Abstract
The observation that the two active forms of proprotein convertase 1/3 (PC1/3) were differentially expressed in beta cells of normal islets raised the possibility that this heterogeneity is lost during type 1 diabetes (T1D) progression. To test this hypothesis, the expression of the convertase was evaluated by confocal microscopy in sections of human pancreas of autoantibody positive (AA+) and T1D donors and compared with that of control. Islets of T1D pancreas were comprised of beta cells expressing either low or high PC1/3 levels and all islets of a pancreatic section contained only one beta cell type. Pancreata of AA+ donors contained either of these two classes of islets intermixed with normal islets comprised of beta cells with heterogeneous PC1/3 expression. This alteration affected the expression of proinsulin and insulin, which in most AA+ and T1D donors were lower than in controls. The present results indicate that the heterogeneity of PC1/3 expression is lost in all beta cells in a subset islets of AA+ donors and in all islets of T1D donors. These findings suggest that the heterogeneity of PC1/3 expression is a biomarker of human beta cell health and that its loss coincides with the initial stages of T1D.
Keywords: insulin synthesizing enzyme, PC1/3, proinsulin, proprotein convertase 1/3, type 1 diabetes
Introduction
Type 1 diabetes (T1D) is an autoimmune disease where auto-aggressive CD8+ T-cells infiltrate the islets causing beta cell death.1–4 The development of autoimmunity is marked by the appearance of autoantibodies in the circulation. These autoantibodies are not believed to be pathogenetic but serve as biomarkers that may predict destructive autoimmunity. It has been reported that patients positive for autoantibodies show an elevation of the proinsulin to insulin ratio in blood, which indicates the presence of alteration in proinsulin processing.5,6 These observations suggest that beta cell deficiency precedes the diagnosis of hyperglycemia.7–9 While the identity of the cellular signals responsible for the appearance of pathological traits in islet cells before T1D diagnosis is currently unknown, there is a current view that the triggering defect lies within the beta cell itself. 10
To gain an insight into possible causes of beta cell dysfunction, the current study sought to ascertain whether the expression of three markers of beta cell activity become abnormal before T1D diagnosis in humans. Those three molecules are the hormone precursor proinsulin, the main enzyme involved in the conversion of proinsulin into insulin termed proprotein convertase 1/3 (PC1/3),11–14 and the mature hormone. We previously reported that normal human beta cells differ in their level of expression of PC1/3. 15 Correlation of the expression of the convertase with that of proinsulin and insulin in individual beta cells revealed the presence of three cell types in human islets. One of these cell types expressed ProIN but lacked PC1/3 (ProIN+ PC1/3–), a second beta cell type had both ProIN and PC1/3 (ProIN+ PC1/3+) expression, with variable levels of the proconvertase, while a third cell type lacked ProIN but expressed the converting enzyme (ProIN– PC1/3+). All three cell types contained insulin, but the ProIN+ PC1/3– cells had low levels of hormone expression. 16 This analysis suggested that the three beta cell types reflected different stages of proinsulin processing and implied the presence of a critical relationship between the levels of the proconvertase and proinsulin to produce the mature hormone.
The present study sought to determine whether the heterogeneity of PC1/3 expression characteristic of normal human beta cells is abrogated in pancreatic islets during progression to T1D diagnosis and if so, whether those alterations are correlated with defects in the expression of insulin and proinsulin. The expression of proSAAS, a regulator of PC1/3 activity,17–19 was also examined.
Methods
Paraffin sections of human pancreas were provided by the network of Pancreatic Organ Donors (nPOD). Immunohistochemical staining of sections was carried out essentially as previously described. 16 Briefly, monoclonal antibody to proinsulin was purchased from Novocastra (PROIN-1G4; Buffalo Grove, IL) and from the Developmental Biology Hybridoma Bank (GS-9A8; Nashville, TN). These two antibodies recognize the junction between B-chain and C-peptide and label both intact proinsulin and the low abundant proinsulin conversion intermediate des-64,65 proinsulin.20,21 Rabbit antibody to PC1/3, a generous gift from Iris Lindberg (University of Maryland), was generated by D.F. Steiner (University of Chicago). This antibody recognizes the active 87 and 66 kDa forms of the enzyme. 22 Guinea pig antibodies to insulin (Linco, EMD Millipore, Burlington, MA) colocalized in beta cells with mouse anti-insulin (05-1108; EMD Millipore). The mouse MAb binds to mature insulin and/or the des-31.32 proinsulin intermediate that has been processed by both PC1/3 and CPE 21 suggesting similar properties for the Linco guinea pig antibody. Rabbit antibody to proSAAS was a generous gift from Dr. I. Lindberg (University of Maryland) who also determined its specificity to that molecule.23,24 Secondary antibodies such as Antirabbit Alexa fluor 488 IgG (green) and goat-anti mouse Alexa fluor 594 (red) were purchased from Molecular Probes (Eugene, OR); donkey-anti guinea pig Cy5 IgG (blue) was purchased from Jackson ImmunoResearch (West Grove, PA).
Sections were heated in 10 mmol/l citrate buffer, pH 6, for 30 min in a water bath at 100C and incubated overnight at 4C with the three primary antibodies. The following day, sections were incubated with the corresponding secondary antibodies for 1 hr at room temperature and cover slipped.
Fluorescence Microscopy
Images were captured with a Leica SP5 confocal microscope and most islets from each stained section were captured. Confocal single plane images were acquired with an Argon (488 nm), HeNe (543 nm), and HeNe(633) laser source using an oil immersion 40 × lens of 1.25 NA. Images were scanned sequentially to prevent crosstalk between the three fluorophores. The same confocal microscope setting for laser power, photomultiplier voltage gain, offset, and pinhole value were used to collect the signals. Characteristics of the donors examined are presented in Table 1.
Table 1.
Donors Characteristics: Autoantibodies, Sex, age and marker expression levels of the Donors.
| GAD | IA2 | mIAA | ZnT8 | Age/sex | PR | ProIN | PC1/3 | IN | |
|---|---|---|---|---|---|---|---|---|---|
| Control | 22.1 ± 1.3 | 17.1 ± 0.6 | 38.4 ± 2 | ||||||
| 6424 | + | – | + | – | 17.6 years, M | PB | 11.8 ± 0.3* | 11.7 ± 0.6* | 27.8 ± 0.8** |
| PT | 9.6 ± 1.0** | 8.5 ± 0.7** | 26.0 ± 1.7** | ||||||
| 6505 | + | – | + | – | 20.5 years, F | PB | 30.2 ± 1.8* | 9.5 ± 0.6** | 34.4 ± 0.3 |
| PT | 16.5 ± 1.2* | 11.9 ± 1.3* | 38.3 ± 2 | ||||||
| 6314 | + | – | – | – | 21 years, M | PB | 17.8 ± 1.0 | 11.8 ± 0.8* | 34.0 ± 1.3 |
| PT | 18.1 ± 0.9 | 26.7 ± 0.9** | 38.1 ± 1.8 | ||||||
| 6450 | + | – | – | – | 20.5 years, F | PH | 14.6 ± 0.8** | 5.54 ± 0.5** | 36.6 ± 2.2 |
| PB | 15.0 ± 1.0** | 12.8 ± 1.2* | 32.0 ± 2.0 | ||||||
| 6517 | + | – | – | + | 22.1 years, M | PB | 21.3 ± 1.4 | 11.7 ± 0.6** | 33.4 ± 1.2 |
| PT | 19.3 ± 1.3 | 13.3 ± 1.0** | 39.3 ± 2.0 | ||||||
| 6123 | + | – | – | – | 23.2 years, F | PB | 23.8 ± 1.1 | 27.6 ± 1.0** | 44.2 ± 1.4** |
| PT | 21.3 ± 0.3 | 29.2 ± 1.0 | 39.6 ± 1.4 | ||||||
| 6397 | + | – | – | – | 21.1 years, F | PB | 13.3 ± 0.9** | 8.9 ± 1.8** | 29.2 ± 1.9** |
| PT | 16.5 ± 0.7** | 14.3 ± 0.6** | 29.5 ± 0.9** | ||||||
| 6429 | + | – | + | – | 22.1 years, M | PB | 15.1 ± 1.0** | 18.1 ± 1.8 | 39.0 ± 1.7 |
| PT | 14.9 ± 1.0** | 20.1 ± 1.4 | 38.5 ± 2.0 | ||||||
| 6520 | + | + | – | + | 21.6 years, M | PB | 12.6 ± 2.3** | 7.9 ± 0.7** | 43.8 ± 2.3 |
| PT. | 3.5 ± 1.5** | 28.9 ± 1.8** | 44.2 ± 3.1 | ||||||
| 6362 | + | – | – | – | 24.9 years, M | PB | 8.7 ± 1.0**. | 29.6 ± 2.4** | 45.2 ± 2.0 |
| PT | 2.4 ± 0.8**. | 15.8 ± 1.6* | 43.0 ± 2.2 | ||||||
| 6325 | + | + | + | – | 20 years, F | PB+ | 11.1 ± 0.72** | 24.0 ± 1.0* | 36.3 ± 3.8 |
| PT | |||||||||
| 5000 | + | – | + | – | 18.7 years, F | PB+ | 12.2 ± 1.7** | 9.8 ± 0.56** | 25.3 ± 3.3 |
| PT |
Fluorescence intensity/islet area of each functional marker. Two regions of the pancreas per donor and 10 to 25 islets per region of pancreas were evaluated. Italics: type 1 diabetic donors. In some of these donors, values from both pancreatic regions are combined. *X = p<0.05;**X = p<0.005
Abbreviations: IA2, tyrosine phosphatase-like insulinoma antigen; GAD65, glutamic acid decarboxylase-65; ZnT8, zinc transporter protein 8; micro mIAA, insulin; PC1/3, proprotein convertase 1/3; PR, pancreas region; ProIN, proinsulin; IN, insulin; PT, pancreas tail; PB, pancreas body; PH, pancreas head.
The insulin, PC1/3, and ProIN positive area of each islet was determined using Image J (National Institute of Health, NIH) and expressed as a percentage of the total islet area. To eliminate the unidentified green cells from the fluorescent intensity of PC1/3, images of islets that were double-labeled for PC1/3 (green) and insulin (blue) were first delineated using the free-hand selection of Image J. The selection included blue/green cells (insulin + PC1/3) cells but avoided green non-insulin cells. Then, using the color-balance tool, the blue component of the image was eliminated, and a measurement of the selected area was determined. At least 15 islets from autoantibody positive donors/tissue section and 5 to 10 islets from T1D donors were evaluated.
Statistical Analysis
Islets from two regions of each pancreas per donor were examined. In several T1D donors, the values of the fluorescence intensity of islets from both pancreatic regions were combined due to the small number of insulin positive islets present. Statistical significance was calculated using the Student’s t-test.
Results
Expression of Beta Cell Functional Markers Is Altered Before T1D Diagnosis
To ascertain whether alterations in beta cell properties develop before hyperglycemia, the expression level of proinsulin, insulin, and PC1/3 was measured in islets of two regions of autoantibody positive and T1D pancreas. The results, shown in Table 1, indicate significant differences in the expression of the markers between islets of several autoantibody positive and controls pancreas. While in some donors, islets displayed a single abnormal marker, pancreatic islets of other donors showed alterations in all three traits. Interestingly, there was a lack of correlation between markers in the presence and/or type of change. Thus, islets of donor #6123 had normal proinsulin levels but an increased level of expression of PC1/3 (PC1/3high) compared with control, while islets of donor #6429 had decreased ProIN and normal PC1/3 levels. These observations indicate that the expression of the markers varied independently of each other.
Comparison of the fluorescent intensity/islet area of each marker with the list of circulating autoantibodies determined in each donor (Table 1) indicates a lack of correlation between the two measurements. For instance, donors #6123 and #6397 have similar profiles of autoantibodies, but they differ in the expression of the three functional markers.
Gradual Loss of PC1/3 Heterogeneous Expression in Islets of Autoantibody Positive Donors
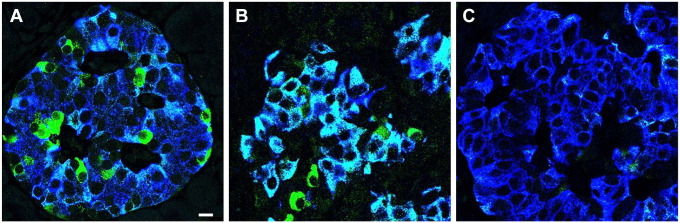
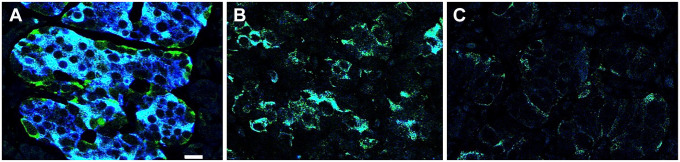
To ascertain whether the heterogeneity in PC1/3 expression found in beta cells of controls is abrogated in T1D, pancreatic islets from four T1D donors were examined (Table 1). A striking characteristic in all these tissues was the homogeneous expression of PC1/3. In pancreas body (pb) and pancreas tail (pt) of donor #5000, pt of donor #6362, and pb of donor #6520, all beta cells showed a dramatic decrease in PC1/3 expression (PC1/3low phenotype). In contrast, PC1/3 levels in beta cells of islets of the pt region of donor #6520, the pb region of donor #6362, and all islets of donors #6325 pt, pb were homogeneously high (PC1/3high phenotype). Representative images of a control islet and islets with the two types of abnormal PC1/3 phenotypes are illustrated in Fig. 1.
Figure 1.
Abnormal PC1/3 phenotypes in beta cells of type 1 diabetes donors. Photomicrographs illustrate T1D islets costained for PC1/3 (green) and insulin (blue). Photomicrographs A to C are representative of all islets of each donor. While expression of PC1/3 in beta cells of islet of control (A) is heterogeneous, the level of the convertase in all beta cells of type 1 diabetes donors is homogeneous and either very high (B) or very low (C). Donor identification is indicated. Cells with green fluorescence remain to be identified. Bar: 12 um. Abbreviations: PC1/3, proprotein convertase 1/3; T1D, type 1 diabetes.
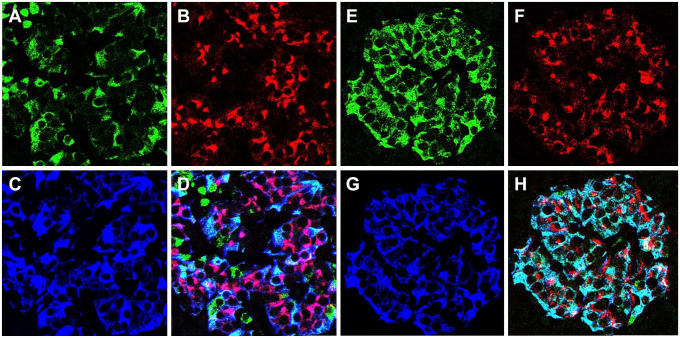
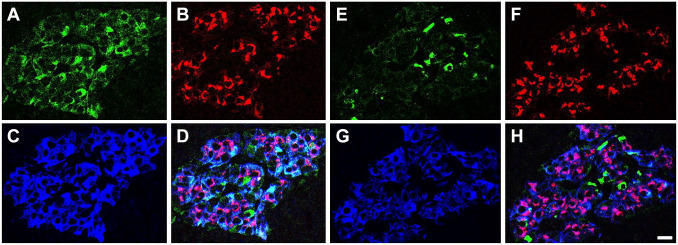
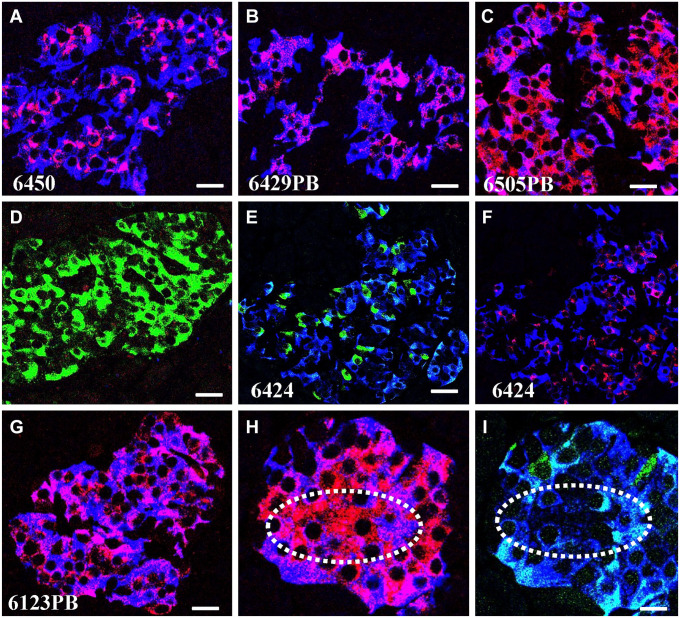
Analysis of pancreata of autoantibody positive donors indicated that PC1/3 heterogeneity in beta cells is lost before T1D diagnosis. Abnormal islets of autoantibody positive donors were concentrated in one pancreatic region (Fig. 2) or distributed in both pancreatic regions examined (Fig. 3), were either PC1/3high or PC1/3low but never a mixture of both abnormal islet types. This observation indicates that the change in the expression of the proconvertase followed the same pattern as in T1D donors. As indicated in Table 1, islets in the pancreas of both autoantibody positive and T1D donors could be PC1/3high in a pancreatic region but PC1/3low in a consecutive area of the pancreas (Table 1), indicating a regional specificity in the defect. Pancreata from PC1/3high donors with T1D also contained islets populated by cells expressing PC1/3 but lacking insulin and/or ProIN (Fig. 4D). Presumably, these represent some of the insulin negative islets described by others.25–28
Figure 2.
Different pancreatic regions contain either normal or abnormal islets. Islets were triple-labeled for PC1/3 (A, E), proinsulin (B, F), and insulin (C, G); D, H illustrate the overlap of the three labels. Photomicrographs of pancreas of donor # 6397 document the presence of normal islets in the tail region (A–D) and PC1/3high islets in the body region (E–H) of the pancreas. Note that the expression of PC1/3 is heterogeneous in A and homogeneously high in E. Bar: 20 um. Abbreviation: PC1/3, proprotein convertase 1/3.
Figure 3.
Coexistence of normal and abnormal islets in the same pancreatic region. Photomicrographs of pancreatic islets of donor #6540 illustrate islets triple-labeled for PC1/3 (A, E), proinsulin (B, F), insulin (C, G). D and H show the overlap of the three markers. Note that PC1/3 expression is heterogeneous in A and homogeneously low in E. Bar: 15 um. Abbreviation: PC1/3, proprotein convertase 1/3.
Figure 4.
Fluorescent intensity and cellular allocation of proinsulin and insulin. Photomicrographs show sections of pancreas immunostained for ProIN (red), PC1/3 (green), and IN (blue). Figures illustrate beta cells with similar ProIN levels but different localization of the prohormone (A, donor #6450; B donor #6429); similar ProIN localization in donors with different number of autoantibodies (B, donor #6429; G, donor #6123); different PC1/3 phenotype but similar ProIN localization (C, donor #6505 and G, donor #6123); high ProIN immunostaining but very low IN and PC1/3 (H illustrates staining for ProIN and IN, I illustrates staining for PC1/3 and insulin, donor #6505); low ProIN and PC1/3 (E illustrates staining for PC1/3 and insulin and F shows immunostaining for ProIN and insulin, donor #6424). (D) Islet of T1D donor stained for insulin, proinsulin and PC1/3. Note that cells express only the proconvertase. Bar: 15 um. Abbreviation: PC1/3, proprotein convertase 1/3.
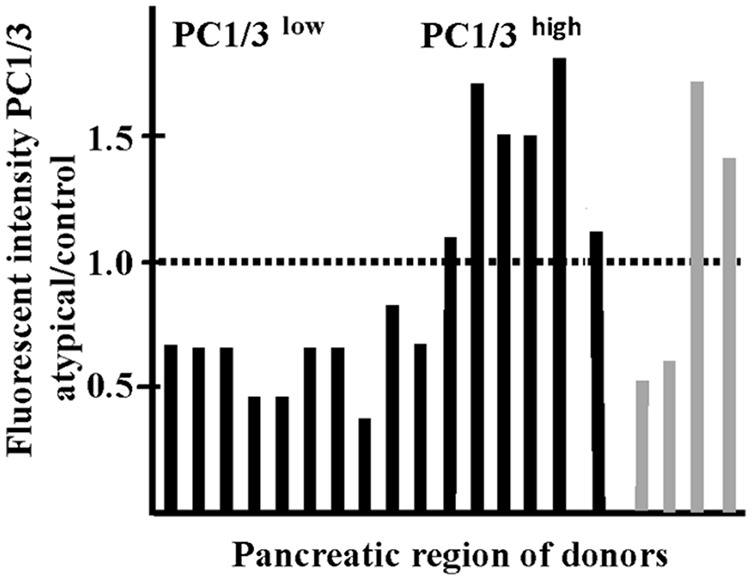
While pancreata of autoantibody are populated by a mixture of abnormal islets and islets populated by normal beta cells with heterogeneous PC1/3 expression (Figs. 2 and 3), all islets in T1D tissues show abnormal expression of the convertase. Evaluation of the ratio of the fluorescent intensity of PC1/3/islet area in islets of autoantibody positive over control and of T1D over control revealed the presence of two groups of islets (Table 2) that reflected the different contributions of islet monotypes (PC1/3low or PC1/3high) and normal islets with heterogeneous expression of the proconvertase. As expected, this ratio was similar for both regions of the pancreas in some donors but different in others. The presence of two groups of islets according to the PC1/3 expression is also illustrated in the histogram in Table 2.
Table 2.
Classification of Donors According to Their PC1/3 Phenotype.
| R < 1 | R > 1 | |||
|---|---|---|---|---|
| 6424 | PB | 0.68 |
|
|
| PT | 0.47 | |||
| 6505 | PB | 0.5 | ||
| PT | 0.7 | |||
| 6314 | PB | 0.7 | ||
| PT | 1.5 | |||
| 6450 | PH | 0.4 | ||
| PB | 0.7 | |||
| 6517 | PB | 0.68 | ||
| PT | 0.76 | |||
| 6123 | PB | 1.7 | ||
| PT | 1.5 | |||
| 6397 | PB | 1.1 | ||
| PT | 0.8 | |||
| 6429 | PB | 1.8 | ||
| PT | 1.1 | |||
| 6520 | PB + PT | 0.46 | ||
| 6362 | PB + PT | 1.7 | ||
| 6325 | PB + PT | 1.4 | ||
| 5000 | PB + PT | 0.5 |
The table illustrates the ratio (R) of the mean fluorescent intensity of PC1/3 per islet area in all islets of each donor over that of control. The same value for control islets was used for all calculations. Note that islets in different regions of the pancreas of autoantibody positive donors are populated by either PC1/3high or PC1/3low beta cells. Islets with PC1/3high and PC1/3low expression do not coexist in the same pancreatic region. At least 15 islets were evaluated for each pancreatic region per donor. Values close to that of control are due to the coexistence of normal and abnormal islets in the same section. Histogram illustrates the values of Table 2. Black lines: autoantibody positive donors; gray lines: type 1 diabetic donors. Note the presence of two abnormal groups according to their PC1/3 phenotype.
Abbreviations: PC1/3, proprotein convertase 1/3; PB, pancreas body; PT, pancreas tail; PH, pancreas head.
The Level of Expression and Subcellular Localization of ProIN Differ Between Donors
ProIN expression decreased significantly relative to control in islets of all T1D donors examined (Table 1). This change was already present before diagnosis as the ratio of proinsulin: islet area in autoantibody positive over control was either similar or lower to controls in pancreatic islets from most donors (Table 3). The exception were islets in a pancreatic section of a donor (#6505) in which the ratio was significantly higher than normal values. In contrast to PC1/3, where islets in different pancreatic regions may express dissimilar phenotypes, in most donors, the fluorescent intensity of ProIN was similar in both regions of the pancreas examined (Table 3).
Table 3.
Classification of Donors According to Their Proinsulin (ProIN) and Insulin (IN) Phenotypes.
| Ratio ProIN | Ratio IN | |||
|---|---|---|---|---|
| <1 | >1 | |||
| 6424 | PB | 0.5 | 0.71 | |
| PT | 0.4 | 0.68 | ||
| 6505 | PB | 1.4 | 0.89 | |
| PT | 0.75 | 1.0 | ||
| 6314 | PB | 0.8 | 0.89 | |
| PT | 0.8 | 1.0 | ||
| 6450 | PH | 0.7 | 0.94 | |
| PB | 0.7 | 0.94 | ||
| 6517 | PB | 0.9 | 0.84 | |
| PT | 0.8 | 0.89 | ||
| 6123 | PB | 0.9 | 1.0 | |
| PT | 1.0 | 1.1 | ||
| 6397 | PB | 0.6 | 1.02 | |
| PT | 0.75 | 0.76 | ||
| 6429 | PB | 0.7 | 0.76 | |
| PT | 0.9 | 1.0 | ||
| 6520 | PB + PT | 0.5 | 1.0 | |
| 6362 | PB + PT | 0.1 | 1.1 | |
| 6325 | PB + PT | 0.1 | 0.9 | |
| 5000 | PB + PT | 0.5 | 0.7 | |
The table illustrates the ratio (R) of the mean fluorescent intensity per islet area of ProIN and IN in islets of each donor over that of control. The same value for control islets was used for all calculations. Note that different pancreatic regions of each donor have similar ProIN phenotype, except for donor #6505. Levels of proinsulin in pancreas of type 1 diabetes donors (italics) are similar or lower than that of autoantibody positive cohort.
Abbreviations: IN, insulin; ProIN, proinsulin; PB, pancreas body; PT, pancreas tail; PH, pancreas head.
The subcellular localization of proinsulin, which varied between autoantibody positive donors, is not correlated with its fluorescent intensity, as revealed by the difference in the location of the prohormone in islets from different pancreas with similar fluorescent intensity (Fig. 4A, B). The difference in the allocation of the prohormone to distinct cytoplasmic compartment also lacks a correlation with the two abnormal PC1/3 traits as donors with either PC1/3low or PC1/3high phenotypes display similar proinsulin localization (Fig. 4C, G). In addition, the displacement of ProIN immunoreactivity from the perinuclear localization to the cytoplasm is not linked to the number of circulating autoantibodies. Thus, donors with single and double autoantibodies were found to display similar prohormone distributions (Fig. 4B, G). Pancreatic islets from some donors contained insulin cells with negligible levels of both ProIN and PC1/3 (Fig. 4E, F). Other tissues with islets displaying abundant cytoplasmic localization of the prohormone lacked PC1/3 and insulin (Fig. 4H, I). These observations suggest that these two latter beta cell types are inactive cells. As cells with cytoplasmic localization of proinsulin were described in T1D islets, 7 the present findings indicate that the presence of these abnormal beta cell types precedes diagnosis.
In common with islets of autoantibody positive donors, changes in the level of expression of PC1/3 in islets of T1D donors were independent from that of ProIN, further supporting the contention that the expression of each marker is regulated independently of the other markers examined.
The Level of Expression of Insulin in Islets Remains Constant During Disease Progression
In contrast with the variability in the expression and cytoplasmic localization of the prohormone and the convertase, the fluorescent intensity/islet area of insulin in islets of autoantibody positive and T1D donors was similar in beta cells from both pancreatic regions examined and was similar or lower than that of controls (Table 3). In view of the observed variations in PC1/3 and proinsulin, the presence of a constant level of the hormone in beta cells is likely to be due to alterations in the regulation of hormone secretion (reviewed in Tsai et al. 29 ).
T1D Also Affects the Expression of proSAAS, a Regulator of PC1/3 Activity
proSAAS is an endogenous binding protein that is a potent inhibitor of the PC1/3 in vitro.17,19,23,24 To determine whether the expression of proSAAS in beta cells is affected by T1D, sections of pancreas from controls and T1D donors from the PC1/3high and PC1/3low groups, respectively, were immunostained for visualization of the granin and insulin. The results show that expression of proSAAS is localized to insulin cells (Fig. 5A), although it also labels unidentified cells that surround the islet. proSAAS expression persists in the PC1/3high group (Fig. 5B) and decreases significantly in the PC1/3low group (Fig. 5C), in agreement with the changes in the expression of the convertase.
Figure 5.
Type 1 diabetes affects proSAAS expression. Photomicrographs show: (A) the localization of proSAAS (green) in an islet of control. proSAAS localize to cells also stained for insulin (blue) and to an unidentified cell type that surrounds the islet. (B) proSAAS expression in an islet of PC1/3high type 1 diabetic donor. (C) proSAAS expression in a PC1/3low type 1 diabetic donor illustrates the near absence of both labels. Bar: 20 um. Abbreviation: PC1/3, proprotein convertase 1/3.
Discussion
It is known that an increase in the proinsulin to insulin ratio in the circulation is a hallmark of the initial stages of type 2 diabetes 30 and is a predictor of abnormal glucose regulation in T1D.9,31,32 While studies on NOD mice, a classical model of T1D, revealed the presence of markers of endoplasmic reticulum stress that are activated under conditions of inflammation31,33,34 leading to abnormal proinsulin processing, the role of endoplasmic reticulum (ER) stress in defects of hormone production in T1D progression in humans remains unclear. However, the results of the present study strongly suggest that alterations in PC1/3, reflected by the loss of its heterogenic expression, are likely to result in the observed decrease in prohormone processing.
The hypothesis suggesting a relationship between proper beta cell function and heterogeneity in the expression of PC1/3 also raises a fundamental question regarding the mechanisms involved in the regulation of convertase expression. The PCSK1 gene encodes the 753-amino acid precursor preproPC1/3, from which the signal peptide is removed in the ER.35–38 The resulting product is a proPC1/3 product of 94 kDa that undergoes propeptide cleavage in the Golgi, yielding an 87-kDa form.37,39 The 87-kDA form is packaged into immature secretory granules, 40 where it generates the 77- and 66-kDa forms.11,41–43
The antibody to PC1/3 used in the studies reported here recognizes the 87 and 74/66 kDa active forms of the enzyme. 22 These forms differ in their enzymatic activity with the 74/66 kDa form more active but less stable than the high molecular mass form.44–46 In vitro analysis indicated that the enzyme exists in multiple ionic forms due to oligomerization and aggregation. 42 It should also be noted that PC1/3 was found to have hysteretic properties, which indicates that it is present in forms with different kinetic properties.47,48 Taken together, those reports suggest that the variation in the expression of the enzyme in normal beta cells may reflect changes not only in its affinity for the antibody but also in its molecular structure that results in differences in the rate of insulin synthesis. If these structural modifications are required for proper proinsulin processing, a perturbation of these changes may play a role in the increase in the proinsulin/insulin ratio in serum characteristic of the disease. 32
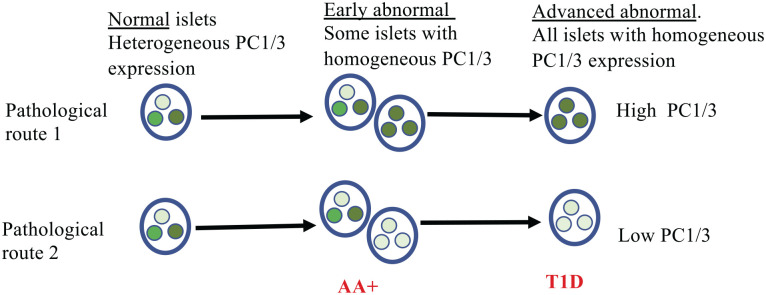
The presence of both normal and abnormal islets in pancreas of autoantibody positive donors but not in tissues from T1D donors raises the hypothesis that there is a gradual change in islet type during progression of the disease from one containing the three beta cell types with heterogeneous PC1/3 expression to monotypes with either high (PC1/3high) or low (PC1/3low) levels of the proconvertase (Fig. 6). This analysis also indicates that alterations in PC1/3 expression is an early indicator of disease progression, and it could be considered as a biomarker of the development of beta cell abnormalities. These alterations in expression also affect proSAAS, a chaperone that functions as a regulator of PC1/3 activity in vitro. ProSAAS, encoded by the mouse gene Pcsk1n, is a 225-residue polypeptide17,49 with broad neuronal distribution.50–52 Notably, in islets of T1D donors, the expression of proSAAS changes in accordance with that of PC1/3, decreasing in PC1/3low and increasing in PC1/3high donors, respectively. This observation suggests that the expression of PC1/3 and proSAAS is coordinated.
Figure 6.
Postulated interpretation of results. Normal islets are populated by three beta cell types displaying variations in PC1/3 expression. Alterations in PC1/3 expression occur in a subset of islets of autoantibody positive donors; the expression of the convertase in beta cells of these abnormal islets is homogeneous, is either high or low, and is characteristic for a pancreatic region. It is hypothesized that there is a gradual change in islet type during progression to type 1 diabetes from one containing three beta cell types with heterogeneous PC1/3 expression to a monotype with either high or low levels of the proconvertase. It is also proposed that the alteration in the expression of the enzyme results in a reduction of its enzymatic activity. Large circle: islet; small circles, beta cells. Shades of green in small circles indicate level of PC1/3 expression. Abbreviation: PC1/3, proprotein convertase 1/3.
Measurement of the level of expression of proinsulin and the proconvertase indicated the presence of significant differences in the values of each of the markers in islets from different autoantibody positive donors. This comparison also indicated that the expression of each marker is differentially affected by diabetogenic signals as islets may have abnormal PC1/3 expression but normal level of ProIN. Another important conclusion from this analysis is that variations in the expression of PC1/3 were specific for a pancreatic region as islets in one region could be PC1/3high while those in other region expressed a PC1/3normal or PC1/3low phenotype. In contrast to the variability in PC1/3 levels reported here, mass spectrometry quantitation of PC1/3 in isolated T1D islets indicated a characteristic reduction in enzyme levels.8,53 However, variation in proconvertase levels between donors and between different pancreatic regions of each donor, such as those reported in the present study, are likely to affect those quantitative results.
As changes in the value of the functional markers examined in this study may occur in response to specific signals, the presence of different values for PC1/3 expression in two regions of the same pancreas suggest that this is the result of two different signaling systems affecting the convertase. The lack of correlation between the changes in PC1/3 and ProIN expression supports the presence of an additional set of signals affecting prohormone levels. Taken together, the results of the present analysis indicate that the position of the islets in the pancreas determines how their properties are modified in disease, and that the incorporation of the positional information of the islets will aid in the identification of possible causes of the change.
While the alteration in PC1/3 expression is an early marker of a beta cell defect, there is recent evidence indicating that alpha cells also become functionally deficient in the prediabetic stage. 54 It is unclear if these defects include the convertase, as the presence of PC1/3 in alpha cells is controversial.55,56 However, in view of the known interactions between alpha and beta cells,57–61 the elucidation of a possible correlation between the alterations in these two islet cell types may provide important insight into causative signals.
In summary, the present study provides evidence indicating that the conversion of PC1/3 expression from a heterogeneous to a homogeneous pattern occurs before T1D and that this conversion may indicate an early beta cell dysfunction during progression of the disease. It is likely that studies analyzing the mechanisms regulating PC1/3 activity will provide invaluable insight into functional beta cell defects that lead to T1D.
Footnotes
Author’s Note: This work was presented at the nPOD meeting in January 2022.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was performed with the support of the Network for Pancreatic Organ Donors with Diabetes (nPOD; RRID:SCR_014641), a collaborative type 1 diabetes research project supported by JDRF (nPOD: 5-SRA-2018-557-Q-R) and The Leona M. & Harry B. Helmsley Charitable Trust (Grant#2018PG-T1D053, G-2108-04793). The content and views expressed are the responsibility of the authors and do not necessarily reflect the official view of nPOD. Organ Procurement Organizations (OPO) partnering with nPOD to provide research resources are listed at http://www.jdrfnpod.org/for-partners/npod-partners/.
Confocal support: National Institute of Health Grant # S10RR026732-01
ORCID iD: Gladys Teitelman  https://orcid.org/0000-0003-0687-3032
https://orcid.org/0000-0003-0687-3032
References
- 1. Roep BO, Tree TI. Immune modulation in humans: implications for type 1 diabetes mellitus. Nat Rev Endocrinol. 2014. Apr;10(4):229–42. doi: 10.1038/nrendo.2014.2. [DOI] [PubMed] [Google Scholar]
- 2. Radenkovic M, Uvebrant K, Skog O, Sarmiento L, Avartsson J, Storm P, Vickman P, Bertilsson PA, Fex M, Korgsgren O, Cilio CM. Characterization of resident lymphocytes in human pancreatic islets. Clin Exp Immunol. 2017. Mar;187(3):418–27. doi: 10.1111/cei.12892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Morgan NG, Leete P, Foulis AK, Richardson SJ. Islet inflammation in human type 1 diabetes mellitus. IUBMB Life. 2014. Nov;66(11):723–34. doi: 10.1002/iub.1330. [DOI] [PubMed] [Google Scholar]
- 4. Atkinson MA, Eisenbarth GS, Michels AW. Type 1 diabetes. Lancet. 2014. Jan 4;383(9911):69–82. doi: 10.1016/S0140-6736(13)60591-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sims EK, Chaudhry Z, Watkins R, Syed F, Blum J, Ouyang F, Perkins SM, Mirmira RG, Sosenko J, DiMeglio LA, Evans-Molina C. Elevations in the fasting serum proinsulin-to-C-peptide ratio precede the onset of type 1 diabetes. Diabetes Care. 2016. Sep;39(9):1519–26. doi: 10.2337/dc15-2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Evans-Molina C, Sims EK, DiMeglio LA, Ismail HM, Steck AK, Palmer JP, Krischer JP, Geyer S, Xu P, Sosenko JM. Type 1, Diabetes TrialNet Study G. β cell dysfunction exists more than 5 years before type 1 diabetes diagnosis. JCI Insight. 2018. Aug 9;3(15):e120877. doi: 10.1172/jci.insight.120877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sims EK, Bahnson HT, Nyalwidhe J, Haataja L, Davis AK, Speake C, DiMeglio LA, Blum J, Morris MA, Mirmira RG, Nadler J, Mastracci TL, Marcovina S, Qian WJ, Yi L, Swensen AC, Yip-Schneider M, Schmidt CM, Considine RV, Arvan P, Greenbaum CJ, Evans-Molina C, T1D Exchange Residual C-peptide Study Group. Proinsulin secretion is a persistent feature of type 1 diabetes. Diabetes Care. 2019. Feb;42(2):258–64. doi: 10.2337/dc17-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sims EK, Syed F, Nyalwidhe J, Bahnson HT, Haataja L, Speake C, Morris MA, Balamurugan AN, Mirmira RG, Nadler J, Mastracci TL, Arvan P, Greenbaum CJ, Evans-Molina C. Abnormalities in proinsulin processing in islets from individuals with longstanding T1D. Transl Res. 2019. Nov;213:90–99. doi: 10.1016/j.trsl.2019.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sims EK, DiMeglio LA. Cause or effect? A review of clinical data demonstrating beta cell dysfunction prior to the clinical onset of type 1 diabetes. Mol Metab. 2019. Sep;27S:S129–38. doi: 10.1016/j.molmet.2019.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Roep BO, Thomaidou S, van Tienhoven R, Zaldumbide A. Type 1 diabetes mellitus as a disease of the beta-cell (do not blame the immune system?). Nat Rev Endocrinol. 2021. Mar;17(3):150–61. doi: 10.1038/s41574-020-00443-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Steiner DF. The proprotein convertases. Curr Opin Chem Biol. 1998. Feb;2(1):31–9. [DOI] [PubMed] [Google Scholar]
- 12. Malide D, Seidah NG, Chretien M, Bendayan M. Electron microscopic immunocytochemical evidence for the involvement of the convertases PC1 and PC2 in the processing of proinsulin in pancreatic beta-cells. J Histochem Cytochem. 1995. Jan;43(1):11–9. [DOI] [PubMed] [Google Scholar]
- 13. Ramzy A, Asadi A, Kieffer TJ. Revisiting proinsulin processing: evidence that human beta-cells process proinsulin with Prohormone Convertase (PC) 1/3 but not PC2. Diabetes. 2020. Apr 14;69(7):1451–62. doi: 10.2337/db19-0276. [DOI] [PubMed] [Google Scholar]
- 14. Seidah NG, Prat A. The biology and therapeutic targeting of the proprotein convertases. Nat Rev Drug Discov. 2012. May;11(5):367–83. doi: 10.1038/nrd3699. [DOI] [PubMed] [Google Scholar]
- 15. Teitelman G. Heterogeneous expression of proinsulin processing enzymes in beta cells of non-diabetic and Type 2 diabetic humans. J Histochem Cytochem. 2019. Jun;67(6):385–400. doi: 10.1369/0022155419831641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Teitelman G. Human islets contain a beta cell type that expresses proinsulin but not the enzyme that converts the precursor to insulin. J Histochem Cytochem. 2020. Oct;68(10):691–702. doi: 10.1369/0022155420961361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fricker LD, McKinzie AA, Sun J, Curran E, Qian Y, Yan L, Patterson SD, Courchesne PL, Richards B, Levin N, Mzhavia N, Devi LA, Douglass J. Identification and characterization of proSAAS, a granin-like neuroendocrine peptide precursor that inhibits prohormone processing. J Neurosci. 2000. Jan 15;20(2):639–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Qian Y, Devi LA, Mzhavia N, Munzer S, Seidah NG, Fricker LD. The C-terminal region of proSAAS is a potent inhibitor of prohormone convertase 1. J Biol Chem. 2000. Aug 4;275(31):23596–601. doi: 10.1074/jbc.M001583200. [DOI] [PubMed] [Google Scholar]
- 19. Cameron A, Fortenberry Y, Lindberg I. The SAAS granin exhibits structural and functional homology to 7B2 and contains a highly potent hexapeptide inhibitor of PC1. FEBS Lett. 2000. May 12;473(2):135–8. doi: 10.1016/s0014-5793(00)01511-8. [DOI] [PubMed] [Google Scholar]
- 20. Sempoux C, Guiot Y, Dubois D, Moulin P, Rahier J. Human type 2 diabetes: morphological evidence for abnormal beta-cell function. Diabetes. 2001. Feb;50(Suppl 1):S172–7. [DOI] [PubMed] [Google Scholar]
- 21. Asadi A, Bruin JE, Kieffer TJ. Characterization of antibodies to products of proinsulin processing using immunofluorescence staining of pancreas in multiple species. J Histochem Cytochem. 2015. Aug;63(8):646–62. doi: 10.1369/0022155415576541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Scopsi L, Gullo M, Rilke F, Martin S, Steiner DF. Proprotein convertases (PC1/PC3 and PC2) in normal and neoplastic human tissues: their use as markers of neuroendocrine differentiation. J Clin Endocrinol Metab. 1995. Jan;80(1):294–301. doi: 10.1210/jcem.80.1.7829629. [DOI] [PubMed] [Google Scholar]
- 23. Fortenberry Y, Hwang JR, Apletalina EV, Lindberg I. Functional characterization of ProSAAS: similarities and differences with 7B2. J Biol Chem. 2002. Feb 15;277(7):5175–86. doi: 10.1074/jbc.M104531200. [DOI] [PubMed] [Google Scholar]
- 24. Lee SN, Prodhomme E, Lindberg I. Prohormone convertase 1 (PC1) processing and sorting: effect of PC1 propeptide and proSAAS. J Endocrinol. 2004. Aug;182(2):353–64. doi: 10.1677/joe.0.1820353. [DOI] [PubMed] [Google Scholar]
- 25. Morgan NG, Richardson SJ. Fifty years of pancreatic islet pathology in human type 1 diabetes: insights gained and progress made. Diabetologia. 2018. Dec;61(12):2499–506. doi: 10.1007/s00125-018-4731-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Campbell-Thompson M, Fu A, Kaddis JS, Wasserfall C, Schatz DA, Pugliese A, Atkinson MA. Insulitis and beta-cell mass in the natural history of type 1 diabetes. Diabetes. 2016. Mar;65(3):719–31. doi: 10.2337/db15-0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Foulis AK, Stewart JA. The pancreas in recent-onset type 1 (insulin-dependent) diabetes mellitus: insulin content of islets, insulitis and associated changes in the exocrine acinar tissue. Diabetologia. 1984. Jun;26(6):456–61. doi: 10.1007/BF00262221. [DOI] [PubMed] [Google Scholar]
- 28. Willcox A, Richardson SJ, Bone AJ, Foulis AK, Morgan NG. Analysis of islet inflammation in human type 1 diabetes. Clin Exp Immunol. 2009. Feb;155(2):173–81. doi: 10.1111/j.1365-2249.2008.03860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tsai EB, Sherry NA, Palmer JP, Herold KC. The rise and fall of insulin secretion in type 1 diabetes mellitus. Diabetologia. 2006. Feb;49(2):261–70. doi: 10.1007/s00125-005-0100-8. [DOI] [PubMed] [Google Scholar]
- 30. Arunagiri A, Haataja L, Pottekat A, Pamenan F, Kim S, Zeltser LM, Paton AW, Paton JC, Tsai B, Itkin-Ansari P, Kaufman RJ, Liu M, Arvan P. Proinsulin misfolding is an early event in the progression to type 2 diabetes. eLife. 2019. Jun 11;8:e44532. doi: 10.7554/eLife.44532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tersey SA, Nishiki Y, Templin AT, Cabrera SM, Stull ND, Colvin SC, Evans-Molina C, Rickus JL, Maier B, Mirmira RG. Islet beta-cell endoplasmic reticulum stress precedes the onset of type 1 diabetes in the nonobese diabetic mouse model. Diabetes. 2012. Apr;61(4):818–27. doi: 10.2337/db11-1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rodriguez-Calvo T, Zapardiel-Gonzalo J, Amirian N, Castillo E, Lajevardi Y, Krogvold L, Dahl-Jorgensen K, von Herrath MG. Increase in pancreatic proinsulin and preservation of beta-cell mass in autoantibody-positive donors prior to type 1 diabetes onset. Diabetes. 2017. May;66(5):1334–45. doi: 10.2337/db16-1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sreenan S, Pick AJ, Levisetti M, Baldwin AC, Pugh W, Polonsky KS. Increased beta-cell proliferation and reduced mass before diabetes onset in the nonobese diabetic mouse. Diabetes. 1999. May;48(5):989–96. doi: 10.2337/diabetes.48.5.989. [DOI] [PubMed] [Google Scholar]
- 34. Chambers KT, Unverferth JA, Weber SM, Wek RC, Urano F, Corbett JA. The role of nitric oxide and the unfolded protein response in cytokine-induced beta-cell death. Diabetes. 2008. Jan;57(1):124–32. doi: 10.2337/db07-0944. [DOI] [PubMed] [Google Scholar]
- 35. Hoshino A, Lindberg I. Prohormone convertases 1/3 and 2. In: Fricker L, Devi L. editors. Peptide biosynthesis. Princeton, NJ: Morgan & Claypool Life Science Publishers; 2012; 1–112 [Google Scholar]
- 36. Benjannet S, Reudelhuber T, Mercure C, Rondeau N, Chretien M, Seidah NG. Proprotein conversion is determined by a multiplicity of factors including convertase processing, substrate specificity, and intracellular environment. Cell type-specific processing of human prorenin by the convertase PC1. J Biol Chem. 1992. Jun 5;267(16):11417–23. [PubMed] [Google Scholar]
- 37. Benjannet S, Rondeau N, Paquet L, Boudreault A, Lazure C, Chretien M, Seidah NG. Comparative biosynthesis, covalent post-translational modifications and efficiency of prosegment cleavage of the prohormone convertases PC1 and PC2: glycosylation, sulphation and identification of the intracellular site of prosegment cleavage of PC1 and PC2. Biochem J. 1993. Sep 15;294(Pt. 3):735–43. doi: 10.1042/bj2940735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shennan KI, Taylor NA, Jermany JL, Matthews G, Docherty K. Differences in pH optima and calcium requirements for maturation of the prohormone convertases PC2 and PC3 indicates different intracellular locations for these events. J Biol Chem. 1995. Jan 20;270(3):1402–7. doi: 10.1074/jbc.270.3.1402. [DOI] [PubMed] [Google Scholar]
- 39. Lindberg I. Evidence for cleavage of the PC1/PC3 pro-segment in the endoplasmic reticulum. Mol Cell Neurosci. 1994. Jun;5(3):263–8. doi: 10.1006/mcne.1994.1030. [DOI] [PubMed] [Google Scholar]
- 40. Stijnen P, Ramos-Molina B, O’Rahilly S, Creemers JW. PCSK1 mutations and human endocrinopathies: from obesity to gastrointestinal disorders. Endocr Rev. 2016. Aug;37(4):347–71. doi: 10.1210/er.2015-1117. [DOI] [PubMed] [Google Scholar]
- 41. Vindrola O, Lindberg I. Biosynthesis of the prohormone convertase mPC1 in AtT-20 cells. Mol Endocrinol. 1992. Jul;6(7):1088–94. doi: 10.1210/mend.6.7.1508222. [DOI] [PubMed] [Google Scholar]
- 42. Hoshino A, Kowalska D, Jean F, Lazure C, Lindberg I. Modulation of PC1/3 activity by self-interaction and substrate binding. Endocrinology. 2011. Apr;152(4):1402–11. doi: 10.1210/en.2010-1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Halban PA. Proinsulin processing in the regulated and the constitutive secretory pathway. Diabetologia. 1994. Sep;37(Suppl 2):S65–72. doi: 10.1007/BF00400828. [DOI] [PubMed] [Google Scholar]
- 44. Zhou Y, Lindberg I. Enzymatic properties of carboxyl-terminally truncated prohormone convertase 1 (PC1/SPC3) and evidence for autocatalytic conversion. J Biol Chem. 1994. Jul 15;269(28):18408–13. [PubMed] [Google Scholar]
- 45. Rufaut NW, Brennan SO, Hakes DJ, Dixon JE, Birch NP. Purification and characterization of the candidate prohormone-processing enzyme SPC3 produced in a mouse L cell line. J Biol Chem. 1993. Sep 25;268(27):20291–8. [PubMed] [Google Scholar]
- 46. Boudreault A, Gauthier D, Rondeau N, Savaria D, Seidah NG, Chretien M, Lazure C. Molecular characterization, enzymatic analysis, and purification of murine proprotein convertase-1/3 (PC1/PC3) secreted from recombinant baculovirus-infected insect cells. Protein Expr Purif. 1998. Dec;14(3):353–66. doi: 10.1006/prep.1998.0964. PMID 9882569. [DOI] [PubMed] [Google Scholar]
- 47. Icimoto MY, Barros NM, Ferreira JC, Marcondes MF, Andrade D, Machado MF, Juliano MA, Judice WA, Juliano L, Oliveira V. Hysteretic behavior of proprotein convertase 1/3 (PC1/3). PLoS ONE. 2011;6(9):e24545. doi: 10.1371/journal.pone.0024545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jiang Y, Li X, Morrow BR, Pothukuchy A, Gollihar J, Novak R, Reilly CB, Ellington AD, Walt DR. Single-molecule mechanistic study of enzyme hysteresis. ACS Cent Sci. 2019. Oct 23;5(10):1691–8. doi: 10.1021/acscentsci.9b00718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Che FY, Yan L, Li H, Mzhavia N, Devi LA, Fricker LD. Identification of peptides from brain and pituitary of Cpe(fat)/Cpe(fat) mice. Proc Natl Acad Sci U S A. 2001. Aug 14;98(17):9971–6. doi: 10.1073/pnas.161542198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Feng Y, Reznik SE, Fricker LD. ProSAAS and prohormone convertase 1 are broadly expressed during mouse development. Brain Res Gene Expr Patterns. 2002. Jan;1(2):135–40. doi: 10.1016/s1567-133x(02)00002-9. [DOI] [PubMed] [Google Scholar]
- 51. Lanoue E, Day R. Coexpression of proprotein convertase SPC3 and the neuroendocrine precursor proSAAS. Endocrinology. 2001. Sep;142(9):4141–9. doi: 10.1210/endo.142.9.8386. [DOI] [PubMed] [Google Scholar]
- 52. Morgan DJ, Wei S, Gomes I, Czyzyk T, Mzhavia N, Pan H, Devi LA, Fricker LD, Pintar JE. The propeptide precursor proSAAS is involved in fetal neuropeptide processing and body weight regulation. J Neurochem. 2010. Jun;113(5):1275–84. doi: 10.1111/j.1471-4159.2010.06706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Rodriguez-Calvo T, Chen YC, Verchere CB, Haataja L, Arvan P, Leete P, Richardson SJ, Morgan NG, Qian WJ, Pugliese A, Atkinson M, Evans-Molina C, Sims EK. Altered beta-cell prohormone processing and secretion in type 1 diabetes. Diabetes. 2021. May;70(5):1038–50. doi: 10.2337/dbi20-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Doliba NM, Rozo AV, Roman J, Qin W, Traum D, Gao L, Liu J, Manduchi E, Liu C, Golson ML, Vahedi G, Naji A, Matschinsky FM, Atkinson MA, Powers AC, Brissova M, Kaestner KH, Stoffers DA, Consortium H. α cell dysfunction in islets from nondiabetic, glutamic acid decarboxylase autoantibody-positive individuals. J Clin Invest. 2022. Jun 1;132(11):e156243. doi: 10.1172/JCI156243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Habener JF, Stanojevic V. Alpha cells come of age. Trends Endocrinol Metab. 2013. Mar;24(3):153–63. doi: 10.1016/j.tem.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 56. Teitelman G. Expression of the glucagon like peptide-1 receptor in human pancreas and incretin therapy. Endocrinology. 2014. Apr;155(4):1175–7. doi: 10.1210/en.2014-1166. [DOI] [PubMed] [Google Scholar]
- 57. Gu W, Anker CCB, Christiansen CB, Moede T, Berggren PO, Hermansen K, Gregersen S, Jeppesen PB. Pancreatic beta cells inhibit glucagon secretion from alpha cells: an in vitro demonstration of alpha-beta cell interaction. Nutrients. 2021. Jun 30;13(7):2281. doi: 10.3390/nu13072281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Rodriguez-Diaz R, Molano RD, Weitz JR, Abdulreda MH, Berman DM, Leibiger B, Leibiger IB, Kenyon NS, Ricordi C, Pileggi A, Caicedo A, Berggren PO. Paracrine interactions within the pancreatic islet determine the glycemic set point. Cell Metab. 2018. Mar 6;27(3):549–558. doi: 10.1016/j.cmet.2018.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Rodriguez-Diaz R, Dando R, Jacques-Silva MC, Fachado A, Molina J, Abdulreda MH, Ricordi C, Roper SD, Berggren PO, Caicedo A. Alpha cells secrete acetylcholine as a non-neuronal paracrine signal priming beta cell function in humans. Nat Med. 2011. Jun 19;17(7):888–92. doi: 10.1038/nm.2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Aslanoglou D, Bertera S, Sanchez-Soto M, Benjamin Free R, Lee J, Zong W, Xue X, Shrestha S, Brissova M, Logan RW, Wollheim CB, Trucco M, Yechoor VK, Sibley DR, Bottino R, Freyberg Z. Dopamine regulates pancreatic glucagon and insulin secretion via adrenergic and dopaminergic receptors. Transl Psychiatry. 2021. Feb 16;11(1):59. doi: 10.1038/s41398-020-01171-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Moede T, Leibiger IB, Berggren PO. Alpha cell regulation of beta cell function. Diabetologia. 2020. Oct;63(10):2064–75. doi: 10.1007/s00125-020-05196-3. [DOI] [PMC free article] [PubMed] [Google Scholar]