Abstract
Terahertz (THz) waves can cover the characteristic spectra of substances such as plasma, organisms, and biomolecules, whereas THz photons have low energy and do not damage biological tissues. Therefore, its absorption characteristics in the THz region can be used to characterize the internal structure of biomolecules. In this study, we designed a microfluidic chip and combined it with THz technology. The spectral intensity in descending order was found to be deionized water, phenylalanine, histidine, glycine and glutamic acid by observing the THz wave transmission in the range of 0.1–1.0 THz, comparing the frequency domain spectra of four amino acid solutions with volume fraction of 2% and deionized water. It is inferred that different molecular structures of amino acids resulted in different numbers of hydrogen bonds formed between them and water molecules, leading to different degrees of absorption of THz waves. In addition, magnetic fields parallel to the THz wave transmission were used to study the variation of different amino acids with magnetic field intensity. It is found that increasing the magnetic field strength decrease the transmission of THz waves. This is because under the action of the magnetic field, on the one hand, the hydrogen bonds formed by water molecules are strengthened and the absorption of THz waves is enhanced; on the other hand, amino acid molecules aggregate and the radius of molecular clusters increases, thus blocking the transmission of THz waves. Finally, we also calculated the electric conductivity of the solutions to prove the accuracy of the experimental results from a theoretical point of view.
Keywords: Terahertz, Microfluidic chip, Magnetic field, Amino acids, Transmission
Terahertz; Microfluidic chip; Magnetic field; Amino acids; Transmission.
1. Introduction
Terahertz (THz) waves are electromagnetic radiation with frequencies range 0.1–10 THz and wavelengths range 30–3000 μm. The THz waveband is between microwave and infrared and has unique properties compared to other bands of waves [1], such as the ability to identify substances. THz spectroscopy has the advantages of being non-destructive, short acquisition time, and simple sample preparation [2]. Because of its good detectability and non-ionizing nature [3], it is useful in the study of structural properties and dynamics of biomolecules. Protein is a biological macromolecule with amino acids as its basic unit, which is the material basis of life. THz work on proteins dates back to 2000, THz spectroscopy was first used to detect resonance patterns of biomolecules in a report by Markelz group [4]. They determined that lyophilized powdered calf thymus DNA samples, bovine serum proteins and collagen all had distinctive characteristic spectra in the 0.06–2.00 THz range. Subsequently, THz-time domain spectroscopy (THz-TDS) studies on amino acids and proteins have been increasingly reported during these 20 years. In 2003, Kutteruf et al. measured the THz absorption properties of amino acids, revealing a dense set of absorption spectra for 20 natural amino acids in the range of 1–15 THz [5]. Subsequently, more and more scholars began to use THz bands for qualitative and quantitative analysis of substances. Zhang H [6] et al. investigated the relationship between the THz dielectric spectra of two amino acids and the volumetric content of the components. In addition, Zang Z [7] et al. used THz-TDS system to measure peptide solutions at different temperatures and pH values and found that significant changes in temperature or pH level had a large effect on the THz absorption coefficient, refractive index, and dielectric constant.
Most biomolecules can only maintain biological activity in a liquid environment [8],so detecting liquid biomolecules is critical. Studies have shown that the use of derivatization methods allows real-time monitoring of changes in the plasma amino acid profile of patients with malignant hematological diseases. It is important for increasing patient transplantation success rates and improving the quality of survival of patients after transplantation [9]. Aromatic amino acids and their metabolites are related to liver, kidney, immune, neuropsychiatric, and cancer diseases. Therefore, detection of amino acid changes in clinical diagnosis is very important and has great potential.
Water is the source of all life and contains a large number of hydrogen bonds. The low-frequency vibrations of hydrogen bonds, including large-amplitude motions of stretching and bending along the actual hydrogen bond coordinates, have been shown to fall mainly in the THz or far-infrared region (0.1–6 THz) [10]. Therefore, the use of the THz technique is proposed in this experiment for the detection of amino acids. However, hydrogen bonds are sensitive to THz waves and have a high absorption of THz waves [11]. This prompted us to develop microfluidic chip technology to address this problem. Microfluidics is a promising tool for medical diagnostics and chemical analysis. It has the advantages of a small sample volume, high sensitivity, low cost, and micron-scale integration [12]. Kun Qian [13] et al. explored the effect of electrolytes on hydrogen bonding of water molecules by observing spectral intensity changes under a THz-TDS system using a microfluidic chip. The microfluidic technique's feasibility was confirmed.
In our previous article [14], we focused on the THz spectra of amino acid solutions in the absence of external conditions. In recent years, however, there has been an increasing interest in magnetic field-guided targeted therapy [15]. At the same time, amino acids, as essential nutrients for the human body, play a vital role in various diseases. If amino acids can be used for targeted therapy, it will be a new breakthrough in medical treatment. In 2014, DeNinno [23] et al. investigated the effect of magnetic fields on the hydration of two solutions of amino acids with different pH values. However, studies on the same amino acids at different magnetic field strengths have not been reported. Therefore, the purpose of this study is to investigate the properties of amino acid solutions under different magnetic field conditions and to provide some reference for the subsequent targeted therapy. In addition, the microstructural changes of amino acid molecules and water molecules under the magnetic field were further revealed.
2. Experimental system
2.1. Experimental apparatus and optical system
The experiment uses a typical THz-TDS system, which mainly consists of a femtosecond laser, a THz generation device, a time delay device, and a THz radiation detection device. The femtosecond laser has a central wavelength of 1550 nm, a pulse width of 75 fs, a repetition frequency of 100 MHz, and a power of 130 mW. The laser is split into two beams after passing through a polarizing beam splitting prism. After being delayed by a mechanical translation stage, one beam is used as a pump circuit and is coupled into the THz generating antenna to generate THz waves. The other beam serves as the detection light and is coupled into the THz detection antenna to detect THz waves. The microfluidic chip is sandwiched in the middle of two off-axis parabolic mirrors. When a THz wave passes through the microfluidic chip with the liquid to be measured, the detection antenna picks up the signal, which is fed to an amplification lock-in amplifier. Finally, a computer is used for data acquisition and processing. The optical path diagram of the experiment is shown in our previous article [14].
2.2. Preparation of microfluidic chips
The sandwich microfluidic chip in this study uses a double-sided adhesive with a thickness of 50 μm as an intermediate layer, and the double-sided adhesive is sheared to produce liquid channels with lengths, widths, and thicknesses of 2 cm, 2 cm, and 50 μm, respectively. Two round holes with a diameter of 2 mm are carved on the cover according to the position of the liquid inlet and outlet on the mold. The chip's substrate and cover plate are made of cyclic olefin copolymer (COC) Zennor 1420R material, which has THz wave transmittance of up to 90% [16]. Additionally, it is transparent to visible light, making it the best material for the preparation of microfluidic chips. The substrate, sandwich, and cover plate bonding, as well as the preparation of the microfluidic chip, were shown in Figure 1.
Figure 1.
Microfluidic chip preparation process.
2.3. External magnetic field device
The external magnetic field device is a multi-layer densely wound coil electromagnet with a 10 cm outer diameter, a 3 cm inner diameter, and a 1.5 cm thickness. The two electromagnets are placed vertically, equally high, and parallel to each other, and the microfluidic chip is placed vertically in the center of the two electromagnets, as shown in Figure 2. A regulated power supply is used to power the electromagnets, generating a horizontal magnetic field of uniform strength in the central of the electromagnets. The magnitude of the applied magnetic field can be adjusted by varying the magnitude of the voltage. In the experiment, the magnetic field device was in an open-air environment. A Gauss meter is placed in the middle of two electromagnets to measure the magnitude of the magnetic field at the location of the microfluidic chip. The magnitude of the voltage applied to the electromagnet and its corresponding magnetic field are shown in Table 1. Simultaneously, the THz wave can pass through the hollow part of the electromagnet, interact with the sample, carry the sample information, and be detected by the detection antenna.
Figure 2.
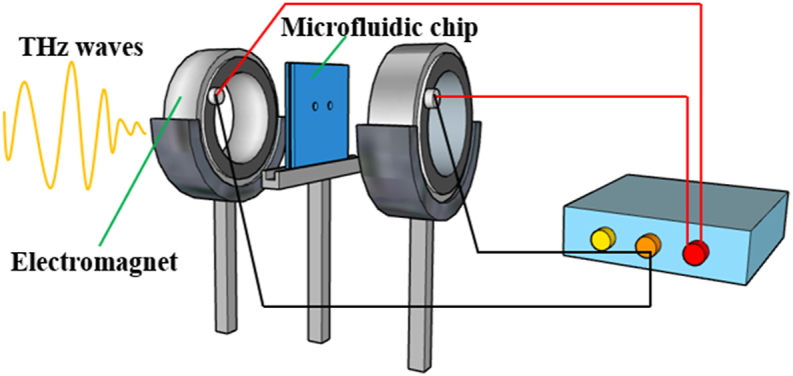
External magnetic field device.
Table 1.
Corresponding values of voltage and magnetic field.
| Voltage (V) | Magnetic Fields (mT) |
|---|---|
| 5 | 6.5 |
| 10 | 15.8 |
| 15 | 21.0 |
| 20 | 30.3 |
| 25 | 38.0 |
3. Experimental results
Because water impurities will affect the experiment, deionized water was selected to configure the solution and perform the experiment. In this experiment, four amino acid solutions with a volume fraction of 2% were used: phenylalanine, histidine, glycine, and glutamic acid. The amino acid solutions were then successively injected into the microfluidic chip and were detected with a THz-TDS system.
3.1. THz transmission intensities of different types of amino acid solutions
First, four amino acid solutions with a volume fraction of 2% and deionized water are separately injected into the microfluidic chip. The measurements were then performed using a THz-TDS system. The transmission intensities of THz waves by different kinds of solutions and empty chips were compared, and the experimental results are shown in Figure 3. The THz transmission levels, in descending order was: deionized water, phenylalanine, histidine, glycine and glutamic acid. Because water contains a large number of hydrogen bonds, which have a strong absorption effect on the THz waves, the THz transmission decreases. The experiment also showed that different types of amino acids have different absorption effects on THz waves. Therefore, it can be tentatively concluded that different types of amino acid solutions have different effects on hydrogen bonds in water, which can be indirectly reflected in the intensity of THz spectra.
Figure 3.
THz transmission intensities of different types of amino acid solutions.
3.2. THz absorption spectra of amino acids in external magnetic fields of different intensities
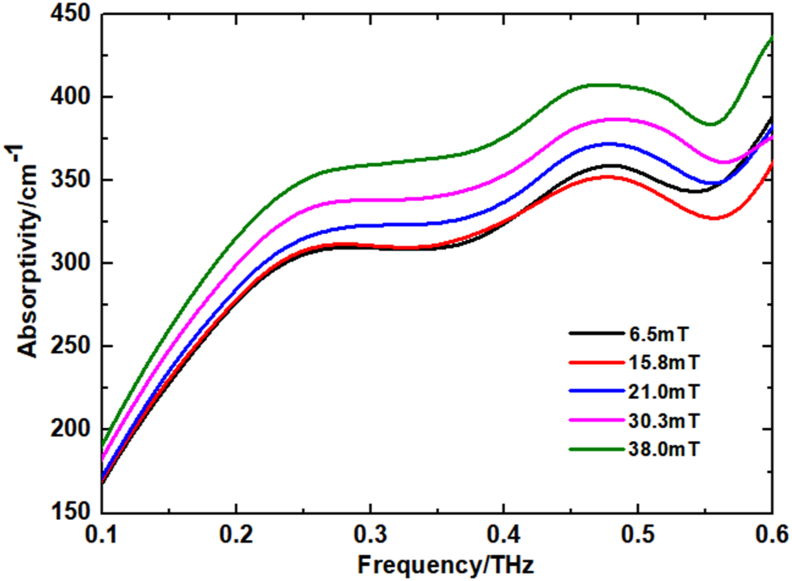
By varying the magnitude of the voltage and thus the magnitude of the uniform horizontal magnetic field generated by the two electromagnets. After each change in the magnitude of the magnetic field, the solution is left to stand for 2 min. After the measurement was completed, the magnitude of the magnetic field was changed again. We calculated the refractive indices and absorption coefficients of the four amino acid solutions with the aid of THz-TDS system and using Eqs. (1), (2), and (3) [17, 18]. Finally, the THz absorption characteristics of the four amino acid solutions under different magnitudes of the applied magnetic field were obtained separately. The experimental results are shown in Figures 4, 5, 6, and 7. The absorption coefficient of amino acid solution increases with the increase of magnetic field intensity.
| (1) |
| (2) |
| (3) |
Where is the phase difference between the sample signal and the reference signal, ρ(ω)is the amplitude ratio between the sample signal and the reference signal (COC Zeonor 1420R material signal), is the angular frequency of THz, is the speed of light, n1 is the COC Zennor 1420R refractive index, ns is the sample refractive index, is the thickness of the sample, αs is the absorption coefficient of the sample. Eq. (1) is used to calculate the refractive index of COC Zennor 1420R. Eq. (2) is used to calculate the sample refractive index. Eq. (3) is used to calculate the sample absorption coefficient.
Figure 4.
THz absorption spectra of phenylalanine in external magnetic fields of different intensities.
Figure 5.
THz absorption spectra of histidine in external magnetic fields of different intensities.
Figure 6.
THz absorption spectra of glutamic in external magnetic fields of different intensities.
Figure 7.
THz absorption spectra of glycine in external magnetic fields of different intensities.
3.3. Error analysis
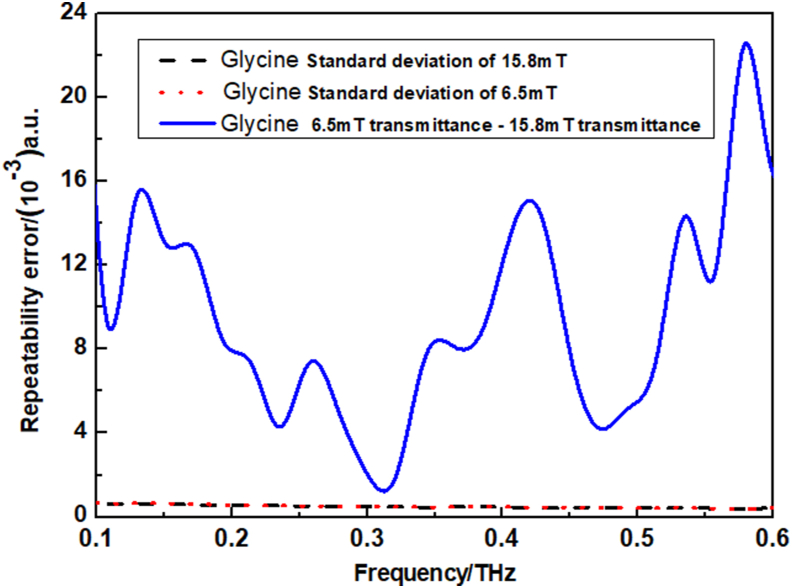
The errors in this study were analyzed by exemplifying the THz transmission characteristics of glycine solutions at magnetic field magnitudes of 6.5 mT and 15.8 mT. The measurements were repeated three times for each set of experiments and therefore standard deviations were obtained. As shown in Figure 8, the difference in the THz transmission characteristics of the glycine solutions at 6.5 mT and 15.8 mT is greater than the standard deviation of the repeated measurements. And all amino acid solutions in this study satisfy the above rule, which indicates the validity of the data.
Figure 8.
Standard deviation analysis of glycine solutions.
4. Simulation
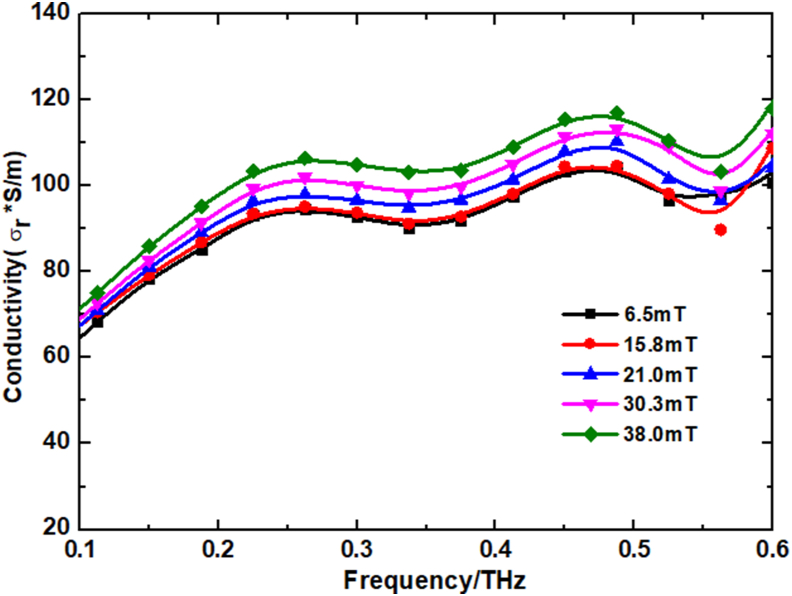
A chip made of COC is constructed for detection by simulation using COMSOL software. Its dimensions are 2 cm in length and width and 50 μm in thickness. THz waves are set to propagate along the z-axis and vibrate in the y-axis through the object, while a magnetic field is applied in the z-axis direction (Reference can be made to Figure 11(a)). The scanning probe is set at the center of the detection and the acquisition time is 0.3375 ps. The parameters of dielectric constant, magnetic permeability and electric conductivity are used for the simulation by setting different solutions. The conductivity in the simulation was obtained by actual measurement with an electric conductivity meter. The electric conductivity of glycine at different magnetic fields is given in the following Table 2. The dielectric constant, magnetic permeability in the simulation do not affect the simulation results too much. Therefore, the relative dielectric constant and magnetic permeability in air are taken in the simulation. The simulated absorption spectra of the glycine solution is shown in Figure 9, and their simulation results remain consistent with the experimental results.
Figure 11.
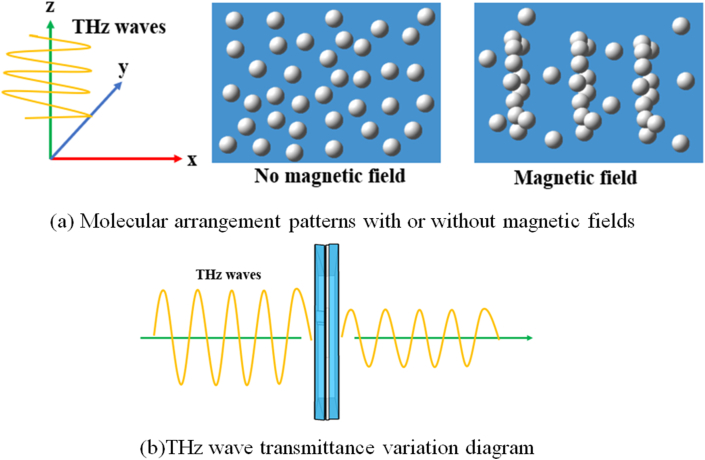
Mechanism of action under magnetic field (a) Molecular arrangement patterns with or without magnetic fields (b)THz wave transmittance variation diagram.
Table 2.
The electric conductivity of glycine solutions at different magnetic fields.
| Voltage (V) | Electric conductivity () |
|---|---|
| 0 | 62 |
| 5 | 65 |
| 10 | 67 |
| 15 | 70 |
| 20 | 74 |
Figure 9.
Simulated absorption spectra of the glycine solutions.
5. Theoretical analyses and discussion
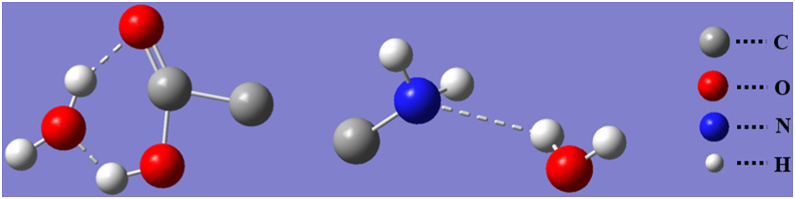
The incorporation of amino acid molecules into water results in collective molecular rotation and weak intermolecular interactions, such as localized molecular translational, oscillatory, and torsional movements, as well as intra- and intermolecular hydrogen bonding, and so on. Water molecules have been shown to form hydrogen bonding networks with the corresponding amino acid residues [19]. Furthermore, Peicheng Xu [20] et al. found that in amino acid solutions, oxygen atoms in water molecules are prone to form hydrogen bonds with hydrogen atoms in carboxyl groups, and hydrogen atoms in water molecules are prone to form hydrogen bonds with oxygen atoms in carbonyl groups. Following the formation of the double hydrogen structure, the structure has the lowest energy and is the most stable structure. Simultaneously, the hydrogen atoms in the amino group also form hydrogen bonds with the oxygen atoms in the water molecule. The intermolecular hydrogen bonding is shown in Figure 10.
Figure 10.
Principle of hydrogen bond formation.
THz spectroscopy is sensitive to intermolecular vibrations, which are usually caused by hydrogen bond stretching. Many studies have also demonstrated that THz spectroscopy is an effective tool for detecting weak bonds (including hydrogen bonds) [21]. Thus, the degree of absorption of THz waves can indirectly reflect the number of hydrogen bonds. The experiments were equipped with the same concentration of four amino acids. When the molecular weight of the amino acid solute increases, the number of its molecules and hydrogen bonds formed decreases. Therefore, the absorption intensity for THz waves is also reduced. According to its molecular weight, it can be compared in descending order was: phenylalanine, histidine, glutamic acid and glycine. However, because glutamic acid has two carboxyl groups in its molecular structure, it can form more hydrogen bonds. Therefore, in our experiments, we observed that the absorption intensity of THz waves in descending order was: glutamic acid, glycine, histidine, phenylalanine.
A magnetic field was applied to the experiment such that its direction was parallel to the THz wave transmission direction. It can be found that for the same amino acid, increasing the strength of the magnetic field reduces the transmission of THz waves. The presence of a magnetic field changes the internal environment of the solution. On the one hand, Kai Tai Chang [22] et al. simulated effects of external magnetic field on the number of hydrogen bonds in a solution using molecular dynamics. The results showed that the structure of water was more stable and the ability of water molecules to form hydrogen bonds in the magnetic field was enhanced. When the magnetic field strength increases, the number of hydrogen bonds increases. On the other hand, DeNinno [23] et al. found that in the presence of a magnetic field, amino acid molecules aggregate, increasing the radius of their molecular clusters and blocking the transmission of THz waves. The principle of the motion is shown in Figure 11.
Also, theoretical electric conductivity calculations for amino acids at different magnetic fields are given in this study. The absorption coefficient in Eq. (3) has the following relationship with the extinction coefficient at the same time [18]:
| (4) |
They are related to the dielectric constant and electric conductivity as follows:
| (5) |
| (6) |
where is the permittivity of vacuum, and are the real and imaginary parts of the dielectric constant, respectively, and are the real and imaginary parts of the electric conductivity, respectively. Figure 12 shows the results of theoretical electric conductivity calculations for glycine at different magnetic fields. The electric conductivity of the glycine solution increases with the increase of the magnetic field strength. The increase in electric conductivity leads to a decrease in THz wave transmission and a lower transmission spectra. This theoretical calculation also further reflects the internal structural changes of amino acid solutions from a microscopic perspective.
Figure 12.
Calculation of theoretical electric conductivity of glycine under different magnetic fields.
6. Conclusion
In this study, the frequency domain spectra of four amino acid solutions at a volume fraction of 2% and deionized water were compared. In the 0.1–1.0 THz range, the transmission of THz waves in descending order was found to be: deionized water, phenylalanine, histidine, glycine and glutamate. Therefore, we can use THz spectroscopy to identify different types of amino acids. Also, the variation of different types of amino acids with the applied magnetic field strength was investigated using a magnetic field parallel to the THz wave transmission. It was found that the intensity of the magnetic field increased the absorption of THz waves by the amino acid solution. Simulations were also performed with the glycine solution and its standard deviation and electric conductivity were analyzed to prove the validity of the experiment. However, the COC material used in the experiment is mainly used to detect inorganic samples and some organic samples, which has some limitations. In the future, we will use THz technology to study the properties of liquid amino acid solutions in depth.
Declarations
Author contribution statement
Ping Ye: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Qinghao Meng: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Guoyang Wang; Haiyun Huang; Yizhou Yang; Cunlin Zhang: Contributed reagents, materials, analysis tools or data.
Bo Su: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
Mr Bo Su was supported by National Natural Science Foundation of China [61575131].
Data availability statement
The data that has been used is confidential.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Cai Y., Wang J.H., Bai Z.C., Su B., Wu R., Cui H.L., Zhang C.L. Terahertz transmission characteristics of water induced by electric field. Spectrosc. Spectr. Anal. 2021;41(6):1683–1687. [Google Scholar]
- 2.Takahashi M. Terahertz vibrations and hydrogen-bonded networks in crystals. Crystals. 2014;4(2):4–103. [Google Scholar]
- 3.Zhang G., Wang F., Liu R. Application and prospect of terahertz technology in medical field. J. Tel. 2021;42(4):6–44. 9. [Google Scholar]
- 4.Markelz A., Roitberg A., Heilweil E. Pulsed terahertz spectroscopy of DNA, bovine serum albumin and collagen between 0.1 and 2.0 THz Chem. Phys. Lett. 2000 8. [Google Scholar]
- 5.Kutteruf M.R., Brown C.M., Iwaki L.K., Campbell M.B., Korter T.M., Heilweil E.J. Terahertz spectroscopy of short-chain polypeptides. Chem. Phys. Lett. 2003;375(3-4):337–343. [Google Scholar]
- 6.Zhang H., Zhang Z.H., Zhao X.Y. Analysis of the relationship between terahertz dielectric spectrum and component content and its application. Infrared Technology. 2014;36(11):7. [Google Scholar]
- 7.Zang Z., Yan S., Han X., Wei D., Cui H.L., Du C. Temperature- and pH-dependent protein conformational changes investigated by terahertz dielectric spectroscopy. Infrared Phys. Technol. 2019;98:260–265. [Google Scholar]
- 8.Shi W., Wang H.Q., Hou L., Dong C.G., Yang L., Zhao H.W., Wang Z.Q., Wang Y.Z. Terahertz nondestructive detection of amino acid fractionated solutions. Sci. China. Phys. Mech. 2021;51(5):90–95. [Google Scholar]
- 9.Li P.F., Tao B.B., Zhang X.D., An Z.L., Zhang X., Li Y.Y., Hao Q.Q., Liu L.H. Determination of 30 amino acids in human by high performance liquid chromatography-tandem mass spectrometry. Anal. Chem. 2013;41(9):1347–1352. [Google Scholar]
- 10.Korter T. American Physical Society; 2005. Terahertz Spectroscopy of Hydrogen-Bonded Molecular Complexes in Solution. [Google Scholar]
- 11.Samanta N., Mahanta D., Choudhury S., Barman A. Collective hydration dynamics in some amino acid solutions: a combined GHz-THz spectroscopic study. Chem. Phys. 2017;146(12) doi: 10.1063/1.4978900. [DOI] [PubMed] [Google Scholar]
- 12.Serita K., Murakami H., Kawayama I., Tonouchi M. A terahertz-microfluidic chip with a few arrays of asymmetric meta-atoms for the ultra-trace sensing of solutions. Photonics. 2019;6(1) [Google Scholar]
- 13.Qian K., Bai Z.C., Wu R., Wang J.H., Su B., Wen Y.W., Zhang C.L. Study of terahertz transmission properties of electrolyte solutions. Spectrosc. Spectr. Anal. 2021;41(7):2018–2022. [Google Scholar]
- 14.Ye P., Wang G.Y., Yang Y.Z., et al. Terahertz absorption properties of two solid amino acids and their aqueous solutions. Int. J. Optoelectron. 2021;39:1–7. [Google Scholar]
- 15.Xiong P., Guo P., Xiang D., et al. Theoretical analysis of magnetic drug targeting therapy under guided magnetic field. ActaPhysica Sinica. 2016;55(8):5. [Google Scholar]
- 16.Fan N., Su B., Wu Y.X., Zhang H.F., Zhang C., Zhang S.B., Zhang C.L. Sandwich-type terahertz microfluidic chip. Spectrosc. Spectr. Anal. 2018;8(5):1362–1367. [Google Scholar]
- 17.Fatemah M., Al-Douseri, Chen Y.Q., Zhang X.-C. THz wave sensing for petroleum industrial applications. Int. J. Infrared Millimet. Waves. 2006;27(4):481–503. [Google Scholar]
- 18.T. D. Dorney, R. G. Baraniuk, D. M. Mittleman, “Material parameter estimation with terahertz time-domain spectroscopy,” J. Opt. Soc. Am.: Optics and Image Sci. Vision, vol. 18, no. 7, pp. [DOI] [PubMed]
- 19.Li X., Chen M.J., Zhang A. Application of special groups in the optimization of drug molecule structure. Chin. J. Med. Chem. 2021;31(7):541–555. [Google Scholar]
- 20.Xu P.C. M.s Thesis, University of Jinan; China: 2017. Amino Acid Hydration and H2O, H2 Study on Hydrogen Bond of S, HF Dimer; p. 30. [Google Scholar]
- 21.Ren G.H., Zong S.Q., Zhu Z.J., Cheng C., Che L.G., Zhou L., Zhang J.B., Liu L.Y., Han J.G., Zhao H.W. Far-infrared terahertz properties of L-cysteine and its hydrochloride monohydrate. Spectrochim. Acta A. 2020;117476 doi: 10.1016/j.saa.2019.117476. [DOI] [PubMed] [Google Scholar]
- 22.Chang K.T., Weng C.I. The effect of an external magnetic field on the structure of liquid water using molecular dynamics simulation. J. Appl. Phys. 2006;100(4):2923. [Google Scholar]
- 23.DeNinno A., Castellano A.C. Influence of magnetic fields on the hydration process of amino acids: vibrational spectroscopy study of L-phenylalanine and L-glutamine. Bioelectromagnetics. 2014;35(2):129–135. doi: 10.1002/bem.21823. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that has been used is confidential.