Abstract
Resident bacteria play an important role in initiating and perpetuating gastrointestinal inflammation. We previously demonstrated that six commensal bacteria including Bacteroides vulgatus caused more aggressive colitis and gastritis in HLA-B27 transgenic rats than did the other five bacteria without B. vulgatus. This study compared the degree of gastrointestinal inflammation in gnotobiotic HLA-B27 transgenic rats monoassociated with either B. vulgatus or Escherichia coli. Gnotobiotic transgenic rats raised in Trexler isolators were selectively colonized with either B. vulgatus or E. coli. Control rats were either germfree or colonized with six common commensal bacteria (Streptococcus faecium, E. coli, Streptococcus avium, Eubacterium contortum, Peptostreptococcus productus, and B. vulgatus [DESEP-B]). After 1 month, all the rats were killed and tissues were prepared for histologic and biochemical evaluation. Colitis induced by B. vulgatus monoassociation was almost equal to that in DESEP-B-colonized rats and was significantly more severe than E. coli-induced colitis, which was absent by histological testing and mild by colonic myeloperoxidase and interleukin-1β concentration determinations. However, gastritis was detectable only in DESEP-B-associated rats. These studies suggest that not all resident bacteria have equal proinflammatory capabilities, since B. vulgatus alone is more active than E. coli alone in inducing colitis, and that colitis and gastritis result from different luminal bacterial stimuli.
Extensive recent data support the major influence of normal resident bacteria on the initiation and perpetuation of intestinal inflammation and extraintestinal manifestations in experimental colitis and human inflammatory bowel disease (25–27). Luminal bacteria have been implicated in the pathogenesis of multiple experimental colitis models (1, 3, 7–9, 20–22, 28, 32, 36). However, not all bacteria have equal abilities to cause inflammation, as demonstrated by their differential responses to antibiotics with narrow specificities and colonization with defined bacterial subsets. Metronidazole, which is selectively active against anaerobic bacteria, is effective in Crohn’s colitis and ileocolitis (31) and also attenuates chronic experimental intestinal inflammation in the indomethacin- and carrageenan-induced models (19, 36) and in HLA-B27 transgenic rats (23, 24). Anaerobic bacterial overgrowth in bypassed jejunoileal segments created to treat morbid obesity leads to systemic inflammation (6), and experimental small intestinal anaerobic bacterial overgrowth through creation of a jejunal self-filling blind loop can induce hepatobiliary inflammation and reactivate quiescent arthritis in genetically susceptible Lewis rats (15, 16). Similarly, experimental cecal anaerobic bacterial overgrowth potentates colitis in HLA-B27 transgenic rats (23). In both human and rat small intestinal bacterial overgrowth models, broad-spectrum antibiotics or metronidazole can treat systemic manifestations (10, 14, 23). These results from experiments with multiple clinical and experimental conditions suggest a dominant proinflammatory role for anaerobic commensal bacteria.
Several resident bacteria have been associated with Crohn’s disease, ulcerative colitis, and chronic inflammation in animal models (26, 27). Luminal concentrations of Bacteroides (13), Eubacterium, Peptostreptococcus, and Coprococcus (33) are increased in Crohn’s patients, while facultative anaerobic bacteria such as Streptococcus faecium (enterococcus, group D streptococcus) are increased in patients with ulcerative colitis (26). Serum antibodies to Eubacterium, Peptostreptococcus, and Coprococcus are specifically increased in Crohn’s disease (2), and functionally altered Escherichia coli has been implicated in the pathogenesis of ulcerative colitis (26). Furthermore, luminal concentrations of E. coli and Enterococcus species correlate with aggressiveness of colitis in B27 transgenic rats (21). Bacteroides vulgatus plays an essential role in the pathogenesis of carrageenan-induced colitis in the guinea pig (20), and purified cell wall polymers from Eubacterium, Peptostreptococcus, and Streptococcus faecium can induce or reactivate quiescent arthritis in genetically susceptible hosts (29, 30). Furthermore, gnotobiotic B27 transgenic rats colonized with a mixture of six different obligate and facultative anaerobic bacteria containing Streptococcus faecium (group D), Streptococcus avium, Peptostreptococcus productus, E. coli, Eubacterium contortum, and B. vulgatus (DESEP-B) developed much more active colitis and gastritis than did littermates colonized with the same mixture without B. vulgatus (22).
These results suggest that B. vulgatus preferentially induces colitis in B27 transgenic rats, but they do not conclusively exclude an adjunctive or even necessary confunctional role for the other five bacterial strains cocolonizing these gnotobiotic rats. The aims of the present study are to compare the abilities of two different common commensal enteric bacteria, B. vulgatus and E. coli, to induce colitis and gastritis in monoassociated HLA-B27 transgenic rats and to compare the inflammatory effects of these single bacterial strains with those of the cocktail of six bacteria previously investigated in this model (22).
MATERIALS AND METHODS
Experimental design.
HLA-B27/β2-microglobulin (HLA-B27) transgenic rats of the 33-3 line on an inbred F344 background (12) raised under germfree (GF) (sterile) conditions (22) were divided into four groups at the age of 2 months. One group remained GF (n = 10) and served as negative controls. The other groups were transferred into separate isolators and then colonized with different bacteria or bacterial cocktails by techniques described previously (22). One group (n = 11) was selectively colonized with B. vulgatus derived from a guinea pig with carrageenan-induced colitis (a gift from A. B. Onderdonk, Harvard University, Cambridge, Mass.). The second group (n = 8) was monoassociated with E. coli derived from a patient with active Crohn’s disease and provided by the Clinical Microbiology Laboratory of the University of North Carolina Hospitals, Chapel Hill, N.C. The third group (n = 12) was colonized with a cocktail of six bacteria (DESEP-B) with previously reported proinflammatory activity in this model (22) and served as a positive control. These bacteria were isolated from patients with Crohn’s disease (except for B. vulgatus) and included the B. vulgatus and E. coli strains used for monoassociation of littermates. Persistent selective bacterial colonization was documented by fecal Gram stain and culture 1 week after colonization and at necropsy. All the rats were clinically observed for evidence of diarrhea and arthritis and were killed at 3 months of age (1 month after colonization) by CO2 asphyxiation within 3 h of removal from the gnotobiotic isolators. The cecal tip and antrum of each rat were fixed in 10% neutral buffered formaldehyde for histological processing. Right colonic segments were taken and snap frozen in methyl-butane at −80°C for biochemical evaluations.
Histological testing.
Tissues were fixed in 10% neutral buffered formaldehyde for 4 to 12 h, transferred into 70% ethanol, and processed the next day by standard techniques (22). Coded samples were examined under light microscopy by a single observer (H. C. Rath). Cecal and gastric inflammation were quantitated by a previously validated histological scoring system ranging from 0 to 4+ (22).
MPO assay.
Methods of tissue preparations and the assay of colonic myeloperoxidase (MPO) activity (units per gram of tissue) were described previously (11).
Enzyme-linked immunosorbent assay.
Interleukin-1β (IL-1β) protein concentrations in the cecal tissue were measured by a rat IL-1β enzyme-linked immunosorbent assay developed by S. Poole (National Laboratory of Biological Standards and Controls, South Mimms, Potters Bar, Hertfordshire, United Kingdom) (22).
Microbial analysis.
Immediately after euthanasia, 1-ml samples from the cecal contents of colonized nontransgenic rats were taken and serially diluted in phosphate-buffered saline. Total bacterial concentrations were determined by using counting chambers. Monoassociation was confirmed by Gram staining and anaerobic culture.
Statistical analysis.
All data are expressed as mean ± standard error of the mean SEM. To test for differences between the groups, analysis of variance was used. Samples with equal distribution were compared by Student’s t test. P < 0.05 was considered statistically significant.
RESULTS
Cecal inflammation.
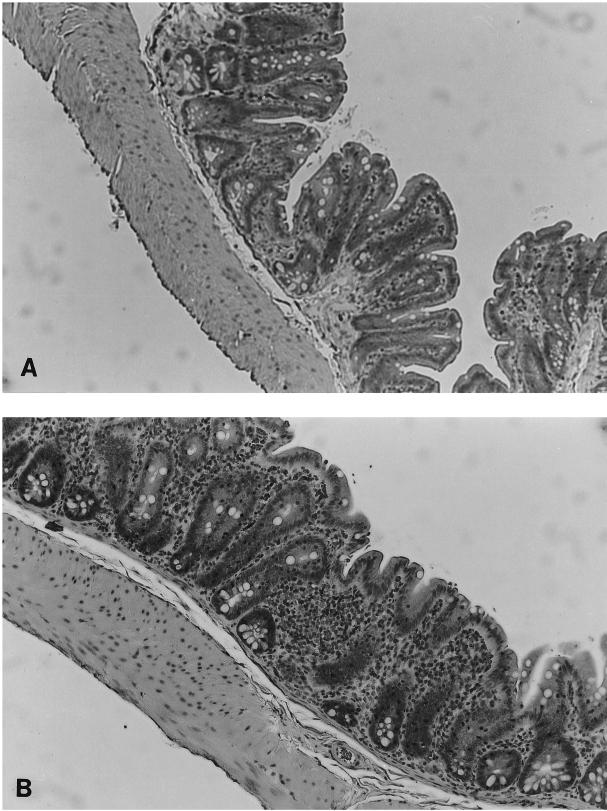
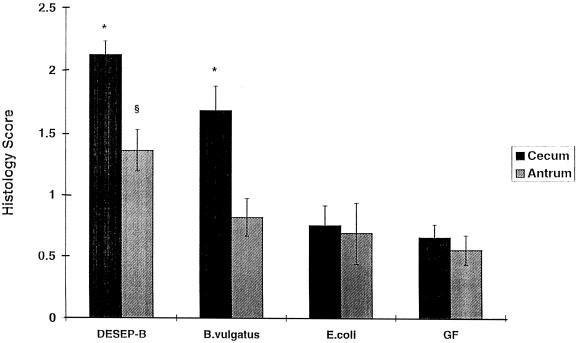
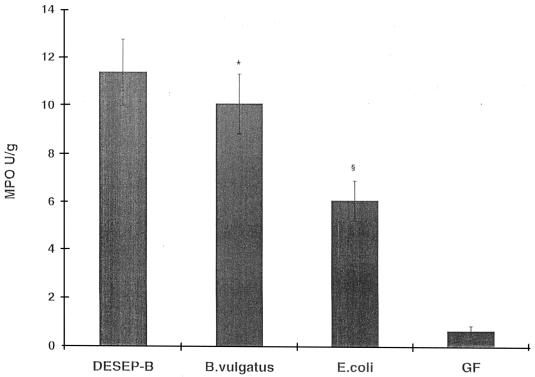
There was no evidence of grossly detectable inflammation in any group. Histologically, cecal inflammation of DESEP-B-colonized rats was characterized as mononuclear cell infiltration limited to the lamina propria with hypertrophy of the mucosa, crypt dilatation, and focal crypt abscesses. Rats monoassociated with B. vulgatus had a similar histologic pattern (Fig. 1B) and by blinded microscopic score had almost as much cecal inflammation as did the positive controls colonized with the six commensals, including B. vulgatus (1.7 ± 0.2 and 2.1 ± 0.1, respectively [P = 0.06]) (Fig. 2). Rats monoassociated with E. coli had almost no cecal inflammation (Fig. 1A), and their histologic scores were nearly identical to those of GF animals (0.8 ± 0.2 and 0.7 ± 0.1, respectively [not significant]) (Fig. 2). Transgenic rats monoassociated with B. vulgatus had more severe cecal inflammation than did those monoassociated with E. coli (P = 0.001). Cecal MPO activity confirmed the lack of difference between DESEP-B- and B. vulgatus-colonized rats and the significantly increased inflammation in both of these groups compared with that in E. coli-monoassociated (P < 0.03) and GF (P < 0.0001) rats (Fig. 3). However, in contrast to the lack of histologic inflammation in E. coli-monoassociated rats, colonic MPO levels were significantly increased in the E. coli group compared with GF controls (6.5 ± 1.0 and 1.1 ± 0.8, respectively [P < 0.0001]). IL-1β protein concentrations in the cecal tissue were not significantly different among the gnotobiotic groups; the concentrations in all groups were significantly elevated compared to those in GF rats (DESEP-B rats, 34.8 ± 4.3 ng/g of tissue; B. vulgatus rats, 37.6 ± 3.3 ng/g; E. coli rats, 25.3 ± 4.6 ng/g [P < 0.0005 with respect to GF rats, which had 5.2 ± 0.6 ng/g]; B. vulgatus versus E. coli was not significant [P = 0.06]). Total luminal bacterial concentrations in the cecum were not different among the groups (1.0 × 104 to 4.4 × 1011 bacteria/ml of cecal contents).
FIG. 1.
Histological features of colons from gnotobiotic HLA-B27/β2-microglobulin transgenic rats monoassociated with B. vulgatus or E. coli for 1 month. (A) Cecal histological section from a transgenic rat monoassociated with E. coli. There is almost no sign of inflammation. (B) Cecal tissue from a transgenic rat monoassociated with B. vulgatus. There is mononuclear cell infiltration of the lamina propria, mucosal thickening, crypt hyperplasia, and focal goblet cell depletion. Magnification, ×40.
FIG. 2.
Blinded histological inflammatory scores of the cecum and antrum in gnotobiotic HLA-B27 transgenic rats. GF transgenic rats, 2 months old, were colonized with DESEP-B, B. vulgatus alone, or E. coli alone or kept GF (negative control). The rats were killed 1 month after bacterial colonization. ∗, P = 0.001 with respect to E. coli and GF rats. §, P < 0.05 with respect to all three other groups.
FIG. 3.
As a marker of neutrophilic infiltration, colonic MPO activity was increased in all colonized rats with respect to GF controls. Homogenized cecal tissues collected 1 month after bacterial colonization were assayed by a colorimetric biochemical assay for MPO (see Materials and Methods). ∗, P < 0.03 with respect to E. coli and P < 0.0001 with respect to GF rats. §, P < 0.0001 with respect to GF rats and P < 0.01 with respect to DESEP-B.
Gastritis.
Mononuclear inflammation in the gastric antrum is a prominent feature in specific-pathogen-free HLA-B27 transgenic rats, although it is absent in GF rats (22). As previously described, transgenic rats colonized with DESEP-B had active antral mucosal inflammation (histological scores of 1.4 ± 0.2 with respect to 0.6 ± 0.1 for GF rats [P < 0.05]) (Fig. 2). However, transgenic rats monoassociated with either B. vulgatus or E. coli had significantly less gastritis (0.8 ± 0.2 and 0.7 ± 0.2, respectively [P < 0.04] with respect to DESEP-B) and were not different from GF rats.
DISCUSSION
In a recent study we demonstrated that six common intestinal commensals isolated from patients or guinea pigs with colitis, including B. vulgatus, could cause colitis and gastritis in HLA-B27 transgenic rats (22). However, the same cocktail minus B. vulgatus caused virtually no intestinal inflammation. These data strongly incriminated B. vulgatus in the pathogenesis of colitis and gastritis in HLA-B27 transgenic rats. However, the question still remained whether this inflammation was an effect of B. vulgatus alone or whether the other bacteria in this cocktail had a synergistic or maybe even an antagonistic influence. To address this question, we monoassociated GF transgenic rats with either B. vulgatus or E. coli, another component of this cocktail. In the present study, we demonstrated that B27 transgenic rats monoassociated with B. vulgatus developed colitis comparable to that in rats colonized with the six commensal bacteria but that E. coli-monoassociated rats had no histological evidence of colitis although slight increases in MPO and IL-1β concentrations in tissue were noted. Differential abilities to induce colitis were not due to colonization efficiency, since colonic luminal concentrations of B. vulgatus and E. coli were almost identical. These observations are consistent with a series of results from our group as well as from other investigators. Onderdonk et al. demonstrated in complex repopulation and monoassociation studies that B. vulgatus was the dominant stimulus in carrageenan-induced colitis in guinea pigs (20), and Dianda et al. showed that T-cell receptor alpha (TCRα)-deficient mice monoassociated with E. coli or Streptococcus faecium had no colitis (9). Moreover, the therapeutic efficacy of metronidazole in Crohn’s disease correlates well with the degree of suppression of fecal Bacteroides concentrations (13). Lichtman et al. showed that chronic metronidazole therapy, which eliminated Bacteroides spp. without significantly altering total luminal bacterial concentrations, prevented hepatobiliary inflammation and reactivation of arthritis in rats with jejunal self-filling blind loops (14, 16). We recently reported that creation of a cecal self-filling blind loop in B27 transgenic rats was associated with 50-fold increased luminal concentrations of Bacteroides species and potentiation of cecal inflammation (23). Metronidazole eliminated luminal Bacteroides and attenuated colitis in this model (23). Furthermore, enterotoxin from certain B. fragilis strains enhances mucosal permeability and epithelial internalization of enteric bacteria (35), and Bacteroides strains stimulate transforming growth factor β1 production and collagen deposition in the colonic wall in decontaminated rats following 2,4,6-trinitrobenzenesulfonic acid (TNBS)-ethanol (18). Finally, Cong et al. (7) documented T-lymphocyte responses to B. vulgatus in C3H/HeJBir mice with colitis. The lack of a significant difference between the cecal inflammation of rats monoassociated with B. vulgatus and those colonized with DESEP-B indicates that none of the other five bacterial species exert a protective influence, especially since there was a strong trend towards more inflammation in the commensal-colonized group. However, the fact that rats raised in a specific-pathogen-free environment have more severe cecal inflammation than those colonized with the cocktail of six defined commensal bacteria (22) suggests that B. vulgatus is not the only resident enteric bacterial strain capable of inducing and/or perpetuating colitis in this model. It is possible that other strains not represented in our cocktail can initiate disease or, alternatively, that B. vulgatus selectively initiates colitis in this model but that once it is initiated, other bacteria may play a role in perpetuating and potentiating chronic intestinal inflammation. The concept of synergistic activities of various resident luminal bacteria in chronic intestinal inflammation is supported by our preliminary observations that the broad-spectrum antibiotic combination of vancomycin and imipenem is superior to metronidazole or ciprofloxacin alone in preventing and treating experimental colitis in HLA-B27 transgenic rats (24). The complex interaction of various luminal bacterial species is further illustrated by the recent correlation of cecal concentrations of E. coli and to a lesser extent Enterococcus species with the degree of colitis in B27 transgenic rats (21). Although the concentrations of anaerobes, including Bacteroides species, were not increased in rats with severe colitis in the study by Onderdonk et al. (21), Bacteroides was one of the most prevalent organisms (109 CFU/g of cecal contents).
Neither of the two groups of monoassociated rats showed any significant gastritis, whereas transgenic rats colonized with DESEP-B manifested chronic antral inflammation. In our previous study of transgenic rats colonized with these commensals excluding B. vulgatus, there was no significant colitis or gastritis (22). In another experiment, HLA-B27 transgenic rats in a specific-pathogen-free environment did not develop colitis or gastritis if the cecum was excluded from the fecal stream, which caused a consequent decrease of cecal bacterial concentrations of about 2 log units (23), even though the bacterial concentration and composition in the gastric lumen did not change. These results correspond to attenuation of distal colitis in TCRα-deficient mice following excision of the cecal tip (“appendectomy”) (17). We hypothesize that the concentration and composition of cecal bacteria are important not only in inducing local colitis but also in stimulating remote inflammation, including the stomach. Cecal luminal bacteria prime lymphocytes in the extensive lymphoid aggregates of the cecal tip, and then these lymphocytes circulate systemically and home to remote mucosal organs, where they are activated if they are exposed to the same bacterial antigens present in the cecum. This hypothesis would explain (i) the lack of gastritis in rats monoassociated with B. vulgatus, since this obligate anaerobe is not present in high concentrations in the partially aerobic stomach; (ii) the lack of gastritis in rats monoassociated with E. coli, since E. coli does not initiate aggressive chronic inflammation in the cecum; and (iii) the presence of gastritis in DESEP-B-colonized rats, since B. vulgatus initiates the cecal inflammation, which enhances the uptake of bacterial antigens from facultative anaerobes present in the cecum as well as in the stomach. This hypothesis is supported by the observation of Aranda et al. that SCID mice raised in a specific-pathogen-free environment develop colitis and gastritis after transfer of CD45RBhigh cells but that “reduced-flora” SCID mice populated with three nonpathogenic Clostridium spp. fail to develop colitis after cell transfer (1). It also explains the observations that HLA-B27 transgenic nude rats raised in a specific-pathogen-free environment develop colitis and gastritis after transfer of lymphocytes from transgenic euthymic rats with colitis (5) whereas GF transgenic rats do not develop intestinal inflammation after receiving the same T cells (24a). Although the failure to transfer colitis to euthymic GF B27 transgenic recipients could be due to resistance of euthymic recipients to colitis mediated by transferred lymphocytes (4), we have shown in preliminary studies that mesenteric lymph node cells from specific-pathogen-free CD3ɛ26 transgenic mice with colitis also do not transfer disease to GF athymic recipients (34).
These results indicate that (i) resident luminal bacteria are important in the initiation and perpetuation of chronic colitis and gastritis in HLA-B27 transgenic rats, (ii) not all bacterial strains have equal ability to cause gastrointestinal inflammation (some bacteria are more aggressive than others), and (iii) different bacteria play different roles in the inflammatory process (B. vulgatus plays a key role in initiating colitis in B27 transgenic rats, while other bacterial strains, although they cannot initiate colitis, play an important role in mediating inflammation in remote organs such as the stomach). These observations have important therapeutic implications for designing clinical trials investigating antibiotics with selective activity in Crohn’s disease and ulcerative colitis.
ACKNOWLEDGMENTS
We gratefully thank Lisa Holt, Toni Grenther, and Julie Vorobiov for expert technical support and Susie May and Beverly Vought for secretarial assistance.
Grant support was provided by USPHS grants DK40249 and DK34989 and Deutsche Forschungsgemeinschaft (DFG) grant Ra 671/1-1 to H. C. Rath.
REFERENCES
- 1.Aranda R, Sydora B C, McAllister P L, Binder S W, Yang H Y, Targan S R, Kronenberg M. Analysis of intestinal lymphocytes in mouse colitis mediated by transfer of CD4+, CD45RBhigh T cells to SCID recipients. J Immunol. 1997;158:3464–3473. [PubMed] [Google Scholar]
- 2.Auer I O, Roder A, Wensinck F, van de Merwe J P, Schmidt H. Selected bacterial antibodies in Crohn’s disease and ulcerative colitis. Scand J Gastroenterol. 1983;18:217–223. doi: 10.3109/00365528309181586. [DOI] [PubMed] [Google Scholar]
- 3.Brandwein S L, McCabe R P, Cong Y, Waites K B, Ridwan B U, Dean P A, Birkenmeier E H, Sundberg J P, Elson C O. Spontaneously colitic C3H/HeJ Bir mice demonstrate selective antibody reactivity to antigens of the enteric bacterial flora. J Immunol. 1997;159:44–52. [PubMed] [Google Scholar]
- 4.Breban M, Hammer R E, Richardson J A, Taurog J D. Transfer of the inflammatory disease of HLA-B27 transgenic rats by bone marrow engraftment. J Exp Med. 1993;178:1607–1616. doi: 10.1084/jem.178.5.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Breban M, Fernandez-Sueiro J L, Richardson J A, Hadavand R R, Maika S D, Hammer R E, Taurog J D. T cells, but not thymic exposure to HLA-B27, are required for the inflammatory disease of the HLA-B27 transgenic rats. J Immunol. 1996;156:794–803. [PubMed] [Google Scholar]
- 6.Brown R G, O’Leary J P, Woodward E R. Hepatic effects of jejunoileal bypass for morbid obesity. Am J Surg. 1974;127:53–58. doi: 10.1016/0002-9610(74)90011-7. [DOI] [PubMed] [Google Scholar]
- 7.Cong Y, Brandwein S L, McCabe R P, Lazenby A, Birkenmeier E H, Sundberg J P, Elson C O. CD4+ T cells reactive to enteric bacterial antigens in spontaneously colitic C3H/HeJBir mice: increased T helper cell type 1 response and ability to transfer disease. J Exp Med. 1998;187:855–864. doi: 10.1084/jem.187.6.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Contractor N V, Bassiri H, Reya T, Park A Y, Baumgart D C, Wasik M A, Emerson S G, Carding S R. Lymphoid hyperplasia, autoimmunity, and compromised intestinal intraepithelial lymphocyte development in colitis-free gnotobiotic IL-2-deficient mice. J Immunol. 1998;160:385–394. [PubMed] [Google Scholar]
- 9.Dianda L, Hanby A M, Wright N A, Sebesteny A, Hayday A C, Owen M J. T cell receptor-alpha beta-deficient mice fail to develop colitis in the absence of a microbial environment. Am J Pathol. 1997;150:91–97. [PMC free article] [PubMed] [Google Scholar]
- 10.Drenick E J, Fisler J, Johnson D. Hepatic steatosis after intestinal bypass—prevention and reversal by metronidazole, irrespective of protein-calorie malnutrition. Gastroenterology. 1982;82:535–548. [PubMed] [Google Scholar]
- 11.Grisham M B, Benoit J N, Granger D N. Assessment of leukocyte involvement during ischemia and reperfusion of intestine. Methods Enzymol. 1990;186:729–742. doi: 10.1016/0076-6879(90)86172-r. [DOI] [PubMed] [Google Scholar]
- 12.Hammer, R. E., S. D. Maika, J. A. Richardson, J. P/ Tang, and J. D. Taurog. 1990. Spontaneous inflammatory disease in transgenic rats expressing HLA-B27 and human beta 2m: an animal model of HLA-B27-associated human disorders. Cell 63:1099–1112. [DOI] [PubMed]
- 13.Krook A, Lindstrom B, Kjellander J, Jarnerot G, Bodin L. Relation between concentrations of metronidazole and Bacteroides spp in faeces of patients with Crohn’s disease and healthy individuals. J Clin Pathol. 1981;34:645–650. doi: 10.1136/jcp.34.6.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lichtman S N, Keku J, Schwab J H, Sartor R B. Hepatic injury associated with small bowel bacterial overgrowth in rats is prevented by metronidazole and tetracycline. Gastroenterology. 1991;100:513–519. doi: 10.1016/0016-5085(91)90224-9. [DOI] [PubMed] [Google Scholar]
- 15.Lichtman S N, Sartor R B, Keku J, Schwab J H. Hepatic inflammation in rats with experimental small intestinal bacterial overgrowth. Gastroenterology. 1990;98:414–423. doi: 10.1016/0016-5085(90)90833-m. [DOI] [PubMed] [Google Scholar]
- 16.Lichtman S N, Wang J, Sartor R B, Zhang C, Bender D E, Dalldorf F G, Schwab J H. Reactivation of arthritis induced by small bowel bacterial overgrowth in rats: role of cytokines, luminal bacteria and bacterial polymers. Infect Immun. 1995;63:2295–2301. doi: 10.1128/iai.63.6.2295-2301.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mizoguchi A, Mizoguchi E, Chiba C, Bhan A K. Role of appendix in the development of inflammatory bowel disease in TCR-alpha mutant mice. J Exp Med. 1996;184:707–715. doi: 10.1084/jem.184.2.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mourelle M, Salas A, Guarner F, Crespo E, Garcia-Lafuente A, Malagelada J R. Stimulation of transforming growth factor beta 1 by enteric bacteria in the pathogenesis of rat intestinal fibrosis. Gastroenterology. 1998;114:519–526. doi: 10.1016/s0016-5085(98)70535-9. [DOI] [PubMed] [Google Scholar]
- 19.Onderdonk A B, Bartlett J G. Bacteriological studies of experimental ulcerative colitis. Am J Clin Nutr. 1979;32:258–265. doi: 10.1093/ajcn/32.1.258. [DOI] [PubMed] [Google Scholar]
- 20.Onderdonk A B, Franklin M L, Cisneros R L. Production of experimental ulcerative colitis in gnotobiotic guinea pigs with simplified microflora. Infect Immun. 1981;32:225–231. doi: 10.1128/iai.32.1.225-231.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Onderdonk A B, Richardson J A, Hammer R E, Taurog J D. A correlation of cecal microflora of HLA-B27 transgenic rats with inflammatory bowel disease. Infect Immun. 1998;66:6022–6023. doi: 10.1128/iai.66.12.6022-6023.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rath H C, Herfarth H H, Ikeda J S, Grenther W B, Hamm T E, Jr, Balish E, Taurog J D, Hammer R E, Wilson K H, Sartor R B. Normal luminal bacteria, especially Bacteroides species, mediate chronic colitis, gastritis, and arthritis in HLA-B27/human beta2 microglobulin transgenic rats. J Clin Investig. 1996;98:945–953. doi: 10.1172/JCI118878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rath H C, Ikeda J S, Linde H-J, Schölmerich J, Wilson K H, Sartor R B. Varying cecal bacterial loads influences colitis and gastritis in HLA-B27 transgenic rats. Gastroenterology. 1999;116:310–319. doi: 10.1016/s0016-5085(99)70127-7. [DOI] [PubMed] [Google Scholar]
- 24.Rath H C, Schultz M, Dieleman L A, Li F, Kölbl H, Falk W, Schölmerich J, Sartor R B. Selective vs. broad spectrum antibiotics in the prevention and treatment of experimental colitis in two rodent models. Gastroenterology. 1998;114:A1067. . (Abstract.) [Google Scholar]
- 24a.Rath, H. C., and R. B. Sartor. Unpublished results.
- 25.Sartor R B. The influence of normal microbial flora on the development of chronic mucosal inflammation. Res Immunol. 1997;148:567–576. doi: 10.1016/s0923-2494(98)80151-x. [DOI] [PubMed] [Google Scholar]
- 26.Sartor, R. B. Microbial factors in the pathogenesis of Crohn’s disease, ulcerative colitis and experimental intestinal inflammation. In J. B. Kirsner (ed.). Inflammatory bowel disease, in press. The Williams & Wilkins Co., Baltimore, Md.
- 27.Sartor R B, Rath H C, Sellon R K. Microbial factors in chronic intestinal inflammation. Curr Opin Gastroenterol. 1996;12:327–333. [Google Scholar]
- 28.Sellon R K, Tonkonogy S L, Schultz M, Grenter W B, Balish E, Rennick D M, Sartor R B. Normal enteric flora are necessary for the development of spontaneous colitis and immune system activation in IL-10-deficient mice. Infect Immun. 1998;66:5224–5231. doi: 10.1128/iai.66.11.5224-5231.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Severijnen A J, van Kleef R, Hazenberg M P, van de Merwe J P. Cell wall fragments from major residents of the human intestinal flora induce chronic arthritis in rats. J Rheumatol. 1989;16:1061–1068. [PubMed] [Google Scholar]
- 30.Stimpson S A, Brown R R, Anderle S K, Klapper D G, Clark R L, Cromartie W J, Schwab J H. Arthropathic properties of cell wall polymers from normal flora bacteria. Infect Immun. 1986;51:240–249. doi: 10.1128/iai.51.1.240-249.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sutherland L, Singleton J, Sessions J, Hanauer S, Krawitt E, Rankin G, Summers R, Mekhjian H, Greenberger N, Kelly M, Levine J, Thomson A, Alpert E, Prokipchuk E. Double blind, placebo controlled trial of metronidazole in Crohn’s disease. Gut. 1991;32:1071–1075. doi: 10.1136/gut.32.9.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taurog J D, Richardson J A, Croft J T, Simmons W A, Zhou M, Fernandez-Sueiro J L, Balish E, Hammer R E. The germfree state prevents development of gut and joint inflammatory disease in HLA-B27 transgenic rats. J Exp Med. 1994;180:2359–2364. doi: 10.1084/jem.180.6.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van de Merwe J P, Schroder A M, Wensinck F, Hazenberg M P. The obligate anaerobic faecal flora of patients with Crohn’s disease and their first-degree relatives. Scand J Gastroenterol. 1988;23:1125–1131. doi: 10.3109/00365528809090179. [DOI] [PubMed] [Google Scholar]
- 34.Veltkamp C, Tonkonogy S L, de Yong Y P, Dieleman L A, Balish E, Terhorst C, Sartor R B. Continuous luminal stimulation is essential for colitis in TGE26 mice after bone marrow transplantation or T cell transfer. Gastroenterology. 1999;116:A838. . (Abstract.) [Google Scholar]
- 35.Wells C L, van de Westerlo E M, Jechorek R P, Feltis B A, Wilkins T D, Erlandsen S L. Bacteroides fragilis enterotoxin modulates epithelial permeability and bacterial internalization by HT-29 enterocytes. Gastroenterology. 1996;110:1429–1437. doi: 10.1053/gast.1996.v110.pm8613048. [DOI] [PubMed] [Google Scholar]
- 36.Yamada T, Deitch E, Specian R D, Perry M A, Sartor R B, Grisham M B. Mechanisms of acute and chronic intestinal inflammation induced by indomethacin. Inflammation. 1993;17:641–662. doi: 10.1007/BF00920471. [DOI] [PubMed] [Google Scholar]