Abstract
The protective antigen (PA83) of Bacillus anthracis is integral to the mechanism of anthrax toxicity. We have isolated a human single-chain Fv antibody fragment (scFv) that blocks binding of a fluorescently tagged protective antigen (PA) moiety to cell surface receptors. Several phage-displayed scFv were isolated from a naive library biopanned against PA83. Soluble, monomeric scFv were characterized for affinity and screened for their capacity to disrupt receptor-mediated binding of PA. Four unique scFv bound to PA83, as determined by surface plasmon resonance, the tightest binder exhibiting a Kd of 50 nM. Two scFv had similar affinities for natural PA83 and a novel, recombinant, 32-kDa carboxy-terminal PA fragment (PA32). Binding of scFv to green fluorescent protein fused to the amino-terminal 32-kDa fragment of B. anthracis edema factor, EGFP-EF32, was used to confirm specificity. Fusion of EGFP to PA32 facilitated development of a novel flow cytometric assay that showed that one of the scFv disrupted PA receptor binding. This method can now be used as a rapid assay for small molecule inhibitors of PA binding to cell receptors. The combined data presented suggest the potential utility of human scFv as prophylactics against anthrax poisoning. Moreover, recombinant PA32 may also be useful as a therapeutic agent to compete with anthrax toxins for cellular receptors during active infection.
The mechanism of anthrax intoxication is relatively well understood (20). The current model suggests that an 83-kDa form of protective antigen (PA83) is secreted from rapidly growing Bacillus anthracis cells and binds to a specific, but as yet unidentified, host cell surface receptor (8). Subsequent cleavage by membrane-bound furin (11, 17) and/or a furin-like protease, possibly PACE4 (12), releases an amino-terminal 20-kDa PA83 fragment, resulting in receptor-bound PA63. The newly exposed surface on PA63 contains a single, high-affinity binding site that is recognized by the amino termini of both the lethal factor and edema factor components of the toxin complexes (23, 25, 33). Endocytosis of the receptor-toxin complex into acidic endosomes elicits a conformational change in PA63 whereby the A subunits (LF or EF) of the toxin are released into the endosome (10). The PA63-receptor complexes then oligomerize into a heptameric ring (30, 31). Lysosomal acidification and subsequent receptor release facilitates irreversible membrane insertion of the oligomeric PA63 pore (2, 18, 52). The pore elicits transport of LF and/or EF into the cytoplasm, where they elicit their respective toxicities. EF is a calcium-calmodulin-dependent adenylate cyclase that is toxic to most cell types and causes local inflammation and edema but is not usually lethal (21, 37). LF is a cell-type-specific metalloprotease that cleaves mitogen-activated protein kinase-kinases (7, 51) and several peptide hormones (14). Lethal factor is the major virulence factor associated with anthrax toxicity and is responsible for systemic shock and death associated with a hyperoxidative burst and cytokine release from macrophages (15, 37). Neither of the toxin A subunits are pathogenic in the absence of cytoplasmic delivery by PA or mechanical means (10).
The crystal structures of PA83 and heptameric PA63 have been solved (36). These structural data support the experimental data (26, 46) that indicate that domain 4, the carboxy terminus of PA63, is responsible for receptor-mediated uptake of the toxin complex. Therefore, antibodies generated against domain 4 of PA could be potential candidates for toxicity neutralization by interfering with PA binding to its host receptor. Additionally, a recombinant PA fragment containing domain 4 might possibly compete with native PA83 for its receptors, thereby inhibiting the first step required for toxin complex formation (22, 26).
Virulent B. anthracis continues to represent a significant health threat. Accordingly, we have set out to search for inhibitors of anthrax toxicity and to develop a rapid screen for the identification of such inhibitors. To these ends, we screened a naive single-chain Fv phagemid library for antibodies that bind native B. anthracis PA83. We have also assessed a soluble, recombinant fragment of PA (i.e., PA32) for use as a potential inhibitor of PA binding to cells. To screen these single-chain FV fragments (scFv) for inhibitory properties, we developed a high-throughput flow cytometric competition assay with a fluorescently tagged form of PA32. In addition to its usefulness in assaying scFv, this fragment may also have therapeutic potential as a novel vaccine candidate or as a competitive inhibitor of anthrax toxins.
Phage display is a powerful tool with which moderate to high-affinity ligands can be rapidly isolated from diverse peptide or antibody libraries (53). Generation of naive antibody libraries, which are synthesized from nonimmunized human rearranged V genes (28, 50), allows selection against a myriad of possible substrates. Isolation of antibody fragments from naive libraries has proven to be highly efficient against numerous targets, including viruses (3, 47), cytokines (19), hormones (6, 50), growth factor receptors (54), and tissue- or tumor-specific markers (16, 34, 42, 48). Phage display-isolated scFv have been used clinically for diagnostic imaging (32), and the potential uses of these recognition elements in both detection and therapeutics are vast.
MATERIALS AND METHODS
Cell culture.
Chinese hamster ovary cells (CHO-K1, ATCC CCL-61) were cultured in minimal essential medium (MEM; Life Technologies) supplemented with 10% (vol/vol) fetal bovine serum (FBS; Hyclone), 100 U of penicillin per ml, and 100 μg of streptomycin (Life Technologies) per ml. A549 human lung carcinoma alveolar epithelial cells (ATCC CCL-185) were cultured in RPMI 1640 culture medium (Life Technologies) supplemented with 10% FBS and the antibiotics mentioned above. Both cell types were released from culture flasks by incubation in 1 mM trypsin-EDTA (Life Technologies) for 3 min at 37°C. Cells were washed once in phosphate-buffered saline (PBS) and diluted to 103/ml in the appropriate culture medium for passage to new flasks. For immunofluorescent microscopy, cells were cultured on LabTec2 (Nalge-Nunc) slides. For flow cytometric assay, cells were released with trypsin-EDTA and washed once in culture medium and once in PBS before subsequent treatments.
Protective antigen and edema factor cloning.
PCR with Pfu polymerase (Stratagene) was carried out on a Perkin-Elmer 9600 thermal cycler at an annealing temperature of 52°C for an extension time of 5 min. The forward and reverse primers for PA32 were 5′-TCGCACTCGAGGGCGCGCATGCCGCCGAAACAACTGCACGTATCATT and 5′-ACTGAGCTCAGCGCTAGCGCCACCAGAACCGCCTCCTATCTCATAGCCTTTTT, respectively (sequences complementary to PA83 are in italics). Template was B. anthracis Sterne strain pXO1 obtained from D. Robertson of Brigham Young University (40). The amplified PCR product was 809 nucleotides long (PA sequence 3346 to 4095; GenBank accession number M22586) and contained restriction sites for XhoI and BssHII at the 5′ end and Bpu1102I and NheI at the 3′ end, respectively. PCR products were gel purified and cut with the appropriate enzyme pairs (i.e., XhoI and Bpu1102I) for ligation into pET15b (Novagen) expression vector. This vector adds a hexahistidine tag to the amino terminus of expressed proteins. DNA sequencing was performed to confirm the correct construction. The BssHII and NheI sites were included for cloning into a different vector not used in these studies. The pET15b-expressed protein contained the B. anthracis mature PA sequence from E486 to G735, which includes the experimentally defined PA receptor binding domain. These constructs were then transformed into Escherichia coli BL21(DE3)(pLysS) (Stratagene). Bacteria were grown in Luria-Bertani broth supplemented with 50 μg of ampicillin and 34 μg of chloramphenicol per ml. Induction by the addition of 1.5 mM isopropyl-β-d-thiogalactopyranoside (IPTG) was performed at 37°C for 4 h. Then, 10 to 20 mg of PA32 was purified from cleared bacterial lysates from a 1-liter mid-log-phase shaker culture passed through a 2-ml Talon metal affinity resin (Clontech) column according to the manufacturer’s protocol.
The sequence encoding the B. anthracis edema factor amino terminus (accession number M23179) was cloned into pET15b, generating EF32. PCR forward and reverse primers were 5′-GCTCGAGAATGAACATTACACTG and 5′-CGCTCAGCACCTTCTTTCTTCAAACTTTC, respectively. They contained XhoI and Bpu1102I restriction sites, respectively (sequences complementary to EF89 are in italics). This edema factor fragment (amino acids N35 to G289) was cloned because the resultant EF fragment retains its ability to bind PA63 yet is enzymatically inactive (52).
EGFP fusion cloning.
The enhanced green fluorescent protein (EGFP) sequence from Clontech was amplified with the forward and reverse primers 5′-GGAATTCCATATGGTGAGCAAGGGCGAGGAGCTGTTCACC and 5′-CCGCTCGAGATCTGAGTACTTGTACAGCTCGTCCATGCC, respectively, and ligated into pET15b/PA32 or pET15b/EF32 between the NdeI and XhoI sites. These chimeric constructs were transformed into E. coli BL21(DE3)(pLysS). Bacteria expressing recombinant proteins were grown and induced as outlined above. Recombinant proteins were purified by immobilized metal affinity chromatography (IMAC) as previously outlined.
PA83 isolation.
PA83 was purified as previously described (39). Briefly, clarified supernatant was collected from a 20-liter culture of pX02-cured Sterne strain of B. anthracis containing mutants LF and EF. A 20% ammonium sulfate precipitation was used to enrich PA83 relative to other secreted proteins. Subsequent fast-performance liquid chromatography purifications were performed by using Mono-Q (Amersham Pharmacia Biotech) and gel filtration (Sephadex G-75; Amersham Pharmacia Biotech) columns. The final protein preparation was >90% pure as determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
scFv display, isolation, purification, and characterization.
A naive scFv phagemid library (6 × 109 diversity [43]) was selected against PA83 according to earlier protocols (41). Three rounds of biopanning were performed in Nunc Immuno-tubes. Washing after each selection round consisted of 20 washes with PBS-Tween 20 (0.1% [vol/vol]), followed by 20 washes with PBS. Phage were eluted with 100 mM triethylamine (pH 12) and then neutralized with 1 M Tris-HCl (pH 7.4). Sandwich enzyme-linked immunosorbent assays (ELISAs) were performed by using 96-well plates coated with 1 μg of PA83. Anti-M13–horseradish peroxidase (HRP) conjugate (Pharmacia Biotech) was used for colorimetric detection with tetramethyl benzidine (Sigma) according to the manufacturer’s protocol. Fingerprint analysis of PCR-amplified antibody variable (Fv) regions from ELISA-positive phage with BstNI restriction enzyme was used to identify unique isolates. Plasmids containing the isolated antibody clones were transformed into E. coli BL21(DE3)(pLysS), and soluble antibody fragment production was induced by stimulation with 1.5 mM IPTG for 6 h at 30°C. A periplasmic preparation was produced as previously described (41), and His-tagged scFv were isolated by IMAC. Monomeric scFv were isolated by size exclusion chromatography with Superdex 75 (Amersham Pharmacia Biotech) as previously described (41).
Binding analysis by SPR.
All protein-binding experiments were conducted in a BIAcore 2000 system (Pharmacia Biosensors). This system characterizes biomolecular interactions by using a noninvasive optical detection principle (i.e., surface plasmon resonance [SPR]). SPR reflects a change in mass concentration at the detector surface as molecules bind or dissociate. One component, the ligand, is covalently attached to a single channel of the flowcell surface. The other component (i.e., analyte) is injected in a continuous flow over the flowcell surface. PA83, PA32, EGFP-PA32, and EGFP-EF32 were coupled to four different channels on a CM5 dextran sensor chip in 10 mM sodium acetate (pH 4.8) via the amine group of lysines onto N-hydroxysuccinimide/N-ethyl-N-(3-diethylamino-propyl)carbodiimide (NHS/EDC)-activated chip surfaces. All ligands were coupled to less than 1,000 resonance units (RU) per channel. Single-chain Fv selected against PA83 were dialyzed extensively against PBS and passed over immobilized ligand at a flow rate of 40 μl/min in PBS. A total of 80 μl of analyte was injected and run over successive channels of ligands in a single flowcell. Four concentrations, ranging from 2 to 100 nM for each analyte, were assessed and used for binding analysis. Curves were fit to a 1:1 stoichiometry of binding with mass transfer compensation. The flowcell was regenerated between samples by using a 4 M MgCl2 solution without a significant change in baseline.
Immunoflourescent microscopy.
Recombinant PA32 was covalently labeled with Texas red as described by the manufacturer (Molecular Probes). Briefly, 1.5 mg of Texas red-X succinimidyl ester was dissolved in 150 μl of dimethyl sulfoxide (final concentration, 12 mM). Then, 4 μl of this soluble dye was added to 50 μl of 100 μM PA32, giving a molar ratio of 10:1. Conjugation proceeded for 30 min at 30°C, followed by another 30 min at 4°C. Unconjugated dye was removed by separation through a Microcon 10 column (Amicon, Inc.). CHO cells were cultured on LabTek2 slides to 80% confluency. Cells were washed twice with cold MEM and stored at 4°C until use. Then, 1 nmol of fluorescently labeled PA32 was added, and the temperature was shifted to 37°C for different time periods. Cells were then fixed in 100% methanol for 15 min, washed once in PBS, and then covered with glycerol mounting medium. PA32 labeling of the cells was photographed by using a ×60 oil immersion objective on a charge-coupled device camera-equipped Axioskop Zeiss microscope (the Zeiss filter for Texas red was 00 and for EGFP was 09). A 100-W Mercury lamp was used for excitation of the fluorophores.
Flow cytometry assay.
Flow cytometry was performed on a Fisher FACSCaliber apparatus with Cellquest software (Becton Dickinson). Cells were diluted to 106/ml, and 10,000 events were collected. The forward-scatter detector was E-1 (gain, 350), the side-scatter detector was set at 350, and the green fluorescence detector (FL1) was set at 500 on a log scale. Cells were washed once in PBS, diluted to 106/ml in PBS containing 1.5% (wt/vol) bovine serum albumin (Sigma), and agitated for 2 h at 4°C to block nonspecific protein binding. Aliquots (0.5 ml) of cells were then dispensed into chilled 1.5-ml Eppendorf tubes. EGFP or EGFP fusion proteins in PBS were added to a 0.2 μM final concentration and allowed to bind for 1 h at 4°C with moderate agitation. Cells were centrifuged at 2,000 × g for 3 min, decanted, resuspended in 1 ml of PBS containing 0.1% (wt/vol) bovine serum albumin, and then analyzed. For competition experiments, nonfluorescent PA83 or PA32 was added to the cells prior to EGFP-PA32. For analysis of scFv inhibition, EGFP-PA32 was incubated with different antibody fragments for 2 h at 4°C prior to the addition to A549 cells.
RESULTS
SDS-PAGE analysis of purified recombinant and natural PA proteins.
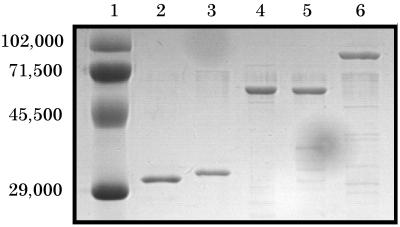
Purification of the native PA83 as described above yielded a >90%-pure protein preparation (Fig. 1, lane 6). Purification of recombinant anthrax proteins was performed by IMAC in a single step. All IMAC-purified proteins were >95% homogeneous after elution as determined by SDS-PAGE (Fig. 1, lanes 2 to 5). A recombinant PA comprised of the carboxy-terminal 32-kDa protein (Fig. 1, lane 3) is highly soluble in E. coli and did not appear to be toxic to the bacteria. PA32 was also cloned as a fusion protein with a green fluorescent protein variant (EGFP) attached to its amino terminus. The EGFP-PA32 fusion (Fig. 1, lane 4) was designed for use in a flow cytometry assay, where inhibitors of PA receptor binding could be analyzed. As controls for the different assays, His-tagged EGFP (full-length 31-kDa band in Fig. 1, lane 2 [kindly donated by Tracy Ruscetti of Los Alamos National Laboratory]) and chimeric EGFP-EF32 (Fig. 1, lane 5) were expressed and purified similarly to the recombinant PA32 proteins.
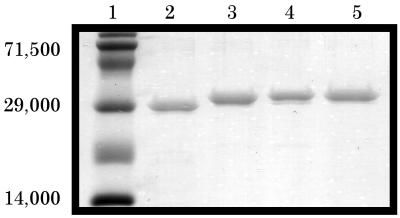
FIG. 1.
Coomassie and SDS-PAGE analysis of purified proteins. Lanes: 1, prestained molecular weight markers; 2, recombinant EGFP; 3, recombinant PA32; 4, recombinant EGFP-PA32 fusion; 5, recombinant EGFP-EF32 fusion; 6, natural PA83. A total of 250 ng of each purified protein was analyzed on an SDS–12% polyacrylamide gel after IMAC (lanes 2 to 5) or Mono-Q and gel filtration chromatography (lane 6).
Characterization of isolated scFv.
Synthetic, recombinant, scFv from a naive phage display library were biopanned against PA83. After three rounds of selection, 60 of 90 isolates showed PA binding ability, as determined by ELISA (data not shown). Fingerprint analysis revealed seven unique isolates, of which five with the highest ELISA scores were chosen for further analysis (Table 1). These scFv were expressed and purified by IMAC and size exclusion chromatography (see Materials and Methods) to isolate monomeric scFv. This was necessary in order to assess the affinity of the antibodies in the absence of avidity effects due to diabody or larger aggregate formation (e.g., one of the antibody fragments, scFv5, showed >90% multimerization and was therefore excluded from subsequent analysis [Table 1]). This procedure yielded >95%-pure antibodies as determined by SDS-PAGE (Fig. 2).
TABLE 1.
Analysis of anti-PA83-selected scFv
| scFv type | ELISA scorea | Monomer/dimer/trimer ratiob | Kd (M)c |
|---|---|---|---|
| scFv1 | 0.75 | 8:2:0 | 1.9 × 10−7 |
| scFv4 | 0.20 | 9:1:0 | 3.1 × 10−7 |
| scFv5 | 1.14 | 1:2:7 | ND |
| scFv12 | 0.27 | 7:3:0 | 1.1 × 10−6 |
| scFv24 | 0.30 | 9:1:0 | 4.3 × 10−7 |
Wells of a 96-well plate were coated with 1 μg of PA83. Anti-M13–HRP conjugated antibody (Pharmacia Biotech) was used to generate signal.
Multimer content was determined by Sephadex-75 separation of IMAC-purified protein as described in Materials and Methods.
Affinity was determined in a BIAcore by using four concentrations of each scFv. ND, not done.
FIG. 2.
Coomassie and SDS-PAGE analysis of anti-PA83 selected single-chain Fv. Lanes: 1, prestained molecular weight markers; 2 to 5, scFv1, scFv4, scFv12, and scFv24, respectively. A total of 500 ng of each purified protein was analyzed on an SDS–12% polyacrylamide gel after IMAC chromatography.
Characterization of scFv binding affinities to PA.
PA83 was coupled to a BIAcore CM5 chip (∼1,000 RU), and four dilutions of each of the purified, monomeric scFv were used to determine equilibrium dissociation constants (Kd). All of the scFv tested showed similar affinities (Table 1). The overall affinities of these antibody fragments are consistent with models that predict submicromolar affinities for naive libraries of this size (35). These scFv were further assessed for their ability to recognize the recombinant PA32 fragment. PA83, EGFP-PA32, PA32, and EGFP-EF32 were coupled to different channels on a single BIAcore CM5 flowcell. Different scFv were sequentially passed over each channel of the chip, and their affinities were determined (Fig. 3). All ligands were coupled at ∼1,000 RU, and a single concentration of analyte was assessed. There is good agreement in the Kd values for the different scFv binding to PA83 when Table 1 and Fig. 3 are compared. Kd values of less than 10−7 M were apparent for all three antibodies directed against PA83. Baseline, nonspecific binding was evident in all three scFv (Kd of ∼10−2 M) passed over nontarget EGFP-EF32. scFv1 and scFv4 showed similar affinities for PA83 and PA32 ligands, whereas scFv12 showed only nonspecific binding to PA32 proteins (Fig. 3). These data indicate that the targets for scFv1 and scFv4 lie within domains 3 or 4 of PA, while the antigenic site for scFv12 is outside this region.
FIG. 3.
Affinities of anti-PA83-selected single-chain Fv determined by SPR. Kd values were determined by SPR in a BIAcore apparatus. Affinities of selected scFv for natural PA83 (solid bars), recombinant EGFP-PA32 (striped bars), and recombinant EGFP-EF32 (open bars) are shown.
Confirmation of PA32 cell binding and internalization.
The above SPR data suggested that the expressed PA32 fragment was correctly folded, at least in terms of epitope presentation, and could be used as a reporter to monitor PA-receptor interaction. Purified, recombinant PA32 was covalently cross-linked to the fluorophore, Texas red. This fluorescent PA32 was added to serum-deficient CHO cell cultures. Receptor internalization was induced by a shift in temperature from 4 to 37°C for different times (Fig. 4). There was a time-dependent internalization of fluorescence into discreet structures, presumably endosomes, within the cytoplasm of these cells. No fluorophore internalization was seen if the cells were kept at 4°C, and the fluorescent label was not observed in nuclei, even at 6 h post-temperature release (data not shown). These data indicate that the PA32 fragment is recognized similarly to natural PA83 and is internalized into cytoplasmic vesicles. To prove specific receptor-mediated uptake, a more quantitative measure was needed.
FIG. 4.
Immunofluorescent microscopy of fluorescently labeled PA32 uptake by CHO cells. Texas red-labeled, recombinant PA32 was incubated for the indicated times at 37°C with adherent CHO cells. After being washed, cells were fixed and photographed. Note the time-dependent cytoplasmic inclusion of labeled PA fragment.
Development of rapid flow assay to assess PA32-receptor interactions.
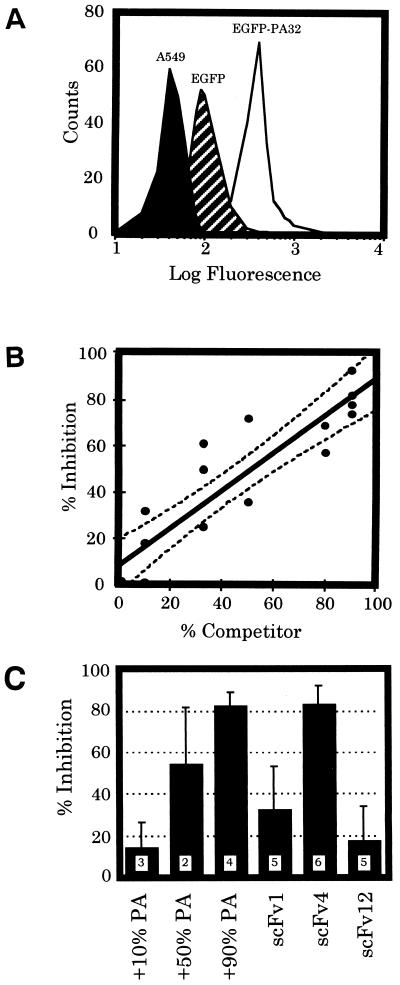
A flow cytometric assay was developed using the EGFP-PA32 fusion protein. Human A549 cells were used as target cells because of their low autofluorescence and minimal phagocytic activity. EGFP alone or the EGFP-EF32 fusion was used to evaluate nonspecific binding by these cells. Initial experiments were performed to ensure significant separation of nonspecific and receptor-mediated protein binding. As shown in Fig. 5A, there was a fourfold-enhanced signal from specific EGFP-PA32 bound to cells compared to nonspecific EGFP binding alone. To confirm that EGFP-PA32 was binding to the PA-specific receptor, competition with different concentrations of natural PA83 or unlabeled PA32 was assessed. There was a statistically significant (P < 0.0001) linear inhibition of fluorescent-PA32 binding by unlabeled PA molecules (Fig. 5B). For a 1:1 stoichiometry of PA-receptor binding, we would expect 50% inhibition by an equimolar concentration of unlabeled PA (i.e., 50% EGFP-PA32, 50% competitor). These data confirm specificity and indicate little or no cooperativity in PA-receptor interactions.
FIG. 5.
Flow cytometric analysis of specific EGFP-PA32 binding by a human epithelial cell line. (A) Green fluorescence signal from nonlabeled (A549, solid area), nonspecifically labeled (EGFP, striped area), and specifically labeled (EGFP-PA32, open area) A549 human epithelial cells. The number of cells is recorded on the ordinate; the log fluorescence intensity is recorded on the abscissa. There is very good separation between nonspecific and specific labeling of cells. (B) Inhibition of EGFP-PA32 signal by unlabeled PA. Linear correlation between the percent unlabeled competitor PA (unlabeled PA/[unlabeled PA plus EGFP-PA32] × 100) and the percent inhibition of mean fluorescent signal. A 95% confidence interval is shown (dotted line). (C) Inhibition of EGFP-PA32- mediated fluorescent signal by unlabeled PA molecules (data from Fig. 5B) or an equimolar concentration of single-chain Fv (scFv1, scFv4, and scFv12). Bars represent the mean and standard error for the indicated number of experiments (boxed numbers).
Inhibition of receptor-mediated EGFP-PA32 binding by scFv targeted to PA.
This flow cytometric analysis was subsequently used to screen scFv for their ability to disrupt PA-receptor interactions. Incubation of scFv4 with EGFP-PA32 at a 1:1 molar ratio was able to significantly (>80%) abolish receptor-mediated binding of EGFP-PA32 to A549 cells (Fig. 5C). A 10-fold molar excess of scFv4 showed little additional inhibition (data not shown) as would be expected for a monovalent competition. The scFv1, which can recognize EGFP-PA32 (Fig. 3), showed minimal inhibition of EGFP-PA32 binding by this assay. This indicates that it does not recognize or mask an essential structure necessary for receptor recognition. The scFv12 did not inhibit binding as expected since it did not recognize the C-terminal PA32 protein (Fig. 3). For comparison, the means of the dose-dependent competition with unlabeled PA are also shown in Fig. 5C. These data indicate that the flow cytometric assay is a sensitive and specific method for identifying molecules which inhibit receptor-mediated anthrax toxin binding and that one of the scFv selected has the potential to inhibit PA binding to cells in a therapeutically useful fashion.
DISCUSSION
The first step in anthrax intoxication is the binding of PA83 to a yet-to-be-characterized host cell surface receptor. Subsequent PA cleavage by a host cellular protease(s) is necessary for toxin A subunit (LF and EF) binding as well as for PA multimerization and pore formation. The main goal of the present study was to generate molecules of potential therapeutic value that can inhibit PA-receptor interactions.
Previous investigations have shown that a vaccine containing only PA83 protected guinea pigs against lethal B. anthracis spore challenge, and PA-specific neutralizing monoclonal antibodies were able to delay the time of death (24). Such evidence suggests the possibility that high-affinity human antibodies generated against PA may offer a significant therapeutic advantage as well. Accordingly, in the first component of our study we set out to isolate several human, single-chain antibody fragments that can bind to PA in a manner that may interfere with PA-receptor binding.
The use of natural and recombinant antibodies or antibody fragments to treat disease is at the forefront of many new therapeutic approaches. A large proportion of new compounds in current clinical trials are human antibody derivatives. Indeed, the first phage display-isolated antibodies (directed against tumor necrosis factor alpha) are now being used as immunoglobulin therapeutics in phase II clinical trials for rheumatoid arthritis (29). There are many methods by which in vitro selection (i.e., the separation of binding clones from nonbinding clones) of displayed antibodies can be performed. These include biopanning of immobilized antigen on various substrates, including plastic solid supports (as described here), columns (13), BIAcore chips (27), fixed cells (5), or even tissue sections (49). By selecting against purified PA83 on a solid plastic support which does not orient the bound protein, we forced selection against all portions of PA83. We isolated several unique, high-affinity clones which were against different epitopes as determined by the ability to bind the PA32 subdomain of PA83. These scFv were used for Western blotting and immunoprecipitations (data not shown) and were screened for the potential to disrupt host cell receptor binding. Isolation of neutralizing scFv from naive libraries is less efficient than from immune libraries, yet we were able to identify an scFv which could inhibit receptor-mediated binding of PA to cells. Another potential use for such scFv is as recognition elements for biosensors. If the affinities of the scFv described above are not sufficiently high for use as biosensor recognition elements, their affinities can be increased by chain shuffling (41) or parsimonious mutagenesis. Alternatively, scFv isolated from immune libraries have generally shown higher affinities. We are therefore in the process of generating an immune scFv library from PA-immunized individuals for use in isolating higher-affinity antibody fragments.
In a second component of our study, we also explored the possibility that a recombinant fragment of PA that lacks the ability to bind toxin A subunits or multimerize but that remains capable of binding to cell surface receptors may also potentially competitively inhibit the binding of intact PA. In this regard, preliminary work by Leppla (22) to generate a recombinant carboxyl-terminal PA fragment indicated that a fragment from T624-G735 could not compete with radiolabeled PA83 for receptors. This work was completed prior to the crystal structure solution by Petosa et al. (36). We postulated that the amino terminus of the recombinant protein should be at a structural domain boundary defined by the crystal structure to retain structural integrity. For this reason, we attempted to clone domain 4 alone (i.e., F596-G735, PA18) and domains 3 and 4 together (i.e., E486-G735, PA32). We were unable to clone domain 4 alone for unknown reasons, but we were able to successfully clone domains 3 and 4 together. We believe the reason PA32 (domains 3 and 4) is able to compete for receptors is that the protein is structurally more stable than the T624-G735 fragment tested by Leppla and coworkers. This hypothesis is supported by our SPR results that show that several of the anti-PA83 scFv also bind to PA32, i.e., the molecule evidently is folded in a manner that preserves epitopes common to native PA. The ability of PA32 to interact with its host cell surface receptor (Fig. 4 and 5A) and be internalized (Fig. 4) favorably supports the possibility that this PA fragment may be effective as an antitoxin treatment during anthrax infection.
The human anthrax vaccine used in the United States and other western countries consists of aluminum hydroxide-adsorbed supernatant material from cultures of toxigenic, nonencapsulated B. anthracis strains. Current protocols for isolating native PA83, the primary immunogen in the vaccine, from culture supernatants are time- and cost-intensive (38). Immunization with this vaccine can cause local edema and erythema, probably due to the presence of trace amounts of LF or EF (9), and frequent boosters are required (4). The recombinant form of PA generated for our studies is safer to manufacture (i.e., no B. anthracis cultures necessary) and simpler to purify and has no chance of “carrying” other toxin components through the manufacturing process. A simple, one-step IMAC separation purifies the recombinant PA more effectively than the standard three-step protocol normally used to purify natural PA83 (39). It has been shown that only immunization with PA, and not LF or EF, can protect against lethal B. anthracis challenge in a guinea pig model (24). It has been suggested that the reduced protection seen with some recombinant PA vaccine preparations may be due to lack of contaminating LF or EF. Dual vaccination with PA32 and LF or EF would be possible since the A toxin subunits cannot interact with recombinant PA32. A similar approach was developed by Singh et al. (44) by using recombinant PA molecules that bind receptors but not LF or EF. The approach of Singh et al. was to mutate the conserved PA83 RKKR protease site to prevent the EF or LF binding site from being exposed by furin cleavage and PA20 release. Immunization of guinea pigs with this cleavage-resistant PA vaccine led to significant protection against otherwise-lethal anthrax infection (45). PA32 offers several advantages over the current human vaccine. The receptor-binding region is a higher proportion of the total immunogenic surface, suggesting that a higher proportion of antibodies will be neutralizing. E. coli expression and IMAC purification are extremely efficient. Being structurally truncated, the PA32 molecule is unable to interact with toxin A subunits and thus is nontoxic, and this PA fragment is unable to form pores due to absence of the D2L2 loop of domain 2 (1). As with the single-chain antibody (scFv4) that interferes with PA-receptor binding, the potential usefulness of PA32 as a therapeutic or prophylactic agent requires further in vitro and in vivo analyses.
ACKNOWLEDGMENTS
We thank Theresa M. Koehler of the University of Texas—Houston Medical School for assistance in producing B. anthracis PA83 and Tracy Ruscetti and Rhiannon Nolan of Los Alamos National Laboratory for the purified EGFP and helpful discussions.
This work was conducted under the auspices of the U.S. Department of Energy and was supported in part by institutional Laboratory Directed Research and Development funds for “Pathogen Detection.”
REFERENCES
- 1.Benson E L, Huynh P D, Finkelstein A, Collier R J. Identification of residues lining the anthrax protective antigen channel. Biochemistry. 1998;37:3941–3948. doi: 10.1021/bi972657b. [DOI] [PubMed] [Google Scholar]
- 2.Blaustein R O, Koehler T M, Collier R J, Finkelstein A. Anthrax toxin: channel-forming activity of protective antigen in planar phospholipid-bilayers. Proc Natl Acad Sci USA. 1989;86:2209–2213. doi: 10.1073/pnas.86.7.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boonham N, Barker I. Strain-specific recombinant antibodies to potato-virus-Y potyvirus. J Virol Methods. 1998;74:193–199. doi: 10.1016/s0166-0934(98)00097-4. [DOI] [PubMed] [Google Scholar]
- 4.Brachman P S, Gold H, Plotkin S A, Fekety F R, Werrin M, Ingraham N R. Field evaluation of a human anthrax vaccine. Am J Public Health. 1962;52:632–645. doi: 10.2105/ajph.52.4.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bradbury A, Persic L, Werge T, Cattaneo A. Use of living columns to select specific phage antibodies. Bio/Technology. 1993;11:1565–1569. doi: 10.1038/nbt1293-1565. [DOI] [PubMed] [Google Scholar]
- 6.Dorsam H, Rohrbach P, Kurschner T, Kipriyanov S, Renner S, Braunagel M, Welschof M, Little M. Antibodies to steroids from a small human naive IgM library. FEBS Lett. 1997;414:7–13. doi: 10.1016/s0014-5793(97)00966-6. [DOI] [PubMed] [Google Scholar]
- 7.Duesbery N S, Webb C P, Leppla S H, Gordon V M, Klimpel K R, Copeland T D, Ahn N G, Oskarsson M K, Fukasawa K, Paull K D, Vandewoude G F. Proteolytic inactivation of MAP-kinase-kinase by anthrax lethal factor. Science. 1998;280:734–737. doi: 10.1126/science.280.5364.734. [DOI] [PubMed] [Google Scholar]
- 8.Escuyer V, Collier R J. Anthrax protective antigen interacts with a specific receptor on the surface of CHO-K1 cells. Infect Immun. 1991;59:3381–3386. doi: 10.1128/iai.59.10.3381-3386.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farchaus J W, Ribot W J, Jendrek S, Little S F. Fermentation, purification, and characterization of protective antigen from a recombinant, avirulent strain of Bacillus anthracis. Appl Environ Microbiol. 1998;64:982–991. doi: 10.1128/aem.64.3.982-991.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Friedlander A M. Macrophages are sensitive to anthrax lethal toxin through an acid-dependent process. J Biol Chem. 1986;261:7123–7126. [PubMed] [Google Scholar]
- 11.Gordon V M, Klimpel K R, Arora N, Henderson M A, Leppla S H. Proteolytic activation of bacterial toxins by eukaryotic cells is performed by furin and by additional cellular proteases. Infect Immun. 1995;63:82–87. doi: 10.1128/iai.63.1.82-87.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gordon V M, Rehemtulla A, Leppla S H. A role for PACE4 in the proteolytic activation of anthrax toxin protective antigen. Infect Immun. 1997;65:3370–3375. doi: 10.1128/iai.65.8.3370-3375.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Griffiths A D, Williams S C, Hartley O, Tomlinson I M, Waterhouse P, Crosby W L, Kontermann R E, Jones P T, Low N M, Allison T J, Prospero T D, Hoogenboom H R, Nissim A, Cox J P L, Harrison J L, Zaccolo M, Gherardi E, Winter G. Isolation of high-affinity human-antibodies directly from large synthetic repertoires. EMBO J. 1994;13:3245–3260. doi: 10.1002/j.1460-2075.1994.tb06626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hammond S E, Hanna P C. Lethal factor active-site mutations affect catalytic activity in vitro. Infect Immun. 1998;66:2374–2378. doi: 10.1128/iai.66.5.2374-2378.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hanna P C, Acosta D, Collier R J. On the role of macrophages in anthrax. Proc Natl Acad Sci USA. 1993;90:10198–10201. doi: 10.1073/pnas.90.21.10198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Henderikx P, Kandilogiannaki M, Petrarca C, Vonmensdorffpouilly S, Hilgers J H M, Krambovitis E, Arends J W, Hoogenboom H R. Human single-chain Fv antibodies to MUC1 core peptide selected from phage display libraries recognize unique epitopes and predominantly bind adenocarcinoma. Cancer Res. 1998;58:4324–4332. [PubMed] [Google Scholar]
- 17.Klimpel K R, Molloy S S, Thomas G, Leppla S H. Anthrax toxin protective antigen is activated by a cell-surface protease with the sequence specificity and catalytic properties of furin. Proc Natl Acad Sci USA. 1992;89:10277–10281. doi: 10.1073/pnas.89.21.10277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koehler T M, Collier R J. Anthrax toxin protective antigen: low-pH-induced hydrophobicity and channel formation in liposomes. Mol Microbiol. 1991;5:1501–1506. doi: 10.1111/j.1365-2958.1991.tb00796.x. [DOI] [PubMed] [Google Scholar]
- 19.Krebs B, Griffin H, Winter G, Rosejohn S. Recombinant human single-chain Fv antibodies recognizing human interleukin-6: specific targeting of cytokine-secreting cells. J Biol Chem. 1998;273:2858–2865. doi: 10.1074/jbc.273.5.2858. [DOI] [PubMed] [Google Scholar]
- 20.Leppla S H. The anthrax toxin complex. In: Alouf J H F J E, editor. Sourcebook of bacterial protein toxins. London, England: Academic Press; 1991. pp. 277–302. [Google Scholar]
- 21.Leppla S H. Anthrax toxin edema factor: a bacterial adenylate-cyclase that increases cyclic-AMP concentrations in eukaryotic cells. Proc Natl Acad Sci USA. 1982;79:3162–3166. doi: 10.1073/pnas.79.10.3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leppla S H. Production and purification of anthrax toxin. Methods Enzymol. 1988;165:103–116. doi: 10.1016/s0076-6879(88)65019-1. [DOI] [PubMed] [Google Scholar]
- 23.Leppla S H, Friedlander A M, Cora E M. Proteolytic activation of anthrax toxin bound to cellular receptors. In: Fehrenbach F, Alouf J E, Falmagne P, Goebel W, Jelaszewics J, Jurgens D, Rappuoli R, editors. Bacterial protein toxins. Stuttgart, Germany: Gustav Fischer Verlag; 1988. pp. 111–112. [Google Scholar]
- 24.Little S F, Ivins B E, Fellows P F, Friedlander A M. Passive protection by polyclonal antibodies against Bacillus anthracis infection in guinea pigs. Infect Immun. 1997;65:5171–5175. doi: 10.1128/iai.65.12.5171-5175.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Little S F, Leppla S H, Burnett J W, Friedlander A M. Structure-function analysis of Bacillus anthracis edema factor by using monoclonal-antibodies. Biochem Biophys Res Commun. 1994;199:676–682. doi: 10.1006/bbrc.1994.1281. [DOI] [PubMed] [Google Scholar]
- 26.Little S F, Novak J M, Lowe J R, Leppla S H, Singh Y, Klimpel K R, Lidgerding B C, Friedlander A M. Characterization of lethal factor-binding and cell-receptor binding domains of protective antigen of Bacillus anthracis using monoclonal antibodies. Microbiology. 1996;142:707–715. doi: 10.1099/13500872-142-3-707. [DOI] [PubMed] [Google Scholar]
- 27.Malmborg A C, Duenas M, Ohlin M, Soderlind E, Borrebaeck C. Selection of binders from phage displayed antibody libraries using the BIAcore biosensor. J Immunol Methods. 1996;198:51–57. doi: 10.1016/0022-1759(96)00159-7. [DOI] [PubMed] [Google Scholar]
- 28.Marks J D, Hoogenboom H R, Bonnert T P, McCafferty J, Griffiths A D, Winter G. Bypassing immunization: human-antibodies from V-gene libraries displayed on phage. J Mol Biol. 1991;222:581–597. doi: 10.1016/0022-2836(91)90498-u. [DOI] [PubMed] [Google Scholar]
- 29.McCafferty J. Antibodies from phage display as genomics tools and therapeutic agents. Coronado, Calif: IBC Antibody Engineering Conference; 1998. [Google Scholar]
- 30.Milne J C, Furlong D, Hanna P C, Wall J, Collier R J. Anthrax protective antigen forms ring-shaped oligomers during intoxication of mammalian cells. FASEB J. 1994;8:A1464. [PubMed] [Google Scholar]
- 31.Milne J C, Furlong D, Hanna P C, Wall J S, Collier R J. Anthrax protective antigen forms oligomers during intoxication of mammalian cells. J Biol Chem. 1994;269:20607–20612. [PubMed] [Google Scholar]
- 32.Neri D, Carnemolla B, Nissim A, Leprini A, Querze G, Balza E, Pini A, Tarli L, Halin C, Neri P, Zardi L, Winter G. Targeting by affinity-matured recombinant antibody fragments of an angiogenesis associated fibronectin isoform. Nat Biotechnol. 1997;15:1271–1275. doi: 10.1038/nbt1197-1271. [DOI] [PubMed] [Google Scholar]
- 33.Novak J M, Stein M P, Little S F, Leppla S H, Friedlander A M. Functional characterization of protease-treated Bacillus anthracis protective antigen. J Biol Chem. 1992;267:17186–17193. [PubMed] [Google Scholar]
- 34.Osbourn J K, Field A, Wilton J, Derbyshire E, Earnshaw J C, Jones P T, Allen D, McCafferty J. Generation of a panel of related human scFv antibodies with high affinities for human CEA. Immunotechnology. 1996;2:181–196. doi: 10.1016/s1380-2933(96)00046-2. [DOI] [PubMed] [Google Scholar]
- 35.Perelson A S, Oster G F. Theoretical studies of clonal selection: minimal antibody repertoire size and reliability of self-non-self discrimination. J Theor Biol. 1979;81:645–670. doi: 10.1016/0022-5193(79)90275-3. [DOI] [PubMed] [Google Scholar]
- 36.Petosa C, Collier R J, Klimpel K R, Leppla S H, Liddington R C. Crystal-structure of the anthrax toxin protective antigen. Nature. 1997;385:833–838. doi: 10.1038/385833a0. [DOI] [PubMed] [Google Scholar]
- 37.Pezard C, Berche P, Mock M. Contribution of individual toxin components to virulence of Bacillus anthracis. Infect Immun. 1991;59:3472–3477. doi: 10.1128/iai.59.10.3472-3477.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Puziss M, Manning L C, Lynch L W, Barclay E, Abelow I, Wright G G. Large-scale production of protective antigen of Bacillus anthracis anaerobic cultures. Appl Microbiol. 1963;11:330–334. doi: 10.1128/am.11.4.330-334.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Quinn C P, Shone C C, Turnbull P C B, Melling J. Purification of anthrax-toxin components by high-performance anion-exchange, gel-filtration and hydrophobic-interaction chromatography. Biochem J. 1988;252:753–758. doi: 10.1042/bj2520753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Robertson D, Bragg T, Simpson S, Kaspar R, Xie W, Tippetts M. Mapping and characterization of the Bacillus anthracis plasmids pXO1 and pXO2. Salisbury Med Bull. 1990;68(Special Suppl.):55–58. [Google Scholar]
- 41.Schier R, Bye J, Apell G, McCall A, Adams G P, Malmqvist M, Weiner L M, Marks J D. Isolation of high-affinity monomeric human anti-c-erbB-2 single-chain Fv using affinity-driven selection. J Mol Biol. 1996;255:28–43. doi: 10.1006/jmbi.1996.0004. [DOI] [PubMed] [Google Scholar]
- 42.Schier R, Marks J D, Wolf E J, Apell G, Wong C, McCartney J E, Bookman M A, Huston J S, Houston L L, Weiner L M, Adams G P. In vitro and in vivo characterization of a human anti-c-erbB-2 single-chain Fv isolated from a filamentous phage antibody library. Immunotechnology. 1995;1:73–81. doi: 10.1016/1380-2933(95)00007-0. [DOI] [PubMed] [Google Scholar]
- 43.Sheets M D, Amersdorfer P, Finnern R, Sargent P, Lindqvist E, Schier R, Hemingsen G, Wong C, Gerhart J C, Marks J D. Efficient construction of a large non-immune phage antibody library: the production of high-affinity human single-chain antibodies to protein antigens. Proc Natl Acad Sci USA. 1998;95:6157–6162. doi: 10.1073/pnas.95.11.6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Singh Y, Chaudhary V K, Leppla S H. A deleted variant of Bacillus anthracis protective antigen is non-toxic and blocks anthrax toxin action in vivo. J Biol Chem. 1989;264:19103–19107. [PubMed] [Google Scholar]
- 45.Singh Y, Ivins B E, Leppla S H. Study of immunization against anthrax with the purified recombinant protective antigen of Bacillus anthracis. Infect Immun. 1998;66:3447–3448. doi: 10.1128/iai.66.7.3447-3448.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Singh Y, Klimpel K R, Quinn C P, Chaudhary V K, Leppla S H. The carboxyl-terminal end of protective antigen is required for receptor-binding and anthrax toxin activity. J Biol Chem. 1991;266:15493–15497. [PubMed] [Google Scholar]
- 47.Takekoshi M, Maeda F, Tachibana H, Inoko H, Kato S, Takakura I, Kenjyo T, Hiraga S, Ogawa Y, Horiki T, Ihara S. Human monoclonal anti-HCMV neutralizing antibody from phage display libraries. J Virol Methods. 1998;74:89–98. doi: 10.1016/s0166-0934(98)00072-x. [DOI] [PubMed] [Google Scholar]
- 48.Tordsson J, Abrahmsen L, Kalland T, Ljung C, Ingvar C, Brodin T. Efficient selection of scFv antibody phage by adsorption to in situ expressed antigens in tissue-sections. J Immunol Methods. 1997;210:11–23. doi: 10.1016/s0022-1759(97)00165-8. [DOI] [PubMed] [Google Scholar]
- 49.van Ewijk W, de Kruif J, Germeraad W T V, Berendes P, Ropke C, Platenburg P P, Logtenberg T. Subtractive isolation of phage displayed single-chain antibodies to thymic stromal cells by using intact thymic fragments. Proc Natl Acad Sci USA. 1997;94:3903–3908. doi: 10.1073/pnas.94.8.3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vaughan T J, Williams A J, Pritchard K, Osbourn J K, Pope A R, Earnshaw J C, McCafferty J, Hodits R A, Wilton J, Johnson K S. Human antibodies with subnanomolar affinities isolated from a large nonimmunized phage display library. Nat Biotechnol. 1996;14:309–314. doi: 10.1038/nbt0396-309. [DOI] [PubMed] [Google Scholar]
- 51.Vitale G, Pellizzari R, Recchi C, Napolitani G, Mock M, Montecucco C. Anthrax lethal factor cleaves the N-terminus of MAP-K-Ks and induces tyrosine/threonine phosphorylation of MAP-Ks in cultured macrophages. Biochem Biophys Res Commun. 1998;248:706–711. doi: 10.1006/bbrc.1998.9040. [DOI] [PubMed] [Google Scholar]
- 52.Wang X M, Wattiez R, Mock M, Falmagne P, Ruysschaert J M, Cabiaux V. Structure and interaction of PA63 and EF (edema toxin) of Bacillus anthracis with lipid-membrane. Biochemistry. 1997;36:14906–14913. doi: 10.1021/bi971661k. [DOI] [PubMed] [Google Scholar]
- 53.Winter G, Griffiths A D, Hawkins R E, Hoogenboom H R. Making antibodies by phage display technology. Annu Rev Immunol. 1994;12:433–455. doi: 10.1146/annurev.iy.12.040194.002245. [DOI] [PubMed] [Google Scholar]
- 54.Witte L, Hicklin D J, Zhu Z P, Pytowski B, Kotanides H, Rockwell P, Bohlen P. Monoclonal-antibodies targeting the vEGF receptor-2 (Flk1/Kdr) as an anti-angiogenic therapeutic strategy. Cancer Metastasis Rev. 1998;17:155–161. doi: 10.1023/a:1006094117427. [DOI] [PubMed] [Google Scholar]