Abstract
Lipid-associated membrane proteins (LAMPs) of Mycoplasma penetrans rapidly induced macrophages to produce proinflammatory cytokines such as tumor necrosis factor alpha (TNF-α). Our analysis showed that the macrophage-stimulating activity of TNF-α production was mainly attributable to a lipid extractable component(s) in the LAMP preparation. Since induction of gene expression is normally preceded by activation of transcriptional factors that bind to their specific recognition elements located in the upstream promoter region, we examined the activity of transcriptional factors, namely, NF-κB and activator protein 1 (AP-1), in thioglycolate exudate peritoneal (TEP) macrophages treated with M. penetrans lipid extract of proteinase K (PK)-digested LAMPs. Initially, in the nuclei of unstimulated TEP cells, there was only a low basal level of active AP-1, and the active form of NF-κB could not be detected. M. penetrans lipid extract of PK-digested LAMPs activated both NF-κB and AP-1 in TEP macrophages within 15 min. The markedly increased activities of both factors gradually declined and dissipated after 2 h. Parallel to the rapid increase of NF-κB and AP-1, the TNF-α transcript also increased significantly 15 min after the stimulation. The high-level expression of TNF-α persisted over 2 h. Dexamethasone blocked the activation of both NF-κB and AP-1 and suppressed the production of TNF-α in TEP macrophages stimulated by M. penetrans lipid extract of PK-digested LAMPs. Our study demonstrates that the M. penetrans lipid extract of PK-digested LAMP is a potent activator for NF-κB and AP-1 in murine TEP macrophages. Our results also suggest that high-level expression of TNF-α in cells induced by M. penetrans lipid extract of PK-digested LAMPs is associated with rapid activation of transcriptional factors NF-κB and AP-1.
Mycoplasmas are a heterogeneous group of the smallest organisms capable of self-replication. Mycoplasmas cause a wide variety of diseases in animals (39). Some mycoplasmas cause respiratory or urogenital diseases in humans (20, 42). However, other mycoplasmas chronically colonize our respiratory and urogenital tracts without apparent clinical significance. There is apparently a significant increase in infections by otherwise unusual species of mycoplasmas in patients with AIDS. Mycoplasma fermentans and Mycoplasma penetrans are the most common AIDS-associated mycoplasmas (18, 19). These mycoplasmas may have a greater propensity to invade systemically from a site of mucosal colonization, and a persistent infection is much more likely to ensue in immunocompromised patients.
Effects of various Mycoplasma species on the functions of macrophages have been studied extensively in vitro. Membrane fractions of certain mycoplasma species are potent inducers of granulocyte-macrophage colony-stimulating factor in bone marrow macrophages (40). Mycoplasma arthritidis induces morphological changes, promotes tumoricidal and listericidal activity, increases uptake of [14C]glucosamine, and enhances acid phosphatase levels in the J774.1 murine macrophage cell line (6). Mycoplasma arginini TUH-14 membrane lipoproteins induce production of interleukin-1 (IL-1), IL-6, and tumor necrosis-factor alpha (TNF-α) by human monocytes (12). M. fermentans-derived high-molecular-weight material induces IL-1, IL-6, TNF-α, nitric oxide, and prostaglandin production in cultured murine macrophages (21, 23). In our laboratory, we have found that a TX-114 preparation of lipid-associated membrane proteins (LAMPs) from M. penetrans and M. fermentans (incognitus) markedly stimulates both human and murine macrophages to produce large amounts of proinflammatory cytokines such as TNF-α, IL-6, and IL-1β. Although many studies have revealed that mycoplasmas or their membrane components can activate production of various cytokines by macrophages from animals or humans (9, 15, 28, 29, 38), the mechanisms that transduce the stimulating signal are largely unknown.
To investigate the molecular mechanism(s) that is responsible for induction of cytokine gene expression in macrophages stimulated by mycoplasmas or their various membrane preparations, we studied TNF-α expression in mouse thioglycolate exudate peritoneal (TEP) macrophages following stimulation by M. penetrans lipid extract of proteinase K (PK)-digested LAMPs. We found that M. penetrans lipid extract of PK-digested LAMPs, like LAMPs, potently induced TNF-α. Since transcriptional factors, such as NF-κB and activator protein 1 (AP-1), are known to play an important role in regulating expression of various genes including cytokine genes (4, 37, 49), we measured the activity and examined the induction kinetics of NF-κB and AP-1 in TEP macrophages following stimulation by M. penetrans lipid extract of PK-digested LAMPs and eventual production of TNF-α. We also examined the effects that dexamethasone, an inhibitor that interferes with the binding activity of NF-κB and AP-1, had on TNF-α production in the macrophages stimulated by M. penetrans lipid extract of PK-digested LAMPs.
(A preliminary report of this research was presented at the 98th General Meeting of the American Society for Microbiology [8arsqb;.)
MATERIALS AND METHODS
Mice.
BALB/c mice purchased from Jackson Laboratory (Bar Harbor, Maine) were kept in the animal facility located in the Armed Forces Institute of Pathology. All mice used for peritoneal macrophages were 8 to 12 weeks old.
Preparation of LAMPs and lipid extract of PK-digested LAMPs from M. penetrans.
M. penetrans was cultured in SP-4 medium to the beginning of the stationary phase and then pelleted by centrifugation. Preparation of LAMPs was performed as previously described (8). A Mycoplasma pellet from 0.5 liter of culture broth was resuspended in 5 ml of Tris-buffered saline (TBS) (50 mM Tris [pH 8.0], 0.15 M NaCl) containing 1 mM EDTA (TBSE), solubilized by adding TX-114 to a final concentration of 2%, and incubated at 4°C for 1 h. To prepare LAMPs, the TX-114 lysate was incubated at 37°C for 10 min for phase separation. After centrifugation at 10,000 × g for 20 min, the upper aqueous phase was removed and replaced with the same volume of 4°C TBSE. The solution was then vortexed and incubated at 4°C for 10 min, and the procedures for phase fractionation were repeated twice. The final TX-114 phase was resuspended in 4°C TBSE to the original volume, and 2.5 volumes of ethanol was added to precipitate membrane components at −20°C overnight. After centrifugation, the pellet was resuspended in phosphate-buffered saline (PBS) by sonication. A Bio-Rad protein assay was performed for protein determination. To prepare the lipid extract of PK-digested LAMPs, 12 mg (determined by the protein assay) of LAMPs was digested with 3 mg of PK in 14 ml of PBS. The digest was then lyophilized, and lipids (including glycolipids and nonpolar lipids) in the lyophilisate were extracted with 7 ml of methanol-chloroform (1:1). The supernatant was transferred to a new tube after centrifugation at 8,000 × g, and the organic solvent was evaporated at 50°C. The dried lipid extract of PK-digested LAMPs was weighed. Thirty-four milligrams of material was derived, indicating that lipid content, which cannot be measured by a protein assay, in the LAMP preparation was more than protein content. The dry lipid extract of PK-digested LAMPs was readily dissolved in PBS, probably due to the presence of the amphiphilic glycolipids forming micelles with nonpolar lipids. For fractionation into a chloroform phase and an aqueous phase, the lyophilized PK-digested LAMPs (originally from 4 mg of protein) were dissolved in 4 ml of chloroform-methanol and incubated for 15 min. Another 2 ml of chloroform and 1.2 ml of water were added to the mixture and emulsified with a pipette. Phases were separated by centrifugation, and the upper aqueous phase was concentrated with a Speed Vac. The lower chloroform phase was air dried and redissolved in PBS containing 0.02% sodium dodecyl sulfate.
Collection and culture of macrophages.
Human peripheral macrophages were derived from buffy coats kindly provided by James W.-K. Shih, Department of Transfusion Medicine, Warren Grant Magnuson Clinical Center, National Institutes of Health. Peripheral blood mononuclear cells were obtained by Ficoll-Paque gradient centrifugation. Cells were suspended in RPMI 1640 culture medium containing 10% fetal bovine serum, glutamine, and penicillin-streptomycin at 2 × 106 cells/ml and incubated for 1 h in 24-well plates. Nonadherent cells were removed, and adherent macrophages were stimulated with mycoplasmal LAMPs at 1 μg/ml for 18 h for cytokine production. Cell lysate was prepared by dissolving macrophages in Triton X-100 lysis buffer containing 0.3 M NaCl, 50 mM Tris (pH 7.5), 0.5% Triton X-100, and 1 mM phenylmethylsulfonyl fluoride. TEP macrophages were obtained from BALB/c mice that had been injected 4 to 5 days previously with 1.5 ml of 4% Brewer’s thioglycolate broth (Difco Laboratories, Detroit, Mich.) by peritoneal lavage with PBS. The cells were pelleted and resuspended in RPMI 1640 culture medium containing 10% fetal bovine serum, glutamine, penicillin-streptomycin, and 5 × 10−5 M 2-mercaptoethanol at 106 cells/ml and plated in 24-well plates. Nonadherent cells were removed after 1 h of incubation at 37°C by washing the cells with culture medium. Adherent macrophages were then stimulated with either LAMPs or M. penetrans lipid extract of PK-digested LAMPs at various concentrations as indicated in Results. The supernatant was collected after 18 h for cytokine enzyme-linked immunosorbent assay (ELISA). For in vivo study, mice were injected intraperitoneally (i.p.) with 100 μg each of the lipid extract of PK-digested LAMPs. TEP cells were obtained at various time periods as indicated in Results. For inhibition experiments, 1 ml of 10−6 M dexamethasone was injected i.p. into mice. After 2 h, mice received 100 μg of M. penetrans lipid extract of PK-digested LAMPs. TEP cells were obtained after 20 min.
Determination of the presence of specific cytokine mRNA by RT-PCR.
Total RNAs were extracted from untreated or treated TEP cells with RNAzol B (Tel-Test, Inc., Friendswood, Tex.) according to the manufacturer’s directions. Detection of specific cytokine messages was done in one reaction tube by reverse transcriptase PCR (RT-PCR). In each reaction tube, total RNAs from 105 macrophages were incubated with a 1 μM final concentration of 5′ and 3′ primers specific for β-actin or TNF-α in the presence of 50 mM KCl, 10 mM Tris HCl (pH 8.3), 0.001% gelatin, 200 μM deoxynucleoside triphosphate, 2.5 mM MgCl2, 1 U of avian myeloblastosis virus RT, 8 U of RNasin, and 1 U of Taq DNA polymerase in a final volume of 40 μl. RT-PCR was performed in a DNA thermal cycler (Perkin-Elmer GeneAmp PCR system 9600). Reverse transcription was first carried out at 42°C for 15 min followed by 30 cycles of DNA amplification: 30 s of denaturation at 94°C, 30 s of annealing at 60°C, and 30 s of extension at 72°C. Primer sequences and sizes of the PCR products were as follows: β-actin (540 bp), 5′-GTG-GGC-CGC-TCT-AGG-CAC-CAA-3′ and 5′-CTC-TTT-GAT-GTC-ACG-CAC-GAT-TTC-3′; TNF-α (446 bp), 5′-AGC-CCA-CGT-CGT-AGC-AAA-CCA-CCA-A-3′ and 5′-ACA-CCC-ATT-CCC-TTC-ACA-GAG-CAA-T-3′ (7). The PCR products were visualized by 2% low-melting-point agarose (NuSieve; FMC) gel electrophoresis and ethidium bromide staining.
Quantitation of cytokines by ELISA.
Cytokines in the supernatant were quantitated with a capture antibody specific for a particular cytokine (Pharmingen, San Diego, Calif.) and a biotinylated detection antibody specific for that particular cytokine (Pharmingen). Quantitation of cytokines was done by following the directions provided by the manufacturers. Briefly, 96-well plates (Nunc, Inc.; Maxisorp) were incubated with capture antibody at 4°C overnight. The plates were then blocked with bovine serum albumin and incubated with samples and standard. Bound cytokine was detected with a biotinylated detecting antibody and peroxidase-labeled avidin (Kirkegaard & Perry Laboratories, Gaithersburg, Md.).
Preparation of nuclear proteins.
Nuclear proteins were prepared by the method of Schreiber et al. (35). Typically, 5 × 106 TEP cells were washed with 10 ml of TBS and pelleted. The pellet was resuspended in 1 ml of TBS and pelleted again by being spun for 15 s in a microcentrifuge. TBS was removed, and the cell pellet was resuspended in 0.8 ml of cold buffer A (10 mM HEPES [pH 7.9], 10 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA, 1 mM dithiothreitol [DTT], 0.5 mM phenylmethylsulfonyl fluoride, 10 mM leupeptin, and 1.5 mM pepstatin). The cells were incubated on ice for 15 min, after which 50 μl of a 10% solution of Nonidet P-40 was added and the tube was vigorously vortexed for 10 s. The homogenate was centrifuged for 30 s in a microcentrifuge, and the supernatant was removed. The nuclear pellet was resuspended in 100 μl of ice-cold buffer C (20 mM HEPES [pH 7.9], 0.4 M NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM DTT, 1 mM phenylmethylsulfonyl fluoride, 10 mM leupeptin, and 1.5 mM pepstatin), and the tube was vigorously rocked at 4°C for 15 min on a shaking platform. The nuclear extract was centrifuged for 5 min in a microcentrifuge at 4°C. The supernatant was frozen in aliquots at −70°C.
Electrophoretic mobility shift assay (EMSA).
EMSA was performed as described by Vincenti et al. (46). A double-stranded oligonucleotide containing a mouse TNF-α enhancer located 510 bp from the start of transcription to the TNF-α gene was used for the binding assay. The sequence of the oligonucleotide including the NF-κB binding site is 5′-CAA-ACA-GGG-GGC-TTT-CCC-TCC-TC-3′ and 3′-GTT-TGT-CCC-CCG-AAA-GGG-AGG-AG-5′. The sequence of the oligonucleotide with the AP-1 binding site is 5′-CGC-TTG-ATG-ACT-CAG-CCG-GAA-3′ and 3′-GCG-AAC-TAC-TGA-GTC-GGC-CTT-5′. For binding reactions, 32P-labeled oligonucleotide fragments (100,000 cpm) were mixed with 2 μg of nuclear protein in a total volume of 20 μl of 25 mM HEPES (pH 7.9)–0.5 mM EDTA–0.5 mM DTT–0.1 M NaCl–10% glycerol–1 μg of bovine serum albumin–2 μg of poly(dI:dC). After 30 min of incubation at room temperature, the reaction mixtures were loaded onto 6% polyacrylamide gels in 0.5× Tris-borate-EDTA. The gels were prerun for 1 h at 150 V and run for 2.5 h at the same voltage. After electrophoresis, the gels were dried and exposed for autoradiography.
RESULTS
Marked induction of TNF-α in TEP macrophages by M. penetrans LAMPs and lipid extract of PK-digested LAMPs.
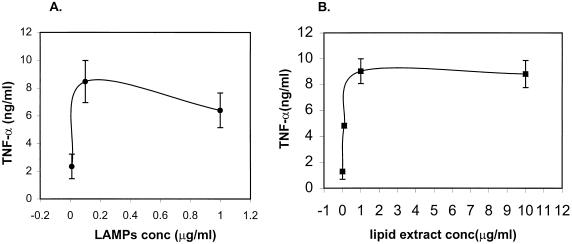
Consistent with many earlier studies of mycoplasmal membranes (9, 15, 29, 38), preparation of LAMPs from M. penetrans and M. fermentans (incognitus) stimulated murine and human macrophages to produce large amounts of proinflammatory cytokines including TNF-α, IL-6, and IL-1β (Table 1). In order to unravel the cellular mechanisms involved in the mycoplasmal activation of cytokines, we concentrated on studying the TNF-α gene expression in murine TEP macrophages induced by M. penetrans LAMPs and their products. A dose-response curve revealed an optimal concentration of LAMPs at 0.1 μg of protein/ml in the induction of TNF-α (Fig. 1A). In an attempt to determine the biochemical nature of the active component in LAMPs, we treated LAMPs with PK without losing the stimulating activity for TNF-α production in macrophages. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis of PK-digested LAMPs verified the complete protein digestion of LAMPs. Lipophilic molecules including lipids, glycolipids (27), and lipopeptides derived from PK-digested LAMPs were extracted with chloroform-methanol. This extract (designated M. penetrans lipid extract of PK-digested LAMPs in this study) was found to be a potent inducer of TNF-α (Fig. 1B). A concentration at 1 μg of lipid (dry weight)/ml would reach the plateau of the stimulatory effect. In an effort to further study the chemical nature of the active component in this lipid extract of PK-digested LAMPs, the lipid extract was fractionated by adding chloroform and water into a chloroform phase (containing mostly hydrophobic nonpolar lipids) and an aqueous phase (containing the more hydrophilic glycolipids). Interestingly, the macrophage-stimulating activity resided mainly in the chloroform phase. The aqueous phase, which normally contains glycolipids, had very little macrophage-stimulating activity. The nonpolar lipid fraction and the M. penetrans lipid extract of PK-digested LAMPs had the same potency in stimulating macrophages. However, detergent was required to dissolve the nonpolar lipids but PBS would readily dissolve the lipid extract of PK-digested LAMPs. For the sake of easy handling, we chose to use the lipid extract of PK-digested LAMPs instead of the nonpolar lipid fraction in this study.
TABLE 1.
Cytokine production by human and murine macrophagesa
| Treatment | IL-1β (ng/ml) in:
|
IL-6 (ng/ml) in supernatant
|
TNF-α (ng/ml) in supernatant
|
|||||
|---|---|---|---|---|---|---|---|---|
| Supernatant
|
Cell lysate
|
|||||||
| Human | Mouse | Human | Mouse | Human | Mouse | Human | Mouse | |
| PBS | 0.1 ± 0.2 | 0 ± 0 | 2.3 ± 2.4 | 0.2 ± 0.1 | 2.2 ± 3.5 | 0 ± 0 | 0.2 ± 0.3 | 0 ± 0 |
| LPS | 12.3 ± 10.4 | 0 ± 0 | 44.0 ± 36.7 | 1.5 ± 0.8 | 70.3 ± 48.6 | 9.5 ± 1.5 | 7.2 ± 6.4 | 7.1 ± 1.1 |
| M. penetrans LAMP | 2.2 ± 1.9 | 0 ± 0 | 51.3 ± 42.8 | 4.9 ± 1.0 | 43.2 ± 28.4 | 5.3 ± 0.8 | 3.5 ± 4.3 | 3.1 ± 0.3 |
| M. fermentans LAMP | 3.0 ± 1.7 | 0 ± 0 | 65.3 ± 61.2 | 7.2 ± 0.4 | 48.3 ± 29.6 | 5.7 ± 0.5 | 3.0 ± 2.0 | 10.6 ± 4.3 |
Macrophages were treated with 1 μg of M. penetrans or M. fermentans (incognitus strain) LAMP per ml. Control cells were treated either with PBS or with 1 μg of lipopolysaccharide (LPS) per ml. Supernatants or cell lysate (for IL-1β only) was prepared 18 h after treatment for cytokine measurement by ELISA. Values represent means ± standard deviations of three separate experiments for mice or five individuals for humans.
FIG. 1.
TNF-α production in M. penetrans LAMP (A)- or M. penetrans lipid extract of PK-digested LAMP (B)-stimulated TEP macrophages. TEP macrophages (106 cells/ml) were cultured in the presence of a 10-fold serial dilution of M. penetrans LAMPs (A) ranging from 0.01 to 1 μg/ml in protein concentration or the lipid extract of PK-digested LAMPs (B) ranging from 0.01 to 10 μg of lipid (dry weight) per ml. TNF-α production was assayed in supernatants harvested after an 18-h stimulation. Results represent means ± standard deviations of three experiments.
Kinetics of in vivo induction of TNF-α transcription.
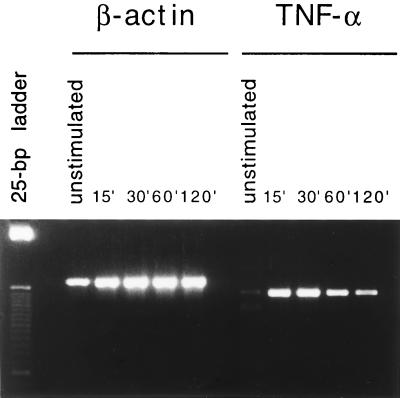
We then examined the effect of M. penetrans lipid extract of PK-digested LAMPs in inducing NF-κB and AP-1 binding activity in correlation with TNF-α expression in an in vivo study. M. penetrans lipid extract of PK-digested LAMPs induced TNF-α expression in TEP cells when injected into the peritoneal cavity of mice bearing a peritoneal exudate (Fig. 2). RT-PCR analysis showed a low basal level of mRNA in the resting TEP cells, which consisted mostly of macrophages. M. penetrans lipid extract of PK-digested LAMPs induced a marked increase in TNF-α transcription as early as 15 min after injection, and this increase persisted for at least 2 h in the treated TEP cells.
FIG. 2.
Kinetics of TNF-α mRNA production in M. penetrans lipid extract of PK-digested LAMP-stimulated TEP cells. Total RNA was prepared from untreated TEP cells or TEP cells isolated 15, 30, 60, or 120 min after i.p. injection of 100 μg of M. penetrans lipid extract of PK-digested LAMPs. Detection of TNF-α mRNA was done by RT-PCR with primers specific for TNF-α. PCR performed with primers specific for β-actin was done in parallel.
M. penetrans lipid extract of PK-digested LAMPs induced both NF-κB and AP-1 binding activities in nuclear extracts of TEP cells.
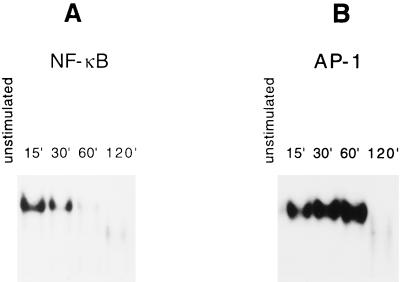
EMSA showed a lack of NF-κB binding activity in nuclear extracts of resting TEP cells. M. penetrans lipid extract of PK-digested LAMPs induced significant NF-κB binding activity in the nuclear extracts of TEP cells as early as 15 min after injection (Fig. 3A). This binding activity appeared to diminish after 30 min. After 2 h, the NF-κB binding activity was completely dissipated and converted to a faster-moving complex that is most likely the p50-p50 homodimer. The kinetics of AP-1 induction was slightly different from that of NF-κB in that a basal level of AP-1 binding activity was present in resting TEP cells (Fig. 3B). However, M. penetrans lipid extract of PK-digested LAMPs induced a significant increase in AP-1 binding activity 15 min after injection. The activity continued to increase and peaked 1 h after injection but dissipated after 2 h. Therefore, closely correlated with induction of TNF-α, M. penetrans lipid extract of PK-digested LAMPs rapidly activated both NF-κB and AP-1 in TEP macrophages. Activation of these nuclear factors subsided after 2 h.
FIG. 3.
Kinetics of NF-κB (A) and AP-1 (B) binding activities in M. penetrans lipid extract of PK-digested LAMP-stimulated TEP cells. Binding activities were monitored by EMSA. Nuclear extracts were prepared from unstimulated TEP cells or TEP cells isolated 15 m, 30 m, 60 m, or 120 min after i.p. injection of 100 μg of M. penetrans lipid extract of PK-digested LAMPs. Each extract (2 μg) was incubated with 32P-labeled oligonucleotide with binding sites for either NF-κB (A) or AP-1 (B). The protein-bound oligonucleotides were separated from free oligonucleotides on native 6% polyacrylamide gels.
Suppression of M. penetrans lipid extract of PK-digested LAMP-induced TNF-α by dexamethasone was linked to suppression of NF-κB and AP-1.
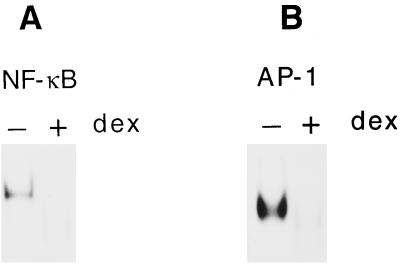
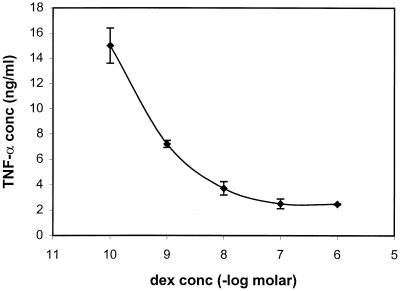
In order to further investigate the link among NF-κB, AP-1 activation, and TNF-α induction, we pretreated TEP cells with dexamethasone (a synthetic glucocorticoid) before stimulation with M. penetrans lipid extract of PK-digested LAMPs. Earlier studies suggested that glucocorticoids inhibit activation of NF-κB and AP-1 through direct interactions between glucocorticoid receptor and AP-1 (14, 36, 48) and between glucocorticoid receptor and NF-κB (30, 34) and through induction of IκBα, an inhibitor of NF-κB (2, 33). Pretreatment with dexamethasone suppressed activation of both NF-κB and AP-1 in M. penetrans lipid extract of PK-digested LAMP-stimulated TEP cells (Fig. 4). Pretreatment with dexamethasone, in turn, suppressed TNF-α induction by the lipid extract of PK-digested LAMP-stimulated TEP cells in a dose-dependent manner (Fig. 5). Maximum suppression was achieved at 10−7 M dexamethasone where a residual amount of TNF-α still remained. Higher concentrations of dexamethasone did not completely inhibit TNF-α production.
FIG. 4.
Suppression of NF-κB (A) and AP-1 (B) binding activities by dexamethasone in M. penetrans lipid extract of PK-digested LAMP-stimulated TEP cells. Mice were injected i.p. with dexamethasone or PBS as control as described in Materials and Methods followed by M. penetrans lipid extract of PK-digested LAMPs after 2 h. Nuclear extracts were prepared from TEP cells 20 min after stimulation. The NF-κB (A) and AP-1 (B) binding activity was monitored by EMSA.
FIG. 5.
Suppression of TNF-α production by dexamethasone in M. penetrans lipid extract of PK-digested LAMP-stimulated TEP macrophages. TEP macrophages (106 cells/ml) were cultured with dexamethasone at a 10-fold dilution starting from 10−6 M. After 2 h, M. penetrans lipid extract of PK-digested LAMPs (5 μg/ml) was added to the cells. Supernatants were collected after 18 h and assayed for TNF-α by ELISA. As a positive control, TNF-α produced by M. penetrans lipid extract of PK-digested LAMP-stimulated TEP macrophages was 17.4 ± 4.2 ng/ml. TNF-α produced by unstimulated TEP macrophages was undetectable.
DISCUSSION
LAMPs prepared from M. penetrans and M. fermentans are potent murine B-cell mitogens (8). It was subsequently found that LAMPs could induce mouse and human macrophages to produce large amounts of IL-1β, IL-6, and TNF-α. To explore the upstream mechanism(s) through which mycoplasmal LAMPs induce high-level production of various cytokines, we used M. penetrans LAMPs or the lipid extract of PK-digested LAMPs to induce TNF-α production in mouse TEP macrophages. Treatment of the LAMPs with PK apparently would not abolish their TNF-α induction activity in macrophages. The chloroform-methanol extract of PK-digested LAMPs retained most of the activity, suggesting the lipid nature of the active component. In fact, our lipid extract of PK-digested LAMPs could be further fractionated into glycolipids and nonpolar lipids. The nonpolar lipid fraction contained most of the macrophage-stimulating activity. The chemical structure of the active component(s) in this M. penetrans lipid extract has not been fully characterized; however, it is likely to be the product of PK-digested LAMPs. Its lipid nature is very much consistent with the earlier finding of a lipopeptide derived from PK-treated M. fermentans-derived high-molecular-weight material by Mühlradt et al. (22). The lipopeptide with the structure of S-(2,3-dihydroxypropyl)-cysteine amino terminus constitutes the principal macrophage-stimulating activity (22, 24). The presence of both ester-bound fatty acids is a prerequisite for biological activity, whereas the amide-bound fatty acid was found to be dispensable (24). This structure is also present in Mycoplasma hyorhinis and may be a general characteristic of the genus Mycoplasma (25).
We demonstrated in this study that M. penetrans lipid extract of PK-digested LAMPs almost instantly activated both NF-κB and AP-1 in murine TEP macrophages. To the best of our knowledge, this is the first time that wall-free mycoplasmas have been found to be potent inducers of NF-κB and AP-1. Activation of these transcriptional factors appeared to markedly enhance expression of the TNF-α gene with a rapid increase of TNF-α mRNA. Interference with the binding activity of these transcriptional factors on the promoter sequences by treatment with dexamethasone significantly blocked production of TNF-α in TEP macrophages. Dexamethasone, however, did not completely abolish the production of TNF-α. Thus, activation of other families of transcriptional factors besides NF-κB and AP-1 might also play a role in the mycoplasmal induction of TNF-α. Since NF-κB and AP-1 binding sites are present in the promoter regions of several cytokine and chemokine genes, activation of both NF-κB and AP-1 may similarly upregulate production of many other cytokine genes in the mycoplasmal LAMP-stimulated macrophages.
If M. penetrans lipid extract of PK-digested LAMPs can rapidly activate NF-κB and AP-1 in mammalian cells, do other species of mycoplasmas also harbor a similar active component(s) in their membranes? We are in the progress of examining many species of human mycoplasmas for their ability to induce these transcriptional factors in mammalian cells. The preliminary results suggest that most, but not all, species of human mycoplasmas have the ability to activate these transcriptional factors and induce production of various cytokines in TEP macrophages. However, different species of mycoplasmas apparently have different degrees of this biological activity. At present, it is not clear if the differences are due to the distinct chemical nature or to the quantities of these active components present in the membranes of these mycoplasmas.
Mycoplasmas have long been implicated in certain inflammatory conditions and/or diseases such as rheumatoid arthritis (13, 32, 43). Since NF-κB regulates the expression of various genes involved in immune functions and inflammatory responses, it has a key role in the host’s responses to infections. The findings of this study may provide a scientific basis for the possible mycoplasmal role in human diseases associated with known aberrant immune responses. Since M. penetrans is one of the most common AIDS-associated mycoplasmas (19), it may be important to note that NF-κB regulates transcription from the human immunodeficiency virus type 1 (HIV-1) long terminal repeat (26). Induction of NF-κB DNA binding activity in T cells and monocytes leads to increased HIV-1 long terminal repeat-directed gene expression (11). Studies showed that HIV-1 cannot productively infect human peripheral blood lymphocytes without prior activation by mitogens in culture (31, 41). However, the non-mitogen-treated peripheral blood lymphocytes become highly susceptible to HIV-1 infection when they are infected by mycoplasmas (31). Infection by mycoplasmas evidently activates many T lymphocytes and renders them susceptible to HIV-1 infection. Furthermore, mycoplasmal infections appear to enhance the HIV cytocidal effect in a culture of human lymphocytes (16, 17). Although the role of mycoplasmas in AIDS is still unclear, L. Montagnier’s laboratory at the Pasteur Institute presented evidence suggesting that infection by M. penetrans infection is associated with disease progression in AIDS (10).
It is also worth noting that activation of NF-κB in cells affects expression of many downstream genes that regulate crucial cell properties, including those that suppress apoptosis (3, 45, 47). 12-O-Tetradecanoylphorbol-13-acetate, a highly potent tumor promoter, and other activators of protein kinase C, can effectively induce cell proliferation and rapidly induce c-jun and c-fos (1). The accumulation of the newly transcribed c-jun and c-fos gene products leads to an increase in AP-1 activity in cells (5). Thus, finding that mycoplasmas or mycoplasmal membrane components are potent activators of NF-κB and AP-1 in mammalian cells could have profound implications other than the induction of TNF-α production. In this context, our laboratory has shown that prolonged infection by mycoplasmas may gradually alter many important biological characteristics of mammalian cells and induce malignant transformation (44). High-level expression of H-ras and c-myc oncogenes as well as c-jun and c-fos is associated with the malignant transforming process (50). Activation of transcriptional factors NF-κB and AP-1 in cells could apparently activate a large group of gene products. Many of these gene products would function cooperatively at various cell cycle checkpoints to suppress cell apoptosis. The process of cell transformation is complex and often requires more time. However, it is only those cells that are not undergoing apoptosis or that have not had their death program initiated that could progress to malignant transformation. Overall, our earlier findings and the current data warrant further studies to elucidate the mycoplasma-mediated processes that could activate a series of genes with powerful oncogenetic activities in the mammalian cells.
ACKNOWLEDGMENTS
This study was supported in part by the American Registry of Pathology.
We thank Douglas J. Wear for his critical review of the manuscript. We are grateful to Jose Rodriguez for his assistance in photography and Susan Ditty for her help in preparing the manuscript.
ADDENDUM IN PROOF
After the paper was accepted for publication, we noticed two reports of similar findings in studies of a different human mycoplasma (J. Garcia, B. Lemercier, S. Roman-Roman, and G. Rawadi, J. Biol. Chem 273:34391–34398, 1998, and G. Rawadi, J. Garcia, B. Lemercier, and S. Roman-Roman, J. Immunol. 162:2193–2203, 1999). These studies show that a synthetic lipopeptide and the membrane lipoproteins derived from Mycoplasma fermentans activate NF-κB and AP-1 in a murine macrophage cell line RAW 264.7.
REFERENCES
- 1.Angel P, Karin M. The role of Jun, Fos and the AP-1 complex in cell-proliferation and transformation. Biochim Biophys Acta. 1991;1072:129–157. doi: 10.1016/0304-419x(91)90011-9. [DOI] [PubMed] [Google Scholar]
- 2.Auphan N, DiDonato J A, Rosette C, Helmberg A, Karin M. Immunosuppression by glucocorticoids: inhibition of NF-kappa B activity through induction of I kappa B synthesis. Science. 1995;270:286–290. doi: 10.1126/science.270.5234.286. [DOI] [PubMed] [Google Scholar]
- 3.Beg A A, Baltimore D. An essential role for NF-kappaB in preventing TNF-alpha-induced cell death. Science. 1996;274:782–784. doi: 10.1126/science.274.5288.782. [DOI] [PubMed] [Google Scholar]
- 4.Collart M A, Baeuerle P, Vassalli P. Regulation of tumor necrosis factor alpha transcription in macrophages: involvement of four κB-like motifs and of constitutive and inducible forms of NF-κB. Mol Cell Biol. 1990;10:1498–1506. doi: 10.1128/mcb.10.4.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Curran T, Franza B R., Jr Fos and Jun: the AP-1 connection. Cell. 1988;55:395–397. doi: 10.1016/0092-8674(88)90024-4. [DOI] [PubMed] [Google Scholar]
- 6.Dietz J N, Cole B C. Direct activation of the J774.1 murine macrophage cell line by Mycoplasma arthritidis. Infect Immun. 1982;37:811–819. doi: 10.1128/iai.37.2.811-819.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ehlers S, Mielke M E, Blankenstein T, Hahn H. Kinetic analysis of cytokine gene expression in the livers of naive and immune mice infected with Listeria monocytogenes. The immediate early phase in innate resistance and acquired immunity. J Immunol. 1992;149:3016–3022. [PubMed] [Google Scholar]
- 8.Feng S-H, Lo S-C. Induced mouse spleen B-cell proliferation and secretion of immunoglobulin by lipid-associated membrane proteins of Mycoplasma fermentans incognitus and Mycoplasma penetrans. Infect Immun. 1994;62:3916–3921. doi: 10.1128/iai.62.9.3916-3921.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8a.Feng S-H, Lo S-C. Abstracts of the 98th General Meeting of the American Society for Microbiology 1998. Washington, D.C: American Society for Microbiology; 1998. Activation of nuclear factor-kappa B and activator protein-1 by Mycoplasma penetrans lipid extract isolated from lipid-associated membrane proteins, abstr. G-14; p. 275. [Google Scholar]
- 9.Gallily R, Salman M, Tarshis M, Rottem S. Mycoplasma fermentans (incognitus strain) induces TNF-α and IL-1 production by human monocytes and murine macrophages. Immunol Lett. 1992;34:27–30. doi: 10.1016/0165-2478(92)90023-h. [DOI] [PubMed] [Google Scholar]
- 10.Grau O, Tuppin P, Slizewicz B, Launay V, Goujard C, Bahraoui E, Delfraissy J F, Montagnier L. A longitudinal study of seroreactivity against Mycoplasma penetrans in HIV-infected homosexual men: association with disease progression. AIDS Res Hum Retroviruses. 1998;14:661–667. doi: 10.1089/aid.1998.14.661. [DOI] [PubMed] [Google Scholar]
- 11.Grilli M, Chiu J J, Lenardo M J. NF-kappa B and Rel: participants in a multiform transcriptional regulatory system. Int Rev Cytol. 1993;143:1–62. doi: 10.1016/s0074-7696(08)61873-2. [DOI] [PubMed] [Google Scholar]
- 12.Herbelin A, Ruuth E, Delorme D, Michel-Herbelin C, Praz F. Mycoplasma arginini TUH-14 membrane lipoproteins induce production of interleukin-1, interleukin-6, and tumor necrosis factor alpha by human monocytes. Infect Immun. 1994;62:4690–4694. doi: 10.1128/iai.62.10.4690-4694.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoffman R W, O’Sullivan F X, Schafermeyer K R, Moore T L, Roussell D, Watson-McKown R, Kim M F, Wise K S. Mycoplasma infection and rheumatoid arthritis: analysis of their relationship using immunoblotting and an ultrasensitive polymerase chain reaction detection method. Arthritis Rheum. 1997;40:1219–1228. doi: 10.1002/1529-0131(199707)40:7<1219::AID-ART5>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 14.Jonat C, Rahmsdorf H J, Park K K, Cato A C, Gebel S, Ponta H, Herrlich P. Antitumor promotion and antiinflammation: down-modulation of AP-1 (Fos/Jun) activity by glucocorticoid hormone. Cell. 1990;62:1189–1204. doi: 10.1016/0092-8674(90)90395-u. [DOI] [PubMed] [Google Scholar]
- 15.Kita M, Ohmoto Y, Hirai Y, Yamaguchi N, Imanishi J. Induction of cytokines in human peripheral blood mononuclear cells by mycoplasmas. Microbiol Immunol. 1992;36:507–516. doi: 10.1111/j.1348-0421.1992.tb02048.x. [DOI] [PubMed] [Google Scholar]
- 16.Lemaitre M, Henin Y, Destouesse F, Ferrieux C, Montagnier L, Blanchard A. Role of mycoplasma infection in the cytopathic effect induced by human immunodeficiency virus type 1 in infected cell lines. Infect Immun. 1992;60:742–748. doi: 10.1128/iai.60.3.742-748.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lo S C, Tsai S, Benish J R, Shih J W, Wear D J, Wong D M. Enhancement of HIV-1 cytocidal effects in CD4+ lymphocytes by the AIDS-associated mycoplasma. Science. 1991;251:1074–1076. doi: 10.1126/science.1705362. [DOI] [PubMed] [Google Scholar]
- 18.Lo S C, Dawson M S, Wong D M, Newton III P B, Sonoda M A, Engler W F, Wang R Y, Shih J W, Alter H J, Wear D J. Identification of Mycoplasma incognitus infection in patients with AIDS: an immunohistochemical, in situ hybridization and ultrastructural study. Am J Trop Med Hyg. 1989;41:601–616. doi: 10.4269/ajtmh.1989.41.601. [DOI] [PubMed] [Google Scholar]
- 19.Lo S C, Hayes M M, Wang R Y, Pierce P F, Kotani H, Shih J W. Newly discovered mycoplasma isolated from patients infected with HIV. Lancet. 1991;338:1415–1418. doi: 10.1016/0140-6736(91)92721-d. [DOI] [PubMed] [Google Scholar]
- 20.Loo V G, Richardson S, Quinn P. Isolation of Mycoplasma pneumoniae from pleural fluid. Diagn Microbiol Infect Dis. 1991;14:443–445. doi: 10.1016/0732-8893(91)90071-m. [DOI] [PubMed] [Google Scholar]
- 21.Mühlradt P F, Frisch M. Purification and partial biochemical characterization of a Mycoplasma fermentans-derived substance that activates macrophages to release nitric oxide, tumor necrosis factor, and interleukin-6. Infect Immun. 1994;62:3801–3807. doi: 10.1128/iai.62.9.3801-3807.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mühlradt P F, Meyer H, Jansen R. Identification of S-(2,3-dihydroxypropyl)cystein in a macrophage-activating lipopeptide from Mycoplasma fermentans. Biochemistry. 1996;35:7781–7786. doi: 10.1021/bi9602831. [DOI] [PubMed] [Google Scholar]
- 23.Mühlradt P F, Schade U. MDHM, a macrophage-stimulatory product of Mycoplasma fermentans, leads to in vitro interleukin-1 (IL-1), IL-6, tumor necrosis factor, and prostaglandin production and is pyrogenic in rabbits. Infect Immun. 1991;59:3969–3974. doi: 10.1128/iai.59.11.3969-3974.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mühlradt P F, Kiess M, Meyer H, Süssmuth R, Jung F. Isolation, structure elucidation, and synthesis of a macrophage stimulatory lipopeptide from Mycoplasma fermentans acting at picomolar concentration. J Exp Med. 1997;185:1951–1958. doi: 10.1084/jem.185.11.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mühlradt P F, Kiess M, Meyer H, Süssmuth R, Jung G. Structure and specific activity of macrophage-stimulating lipopeptides from Mycoplasma hyorhinis. Infect Immun. 1998;66:4804–4810. doi: 10.1128/iai.66.10.4804-4810.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nabel G, Baltimore D. An inducible transcription factor activates expression of human immunodeficiency virus in T cells. Nature. 1987;326:711–713. doi: 10.1038/326711a0. . (Erratum, 344:178, 1990.) [DOI] [PubMed] [Google Scholar]
- 27.Neyrolles O, Brenner C, Prevost M C, Fontaine T, Montagnier L, Blanchard A. Identification of two glycosylated components of Mycoplasma penetrans: a surface-exposed capsular polysaccharide and a glycolipid fraction. Microbiology. 1998;144:1247–1255. doi: 10.1099/00221287-144-5-1247. [DOI] [PubMed] [Google Scholar]
- 28.Quentmeier H, Schmitt E, Kerchhoff H, Grote W, Mühlradt P F. Mycoplasma fermentans-derived high-molecular-weight material induces interleukin-6 release in cultures of murine macrophages and human monocytes. Infect Immun. 1990;58:1273–1280. doi: 10.1128/iai.58.5.1273-1280.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rawadi G, Roman-Roman S. Mycoplasma membrane lipoproteins induce proinflammatory cytokines by a mechanism distinct from that of lipopolysaccharide. Infect Immun. 1996;64:637–643. doi: 10.1128/iai.64.2.637-643.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ray A, Prefontaine K E. Physical association and functional antagonism between the p65 subunit of transcription factor NF-kappa B and the glucocorticoid receptor. Proc Natl Acad Sci USA. 1994;91:752–756. doi: 10.1073/pnas.91.2.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sasaki Y, Honda M, Makino M, Sasaki T. Mycoplasmas stimulate replication of human immunodeficiency virus type 1 through selective activation of CD4+ T lymphocytes. AIDS Res Hum Retroviruses. 1993;9:775–780. doi: 10.1089/aid.1993.9.775. [DOI] [PubMed] [Google Scholar]
- 32.Schaeverbeke T, Vernhes J P, Lequen L, Bannwarth B, Bebear C, Dehais J. Mycoplasmas and arthritides. Rev Rhum Engl Ed. 1997;64:120–128. [PubMed] [Google Scholar]
- 33.Scheinman R I, Cogswell P C, Lofquist A K, Baldwin A S., Jr Role of transcriptional activation of I kappa B alpha in mediation of immunosuppression by glucocorticoids. Science. 1995;270:283–286. doi: 10.1126/science.270.5234.283. [DOI] [PubMed] [Google Scholar]
- 34.Scheinman R I, Gualberto A, Jewell C M, Cidlowski J A, Baldwin A S., Jr Characterization of mechanisms involved in transrepression of NF-κB by activated glucocorticoid receptors. Mol Cell Biol. 1995;15:943–953. doi: 10.1128/mcb.15.2.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schreiber E, Matthias P, Muller M M, Schaffner W. Rapid detection of octamer binding proteins with ’mini-extracts’, prepared from a small number of cells. Nucleic Acids Res. 1989;17:6419. doi: 10.1093/nar/17.15.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schule R, Rangarajan P, Kliewer S, Ransone L J, Bolado J, Yang N, Verma I M, Evans R M. Functional antagonism between oncoprotein c-Jun and the glucocorticoid receptor. Cell. 1990;62:1217–1226. doi: 10.1016/0092-8674(90)90397-w. [DOI] [PubMed] [Google Scholar]
- 37.Shakhov A N, Collart M A, Vassalli P, Nedospasov S A, Jongeneel C V. Kappa B-type enhancers are involved in lipopolysaccharide-mediated transcriptional activation of the tumor necrosis factor alpha gene in primary macrophages. J Exp Med. 1990;171:35–47. doi: 10.1084/jem.171.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sher T, Yamin A, Rottem S, Gallily R. In vitro induction of tumor necrosis factor α, tumor cytolysis and blast transformation by Spiroplasma membranes. J Natl Cancer Inst. 1990;82:1142–1145. doi: 10.1093/jnci/82.13.1142. [DOI] [PubMed] [Google Scholar]
- 39.Simecka J W, Davis J K, Davidson M K, Ross S E, Stadtlander C T K-H, Cassell G H. Mycoplasma diseases of animals. In: Basement J B, Finch L R, Maniloff J, McElhaney R N, editors. Mycoplasmas: molecular biology and pathogenesis. Washington, D.C: American Society for Microbiology; 1992. pp. 391–416. [Google Scholar]
- 40.Stuart P M, Cassell G H, Woodward J G. Differential induction of bone marrow macrophage proliferation by mycoplasmas involves granulocyte-macrophage colony-stimulating factor. Infect Immun. 1990;58:3558–3563. doi: 10.1128/iai.58.11.3558-3563.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tang S, Patterson B, Levy J A. Highly purified quiescent human peripheral blood CD4+ T cells are infectible by human immunodeficiency virus but do not release virus after activation. J Virol. 1995;69:5659–5665. doi: 10.1128/jvi.69.9.5659-5665.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taylor-Robinson D. Genital mycoplasma infections. Clin Lab Med. 1989;9:501–523. [PubMed] [Google Scholar]
- 43.Taylor-Robinson D, Schaeverbeke T. Mycoplasmas in rheumatoid arthritis and other human arthritides. J Clin Pathol. 1996;49:781–782. doi: 10.1136/jcp.49.10.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tsai S, Wear D J, Shih J W, Lo S C. Mycoplasmas and oncogenesis: persistent infection and multistage malignant transformation. Proc Natl Acad Sci USA. 1995;92:10197–10201. doi: 10.1073/pnas.92.22.10197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van Antwerp D J, Martin S J, Kafri T, Green D R, Verma I M. Suppression of TNF-alpha-induced apoptosis by NF-kappaB. Science. 1996;274:787–789. doi: 10.1126/science.274.5288.787. [DOI] [PubMed] [Google Scholar]
- 46.Vincenti M P, Burrell T A, Taffet S M. Regulation of NF-kappa B activity in murine macrophages: effect of bacterial lipopolysaccharide and phorbol ester. J Cell Physiol. 1992;150:204–213. doi: 10.1002/jcp.1041500127. [DOI] [PubMed] [Google Scholar]
- 47.Wang C Y, Mayo M W, Korneluk R G, Goeddel D V, Baldwin A S., Jr NF-kappaB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science. 1998;281:1680–1683. doi: 10.1126/science.281.5383.1680. [DOI] [PubMed] [Google Scholar]
- 48.Yang-Yen H F, Chambard J C, Sun Y L, Smeal T, Schmidt T J, Drouin J, Karin M. Transcriptional interference between c-Jun and the glucocorticoid receptor: mutual inhibition of DNA binding due to direct protein-protein interaction. Cell. 1990;62:1205–1215. doi: 10.1016/0092-8674(90)90396-v. [DOI] [PubMed] [Google Scholar]
- 49.Yao J, Mackman N, Edgington T S, Fan S T. Lipopolysaccharide induction of the tumor necrosis factor-alpha promoter in human monocytic cells. Regulation by Egr-1, c-Jun, and NF-kappaB transcription factors. J Biol Chem. 1997;272:17795–17801. doi: 10.1074/jbc.272.28.17795. [DOI] [PubMed] [Google Scholar]
- 50.Zhang B, Shih J W, Wear D J, Tsai S, Lo S C. High-level expression of H-ras and c-myc oncogenes in mycoplasma-mediated malignant cell transformation. Proc Soc Exp Biol Med. 1997;214:359–366. doi: 10.3181/00379727-214-44104. [DOI] [PubMed] [Google Scholar]