Abstract
We have used expression screening of a genomic Mycobacterium tuberculosis library with tuberculosis (TB) patient sera to identify novel genes that may be used diagnostically or in the development of a TB vaccine. Using this strategy, we have cloned a novel gene, termed mtb39a, that encodes a 39-kDa protein. Molecular characterization revealed that mtb39a is a member of a family of three highly related genes that are conserved among strains of M. tuberculosis and Mycobacterium bovis BCG but not in other mycobacterial species tested. Immunoblot analysis demonstrated the presence of Mtb39A in M. tuberculosis lysate but not in culture filtrate proteins (CFP), indicating that it is not a secreted antigen. This conclusion is strengthened by the observation that a human T-cell clone specific for purified recombinant Mtb39A protein recognized autologous dendritic cells infected with TB or pulsed with purified protein derivative (PPD) but did not respond to M. tuberculosis CFP. Purified recombinant Mtb39A elicited strong T-cell proliferative and gamma interferon responses in peripheral blood mononuclear cells from 9 of 12 PPD-positive individuals tested, and overlapping peptides were used to identify a minimum of 10 distinct T-cell epitopes. Additionally, mice immunized with mtb39a DNA have shown increased protection from M. tuberculosis challenge, as indicated by a reduction of bacterial load. The human T-cell responses and initial animal studies provide support for further evaluation of this antigen as a possible component of a subunit vaccine for M. tuberculosis.
Approximately 1.7 billion people are infected with Mycobacterium tuberculosis (21), the causative agent of tuberculosis (TB). TB is a leading cause of infectious mortality worldwide, accounting for 2.9 million deaths annually (5). The emergence of drug-resistant strains and coinfections with human immunodeficiency virus (HIV) has led to an increased mortality of TB cases (7, 27, 40). The only currently available vaccine to prevent TB is Mycobacterium bovis BCG, a live attenuated mycobacterial strain first developed in 1921 (11). However, individual trials with BCG have yielded highly variable results (6), and a recent meta-analysis of BCG as a vaccine to prevent TB has shown it to have limited efficacy, providing only 50% protection from pulmonary TB (14). In addition, BCG can cause disseminated disease in immunocompromised individuals (10, 43, 44). The variable efficacy of BCG, coupled with the inherent difficulties of standardization and safety of a live vaccine, has generated interest in the development of a subunit vaccine based on immunodominant M. tuberculosis antigens.
Immunity to M. tuberculosis depends upon the cellular immune response. In studies in the mouse model for TB, cellular depletion and adoptive transfer experiments have demonstrated a protective role for both CD4+ and CD8+ T lymphocytes (26, 29, 30, 34). In addition, gamma interferon (IFN-γ) is critical for immunity to TB since disruption of the IFN-γ gene in mice resulted in increased susceptibility to TB infection (16, 18). In humans, evidence for the need of an intact cellular immune response for resistance to TB also exists. Impaired cellular immunity resulting from HIV infection leads to increased likelihood of active tuberculosis (9, 12), and the need for IFN-γ is suggested by increased susceptibility to atypical mycobacteria in individuals with a mutated IFN-γ receptor (20, 28).
The identification of M. tuberculosis antigens capable of eliciting T-cell responses has been pursued by a variety of procedures, including the biochemical fractionation and purification of M. tuberculosis proteins (4, 36) and the utilization of mycobacterium-specific monoclonal antibodies to screen genomic M. tuberculosis expression libraries (13, 17, 41). A strategy that has been less widely exploited is expression cloning of M. tuberculosis genomic libraries by using sera from patients with active or recently treated infections (2). The rationale for this approach is based on the hypothesis that some M. tuberculosis T-cell antigens are capable of generating a detectable humoral response in individuals with active disease. Since serological responses to protein antigens are T cell dependent and an increase in mycobacterial antigen-specific immunoglobulin G in sera from TB patients compared to sera from healthy purified protein derivative (PPD)-positive controls has previously been reported (39), we reasoned that pools of sera from individuals with active or recently treated disease could be used as a tool to identify T-cell antigens.
In this study, we have used serological expression cloning to identify a novel T-cell antigen from M. tuberculosis, referred to as Mtb39A. Recombinant Mtb39A protein elicited strong T-cell responses in 75% of healthy PPD-positive individuals, and mtb39a DNA immunization of mice provided partial protection against M. tuberculosis challenge. These results support further evaluation of the antigen as a component of a subunit vaccine.
MATERIALS AND METHODS
Bacterial strains.
M. tuberculosis strains H37Rv, H37Ra, and Erdman were gifts from the Seattle VA Hospital; strain C was a gift from Lee Riley, University of California, Berkeley; and Mycobacterium bovis BCG and Mycobacterium leprae (Pasteur) were obtained from Genesis Corp., Auckland, New Zealand. The following other species of mycobacteria were obtained from the American Type Culture Collection (Rockville, Md.): M. vaccae (ATCC 15483), M. avium avium (ATCC 35718), M. chelonae (ATCC 14472), M. fortuitum (ATCC 6841), M. gordonae (ATCC 14470), M. scrofulaceum (ATCC 19981), and M. smegmatis (ATCC 19420).
Patient sera and peripheral blood mononuclear cells (PBMCs).
A pool of M. tuberculosis patient sera was made from three samples. Patient 1 serum was obtained from a 52-year-old male with pulmonary TB (4+ acid-fast bacillus [AFB]) 2 months after initiation of treatment (16-mm PPD; patient had a history of vaccination with BCG). Patient 2 serum was obtained from an 18-year-old female with pulmonary TB (2+ AFB) 3 months after initiation of treatment (19-mm PPD; patient’s pulmonary TB 5 years earlier was treated for 1 year). Patient 3 serum was obtained from a 46-year-old female with pulmonary TB (3+ AFB) 2 months after initiation of treatment (16-mm PPD; no history of BCG vaccination).
PBMC were obtained from either blood or apheresis product from healthy PPD-positive or -negative individuals by density centrifugation over Ficoll. None of the 12 PPD-positive donors had a history of BCG immunization.
Isolation of M. tuberculosis clones.
M. tuberculosis H37Ra genomic DNA was isolated and sheared by sonication to a size range of 1 to 4 kilobases. M. tuberculosis H37Rv genomic DNA was partially digested with Sau3AI. Libraries were constructed in Lambda ZapII (Stratagene, La Jolla, Calif.) by using EcoRI adaptors. Expression screening was performed using a pool of patient sera preadsorbed with Escherichia coli (38). This resulted in the identification and purification of the following seven immunoreactive clones: TbH2, TbH4, TbH5, TbH8, TbH9, TbH12, and TbH16. TbH2, TbH5, TbH8, TbH9, and TbH16 were recovered from the M. tuberculosis H37Ra library, and TbH4 and TbH12 were recovered from the M. tuberculosis H37Rv library.
Cloning of full-length genes mtb39a and mtb39b and the partial mtb39c was accomplished by isolating the 5′ portion (approximately 500 bp) of the TbH9 insert, random labeling with [32P]dCTP, and screening approximately 75,000 PFU. DNA screening was performed as described previously (38). Seven positive clones were recovered, the insert sizes were determined by restriction digests, and the three largest were subjected to DNA sequence analysis (Applied Biosystems, Foster City, Calif.).
Expression of recombinant M. tuberculosis antigens.
The recombinant antigens encoded by TbH4, TbH5, TbH9, TbH12, and TbH16 were expressed and purified. All of these antigens represented fusions with the N-terminal 4-kDa β-galactosidase, except rTbH4, which initiated within the clone. Induced bacterial pellets were lysed, and rTbH4, rTbH5, rTbH9, rTbH12, and rTbH16 were recovered from the inclusion bodies. The recombinant proteins were purified by ammonium sulfate precipitation and preparative gel separation by sodium dodecyl sulfate–10% polyacrylamide gel electrophoresis (SDS–10% PAGE). Recombinant proteins were eluted from the gels and dialyzed in either phosphate-buffered saline (PBS) or 10 mM Tris (pH 7.4), the protein concentration was measured by the Pierce (Rockford, Ill.) bicinchoninic acid assay, and purity was assessed by SDS-PAGE followed by Coomassie blue staining.
The insert of the TbH9 clone was engineered for expression by PCR, utilizing a primer containing an NdeI site, an N-terminal histidine tag, and a primer encoding a termination site followed by a HindIII site. Amplified product was digested with NdeI and HindIII and ligated into pET17b. This clone was termed pETΔMtb39A, and the recombinant protein encoded was termed rΔMtb39A. This recombinant protein contained the N-terminal residues encoded by the vector sequence MHHHHHHPGCR and followed by residues encoded by the M. tuberculosis DNA sequence.
The full-length mtb39a gene was engineered for expression as follows. PCR was performed by using primers which included flanking restriction sites NdeI and HindIII and DNA sequence encoding an N-terminal 6-histidine tag. The PCR product was digested with NdeI and HindIII and ligated into pET17b.
Expression and purification of rMtb39A and rΔMtb39A was performed as follows. Induced E. coli BL-21(pLysE) pellets were lysed, and the recombinant proteins were recovered in the inclusion bodies. Purification was accomplished by solubilization of pellets in binding buffer (8 M urea–0.1 M NaPO4–10 mM Tris [pH 8.0]) and mixed with Ni2+ nitrilotriacetic acid-agarose by rocking at room temperature. The mixture was placed in a column and washed with 8 M urea–0.1 M NaPO4–10 mM Tris (pH 6.3), and recombinant protein was then eluted with 8 M urea–0.1 M NaPO4–10 mM Tris (pH 4.5). Fractions containing protein were combined and dialyzed against 10 mM Tris (pH 7.4). Under these purification conditions, complete solubility as assessed by microfiltration (0.2 μM) was maintained at concentrations up to 1 mg/ml. Purity of the two recombinants was assessed by SDS-PAGE followed by Coomassie blue staining and by high-performance liquid chromatography (HPLC) analysis, which demonstrated greater than 95% purity. N-terminal sequencing using traditional Edman chemistry with a Procise 494 protein sequencer (Perkin-Elmer, Applied Biosystems Division, Foster City, Calif.) was performed to confirm recombinant protein identities. Endotoxin was determined to be less than 100 EU/mg by Limulus amoebocyte lysate (LAL) assay (BioWhittaker, Walkersville, Md.).
The DNA sequence encoding the M. tuberculosis 85B protein was engineered by PCR amplification of genomic DNA isolated from the H37Ra strain by using primers designed to amplify the entire mature secreted sequence (25), with the 5′ primer encoding 6-histidine residues. The PCR product was digested with NdeI and EcoRI and ligated into the pET 17b plasmid. Plasmids were subsequently transformed into competent E. coli BL-21(pLysE) cells for expression of the recombinant protein. Purification of recombinant M. tuberculosis 85B (r85B) was performed in a manner similar to that described for rMtb39A and rΔMtb39A.
All DNA manipulations of the various clones were confirmed by DNA sequencing to eliminate the possibility of the introduction of mutations by restriction, ligation, and PCR.
Mtb39A peptide synthesis.
Mtb39A peptides were synthesized on a Rainin/PTI Symphony peptide synthesizer by using 9-fluorenylmethoxycarbonyl batch chemistry with HBTU activation. Peptides were analyzed by reverse-phase HPLC using a Vydac C18 column. Peptide molecular weights were verified by using a matrix-assisted laser desorption/ionization time-of-flight mass spectrometer.
Molecular analysis of M. tuberculosis clones.
DNA was prepared by following the manufacturers’ protocols (Qiagen, Chatsworth, Calif.; Promega, Madison, Wis.). DNA sequencing was performed with an Automated Sequencer (model 373; Applied Biosystems). DNA sequences and deduced amino acid sequences were used in database searches (EMBL, GenBank, and Swiss and PIR and Translated Release 97).
Genomic DNA from mycobacterial strains was digested with PstI, separated by agarose gel electrophoresis, and blotted on Nytran (Schleicher & Schuell, Keene, N.H.). The mtb39a gene was labeled with [32P]dCTP by random oligonucleotide primers (Boehringer Mannheim, Indianapolis, Ind.) and used as a probe. Hybridization was performed at 65°C in 0.2 M Na-H2PO4–3.6 M NaCl–0.2 M EDTA overnight and washed to a stringency of 0.075 M NaCl–0.0075 M sodium citrate (pH 7.0)–0.5% SDS at the temperature of hybridization.
Immunoblot analysis.
Antisera to rTbH9 and r85B were raised by using adult New Zealand White rabbits (R & R Rabbitry, Stanwood, Wash.) by an initial subcutaneous (s.c.) delivery of 100 to 200 μg of recombinant antigen in 1 ml of incomplete Freund’s adjuvant (IFA) (Bethesda Research Laboratories, Gaithersburg, Md.) together with 100 μg of muramyl dipeptide (Calbiochem, La Jolla, Calif.), followed by two successive s.c. immunizations of 75 to 100 μg of antigen in 1 ml of IFA at 3-week intervals. A final intravenous boost of 75 to 100 μg of antigen was delivered after four additional weeks, and serum was collected 2 weeks later.
M. tuberculosis H37Rv lysate, culture filtrate proteins (CFP), PPD, and purified rMtb39A were subjected to SDS-PAGE in either a 7.5 or 12% polyacrylamide gel and transferred to nitrocellulose. Filters were blocked with PBS (pH 7.4) containing 5% nonfat milk at 4°C overnight, washed three times in PBS–0.1% Tween 20 (PBS-T), and incubated for 1 h in rabbit sera (diluted 1:250 in PBS-T) on a rocker at room temperature. Filters were washed three times with PBS-T, and bound antibody was detected with 105 cpm of 125I-labeled protein A/ml followed by autoradiography.
Proliferation and cytokine production assays.
PBMC were cultured in 96-well round-bottom plates (Corning Costar, Cambridge, Mass.) at 2 × 105 cells/well in a volume of 200 μl. Antigens were tested in triplicate at 10 μg/ml and in some assays, antigens were titrated. Culture medium consisted of RPMI medium with 10% pooled human serum and 50 μg of gentamicin/ml. After 5 days of culture at 37°C in 5% CO2, 50 μl of culture supernatant was carefully aspirated for determination of IFN-γ levels, and the plates were pulsed with 1 μCi of tritiated thymidine/well. After culture for a further 18 h, cells were harvested, and tritium uptake was determined by using a gas scintillation counter. IFN-γ levels in culture supernatants were determined by enzyme-linked immunosorbent assay ELISA, as described previously (42).
Evaluation of Mtb39A in Mtb-infected DC.
Monocyte-derived dendritic cells (DC) were prepared essentially according to the method of Romani et al. (37), by culture of adherent PBMC in RPMI–10% human serum containing 10 ng of interleukin 4 (Immunex Corporation, Seattle, Wash.)/ml and 30 ng of granulocyte-macrophage colony-stimulating factor (Immunex)/ml for 5 to 7 days. Cells were harvested with cell-dissociation medium (Sigma, St. Louis, Mo.) and seeded at 2 × 104 cells per well in 96-well flat-bottom plates (Corning Costar) in 100 μl of RPMI–10% HS. Where indicated, M. tuberculosis was subsequently added in 25 μl of medium. After 18 h, 5 × 104 CD4+ D160TbH9-9 T cells were added in 100 μl of medium, and supernatants were harvested after 18 to 24 h for determination of IFN-γ levels. D160TbH9-9 is an Mtb39A-specific T-cell clone generated by limiting dilution cloning of D160 PBMC stimulated with rMtb39A.
Naked DNA immunization.
C57BL/6 mice were immunized intramuscularly (i.m.), three times, 1 month apart, with 100 μg of mtb39a DNA engineered in the pJA4304 vector (generous gift of James I. Mullins and Jim Arthos, University of Washington School of Medicine, Seattle, Wash.) lacking the tPA signal peptide, or with control pJA4304 vector. The plasmid for DNA immunizations was generated by PCR cloning of the mtb39a coding sequence into the expression vector JA4304. The 5′ PCR primer contained a consensus Kozak sequence (GGCCACC) just upstream of the ATG initiator codon to allow efficient initiation of eukaryotic translation. The PCR fragment was inserted as a HindIII-BglII fragment into the corresponding restriction sites of the vector. Plasmid DNA preparations were done on a Qiagen column. Expression of the mtb39a gene was confirmed upon transient transfection into Cos cells. Residual endotoxin content was measured by the LAL assay and was below 0.001 EU/μg. In addition, groups of mice were also immunized with 104 BCG (once, s.c.) or simply injected with saline. Thirty days after the last immunization, the mice were challenged by the aerosol route with approximately 100 CFU of M. tuberculosis Erdman. Protection was measured by enumerating the bacteriological burden (CFU) in the mouse lungs.
RESULTS
Isolation and molecular characterization of mtb39a.
Approximately 126,000 recombinant phage from M. tuberculosis H37Ra and M. tuberculosis H37Rv genomic libraries were screened by using a pool of TB patient sera (see Materials and Methods). This resulted in the identification and purification of seven serologically reactive clones. The recombinant antigens encoded by five of these clones were expressed and purified.
Preliminary cellular assays with the five purified recombinant M. tuberculosis antigens, designated rTbH4, rTbH5, rTbH9, rTbH12, and rTbH16, demonstrated that only rTbH9 was effective in eliciting T-cell proliferation and production of IFN-γ using purified PBMC from healthy PPD-positive donors (data not shown). DNA sequence analysis of the region encoding the entire open reading frame (ORF) of TbH9 revealed the presence of 22 carboxy-terminal residues derived from λ phage sequence, presumably due to an unusual ligation or recombination event during the formation of the library. The TbH9 clone was reengineered to remove both the majority of the 5′ lacZ sequence and contaminating phage sequence and to add a six-histidine tag. This recombinant protein (rΔMtb39A) was expressed and purified on a nickel column (Fig. 1).
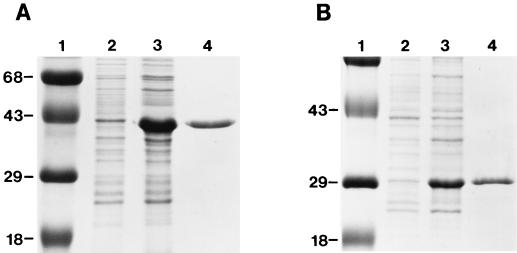
FIG. 1.
Purification of rMtb39A and rΔMtb39A proteins. Expression and purification of rMtb39A (A) and rΔMtb39A (B) are shown with uninduced (lane 2) and induced (lane 3) E. coli lysates and 5 μg of purified proteins (lane 4). Molecular mass markers are shown in kDa (lane 1).
The TbH9 insert was used as a probe to clone the full-length gene from an M. tuberculosis H37Rv genomic library. Three clones that were recovered were sequenced, one of which encoded the full-length gene consisting of 391 amino acids, with a predicted mass of 39,162 Da (Fig. 2), and the gene was designated mtb39a. Interestingly, the other two clones contained DNA sequences that were highly related to, but nonidentical with, mtb39a and each other (data not shown), indicating the presence of a family of at least three highly related genes. The genes of these other family members were designated mtb39b and mtb39c. The DNA sequence analysis of these two clones revealed the full-length ORF encoded by mt39b was present within one clone, but mtb39c was incomplete, lacking the C-terminal portion. The sequence comparison of the proteins encoded by mtb39a, mtb39b, and the N-terminal portion of mtb39c, referred to as Mtb39A, Mtb39B, and Mtb39C respectively, revealed amino acid identity ranging from 82 to 88% (Fig. 2). Database searches with the DNA sequences of mtb39a, mtb39b, and the N-terminal portion of mtb39c using the EMBL and GenBank database revealed identities with recently deposited sequences derived from the Sanger Center (Cambridge, United Kingdom). These sequences were located on cosmids I364, SCY02B10, and SCY13E12, respectively. The amino acid sequences from Mtb39A, Mtb39B, and Mtb39C have identity to hypothetical ORFs encoded within these cosmids (accession no. e311073, e250360, and e316074, respectively). The hypothetical ORF identical to the partial Mtb39C amino acid sequence recovered by cloning demonstrates that this family member is of approximately the same size as Mtb39A and Mtb39B, and the C-terminal portion is included for comparative purposes (Fig. 2). All three of the Mtb39 family members belong to a group of 68 M. tuberculosis proteins referred to as the PPE family, based on the presence of this amino acid sequence within the members, usually located near the N terminus (15). The cellular location of the PPE family of proteins has not been demonstrated, nor has a function been assigned to any of the family members except one, a lipase (15). Hydropathy analysis (22) of the Mtb39 amino acid sequences demonstrated the presence of several extended hydrophobic regions in the three family members that could potentially serve as transmembrane domains (data not shown).
FIG. 2.
Comparison of amino acid sequences encoded by mtb39 genes. Identical residues are indicated by dots (.) and deletions are indicated by dashes (-). Amino acid residues present in rTbH9 are underlined. The carboxy-terminal portion of the Mtb39C ORF was recovered by database searching and extends from residues 360 to 393.
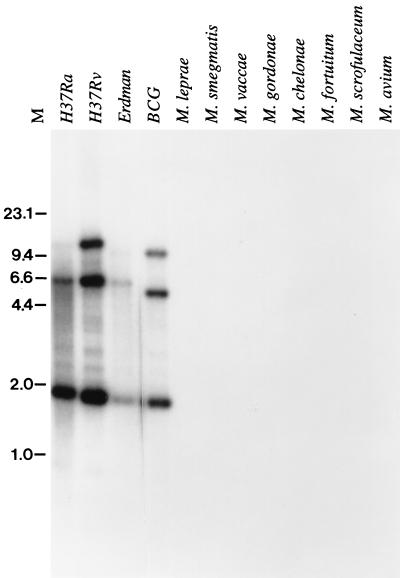
Genomic DNA from a number of mycobacterial species was analyzed by Southern blotting by using the mtb39a gene as a probe. The results demonstrate the presence of three highly related members of the mtb39 gene family in various M. tuberculosis strains, consistent with the number of genes recovered from the M. tuberculosis H37Rv genomic library (Fig. 3). Based on restriction enzyme digest patterns, all three family members appear to be conserved in M. tuberculosis isolates, including clinical isolates M. tuberculosis Erdman (Fig. 3) and M. tuberculosis C (data not shown). Hybridization consistent with the presence of the three family members was also observed in M. bovis BCG, but no hybridization was detected in M. leprae, M. smegmatis, M. vaccae, M. gordonae, M. chelonae, M. fortuitum, M. scrofulaceum, and M. avium.
FIG. 3.
Southern blot analysis of mtb39 genes. Genomic DNA (2.5 μg) from mycobacterial strains was digested with PstI, separated by agarose gel electrophoresis, and blotted onto Nytran. The mtb39a gene was labeled with [32P]dCTP by random oligonucleotide primers and used as a probe. Molecular sizes in kilobases are shown.
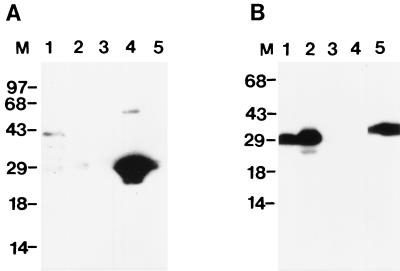
To further characterize native and recombinant Mtb39A, full-length recombinant Mtb39A (rMtb39A) was expressed and purified (Fig. 1). To characterize the native M. tuberculosis protein encoded by mtb39a, a rabbit antiserum was raised to rTbH9 and used in an immunoblot of M. tuberculosis lysate. Mtb39A protein could be detected in M. tuberculosis lysate at approximately 40 kDa, in agreement with the predicted mass. However, no protein could be detected in M. tuberculosis CFP (Fig. 4). Integrity of the CFP was verified by Coomassie staining (data not shown) and by immunoblotting using a rabbit antiserum raised against purified r85B, a known secreted M. tuberculosis protein (Fig. 4).
FIG. 4.
Characterization of native Mtb39A. M. tuberculosis H37Rv lysate (2.5 μg; lane 1), 2.5 μg of culture filtrate protein (lane 2), 50 ng of purified rMtb11 (recombinant 11-kDa M. tuberculosis antigen [unpublished results]) (lane 3), 50 ng of purified rΔMtb39A (lane 4), and 50 ng of purified recombinant antigen 85B (lane 5) were subjected to SDS-PAGE, transferred to nitrocellulose, and reacted with rabbit antisera generated against rΔMtb39A (A) or recombinant antigen 85B (B). Molecular markers in kDa are indicated.
PBMC responses to Mtb39A.
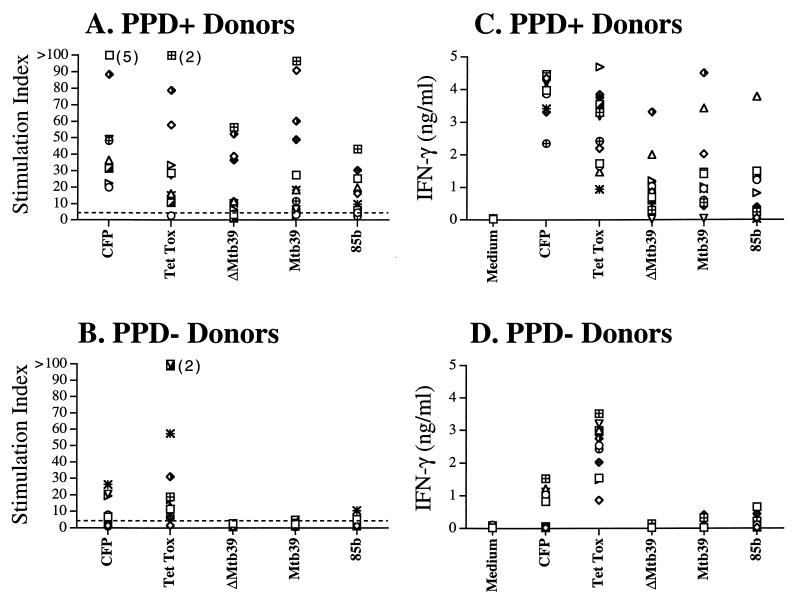
PBMC from 12 PPD-positive and 12 PPD-negative donors, all with no history of TB, were analyzed for T-cell proliferation and IFN-γ production in response to CFP, tetanus toxoid, purified rΔMtb39A, purified rMtb39A, and, for comparative purposes, r85B. The proliferation results (Fig. 5A and B) indicate responses by the majority of the 12 PPD-positive donors to rΔMtb39A, while only very weak or negative responses were observed in the PPD-negative donors. Using an arbitrary cutoff of a stimulation index (SI) of 5 for a positive response, the full-length protein rMtb39A elicited proliferative responses in 9 of 12 PPD-positive donors tested, with responses seen in none of the 12 PPD-negative donors. In addition, the majority of PPD-positive individuals responding to both rΔMtb39A and rMtb39A, had greater responses to rMtb39A (the mean SI for rMtb39A was 32.4, compared with 19.4 for rΔMtb39A), suggesting the recognition of multiple T-cell epitopes within rMtb39A, with some located in the N-terminal and/or C-terminal regions not present within rΔMtb39A. A similar number of donors (8 of 12) responded to r85B, although the mean SI was considerably lower with r85B (15.3) compared with that of Mtb39A (32.4). In addition, r85B induced a response (SI, >5) in 2 of 12 of the PPD-negative donors. The response to tetanus toxoid was similar in both groups, with 11 of 12 PPD-positive and -negative donors responding with SIs of greater than 5.
FIG. 5.
Proliferation and IFN-γ production by PBMC from PPD-positive and -negative donors to CFP, tetanus toxoid, rΔMtb39A, rMtb39A, and r85B. Proliferation was measured in PBMC from PPD-positive donors (n = 12) and PPD-negative donors (n = 12). PBMC (2 × 105 cells/well) were cultured in the presence of antigen (10 μg/ml) for 5 days. After 5 days, 50 μl of culture supernatant was carefully aspirated for determination of IFN-γ levels by ELISA, and the plates were pulsed with tritiated thymidine. After culture for a further 18 h, cells were harvested, and tritium uptake was determined by using a gas scintillation counter. Proliferation results are reported as an SI (counts per minute of cultures with antigen/counts per minute of medium control cultures). The number of responses that have an SI of greater than 100 is indicated in parentheses.
The IFN-γ production responses (Fig. 5C and D) were similar to the proliferative responses, with the majority of PPD-positive individuals responding to both rΔMtb39A and rMtb39A, and low or no response observed in the PPD-negative donors.
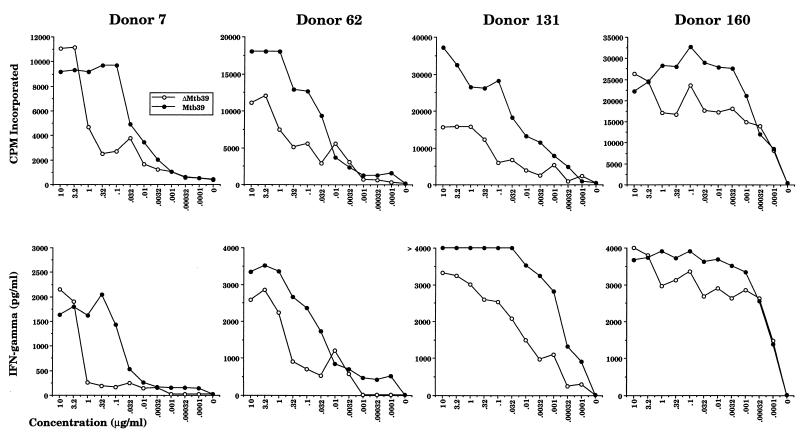
Four of the strongest responders to rMtb39A were selected for more detailed analysis. The results of antigen titration experiments indicated that very low amounts of rMtb39A protein were sufficient to elicit significant responses, such that in some donors as little as 1 ng of antigen/ml elicited a maximal response (Fig. 6). PBMC from these donors all made strong IFN-γ responses to rMtb39A that correlated well with their proliferative responses. Stronger proliferation and IFN-γ responses to the full-length protein were noted over the entire titration curve with several donors, again suggesting the presence of additional T-cell epitopes in the N-terminal or C-terminal region of rMtb39A that were not present in rΔMtb39A.
FIG. 6.
Dose response of PBMC from PPD-positive donors to rΔMtb39A and rMtb39A. Proliferation and IFN-γ production to rΔMtb39A and rMtb39A were measured in PBMC from four PPD-positive donors. After 5 days, 50 μl of culture supernatant was carefully aspirated for determination of IFN-γ levels by ELISA, and the plates were pulsed with tritiated thymidine. After culture for a further 18 h, cells were harvested, and tritium uptake was determined by using a gas scintillation counter.
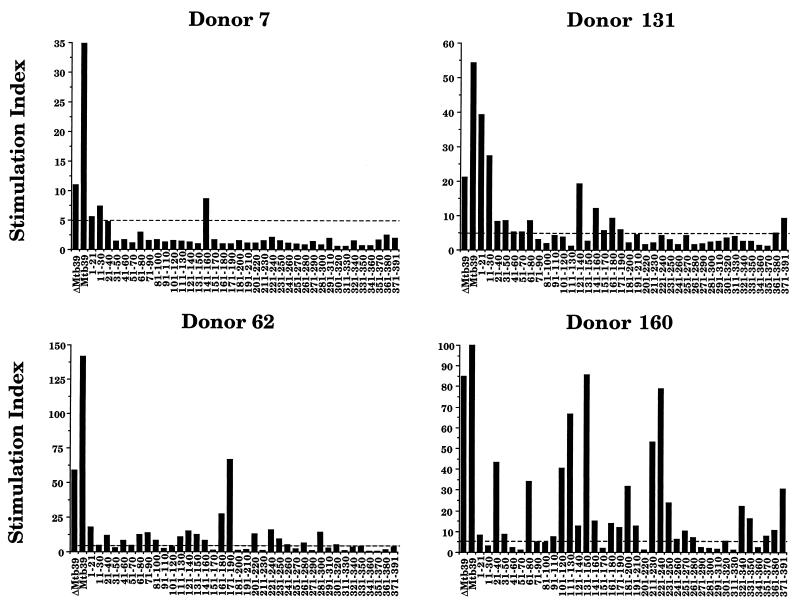
To determine the complexity of T-cell epitopes being recognized by the PPD-positive donors, overlapping peptides across the entire ORF of Mtb39A were synthesized and analyzed for the ability to elicit T-cell proliferation from PBMC (Fig. 7). Responses by the four donors tested indicated the presence of at least 10 distinct T-cell epitopes within rMtb39A. The intensity and complexity of responses varied greatly among the different donors, from the restricted response observed with donor 7 (responding to only three peptides) to the broad response observed with donor 160, where proliferative responses to 26 of the 38 peptides were observed. All peptides were tested on PBMC from eight PPD-negative donors and none of the peptides elicited a response (SI, >5) from any of the donors (data not shown).
FIG. 7.
PBMC responses from PPD-positive donors to rΔMtb39A, rMtb39A, and peptides derived from the amino acid sequence. Proliferation to rΔMtb39A, rMtb39A and peptides based on Mtb39A amino acid sequence was measured in PBMC from four PPD-positive donors. PBMC (2 × 105 cells/well) were cultured in the presence of antigen (10 μg/ml) for 5 days. After 5 days, 50 μl of culture supernatant was carefully aspirated for determination of IFN-γ levels by ELISA, and the plates were pulsed with 1 μCi of tritiated thymidine/well. After culture for a further 18 h, cells were harvested, and tritium uptake was determined by using a gas scintillation counter. Proliferation results to the antigens are reported as an SI (counts per minute of cultures with antigen/counts per minute of medium control cultures).
The peptide results are also consistent with the differential responses to rΔMtb39A and rMtb39A in donor 7, donor 62, and donor 131 that were observed. In all three donors, PBMC yielded greater proliferative responses to the full-length protein than to the truncated portion. Examination of the profile of stimulatory peptides for these three donors revealed that all recognize additional peptides in the N-terminal or C-terminal region of Mtb39 not included in rΔMtb39A. The response to rΔMtb39A was not significantly reduced in donor 160, where the majority of stimulatory peptides are located within regions present in both recombinant proteins and the overwhelming response to these epitopes likely masks any additivity by reactive N-terminal and C-terminal peptides.
Mtb39A is presented by M. tuberculosis-infected DC.
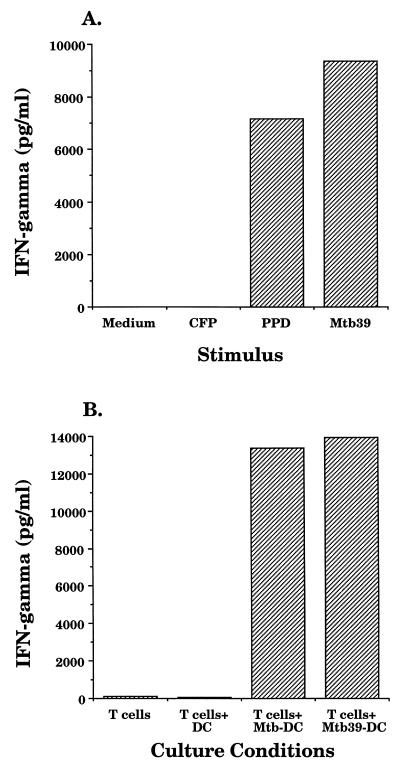
An Mtb39A-specific CD4+ T-cell clone, termed D160TbH9-9, was utilized to confirm the immunoblot results indicating that Mtb39A was not detectable in M. tuberculosis CFP. Blood-derived DC were chosen for these studies because of their superior antigen-presenting capabilities compared with monocytes and because they are amenable to infection with M. tuberculosis (24). Autologous DC were pulsed with PPD, CFP, or rMtb39A and incubated with D160TbH9-9 cells, and IFN-γ production was measured. Production of IFN-γ was observed when the dendritic cells were pulsed with PPD or rMtb39A but not when pulsed with CFP (Fig. 8A), consistent with the previous immunoblot data indicating that Mtb39A was not present in CFP. An Mtb39A-specific CD4+ T-cell clone derived from donor 131 also showed strong reactivity with PPD but no reactivity with CFP (data not shown). The D160TbH9-9 clone was then used to determine if Mtb39A could be detected in M. tuberculosis-infected DC. When autologous DC infected with M. tuberculosis were incubated with D160TbH9-9 cells, production of IFN-γ was observed (Fig. 8B), indicating that Mtb39A expressed by M. tuberculosis can be processed and presented by infected DC.
FIG. 8.
Mtb39A is present in Mtb-infected DC. A total of 5 × 104 Mtb39A-specific CD4+ T-cell clone D160TbH9-9 cells were incubated with 2 × 104 autologous DC that had either been incubated with PPD, CFP, or rMtb39A at a concentration of 10 μg/ml (A) or incubated for 18 h with M. tuberculosis H37Rv (MOI, 50) (B). Supernatants were collected after 18 h and assessed for the presence of IFN-γ by ELISA.
Responses to Mtb39A DNA immunization in murine protection model.
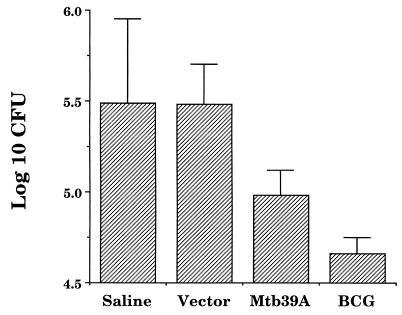
Because immunity to tuberculosis is apparently dependent on both CD4+ and CD8+ T-cell responses, experiments were designed to investigate the protection potential of mtb39a delivered in a naked DNA format. These experiments were supported by previous immunogenicity studies which demonstrated that mtb39a engineered in the expression vector pJA4304 under the CMV promoter induced both CD4+ and CD8+ T-cell responses (data not shown). C57BL/6 mice were immunized three times with pJA4304 vector DNA or the mtb39a construct and challenged with aerosolized viable and virulent M. tuberculosis. Resistance was assessed 4 weeks after infection by enumerating the bacteriological burden in the lungs. Figure 9 illustrates the results and shows that mice immunized with mtb39a DNA were able to develop lung protection against tuberculosis. This protection was not as strong as that induced by BCG vaccination but was significant in that the bacteriological burden in the lungs of mtb39a DNA immunized mice was five times smaller than that observed for mice infected with control DNA or with saline.
FIG. 9.
mtb39a DNA immunization in the murine protection model. Groups of five C57BL/6 mice were immunized i.m. three times 1 month apart with 100 μg of mtb39a DNA or with control DNA (Vector). In addition, groups of five mice were also immunized with BCG (once) or simply injected with saline. Thirty days after the last immunization, the mice were challenged by the aerosol route with approximately 100 CFU of M. tuberculosis Erdman. Protection was measured by enumerating the bacteriological burden (CFU) in the mouse lungs, shown as log10 CFU.
DISCUSSION
An initial requirement a TB candidate vaccine antigen must meet is that it is recognized by the immune system during the course of infection in the majority of individuals of the target population. To assess candidate M. tuberculosis antigens, we have used a panel of PPD-positive donors, with no history of tuberculosis disease. We have purposely chosen an ethnically diverse group, and have used T-cell proliferation and production of IFN-γ from in vitro-stimulated PBMC to measure recognition of the relevant antigen during previous exposure to M. tuberculosis. The presumption that strong IFN-γ responses are required for protective immunity is supported by the fact that both mice and humans that are deficient in IFN-γ production or signaling in vivo are susceptible to mycobacterial infections (16, 18, 20, 28). This preliminary screening of candidate antigens has identified Mtb39A as an antigen recognized by the majority of M. tuberculosis infected individuals without evident disease, with strong T-cell proliferation and IFN-γ production observed in 9 of 12 donors tested. Often, the responses to this single recombinant antigen that were observed were similar in magnitude to that elicited by the complex mixture of proteins present in CFP and were superior to those elicited by r85B. Equally striking is the ability of Mtb39A to elicit responses from PPD-positive donors at exceedingly low antigen concentrations, suggesting that M. tuberculosis antigens expressed at low levels retain the ability to trigger potent immune responses.
It is likely that a candidate vaccine antigen for M. tuberculosis will need to contain multiple T-cell epitopes to afford protection in an outbred human population. Experiments using overlapping peptides on a small panel of donors confirmed the presence of at least 10 distinct T-cell epitopes contained within Mtb39A recognized by PBMC from human PPD-positive donors. The recovery of three highly related Mtb39 family members poses the question of antigen immunogenicity versus a cross-reactive elicitation of a T-cell response. A broad response, such as that observed in donor 160, indicates that Mtb39A was the antigen against which the immune system developed a response during the infection by M. tuberculosis. Less clear are more restricted responses, as was observed in donor 7, in which only three peptides appeared to be recognized. A complete understanding of the potential contributions by all three family members will rely on the synthesis and testing of overlapping peptides derived from all three amino acid sequences in the regions of divergence.
The data generated with the rMtb39A and derived peptides indicate an abundance of T-cell epitopes recognized by healthy PPD-positive donor PBMC. However, these data are based entirely on either synthetic or recombinant molecules. Indeed, native Mtb39A may contain additional epitopes that cannot be detected by using these synthetic or recombinant forms, since the possibility of differential processing due to potential conformational differences between these forms and native Mtb39A exists. The low level of expression of native Mtb39A in M. tuberculosis cultures makes testing this a difficult proposition, since purification will likely result in some contaminating M. tuberculosis proteins which could contribute to the response in T-cell assays.
Recent research with the goal of identifying TB antigens recognized by human T cells has focused on antigens present in M. tuberculosis CFP. This focus has arisen from the observation that live mycobacteria, but not heat-killed preparations, elicit protective immunity in animal models (31). Subsequent experiments in mice and guinea pigs demonstrated that the mixture of antigens that comprise CFP could confer protection (3, 32, 33). These protection experiments indicate that immunologically relevant antigens are present in CFP, and efforts have resulted in the identification of a number of these antigens capable of eliciting CD4+ T-cell recall responses, including the Ag 85 complex, ESAT-6, MPT64, and the 10-kDa antigen (1, 8, 19, 23, 35). The results presented here provide evidence that potent T-cell antigens from TB are not exclusively present in CFP. This assertion is based upon two lines of evidence. First, data demonstrated that Mtb39A is a very potent antigen based upon its ability to stimulate strong T-cell proliferation and IFN-γ production by PBMC from PPD-positive but not PPD-negative individuals. Second, Mtb39A could not be detected in CFP by either a high-titer polyclonal Mtb39A antiserum or by a very sensitive CD4+ T-cell clone cultured with DC pulsed with CFP.
More important than whether an antigen is present within CFP or PPD is whether the antigen is expressed, processed, and presented by major histocompatibility complex molecules upon infection of cells with TB. We have demonstrated that the Mtb39A-specific CD4+ T-cell clone could detect the presence of Mtb39A upon infection of autologous DC with TB. Although the data presented here are insufficient to claim that M. tuberculosis expresses Mtb39A during infection of DC, they do demonstrate the ability of the protein to be processed and presented in the context of an infection, either by the infected DC or uninfected neighboring DC.
Utilization of the host immune response provides a direct method to distinguish relevant antigens in a complex lysate or gene expression library. We have used a two-step expression cloning approach, initially screening with antisera from individuals with active TB, followed by evaluation of purified recombinant proteins based on T-cell stimulation, in an attempt to isolate novel T-cell antigens. Although the majority of M. tuberculosis antigens recovered by their reactivity with TB patient sera did not elicit significant cellular responses, one potent T-cell antigen, Mtb39A, was identified through this approach. We have utilized a process that starts with human reactivity (both T cell and serological) to identify candidate antigens, and proceeded by characterizing these by testing human T-cell responses from protected individuals. We then proceeded to animal studies, and evaluated both immunogenicity and protective characteristics of the antigens. This approach clearly differs from that used by many other researchers in this field, where initial tests of candidate antigens involve animal immunogenicity and protection studies using native M. tuberculosis antigens, followed by evaluation of human responses to the antigens. It is worth noting that the validity of either of these approaches to recover relevant antigens that would comprise an effective M. tuberculosis vaccine for humans is as yet uncertain. Presumably, the best candidate vaccine antigens would be those that are able to provide protection in at least one of the animal models and are known to be recognized by the immune systems of humans infected with M. tuberculosis.
ACKNOWLEDGMENTS
We thank Paul Sleath for assistance with synthetic peptides, Tom Vedvick for N-terminal sequencing of recombinant proteins, and Steve Johnson and John Webb for M. tuberculosis H37Ra and H37Rv genomic libraries. We thank James I. Mullins and Jim Arthos for kindly providing the pJA4304 plasmid. We thank Dan Hoppe for DNA sequencing efforts and Karen Kinch for assistance in manuscript preparation.
Murine protection experiments were performed on NIH program AI-75320.
REFERENCES
- 1.Abou Zeid C, Ratliff T L, Wiker H G, Harboe M, Bennedsen J, Rook G A. Characterization of fibronectin-binding antigens released by Mycobacterium tuberculosis and Mycobacterium bovis BCG. Infect Immun. 1988;56:3046–3051. doi: 10.1128/iai.56.12.3046-3051.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amara R R, Satchidanandam V. Analysis of a genomic DNA expression library of Mycobacterium tuberculosis using tuberculosis patient sera: evidence for modulation of the host immune response. Infect Immun. 1996;64:3765–3771. doi: 10.1128/iai.64.9.3765-3771.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andersen P. Effective vaccination of mice against Mycobacterium tuberculosis infection with a soluble mixture of secreted mycobacterial proteins. Infect Immun. 1994;62:2536–2544. doi: 10.1128/iai.62.6.2536-2544.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andersen P, Andersen A B, Sorensen A L, Nagai S. Recall of long-lived immunity to Mycobacterium tuberculosis infection in mice. J Immunol. 1995;154:3359–3372. [PubMed] [Google Scholar]
- 5.Arachi A. The global tuberculosis situation and the new control strategy of the World Health Organization. Tubercle. 1991;72:1–6. doi: 10.1016/0041-3879(91)90017-m. [DOI] [PubMed] [Google Scholar]
- 6.Baily G V. BCG vaccination after the Madras study. Lancet. 1981;i:309–310. [PubMed] [Google Scholar]
- 7.Barnes P F, Barrows S A. Tuberculosis in the 1990s. Ann Intern Med. 1993;119:400–410. doi: 10.7326/0003-4819-119-5-199309010-00009. [DOI] [PubMed] [Google Scholar]
- 8.Barnes P F, Mehra V, Rivoire B, Fong S J, Brennan P J, Voegtline M S, Minden P, Houghten R A, Bloom B R, Modlin R L. Immunoreactivity of a 10-kDa antigen of Mycobacterium tuberculosis. J Immunol. 1992;148:1835–1840. [PubMed] [Google Scholar]
- 9.Barnes P F, Bloch A B, Davidson P T, Snider D E., Jr Tuberculosis in patients with human immunodeficiency virus infection. N Engl J Med. 1991;324:1644–1650. doi: 10.1056/NEJM199106063242307. [DOI] [PubMed] [Google Scholar]
- 10.Braun M M, Cauthen G. Relationship of the human immunodeficiency virus epidemic to pediatric tuberculosis and bacillus Calmette-Guerin immunization. Pediatr Infect Dis J. 1992;11:220–227. doi: 10.1097/00006454-199203000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Calmette A, Guerin C, Negre L, Boquet A. Prémunition des nouveaux-nés contre la tuberculose par le vaccin BCG, 1921–1926. Ann Inst Pasteur (Paris) 1926;40:89–133. [Google Scholar]
- 12.Chaisson R E, Schecter G F, Theuer C P, Rutherford G W, Echenberg D F, Hopewell P C. Tuberculosis in patients with the acquired immunodeficiency syndrome. Clinical features, response to therapy, and survival. Am Rev Respir Dis. 1987;136:570–574. doi: 10.1164/ajrccm/136.3.570. [DOI] [PubMed] [Google Scholar]
- 13.Coates A R M, Hewitt J, Allen B W, Ivanyi J, Mitchison D A. Antigenic diversity of Mycobacterium tuberculosis and Mycobacterium bovis detected by means of monoclonal antibodies. Lancet. 1981;ii:167–169. doi: 10.1016/s0140-6736(81)90355-x. [DOI] [PubMed] [Google Scholar]
- 14.Colditz G A, Brewer T F, Berkey C S, Wilson M E, Burdik E, Fineburg H V, Mosteller F. Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. JAMA. 1994;271:698–702. [PubMed] [Google Scholar]
- 15.Cole S T, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, Gordon S V, Eiglmeier K, Gas S, Barry III C E, Tekaia F, Badcock K, Basham D, Brown D, Chillingworth T, Conner R, Davies R, Devlin K, Feltwell T, Gentles S, Hamlin N, Holroyd S, Hornsby T, Jagels K, Krogh A, McLean J, Moule S, Murphy L, Oliver K, Osborne J, Quail M A, Rajandream M-A, Rogers J, Rutter S, Seeger K, Skelton J, Squares R, Squares S, Sulston J E, Taylor K, Whitehead S, Barrell B G. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 1998;393:537–544. doi: 10.1038/31159. [DOI] [PubMed] [Google Scholar]
- 16.Cooper A M, Dalton D K, Stewart T A, Griffen J P, Russel D G, Orme I M. Disseminated tuberculosis in interferon-γ gene-disrupted mice. J Exp Med. 1993;178:2243–2247. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Engers H D, Houba V, Bennedsen J, Buchanan T M, Chapars S D, Kadival G, Closs O, David J R, van Embden J D A, Godal T, Mustafa S A, Ivanyi J, Young D B, Kaufmann S H E, Komenko A G, Kolk A H J, Kubin M, Louis J A, Minden P, Shinnick T M, Trnka L, Young R A. Results of a World Health Organization sponsored workshop to characterize antigens recognized by mycobacterium-specific monoclonal antibodies. Infect Immun. 1986;51:718–720. doi: 10.1128/iai.51.2.718-720.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flynn J L, Chan J, Triebold K J, Dalton D K, Stewart T A, Bloom B R. An essential role for IFN-γ in resistance to M. tuberculosis infection. J Exp Med. 1993;178:2248–2253. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haslov K, Andersen A, Nagal S, Gottschau A, Sorensen T, Andersen P. Guinea pig cellular immune responses to proteins secreted by Mycobacterium tuberculosis. Infect Immun. 1995;63:804–810. doi: 10.1128/iai.63.3.804-810.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jouanguy E, Altare F, Lamhamedi S, Revy P, Emile J, Newport M, Levin M, Blanche S, Seboun E, Casanova J. Interferon-gamma-receptor deficiency in an infant with fatal Bacille Calmette-Guerin infection. N Engl J Med. 1996;335:1956–1961. doi: 10.1056/NEJM199612263352604. [DOI] [PubMed] [Google Scholar]
- 21.Kochi A. The global tuberculosis situation and the new control strategy of the World Health Organization. Tubercle. 1991;72:1–6. doi: 10.1016/0041-3879(91)90017-m. [DOI] [PubMed] [Google Scholar]
- 22.Kyte J, Doolittle R F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982;157:105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- 23.Launois P, DeLeys R, Niang M N, Drowart A, Andrien M, Dierckx P, Cartel J L, Sarthou J L, Van Vooren J P, Huygen K. T-cell-epitope mapping of the major secreted mycobacterial antigen Ag85A in tuberculosis and leprosy. Infect Immun. 1994;62:3679–3687. doi: 10.1128/iai.62.9.3679-3687.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lewinsohn D M, Alderson M R, Briden A L, Riddell S R, Reed S G, Grabstein K H. Characterization of human CD8+ T cells reactive with Mycobacterium tuberculosis-infected antigen-presenting cells. J Exp Med. 1998;187:1633–1640. doi: 10.1084/jem.187.10.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsuo K, Yamaguchi R, Yamazaki A, Tasaka H, Yamada T. Cloning and expression of the Mycobacterium bovis BCG gene for extracellular alpha antigen. J Bacteriol. 1988;170:3847–3854. doi: 10.1128/jb.170.9.3847-3854.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Müller I, Cobbold S P, Waldmann H, Kaufmann S H E. Impaired resistance to Mycobacterium tuberculosis infection after selective in vivo depletion of L3T4+ and Lyt-2+ T cells. Infect Immun. 1987;55:2037–2041. doi: 10.1128/iai.55.9.2037-2041.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Narrain J P, Raviglione M C, Kochi A. HIV-associated tuberculosis in developing countries: epidemiology and strategies for prevention. Tuberc Lung Dis. 1992;73:311–321. doi: 10.1016/0962-8479(92)90033-G. [DOI] [PubMed] [Google Scholar]
- 28.Newport M J, Huxley C M, Huston S, Hawrylowicz C M, Oostra B A, Williamson R, Levin M. A mutation in the interferon-gamma-receptor gene and susceptibility to mycobacterial infection. N Engl J Med. 1997;335:1941–1949. doi: 10.1056/NEJM199612263352602. [DOI] [PubMed] [Google Scholar]
- 29.Orme I M, Collins F M. Adoptive protection of the Mycobacterium tuberculosis-infected lung. Dissociation between cells that passively transfer protective immunity and those that transfer delayed type hypersensitivity to tuberculin. Cell Immunol. 1984;84:113–120. doi: 10.1016/0008-8749(84)90082-0. [DOI] [PubMed] [Google Scholar]
- 30.Orme I M. The kinetics of emergence and loss of mediator T lymphocytes acquired in response to infection with Mycobacterium tuberculosis. J Immunol. 1987;138:293–298. [PubMed] [Google Scholar]
- 31.Orme I M. Induction of nonspecific acquired resistance and delayed-type hypersensitivity, but not specific acquired resistance in mice inoculated with killed mycobacterial vaccines. Infect Immun. 1988;56:3310–3312. doi: 10.1128/iai.56.12.3310-3312.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Orme I. Protective and memory immunity in mice infected with Mycobacterium tuberculosis. Immunobiology. 1994;191:503–508. doi: 10.1016/S0171-2985(11)80456-0. [DOI] [PubMed] [Google Scholar]
- 33.Pal P G, Horwitz M A. Immunization with extracellular proteins of Mycobacterium tuberculosis induces cell-mediated immune responses and substantial protective immunity in a guinea pig model of pulmonary tuberculosis. Infect Immun. 1992;60:4781–4792. doi: 10.1128/iai.60.11.4781-4792.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pedrazanni T, Hug K, Louis J A. Importance of L3T4+ and Lyt-2+ T cells in the immunologic control of infection with Mycobacterium bovis strain bacillus Calmette-Guerin in mice. J Immunol. 1987;139:2032–2037. [PubMed] [Google Scholar]
- 35.Roche P W, Triccas J A, Avery D T, Fifis T, Billman-Jacobe H, Britton W J. Differential T cell responses to mycobacteria-secreted proteins distinguish vaccination with Bacille Calmette-Guerin from infection with Mycobacterium tuberculosis. J Infect Dis. 1994;170:1326–1330. doi: 10.1093/infdis/170.5.1326. [DOI] [PubMed] [Google Scholar]
- 36.Romain F, Augier J, Pescher P, Marchal G. Isolation of a proline-rich mycobacterial protein eliciting delayed-type hypersensitivity reactions only in guinea pigs immunized with living mycobacteria. Proc Natl Acad Sci USA. 1993;90:5322–5326. doi: 10.1073/pnas.90.11.5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Romani N, Gruner S, Brang D, Kampgen E, Lenz A, Trockenbacher B, Konwalinka G, Fritsch P O, Steinman R M, Schuler G. Proliferating dendritic cell progenitors in human blood. J Exp Med. 1994;180:83–93. doi: 10.1084/jem.180.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 39.Sanchez F O, Rodriguez J I, Agudelo G, Garcia L F. Immune responsiveness and lymphokine production in patients with tuberculosis and healthy controls. Infect Immun. 1994;62:5673–5678. doi: 10.1128/iai.62.12.5673-5678.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schaaf H S, Botha P, Beyers N, Gie R P, Vermeulen H A, Groenwald P, Coetzee G J, Donald P R. The 5-year outcome of multidrug resistant tuberculosis patients in the Cape Province of South Africa. Trop Med Int Health. 1996;1:718–722. doi: 10.1111/j.1365-3156.1996.tb00100.x. [DOI] [PubMed] [Google Scholar]
- 41.Shinnick T M. The 65-kilodalton antigen of Mycobacterium tuberculosis. J Bacteriol. 1987;169:1080–1088. doi: 10.1128/jb.169.3.1080-1088.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Skeiky Y A W, Guderian J A, Benson D R, Bacelar O, Carvalho E M, Kubin M, Badaro R, Trinchieri G, Reed S G. A recombinant Leishmania antigen that stimulates human peripheral blood mononuclear cells to express a Th1-type cytokine profile and to produce interleukin 12. J Exp Med. 1995;181:1527–1537. doi: 10.1084/jem.181.4.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Von Reyn C F, Clements C J, Mann J M. Human immunodeficiency virus infection and routine childhood immunization. Lancet. 1987;ii:669–672. doi: 10.1016/s0140-6736(87)92451-2. [DOI] [PubMed] [Google Scholar]
- 44.Weltman A C, Rose D N. The safety of Bacille Calmette-Guerin vaccination in HIV infection and AIDS. AIDS. 1993;7:149–157. doi: 10.1097/00002030-199302000-00001. [DOI] [PubMed] [Google Scholar]