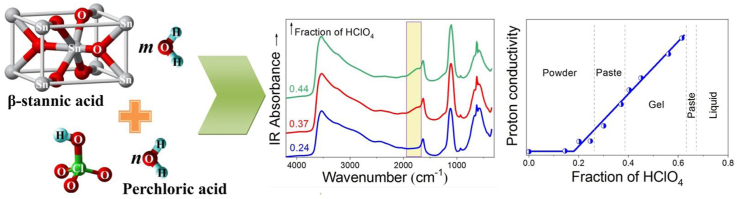
Abstract
The β-stannic acid powders(I) containing various amounts of perchloric acid with an initial concentration of 56.2 wt% HClO4 were studied by IR and Raman spectroscopy combined with XRD analysis and impedance spectroscopy. The addition of aqueous perchloric acid to I at room temperature leads to the adsorption compound formation. The degree of ionization of HClO4 is 100%. Based on these studies, a structural model has been proposed, according to which the hydrated H+ and ClO4¯ ions fill the pores/capillaries of I. The structure of I does not change during the adsorption process of ions. The finely dispersed powder of I turns into a viscous transparent gel with an optimal ratio of reagents, which indicates its plasticization. The electrochemical properties of the plasticized samples were investigated by impedance method. It was found that plasticization can increase the proton conductivity by more than two orders of magnitude. The plasticized samples have sensory properties. The plot of conductivity 25 versus weight fractions of perchloric acid solution Wpa reveals a linear dependence at RH = 32% and t = 25 °C in a wide range of Wpa values. The maximum value of 25 = 0.43 S cm−1 is achieved at Wpa = 0.61. The straight line has a break at the very beginning due to the perchloric acid flow threshold.
Keywords: Perchloric acid, Stannic acid, Adsorption compound, Vibrational spectra, Impedance spectra
Graphical abstract
Perchloric acid; Stannic acid; Adsorption compound; Vibrational spectra; Impedance spectra.
1. Introduction
Examples of the stannic acid practical use are few, which, however, do not diminish its value as a material with pronounced ion-exchange properties. In particular, this acid is used to concentrate and separate of isotopes [1]. The extraction of platinides, lanthanides and some other heavy metals from complexing solutions [2] is another direction of its practical use as an adsorbent. One more direction is the oxidative dehydrogenation catalysis of organic substances [3]. The acid is also used as a precursor in tin dioxide synthesis [1, 4, 5]. In addition, stannic acid (tin dioxide hydrate) is a solid-state proton conductor [6] which makes it a promising substance for some electrochemical applications.
Hydrolytic decomposition of SnCl4 with an aqueous ammonia solution can produce two forms of stannic acid (α and β), while the reaction of concentrated nitric acid with metallic tin can produce only one form (β) [7, 8, 9]. A freshly precipitated gel contains the α-form which takes a lot of water with it. To prepare the β-form, the gel is subjected to aging or heating. As a result an irreversible process of gradual enlargement (polymerization) of colloidal particles occurs. The process is accompanied by the separation of the liquid phase part initially entrained by ions. An increase in the size of colloidal particles naturally affects their properties.
The transition α → β occurs spontaneously even when the gel is kept under the layer solution. Intermolecular or valence forces acting between colloidal particles cause the formation of a loose spatial network. The two forms of stannic acid can be recognized by a solubility test: the α-form dissolves in concentrated acids and alkalis, whereas the β-form is practically insoluble. In addition, the α-form does not exhibit a microcrystalline structure, while the β-form does [9]. In the reference literature and original papers, different formulas of the stannic acid were used, which can be explained by uncertainty as to its chemical composition. Some versions are presented in Table 1.
Table 1.
Formulas of the stannic acid.
Formula 1 in the first column and Formula 2 in the second column correspond to the stannate formulas (Na2SnO3⋅3H2O, in particular). Formula 2 in the first column and similar formula 3 in the first and second columns represent α- and β-stannic acids as stannous dioxide hydrate. In up-to-date publications, formula 3 represents, as a rule, insoluble gels belonging to the β-form, although sometimes it is also used to represent the α-form [5]. In some works [5, 11] the formula H4SnO4 used, which is equivalent to formula 4. Moreover, stannic acid is sometimes represented even as a mixture of acids: H4SnO4, H2SnO3 and H10Sn5O15 [12]. The authors of the monograph [1] use various variants of the formulas, analogous to the above formulas of stannic acid. In addition, they also use formula H2Sn(OH)6.
The isoelectric point of the freshly precipitated gel, according to the literature data, is near pH = 10. The stannic acid formation goes through a series of stages. The first stage should be the formation of Sn(OH)4 which is obviously unstable. The stage of hydroxide transformation into a stannic acid gel, according to the scheme Sn(OH)4·mH2O ≡ H4SnO4·mH2O → SnO2·2H2O + (m– 2)H2O, presumably should be very fast. Some hydroxide ions remain in the structure of the SnO2·2H2O gel. The analysis of SnO2·mH2O samples by proton magnetic resonance [1, 13] allowed the identification of the terminal and bridging anionic hydroxyl groups (≡Sn–OH and ≡Sn–(OH)–Sn≡, respectively). Based on these results, a model was proposed according to which the colloidal particle core consists of crystalline tin dioxide, and the surface layer is tin hydroxide or oxyhydroxide.
Some ambiguity still exists concerning the possible structural differences between the α-and β-forms. The IR spectra attributed to the α-form turned out to be identical to the spectra of the β-form [14, 15, 16].
The high sorption capacity for water and ions is due to the porosity of the material and, in addition, to the presence of a large number of acidic and basic adsorption sites. The value of the acid dissociation constant K1 = 4 · 10−10 for Sn(OH)4 is given [17]. Since tin (IV) hydroxide is not stable, the above K1 value should be referred, in fact, to SnO2·2H2O. For SnO2·2H2O pellets the value of volume specific conductivity 25 is 4 · 10−4 S cm−1 [6]. Taking into account the fact that stannic acid is a very weak electrolyte, the level of electrical conductivity established for it can be regarded generally speaking as rather high.
The goal of this work was to study vibrational spectra and electrochemical properties of the stannic acid doped or modified by ions of a strong electrolyte, such as perchloric acid. The β-form samples were taken as the basis because they dissolve neither in acids nor in alkalis (more precisely, they dissolve but only slightly). In the chemical literature, we did not find data on the behavior of stannic acid in mixtures with any strong acid.
The known chemical properties of these two acids left little chance that the study of their mixtures would give new results. The behavior of a number of weak inorganic and organic acids in anhydrous HClO4 solutions was thoroughly studied more than half a century ago by the available methods of analytical and physical chemistry. Nitric, phosphoric, selenic, acetic and some other acids in mixtures with anhydrous HClO4 exhibit the properties of bases, which leads to the formation of liquid and crystalline compounds –acylperchlorates [18]. The acylperchlorate is not known for the stannic acid, most likely because it is unstable. In addition, the function of any soluble weak acid as a base cannot be realized in diluted HClO4–H2O solutions due to the competition with water molecules. Meanwhile, the specific nature of the β-stannic acid samples made it possible, starting from a heterogeneous system “SnO2·mН2О-HClO4–H2O”, to obtain a homogeneous plastic material with characteristic features of an adsorption compound, which is sufficiently stable. It is high conductive at room temperature due to the aqueous perchloric acid presence. The conductivity of the obtained material is commensurate with the aqueous perchloric acid conductivity. Among the perchloric acid crystalline hydrates, the closest structural analogue of this adsorption compound is the glass-crystalline solid solution HClO4·nH2O, which has an open maximum at the point with coordinates n = 5.5, t = - 44.7 °C in the HClO4–H2O melting diagram [18, 19]. The analogy arises due to the proton inclusion in the network of hydrogen bonds of water. This solid solution belongs to the aqueous clathrates type [20] and has an appreciable proton conductivity [21].
Crystal hydrates of perchloric acid with n = 1 and 2 belong to a completely different structural type. Their structure contains the discrete H3O+ ions in the first case and H5O2+ in the second. The rather high proton conductivity of crystals with n = 1, namely, 3.41 ·10−4 S cm−1 at 25 °С reported by the authors of the paper [22] needs, in our opinion, verification judging by the nature of the IR spectra published in some other articles of this research team. In particular, rather intense bands at 3220 and 3340 cm−1 in the IR absorption spectra of the low-temperature monoclinic phase H3O+ClO4- were misinterpreted [23, Figure 4b] and [24] by belonging to the ν3H3O+ and 2ν4H3O+ vibrations, while these two bands actually belong to the OH stretching vibrations of H5O2+. It is known that H5O2+ClO4− crystals have mp. – 20.65 °C [18], and the melting point for the eutectic mixture H5O2+ClO4- with H3O+ClO4- is even lower. Conductivity was measured [22] at 25 °С and additionally at negative temperatures. From this it follows that the surface liquid H5O2+ClO4- film could affect the measured conductivity of H3O+ClO4- solid samples at room and sub-zero temperatures. Also, some indirect evidences suggest that there are no conditions for proton transfer in the similar crystals provided n = 1. In particular, the following dependence of the values of proton conductivity and activation energy on n was established for the H4SiW12O40·nH2O crystalline hydrates [25]:
| n | 3.3 | 4.6 | 7 | 9 | 12 | 14.8 | 15.8 | 17 | 22 |
| σ20, cm-1 | 2.15·10−8 | 2.14·10−7 | 8·10−4 | 1.8·10−3 | 3.2·10−3 | 1.0·10−2 | 0.55·10−2 | 1.2·10−2 | 2.9·10−2 |
| E, eV | 0.53 | 0.51 | 0.335 | 0.334 | 0.370 | 0.355 | 0.334 | 0.357 | 0.342 |
Figure 4.
Raman spectra of β-SnO2·mH2O: pressed air-dried white powder, m = 1.3 (a) and pressed translucent sample, m = 2 (b).
It follows, in particular, that at room temperature these silicotungstic acid crystalline hydrates are dielectrics when they contain predominantly H3O+ ions (n/4 ≤ 1). The conductivity increases by several orders of magnitude, while the activation energy decreases by a factor of 1.6 when H5O2+ ions (n/4≅2) appear in the crystal structure. Similar data are given in the article devoted to inelastic neutron -scattering study of proton dynamics in β – alumina [26] where it is said that conductivity due to jump H3O+ ions is very weak under normal conditions: the measured conductivity is ∼10−11 Scm−1 with an activation energy ∼0.8 eV. There are also other examples of the very low proton conductivity in compounds containing only H3O+ ions, but not having H5O2+ or higher proton hydrates in their structure [27, 28]. The reason for the low conductivity in all these examples should be sought in the formation of rather strong hydrogen bonds with the anion, which, on the one hand, stabilize the H3O+ ion in the crystal structure and, on the other hand, prevent its migration from anion to anion. Due to the fact that the vibrational spectra are not given directly in [22], of course, we cannot state with certainty that the conductivity measurements were made not for clear samples but for samples with the H5O2+ClO4- contamination and that the conductivity of pure samples of the orthorhombic phase H3O+ClO4- at 25 °С is extremely small in reality. Only one thing is quite obvious: all the IR spectra of the high-temperature orthorhombic phase and the low-temperature monoclinic phase, which were published in different years by the above research team, contain H5O2+ClO4- impurity bands.
Analyzing mixtures of stannic and perchloric acids, we actually obtained a gel electrolyte. Generally speaking, it can be considered as an inorganic analog of polymer gel electrolytes with high values of protonic conductivity. Many examples of the polymer electrolytes gel use in fuel cells, electrochromic devices, solid state batteries, supercapacitors, etc. are given in the reviews [27, 28]. The presence of perchloric acid in the stannic acid gel electrolyte makes it apparently unpromising for these purposes. There is, however, a potential for its practical use in the hydrogen sensors, as described in our work. A similar but heterogeneous proton-conducting material (composite) was previously obtained for a SnO2·1.5 H2O – H3PW12O40 ·nH2O system and studied by scanning tunneling spectroscopy and impedance spectroscopy [29].
2. Materials and methods
2.1. Materials and reagents
β-Stannic acid (hydrate of tin dioxide) was synthesized using a slightly modified procedure [9]. For this purpose, tin tetrachloride was hydrolyzed by an aqueous ammonia solution. In another series of experiments, this solution was replaced with an aqueous solution of sodium hydroxide. A 175.28 g portion of SnCl4·5H2O was dissolved in 200 g of H2O, filtered off, and 200 ml of 35 wt% hydrochloric acid was added to lighten the solution. Then 450 g of 7.4 wt% aqueous NaOH solution was added to the resulting solution, mixed with a magnetic stirrer, heated to t = 100 °C and kept at this temperature for 1 h, whereupon the pH was brought to 7–8. The deposited gel was washed six times with distilled water in a centrifuge (8.5 thousand rpm, 15 min). The washed precipitate was dried in air at a fixed temperature t = 35 °C for 2 days. Then it was ground and subjected to additional drying in air under the same conditions for a day. The yield of the final product is 55.21 g. An air-dried β-stannic acid precipitate is a colorless powder. The TG and DSC curves of the non - plasticized β-stannic acid samples used in the experiments with perchloric acid are shown in Figure 1. It corresponds to the chemical formula SnO2·mH2O, where m = 1.3.
Figure 1.
The TG and DSC curves of the β - stannic acid samples whose composition corresponds to the chemical formula SnO2·mH2O, where m = 1.3.
The purity of the samples was checked by vibrational spectroscopy. Gels precipitated with an ammonia solution contain adsorbed ammonium ions, traces of which are detected by spectroscopic methods even during the most thorough cleaning. Gels specifically for spectroscopic studies were precipitated with an aqueous solution of sodium hydroxide, which was necessary to eliminate the NH4+ maxima which screen some of the weak bands of the hydroxyl group.
Additionally α-stannic acid samples were synthesized to compare their properties with those of the β-form samples. The synthesis procedure was similar to the previous procedure. The only difference is that all operations were carried out at 0 °C.
2.2. Plasticization of β-stannic acid powder by aqueous perchloric acid
The β-SnO2·mH2O powder with m = 1.3 was plasticized by a calculated amount of an aqueous solution of perchloric acid with a concentration of 56.2 wt% HClO4. The suspension formed by mixing the powder with the solution was vigorously stirred at room temperature until the residual traces of the liquid phase disappeared. During the exothermic reaction, the formation of a transparent mass was observed. The degree of its transparency depends on the perchloric acid content. At a low content, a white powder is formed. It looks like the pure β-stannic acid, except that it consists of somewhat larger grains. The SnO2/HClO4/H2O molar ratio was estimated based on the weighed portions of the initial reagents. A number of additional experiments were performed with concentrations of 20 and 10 wt% HClO4.
Note that the term plasticization in the scientific literature refers to the introduction of low-volatile low-molecular substances (plasticizers) into polymers that increase their plasticity and elasticity. Plasticization affects the mechanical properties, dielectric properties and electrical conductivity of the polymer. Stannic acid can in principle be considered as a kind of inorganic polymer. It is a free-flowing crystalline powder consisting of rather hard grains, which, however, is well compressed into pellets. When mixed with a known amount of HClO4– H2O solution, the grains of the powder spontaneously stick together, forming a very viscous, plastic and transparent material, and the liquid phase disappears. This phenomenon, found by visual examination of β-stannic acid- HClO4– H2O mixtures, is not observed when the stannic acid is replaced with a SnO2 crystalline powder, see Section 3.1. From this point of view, the term plasticization seems to be quite justified in relation to β-stannic acid, the physical properties of which undergo changes under the influence of perchloric acid. The plasticization effect gives reason to consider the interaction products of two acids at room temperature as a single-phase chemical material, and not as a simple mechanical mixture of two phases.
2.3. Characterization techniques
Samples of stannic acid and its plasticization products were studied by physical analysis methods such as X-ray diffraction (XRD) (diffractometer AERJS PAN ANALYTICA), IR absorption spectroscopy (vacuum FTIR spectrometer Bruker model Vertex 70V, resolution 4 cm−1), ATR IR spectroscopy (Vertex 70V with diamond Platinum – ATR accessory) and Raman spectroscopy (Vertex 70V with RAMII accessory, λexc = 1064 nm, resolution 4 cm−1). The presence of water in the non - plasticized stannic acid samples was searched by the DSC method on Netzsch STA 409 PC Luxx® synchronous thermal analyzer and QMS 403 C Aëolos instruments. The decomposition curves were recorded in the temperature range 25–700 °C, the heating rate was 5 °C/min.
IR absorption spectra of the films on a silicon plate and ATR IR spectra of the powders and gels on a diamond crystal were recorded in the range of 350–4000 cm−1 as a rule at atmospheric pressure to avoid dehydration. And only in some cases the rapid recording was done under vacuum (<1 hPa) in order to study the long-wavelength range from 50 to 350 cm−1.
Powders of stannic acid with a low content of perchloric acid (weight fraction of the solution 0.14–0.20) have a poor adhesion to silicon plate. For this reason, they were pre-moistened with a small amount of distilled water to prepare a thin layer on the plate before recording the IR absorption spectra. The paste formed upon moistening was ground on the rough side of this plate until a thin layer was formed, and then dried in air. Plastic samples and pastes with a significant content of perchloric acid have a good adhesion. So they needed no moistening. Raman spectra were recorded for the powders and pastes pressed into a hole with a 2 mm diameter in a small stainless steel rim.
Electrochemical cells were made by sequential pressing of pellets from the material under study and the material of electrodes in a Teflon cage; the conductivity (σ) and Faraday resistance RF were investigated by the electrochemical impedance method on a Z-500PX device (ELINS, Russia) in the frequency range 14 mHz–500 kHz with a voltage amplitude of 5 mV.
2.4. Electrochemical measurements
The conductive and electrocatalytic properties of the plasticized β-stannic acid pellets were studied by the electrochemical impedance method using symmetric electrochemical cells of two types:
| Ti(C) / Sample/ Ti(C) I |
| Ti(C, Pt, air, H2) / Sample/ Ti(C, Pt, air, H2) II |
Carbon paper electrodes (I) or with a platinum catalyst on the carbon paper (II) were used. Prior to measurements, the gel samples were kept for several days in a desiccator with a fixed humidity (RH = 32%). Electrochemical cells were made by successive pressing in a Teflon holder of pellets from the test material with a diameter of 3 mm for (I) and 5 mm for (II) and the material of the electrodes. The compacting pressure varied from 1 t/cm2 to weak manual pressing, depending on the Stannic acid - HClO4 - H2O system composition. The specific conductivity of the pellets was calculated using the equation:
| (1) |
where Rel is the measured bulky resistance of the solid electrolyte, which is proportional to the pellet thickness(l) and inversely proportional to its area (s). For cells (II) with active electrodes, the charge transfer resistance (Faraday resistance RF) was additionally measured, which characterizes the reversibility of the ongoing electrochemical reaction at the electrode-solid electrolyte membrane interface. From this it follows that RF resistance has a surface character. It is inversely proportional to the s value. The RF value includes series-connected resistances of two identical boundaries Ti(C, Pt, air, H2)/Sample of the cell (II) under study. Respectively, to calculate the Faraday resistivity the measured RF value must be divided by 2. The specific Faraday resistance was calculated using Eq. (2):
| (2) |
Where and are the measured and the specific Faraday resistance, respectively.
3. Results and discussion
3.1. The composition and physical properties of the β-stannic acid plasticized with perchloric acid
Mixing SnO2·mH2O (m = 1.3) powder with a solution of HClO4·nH2O (n = 4.3) at room temperature leads initially to the formation of non-transparent suspension, with the viscosity very low. After a short time, the reaction mixture becomes more and more transparent, viscous and adhesive. The process is accompanied by a positive heat effect. A transparent plastic sample without traces of a liquid phase can be obtained at the optimal ratio of reagents. Namely, the weight of solution in the initial mixture should be approximately equal to the powder weight and in no case should exceed it by more than one and a half times (Table 2). The HClO4–H2O solution acts on the β-stannic acid powder in this case as a plasticizer. In turn, the β-stannic acid acts as a thickener for the solution. Dilution of HClO4 to 20 wt% (n = 22.3) and 10 wt% (n = 50.2) at the fixed ratio of HClO4/SnO2 almost does not affect the results. Only an additional time is required to evaporate the liquid water excess. Although some influence on the optical properties is observed: after excess water evaporation, the samples do not become entirely transparent. They continue to remain turbid presumably due to the microscopic inclusions of colloidal solution drops.
Table 2.
The composition and aggregate state of the β-stannic acid samples plasticized with perchloric acid having a concentration of 56.2 wt% HClO4.
| Wpa | SnO2:H2O:HClO4 | Sample description |
|---|---|---|
| 0.142 | 1:2.4:0.170 | White powder |
| 0.197 | 1:2.8:0.251 | White powder |
| 0.243 | 1:3.1:0.329 | White powder |
| 0.296 | 1:3.5:0.430 | Nontransparent paste |
| 0.365 | 1:4.2:0.589 | Semitransparent paste |
| 0.397 | 1:4.6:0.674 | Transparent gelatinous sample |
| 0.444 | 1:5.2:0.818 | Transparent gelatinous sample |
| 0.481 | 1:5.7:0.949 | Transparent gelatinous sample |
| 0.555 | 1:7.1:1.28 | Transparent gelatinous sample |
| 0.609 | 1:8.5:1.59 | Transparent gelatinous sample |
| 0.643 | 1:9.5:1.84 | Semitransparent paste |
| 0.674 | 1:11:2.12 | Nontransparent viscous liquid |
After some time, cracks appear in the transparent sample, but it remains plastic for an unlimited time. After a long-term storage in air at room temperature transparent colorless samples turn yellow, but we did not find any other indications of decomposition. Note that such samples are low hygroscopic. If the powder weight exceeds the standard HClO4–H2O solution weight by ≥ 3 times, the final product is a non-hygroscopic colorless powder. Unlike transparent samples, this powder does not turn yellow after storage, despite the fact that it contains perchloric acid. Visually, it resembles a white pure β-form powder, but it consists of larger grains. A non-transparent viscous or semi-liquid paste was obtained when the weight of perchloric acid with a concentration of 56.2 wt% HClO4 exceeded the powder mass by 1.8 times.
The weight fraction of HClO4 aqueous solution in the plasticized β-stannic acid was calculated by the formula:
| Wpa = wpa / (wpa + wβ), | (3) |
where wpa is a weight of perchloric acid sample and wβ is a weight of β-stannic acid sample. The formula to calculate the HClO4·nH2O molar fraction in the plasticized β-stannic acid has a similar form:
| Xpa = npa / (npa + nβ), | (4) |
where npa is the number of moles of the HClO4·nH2O solution and nβ is the number of moles of β-stannic acid SnO2·mH2O.
The molecular weight of SnO2·mH2O at m = 1.3 is close to the molecular weight of HClO4·nH2O at n = 4.3. In this case, Wpa ∼ Xpa. On average, Wpa = Xpa – 0.005. The Wpa and Xpa values refer to the really existing metastable binary subsystem SnO2·mH2O–HClO4·nH2O. For the conventional ternary system SnO2–H2O–HClO4, the molecular composition of a transparent sample (gel) can be characterized by the chemical formula SnO2·zH2O·yHClO4, where z = m + n·y. Stoichiometric coefficients z and y are calculated from the weighed portions of initial reagents. The y value does not depend on the ambient humidity. While the values of m, n and z depend on the humidity of the environment within certain limits. It can be shown that Xpa = y/(1 + y). The calculated values of z and y (molar ratios of the components for the ternary system) are given in Table 2.
Note that stannic acid is not formed under normal conditions in the stable SnO2–H2O–HClO4 ternary system when its components are crystalline tin dioxide, water, and perchloric acid. In control experiments, tin dioxide hydrate powder was replaced with highly dispersed crystalline tin dioxide powder (cassiterite) which is chemically inert with respect to aqueous solutions of perchloric acid at room temperature. It was found that mixing of 56.2% perchloric acid with SnO2 powder in any proportions gives a non-transparent milk-white suspension. This suspension does not disappear with an arbitrarily long stirring, and its viscosity remains small all this time. After stirring is stopped, the liquid phase i.e. initial H2O–HClO4 solution is detected by sight due to the rapid sedimentation of SnO2 crystalline particles. Thus, a SnO2–H2O–HClO4 mixture based on the crystalline tin dioxide does not possess the properties of a metastable binary subsystem SnO2·mH2O–HClO4·nH2O, which can also be represented as a ternary subsystem SnO2·mH2O–H2O–HClO4.
The α-stannic acid freshly precipitated at 0 °C differs in its properties from the β-stannic acid in that it completely dissolves in 56.2% perchloric acid. As a result, a transparent colloidal solution is formed. However, after a few hours of exposure at room temperature, the viscosity of this solution increases to give a transparent gelatinous mass. Hence, it follows that the irreversible process α → β occurs in a colloidal solution.
The Wpa values in Table 2 were calculated by formula 3. The values of Xpa calculated by formula 4 are very close to the values of Wpa.
3.2. Vibrational data for the starting reagents
The β-stannic acid–aqueous perchloric acid system is in a metastable state at room temperature. Both acids interact with each other only through the formation of hydrogen bonds. As a consequence, their IR and Raman spectra change insignificantly under the hydrogen bonds influence. Formally, to track the changes, it would be necessary to decompose the contours of the overlapping bands and lines. However, their contours are very asymmetric, which greatly complicates solving problem. A more efficient approach, as we see it, consist in a detailed study of the spectra of each component separately for the purpose of subsequent comparison with the spectra of the final products.
3.2.1. ATR IR and IR absorption data for the β-stannic acid
The ATR IR spectra and IR absorption of the β-Sn O 2·mH2O samples are shown in Figure 2. All of them are similar to the known IR absorption spectra for KBr pellets of SnO2·mH2O (m = 1.25 [15], 1.5 [30, 31]). Some other values of m from 0.5 to 4 were reported earlier [6, 13, 32]. The m variation is explained by the dependence on the synthesis conditions and environment humidity. A significant part of the available water molecules are physically adsorbed. Physical adsorption occurs, in particular, due to the formation of hydrogen bonds between H2O molecules and anionic hydroxyl groups Sn–OH inside the stannic acid capillaries/pores. Such molecules are easily removed under vacuum at room temperature. A strong broad band at 3430 ± 10 cm−1 in the IR spectrum for KBr pellets mainly refers to the stretching vibrations ν(OH) of these molecules and to a lesser degree to the stretching vibrations ν(OH) of anionic Sn–OH hydroxyl groups. It has a high degree of asymmetry, which is explained by the overlapping of a very broad band at ∼2990 cm−1. The overlapped band was assigned to the stretching vibrations ν(OH) of the tin (IV)-coordinated water molecules in [30, 31]. The coordination is implemented on the surface of colloidal particles aggregates through the formation of metal–oxygen bonds (Sn–OH2) with chemisorbed water molecules. This kind of molecules can be removed at temperatures above 300 °С [13]. Therefore, tin (IV)-coordinated water molecules can be considered as chemisorbed. A medium intensity narrow band at 1641 ± 6 cm−1 was assigned to the bending δ(HOH) vibration of the physically adsorbed and chemisorbed H2O molecules. Weak bands with diffuse maxima at 1020 ± 60 cm−1 and 1220 ± 20 cm−1 were assigned for KBr pellets to the bending δ(SnOH) vibrations of anionic hydroxyl groups [14, 30]. The strong band with a maximum at 565 ± 5 cm−1 (shoulder at 665 ± 5 cm−1) belongs to the SnO stretching vibrations [30].
Figure 2.
ATR IR spectra of β-SnO2·mH2O: air-dried white powder, m = 1.3 (a) and ground to white powder originally translucent sample, m ∼ 2 (b). IR absorption spectrum of the β-SnO2·mH2O (m ∼ 2) translucent film obtained at a silicon window (c).
All these bands previously found for KBr pellets are also present in the ATR spectra of β-stannic samples. In particular, the broad asymmetric ν(OH) band at 3212 cm−1 (shoulder at 3278 cm−1) was found in the spectra of air-dried powders SnO2·mH2O (m = 1.3) recorded at atmospheric pressure. The ATR spectrum recorded during exposure of the same sample under vacuum is shown in Figure 2a. In this case, the ν(OH) band is observed at 3205 cm−1 (shoulder at ∼ 3311 cm−1) and δ(HOH) band at 1640 cm−1. Small frequency changes are explained by exposure the sample under vacuum. Two weak δ(SnOH) bands are observed at 1019 and 1240 cm−1. The ν(SnO) strong band is observed at 500 cm−1 (shoulder 614 cm−1). In the long-wavelength ATR IR spectrum, a medium intensity doublet with maxima at 241 ± 2 cm−1 and 274 ± 2 cm−1 was found related to the SnO deformation vibrations. Note that in the ATR IR spectrum of crystal powder SnO2 (cassiterite) the ν(SnO) vibrations give a strong band at 575 cm−1 (shoulders 480 and 589 cm−1), whereas δ(ОSnО) vibrations give rise to a medium intensity doublet with maxima at 238 and 283 cm−1.
To analyze the dependence of the SnO2·mH2O spectra on the sample preparation procedure method, the β-stannic acid powder (m = 1.3) was treated with an aqueous solution of perchloric acid, followed by thorough washing with distilled water and drying in air at room temperature. As a result, the value m = 2 was obtained, which was established by the DSC method. The increase in m is probably due to a change in the capillaries/pores size. The washed sample looked like a translucent mass. Its ATR IR spectrum is shown in Figure 2b. The difference between this spectrum and the spectrum in Figure 2a is generally small. However, only one of the two δ(SnOH) bands (at 1240 cm−1) shifts slightly to 1237 cm−1, while the other one (at 1019 cm−1) shifts to 957 cm−1 (Figure 2b).
The IR absorption spectrum of the β-stannic acid film on the Si plate is shown in Figure 2c for comparison with the ATR IR spectrum in Figure 2b. To obtain a sample of the required thickness for the IR absorption spectrum recording, the β-stannic acid powder was placed on the Si plate and pretreated with perchloric acid. The finally formed thin film was washed from perchloric acid with a large amount of distilled water and then dried in air at room temperature. The IR absorption spectrum as a whole is similar to the ATR IR spectrum. The positions of ν(OH) and of two δ(SnOH) bands depend more than other on the sample preparation method. In the absorption spectrum, the ν(OH) band is observed at 3375 cm−1 and δ(SnOH) bands are observed at 1079 and 1228 cm−1. Also the ν(SnO) frequency at 553 cm−1 is noticeably different. It increases by 36 cm−1 on going from the ATR spectrum to the IR absorption spectrum. As a rule, this frequency does not go beyond the values of 490–549 cm−1 when using the ATR IR spectroscopy method, regardless of the sample preparation method.
Note that the prepared pure β-stannic acid samples contain the proton hydrates in very small amounts that cannot be detected by vibrational spectroscopy methods. Broad bands of water molecules and hydroxyl groups produce a significant background, thereby preventing their detection. A similar difficulty also arises when the β-stannic acid powder adsorbs small traces of aqueous perchloric acid.
For the β-stannic acid spectroscopic determination in the presence of perchloric acid, the ν(SnO) band is of analytical significance. Its other bands are shielded to a large extent or completely by the IR bands of proton hydrates, perchlorate ions and free water molecules belonging to perchloric acid.
3.2.2. ATR IR data for the α-stannic acid
The ATR IR spectrum of the gel freshly precipitated at 0 °C (Figure 3a), is similar to the ATR IR spectrum of the β-stannic acid. Two bands of δ(SnOH) bending vibrations at 1003 and 1172 cm−1 appear more pronounced after short (1 min) vacuum drying (<1 hPa) of a freshly precipitated α-stannous acid gel due to the removal of a part of physically adsorbed Н2О molecules (Figure 3b). The results of IR spectroscopic studies of the α-stannic acid are consistent with the literature data [14, 15, 16] from which it follows that its spectrum practically does not differ from the spectrum of the β-form.
Figure 3.
ATR IR spectra of α-SnO2·mH2O: freshly precipitated at 0 °C milky - white gel, m = 6.5 (a), powder held for several minutes at room temperature under vacuum, m < 6 (b).
3.2.3. Raman data for the β-stannic acid
The single strong line ν(SnO) is found in the powder spectrum of β-stannic acid (SnO2·mH2O) at 573 ± 2 cm−1 (shoulders at 500 ± 3 cm−1 and 445 ± 5 cm−1) (Figure 4a). The complication of its contour can be explained by the existence of various types of tin–oxygen bonds. Some bonds should belong to the core of the colloidal particle SnO2, others to hydroxyl groups (Sn–OH) and to coordinated water molecules (Sn–OH2). Note that the SnO2·mH2O deuteration causes a shift of the ν(SnO) maximum to 565 ± 3 cm−1. A weak line δ(OSnO) is observed as a barely visible shoulder at 190 ± 3 cm−1 on the exciting line wing. The very weak broad bands of water molecules and hydroxyl groups practically merge with the general background. The very weak broad line (band) with a maximum at ∼3438 cm−1 presumably corresponds to the OH stretching vibrations of physically adsorbed water molecules. Another weak band, with a maximum at ∼ 2929 cm−1, probably corresponds to the stretching vibrations of chemisorbed water molecules. Besides two very weak bands at ∼1275 and ∼1066 cm−1 were found in the frequency range of bending vibrations δ (SnOH) of hydroxyl groups.
The ν(SnO) line is detected at 572 cm−1 in the Raman spectrum of the β-form treated with perchloric acid and then washed it off (Figure 4b). This line is suitable for the β-stannic acid determination in the presence of perchloric acid by Raman spectroscopy. Whereas all other lines are very weak.
3.2.4. Raman data for the α-stannic acid
The Raman spectrum of the freshly precipitated at 0 °C α-stannic acid gel is shown in Figure 5а. It does not practically differ from the spectrum of β-stannic acid. In this case the ν(SnO) line is observed at 578 cm−1 with shoulder at 457 cm−1. An air-dried sample of α-stannic acid has a ν(SnO) line at 573 cm−1 (Figure 5b).
Figure 5.
Raman spectra of α-SnO2·mH2O: freshly precipitated at 0 °C milky-white gel, m = 6.5 (a) and pressed air-dried powder, m < 6 (b).
3.2.5. IR absorption data for the aqueous perchloric acid
Figure 6 shows the IR absorption spectra of aqueous perchloric acid recorded for concentrations 56.2, 20 and 10 wt% HClO4. The results of IR spectroscopic studies of the HClO4–H2O solutions are consistent with the data [33]. Perchloric acid, predominantly with the concentration 56.2 wt% HClO4, was used to plastisize stannous acid in most of our experiments. Higher concentrations could cause a destructive chemical reaction, while lower concentrations should lengthen the experiment time due to the need of solvent excess evaporation. Nevertheless some experiments were also carried out using much lower HClO4 concentrations.
Figure 6.
IR absorption spectra of aqueous perchloric acid. The concentration is 56.2 (a), 20 (b), and 10 (c) wt% HClO4.
The structures of an aqueous solution of perchloric acid and its crystalline hydrate should have a number of common details, provided that their compositions coincide or differ slightly. The closest structural analogue of liquid acid with a concentration of 56.2 wt% HClO4 is a solid solution, for which the coordinates of the maximum point on the melting diagram of HClO4–H2O system are C = 50.45 ± 0.15 wt% HClO4 and t = -44, 7 ± 0.7 °C and the coordinates of two extremal points are equal to С = 48.0 ± 0.2 wt% HClO4, t = -60.0 ± 0.6 °C and С = 53.2 ± 0.2 wt% HClO4 and t = -60.0 ± 0.6 °C. These most accurate coordinates were obtained by the crystal-optical method in [19]. The open maximum on the melting curve corresponds to the molar ratio n = H2O/HClO4 = 5.5 of the components of the HClO4–H2O system. According to the data of crystal-optical studies, the solid solution has a glass-transparent plastic phase. The plastic phase is formed by cubic crystals due to their spontaneous coalescence. The structure of these crystals has no orientational long-range order. The information on the properties of the glass-transparent plastic phase provides additional arguments to compare it with glassy and liquid solutions. The XRD study showed that the HClO4·5.5H2O cubic crystals have the clathrate structure [20]. The low-temperature IR and Raman spectra were recorded for the maximum point and for the two extremal points of the perchloric acid solid solution. Some similarity was found between the spectra of plastic, glassy and liquid phases [34].
It is noteworthy that the clathrate structure appearance in dilute aqueous solutions of strong electrolytes was predicted by the theoretical model [35]. In the case of very high concentrations, the liquid perchloric acid structure correlates to a greater extent with the structure of crystalline hydrates HClO4·nH2O for which n < 3.5, as supported by IR and Raman spectroscopy data [33, 36, 37, 38, 39]. A review of X-ray structural studies of a large number of perchloric acid crystalline hydrates with n = 1.0, 2.0, 2.5, 3.0 and 3.5 was earlier published in [40].
In relation to the dilute perchloric acid, the model [35] predicts the ClO4- localisation inside the suitable voids of the water network. The proton is not localized, but is distributed between the poles of H2O molecules, so that the so-called "proton gas" is formed. Model [35] does not impose any restrictions on the number of water molecules between which the proton is distributed in a dilute aqueous solution, although physicochemical data indicate that such restrictions actually exist. The hydration numbers of the proton h in perchloric acid were estimated using ATR IR spectroscopy method [41] and from the values of the thermodynamic activity of water [42]. Calculated on the basis of thermodynamic data the h values were plotted versus the molar ratio n = H2O/HClO4. In this way, it was found in particular, that h is limited to a value of 6.4 at n→ ∞. Based on the difference n–h, the number of liquid water molecules not bound by a proton can be estimated.
Recalculation of the 56.2 wt% HClO4 concentration to n gives the value 4.3. Based on the graph h(n) [33], we find that this value of n corresponds to h = 4. Then n–h = 0.3. Such a small amount of free water molecules is not determined by IR absorption spectroscopy, as evidenced by Figure 6a. In this case the proton influence extends to only four water molecules. Four molecules are quite enough to form a clathrate structure similar to that of solid solution. Although at n = 4.3, the cavities inside which the anion is located may not be completely formed. The wall contacts of the H2O molecules with intracavitary ClO4- anions lead to a rather weak hydrogen bonding. Weak hydrogen bonds are responsible for the strong ν(OH) band at 3541 cm−1 . with a moderately broad contour and for the weak δ(HOH) band at 1634 cm−1 with a narrow contour. Strong hydrogen bonds are responsible for the strong continuous ν(OH) band at ∼2800 cm−1 and for the medium intensity δ (HOH) band at 1750 ± 10 cm−1 with a relatively broad contour. A strong band at 1119 ± 2 cm−1 refers to the triple degenerate antisymmetric stretching vibration ν3 of the ClO4- anion. A medium intensity band at 627 ± 1 cm−1 refers to the triple degenerate deformation vibration ν4. A weak sharp band at 929 ± 2 cm−1 refers to the totally symmetric vibration ν1 which is IR forbidden for the ClO4- point symmetry group Td. A very weak forbidden band ν2 of the double degenerate deformation vibration is not observed. The forbidden band ν1 appearance means that the ClO4- tetrahedral symmetry is violated to a small extent due to the formation of the hydrogen bonds between the proton hydrates and anion. The ν3 band is five times broader than the ν4 band. The reason is the well-known features of the degenerate vibrational levels perturbation by hydrogen bonds. As a rule, the perturbation of degenerate stretching vibrations is much stronger than the perturbation of degenerate deformation vibrations.
For the 20 wt% HClO4 concentration, the value of n is 22.3. It corresponds to the value h = 6.4 that follows from the graph h(n). Then n–h = 15.9. In this case the number of free molecules increased by more than 50 times. The increase in the number of free water molecules is confirmed by the broad band appearance at ∼3417 cm−1 against the background of continuous band of proton hydrates (Figure 6b). Its contour is complicated by a shoulder at ∼ 3273 cm−1. Similar complication of the ν(OH) strong broad band is also observed in the liquid water IR spectrum. An increase in the number of free molecules leads also to an intensity increase of the narrow band at 1640 ± 6 cm−1, while the intensity of the broad band at 1734 ± 10 cm−1 decreases very markedly. The ClO4- anion ν3 band is observed at 1114 cm−1. Its intensity is still quite high despite a significant decrease in the concentration of perchloric acid. A medium intensity of the ν4 band is observed at 627 ± 1 cm−1. A very weak band ν1 of the IR forbidden vibration is found at 931 ± 2 cm−1.
For the 10 wt% HClO4 the value n is 50.2. It corresponds to the value h = 6.4 as evidenced by the graph h(n). Now n–h = 43.8. The free water molecules number increases by 2.8 times compared to the previous case. The intensity of the broad band ν(OH) at 3425 ± 10 cm−1 (shoulder at 3283 ± 10 cm−1) and the narrow band δ(HOH) at 1646 ± 3 cm−1 increases by the same number of times. As a consequence, a considerable screening of the bands at 3527 cm−1, ∼2800 cm−1 and ∼1734 cm−1 is observed. In addition, a broad band appears at ∼ 600 cm−1, which refers to libration vibrations τ(H2O) of free liquid water molecules (Figure 6c). IR spectra of liquid water and references to the papers where they were published are given in the monograph [43]. A medium intensity band at 1112 cm−1 refers to the ν3 vibration of ClO4- anion. A weak band at 629 cm−1 refers to the ν4 vibration. A very weak ν1 band is not detected, since it is screened by the broad τ(H2O) band. A decrease in the concentration is accompanied by a narrowing of the contours of ν3 and ν4 bands (compare the spectra in Figure 6a-c). This phenomenon implies a weakening of the ClO4- anion interaction with its positively charged hydration shell.
It can be noted that spectrum shown in Figure 6a only becomes less intense at a large acid dilution, but does not undergo qualitative changes. This observation implies that the positive charge is distributed among a limited number of h water molecules mainly in the nearest environment of the anion. Based on the evaluation of the number of water molecules required to create a cavity in the crystal structure of HClO4·5.5H2O around the ClO4- anion [20], we can conclude that at 56.2 wt% HClO4 and at lower concentrations this anion should be surrounded approximately by twenty H2O molecules. This number is more than three times larger than the maximal proton hydration number h. The arising contradiction can be resolved by assuming a fast exchange of protons in the hydration shell of the anion. Information about the fast (in the time scale of the NMR method) exchange of protons in an aqueous solution of acids is given in the monograph [44]. The discussion of the structure of dilute aqueous perchloric acid leads, in particular, to the conclusion that any proton complex with water molecules has a short lifetime in the NMR time scale. It disappears and reappears as a result of proton migration from one water molecule to another along the hydrogen bond network. Conceivably it migrates between H2O molecules around the anion in the absence of an external electric field. However, in the time scale of vibrational spectroscopy methods, the properties of neutral molecules and molecules bound by a proton are very different. Therefore, extrapolation the NMR and IR spectroscopic data to low concentrations can give generally speaking, two different models of the ion pair isolated by liquid water molecules. In the first case, ClO4- is inside the spherically symmetric cavity with H+ uniformly shared between the water molecules forming this cavity. In the second case, the symmetry is distorted due to the real non-uniformity of H+ sharing.
When the β-stannic acid powder is treated with perchloric acid with a concentration of 56.2 wt% HClO4, the bands of the initial solution (Figure 6a) pass with slight changes into the spectra of the interaction products of these two acids.
3.2.6. Raman data for the aqueous perchloric acid
Raman spectroscopic studies of the HClO4–H2O solutions (Fig. 7a-c) are consistent with the data [36]. All four types of fundamental vibrations of the ClO4- anion are active in the Raman spectrum. Figure 7a shows the Raman spectrum corresponding to concentration 56.2 wt% HClO4. The very strong line at 935 ± 1 cm−1 refers to ν1. The lines of medium intensity at 462 ± 1 cm−1, 1106 ± 6 cm−1 and 629 ± 1 cm−1 refer to ν2, ν3 and ν4, respectively. All of them have rather narrow contours. The symmetry perturbation of the ClO4- anion is too weak to cause splitting of double and triple degenerate vibrational levels. Only some broadening of the ν3 levels is observed instead of splitting. The ν3 line is five times broader than the ν4 line due to the higher sensitivity of the stretching vibration than the bending vibration to perturbations of the anion symmetry. The ν1, ν2, ν3 and ν4 lines become narrower at 20 and 10 wt% HClO4 (Fig. 7b,c).
Figure 7.
Raman spectra of aqueous perchloric acid. The concentration is 56.2 (a), 20 (b), and 10 (c) wt% HClO4.
3.3. Vibrational spectra of the β-stannic acid plasticized with perchloric acid
3.3.1. IR absorption data
IR absorption spectra of the plasticized β-stannic acid samples are shown in Figure 8. The samples were plasticised using perchloric acid with a concentration of 56.2 wt% HClO4. The sample composition is described by the formula SnO2·zH2O·yHClO4, where z = 3.1–5.2 and y = 0.33–0.82. Specific values of x and y are given in Table 2. Samples with z ≤ 3.1 and y ≤ 0.33 are white powder. All the samples for which z = 4.6–8.5 and y = 0.7–1.6 are plastic and optically transparent. During plasticization, the liquid phase completely disappears, but all of its bands continue to be observed due to the special structure of such samples. The spectrum of the initial HClO4·nH2O liquid phase is superimposed on the spectrum of the SnO2·mH2O solid phase, as if these phases formed a simple mechanical mixture between themselves. The proton hydrates are characterized by an intense broad band ν(OH) at 3536 ± 3 cm−1, a medium intensity narrow band δ(HOH) at 1633 ± 2 cm−1, an intense continuous band ν(OH) at ∼3000 cm−1 (Fig. 8a-c), as well as a moderately broad band δ(HOH) at 1737 ± 7 cm−1 of medium intensity (Fig. 8b,c). All these bands are also present in the IR spectrum of perchloric acid with a concentration of 56.2 wt% HClO4 (Figure 6a).
Figure 8.
IR absorption spectra of β-stannic acid samples (SnO2∙mH2O, m = 1.3) plasticized with aqueous perchloric acid. Used HClO4 concentration is 56.2 wt% (or HClO4∙nH2O, n = 4.3) and the weight fraction of the solution is Wpa = 0.24 (a), 0.37 (b) and 0.44 (c).
Whereas H2O molecules physically adsorbed by SnO2·mH2O powders have their own narrow band of OH bending vibration at 1643 ± 6 cm−1 as was already mentioned in Section 3.1.1 (see Figure 2). The intensities of two mutually overlapping bands δ (НОН), one of which belongs to H2O molecules adsorbed by the SnO2·mH2O, and the other to H2O molecules in the HClO4·nH2O, are eventually superimposed. The superimposition effect is most noticeable in Figure 8a, which shows the IR spectrum of the β-form plasticized with a relatively small amount of 56.2 wt% HClO4. In this case, the contribution of H2O molecules physically adsorbed by the β-form to the total intensity of δ(НОН) band is several times greater than the contribution of neutral H2O molecules belonging to the water solution of perchloric acid.
The ClO4- anion has an intense ν3 band at 1114 ± 4 cm−1 and a medium intensity ν4 band at 627 ± 1 cm−1, as well as a weak narrow ν1 band at 930 ± 1 cm−1 (Figure 8c). Note that in plasticized samples the band of the IR forbidden vibration ν1 is weak as in the perchloric acid solution. The degenerate vibration levels are not split. It follows that the ClO4- anion is not coordinated by the Sn4+ cation.
The presence of the β-form in the plasticized samples is confirmed by the registration of an intense ν(SnO) band with a maximum at 579 ± 5 cm−1, which has a shoulder at 665 ± 3 cm−1. In the long-wavelength region of the absorption spectrum (recorded under vacuum conditions), the δ(OSnO) band is observed with a maximum at 300 ± 6 cm−1 (shoulder 252 ± 5 cm−1). Plasticization with perchloric acid leads to an increase in the vibration frequency ν(SnO) and δ(OSnO) by 20 cm−1 on average. As the plasticizer amount increase, the ν(SnO) band intensity gradually decreases, while the intensity of the ν4 ClO4- band increases (Figure 8a–c).
Denote by D626/D579 the ratio of optical densities at the maxima of the bands, the positions of which are indicated in the subscripts. The graph of D626/D579 versus Wpa is non-linear due to the τ(H2O) band superimposition on the ν(SnO) band.
The δ(SnOH) bands are screened by an intense band ν3 ClO4-. The experimental data do not confirm the assumption of possible neutralization of hydroxyl groups by proton hydrates. If the neutralization reaction flows according to the scheme Sn4+ –ОН- + Н+ + ClO4- → Sn4+ –О– ClO3- + Н2О the formation of a metal–oxygen bond Sn4+ – O – ClO3-.should be expected, which, however, does not actually occur.
Comparing the IR absorption spectra in Figures 6a and 8a–c, it can be seen that after the inclusion of large or small amounts of perchloric acid with a concentration of 56.2 wt% HClO4 into the β-stannic acid samples, the contours of its bands in all cases change little. With small amounts the continuous ν(OH) band of strong hydrogen bonds of proton hydrates extending approximately from 1500 to 3500 cm−1 becomes less intense. Accordingly, a relief appears at 2042, 2438, 3068 and 3269 cm−1 on its background from the superposition of β-stannic acid bands.
3.3.2. Raman data
The Raman spectra of plasticized samples of the composition SnO2·zH2O·yHClO4 (z = 3.1–5.2 and y = 0.33–0.82) are shown in Figure 9. Extremely weak bands (or lines) ν(OH), δ(HOH), δ(SnOH) and δ(SnOH2) failed to be recorded, despite multiple rescanning of spectra with accumulation of weak signals. The reason is that a too long wavelength λ = 1064 nm of the excitation band was used. In the expected frequency range, only a weakly pronounced relief is observed.
Figure 9.
Raman spectra of β-stannic acid samples (SnO2·mH2O, m = 1.3) after plasticization with aqueous perchloric acid. Used HClO4 concentration is 56.2 wt% (or HClO4·nH2O, n = 4.3) and the weight fraction of solution is Wpa = 0.14 (a), 0.24 (b) and 0.40 (c).
The free ClO4- anion with Td point symmetry should have four fundamental vibrations that are active in the Raman spectrum. In accordance with the selection rules, all four lines were found: a very intense line is at 935 ± 1 cm−1 (ν1) and three lines of medium intensity at 461 ± 1 cm−1 (ν2), 1114 ± 10 cm−1 (ν3) and 628 ± 1 cm−1 (ν4). The line ν2 of double degenerate vibrations and the lines ν3 and ν4 of triple degenerate vibrations show no evidence of splitting, which indicates no ClO4– anion coordination by the Sn4+ cation. The absence of this coordination was also found by the IR spectroscopy method (Section 3.3.1). All lines in the Raman spectrum have narrow contours, with the exception of ν3 and ν(SnO). The ν3 line is five times broader than the ν4 line. The difference can be due to the unequal sensitivity of stretching and deformation vibrations to the anion symmetry perturbation by hydrogen bonds.
The medium intensity line at 586 ± 3 cm−1 refers to the stretching ν(SnO) vibration. It has a moderately broad contour, but there are narrow lines ν2 and ν4 of the ClO4–.anion on both sides of it. The intensity of the ν2 and ν4 lines successively increases, and the intensity of the ν(SnO) line decreases with an increase of the HClO4 solution content in plasticized samples (Figure 9 a - c).
A linear relationship exists between I628/I586 and Wpa as well as between I461/I586 and Wpa where I628/I586 and I461/I586 are the ratio of intensities at the maxima which indicated by subscripts. The initial β-form powder has an intense ν(SnO) line in the Raman spectrum at 576 ± 2 cm−1. Thus, plasticization causes a 10 cm−1 increase of the ν(SnO) vibration frequency. The weak line δ(OSnO) is represented by a barely noticeable shoulder in the region of ∼200 cm−1 against the background of the exciting line.
The registration of all four ClO4– lines (Figure 9a - c), which are practically indistinguishable from the lines in the spectrum of the initial perchloric acid samples (Figure 7a), is an additional source of information on the structure of plasticization products.
3.4. Powder XRD data
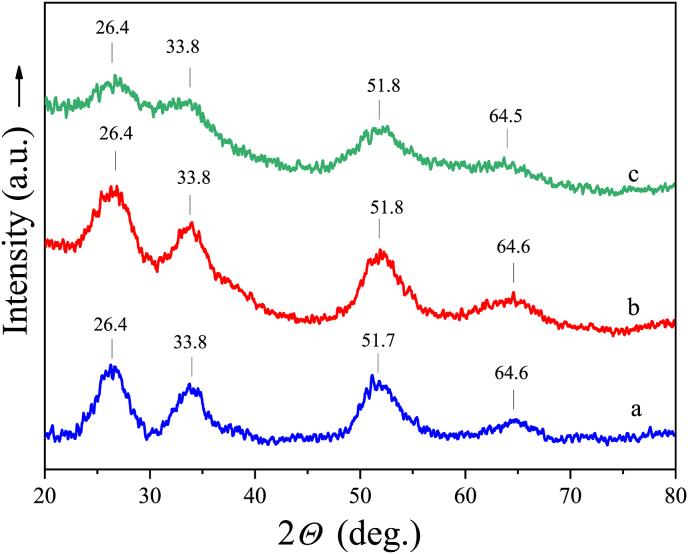
Figure 10a shows an XRD pattern of the non-plasticized β-stannic acid. Similar data for the tin dioxide hydrate were published in [14]. The patterns of the samples, first plasticized and then washed from perchloric acid are of similar appearance (Figure 10b).
Figure 10.
XRD patterns of β-stannic acid (a, b) and α-stannic acid (c) samples. Sample (a) is an air-dried white powder (m = 1.3); translucent sample (b) is obtained by washing perchloric acid from plasticized β-stannic acid (m = 2); sample (c) is a milky - white gel freshly precipitated at 0 °C (m = 6.5).
The XRD pattern of the gel freshly precipitated at 0 °C was obtained in a separate series of experiments (Figure 10c). In this case, the diffraction lines become less intense due to the partial formation of an amorphous α-stannic acid.
Figure 11 shows the XRD patterns of the β-form plasticized with perchloric acid (56.2 wt% HClO4). The values of interplanar spacing are collected in Table 3. In fact they are the same for all samples, regardless of the content of the plasticizer. The positions of broad diffraction lines correspond to the mean values of 1.45, 1.76, 2.66, and 3.38 Å. Consequently, the microcrystalline structure does not undergo noticeable changes in the process of β-form plasticization.
Figure 11.
XRD patterns of β-stannic acid samples (m = 1.3) plasticized with aqueous perchloric acid. HClO4 concentration used is 56.2 wt%. The weight fraction of solution is Wpa = 0.20 (a), 0.37 (b) and 0.45 (c).
Table 3.
XRD data for the β-stannic acid samples non-plasticized and plasticized with 56.2 wt% HClO4.
| Wpa | 2θ, deg | d, Ǻ |
|---|---|---|
| 0 | 26.4 34.0 51.4 64.5 |
3.38 2.68 1.78 1.45 |
| 0.17 | 26.2 33.6 51.9 64.7 |
3.39 2.67 1.76 1.44 |
| 0.44 | 26.4 33.7 52.2 64.4 |
3.38 2.66 1.75 1.45 |
Approximate estimation of the crystalline nanoparticles size from the XRD patterns in Figure 9 using the Scherrer formula gives the values (Ǻ): 27 ± 1 (a), 25 ± 2 (b), and 23 ± 1(c). A similar estimation from the XRD patterns in Figure 10 gives the values (Ǻ): 26 ± 1 (a), 42 ± 4 (b), and 44 ± 1(c).
3.5. Structural model and mechanism of plasticization of β-stannic acid powders
Comparing the spectra of plasticized samples (Figures 8 and 9) with the spectra of the starting components before plasticization (Figures 2 and 6a, 4 and 7a), it is easy to see that the former could be simulate by summing the starting components spectra with the optimal choice of coefficients. However, this does not mean that the material obtained in our work is a composite or a mechanical mixture of two phases. The experiments with the transparent plasticized samples showed that plasticization products do not contain any liquid phase as described in Section 3.1. It clearly follows from our data that β-stannic acid is capable of accumulating the large amounts of aqueous perchloric acid at room temperature.
It is well known that the stannic acid samples have a large specific surface, which can exceed 100 m2/g [1]. Physical sorption of water vapor by air-dry powders of this acid is carried out due to the weak binding of H2O molecules by hydroxyl groups inside the pores. Estimation of specific surface area, pores volume, and pores size (the most frequently occurring) gives values of Ssp = 27.5 m2/g, Vpore = 0.021 cm3/g, and rpore = 26.3 Å, respectively for the β– form samples [45]. The same samples were used also in our work. Summarizing the vibrational spectroscopy, powder X-diffraction and visual observations data we conclude that in the plasticization process, the β-form pores/capillaries are filled with a HClO4–H2O solution. Thus neither the structure of stannic acid nor the structure of the perchloric acid solution undergo significant changes when an adsorption compound is formed. It follows that the interaction between both components of the adsorption compound should be rather weak. The concentration of HClO4 inside the pores naturally decreases to some extent due to the dilution with water molecules belonging to the β-form. Perchloric acid ions retain a sufficiently large number of water molecules and cause the β-form powder to transform into a plastic transparent mass. The participation of H2O molecules in the plasticization process is confirmed by observations, according to which, after 100% washing off the perchloric acid with distilled water, the samples remain semitransparent for some time. At that, the water content in the washed samples increases by approximately one and a half time as compared with that in the initial powder of the β-form. The transparency then decreases because of significant water losses in the absence of perchloric acid ions.
3.6. Data of impedance spectroscopy
The impedance spectra of cells I and II are shown in Figures 12 and 13. Measurements in air give the Nyquist plots typical of an irreversible electrochemical system. In addition, Figure 13 shows the hodographs of cell II with catalytically active Pt electrodes for electrode processes in an atmosphere containing hydrogen. The solid electrolyte serves as a proton-conducting membrane. At the interface between the active electrode and the solid electrolyte a compromise potential is established, which is determined by the ratio of the rates of the two reactions:
| Н2 → 2H+ + 2e− | (5) |
| O2 + 4H+ +4e− → 2H2O | (6) |
Figure 12.
Typical impedance spectra of cell I in air (t = 25 °C, RH = 32%) obtained for the β-stannic acid samples (m = 1.3) plasticized with aqueous perchloric acid. The HClO4 concentration is 56.2 wt%. The weight fraction of solution is Wpa = 0.30 (1), 0.37 (2) and 0.45 (3), s = 0.196 cm2; thickness l = 2.6 (1), 2.6 (2) and 2.1 (3) mm.
Figure 13.
Typical impedance spectra of cell II in an air atmosphere (solid points) and in a mixture of air and 2% of hydrogen (open points) obtained at t = 25 °C and RH = 32% for the β-stannic acid samples (m = 1.3) before and after plasticization with aqueous perchloric acid. Used HClO4 concentration is 56.2 wt%.
In the presence of hydrogen, the impedance spectrum takes the form of a semicircle, characteristic of a reversible process, when, along with the charging of the double layer, an electrochemical reaction proceeds at a noticeable rate, as evidenced by the finite RF value. This value is determined from the difference between the low-frequency and high-frequency cutoffs on the real resistance axis. The specific Faraday resistance was calculated using Eq. (2).
-
1
Wpa = 0.24, s = 0.07cm2, l = 0.2cm, = 0.041 S·cm−1; F = 14.8 Ohm ·cm2;
-
2
Wpa = 0, s = 0.07cm2, l = 0.147cm; = 0.0036 S·cm−1; F = 94 Ohm ·cm2.
Note that the adsorbed perchloric acid increases the conductivity of the β-form by an order of magnitude already at a small value of Wpa = 0.24 near the break point on the linear graph of conductivity. In addition, it reduces the resistance of the Faraday reaction RF by a factor of 6, despite the fact that the measurements were carried out at a minimum acid content near the percolation threshold. It follows that perchloric acid adsorbed by the stannic acid has a catalytic effect on the hydrogen oxidation process in air. This feature makes the obtained material promising from the point of view of its possible use in electrochemical devices, such as hydrogen sensors. For the latter, the conductivity of the material is not as important as the response speed, which is largely determined by the charge transfer resistance.
A plot of the specific proton conductivity 25 (t = 25°С, RH = 32%) against the weight fractions of perchloric acid is shown in Figure 14. The specific proton conductivity 25 was calculated using Eq. (1).
Figure 14.
Dependence of volumetric specific proton conductivity of the plasticized β-stannic acid samples (m = 1.3) on weight fractions of perchloric acid solution. HClO4 concentration is 56.2 wt%. Based on impedance measurements for the cell I at t = 25 °C and RH = 32%.
A linear dependence of 25 is observed in a wide range of concentrations. The maximum value of 25 = 0.43 S cm−1 is achieved at Wpa = 0.61. A break in a straight line at Wpa = 0.18 indicates the existence of a perchloric acid percolation threshold. At lower concentrations, the contribution of the β-stannic acid to the conductivity becomes decisive. The threshold can arise due to the rupture of hydrogen bonds H2O…H2O under the influence of hydrogen bonds formation with the β-stannic acid pores/capillaries walls. The destructive effect of the walls cannot be decisive when the capillaries/pores are filled with a large amount of adsorbed solution, but it can come to the fore when this amount decreases to a threshold value.
The linear extrapolation of the conductivity to zero weight fraction of stannic acid gives a conductivity higher than according to the data [46] should have perchloric acid with the concentration 56.2 wt% (0.81 Ω-1·cm−1 and 0.55 Ω-1·cm−1, respectively). This result can be explained from the data of spectral studies by the integration in the gel of perchloric acid ions and "free" molecules of water physically sorbed by β-stannic acid. Therefore, extrapolation leads to a more dilute hydrate with higher conductivity. In addition, the fact of an increase in ionic conductivity upon heterogeneous doping of an ionic conductor with nanosized oxides is widely known, see for example [47].
From the literature data [27, 28] it follows that β-stannic acid itself is a protonic conductor. In addition, the protonic nature of the conductivity of perchloric acid is obvious. The decisive contribution of perchloric acid to the conductivity of the studied plasticized samples clearly indicates the protonic nature of their conductivity. This is also evidenced by the effect of hydrogen on the electrode processes in cell (II) (Figure 13).
4. Conclusions
Samples of the “Stannic acid - HClO4 - H2O” system were analyzed in detail using the methods of vibrational spectroscopy, impedance spectroscopy, and X-ray diffraction analysis. The addition of aqueous perchloric acid to the β-stannic acid powder results in transparent gel electrolyte formation with high value of proton conductivity, subject to the optimal choice of the amount and concentration of the perchloric acid. The variation of conductivity as a function of the aqueous perchloric acid weight fraction has been observed to be different in the regions of small and large amounts of the added aqueous solution. The linear plot of conductivity versus weight fraction has a break indicating the existence of a percolation threshold. Based on this, a conclusion can be drawn about the implementation of two different conduction mechanisms in these regions. The transparent gel electrolyte conductivity has been found to be higher than that of the aqueous perchloric acid. A plastic sample becomes transparent in the process of adsorption of sufficiently large amounts of aqueous perchloric acid by β-stannic acid powder. The results of studies by vibrational spectroscopy and X-ray phase analysis performed on transparent plasticized samples indicate the formation of a gel-like adsorption compound of variable composition between stannic acid and aqueous perchloric acid. Transparency indicates the plasticizing effect of perchloric acid on the powder of β-stannic acid. Opaque samples contain a minimal amount of perchloric acid solution. In addition, they are quite hard and stable and also have the necessary catalytic properties in order to be recommended for practical use in sensor devices based on the results of electrochemical measurements. Obviously, perchloric acid has catalytic properties. From this point of view, there should not be much difference between the electrochemical behavior of the material under study and finely dispersed powder of crystalline (cassiterite) tin dioxide or silica gel grains if they are treated with an aqueous solution of perchloric acid. Examples of the cassiterite supported perchloric acid use as a catalyst are not known to us. With regard to silica gel supported perchloric acid, it can be noted that in the literature it is characterized as a green, reusable, and highly efficient heterogeneous catalyst for the synthesis of many organic substances. Numerous publications on this topic can be easily found online.
Declarations
Author contribution statement
Alexander I. Karelin: Conceived and designed the experiments; Wrote the paper.
Lyudmila S. Leonova: Analyzed and interpreted the data; Wrote the paper.
Nelly S. Tkacheva: Analyzed and interpreted the data.
Svetlana E. Nadkhina: Performed the experiments.
Yury A. Dobrovolsky: Contributed reagents, materials, analysis tools or data.
Funding statement
This work was supported by Ministry of Science and Higher Education of the Russian Federation (AAAA-A19-119061890019-5).
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Pletnev R.N., Ivakin A.A., Kleshchev D.G., Denisova T.A., Burmistrov V.A. Nauka; Moscow: 1986. Hydrated Oxides of the Groups IV and V Elements. (in Russian) [Google Scholar]
- 2.Pechenyuk S.I. Sorption properties of hydrogels of transition and p-metal oxide hydroxides. Russ. Chem. Bull. 1999;48:229–238. [Google Scholar]
- 3.Hattori T., Athoh S., Tagawa T., Murakami J. In: Preparation of Catalysts IV. Delmon at al. B., editor. Elsevier; Amsterdam: 1987. p. 113. [Google Scholar]
- 4.Wu N.-L., Wu L.-F., Rusakova I.A., Hamed A., Litvinchuk A.P. Evolution in structural and optical properties of stannic oxide xerogel upon heat treatment. J. Am. Ceram. Soc. 1999;82:67–73. [Google Scholar]
- 5.Ivanov V.V., Sidorak I.A., Shubin A.A., Denisova L.T. Synthesis of SnO2 powders by decomposition of the thermally unstable compounds. J. Sib. Fed. Univ. Eng. Technol. 2010;2:189–203. [Google Scholar]
- 6.England W.A., Cross M.G., Hamnett A., Wieseman P.J., Goodenaugh J.B. Fast proton conduction in inorganic ion-exchange compounds. Solid State Ion. 1980;1:231–243. [Google Scholar]
- 7.Brief Сhemical Encyclopedia, V. 3. Publishing House "Soviet Encyclopedia; Moscow: 1964. p. 738. (In Russian) [Google Scholar]
- 8.Сhemical Encyclopedia, V. 3. Publishing House "Extensive Russian Encyclopedia; Moscow: 1992. p. 383. (In Russian) [Google Scholar]
- 9.Brauer G., editor. Handbook der Preparativen Anorganischen Chemie in drei Banden. Ferdinand Enke Verlag; Stuttgart: 1975. [Google Scholar]
- 10.Mellor J.W. Green & Co., Ltd.; London: 1927. Comprehensive Treatise on Inorganic and Theoretical Chemistry. V. VII, Longmans. [Google Scholar]
- 11.Wang J., Yang M., Li Y., Chen L., Zhang Y., Ding B. Synthesis of Fe-doped nanosized SnO2 powders by chemical Co-precipitation method. J. Non-Cryst. Solids. 2005;351:228–232. [Google Scholar]
- 12.Prosanov I.V., Chesalov Yu.A., Gerasimov K.B., Bulina N.V. Hybrid material polyvinyl alcohol-stannic acid/stannic oxide. Phys. Solid State. 2013;55:1416–1421. [Google Scholar]
- 13.Sharygin L.M., Denisova T.A., Vovk S.M., Perehozeva T.N., Pletnev R.N., Gonchar V.F. Distribution of various water forms in hydrated tin dioxide. Zh. Neorg. Khim. 1985;30:1968–1973. (In Russian) [Google Scholar]
- 14.Sharygin L.M., Vovk S.M., Gonchar V.F., Baribin V.I., Perehozeva T.N. Study of tin hydrated dioxide by the vibrational spectroscopy method. Zh. Neorg. Khim. 1983;28:576–580. (In Russian) [Google Scholar]
- 15.Lepatey M., Sautereay J., Bloch J.-M. Etude des proprieties physicochimiques de composes hydroxylуes de L’etain (IV): les acides stanniques. Compt. Rend. Acad. Sci. Paris, Ser. C. 1971;273:755–758. [Google Scholar]
- 16.Gavrilenko O.N., Pashkova E.V., Belous A.G. Effect of synthesis methods on the morphology of nanosized tin dioxide particles. Russ. J. Inorg. Chem. 2007;52:1835–1839. [Google Scholar]
- 17.Nekrasov B.V. Vol. 2. Publishing House «Khimia»; Moscow: 1967. p. 138. (Basic Foundation of General Chemistry). In Russian. [Google Scholar]
- 18.Rosolovsky V.Ya. Publishing House «Nauka»; Moscow: 1968. Сhemistry of Anydrous Perchloric Acid; p. 69. In Russian. [Google Scholar]
- 19.Karelin A.I. Solid solutions and plastic phases based on HClO4·5.5H2O in the system perchloric acid–water. Russ. Chem. Bull. 1988;37:6–10. [Google Scholar]
- 20.Mootz D.E., Oellers E.-J., Wiebcke M. First examples of type I clathrate hyrates of strong acides: polyhydrates of hexafluorophosphoric, tetrafluoroboric and perchloric acid. J. Am. Chem. Soc. 1987;109:1200–1202. [Google Scholar]
- 21.Huang T.-H., Davis R.A., Frese U., Stimming U. Proton mobility in liquid and frozen HClO4·5.5H2O: NMR and conductivity measurements. J. Phys. Chem. 1988;92:6874–6876. [Google Scholar]
- 22.Potier A., Rousselet D. Conductivité électrique et diffusion du proton dans le perchlorate d’oxonium. J. Chim. Phys. 1973;70:873–878. [Google Scholar]
- 23.Pham Thi M., Herzog J.F., Herzog -Cance M.H., Potier A. Dynamic aspects of a fast protonic conductor: oxonium perchlorate. Part I, Raman and inelastic neutron scattering spectra. J. Mol. Struct. 1989;195:293–310. [Google Scholar]
- 24.Fournier M., Masherpa G., Rousselet D., Potier J. Attribution des frequencies de vibration de l ion oxonium. C.R. Acad. Sc. Paris, Serie C. 1969;269:279–282. [Google Scholar]
- 25.Karelin A.I., Leonova L.S., Kolesnikova A.M./, Vakulenko A.M. Structure of a proton- conducting complex of silicotungstic acid. Russ. J. Inorg. Chem. 2003;48:885–896. [Google Scholar]
- 26.Colomban Ph., Fillaux F., Tomkinson J., Kearley J. Inelastic neutron – scattering study of proton dynamics in β-alumina. Solid State Ion. 1995;77:45–500. [Google Scholar]
- 27.Colomban Ph., Novak A. Proton Conductors, Solids, Membranes and Gels-Materials and Devices. Cambridge University Press; Cambridge: 1992. Proton conductors and conductivity; pp. 38–56. [Google Scholar]
- 28.Colomban Ph. Proton conductors and their applications: a tentative historical overview of the early researches. Solid State Ion. 2019;334:125–144. [Google Scholar]
- 29.Treglazov I.V., Dobrovolsky Yu.A., Leonova L.S., Vasil’ev S. Yu. Conductivity and morphology of composite solid electrolytes SnO2·nH2O – heteropolycompounds. Russ. J. Electrochem. 2003;39:501–506. [Google Scholar]
- 30.Karelin A.I., Leonova L.S., Arsatov A.V., Dobrovol'sky Yu.A. Vibrational spectra, structure, and proton conduction in hydrous tin dioxide. Russ. J. Inorg. Chem. 2013;58:711–718. [Google Scholar]
- 31.Karelin A.I., Leonova L.S., Arsatov A.V., Dobrovol'sky Yu.A. Water adsorption on tin hydrodioxide and its effect on the contour of the infrared absorption band ν(OH) Russ. J. Inorg. Chem. 2013;58:563–569. [Google Scholar]
- 32.Kostrikin A.V., Spiridonov F.M., Lin'ko I.V., Kosenkova O.V., Kuznetsova R.V., Komissarova L.N. Structure and dehydratation of hydrous tin dioxide xerogel. Russ. J. Inorg. Chem. 2007;52:1098–1104. [Google Scholar]
- 33.Leuchs M., Zundel G. Easily polarisable hydrogen bonds in aqueous solutions of acids – perchloric acid and trifluoromethane sulfonic acid. J. Chem. Soc., Faraday Trans. 1978;2(74):2256–2267. [Google Scholar]
- 34.Karelin A.I. Solid solutions, clathrate structures and vibrational spectra of plastic crystals of HClO4·5.5H2O, Russian. J. Struct. Chem. 1991;32:199–208. [Google Scholar]
- 35.Samoilov O.Ya. Consultants Bureau; New York: 1965. Structure of Aqueous Electrolyte Solutions and the Hydration of Ions. [Google Scholar]
- 36.Ratcliffe С.I., Irish D.E. Vibrational spectral studies of solutions at elevated - temperatures and pressures. VI. Raman studies of perchloric acid. Can. J. Chem. 1984;62:1134–1144. [Google Scholar]
- 37.Karelin A.I. Mechanism of the effect of proton hydrates on the parameters ClO4-…HClO4 hydrogen bond in liquid oxonium and dioxonium perchlorates. Russ. J. Inorg. Chem. 2002;47:1541–1550. [Google Scholar]
- 38.Karelin A.I., Nikitina Z.K. Infrared spectroscopic study of the complex formation between [H2n+1On]+ and ClO4−Ions. J. Coord. Chem. 1983;9:1458–1469. (In Russian) [Google Scholar]
- 39.Karelin A.I., Nikitina Z.K. Synthesis, vibrational spectra and bonding in the complex [OH3(ClO4)3]2− anion. J. Coord. Chem. 1982;8:303–310. (In Russian) [Google Scholar]
- 40.Lundgren J.-O., Olovsson I. In: Schuster P., Zundel G., Sandorfy C., editors. Vol. 2. North – Holland Publ. Co; Amsterdam: 1976. The hydrated proton in solids; pp. 473–522. (The Hydrogen Bond). [Google Scholar]
- 41.Stoyanov E.S., Stoyanova I.V., Reed Ch.A. The unique nature of H+ in water. Chem. Sci. 2011;2:462–472. [Google Scholar]
- 42.Karelin A.I., Tarasenko V.A. Hydration numbers of perchloric acid: estimation method based on the raoult law. Chem. Phys. 2019;523:211–221. [Google Scholar]
- 43.Zundel G. Academic Press; New York and London: 1969. Hydration and Intermolecular Interaction. [Google Scholar]
- 44.Bell R.P. second ed. Chapman & Hall; London: 1973. The Proton in Chemistry. [Google Scholar]
- 45.Karelin A.I., Tkacheva N.S., Nadkhina S.E., Leonova L.S., Kolesnikova A.M., Usacheva L.S., Levchenko A.V., Yu A. Dobrovol'sky, structure of hydrated tin dioxide doped with Sb(III) ions. Russ. J. Inorg. Chem. 2016;61:1144–1152. [Google Scholar]
- 46.Brickwedde L.H. Properties of aqueous solutions of perchloric acid. J. Res. NBS. 1949;42:309–329. [Google Scholar]
- 47.F Uvarov N. Ionics of nano heterogeneous materials. Russ. Chem. Rev. 2007;76(5):415–433. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.