extraspinal bone metastasis foci, visceral metastasis, preoperative Frankel grade, targeted therapy, radiotherapy | Age, extraspinal bone metastasis foci, visceral metastasis, preoperative Frankel grade, chemotherapy, targeted therapy |
| Truong VT | Tobacco use, preoperative and postoperative ASIA score, postoperative ambulatory status, postoperative radiotherapy, and postoperative chemotherapy | Tobacco use, postoperative ambulatory status, postoperative radiotherapy, and postoperative chemotherapy |
| Tancioni F | Control of the primary tumor, the delay between the cancer diagnosis and the occurrence of spine metastases, visceral metastases, number of involved vertebrae, extraspinal bone metastasis foci | – |
| Jung JM | Charlson comorbidity index, number of involved vertebrae | – |
ECOG Eastern Cooperative Oncology Group, PS performance status, ASIA American Spinal Injury Association
Results of quantitative meta-analysis
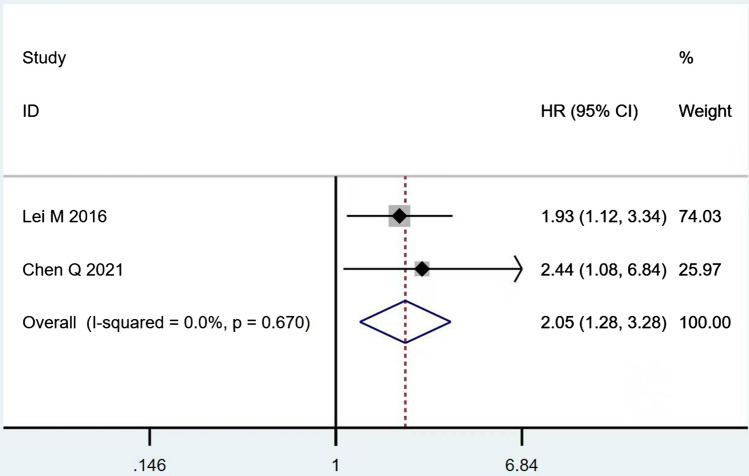
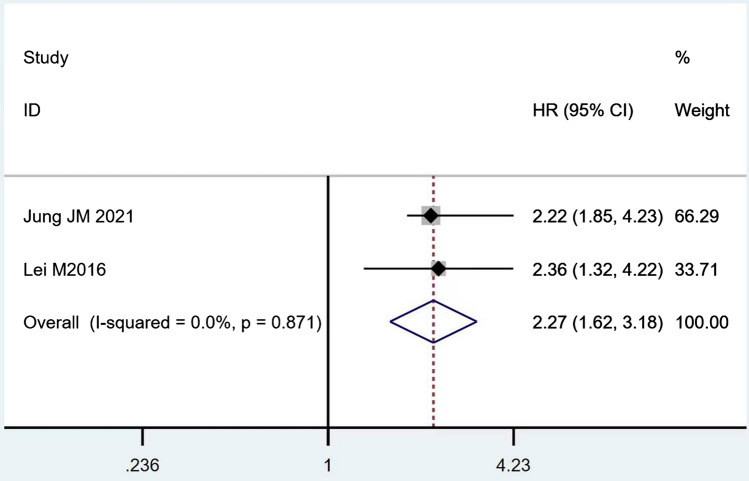
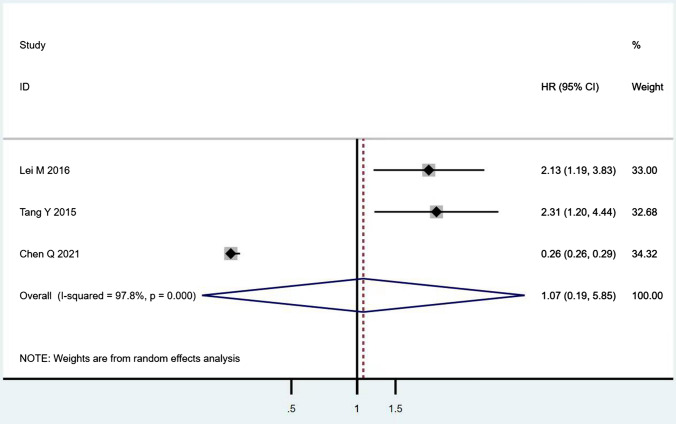
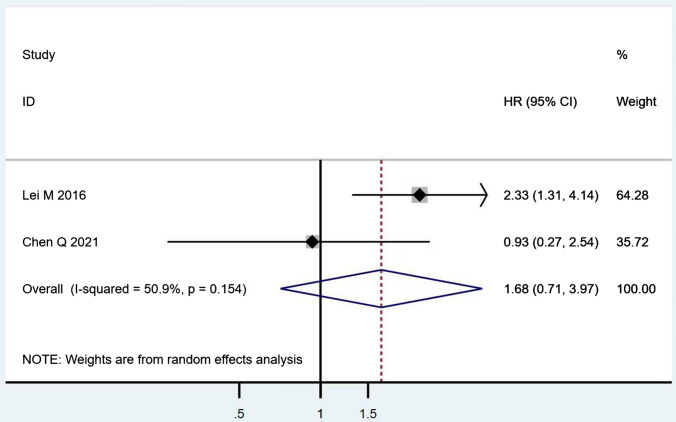
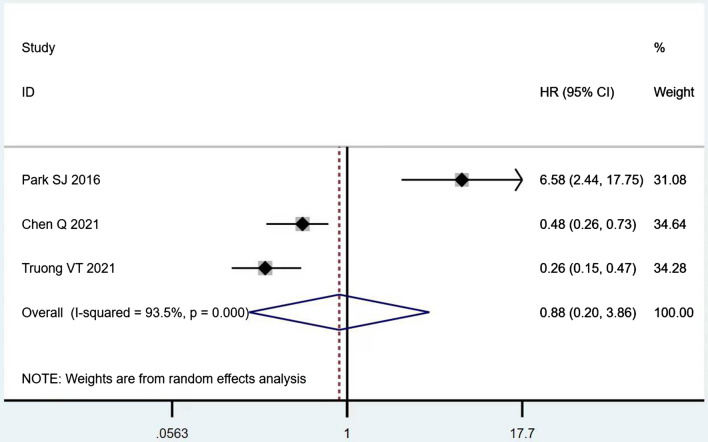
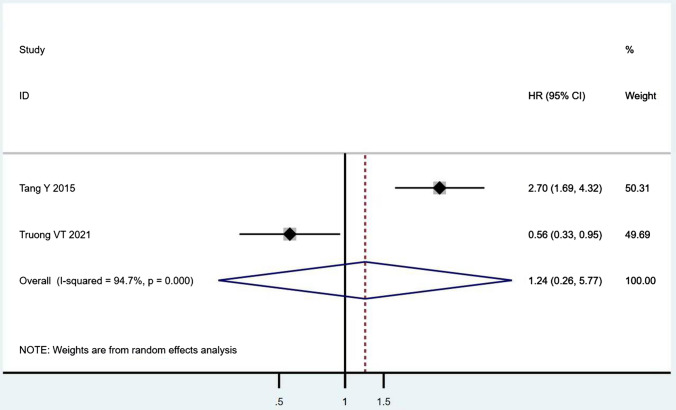
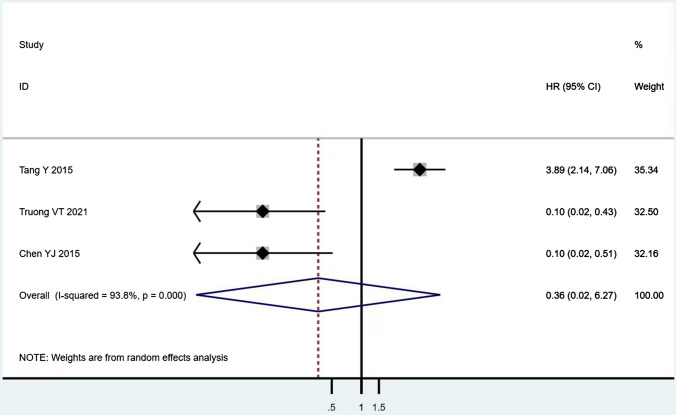
According to Table 8, the prognostic effects of the factors are represented with HR and 95% CI in 7 studies. The pooled HR of preoperative ambulatory status (HR = 2.05, 95% CI 1.28–3.28, p = 0.003; Fig. 2) and the number of involved vertebrae (HR = 2.27, 95% CI 1.62–3.18, p < 0.001; Fig. 3) demonstrated statistical significance, while no significant prognostic effect on overall survival was found for targeted therapy (HR = 1.07, 95% CI 0.19–5.85, p = 0.939; Fig. 4), visceral metastases (HR = 1.68, 95% CI 0.71–3.97, p = 0.238; Fig. 5), chemotherapy (HR = 0.88, 95% CI 0.20–3.86, p = 0.863; Fig. 6), radiotherapy (HR = 1.24, 95% CI 0.26–5.77, p = 0.788; Fig. 7) or postoperative ambulatory status (HR = 0.36, 95% CI 0.02–6.27, p = 0.487; Fig. 8).
Table 8.
The prognostic effects of identified factors (HR, 95% CI)
| Variable | Lei M | Chen Q | Park SJ | Tang Y | Truong VT | Chen YJ | Jung JM |
|---|---|---|---|---|---|---|---|
| Preoperative ambulatory status | 1.93, 1.12–3.34 | 2.437, 1.081–6.839 | – | – | – | – | 2.36, 1.32–4.22 |
| Targeted therapy | 2.13, 1.19–3.82 | 0.264, 0.256–0.291 | – | 2.31, 1.20–4.44 | – | – | |
| Number of involved vertebrae | – | – | – | – | – | – | 2.22, 1.85–4.23 |
| Visceral metastases | 2.33, 1.31–4.14 | 0.932, 0.271–2.541 | – | – | – | – | – |
| Chemotherapy | – | 0.48, 0.265–0.734 | 6.58, 2.44–17.75 | – | 0.26, 0.15–0.47 | – | – |
| Radiotherapy | – | – | – | 2.70, 1.69–4.32 | 0.56, 0.33–0.95 | – | – |
| Postoperative ambulatory status | – | – | – | 3.89, 2.14–7.06 | 0.10, 0.02–0.43 | 0.10, 0.02–0.51 | – |
The hazard ratio (HR) and 95% confidence interval (95% CI) were reported by 7 primary studies
HR hazard ratio, 95% CI confidence interval
Fig. 2.
The forest plot for preoperative ambulatory status (ambulatory vs. non-ambulatory)
Fig. 3.
The forest plot for number of involved vertebrae (1–2 vs. ≥ 3)
Fig. 4.
The forest plot for targeted therapy (with vs. without)
Fig. 5.
The forest plot for visceral metastases (without vs. with)
Fig. 6.
The forest plot for chemotherapy (with vs. without)
Fig. 7.
The forest plot for radiotherapy (with vs. without)
Fig. 8.
Postoperative ambulatory status (ambulatory vs. non-ambulatory)
Discussion
Unsatisfactory outcomes are expected in patients with spinal metastases secondary to lung cancer compared with other primary cancers like breast cancer and prostate cancer. In the landmark study of Patchell [36], surgery with adjuvant radiotherapy achieved superior neurological function improvements compared with radiotherapy alone. Nevertheless, Rades [37] concluded the otherwise, i.e., no significant clinical outcome difference between patients receiving radiotherapy alone and those receiving surgery and radiotherapy. At present, no consensus has been reached on the most appropriate treatment for patients with spinal metastasis from lung cancer. Morgen [2] found a statistically significant improvement in the one-year survival rates of patients with spinal metastasis from lung cancer. Another study drew a conclusion that actual survival times were longer than that predicted by Tokuhashis score [33]. The above findings demonstrated that the current survival time predictions of metastatic spinal diseases might not be accurate enough for patients with lung cancer, which might influence surgical decisions. Thus, the current study was designed to provide a more precise summary of surgical outcomes from existing research and identify the independent prognosis for spinal metastasis from lung cancer. Among them, in Wu [38] and da Silva GT’s [39] study, there was no qualification in the choice of treatment. However, the current study was specific to the surgical intervention and our results were applicable to patients’ undergoing surgery. In Hsieh MK’s review, eleven studies were included and a significant correlation with survival was found in the numbers of extraspinal bone metastases [40]. Compared with this study, we updated systematic review of qualitative evaluation and added meta-analysis of pooled HR and 95% CI to identify the independent prognosis more accurately.
The significant effects of surgical intervention in pain relief and neurological function improvement were proven beyond doubt. In 5 identified articles, the VAS scores decreased from 7.2 ± 1.8 to 2.8 ± 1.6 after surgical intervention. In 10 included studies, 145 patients regained ambulation, accounting for 63.3% of the total preoperative non-ambulatory patients. Tang et al. reported the most significant pain relief and the highest ambulation recovery ratio (91.8%), where 17 patients received total en bloc spondylectomy while the remaining patients underwent multiple palliative surgeries. Further, the reported patients were treated postoperatively with chemotherapy, target therapy, radiation therapy, and bisphosphonate treatment. After all, diversified therapy strategies would bring more opportunities to more patients [41]. In this regard, we found that radiotherapy was the most common adjuvant therapy for surgery, which was reported in 10 studies involving 367 patients. Rades D [42] found that neurological function improvement occurred more often in patients receiving direct decompressive surgery plus stabilization of involved vertebrae with radiotherapy than those receiving radiotherapy alone. Historically, the majority of surgically treated spinal metastases were managed via decompressive posterior laminectomy-a finding corroborated by this study. We found that decompressive laminectomy is the most common surgery (55.8%) in 12 included studies and instrumented fusion was used in most cases. Decompressive laminectomy without instrumentation has many disadvantages including a failure to resolve anterior compression and the introduction or exacerbation of spinal instability. In a review of literature, Moline [43] identified seven studies of patients with metastatic spinal cord compression undergoing laminectomy with or without posterior stabilization. These seven studies comprising 853 patients reported a 48% improvement in the rate of neurologic improvement based on Frankel or ASIA scale. A stand-alone laminectomy is generally considered only with metastatic involvement of the epidural space and lamina but is limited because it does not allow for decompression of ventral metastatic epidural spinal cord compression. In an epidemiologic study using national administrative data from the MarketScan database, corpectomy was identified as the second most common surgery type after laminectomy, which similar to our findings. [44] The transpedicular corpectomy could ventrally decompress the spinal cord and increase the space between the lesion and the spinal cord for safer delivery of stereotactic radiosurgery, which is a significant advantage because the majority of spinal metastases occur ventrally in the vertebral body [45]. Today, surgical approaches are mostly chosen by their practicality in relation to the region of the affected spine and the goals of surgery. For example, metastatic spine lesions occurring ventrally in the subaxial cervical spine are best approached anteriorly. Regrettably, we failed to analyze the outcomes of specific surgery type and make further comparisons because the relevant data wasn’t described separately in most included articles.
In this study, the median survival varied between 3.6 and 33.6 months. Considering the differences in the involved surgical procedures and adjuvant therapies, the wide variation in survival time was not surprising. Additionally, 2 included articles reported patients with spinal metastases originating from multiple primary tumor sites, in which the patients with primary lung cancer had lower survival rates than those with other primary tumors [22, 35]. Considering the small sample sizes and different factors included in each study, the two identified prognostic factors were inconsistent. To summarize the evidence for the prognostic value of these factors, we re-analyzed the prognostic effects by quantitative meta-analysis and identified two statistically significant factors: preoperative ambulatory status and the number of involved vertebrae. In several classical prognostic scoring systems like the modified Tokuhashi score and the Tomita score, the number of involved vertebrae was considered an important prognostic factor [17, 46]. Multiple involved vertebrae usually reflect a more advanced stage of the disease and significantly reduced patient survival times. In addition, more involved vertebrae implied increased vertebra segments for operation, which may bring greater intraoperative and postoperative complications risks.
In this study, preoperative ambulatory status was identified as a prognostic factor, which has been controversial. Classical prognostic scoring systems such as Bauer, Van der Linden, and Tomita did not include the neurological function as a prognostic factor [47, 48]. Chen [24] found no significant association between survival and preoperative or postoperative ambulatory status. They argued that neurologic deficit could not reflect the disease severity that might affect the anatomical location or size of spinal metastasis. Neurologic deficits were attributed to the spinal cord compression instead of the number of involved vertebrae. Thus, patients with neurologic deficits could achieve long survival through appropriate treatment like decompression surgery. However, some held contrary opinions that neurologic function was still a factor related to survival. Lei [23] presented a novel scoring system for predicting the survival of patients after decompression surgery, which included the preoperative ambulatory status. In 2015, NESMS was developed based on the modified Bauer score, preoperative ambulatory status, and albumin level[12]. The team performed multiple follow-up studies including prospective studies and validated the superior prognostic utility of NESMS for either short-term or long-term patients survival [5, 18, 49]. This might be explained by the fact that poorer neurologic function affected patients’ daily activities and quality of life and may lead to long-term bedridden complications, thereby negatively influenced survival [50]. Based on the meta-analysis, no statistical significance was found in postoperative ambulatory status, which was reported as a prognostic factor in previous studies. Indeed, the result of this study was also supported by other studies. Admittedly, postoperative neurologic functions are tightly associated with preoperative neurologic functions and affect post-surgery patient status more directly. However, preoperative neurologic functions reflect the development and progression of cancers to a greater extent than postoperative neurologic functions. In addition to its prognostic values, preoperative ambulatory status could also serve as a basis for deciding whether to operate.
Although, histological type is an important prognostic factor for lung cancer, its prognostic role for spinal metastasis is s still unclear. According to Armstrong’s [51] systematic review of interventions and outcomes in lung cancer metastases to the spine comprising 1925 patients, there was no statistically significant survival difference between different types of primary lung cancer. Additionally, in 7 studies included in our review involving prognostic factor, histological type of primary lung cancer wasn’t identified as a significant prognostic factor in any of them. However, other authors have reached different conclusions [52, 53]. Although 6 included studies focused on patients with NSCLC, we failed to make further comparisons between them with other studies involving different histological type of lung cancer due to the heterogeneity of outcomes assessment tools. This question remains to be addressed in future studies by more high-quality clinical studies.
Nowadays, the management of spinal metastases should remain a multidisciplinary collaboration. The most contentious issue discussed by scholars was the selection of the most appropriate treatment. As mentioned earlier, Rades D [37] believe that radiotherapy could achieve a satisfactory curative effect, not inferior to surgery. Rades [54] also reported that long-course radiotherapy achieved a better one-year local control of spinal metastasis than short-course radiotherapy, which further proved the therapeutic value of radiotherapy. Nevertheless, a systematic review involving 33 studies revealed that paraplegic patients had a fourfold greater functional ambulation recovery rate through surgical intervention than radiotherapy alone, concluding that surgery could provide a valuable advantage over radiotherapy in ambulatory function restoration and pain reduction [55]. Although laminectomy was not included, this review still demonstrated the superior efficacy of improved surgical techniques in ambulatory function recovery and pain reduction. In summary, it did not provide a solid basis to judge which treatment was more effective. In recent years, modern surgical techniques for spine lesions have consistently demonstrated improved results. We believe that patients with sufficient expected survival time could benefit from surgery, and combining multiple adjuvant therapies is necessary, especially with radiotherapy.
As with other studies of this sort, our study is not without limitations. Most of the included studies were retrospective, and only one was prospective. Therefore, more high-quality, large-sample size prospective studies should be conducted to provide the best evidence-based information. Most of the included studies focused largely on survival and the related prognostic factors, while surgical details were not described in several studies. We could not perform further pooled analysis based on inadequate and dispersal data. Furthermore, due to the limitations of primary lesion and treatment, the number of patients included was not large enough, and the surgical intentions of the included cases were mixed inevitabley, as were the adjuvant therapies. As a result, we could not identify the detailed clinical outcomes of specific adjuvant therapy or surgical procedure. Similarly, the number of included articles was limited. Only 2 statistically significant prognostic factors were identified, and some needed variables were missing in the included articles. Although receiving several adjuvant therapies had less impact on the prognosis than the preoperative ambulatory status or the number of involved vertebrae, its treatment value was non-negligible.
Conclusion
In this study, we provided a comprehensive and targeted systematic review of the prognostic factors and outcomes of surgical intervention for patients with spinal metastases secondary to lung cancer. In 5 identified articles, the VAS scores decreased from 7.2 ± 1.8 to 2.8 ± 1.6 after surgical intervention. In 10 studies, 145 patients regained ambulation, reaching an ambulation recovery ratio of 63.3%. For patients receiving surgery for spinal metastasis from lung cancer, preoperative ambulatory status and the number of involved vertebrae were significant prognostic factors associated with survival during surgical treatments. We believe that patients with sufficient expected survival time could benefit from surgery, especially with adjuvant therapies.
Supplementary Information
Below is the link to the electronic supplementary material.
Appendix 1: PRISMA checklist (DOCX 52 kb)
Appendix 2: Searching strategies of PubMed and Embase (DOCX 11 kb)
Acknowledgements
We would like to thank the reviewers for their thorough review of our manuscript, especially under the severe circumstance of worldwide epidemic COVID-19, and we wish that everybody pulls through safe and sound.
Funding
The authors declare that they have received any funding support.
Data availability
All supporting data can be provided upon request to the authors.
Declarations
Conflict of interest
We declare that we have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jiale Zheng, Xing Ding and Jinze Wu have been contributed equally to this work and co-first authors.
Contributor Information
Jiale Zheng, Email: 1723515060@qq.com.
Xing Ding, Email: dingxing2006@126.com.
Jinze Wu, Email: wujinze@126.com.
Lin Li, Email: lilin@126.com.
Xin Gao, Email: gaoxin@126.com.
Quan Huang, Email: huangquan@126.com.
Zhengwang Sun, Email: specialsamsun@126.com.
Junming Ma, Email: junming236@aliyun.com.
Mengchen Yin, Email: yinmengchen0513@126.com.
References
- 1.Wadowska K, Bil-Lula I, Trembecki L, Sliwinska-Mosson M. Genetic markers in lung cancer diagnosis: a review. Int J Mol Sci. 2020 doi: 10.3390/ijms21134569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morgen SS, Lund-Andersen C, Larsen CF, Engelholm SA. Dahl B (2013) Prognosis in patients with symptomatic metastatic spinal cord compression: survival in different cancer diagnosis in a cohort of 2321 patients. Spine (Phila Pa 1976) 1976;38:1362–1367. doi: 10.1097/BRS.0b013e318294835b. [DOI] [PubMed] [Google Scholar]
- 3.Westhoff PG, de Graeff A, Monninkhof EM, Bollen L, Dijkstra SP, van der Steen-Banasik EM, van Vulpen M, Leer JW, Marijnen CA, van der Linden YM, Dutch Bone Metastasis Study G An easy tool to predict survival in patients receiving radiation therapy for painful bone metastases. Int J Radiat Oncol Biol Phys. 2014;90:739–747. doi: 10.1016/j.ijrobp.2014.07.051. [DOI] [PubMed] [Google Scholar]
- 4.Bollen L, de Ruiter GC, Pondaag W, Arts MP, Fiocco M, Hazen TJ, Peul WC, Dijkstra PD. Risk factors for survival of 106 surgically treated patients with symptomatic spinal epidural metastases. Eur Spine J. 2013;22:1408–1416. doi: 10.1007/s00586-013-2726-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schoenfeld AJ, Ferrone ML, Schwab JH, Blucher JA, Barton LB, Tobert DG, Chi JH, Shin JH, Kang JD, Harris MB. Prospective validation of a clinical prediction score for survival in patients with spinal metastases: the New England Spinal Metastasis Score. Spine J. 2021;21:28–36. doi: 10.1016/j.spinee.2020.02.009. [DOI] [PubMed] [Google Scholar]
- 6.Yoshihara H, Yoneoka D. Trends in the surgical treatment for spinal metastasis and the in-hospital patient outcomes in the United States from 2000 to 2009. Spine J. 2014;14:1844–1849. doi: 10.1016/j.spinee.2013.11.029. [DOI] [PubMed] [Google Scholar]
- 7.Xiong GX, Collins JE, Ferrone ML, Schoenfeld AJ. Prospective comparison of 1-year survival in patients treated operatively and non-operatively for spinal metastatic disease: results of the prospective observational study of spinal metastasis treatment (POST) Spine J. 2022 doi: 10.1016/j.spinee.2022.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quraishi NA, Rajagopal TS, Manoharan SR, Elsayed S, Edwards KL, Boszczyk BM. Effect of timing of surgery on neurological outcome and survival in metastatic spinal cord compression. Eur Spine J. 2013;22:1383–1388. doi: 10.1007/s00586-012-2635-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dea N, Versteeg A, Fisher C, Kelly A, Hartig D, Boyd M, Paquette S, Kwon BK, Dvorak M, Street J. Adverse events in emergency oncological spine surgery: a prospective analysis. J Neurosurg Spine. 2014;21:698–703. doi: 10.3171/2014.7.SPINE131007. [DOI] [PubMed] [Google Scholar]
- 10.Xiong GX, Fisher MWA, Schwab JH, Simpson AK, Nguyen L, Tobert DG, Balboni TA, Shin JH, Ferrone ML. Schoenfeld AJ (2022) A natural history of patients treated operatively and nonoperatively for spinal metastases over 2 years following treatment: survival and functional outcomes. Spine (Phila Pa 1976) 1976;47:515–522. doi: 10.1097/BRS.0000000000004322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Turner I, Kennedy J, Morris S, Crockard A, Choi D. Surgery and radiotherapy for symptomatic spinal metastases is more cost effective than radiotherapy alone: a cost utility analysis in a U.K. Spinal Center World Neurosurg. 2018;109:e389–e397. doi: 10.1016/j.wneu.2017.09.189. [DOI] [PubMed] [Google Scholar]
- 12.Ghori AK, Leonard DA, Schoenfeld AJ, Saadat E, Scott N, Ferrone ML, Pearson AM, Harris MB. Modeling 1-year survival after surgery on the metastatic spine. Spine J. 2015;15:2345–2350. doi: 10.1016/j.spinee.2015.06.061. [DOI] [PubMed] [Google Scholar]
- 13.Bartels RH, van der Linden YM, van der Graaf WT. Spinal extradural metastasis: review of current treatment options. CA Cancer J Clin. 2008;58:245–259. doi: 10.3322/CA.2007.0016. [DOI] [PubMed] [Google Scholar]
- 14.Liu Y, Li L, Jiang D, Yang M, Gao X, Lv K, Xu W, Wei H, Wan W. Xiao J (2021) A novel nomogram for survival prediction of patients with spinal metastasis from prostate cancer. Spine (Phila Pa 1976) 1976;46:E364–E373. doi: 10.1097/BRS.0000000000003888. [DOI] [PubMed] [Google Scholar]
- 15.Hibberd CS, Quan GMY. Accuracy of preoperative scoring systems for the prognostication and treatment of patients with spinal metastases. Int Sch Res Not. 2017;2017:1320684. doi: 10.1155/2017/1320684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morgen SS, Fruergaard S, Gehrchen M, Bjorck S, Engelholm SA, Dahl B. A revision of the Tokuhashi revised score improves the prognostic ability in patients with metastatic spinal cord compression. J Cancer Res Clin Oncol. 2018;144:33–38. doi: 10.1007/s00432-017-2519-y. [DOI] [PubMed] [Google Scholar]
- 17.Tomita K, Kawahara N, Kobayashi T, Yoshida A, Murakami H. Akamaru T (2001) Surgical strategy for spinal metastases. Spine (Phila Pa 1976) 1976;26:298–306. doi: 10.1097/00007632-200102010-00016. [DOI] [PubMed] [Google Scholar]
- 18.Schoenfeld AJ, Ferrone ML, Blucher JA, Agaronnik N, Nguyen L, Tobert DG, Balboni TA, Schwab JH, Shin JH, Sciubba DM, Harris MB. Prospective comparison of the accuracy of the New England Spinal Metastasis Score (NESMS) to legacy scoring systems in prognosticating outcomes following treatment of spinal metastases. Spine J. 2022;22:39–48. doi: 10.1016/j.spinee.2021.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohen JF, Deeks JJ, Hooft L, Salameh JP, Korevaar DA, Gatsonis C, Hopewell S, Hunt HA, Hyde CJ, Leeflang MM, Macaskill P, McGrath TA, Moher D, Reitsma JB, Rutjes AWS, Takwoingi Y, Tonelli M, Whiting P, Willis BH, Thombs B, Bossuyt PM, McInnes MDF. Preferred reporting items for journal and conference abstracts of systematic reviews and meta-analyses of diagnostic test accuracy studies (PRISMA-DTA for Abstracts): checklist, explanation, and elaboration. BMJ. 2021;372:n265. doi: 10.1136/bmj.n265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Noteboom EA, May AM, van der Wall E, de Wit NJ, Helsper CW. Patients’ preferred and perceived level of involvement in decision making for cancer treatment: a systematic review. Psychooncology. 2021;30:1663–1679. doi: 10.1002/pon.5750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hayden JA, van der Windt DA, Cartwright JL, Cote P, Bombardier C. Assessing bias in studies of prognostic factors. Ann Intern Med. 2013;158:280–286. doi: 10.7326/0003-4819-158-4-201302190-00009. [DOI] [PubMed] [Google Scholar]
- 22.Chaichana KL, Pendleton C, Sciubba DM, Wolinsky JP, Gokaslan ZL. Outcome following decompressive surgery for different histological types of metastatic tumors causing epidural spinal cord compression. Clinical article J Neurosurg Spine. 2009;11:56–63. doi: 10.3171/2009.1.SPINE08657. [DOI] [PubMed] [Google Scholar]
- 23.Lei M, Liu Y, Liu S, Wang L, Zhou S, Zhou J. Individual strategy for lung cancer patients with metastatic spinal cord compression. Eur J Surg Oncol. 2016;42:728–734. doi: 10.1016/j.ejso.2016.01.018. [DOI] [PubMed] [Google Scholar]
- 24.Chen YJ, Chen HT, Hsu HC. Preoperative palsy score has no significant association with survival in non-small-cell lung cancer patients with spinal metastases who undergo spinal surgery. J Orthop Surg Res. 2015;10:149. doi: 10.1186/s13018-015-0291-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park SJ, Lee CS, Chung SS. Surgical results of metastatic spinal cord compression (MSCC) from non-small cell lung cancer (NSCLC): analysis of functional outcome, survival time, and complication. Spine J. 2016;16:322–328. doi: 10.1016/j.spinee.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 26.Zairi F, Karnoub MA, Vieillard MH, Bouras A, Marinho P, Allaoui M, Devos P, Assaker R. Evaluation of the relevance of surgery in a retrospective case series of patients who underwent the surgical treatment of a symptomatic spine metastasis from lung cancer. Eur Spine J. 2016;25:4052–4059. doi: 10.1007/s00586-016-4397-4. [DOI] [PubMed] [Google Scholar]
- 27.Tang Y, Qu J, Wu J, Li S, Zhou Y, Xiao J. Metastatic spinal cord compression from non-small-cell lung cancer treated with surgery and adjuvant therapies: a retrospective analysis of outcomes and prognostic factors in 116 patients. J Bone Joint Surg Am. 2015;97:1418–1425. doi: 10.2106/JBJS.N.01124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin HH, Chiu CH, Chou PH, Ma HL, Wang JP, Wang ST, Liu CL, Chang MC. Functional outcomes and survival after surgical stabilization for inoperable non-small-cell lung cancer with spinal metastasis of the thoracic and lumbar spines: a retrospective comparison between epidermal growth factor receptor-tyrosine kinase inhibitor and platinum-based chemotherapy groups. Spinal Cord. 2020;58:194–202. doi: 10.1038/s41393-019-0352-7. [DOI] [PubMed] [Google Scholar]
- 29.Jung JM, Hyun SJ, Kim KJ. Surgical impacts of metastatic non-small cell lung cancer to the thoracic and lumbar spine. J Korean Med Sci. 2021;36:e52. doi: 10.3346/jkms.2021.36.e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen Q, Chen X, Zhou L, Chen F, Hu A, Wang K, Liang H, Jiang L, Li X, Dong J. The emergence of new prognostic scores in lung cancer patients with spinal metastasis: a 12-year single-center retrospective study. J Cancer. 2021;12:5644–5653. doi: 10.7150/jca.60821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang C, Han X, Li L, Zhang C, Ma Y, Wang G. Posterior decompression surgery and radiofrequency ablation followed by vertebroplasty in spinal metastases from lung cancer. Med Sci Monit. 2020;26:e925169. doi: 10.12659/MSM.925169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fukuhara A, Masago K, Neo M, Fujibayashi S, Fujita S, Hatachi Y, Irisa K, Sakamori Y, Togashi Y, Kim YH, Mio T, Mishima M. Outcome of surgical treatment for metastatic vertebra bone tumor in advanced lung cancer. Case Rep Oncol. 2010;3:63–71. doi: 10.1159/000299385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hessler C, Vettorazzi E, Madert J, Bokemeyer C. Panse J (2011) Actual and predicted survival time of patients with spinal metastases of lung cancer: evaluation of the robustness of the Tokuhashi score. Spine (Phila Pa 1976) 1976;36:983–989. doi: 10.1097/BRS.0b013e3181e8f7f8. [DOI] [PubMed] [Google Scholar]
- 34.Truong VT, Shedid D, Al-Shakfa F, Hattou L, Shen J, Boubez G, Yuh SJ, Wang Z. Surgical intervention for patients with spinal metastasis from lung cancer: a retrospective study of 87 cases. Clin Spine Surg. 2021;34:E133–E140. doi: 10.1097/BSD.0000000000001062. [DOI] [PubMed] [Google Scholar]
- 35.Tancioni F, Navarria P, Pessina F, Attuati L, Mancosu P, Alloisio M, Scorsetti M, Santoro A, Baena RR. Assessment of prognostic factors in patients with metastatic epidural spinal cord compression (MESCC) from solid tumor after surgery plus radiotherapy: a single institution experience. Eur Spine J. 2012;21(Suppl 1):S146–148. doi: 10.1007/s00586-012-2232-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patchell RA, Tibbs PA, Regine WF, Payne R, Saris S, Kryscio RJ, Mohiuddin M, Young B. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet. 2005;366:643–648. doi: 10.1016/S0140-6736(05)66954-1. [DOI] [PubMed] [Google Scholar]
- 37.Rades D, Huttenlocher S, Dunst J, Bajrovic A, Karstens JH, Rudat V, Schild SE. Matched pair analysis comparing surgery followed by radiotherapy and radiotherapy alone for metastatic spinal cord compression. J Clin Oncol. 2010;28:3597–3604. doi: 10.1200/JCO.2010.28.5635. [DOI] [PubMed] [Google Scholar]
- 38.Wu XG, Zhu BQ, Li AM, Zhang DY. Prognostic factors affecting overall survival in patients with spinal metastasis due to lung cancer: a systematic review and meta-analysis. Eur Rev Med Pharmacol Sci. 2022;26:1683–1694. doi: 10.26355/eurrev_202203_28237. [DOI] [PubMed] [Google Scholar]
- 39.da Silva GT, Bergmann A, Santos Thuler LC. Prognostic factors in patients with metastatic spinal cord compression secondary to lung cancer: a systematic review of the literature. Eur Spine J. 2015;24:2107–2113. doi: 10.1007/s00586-015-4157-x. [DOI] [PubMed] [Google Scholar]
- 40.Hsieh MK, Bowles DR, Canseco JA, Sherman MB, Schroeder GD, Vaccaro AR. Is open surgery for metastatic spinal cord compression secondary to lung cancer really beneficial? A systematic review. World Neurosurg. 2020;144:e253–e263. doi: 10.1016/j.wneu.2020.08.098. [DOI] [PubMed] [Google Scholar]
- 41.Yang H. Targeted nanosystems: advances in targeted dendrimers for cancer therapy. Nanomedicine. 2016;12:309–316. doi: 10.1016/j.nano.2015.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rades D, Huttenlocher S, Bajrovic A, Karstens JH, Adamietz IA, Kazic N, Rudat V, Schild SE. Surgery followed by radiotherapy versus radiotherapy alone for metastatic spinal cord compression from unfavorable tumors. Int J Radiat Oncol Biol Phys. 2011;81:e861–868. doi: 10.1016/j.ijrobp.2010.11.056. [DOI] [PubMed] [Google Scholar]
- 43.Molina C, Goodwin CR, Abu-Bonsrah N, Elder BD, De la Garza RR, Sciubba DM. Posterior approaches for symptomatic metastatic spinal cord compression. Neurosurg Focus. 2016;41:E11. doi: 10.3171/2016.5.FOCUS16129. [DOI] [PubMed] [Google Scholar]
- 44.Azad TD, Varshneya K, Ho AL, Veeravagu A, Sciubba DM, Ratliff JK. Laminectomy versus corpectomy for spinal metastatic disease-complications, costs, and quality outcomes. World Neurosurg. 2019;131:e468–e473. doi: 10.1016/j.wneu.2019.07.206. [DOI] [PubMed] [Google Scholar]
- 45.Sciubba DM, Petteys RJ, Dekutoski MB, Fisher CG, Fehlings MG, Ondra SL, Rhines LD, Gokaslan ZL. Diagnosis and management of metastatic spine disease. A review J Neurosurg Spine. 2010;13:94–108. doi: 10.3171/2010.3.SPINE09202. [DOI] [PubMed] [Google Scholar]
- 46.Tokuhashi Y, Matsuzaki H, Oda H, Oshima M. Ryu J (2005) A revised scoring system for preoperative evaluation of metastatic spine tumor prognosis. Spine (Phila Pa 1976) 1976;30:2186–2191. doi: 10.1097/01.brs.0000180401.06919.a5. [DOI] [PubMed] [Google Scholar]
- 47.van der Linden YM, Dijkstra SP, Vonk EJ, Marijnen CA, Leer JW, Dutch Bone Metastasis Study Prediction of survival in patients with metastases in the spinal column: results based on a randomized trial of radiotherapy. Cancer. 2005;103:320–328. doi: 10.1002/cncr.20756. [DOI] [PubMed] [Google Scholar]
- 48.Bauer HC, Wedin R. Survival after surgery for spinal and extremity metastases. Prognostication in 241 patients. Acta Orthop Scand. 1995;66:143–146. doi: 10.3109/17453679508995508. [DOI] [PubMed] [Google Scholar]
- 49.Schoenfeld AJ, Le HV, Marjoua Y, Leonard DA, Belmont PJ, Jr, Bono CM, Harris MB. Assessing the utility of a clinical prediction score regarding 30-day morbidity and mortality following metastatic spinal surgery: the New England Spinal Metastasis Score (NESMS) Spine J. 2016;16:482–490. doi: 10.1016/j.spinee.2015.09.043. [DOI] [PubMed] [Google Scholar]
- 50.Gao ZY, Zhang T, Zhang H, Pang CG, Jiang WX. Prognostic factors for overall survival in patients with spinal metastasis secondary to prostate cancer: a systematic review and meta-analysis. BMC Musculoskelet Disord. 2020;21:388. doi: 10.1186/s12891-020-03412-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Armstrong V, Schoen N, Madhavan K, Vanni S. A systematic review of interventions and outcomes in lung cancer metastases to the spine. J Clin Neurosci. 2019;62:66–71. doi: 10.1016/j.jocn.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 52.Uei H, Tokuhashi Y. Prognostic factors in patients with metastatic spine tumors derived from lung cancer-a novel scoring system for predicting life expectancy. World J Surg Oncol. 2018;16:131. doi: 10.1186/s12957-018-1439-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tan JH, Tan KA, Zaw AS, Thomas AC, Hey HW, Soo RA. Kumar N (2016) Evaluation of scoring systems and prognostic factors in patients with spinal metastases from lung cancer. Spine (Phila Pa 1976) 1976;41:638–644. doi: 10.1097/BRS.0000000000001279. [DOI] [PubMed] [Google Scholar]
- 54.Rades D, Lange M, Veninga T, Stalpers LJ, Bajrovic A, Adamietz IA, Rudat V, Schild SE. Final results of a prospective study comparing the local control of short-course and long-course radiotherapy for metastatic spinal cord compression. Int J Radiat Oncol Biol Phys. 2011;79:524–530. doi: 10.1016/j.ijrobp.2009.10.073. [DOI] [PubMed] [Google Scholar]
- 55.Kim JM, Losina E, Bono CM, Schoenfeld AJ, Collins JE, Katz JN. Harris MB (2012) Clinical outcome of metastatic spinal cord compression treated with surgical excision +/– radiation versus radiation therapy alone: a systematic review of literature. Spine (Phila Pa 1976) 1976;37:78–84. doi: 10.1097/BRS.0b013e318223b9b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 1: PRISMA checklist (DOCX 52 kb)
Appendix 2: Searching strategies of PubMed and Embase (DOCX 11 kb)
Data Availability Statement
All supporting data can be provided upon request to the authors.