Abstract
Microbial enhanced oil recovery (MEOR), characterized with the virtues of low cost and environmental protection, reflects the prevalent belief in environmental protection, and is attracting the attention of more researchers. Nonetheless, with the prolonged slump in global oil prices, how to further reduce the cost of MEOR has become a key factor in its development. This paper described the recent development of MEOR technology in terms of mechanisms, mathematical models, and field application, meanwhile the novel technologies of MEOR such as genetically engineered microbial enhanced oil recovery (GEMEOR) and enzyme enhanced oil recovery (EEOR) were introduced. The paper proposed three possible methods to decrease the cost of MEOR: using inexpensive nutrients as substrates, applying a mixture of chemical and biological agents, and utilizing crude microbial products. Additionally, in order to reduce the uncertainty in the practical application of MEOR technology, it is essential to refine the reservoir screening criteria and establish a sound mathematical model of MEOR. Eventually, the paper proposes to combine genetic engineering technology and microbial hybrid culture technology to build a microbial consortium with excellent oil displacement efficiency and better environmental adaptability. This may be a vital part of the future research on MEOR technology, which will play a major role in improving its economic efficiency and practicality.
Keywords: Microbial enhanced oil recovery, MEOR mechanisms, Biosurfactants, Biopolymers, Field trials
Highlights
-
•
Mechanisms of microbial enhanced oil recovery.
• The novel technology of microbial enhanced oil recovery.
• Field trails of microbial enhanced oil recovery.
Microbial enhanced oil recovery; MEOR mechanisms; Biosurfactants; Biopolymers, Field trials.
1. Introduction
Traditional oil recovery technology consists of two stages: primary and secondary oil recovery (Hadia et al., 2019). Primary oil recovery is the use of formation pressure to extract oil and gas from the reservoirs, with a 5–10% recovery rate of the original oil in place. In the stage of secondary oil recovery, people increase the reservoir pressure by injecting gas or water into the reservoir to replenish elastic energy for the rock and fluid in the formation, with the recovery rate ranging from 10% to 40% of the original oil in place (Hadia et al., 2019; Patel et al., 2015). However, more than 60% of crude oil remains trapped even though the reservoir has been tapped twice by traditional oil recovery technology (Wang et al., 2022; Niu et al., 2020). The tertiary oil recovery technology uses various physical, chemical and biological techniques to improve reservoir seepage characteristics and residual oil mobility, triggering the improvement of oil recovery (Haq et al., 2020). Gas enhanced oil recovery (GEOR) and chemical enhanced oil recovery (CEOR) are popular methods of enhanced recovery in tertiary oil recovery technology (Hadia et al., 2019; Massarweh and Abushaikha, 2021). However, they are both cost-effective and high-risk technologies, and even contain toxic chemicals potentially damaging the environment and human health. In recent years, with the global oil price depression, how to extract crude oil economically and efficiently has become a hot topic (Safdel et al., 2017).
Microbial enhanced oil recovery (MEOR) attracts broad attention with the advantages of environment-friendliness and low-cost (Wu et al., 2022). Microbial enhanced oil recovery (MEOR) is one of the tertiary oil recovery approaches which uses ex-situ/in-situ microorganisms and their metabolites like biopolymers, biosurfactants, bio-enzymes, biogases, solvents, and biogenic acids to modify the flow characteristics of residual oil in the reservoirs to enhance oil recovery (Niu et al., 2020; Patel et al., 2015). However, it has not been widely used in the petroleum industry due to the complexity of its mechanism and the uncontrollable reservoir environment. Meanwhile, its success has been inconsistent, even in some field trials of enhanced oil recovery (EOR). Therefore, it is necessary to summarize and analyze the research of MEOR technology in recent years to reduce the additional risk cost caused by its uncontrollable factors. This paper reviewed MEOR technology from three aspects: mechanisms, mathematical model, and field application. Moreover, two novel technologies of MEOR, including genetically engineered microbial enhanced oil recovery (GEMEOR) and enzyme enhanced oil recovery (EEOR), are introduced. In the paper, we analyzed the research on microbial enhanced recovery in recent years and proposed possible solutions on how to further reduce the cost of MEOR and enhance microbial flooding efficiency.
2. MEOR mechanisms
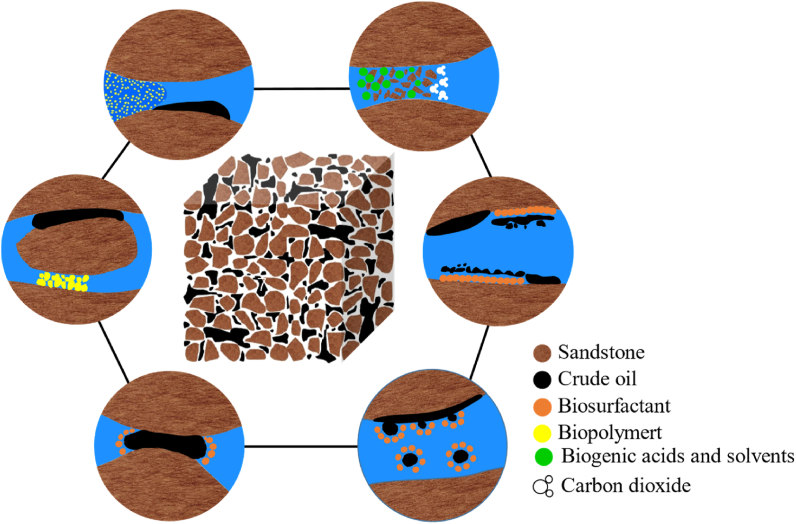
MEOR is usually achieved via the interaction of microbial metabolites with reservoirs, which mainly includes increasing swept volume and enhancing oil displacement efficiency (Wang et al., 2019). The mechanisms of MEOR based on microbial metabolites can be classified into the following parts: (1) Biosurfactants are mainly used to reduce the oil/water interfacial tension, modify the porous media wettability, emulsify residual oil, and improve the migration ability of bacteria (Hajibagheri et al., 2017; Zhang et al., 2022); (2) Biopolymers and microorganisms plug the high-permeability porous media selectively, beyond which the biopolymers are also used as tackifiers to increase the aqueous phase viscosity (Elshafie et al., 2017; Gao, 2018; Qi et al., 2018); (3) Biogases, solvents and biogenic acids can dissolve the carbonate rocks in oil reservoirs, making it easier for water to enter the pores of rocks and contact with the residual oil. Meanwhile, the gas produced by some of the dissolved carbonate rocks can increase the reservoir pressure (Rathi et al., 2018); (4) The microorganisms in reservoirs live on the crude oil as carbon source, which can degrade the long-chain saturated hydrocarbons, reduce the viscosity and improve the fluidity of crude oil (Tao et al., 2017). Particularly, the first two mechanisms are believed to have the greatest effect on improving oil recovery (Patel et al., 2015). Figure 1 illustrated the mechanisms of microbial metabolites in the process of MEOR.
Figure 1.
The role of microbial products in the process of MEOR.
2.1. Role of biosurfactants
Biosurfactants contain hydrophilic and hydrophobic groups which are a kind of amphiphilic compounds (Alvarez Yela et al., 2016). In oil reservoirs, the hydrophilic and hydrophobic groups of biosurfactants will be soluble in aqueous phase and oil phase respectively. As shown in Figure 1, This unique property can eliminate the repulsive force between oil and water, change the wettability and emulsify crude oil. Compared with the chemical surfactants, the advantages of biosurfactants are exhibited as biodegradability, low toxicity, and excellent stability (Cameotra and Makkar, 2004). Biosurfactants can be roughly classified into six categories, including the lipopeptides, glycolipids, phospholipids, fatty acids, polymeric surfactants, and particulate biosurfactants (Geetha et al., 2018). Some of them, such as lipopeptides and glycolipids, have been widely used to enhance the oil recovery (Dhanarajan et al., 2017). As of now, a variety of microorganisms have been identified as the producing surfactant, including Bacillus, Pseudomonas, Saccharomyces, Rhodococcus and Acinetobacter. Table 1 provides the details about biosurfactants classification and producing microbes.
Table 1.
Biosurfactants classification and producing microbes.
| Class | Biosurfactants | Microbes | Yield (g/L) | ST (mN/m) | Reference |
|---|---|---|---|---|---|
| Lipopeptides | surfactin | B. subtilis AB2.0 | 0.01 | 24.70 | Alvarez et al. (2020) |
| B. subtilis MG495086 | 6.30 | 29.85 | Datta et al. (2018) | ||
| B. subtilis AnPL-1 | 0.15 | 28.50 | Zhao et al. (2021) | ||
| Bacillus tequilensis MK 729017 | 7.46 | 30.00 | Datta et al. (2020) | ||
| Bacillus licheniformis L20 | 1.22 | Liu et al. (2022) | |||
| lichenysin |
B. licheniformis Staphylococcus sp. CO100 |
2.15 | / |
Qiu et al. (2014) Hentati et al. (2021) |
|
| fengycin | B. subtilis K1 | 2.20 | 27.00 | Pathak and Keharia (2014) | |
| Glycolipids | Rhamnolipids | Achromobacter sp. TMB1 | / | / | Haloi et al. (2021) |
| P. aeruginosa #112 | 3.20 | 29.80 | Gudina et al. (2015) | ||
| P. aeruginosa NCIM 5514 | 3.15 | 29.14 | Varjani and Upasani (2019) | ||
| Sophorolipids | Candida bombicola ATCC 22214 | 2.42 | 28.56 | Elshafie et al. (2015) | |
| Trehalose lipids | Gordonia amarae | / | / | Dogan et al. (2006) | |
| Phospholipids and fatty acids | phospholipids | Klebsiella pneumoniaeWMF02 | / | 25.70 | Jamal et al. (2012) |
| fatty acids | Candida ingens | 1.00 | 25.00 | Amezcua-Vega et al. (2007) | |
| Polymeric surfactants | Emulsan | Acinetobacter venetianus RAG-1 | / | / | Fondi et al. (2012) |
| Aeribacillus pallidus SL-1 | 0.90 | / | Tao et al. (2020) | ||
| Liposan | Candida lipolytica | 10.0 | 31.00 | Asfora Sarubbo et al. (2006) | |
| Lipid | Rhodococcus sp. TA6 | / | 29.80 | Shavandi et al. (2011) | |
| Particulate biosurfactants | Vesicles and fimbriae | Acinetobacter junii B6 | / | 36.00 | Ohadi et al. (2018) |
Biosurfactants exhibit the ability to reduce oil-water interfacial tension and emulsify crude oil, which have been shown to be closely related to their molecular mass. Low-molecular-weight biosurfactants, for example, the surfactin, can reduce the oil-water interfacial tension and change the wettability (Alvarez et al., 2020; Varjani and Upasani, 2019). While biosurfactants with high molecular weight, such as emulsan, perform well at emulsifying (Asfora Sarubbo et al., 2006; Tao et al., 2020). Furthermore, studies have shown that the concentration of biosurfactant can affect its function on crude oil as well. When the biosurfactants are at a low concentration, they tend to disperse the crude oil into minor particles to improve the utilization of crude oil by microorganisms. On the contrary, when the biosurfactants are at a high concentration, the hydrophobic groups of them tend to form micelles with crude oil and stabilize (Sharma and Pandey, 2020). Therefore, the concentration of biosurfactants should be configured according to the actual demand.
In practical EOR applications, biosurfactants are usually used at concentrations above their critical micellar concentration (CMC) values to achieve excellent emulsification of crude oil. Some studies indicate that the CMC of biosurfactants is obviously lower than that of chemical surfactants. This means that we can use much smaller amounts of biosurfactants than chemical surfactants to achieve the desired effect (Hadia et al., 2019). Nevertheless, high production cost still partly limits the application of biosurfactants. Therefore, it is urgent to reduce the production cost of biosurfactants. This paper summarized three possible methods. Firstly, the most promising approach is to use the abundant and cheap agro residue as the primary substances. Such as molasses, corn steep liquor and rice mill polishing residue played well in the production of biosurfactants (Al-Bahry et al., 2013; Gurjar and Sengupta, 2015). The mixture of biosurfactants and chemical surfactants is another potential method to reduce the cost in MEOR. Studies have shown that mixing biosurfactant with a green non-ionic surfactant is a practical approach to enhance oil recovery (Haq et al., 2020). The third method for reducing costs is to directly extract the biosurfactants from nature. In oil reservoirs with bacteria that produce surfactant, researchers believe there is probably a large amount of biosurfactants. Ethylenediamine (EDA) is used to extract biosurfactants (such as anionic surfactants) from crude oil into the aqueous phase, resulting in a cationic surfactant complex with surface tension (ST) reduced to 48 mN/m (Nasiri and Biria, 2020).
In general, biosurfactants exhibit excellent application prospect in enhancing oil recovery. But at present, how to further reduce the cost of obtaining and using biosurfactants have become particularly important. The three possible implementation methods proposed in this paper: the application of cheap substrates to produce biosurfactants, the use of a mix of biosurfactants and chemical surfactants, and the extraction of the surfactants from nature. This may make biosurfactants exhibit better practicality and applicability in MEOR.
2.2. Role of biopolymers and biomass
Biopolymers and biomass have been used in the MEOR field mainly for plugging of high-permeability zones and realizing permeability modification (Sen, 2008). Compared with synthetic polymers, biopolymers exhibit excellent stability, even in conditions of high temperature, hyper salinity and high shear rate (Couto et al., 2019). Besides, biopolymers have no pollution and the rejectamenta could be broken down by microorganisms (Zhao et al., 2018).
The actual reservoirs are mostly heterogeneous. Fluids enter the high-permeability zones easily, but it is hard to immerge low-permeability zones and move the residue oil. In the MEOR process, the microorganisms and nutrients that produce polymers will flow into the high permeability areas with the fluid. Biopolymers and bacteria clump together through continuous metabolic reproduction to block areas with high permeability, improving the efficiency of water drive sweep (Patel et al., 2015). Moreover, studies have shown that the extracellular polysaccharides produced by bacteria protect them from drying and predation, as well as increasing the adhesion of bacteria, so this type of bacteria has a better plugging effect than others (Sen, 2008). In addition, the plugging efficiency of biomass is related to the permeability of reservoirs and the radius of throats. The pore throat radius of reservoir is generally larger and the plugging ability of bacteria is weaker. With low permeability, the sieving effect becomes more prominent when the pore throat radius equals the diameter of the bacteria, and the sealing efficiency of the bacteria for the reservoir increases significantly (Bi et al., 2016; Sen, 2008).
In addition, biopolymers are often used as thickeners to enhance crude oil recovery by increasing the viscosity of the aqueous phase and expanding the sweep efficiency (Jang et al., 2015). Such polymers usually exhibit typical pseudoplasticity besides excellent stability in the reservoir environment. In the near-well zone, the polymer solution flows fast, with high shear rate and low viscosity, which contributes to promoting deeper into the reservoir. As the polymer solution moves away from the injection side, the shear rate decreases and the viscosity increases, which facilitates the repulsion of the remaining oil.
Xanthan gum, as a common biopolymer, has been widely used in petroleum, cosmetic and pharmaceutical fields due to its special molecular structure (Palaniraj and Jayaraman, 2011). It is a polymeric extracellular polysaccharide produced by Xanthomonas, whose cellulose backbone consists of five monosaccharides (two glucose units, two mannose units and one glucuronide unit), to form a pentasaccharide repeating unit (Xu et al., 2014; Ramos de Souza et al., 2022). Compared with synthetic polymer hydrolyzed polyacrylamide, xanthan gum shows a better resistance to the heat and salt but processes a slightly higher cost (Jang et al., 2015; Rellegadla et al., 2017). Except xanthan gum, many kinds of biopolymers have been used in petroleum filed as well. Table 2 listed some biopolymers and their producing bacteria with potential applications in the field of MEOR. Others, such as Rhizobium viscosum CECT 908, produced a biopolymer which was found to maintain excellent stability even at high shear rates, temperatures, and salinities (Couto et al., 2019). In the oil recovery tests of heavy crude oil, the R. viscosum biopolymer exhibits better performance than xanthan gum, the former can achieve a recovery rate of 25.7%, while the latter is less than 20%. Moreover, other studies have shown that the combination of biopolymer and biosurfactant flooding is better than their single use in enhancing oil recovery (Ji et al., 2022). For example, the combination of Enterobacter cloacae (biopolymer-producing strain) and Pseudomonas aeruginosa (biosurfactant-producing strain) were used to profile control and flooding, the oil recovery up to 17.4%, as against to 10.4% and 7.9% for them, respectively, when used alone (Bi et al., 2019). This provides a sound idea on the follow-up polymer drive studies.
Table 2.
Biopolymers used in MEOR with their producing microbes.
| Biopolymers | Microbes | Reference |
|---|---|---|
| Xanthan gum | Xanthomonas campestris | Jang et al. (2015) |
| Diutan gum | Sphingomonas sp. | Li et al. (2017) |
| Dextran | Pseudomonas stutzeri | Zhao et al. (2018) |
| Levan | Bacillus licheniformis | Dhanarajan et al. (2017) |
| Pullulan | Aureobasidium pullulans | Elshafie et al. (2017) |
| Scleroglucan | Sclerotium fungi | Castillo et al. (2015) |
| Welan gum | Alcaligenes sp | Xu et al. (2014) |
| — | Rhizobium viscosum | Couto et al. (2019) |
Most of the current research on novel microbial polymers remains in the laboratory stage, except the Xanthan gum. This is mainly due to its production difficulty and high production cost. In view of this, in the follow-up research work, we should simplify its production process as much as possible and reduce its production cost. Moreover, the mixed use of biopolymers with other oil displacement reagents is also a promising research direction.
2.3. Role of biogases, biogenic acids and solvents
Biogases, organic solvents and acids produced by microbial metabolism play a vital role in microbial recovery enhancement technologies. Gases are metabolized by microorganisms, such as carbon dioxide, nitrogen and methane, which can increase formation pressure in the reservoir (Niu et al., 2020). Besides, the gas dissolved in the crude oil may swell the volume of the crude oil, reduce the viscosity of the crude oil and improve the flow properties. Microorganisms produced acids and organic solvents, such as acetic acid, propionic acid, butyric acid, ethanol, acetone, butanol and isopropanol, which can dissolve the carbonate rocks in the reservoir, thus increasing the porosity and permeability of the reservoir (Sen, 2008; Al-Sulaimani et al., 2011). In addition, biogas, organic solvents, and acids, which metabolite in the process of microbial enhanced oil recovery, usually have certain synergistic effects. Researchers enriched the thermophilic anaerobic bacteria from heavy oil samples, which proved that metabolites produced by native microorganisms, such as carbon dioxide, ethanol, acetic acid and biosurfactants exhibit the ability to improve heavy oil recovery in carbonate porous media, and the oil recovery was up to 12% (Castorena-Cortés et al., 2012). Rathi et al. enriched methanogenic bacteria from high-temperature reservoirs, which can produce methane (8.08 mmol/L), carbon dioxide (4.25 mmol/L) and volatile fatty acids (1957.11 mg/L), recover 8.3% of crude oil in the core of sandstone filling (Rathi et al., 2018).
Overall, the utilization of biogas, organic solvents, and acids generated by microbial metabolism are mainly tend to inject cheap nutrients into the reservoir and activate endogenous microorganisms to improve the recovery of depleted reservoirs.
2.4. Biodegradation
In the petroleum industry, microbial degradation commonly used to storage tanks cleaning, wellhead wax cleaning and wax prevention, or to enhance recovery of dead reservoirs. During microbial degradation, the bacteria can degrade long chain hydrocarbons to short linear alkanes of crude oil, which essentially change the physicochemical properties of crude oil, especially the viscosity, and improve the fluidity of residual oil, thus the oil becomes easier to be cleaned or extracted (Muthukumar et al., 2022). Crude oil, on the other hand, is usually categorized into four major groups: saturated hydrocarbons, aromatic hydrocarbons, asphaltenes and resins (Varjani, 2017). Different microorganisms can degrade different components of crude oil. The simpler compounds in crude oil can be degraded by various microorganisms, but complex compounds (e like PAHs, asphaltenes and resins) can be degraded by few microorganisms. For example, the Bacillus, Pseudomonas, Rhodococcus, Immunobacterium, and Saccharomyces can effectively degrade the saturated and monoaromatic hydrocarbons of crude oil, and a few Rhodococcus, Mycobacterium, Pseudomonas, and Mycobacterium contribute to the degradation of the complex compounds in crude oil.
Therefore, to adequately degrade the components of the residual oil, the idea of using microbial consortium to degrade crude oil was initiated. The researchers constructed a mixed consortium of microorganisms with Rhodococcus erythropolis, Serratia proteamaculans, Alcaligenes sp., and Rhizobium sp., and the consortium consumed 85.26% of the crude oil within 15 days, with significantly higher consumption efficiency than that of individual strains, which confirmed the feasibility of the concept (Xia et al., 2019).
Besides the use of microbial consortia to degrade diverse crude oil components, mutually beneficial symbiotic relationships may exist between individual members of the consortium. During the degradation of petroleum hydrocarbons, some bacteria showed the ability to degrade metabolites accumulated by other members, alleviating the inhibitory effect of metabolites on the degradation ability of such bacteria, which consequently improved the degradation efficiency (Sun et al., 2021; Zhong et al., 2011). For instance, the consortium containing Rhodococcus sp. WB9 and Mycobacterium sp. WY10 showed relatively high phenanthrene-degradation efficiency. It was because that strain WB9 degraded phenanthrene to produce 1-hydroxy-2-naphthoic acid which suppressed its degrading activity of phenanthrene, but strain WY10 could degrade 1-hydroxy-2-naphthoic acid and the 1-hydroxy-2-naphthoic acid repression on phenanthrene degradation when strain WB9 was relieved (Sun et al., 2021). Furthermore, the researchers found that metabolites produced by the microorganisms also promoted the degradation of other members in the consortium. For instance, when the biosurfactant produced by yeast Pseudozyma sp. was added to Pseudomonas putida, the degradation of crude oil by the bacteria showed considerable improvement, especially for alkanes (C10–C24) increasing about 46% (Sajna et al., 2015). This may be explained by the fact that biosurfactants modulate the content of cell surface proteins and change the hydrophobicity of cell membranes, which makes it easier for cells to adhere to hydrocarbons, hence improving the degradation of crude oil by microorganisms (Sharma and Pandey, 2020).
As a conclusion, the microbial consortium combined with the characteristics of each member cleverly is technically challenging, yet shows promising potential for applications on tank cleaning, wellhead wax cleaning and wax prevention, or for improving the recovery of depleted reservoirs.
3. GEMEOR
Currently, the microorganisms used in oil recovery possess limitations, although they have their specific properties (Niu et al., 2020, Tatar, 2018). The microorganisms commonly used in MEOR are almost screened from endogenous microbes of reservoirs. These natural microorganisms are more or less defective, like poor salt tolerance or temperature resistance. With the development of genetic engineering technology, rapid progress has been made in genetically engineered microbial enhanced oil recovery (GEMEOR) technology. GEMEOR mainly uses genetic recombination, protoplast fusion and mutagenesis to create oil microorganisms with excellent performance. For example, Enterobacter cloacae can produce water-insoluble biopolymer at the optimum temperature of 30 °C. Protoplast fusion of Enterobacter cloacae and thermophilic Geobacillus strain can construct high-temperature resistant polymeric engineering bacteria, which can produce extracellular polysaccharide at 45 °C and perform well in core displacement experiment, with an improvement oil recovery by 11.3% (Sun et al., 2013). As another example the new strain FA-2 was constructed by protoplast fusion using Bacillus mojavensis which can produce lipopeptide under aerobic conditions and Pseudomonas stutzeri which can grow rapidly under anaerobic conditions. This novel strain showed excellent adaptability, and produced up to 382 mg/L of lipopeptides under extreme conditions (anaerobic conditions, pH 4.5–10.0 and salinity up to 100 g/L) (Liang et al., 2017). In addition, special functional strains can be constructed by gene knockout technology. For example, the fructose-1,6-diphosphatase (FBP) encoding gene in Enterobacter was knocked out to construct a new strain that can produce cellulose under specific conditions. Scanning electron microscopy showed that the new strain produced bacterial cellulose using glucose instead of glycerol as the sole carbon source. Bacterial concentration and cellulose production at different locations in core experiments showed that the plugging position of new strain was better than the original strain. Moreover, enhanced oil recovery by the new strain was 12.09%, 3.86% higher than the original strain (Gao et al., 2020).
The current research on GEMEOR remains focused on the construction of functional strains with good performance in the laboratory, but lacks practical field application. Therefore, in the subsequent research, we need to construct functional bacteria that can be applied to the target reservoirs for the better field test.
4. EEOR
Enzyme, produced by viable cells, is a kind of organic substances with catalytic activity and high selectivity. Enzyme enhanced oil recovery (EEOR) is a new method of microbial enhanced oil recovery in recent years. In the early oil and gas industry, enzymes were mainly used for hydrocarbon desulfurization and polymer pretreatment (Patel et al., 2015). It was later shown that enzymes produced by microorganisms have the resemblance with biosurfactants, which can change rock wettability and reduce oil-water interfacial tension (Rahayyem et al., 2019). In addition, bio-enzyme can break down the heavy components of crude oil, such as asphaltenes and paraffins, into lower molecular weight components, to reduce the viscosity and fluidity (Parthipan et al., 2017). The most common bio-enzymes used in EEOR are proteinases, dehydrogenases, esterase and lipases, etc (Rahayyem et al., 2019). In practical application, enzymes are usually mixed with other enzymes or surfactants. For example, bio-enzyme DGE mainly consists of protease, ethanol dehydrogenase and xylanase. Studies show that bio-enzyme DGE possesses the ability of reducing oil-water interfacial tension and changing the rock wettability. Meanwhile, the high interfacial activity qualified by DGE solution has a certain emulsification effect on crude oil during EEOR process, improves oil-water phase flow ratio and increases the oil drive efficiency (Daoshan et al., 2009). EEOR technology is now better applied in field trials, such as China, Myanmar, United Arab Emirates, etc (Rahayyem et al., 2019). Huff and puff test was conducted in the west oilfield of Dagang, China, by injecting enzyme solution with a mass fraction of 6%. The pilot achieved positive results, with daily oil increase of 6.49 t in the well group, water content decreased of 14.7%, and input production ratio over 1:7 (Feng et al., 2008). In addition, it has been shown that the alternate use of conventional MEOR technology and EEOR can significantly improve the recovery of crude oil (Gao et al., 2017).
EEOR is a microbial oil recovery technology with great potential, but still has many shortcomings. Screening and designing cost-effective enzyme-producing strains, selecting inexpensive formulations and shortening the production cycle are effective measurements to promote the development of EEOR technology. In addition, some factors need to be considered in the field test study, such as preventing enzyme degradation, improving emulsion formation, and reducing enzyme loss in the reservoir.
5. Numerical modeling of MEOR
MEOR is a comprehensive technology that integrates multiple disciplines of petroleum geology, biological porous flow and interface physical chemistry. The complex theory basis contributes to much uncertainty in practical applications. In order to accurately predict the production trend of the reservoir during microbial enhanced recovery, optimize the field plan, reduce the implementation risk and achieve economic and efficient extraction. Since the late 1980s, many researchers have conducted numerical simulation studies of MEOR based on the mechanism of MEOR action (Yao et al., 2012; Sivasankar and Suresh Kumar, 2019; Wang et al., 2019; Chakraborty et al., 2020). So far, some distinctive MEOR mathematical models have been formed (Table 3). The contents covered the transport of oil, water, microorganisms, nutrients, and metabolites in the reservoir, the growth and death of microorganisms, nutrient consumption, product generation, and the effects of different products on the physical properties of the reservoir and crude oil.
Table 3.
The mathematical models used for MEOR.
| Models | Model features | Reference |
|---|---|---|
| A three-dimensional, three-phase, multiple component numerical model | Considering the change of reservoir permeability and oil phase physical property by microorganism | Islam (1990) |
| Three - dimensional three - phase five - component Model based on Black Oil Model | A comprehensive description of the biological behavior of microorganisms and nutrients in the formation (growth, death, adsorption, chemotaxis, and nutrient consumption) | Chang et al. (1991) |
| One - dimensional three - phase multi - component model based on Islam Model | Decrease of formation permeability due to microbial retention on pore surface and pore throat blockage; Monod equation with two restricted nutrients | Xu et al. (1992) |
| Mathematical model of indigenous microbial flooding | Carbon source and oxygen as nutrient source for controlling microbial growth | Yao et al. (2012) |
| One-dimensional inhomogeneous, isotropic, and incompressible non-isothermal mathematical model | Study on coupled heat and mass transfer of microorganisms and their Nutrients in reservoirs | Sivasankar and Suresh Kumar (2019) |
| One-dimensional two-phase five-component mathematical model | The effects of bacteria and their products on porosity, permeability and water viscosity are described in detail. | Wang et al. (2018) |
| Mathematical model of microbial enhanced oil recovery by double-bacterial competition mechanism | The effect of double-bacterial competition mechanism and product interaction on enhanced oil recovery was analyzed. | Wang et al. (2019) |
Islam model, Change model and Zhang model are the most classical models in numerical modeling of MEOR, which described the various physical and chemical activities and the variations of reservoir characteristics in details. The Islam model is based on the transport of microorganisms and nutrients in the formation along with the kinetics of microbial growth, which considers the metabolism of microorganisms in the reservoir, the blockage of the reservoir by transport, and the change in the physical properties of the oil phase (Islam, 1990). Change model is based on the black oil model, which covers all the activities of microorganisms and nutrients in the reservoir and provides sound reference value (Chang et al., 1991). The Zhang model, on the other hand, is developed from the Islam model based on two limiting nutrient Monod equations and considers the effect of microbial adsorption on the pore surface of reservoirs (Xu et al., 1992).
Since then, many researchers have improved the above model and established numerical models of MEOR considering more comprehensively and more closely to the real reservoir environment. For instance, in order to simulate endogenous microbial enhanced oil recovery (EMEOR) process researchers have established a mathematical model of EMEOR by taking carbon and oxygen sources as nutrient sources to control microbial growth and considering the effects of microbial action on parameters such as porosity, permeability, viscosity, and surface tension. The optimal nutrient injection concentration, injection concentration, gas-liquid ratio, injection period and other parameters of the target block were calculated by the model and then compared with the experimental data in field to verify the reliability of simulation results (Yao et al., 2012). To explore the role of microorganisms in real reservoir on enhanced oil recovery, the researchers established a mathematical model of microbial oil recovery with competition mechanism of dual bacteria to analyze the effect of dual bacteria competitive growth and product interaction on enhanced oil recovery (Wang et al., 2019). It is shown that the double-bacterial competition model is more appropriate to the actual reservoir and has better accuracy when compared with the single-bacterial model. In addition to the competition among microorganisms, the complex reservoir environment is an extreme challenge for the growth of microorganisms. The researchers studied the effects of reservoir temperature, mineralization, and pH on microbial enhancement of recovery by building the corresponding mathematical models and obtained the following conclusions: (1) reservoir temperature and seepage velocity have a combined effect in oil recovery; (2) The injection water with a salinity closed to the optimum for microbial growth can significantly improve the oil displacement efficiency; (3) When changing the reservoir pH from high acid to low base (pH 5–8), the biosurfactants have the best effect on reducing the interfacial tension, which can significantly increase the oil driving efficiency (Sivasankar and Suresh Kumar, 2017, Sivasankar and Suresh Kumar, 2018, Sivasankar and Suresh Kumar, 2019).
Usually, the focus of mathematical models is different in different reservoir environments, leading to differences in composition, diffusion, adsorption, migration mechanism and so on. In general, the establishment of mathematical model is a process of continuous improvement, only for closer to the real reservoir environment, to provide a certain reference basis for actual production.
6. Field application
6.1. Screening criteria
The reservoir environment plays a decisive role in the effectiveness of MEOR, such as reservoir temperature, pressure, mineralization, pH, permeability, porosity, and crude oil viscosity. Temperature of the reservoir is the most important element, which shows a considerable influence on the growth and metabolism of microorganisms. Extremely low or high temperatures will cause slow growth or even death of microorganisms, thus affecting the synthesis of microbial products. Pressure is an extremely important parameter in the MEOR process, and excessive pressure will affect the growth of microorganisms. Variable pressure also leads to changes in the solubility of reservoir gas, resulting in changes in crude oil viscosity and potentially affecting oil displacement efficiency. The high mineralization is in addition to affecting the growth of microorganisms, it also tends to form precipitation with other substances and cause blocking (Gao, 2018). Besides affecting the metabolic activity of microorganisms, pH also affects the performance of biosurfactants. A low pH will lead to aggregation and sedimentation of biosurfactants, which is commonly used in experiments for the crude purification of surfactants produced by microorganisms (Pereira et al., 2013; Safdel et al., 2017). Permeability and porosity of the reservoir affect microbial transport, the smaller the pore radius, the greater the resistance in the process of microbial migration (Sen, 2008). Due to the wide variation in technology and reservoir conditions in different countries and regions, the criteria used to screen reservoirs for MEOR technology considerably differ. Table 4 lists reservoir screening criteria including the Institute of Reservoir Research, the US Department of Energy and CNPC.
Table 4.
Reservoir screening criteria for MEOR.
| Parameters | CNPC (Guo et al., 2015) | IRS (Patel et al., 2015) | US DOE (Patel et al., 2015) | Bryant (1991) | Al-Adasani and Bai (2010) | Sheng (2013) |
|---|---|---|---|---|---|---|
| Type of formation | Sandstone | Sandstone | Sandstone | - | Sandstone | - |
| Temperature, °C | 30–60 | <90 | <71 | <77 | 86–90 | <98 |
| Pressure, kg/cm2 | - | <300 | - | - | - | 105–200 |
| Salinity, g/L | <100 | <10 | <10 | <100 | - | <150 |
| pH value | - | 6–9 | - | - | - | 4–9 |
| Permeability, mD | ≥150 | >50 | >100 | >75 | 60–200 | >50 |
| Porosity, % | 17–25 | - | - | - | 12–26 | >15 |
| Viscosity, cp | 30–150 | <20 | - | - | 1.7–8900 | 5–50 |
| °API gravity | - | >20 | 18–40 | >15 | 12–33 | >15 |
| Water cut, % | 60–85 | 30–90 | - | - | - | - |
| Depth, ft (m) | - | <8000 | <10000 | <8000 | 1572–3464 | <3500 |
| Wax content, % | ≥7 | - | - | - | - | - |
| Oil saturation, % | - | >25 | >25 | >25 | 55–65 | >25 |
6.2. Field trials
Since Beckman first proposed the idea of MEOR in 1926, many researchers have conducted specific studies on it. In particular, the oil crisis of the 1970s greatly stimulated the desire of oil workers for cheap and efficient means of exploitation. MEOR technology has developed rapidly and numerous field trials have been conducted. According to application, the process of MEOR can be classified into four categories: microbial flooding recovery (MFR), microbial selective plugging recovery (MSPR), cyclic microbial recovery (CMR), and microbial wax removal (MWR). Table 5 lists the features and limitations of four commonly-used microbial oil recovery techniques and together with two novel technologies, GEMEOR and EEOR. Table 6 lists some field tests of MEOR in various countries, including the technical tools, microorganisms and nutrients used.
Table 5.
The features of different MEOR technologies.
| Types | Features | Limitations | Reference |
|---|---|---|---|
| MFR | Wide range of action Long duration |
Only suitable for specific reservoir environments | Liu et al. (2005) |
| MSPR | Plugging high permeability areas | May block other seepage channel | Bi et al., (2016), Bi et al. (2019) |
| CMR | Low cost Reusable |
Only suitable for single well | Gao (2018) |
| MWR | Wellbore wax removal and wax prevention | / | She et al. (2019) |
| GEMEOR | Combining the characteristics of dominant bacteria to construct new strain | High difficulty in operation | Niu et al. (2020) |
| EEOR | Low cost of application Reusable |
High cost of production | Aurepatipan et al. (2018) |
Table 6.
The field trials of MEOR around the world.
| Country | Technology | Microbial systems | Nutrients | Effects | References |
|---|---|---|---|---|---|
| China | MFR, MSPR CMR, MWR |
Mixed suspension of Arthrobacter, Pseudomonas and Bacillus | Phosphate salts, ammonium salts, yeast extract, peptone | Increased about 8700 t crude oil | Liu et al. (2005) |
| Brevibacillus brevis and Bacillus cereus | Yeast extract, phosphate salts, ammonium salts etc. | The 60 Wells have an effective rate of 71.7%, with a cumulative oil increase of 9175.5t | Guo et al. (2007) | ||
| Bacillus bacteria and filamentous bacteria | Yeast extract, phosphate salts, ammonium salts etc. | Increased about 1300 t crude oil | Jun et al. (2007) | ||
| Indigenous microorganisms | Corn steep powder, sodium nitrate, diammonium hydrogen phosphate | Increased about 3068 t crude oil | Le et al. (2014) | ||
| Mixed strains of Pseudomonas, Bacillus and Dietz monocytogenes etc. | Molasses 1%, phosphorus source 0.5%, nitrogen source 0.3% | Increased about 3464 t crude oil in 405 adys | Wang et al. (2016) | ||
| Two Bacillus strains | Molasses 0.545%, phosphorus and nitrogen source | The average oil production was improved from 2.2 to 3.5 t/day after microbial treatment | Sun et al. (2017) | ||
| Luteimonas huabeiensis | Glucose, peptone, yeast powder, phosphate salts, ammonium salts etc. | 210,000 tons of crude oil produced over 43 months from 169 production wells | Ke et al. (2018) | ||
| Russia | MFR | Indigenous microorganisms | Aeration, phosphorus, and nitrogen salts | The additional oil reached to 41.08 t | Ivanov et al. (1993) |
| Hydrocarbon-oxidizing bacteria | Nitrogen and phosphorous source | A total of 1250 t additional oil was recovered | Nazina et al. (2020) | ||
| USA | MSPR, CMR MFR |
In-situ microbial populations | Molasses and ammonium nitrate | Not yet reported | Coates et al. (1993) |
| Mixed strains of Bacillus, Clostridium etc. | Molasses 4% | The oil production rate was improved to 19.6% | Bryant et al. (1994) | ||
| Indigenous microbes | Adaptable nutrients | The application on a producing well led to an increase in well tests from 20 to over 80t |
Zahner et al. (2010) Akintunji et al. (2012) |
||
| Argentina | MFR | Hydrocarbon degrading anaerobic facultative microorganisms | Inorganic nutrients (containing potassium, phosphorus, nitrogen etc.) | The oil production rate was improved to 26% | Strappa et al. (2004) |
| Azerbaijan | MFR | Indigenous microorganisms | Molasses and milk whey | The oil production of single well increased from the 0.7 t/d to 1.8 t/d | Ibragimov et al. (2015) |
| Canada | MFR | In-situ microbial populations | Salts, ammonium nitrate, and organic compounds | The daily oil production per well increased from 1.4 to more than 8 m3 | Town et al. (2010) |
| Indonesia | CMR | In-situ microbial populations | phosphorus and nitrogen source, potassium chloride etc. | The well MJ-125 average oil rate gain is about 20% | Ariadji et al. (2017) |
| Molasses, Diammonium Phosphate, NPK fertilizer, sodium nitrate | The water cut were decreased from 99% to 92%, and incremental oil was gained by 1395 barrels | Ariadji et al. (2019) | |||
| Malaysia | CMR | Adaptive microorganisms | Adaptable nutrients | 90 bopd per well incremental oil | Sabut et al. (2003) |
| Myanmar | EEOR | Adaptive microorganisms | Adaptable nutrients | Approximately 1636 barrels of incremental oil | Ott et al. (2011) |
| Peru | MFR | Mixed strains | Inorganic nutrients (containing potassium, phosphorus, nitrogen etc.) | Increased about 13907 bbls of crude oil | Maure et al. (2005) |
In 1954, the first field test of microbial enhanced oil recovery was conducted in Arkansas, USA (Ghadimi and Ardjmand, 2006). In 1992, researchers conducted field tests in Oklahoma to determine whether microorganisms preferentially seal high permeability zones to improve water drive efficiency. The results of the test indicated that large-scale injection of readily metabolizable carbohydrates would not adversely affect ongoing operations in the field, but would alter existing flow patterns and reduce propagation rates within the test area (Coates et al., 1993). Similarly in Oklahoma, researchers injected microbes and molasses into wells from a centralized injection station and increased oil production by 19.6% from 1990 to 1993 (Bryant et al., 1994). In 2007, endogenous microbial flooding was carried out in California. The researchers injected amount of nutrient mixture into the reservoir to activate the original microorganisms in the reservoir. The test results showed a positive increase of 6% in crude oil recovery (Zahner et al., 2010). In addition, a field test of endogenous microbial flooding in Texas has shown that microbial flooding technology may be used in reservoirs with permeability below 50 mD (Akintunji et al., 2012).
In China, many field trials have been conducted in recent years, involving oil fields such as Daqing, Xinjiang, Dagang, Shengli, North China, etc., and all achieved positive results (Gao, 2018). Microbial huff and puff and microbial enhanced water flooding tests have been carried out successively in Daqing Oilfield. The cumulative oil increase of microbial huff and puff test was 9175.5 t, and the cumulative oil increase of microbial enhanced water flooding over 5800 t, confirming the feasibility of microbial oil recovery technology in Daqing permeable reservoirs (Guo et al., 2007). Besides, some studies have shown that microbial oil recovery technology also has a positive effect on the extraction of heavy oil. The researchers combined the screened viscosity-reducing microorganisms for heavy oil with reservoir endogenous microorganisms and found that the wax and asphaltene content of the produced oil could be effectively reduced and the average daily production of the wells increased significantly (Sun et al., 2017). Usually, it is difficult to effectively recover the reservoir after polymer drive, while researchers conducted microbial modification tests on this type of reservoir and found that with the injection of bacteria, the water content of the extracted fluid decreased significantly and the recovery was enhanced (Jun et al., 2007).
In Russia, it was used to enhance oil recovery by activating microbial colonies in the reservoir. Microorganisms producing organic acids, surfactants, carbonic acid, and methane were discovered during the test, which resulted in a cumulative oil increase of 41,080 t in the Romashkino field (Ivanov et al., 1993). In Argentina, parthenogenic anaerobic microorganisms that degrade hydrocarbons have been successfully used to enhance crude oil recovery, with an increase in short-chain hydrocarbons and enhancement mobility of crude oil in the test (Strappa et al., 2004). In Canada, microbial treatment of less economical and idle wells yielded positive results and effectively extended the life of these wells (Town et al., 2010). In Indonesia, researchers used molasses and commercial fertilizers to activate microbes in reservoirs, increasing crude oil production by about 1750 barrels. It was found that the abundance of total aerobic bacteria in the reservoir increased 10,000 times, the abundance of anaerobic bacteria increased 10 times, and the abundance of hydrocarbon degrading bacteria increased 1000 times after nutrient injection. The increase in the number of beneficial microorganisms reduced the oil-water interfacial tension by about 47 % and the viscosity by about 24 % (Ariadji et al., 2017, 2019). Microbial enzymatic oil recovery experiments were conducted in Mann Field, Myanmar. Testers injected 2 % of the bio-enzyme concentrate into two test wells, resulting in an increase of 2166 barrels (Ott et al., 2011). Additionally, in Azerbaijan, Malaysia, Romania, Peru and other countries, MEOR field tests showed positive results (Bybee and Karen, 2006; Ghazali et al., 2001; Ibragimov et al., 2015; Ibrahim et al., 2004; Lazar et al., 1999; Maure et al., 2005; Sabut et al., 2003).
7. Conclusions and outlooks
As global oil prices have prolonged slump in recent years, it is urgent to decrease the cost of MEOR further in order to seek better returns. Compared with other three recovery techniques, the microbial oil recovery technology takes advantage of relatively inexpensive, environment-friendly, and pollution-free. This paper reviewed abundant field trials, which confirmed the superiority and feasibility of MEOR technology. But meanwhile, some factors still strict the widespread application of MEOR technology, including the intricacy of the MEOR process, the unstable success rate, the low product yield and the low oil recovery. Therefore, future research work should focus on solving these urgent problems. The first issue is the cost of microbial products. Through the study of relevant literature, this paper summarizes the following possible methods to reduce the cost: (1) Using abundant and cheap agro residue or industrial waste as a substrate for bacterial growth whenever possible; (2) Using biological products in combination with chemical products; (3) Using crude microbial products whenever possible, eliminating the cost of expensive purification. The next issue is that the complex reservoir environment has a great impact on microbial growth and the success rate of microbial displacement technology. Therefore, before microbial flooding, it is necessary to structure a more systematic reservoir screening criteria, analyze the reservoir characteristics and microbial diversity of each well systematically, and establish the corresponding mathematical model of MEOR. At the same time, it is also feasible to construct strains with outstanding tolerance to harsh reservoir environment by genetic engineering technology. Moreover, the application of genetic engineering technology and microbial consortium construction technology may be a breakthrough for microbial oil recovery in the future. The former enables the creation of functional oil recovery bacteria with excellent performance, while the latter creates perfect microbial consortia. The combination of both may enhance the practicality of MEOR technology.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Al-Adasani A., Bai B. Soc. Pet. Eng. J.; 2010. Recent developments and updated screening criteria of enhanced oil recovery techniques. (International Oil and Gas Conference and Exhibition in China). [Google Scholar]
- Akintunji A.A., Sheehy A., Marcotte B.W.G., Govreau B.R. Soc. Pet. Eng. J.; 2012. A Texas MEOR application shows outstanding production improvement due to oil release effects on relative permeability. (SPE Improved Oil Recovery Symposium). [Google Scholar]
- Al-Bahry S.N., Al-Wahaibi Y.M., Elshafie A.E., Al-Bemani A.S., Joshi S.J., Al-Makhmari H.S., Al-Sulaimani H.S. Biosurfactant production by Bacillus subtilis B20 using date molasses and its possible application in enhanced oil recovery. Int. Biodeterior. Biodegrad. 2013;81:141–146. [Google Scholar]
- Al-Sulaimani H., Joshi S., Al-Wahaibi Y., Al-Bahry S., Elshafie A., Al-Bemani A. Microbial biotechnology for enhancing oil recovery: current developments and future prospects. Biotechnol. Bioinf. Bioeng. 2011;1(2):147–158. [Google Scholar]
- Alvarez V.M., Guimarães C.R., Jurelevicius D., de Castilho L.V.A., de Sousa J.S., da Mota F.F., Freire D.M.G., Seldin L. Microbial enhanced oil recovery potential of surfactin-producing Bacillus Subtilis AB2.0. Fuel. 2020;272:117730. [Google Scholar]
- Alvarez Yela A.C., Tibaquirá Martínez M.A., Rangel Piñeros G.A., López V.C., Villamizar S.H., Núñez Vélez V.L., Abraham W.-R., Vives Flórez M.J., González Barrios A.F. A comparison between conventional Pseudomonas aeruginosa rhamnolipids and Escherichia coli transmembrane proteins for oil recovery enhancing. Int. Biodeterior. Biodegrad. 2016;112:59–65. [Google Scholar]
- Amezcua-Vega C., Poggi-Varaldo H.M., Esparza-Garcia F., Rios-Leal E., Rodriguez-Vazquez R. Effect of culture conditions on fatty acids composition of a biosurfactant produced by Candida ingens and changes of surface tension of culture media. Bioresour. Technol. 2007;98(1):237–240. doi: 10.1016/j.biortech.2005.11.025. [DOI] [PubMed] [Google Scholar]
- Ariadji T., Astuti D.I., Aditiawati P., Purwasena I.A., Persada G.P., Soeparmono M.R., Amirudin N.H., Ananggadipa A.A., Sasongko S.Y., Abqory M.H. Soc. Pet. Eng. J.; 2017. Microbial huff and puff project at Mangunjaya field wells: the first. (SPE Asia Pacific Oil and Gas Conference and Exhibition). [Google Scholar]
- Ariadji T., Astuti D.I., Priharto N., Ananggadipa A.A., Persada G.P., Subiantoro E., Erwanto P.E., Abqory M.H. Soc. Pet. Eng. J.; 2019. Field implementation of nutrient huff and puff in Bentayan field, South Sumatera: towards a low-cost EOR. (SPE/IATMI Asia Pacific Oil and Gas Conference and Exhibition). [Google Scholar]
- Asfora Sarubbo L., Moura De Luna J., De Campos-Takaki G.M. Production and stability studies of the bioemulsifier obtained from a new strain of Candida glabrata UCP 1002. Electron. J. Biotechnol. 2006;9(4):400–406. [Google Scholar]
- Aurepatipan N., Champreda V., Kanokratana P., Chitov T., Bovonsombut S. Assessment of bacterial communities and activities of thermotolerant enzymes produced by bacteria indigenous to oil-bearing sandstone cores for potential application in Enhanced Oil Recovery. J. Petrol. Sci. Eng. 2018;163:295–302. [Google Scholar]
- Bi Y.Q., Yu L., Huang L.X., Ma T., Xiu J.L., Yi L.N. Microscopic profile control mechanism and potential application of the biopolymer-producing strain FY-07 for microbial enhanced oil recovery. Petrol. Sci. Technol. 2016;34(24):1952–1957. [Google Scholar]
- Bi Y., Xiu J., Ma T. Application potential analysis of enhanced oil recovery by biopolymer-producing bacteria and biosurfactant-producing bacteria compound flooding. Appl. Sci. 2019;9(23):5119. [Google Scholar]
- Bryant R.S. MEOR screening criteria fit 27% of U. S. oil reservoirs. Oil Gas J. 1991;89(15):56–59. [Google Scholar]
- Bryant R.S., Stepp A.K., Bertus K.M., Burchfield T.E. Soc. Pet. Eng. J.; 1994. Dennis M microbial enhanced waterflooding field tests. (SPE/DOE Improved Oil Recovery Symposium). [Google Scholar]
- Bybee, Karen MEOR in Northwest Peru. J. Petrol. Technol. 2006;58(1):48–49. [Google Scholar]
- Cameotra S.S., Makkar R.S. Recent applications of biosurfactants as biological and immunological molecules. Curr. Opin. Microbiol. 2004;7(3):262–266. doi: 10.1016/j.mib.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Castillo N.A., Valdez A.L., Farina J.I. Microbial production of scleroglucan and downstream processing. Front. Microbiol. 2015;6:1106. doi: 10.3389/fmicb.2015.01106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castorena-Cortés G., Zapata-Peñasco I., Roldán-Carrillo T., Reyes-Avila J., Mayol-Castillo M., Román-Vargas S., Olguín-Lora P. Evaluation of indigenous anaerobic microorganisms from Mexican carbonate reservoirs with potential MEOR application. J. Petrol. Sci. Eng. 2012;81:86–93. [Google Scholar]
- Chakraborty S., Govindarajan S.K., Gummadi S.N. Influence of crucial reservoir properties and microbial kinetic parameters on enhanced oil recovery by microbial flooding under nonisothermal conditions: mathematical modelling and numerical simulation. J. Petrol. Sci. Eng. 2020 [Google Scholar]
- Chang M.M., Chung F.T.H., Bryant R.S., Gao H.W., Burchfield T.E. Soc. Pet. Eng. J; 1991. Modeling and laboratory investigation of microbial transport phenomena in porous media. (SPE Annual Technical Conference and Exhibition). SPE 22845-MS. [Google Scholar]
- Coates J.D., Chisholm J.L., Knapp R.M., McInerney M.J., Menzie D.E., Bhupathiraju V.K. In: Premuzic E.T., Woodhead A., editors. vol. 39. Elsevier; 1993. Microbially enhanced oil recovery field pilot, Payne county, Oklahoma; pp. 197–205. (Developments in Petroleum Science). [Google Scholar]
- Couto M.R., Gudina E.J., Ferreira D., Teixeira J.A., Rodrigues L.R. The biopolymer produced by Rhizobium viscosum CECT 908 is a promising agent for application in microbial enhanced oil recovery. Nat. Biotechnol. 2019;49:144–150. doi: 10.1016/j.nbt.2018.11.002. [DOI] [PubMed] [Google Scholar]
- Daoshan L., Mingyi S., Fangtian N.J.P.G., Efficiency R. Research on pilot test of biologic-enzyme enhanced oil recovery in Gangxi oil reservoir of Dagang Oilfield. Pet. Geo. Rec. Eff. 2009 [Google Scholar]
- Datta P., Tiwari P., Pandey L.M. Isolation and characterization of biosurfactant producing and oil degrading Bacillus subtilis MG495086 from formation water of Assam oil reservoir and its suitability for enhanced oil recovery. Bioresour. Technol. 2018;270:439–448. doi: 10.1016/j.biortech.2018.09.047. [DOI] [PubMed] [Google Scholar]
- Datta P., Tiwari P., Pandey L.M. Oil washing proficiency of biosurfactant produced by isolated Bacillus tequilensis MK 729017 from Assam reservoir soil. J. Petrol. Sci. Eng. 2020;195 [Google Scholar]
- Dhanarajan G., Rangarajan V., Bandi C., Dixit A., Das S., Ale K., Sen R. Biosurfactant-biopolymer driven microbial enhanced oil recovery (MEOR) and its optimization by an ANN-GA hybrid technique. J. Biotechnol. 2017;256:46–56. doi: 10.1016/j.jbiotec.2017.05.007. [DOI] [PubMed] [Google Scholar]
- Dogan I., Pagilla K.R., Webster D.A., Stark B.C. Expression of Vitreoscilla hemoglobin in Gordonia amarae enhances biosurfactant production. J. Ind. Microbiol. Biotechnol. 2006;33(8):693–700. doi: 10.1007/s10295-006-0097-0. [DOI] [PubMed] [Google Scholar]
- Elshafie A., Joshi S., Al-Wahaibi Y., Al-Bahry S., Al-Bemani A., Al-Hashmi A., Al-Mandhari M. Soc. Pet. Eng. J.; 2017. Isolation and characterization of biopolymer producing Omani Aureobasidium pullulans strains and its potential applications in microbial enhanced oil recovery. (SPE Oil and Gas India Conference and Exhibition). [Google Scholar]
- Elshafie A.E., Joshi S.J., Al-Wahaibi Y.M., Al-Bemani A.S., Al-Bahry S.N., Al-Maqbali D., Banat I.M. Sophorolipids production by Candida bombicola ATCC 22214 and its potential application in microbial enhanced oil recovery. Front. Microbiol. 2015;6:1324. doi: 10.3389/fmicb.2015.01324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Q.X., Ma X.P., Zhou L.H., Shao D.B., Wang X.L., Qin B.Y. 2008. EOR pilot tests with modified enzyme in China. (Fault-Block Oil Gas Field). [Google Scholar]
- Fondi M., Orlandini V., Emiliani G., Papaleo M.C., Maida I., Perrin E., Vaneechoutte M., Dijkshoorn L., Fani R. Draft genome sequence of the hydrocarbon-degrading and emulsan-producing strain Acinetobacter venetianus RAG-1T. J. Bacteriol. 2012;194(17):4771–4772. doi: 10.1128/JB.01019-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao C. Experiences of microbial enhanced oil recovery in Chinese oil fields. J. Petrol. Sci. Eng. 2018;166:55–62. [Google Scholar]
- Gao H., Lai H., Zhang J., Xue Q. Effects of bacterial cell density and alternating microbial- and enzymolysis-enhanced oil recovery on oil displacement efficiency. Chem. Eng. J. 2017;327:28–38. [Google Scholar]
- Gao G., Ji K., Zhang Y., Liu X., Dai X., Zhi B., Cao Y., Liu D., Wu M., Li G., Ma T. Microbial enhanced oil recovery through deep profile control using a conditional bacterial cellulose-producing strain derived from Enterobacter sp. FY-07. Microb. Cell Fact. 2020;19(1) doi: 10.1186/s12934-020-01314-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geetha S.J., Banat I.M., Joshi S.J. Biosurfactants: production and potential applications in microbial enhanced oil recovery (MEOR) Biocatal. Agric. Biotechnol. 2018;14:23–32. [Google Scholar]
- Ghadimi M.R., Ardjmand M. Soc. Pet. Eng. J.; 2006. Simulation of microbial enhanced oil recovery. (International Oil Conference and Exhibition in Mexico). [Google Scholar]
- Ghazali Abd., Karim M., Hj Salim M.A., Zain Md., Talib N.N. Soc. Pet. Eng. J.; 2001. Microbial enhanced oil recovery (MEOR) technology in Bokor field, Sarawak. (SPE Asia Pacific Improved Oil Recovery Conference). [Google Scholar]
- Gudina E.J., Rodrigues A.I., Alves E., Domingues M.R., Teixeira J.A., Rodrigues L.R. Bioconversion of agro-industrial by-products in rhamnolipids toward applications in enhanced oil recovery and bioremediation. Bioresour. Technol. 2015;177:87–93. doi: 10.1016/j.biortech.2014.11.069. [DOI] [PubMed] [Google Scholar]
- Guo W.K., Hou Z.W., Shi M., Wu X.L. Recovery mechanism and application of Brevibacillus brevis and Bacillus cereus in extra-low permeability reservoir of Daqing. Petrol. Explor. Dev. 2007;34(1):73–78. [Google Scholar]
- Guo H., Li Y., Yiran Z., Wang F., Xian G. Soc. Pet. Eng. J.; 2015. Progress of microbial enhanced oil recovery in China. (SPE Asia Pacific Enhanced Oil Recovery Conference). [Google Scholar]
- Gurjar J., Sengupta B. Production of surfactin from rice mill polishing residue by submerged fermentation using Bacillus subtilis MTCC 2423. Bioresour. Technol. 2015;189:243–249. doi: 10.1016/j.biortech.2015.04.013. [DOI] [PubMed] [Google Scholar]
- Hadia N.J., Ottenheim C., Li S., Hua N.Q., Stubbs L.P., Lau H.C. Experimental investigation of biosurfactant mixtures of surfactin produced by Bacillus Subtilis for EOR application. Fuel. 2019;251:789–799. [Google Scholar]
- Hajibagheri F., Lashkarbolooki M., Ayatollahi S., Hashemi A. The synergic effects of anionic and cationic chemical surfactants, and bacterial solution on wettability alteration of carbonate rock: an experimental investigation. Colloid Surface A. 2017;513:422–429. [Google Scholar]
- Haloi S., Saikia M.D., Gogoi S.B., Mohan R., Medhi T. Aggregation and static adsorption behaviour of Achromobacter sp. TMB1 produced rhamnolipids on sandstone core in relation to microbial enhanced oil recovery. J. Petrol. Sci. Eng. 2021;205 [Google Scholar]
- Haq B., Liu J., Liu K., Al Shehri D. The role of biodegradable surfactant in microbial enhanced oil recovery. J. Petrol. Sci. Eng. 2020;189 [Google Scholar]
- Hentati D., Cheffi M., Hadrich F., Makhloufi N., Rabanal F., Manresa A., Sayadi S., Chamkha M. Investigation of halotolerant marine Staphylococcus sp. CO100, as a promising hydrocarbon-degrading and biosurfactant-producing bacterium, under saline conditions. J. Environ. Manage. 2021;277 doi: 10.1016/j.jenvman.2020.111480. [DOI] [PubMed] [Google Scholar]
- Ibragimov K.M., Abdullayeva F.Y., Guseynova N.İ. Soc. Pet. Eng. J.; 2015. Experience of microbial enhanced oil recovery methods at Azerbaijan fields. (The Second SPE Annual Caspian Technical Conference and Exhibition). [Google Scholar]
- Ibrahim Z., Omar M.I., Foo K.S., Elias E.J., Othman M. Soc. Pet. Eng. J.; 2004. Simulation analysis of microbial well treatment of Bokor field, Malaysia. (SPE Asia Pacific Oil and Gas Conference and Exhibition). [Google Scholar]
- Islam M.R. Soc. Pet. Eng. J.; 1990. Mathematical modeling of microbial enhanced oil recovery. (SPE Annual technical conference and exhibition). [Google Scholar]
- Ivanov M.V., Belyaev S.S., Borzenkov I.A., Glumov I.F., Ibatullin R.R. In: Premuzic E.T., Woodhead A., editors. vol. 39. Elsevier; 1993. Additional oil production during field trials in Russia; pp. 373–381. (Developments in Petroleum Science). [Google Scholar]
- Jamal P., Wan M.F., Wan N., Alam M.Z. Optimum medium components for biosurfactant production by Klebsiella pneumoniae WMF02 utilizing sludge palm oil as a substrate. Aust. J. Basic App. Sci. 2012;6(1):100–108. [Google Scholar]
- Jang H.Y., Zhang K., Chon B.H., Choi H.J. Enhanced oil recovery performance and viscosity characteristics of polysaccharide xanthan gum solution. J. Ind. Eng. Chem. 2015;21:741–745. [Google Scholar]
- Ji S., Wei F., Li B., Li P., Li H., Li S., Wang J., Zhu H., Xu H. Synergistic effects of microbial polysaccharide mixing with polymer and nonionic surfactant on rheological behavior and enhanced oil recovery. J. Petrol. Sci. Eng. 2022;208 [Google Scholar]
- Jun S., Fenglan W., Zhiyao W., Bian Y., Zhenyu Y., Menghua G., Wei L., Zhaowei H. Soc. Pet. Eng. J.; 2007. Study on the pilot test with microbial profile modification after polymer flooding in Daqing oil field. (SPE Saudi Arabia Section Technical Symposium). [Google Scholar]
- Ke C.-Y., Sun W.-J., Li Y.-B., Lu G.-M., Zhang Q.-Z., Zhang X.-L. Microbial enhanced oil recovery in Baolige Oilfield using an indigenous facultative anaerobic strain Luteimonas huabeiensis sp. nov. J. Petrol. Sci. Eng. 2018;167:160–167. [Google Scholar]
- Lazar I., Voicu A., Nicolescu C., Mucenica D., Sandulescu L. The use of naturally occurring selectively isolated bacteria for inhibiting paraffin deposition. J. Petrol. Sci. Eng. 1999;22(1):161–169. [Google Scholar]
- Le J., Liu F., Zhang J., Bai L., Wu X.J.A.P.S. A field test of activation indigenous microorganism for microbial enhanced oil recovery in reservoir after polymer flooding. Act. Petrol. Sci. 2014;35(1):99–106. [Google Scholar]
- Li Y., Xu L., Gong H., Ding B., Dong M., Li Y. A microbial exopolysaccharide produced by Sphingomonas species for enhanced heavy oil recovery at high temperature and high salinity. Energy Fuel. 2017;31(4):3960–3969. [Google Scholar]
- Liang X., Shi R., Radosevich M., Zhao F., Zhang Y., Han S., Zhang Y. Anaerobic lipopeptide biosurfactant production by an engineered bacterial strain for in situ microbial enhanced oil recovery. RSC Adv. 2017;7(33):20667–20676. [Google Scholar]
- Liu J., Ma L., Mu B., Liu R., Ni F., Zhou J. The field pilot of microbial enhanced oil recovery in a high temperature petroleum reservoir. J. Petrol. Sci. Eng. 2005;48(3–4):265–271. [Google Scholar]
- Liu Q., Niu J., Liu Y., Li L., Lv J. Optimization of lipopeptide biosurfactant production by Bacillus licheniformis L20 and performance evaluation of biosurfactant mixed system for enhanced oil recovery. J. Petrol. Sci. Eng. 2022;208 [Google Scholar]
- Massarweh O., Abushaikha A.S. A review of recent developments in CO2 mobility control in enhanced oil recovery. Petroleum. 2021 [Google Scholar]
- Maure M.A., Saldana A.A., Ramirez J. Soc. Pet. Eng. J.; 2005. A biotechnology applications to EOR in Talara off-shore oil fields, Northwest Peru. (Latin American and Caribbean Petroleum Engineering Conference). [Google Scholar]
- Muthukumar B., Al Salhi M.S., Narenkumar J., Devanesan S., Tentu Nageswara R., Kim W., Rajasekar A. Characterization of two novel strains of Pseudomonas aeruginosa on biodegradation of crude oil and its enzyme activities. Environ. Pollut. 2022;304 doi: 10.1016/j.envpol.2022.119223. [DOI] [PubMed] [Google Scholar]
- Nasiri M.A., Biria D. Extraction of the indigenous crude oil dissolved biosurfactants and their potential in enhanced oil recovery. Colloid Surface A. 2020;603 [Google Scholar]
- Nazina T., Sokolova D., Grouzdev D., Semenova E., Babich T., Bidzhieva S., Serdukov D., Volkov D., Bugaev K., Ershov A., Khisametdinov M., Borzenkov I. The potential application of microorganisms for Sustainable petroleum recovery from heavy oil reservoirs. Sustainability. 2020;12(1):23. [Google Scholar]
- Niu J., Liu Q., Lv J., Peng B. Review on microbial enhanced oil recovery: mechanisms, modeling and field trials. J. Petrol. Sci. Eng. 2020;192:107350. [Google Scholar]
- Ohadi M., Dehghannoudeh G., Forootanfar H., Shakibaie M., Rajaee M. Investigation of the structural, physicochemical properties, and aggregation behavior of lipopeptide biosurfactant produced by Acinetobacter junii B6. Int. J. Biol. Macromol. 2018;112:712–719. doi: 10.1016/j.ijbiomac.2018.01.209. [DOI] [PubMed] [Google Scholar]
- Ott W.K., Nyo T., Aung W.N., Khaing A.T. Soc. Pet. Eng. J.; 2011. EEOR success in Mann field, Myanmar. (SPE Enhanced Oil Recovery Conference). [Google Scholar]
- Palaniraj A., Jayaraman V. Production, recovery and applications of xanthan gum by Xanthomonas campestris. J. Food Eng. 2011;106(1):1–12. [Google Scholar]
- Parthipan P., Preetham E., Machuca L.L., Rahman P.K., Murugan K., Rajasekar A. Biosurfactant and degradative enzymes mediated crude oil degradation by bacterium Bacillus subtilis A1. Front. Microbiol. 2017;8:193. doi: 10.3389/fmicb.2017.00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel J., Borgohain S., Kumar M., Rangarajan V., Somasundaran P., Sen R. Recent developments in microbial enhanced oil recovery. Renew. Sust. Energy Rev. 2015;52:1539–1558. [Google Scholar]
- Pathak K.V., Keharia H. Application of extracellular lipopeptide biosurfactant produced by endophytic Bacillus subtilis K1 isolated from aerial roots of banyan (Ficus benghalensis) in microbially enhanced oil recovery (MEOR) Biotech. 2014;4(1):41–48. doi: 10.1007/s13205-013-0119-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira J.F.B., Gudiña E.J., Costa R., Vitorino R., Teixeira J.A., Coutinho J.A.P., Rodrigues L.R. Optimization and characterization of biosurfactant production by Bacillus subtilis isolates towards microbial enhanced oil recovery applications. Fuel. 2013;111:259–268. [Google Scholar]
- Qi Y.B., Zheng C.G., Lv C.Y., Lun Z.M., Ma T. Compatibility between weak gel and microorganisms in weak gel-assisted microbial enhanced oil recovery. J. Biosci. Bioeng. 2018;126(2):235–240. doi: 10.1016/j.jbiosc.2018.02.011. [DOI] [PubMed] [Google Scholar]
- Qiu Y., Xiao F., Wei X., Wen Z., Chen S. Improvement of lichenysin production in Bacillus licheniformisby replacement of native promoter of lichenysin biosynthesis operon and medium optimization. Appl. Microbiol. Biotechnol. 2014;98(21):8895–8903. doi: 10.1007/s00253-014-5978-y. [DOI] [PubMed] [Google Scholar]
- Rahayyem M., Mostaghimi P., Alzahid Y.A., Halim A., Evangelista L., Armstrong R.T. Soc. Pet. Eng. J.; 2019. Enzyme enhanced oil recovery EEOR: a microfluidics approach. (SPE Middle East Oil and Gas Show and Conference). [Google Scholar]
- Ramos de Souza E., Rodrigues P.D., Sampaio I.C.F., Bacic E., Crugeira P.J.L., Vasconcelos A.C., dos Santos Silva M., dos Santos J.N., Quintella C.M., Pinheiro A.L.B., Almeida Pfd. Xanthan gum produced by Xanthomonas campestris using produced water and crude glycerin as an environmentally friendlier agent to enhance oil recovery. Fuel. 2022;310:122421. [Google Scholar]
- Rathi R., Lavania M., Kukreti V., Lal B. Evaluating the potential of indigenous methanogenic consortium for enhanced oil and gas recovery from high temperature depleted oil reservoir. J. Biotechnol. 2018;283:43–50. doi: 10.1016/j.jbiotec.2018.06.347. [DOI] [PubMed] [Google Scholar]
- Rellegadla S., Prajapat G., Agrawal A. Polymers for enhanced oil recovery: fundamentals and selection criteria. Appl. Microbiol. Biotechnol. 2017;101(11):4387–4402. doi: 10.1007/s00253-017-8307-4. [DOI] [PubMed] [Google Scholar]
- Sabut B., Salim M.A.H., Hamid A.S.A., Khor S.F. Soc. Pet. Eng. J.; 2003. Further evaluation of microbial treatment technology for improved oil production in Bokor field, Sarawak. (SPE International Improved Oil Recovery Conference). [Google Scholar]
- Safdel M., Anbaz M.A., Daryasafar A., Jamialahmadi M. Microbial enhanced oil recovery, a critical review on worldwide implemented field trials in different countries. Renew. Sust. Energy Rev. 2017;74:159–172. [Google Scholar]
- Sajna K.V., Sukumaran R.K., Gottumukkala L.D., Pandey A. Crude oil biodegradation aided by biosurfactants from Pseudozyma sp. NII 08165 or its culture broth. Bioresour. Technol. 2015;191:133–139. doi: 10.1016/j.biortech.2015.04.126. [DOI] [PubMed] [Google Scholar]
- Sen R. Biotechnology in petroleum recovery: the microbial EOR. Prog. Energy Combust. 2008;34(6):714–724. [Google Scholar]
- Sharma S., Pandey L.M. Production of biosurfactant by Bacillus subtilis RSL-2 isolated from sludge and biosurfactant mediated degradation of oil. Bioresour. Technol. 2020;307 doi: 10.1016/j.biortech.2020.123261. [DOI] [PubMed] [Google Scholar]
- Shavandi M., Mohebali G., Haddadi A., Shakarami H., Nuhi A. Emulsification potential of a newly isolated biosurfactant-producing bacterium, Rhodococcus sp. strain TA6. Colloid Surface B. 2011;82(2):477–482. doi: 10.1016/j.colsurfb.2010.10.005. [DOI] [PubMed] [Google Scholar]
- She H., Kong D., Li Y., Hu Z., Guo H. Recent Advance of Microbial Enhanced Oil Recovery (MEOR) in China. Geofluids. 2019;2019:1871392. [Google Scholar]
- Sheng J.J. In: Enhanced Oil Recovery Field Case Studies. Sheng J.J., editor. Gulf Professional Publishing; Boston: 2013. Chapter 19 - introduction to MEOR and its field applications in China; pp. 543–559. [Google Scholar]
- Sivasankar P., Suresh Kumar G. Influence of pH on dynamics of microbial enhanced oil recovery processes using biosurfactant producing Pseudomonas putida: mathematical modelling and numerical simulation. Bioresour. Technol. 2017;224:498–508. doi: 10.1016/j.biortech.2016.10.091. [DOI] [PubMed] [Google Scholar]
- Sivasankar P., Suresh Kumar G. Modelling the influence of interaction between injection and formation brine salinities on in-situ microbial enhanced oil recovery processes by coupling of multiple-ion exchange transport model with multiphase fluid flow and multi-species reactive transport models. J. Petrol. Sci. Eng. 2018;163:435–452. [Google Scholar]
- Sivasankar P., Suresh Kumar G. Influence of bio-clogging induced formation damage on performance of microbial enhanced oil recovery processes. Fuel. 2019;236:100–109. [Google Scholar]
- Strappa L., Lucia J.P., Maure A., Llopiz M.L. Soc. Pet. Eng. J.; 2004. A novel and successful MEOR pilot project in a Strong water-drive reservoir Vizcacheras field. (SPE-DOE Improved Oil Recovery Symposium Proceedings). [Google Scholar]
- Sun S., Luo Y., Cao S., Li W., Zhang Z., Jiang L., Dong H., Yu L., Wu W. Construction and evaluation of an exopolysaccharide-producing engineered bacterial strain by protoplast fusion for microbial enhanced oil recovery. Bioresour. Technol. 2013;144:44–49. doi: 10.1016/j.biortech.2013.06.098. [DOI] [PubMed] [Google Scholar]
- Sun S., Luo Y., Zhou Y., Xiao M., Zhang Z., Hou J., Wei X., Xu Q., Sha T., Dong H., Song H., Zhang Z. Application of Bacillus spp. in pilot test of microbial huff and puff to improve heavy oil recovery. Energy Fuel. 2017;31(12):13724–13732. [Google Scholar]
- Sun S., Wang H., Yan K., Lou J., Ding J., Snyder S.A., Wu L., Xu J. Metabolic interactions in a bacterial co-culture accelerate phenanthrene degradation. J. Hazard Mater. 2021;403:123825. doi: 10.1016/j.jhazmat.2020.123825. [DOI] [PubMed] [Google Scholar]
- Tao K., Liu X., Chen X., Hu X., Cao L., Yuan X. Biodegradation of crude oil by a defined co-culture of indigenous bacterial consortium and exogenous Bacillus subtilis. Bioresour. Technol. 2017;224:327–332. doi: 10.1016/j.biortech.2016.10.073. [DOI] [PubMed] [Google Scholar]
- Tao W., Lin J., Wang W., Huang H., Li S. Biodegradation of aliphatic and polycyclic aromatic hydrocarbons by the thermophilic bioemulsifier-producing Aeribacillus pallidus strain SL-1. Ecotoxicol. Environ. Saf. 2020;189 doi: 10.1016/j.ecoenv.2019.109994. [DOI] [PubMed] [Google Scholar]
- Tatar A. In: Fundamentals of Enhanced Oil and Gas Recovery from Conventional and Unconventional Reservoirs. Bahadori A., editor. Gulf Professional Publishing; 2018. Chapter ten - microbial enhanced oil recovery: microbiology and fundamentals; pp. 291–508. [Google Scholar]
- Town K., Sheehy A.J., Govreau B.R. MEOR success in Southern Saskatchewan. Soc. Pet. Eng. J. 2010 [Google Scholar]
- Varjani S.J. Microbial degradation of petroleum hydrocarbons. Bioresour. Technol. 2017;223:277–286. doi: 10.1016/j.biortech.2016.10.037. [DOI] [PubMed] [Google Scholar]
- Varjani S., Upasani V.N. Evaluation of rhamnolipid production by a halotolerant novel strain of Pseudomonas aeruginosa. Bioresour. Technol. 2019;288 doi: 10.1016/j.biortech.2019.121577. [DOI] [PubMed] [Google Scholar]
- Wang X., Yang Y., Xi W. Microbial enhanced oil recovery of oil-water transitional zone in thin-shallow extra heavy oil reservoirs: a case study of Chunfeng Oilfield in western margin of Junggar Basin, NW China. Petrol. Explor. Dev. 2016;43(4):689–694. [Google Scholar]
- Wang T., Yu L., Xiu J., Ma Y., Lin W., Ma T., Wang X., Wang L. A mathematical model for microbial enhanced oil recovery using biopolymer-producing microorganism. Fuel. 2018;216:589–595. [Google Scholar]
- Wang T., Yu L., Xiu J., Huang L., Cui Q., Ma Y. A mathematical model for microbial enhanced oil recovery considering the double-bacterial competition mechanism. J. Petrol. Sci. Eng. 2019;178:336–343. [Google Scholar]
- Wang X.-T., Liu B., Li X.-Z., Lin W., Li D.-A., Dong H., Wang L. Biosurfactants produced by novel facultative-halophilic Bacillus sp. XT-2 with biodegradation of long chain n-alkane and the application for enhancing waxy oil recovery. Energy. 2022;240 [Google Scholar]
- Wu B., Xiu J., Yu L., Huang L., Yi L., Ma Y. Biosurfactant production by Bacillus subtilis SL and its potential for enhanced oil recovery in low permeability reservoirs. Sci. Rep. 2022;12(1) doi: 10.1038/s41598-022-12025-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia M., Fu D., Chakraborty R., Singh R.P., Terry N. Enhanced crude oil depletion by constructed bacterial consortium comprising bioemulsifier producer and petroleum hydrocarbon degraders. Bioresour. Technol. 2019;282:456–463. doi: 10.1016/j.biortech.2019.01.131. [DOI] [PubMed] [Google Scholar]
- Xu Z., Knapp R.M., Mclnerney M.J. Soc. Pet. Eng. J; 1992. A mathematical model for microbially enhanced oil recovery process. (SPE Annual Technical Conference and Exhibition). SPE 24202-MS. [Google Scholar]
- Xu L., Xu G., Yu L., Gong H., Dong M., Li Y. The displacement efficiency and rheology of welan gum for enhanced heavy oil recovery. Polym. Adv. Technol. 2014;25(10):1122–1129. [Google Scholar]
- Yao C., Lei G., Ma J., Zhao F., Cao G. Laboratory experiment, modeling and field application of indigenous microbial flooding. J. Petrol. Sci. Eng. 2012;90-91:39–47. [Google Scholar]
- Zahner R.L., Govreau B.R., Sheehy A. Soc. Pet. Eng. J.; 2010. MEOR success in Southern California. (SPE Improved Oil Recovery Symposium). [Google Scholar]
- Zhang J., Feng W., Xue Q. Biosurfactant production and oil degradation by Bacillus siamensis and its potential applications in enhanced heavy oil recovery. Int. Biodeter. Biodegr. 2022;169 [Google Scholar]
- Zhao F., Guo C., Cui Q., Hao Q., Xiu J., Han S., Zhang Y. Exopolysaccharide production by an indigenous isolate Pseudomonas stutzeri XP1 and its application potential in enhanced oil recovery. Carbohyd. Polym. 2018;199:375–381. doi: 10.1016/j.carbpol.2018.07.038. [DOI] [PubMed] [Google Scholar]
- Zhao F., Zhu H., Cui Q., Wang B., Su H., Zhang Y. Anaerobic production of surfactin by a new Bacillus subtilis isolate and the in situ emulsification and viscosity reduction effect towards enhanced oil recovery applications. J. Petrol. Sci. Eng. 2021;201 [Google Scholar]
- Zhong Y., Luan T., Lin L., Liu H., Tam N.F.Y. Production of metabolites in the biodegradation of phenanthrene, fluoranthene and pyrene by the mixed culture of Mycobacterium sp. and Sphingomonas sp. Bioresour. Technol. 2011;102(3):2965–2972. doi: 10.1016/j.biortech.2010.09.113. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.