Abstract
Cysteine proteinases have been emphasized in the virulence of Porphyromonas gingivalis in chronic periodontitis. These hydrolases may promote the degradation of extracellular matrix proteins and disrupt components of the immune system. In this study it was shown that purified Arg-gingipain and Lys-gingipain inhibited expression of class II major histocompatibility complex (MHC) proteins in response to the stimulation of endothelial cells with human gamma interferon (IFN-γ). Treatment with the cysteine proteinases resulted in a rapid shift in the apparent molecular size of IFN-γ from 17 to 15 kDa, as shown by Western blot analysis, a response which also occurred in the presence of serum. Further, glycosylated natural IFN-γ from human leukocytes and unglycosylated recombinant IFN-γ from Escherichia coli were both digested by the cysteine proteinases. Immunoblot analysis indicated that cleavage within the carboxyl terminus of recombinant IFN-γ correlated with the loss of induction of MHC class II expression as monitored by analytical flow cytometry. No hydrolysis of MHC class II molecules or human IFN-γ receptor by these proteinases was detected by Western blot analysis. These findings suggest that P. gingivalis cysteine proteinases may alter the cytokine network at the point of infection through the cleavage of IFN-γ. Degradation of IFN-γ could have important consequences for the recruitment and activation of leukocytes and therefore may contribute significantly to the destruction of the periodontal attachment.
The gram-negative anaerobic pathogen Porphyromonas gingivalis has been implicated as a key etiological agent of destructive periodontal disease (21, 37, 43). The major proteinases released by the bacterium hydrolyze peptide bonds after arginyl (gingipain-R; RgpA) or lysyl residues (gingipain-K; Kgp) (36). A polypeptide product of one gingipain locus, RgpA, consists of a pre-pro-fragment, a 50-kDa catalytic domain, and hemagglutinin domains (34). The kgp gene also encodes a pre-pro-fragment, a 60-kDa catalytic domain, and hemagglutinin domains (35). Gingipains isolated from P. gingivalis are potent enzymes with activity against a wide range of substrates, including matrix metalloproteinases (9), complement factors (10, 52), immunoglobulins (25, 44), fibronectin (29, 51), proteinase inhibitors (5, 20), coagulation factors (32), the fibrinogen/fibrin pathway (28), and the kallikrein-kinin system (23, 24). These proteinases participate in the degradation of periodontal tissues directly or indirectly as activators or inactivators of the host immune system. There is considerable evidence to suggest that proinflammatory cytokines, including interleukin 1β (IL-1β), IL-6, and tumor necrosis factor alpha (TNF-α) are degraded by P. gingivalis hydrolases (4, 12, 13).
The progression of periodontal disease is not clearly understood but is characterized by a local accumulation of activated leukocytes (33). Cytokines produced locally probably have an influence on the development of this immune response (14). Of these cytokines, IL-1, IL-2, IL-4, IL-5, IL-6, IL-8, gamma interferon (IFN-γ), transforming growth factor-β, and TNF-α are all implicated (26, 50). The synergistic action of IFN-γ and TNF-α in inflammation is well established where IFN-γ enhances TNF-α production and/or activity.
IFN-γ plays an essential role in the regulation of variety of immune functions. It is produced by antigen-specific T cells and natural killer cells recruited by IL-2, and it has been shown to occur in a pattern similar to that of a controlled delayed-type hypersensitivity response in the gingivitis lesion (41). Lower levels of IFN-γ in periodontal disease lesions may result in decreased Th1 phenotype responses (16). It has been suggested that the stable and progressive lesions are regulated by antigen-specific Th1 phenotype and Th2 phenotype cells, respectively (18, 19).
Major histocompatibility complex class II (MHC-II) molecules are heterodimeric transmembrane glycoproteins consisting of α and β chains (2). The different MHC-II isotypes (HLA-DR, -DQ, and -DP in humans) are encoded by distinct α-chain and β-chain genes (47). MHC-II molecules are essential in order to present peptides generated in the intracellular vesicles of endothelial cells, macrophages, and other antigen-presenting cells to CD4+ T helper lymphocytes (38). A lack of MHC-II expression is known to result in severe immunodeficiency (31).
IFN-γ is a pleiotropic cytokine with immunomodulatory effects on a variety of immune cells (11). IFN-γ is required to upregulate MHC class II proteins and Fcγ receptor expression on macrophages and many other cells, including endothelial cells, lymphoid cells, mast cells and fibroblasts to influence the ability of these cells to present antigen during the induction phase of immune responses (3, 53). IFN-γ is also known as the main factor regulating immunoglobulin G2 (IgG2) switching in mouse B cells challenged with lipopolysaccharide. In periodontitis subjects with progressive lesions, low-avidity antibodies, particularly of the IgG2 class, which lack strong complement fixation and opsonization properties, appear to dominate (49). IFN-γ has been detected by various means in cases of periodontitis (16, 17, 19), but the biological activity of the measured protein was not presented in these studies.
We present here evidence that P. gingivalis proteinases are able to cleave the human IFN-γ molecule but not the HLA-DR molecule or the human IFN-γ receptor α and β chains on human umbilical vein endothelial (HUVE) cells. Also, we demonstrate that degradation occurs at the carboxyl terminal of the IFN-γ in the absence or presence of serum to inactivate the ability of IFN-γ to induce HLA-DR expression in endothelial cells.
MATERIALS AND METHODS
Chemicals and reagents.
Leupeptin, antipain, tosyl-l-phenylalanyl chloromethyl ketone (TPCK), phenylmethylsulfonyl fluoride, iodoacetamide, Nα-tosyl-l-lysine chloromethyl ketone (TLCK), EDTA, pepstatin A, sodium dodecyl sulfate (SDS), N-ethylmaleimide, α1 antitrypsin, Trizma base, Tris-hydrochloride (Tris-HCl), magnesium chloride (MgCl2), l-arginine, l-lysine, l-cysteine, tosyl-Gly-l-Pro-l-Arg p-nitroanilide (GPR-pNA), tosyl-Gly-l-Pro-l-Lys p-nitroanilide (GPK-pNA), collagenase type 1A, endothelial cell growth factor, trypsin, recombinant IFN-γ (rIFN-γ), and native IFN-γ (nIFN-γ) were purchased from Sigma Chemical Co., St. Louis, Mo. Mercuric chloride (HgCl2), Tween 20, and M199 medium were obtained from ICN Biochemicals, Irvine, Calif. 3-[(3-Cholamidopropyl)-dimethylammonio]-1-propanesulfonate (CHAPS) was purchased from Calbiochem, La Jolla, Calif. Phosphate-buffered saline (PBS) was purchased from Oxoid. All reagents for electrophoresis and Western blotting were from Bio-Rad, Richmond, Calif.
RgpA and Kgp isolation.
P. gingivalis (ATCC 33277) cells were grown in enriched Trypticase soy broth (Difco, Detroit, Mich.) under anaerobic conditions for 48 h (7). The bacterial pellet was then extracted in 0.05 M Tris–1 mM CaCl2 (pH 7.5) (Tris buffer) with 1% CHAPS, a nondenaturing zwitterionic detergent, by gentle mixing for 2 h (8). Insoluble material was separated from the detergent extract by centrifugation (8,000 × g, 15 min, 4°C), and the supernatant was passed over a Mono-Q fast-protein liquid chromatography column (Pharmacia, Uppsala, Sweden) equilibrated with Tris buffer containing 1% CHAPS at a flow rate of 60 ml h−1. After being loaded and washed, the proteins were eluted from the column with the same buffer containing 1 M sodium chloride. The Mono-Q eluant was dialyzed against Tris buffer, and final purification was achieved by affinity chromatography over an arginine-Sepharose column (XK 26; Pharmacia) previously equilibrated with Tris buffer. The dialyzed sample was applied at a flow rate of 60 ml h−1, and then the column was washed with 0.5 M NaCl in the same buffer. Kgp was eluted with 0.75 M l-lysine (pH 7.4). After re-equilibration, RgpA was eluted with 1 M l-arginine (pH 7.4).
Enzyme activity assays.
The amidolytic activities of the purified RgpA and Kgp were measured with the substrates GPR-pNA (1 mM) and GPK-pNA (1 mM). Then, 1 μg of RgpA or Kgp was preincubated in Tris buffer containing 5 mM cysteine for 5 min at room temperature. The enzyme and the substrate were combined, and the rates of hydrolysis were measured at 37°C on the basis of the increase in A414 as measured with a Titertek Twinreader PLUS photometer (Flow Lab). To measure the effect of stimulating agents or inhibitors on the activated gingipains, the compounds were preincubated with enzyme at room temperature for up to 30 min in assay buffer prior to assay for residual amidolytic activities.
Measurement of kinetic constants for Arg-gingipain and Lys-gingipain.
Experiments were carried out in which RgpA or Kgp was preincubated in Tris buffer containing 5 mM l-cysteine for 15 min at 37°C. The activated RgpA or Kgp (160 fM each) was then added to the stock substrate solution (160 μM) at 37°C for 10 min, and the reaction was stopped in aliquots with TLCK (2 mM). Aliquots were resolved by 14% polyacrylamide gels by SDS-polyacrylamide gel electrophoresis (PAGE) for Western blot analysis with rabbit anti-human IFN-γ polyclonal antibodies (Endogen). Hydrolysis of rIFN-γ was measured as the cleaved rIFN-γ product, which is proportional to the increase in density as determined by densitometry, thereby allowing determination of the kinetic parameters Km and Vmax.
Endothelial cell isolation and culture.
HUVE cells were isolated and cultured as described previously (46). Briefly, the cells were obtained by treatment of fresh human umbilical cord with collagenase type 1A and then serially cultured. Culture medium M199 was supplemented with 20% fetal calf serum (FCS) (Trace Biosciences, Ltd.), 10 U of heparin (DBL) per ml, 30 μg of endothelial cell growth factor per ml, 50 U of penicillin per ml, and 50 μg of streptomycin per ml. Cells used in these experiments were confluent and at passage levels 4 through 6. Endothelial cells were identified by reaction with Ulex agglutinin (Dako).
Endothelial cell assay conditions.
HUVE cells were seeded at a density of 105 cells/cm2 in supplemented medium containing 20% FCS in 12-well flat-bottomed tissue culture plates (Costar, Cambridge, Mass.). rIFN-γ or nIFN-γ (6.7 × 105 IU/nM) were added, along with various concentrations of RgpA or Kgp, to the culture wells and then incubated for various times as described in the figure legends. At the end of each experimental culture period the cells were treated with 0.05% trypsin–0.02% EDTA to produce a monodispersed cell suspension. Cells were collected by brief centrifugation and prepared for flow cytometry analysis with three washes with PBS containing 2% FCS. Alternatively, the cells were washed twice in PBS alone for protein analysis by Western blotting. Previous experiments established that this method of cell harvesting does not decrease the surface expression of the HLA-DR antigen studied.
Flow cytometric analysis for MHC-II antigen.
Endothelial cell surface antigen expression of HLA-DR was determined by indirect immunofluorescence. Harvested endothelial cells were incubated with a saturating 1:200 concentration of primary mouse anti-human HLA-DR β-chain monoclonal antibody (Dako), labeled with a 1:50 concentration of rabbit anti-mouse secondary antibody (fluorescein isothiocyanate conjugated) (Dako), and quantitated by using a Becton Dickson FACSCAN analyzer. Incubations were for 45 min at 4°C. Volume gates were set to include the entire endothelial cell population. Data are presented as histograms of relative fluorescence in a logarithmic scale on the x axis and the cell number as a linear scale on the y axis.
SDS-PAGE and Western blot analysis.
Proteins were resolved in 14% polyacrylamide gels (SDS-PAGE) (27) and transferred to polyvinylidene difluoride membrane essentially as described by Towbin et al. (48). The membranes were then incubated for 2 h in 20 mM Tris with 500 mM NaCl buffer (Tris-buffered saline) containing 0.1% Tween 20 and 4% skim milk (blocking buffer). IFN-γ was detected with either polyclonal rabbit anti-human IFN-γ (Endogen) or polyclonal goat anti-human IFN-γ, which recognizes the epitope corresponding to amino acids 148 to 166 mapping at the carboxy terminus of the IFN-γ precursor (Santa Cruz Biotechnology). Either alkaline phosphatase goat anti-rabbit (Dako) or biotin-labeled rabbit anti-goat IgG (Dako) was used as the secondary antibody accordingly. Biotin was detected with streptavidin-alkaline phosphatase (Dako), and color was developed in a solution containing nitroblue tetrazolium chloride (1.65 mg) and 5-bromo-4-chloro-3-indolylphosphate p-toluidine salt (0.8 mg) in 5 ml of 100 mM Tris-HCl (pH 9.5) containing 100 mM NaCl and 50 mM MgCl2. Membranes were washed three times in Tris-buffered saline–0.1% Tween after each step. The HLA-DR was detected with monoclonal mouse anti-human HLA-DR antibodies which recognize the α or β chains, respectively (Dako). The IFN-γ receptor (R) was detected with polyclonal antibody specific for amino acids 466 to 485 mapping at the COOH-terminal domain of the human IFN-γ R α chain or detected with polyclonal antibody specific for the amino acids 318 to 337 mapping at the COOH-terminal domain for IFN-γ R β chain (Santa Cruz Biotechnology).
RESULTS
Characterization of the proteinase preparations.
The RgpA and Kgp isolated from the cellular fraction of P. gingivalis had activity and inhibition profiles characteristic of the gingipains previously published (Tables 1 and 2). Based on activity profiles, RgpA preparation was ∼99% pure with 1% Kgp contamination, whereas Kgp preparation was ∼85% pure with 15% RgpA contamination. Boiling and reduction of the gingipains (denaturation) resulted in more complex SDS-PAGE banding patterns that were characteristic of gingipains previously described (Fig. 1). NH2-terminal sequencing confirmed the identity of the peptide fragments as RgpA and Kgp domains (data not shown).
TABLE 1.
Inhibition profiles of gingipain-R and gingipain-K on the hydrolysis of GPR-pNA and GPK-pNA
| Inhibitor | Concn (mM) | % Residual activity of:
|
|
|---|---|---|---|
| Gingipain-R | Gingipain-K | ||
| Leupeptin | 0.1 | 0 | 100 |
| TPCK | 1 | 0 | 0 |
| Phenylmethylsulfonyl fluoride | 10 | 100 | 85 |
| Iodoacetamide | 10 | 0 | 0 |
| 1 | 50 | 50 | |
| TLCK | 2 | 0 | 0 |
| HgCl2 | 2 | 0 | 0 |
| EDTA | 5 | 10 | 100 |
| Pepstatin A | 0.1 | 92 | 100 |
| SDS | 10 | 30 | 50 |
| N-Ethylmaleimide | 5 | 0 | 0 |
| α1-Antitrypsin | 0.01 | 90 | 82 |
TABLE 2.
Activity profiles of gingipain-R and gingipain-K on the substrate GPR-pNA and GPK-pNA in the absence or presence of 5 mM l-cysteine
| Assay | Activity profiles (μM/μg/min) of:
|
|||
|---|---|---|---|---|
| RgpA
|
Kgp
|
|||
| +Cys | −Cys | +Cys | −Cys | |
| GPR-pNA | 9.77 | 0.1 | 0.84 | 0 |
| GPK-pNA | 0.1 | 0.14 | 5.4 | 0.14 |
FIG. 1.
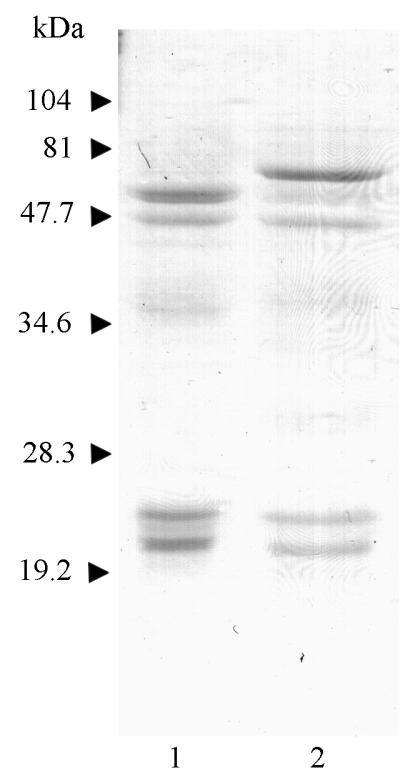
SDS-PAGE of purified RgpA and Kgp. Gingipains were isolated as described in Materials and Methods, denatured by boiling in SDS loading buffer, and then resolved by SDS-PAGE. Lane 1, RgpA; lane 2, Kgp.
Cotreatment of HUVE cells with rIFN-γ and gingipains.
Nonstimulated HUVE cells in culture did not express HLA-DR antigen, as measured by flow cytometric analysis (Fig. 2a), but they could be induced to express DR antigen by incubation with rIFN-γ (Fig. 2b). RgpA or Kgp (the effect of Kgp is not shown) at concentrations of 60 nM in serum-containing culture medium eliminated the surface expression of HLA-DR to control levels on HUVE cells when added simultaneously with 3 nM rIFN-γ (Fig. 2c). When the gingipains were added either 1 or 2 days after the rIFN-γ, the HLA-DR expression was limited in a time-dependent manner (Fig. 2d and e). This activity of RgpA and Kgp was destroyed when the gingipains were heated for 30 min at 80°C, indicating that the effect was structurally labile and was not due to toxicity (data not shown). The cysteine proteinase inhibitor TLCK (2 mM, final concentration) was able to block the effect of both RgpA and Kgp (the effect of Kgp is not shown) (Fig. 3). Finally, RgpA and Kgp each had a similar dose-dependent effect of limiting HLA-DR expression in HUVE cell cultures (Fig. 4). The dose effects were linear to a concentration of 35 nM gingipain in the culture medium with a maximal effect near 75 nM. These data suggest that the effect of RgpA and Kgp on HLA-DR expression is associated with a free thiol and probably with protease activity of the gingipains.
FIG. 2.
Time-dependent inhibition by RgpA of HLA-DR expression induced in HUVE cells. HUVE cells were seeded subconfluently at a density of 105 cells/cm2 and maintained for 4 days in supplemented medium containing 20% FCS as described in Materials and Methods. At the start of the incubation, rIFN-γ (3 nM) was added to some wells (b to e). RgpA (60 nM) was added either at the time of (c), 1 day after (d), or 2 days after (e) the addition of the rIFN-γ. Four days after the start of the incubation, cells were removed and analyzed for HLA-DR expression by flow cytometric analysis as described in Materials and Methods. The cell number (y axis) versus logarithm of fluorescence (x axis) is represented. The data are representative of four separate experiments.
FIG. 3.
Inhibition by RgpA of HLA-DR expression induced in HUVE cells is thiol mediated. RgpA was preincubated with or without the thiol-protease inhibitor TLCK for 1 h at 37°C and then dialyzed exhaustively against PBS. rIFN-γ (3 nM) and the TLCK-treated RgpA (60 nM) were simultaneously added to HUVE cells seeded at a density of 105 cells/cm2 and then incubated for 4 days in supplemented medium containing 20% FCS as described in Materials and Methods. After 4 days of culture, cells were removed and analyzed for HLA-DR expression by flow cytometric analysis. The cell number (y axis) versus the logarithm of fluorescence (x axis) is represented. The data are representative of three separate experiments.
FIG. 4.
Dose-dependent inhibition by RgpA and Kgp of HLA-DR expression induced in HUVE cells. HUVE cells were seeded subconfluently at a density of 105 cells/cm2 and maintained for 4 days in supplemented medium containing 20% FCS. At the start of the incubation, rIFN-γ (3 nM) and various concentrations of RgpA or Kgp were added simultaneously, and the cultures were then maintained for 4 days. After 4 days, cells were removed and analyzed for HLA-DR expression by flow cytometric analysis. Error bars show the means and standard errors of the means for three separate experiments, which were representative of multiple experiments. Symbols: ●, RgpA; ▴, Kgp.
The possibility that the decreased measurement of HLA-DR expression was due to the proteolytic activity of the RgpA or Kgp on HLA-DR molecules directly was examined. We incubated HUVE cells with rIFN-γ for 3 days to induce HLA-DR expression and then washed the cells and added 60 nM RgpA or Kgp for a subsequent 24 h in culture in the presence of 5 mM l-cysteine. With specific antibodies against either the α-chain or β-chain monomorphic regions, no hydrolysis of the HLA-DR complex was detected after incubation of the cells with either RgpA or Kgp (Fig. 5).
FIG. 5.
RgpA or Kgp do not cleave the HLA-DR molecule on HUVE cells. HUVE cells were seeded at a density of 105 cells/cm2 and incubated for 3 days in supplemented medium containing 3 nM rIFN-γ to induce HLA-DR expression. Cells were then washed and cultured for an additional 24 h in serum-containing supplemented medium with 60 nM RgpA or Kgp. The cells were subsequently removed, washed, and solubilized in SDS; the proteins were then resolved by SDS-PAGE and subjected to Western blot analysis. (A) Detection of the HLA-DR α chain. (B) Detection of the HLA-DR β chain. Lane 1, untreated HUVE cells; lane 2, RgpA treatment of HUVE cells; lane 3, Kgp treatment of HUVE cells. The data are representative of three separate experiments.
Since the gingipains limited the expression of HLA-DR which had been experimentally induced through the IFN-γ pathway, we also examined whether the RgpA and Kgp were acting on the IFN-γ receptor α and β chains of HUVE cells. As shown in Fig. 6, we were not able to detect any hydrolysis of the IFN-γ receptor α or β chains resulting from the addition of cysteine-activated RgpA and Kgp to HUVE cell cultures. These results suggested that the HLA-DR molecule and the IFN-γ receptor α and β chains are resistant to proteolytic digestion by the gingipains.
FIG. 6.
Lack of RgpA or Kgp effect on the IFN-γ receptor molecule. Confluent HUVE cells seeded at a density of 105 cells/cm2 in supplemented medium containing 20% FCS were incubated for 1 or 4 days with 60 nM RgpA or Kgp. The cells were removed, washed, and solublized in SDS; the proteins were then resolved by SDS-PAGE and subjected to Western blot analysis. (A) Detection of the IFN-γ R α chain. Lanes 1 to 5 show HUVE cells treated as follows: lane 1, Kgp for 1 day; lane 2, RgpA for 1 day; lane 3, Kgp for 4 days; lane 4, RgpA for 4 days; lane 5, medium only. (B) Detection of the IFN-γ R β-chain. Lanes 1 to 3 show HUVE cells treated as follows: lane 1, Kgp for 1 day; lane 2, RgpA for 1 day; lane 3, medium only. The data are representative of three separate experiments.
Digestion of IFN-γ by RgpA or Kgp in the absence or presence of serum.
Both nIFN-γ and rIFN-γ were partially hydrolyzed by the gingipains in separate reactions, as determined by Western blot analysis. Proteolytic processing of nIFN-γ in the presence of serum (Fig. 7) was rapid with a 1:1 enzyme-substrate (E:S) molar ratio but did not progress beyond the initial cleavage generating the 15-kDa fragment, even after an overnight incubation at 37°C. Incubation with either RgpA or Kgp gave similar results.
FIG. 7.
Time course of native IFN-γ degradation by RgpA and Kgp in serum. RgpA or Kgp (4.7 pM each) was preincubated for 15 min at 37°C with 5 mM l-cysteine. The activated gingipains were then mixed with whole bovine serum and combined with an equimolar ratio of native IFN-γ (4.7 pM in each reaction) for a final serum concentration of 20%. Digestions were incubated at 37°C for various times and then stopped in aliquots with TLCK (2 mM, final concentration). Aliquots were resolved by SDS-PAGE for Western blot analysis with polyclonal antibodies against IFN-γ as described in Materials and Methods. Control samples incubated without gingipains are labeled IFN-γ. (A) Digestion with RgpA. (B) Digestion with Kgp. The data are representative of three separate experiments.
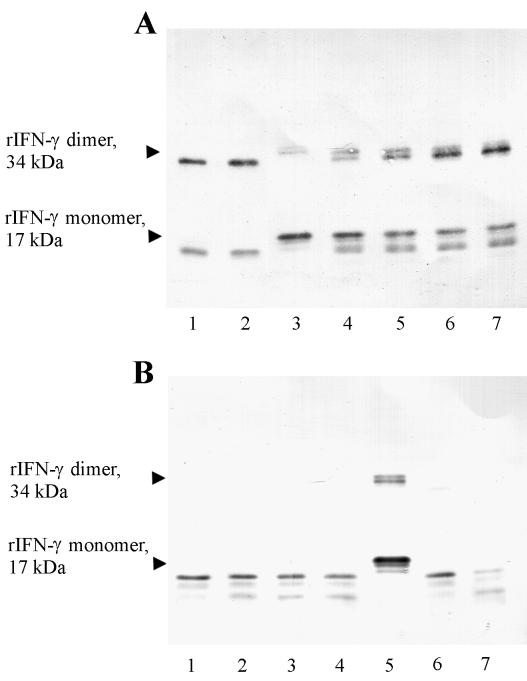
Proteolytic processing of rIFN-γ also was similar with both the RgpA and the Kgp. As the reaction progressed over time, however, lower-molecular-size fragments were detected in serum-free conditions, with gradual loss of the remaining 15 kDa fragment at an E:S molar ratio of 1:28 (see Fig. 9). Hydrolysis of the rIFN-γ was approximately 10-fold slower in the presence of 20% serum than in the absence of serum.
FIG. 9.
Degradation of recombinant IFN-γ by RgpA or Kgp in the presence of leupeptin. RgpA or Kgp was preincubated with 5 mM l-cysteine for 15 min and then with or without 0.1 mM leupeptin for 15 min at 37°C. The gingipains were then combined with rIFN-γ for a final E:S ratio of 1:28 (0.54 pM gingipains with 15 pM rIFN-γ in each reaction). Digestions were incubated at 37°C for various times and then stopped in aliquots with TLCK (2 mM, final concentration). Aliquots were resolved by SDS-PAGE for Western blot analysis with rabbit anti-human IFN-γ polyclonal antibodies as described in Materials and Methods. (A) Digestion with RgpA. Lanes 1 and 2, cysteine (5 mM), at 10 min and 80 min respectively; lane 3, human rIFN-γ as a control; lanes 4 to 7, cysteine (5 mM) and leupeptin (0.1 mM) at 10, 20, 40, and 80 min, respectively. (B) Digestion with Kgp. Lanes 1 to 4, cysteine (5 mM) and leupeptin (0.1 mM) at 10, 20, 40, and 80 min, respectively; lane 5, human rIFN-γ as a control; lanes 6 and 7, cysteine (5 mM) at 10 and 80 min, respectively.
More-limited proteolysis of recombinant IFN-γ (Fig. 8) in the absence of serum by RgpA or Kgp occurred at an E:S molar ratio of 1:1,000 and resulted in partial conversion of the 17-kDa rIFN-γ to a 15-kDa fragment after 10 min of incubation. Importantly, IFN-γ dimers demonstrated rates of hydrolysis similar to the monomers.
FIG. 8.
Time course of rIFN-γ degradation by RgpA and Kgp in the absence of serum. RgpA or Kgp was preincubated for 15 min at 37°C with 5 mM l-cysteine. The activated gingipains were then incubated with rIFN-γ at a final S:E ratio of 1,000:1 (15 pM rIFN-γ with 15 fM gingipains in each reaction). Digestions were incubated at 37°C for various times and then stopped in aliquots with TLCK (2 mM, final concentration). Aliquots were resolved by SDS-PAGE for Western blot analysis with rabbit anti-human IFN-γ polyclonal antibodies as described in Materials and Methods. Control samples incubated without gingipain are labeled IFN-γ. (A) Digestion with RgpA. (B) Digestion with Kgp. The data are representative of three separate experiments.
Degradation of rIFN-γ by RgpA or Kgp in the presence of leupeptin and the kinetic characteristics.
To determine the relative roles of RgpA and Kgp in IFN-γ hydrolysis, reactions with RgpA and Kgp were carried out in the presence of 5 mM l-cysteine and 0.1 mM leupeptin, an inhibitor of RgpA and not Kgp (Fig. 9). Although the RgpA preparation was shown to contain a low level of Kgp (Tables 1 and 2), adding leupeptin to the RgpA preparation almost completely abolished IFN-γ hydrolysis, demonstrating that RgpA cleaves IFN-γ. In the case of Kgp, complete degradation of rIFN-γ occurred within 10 min in the presence or absence of 0.1 mM leupeptin, demonstrating that Kgp also cleaves IFN-γ.
The Km and Vmax values for Arg-gingipain and Lys-gingipain were determined by measuring the accumulation of 15-kDa cleavage product in Western Blot analysis. The Km values for the formation of rIFN-γ cleavage product were 13 μM for Arg-gingipain and 3 μM for Lys-gingipain. The Vmax values for Arg-gingipain and Lys-gingipain were 442 and 227 nM/min, respectively. (The reaction with Lys-gingipain was carried out in the presence of 0.1 mM leupeptin to compensate for the percentage of RgpA in the Kgp preparation [see Tables 1 and 2].) KGP exhibited a higher affinity for the rIFN-γ as evidenced by lower Km value, while RgpA cleaved the rIFN-γ more efficiently, with a higher Vmax value. These data provide supportive evidence that both RgpA and Kgp cleave rIFN-γ efficiently.
Cleavage of rIFN-γ COOH-terminal epitope by RgpA or Kgp results in loss of HLA-DR induction.
Since human IFN-γ has a number of basic amino acid residues (1) in the COOH-terminal portion which may be processed by tryptic-like protease(s), we suspected that the conversion of the 17-kDa molecule to the 15-kDa fragment by gingipains was due to proteolysis in the COOH-terminal region of IFN-γ and was associated with a loss of biological activity. To demonstrate this, the rIFN-γ was incubated with various concentrations of RgpA or Kgp for different periods of time, and the reactions were stopped with TLCK. Aliquots of the digests were analyzed by Western blot by using a polyclonal antibody directed against the COOH terminus of the rIFN-γ molecule. To replicate the incubation condition in the HUVE cell cultures, aliquots of the same digests were also dialyzed and applied to HUVE cell cultures to assess HLA-DR induction. Loss of the COOH terminus detected by Western blot analysis (Fig. 10a to c) corresponded with loss of HLA-DR induction (Fig. 10d and e).
FIG. 10.
Loss of COOH-terminal epitope resulting from digestion of rIFN-γ by RgpA or Kgp results in loss of HLA-DR induction. Western blots of rIFN-γ (4.7 pM in each reaction) incubated with RgpA (A) or Kgp (B) (0.67 pM each) for various times in the absence of serum or with RgpA in the presence of 20% FCS (C). Digestions were stopped in aliquots with TLCK (2 mM, final concentration), and the products were resolved by SDS-PAGE for Western blot analysis with polyclonal antibody corresponding to amino acids 148 to 166 mapping at the COOH terminus of the rIFN-γ. Control samples incubated without gingipain are labeled IFN-γ. (D and E) Flow cytometric analysis of HLA-DR expression induced by gingipain-treated rIFN-γ. Samples of rIFN-γ (D) or nIFN-γ (E) treated with RgpA or Kgp for 15 min in the absence of serum as described above were dialyzed against PBS and then incubated with HUVE cells seeded at a density of 105 cells/cm2 in supplemented medium containing 20% FCS for 4 days. The samples were then analyzed for HLA-DR expression by flow cytometric analysis. The cell number (y axis) versus the logarithm of fluorescence (x axis) is represented. The data are representative of three separate experiments.
DISCUSSION
The results of this study demonstrated that both the Arg-gingipain and Lys-gingipain of P. gingivalis could rapidly cleave and inactivate the IFN-γ molecule. Treatment of native glycosylated human IFN-γ with P. gingivalis cysteine proteinases resulted in the generation of a fragment which displays an Mr of 15,000, with some further degradation occurring after prolonged incubation. Both the cysteine-activated RgpA and Kgp cleaved the human IFN-γ molecule within 10 min, with the further degradation of IFN-γ by Kgp occurring at a slower rate. Proteolysis also occurred in the presence of serum inhibitors, a finding which is consistent with the findings from flow cytometric analysis, where the P. gingivalis cysteine proteinases decreased the expression of HLA-DR molecules on HUVE cells in the presence of 20% FCS in a dose- and time-dependent manner. The proteinase inhibitors α2-macroglobulin and α1-antitrypsin, which are responsible for most of the total proteinase-inhibitory capacity of plasma (5), may play significant roles in protecting tissues from proteolytic enzymes released in infected sites. Resistance to plasma inhibitors and preferential cleavage of IFN-γ in the presence of plasma proteins, which is possibly related to IFN-γ structure, implies a role for the gingipains in driving an ineffective immune response.
Cleavage rates of rIFN-γ by gingipain-R and gingipain-K exhibit very similar kinetics. Although gingipain-R proceeded with a higher kinetic constant than gingipain-K, this was balanced by the higher affinity constant of gingipain-K. In relation to the high conservation of the noncatalytic regions of these enzymes, the detected differences are potentially attributable to structural heterogeneity within the catalytic domains.
The regulatory effects of IFN-γ include induction of HLA-DR expression, activation of macrophages to enhance phagocytic capability as well as activation and growth enhancement of cytotoxic T lymphocytes and natural killer cells. Cleavage of IFN-γ may disrupt locally the host’s defense against microbial pathogens by affecting the antigen-presenting activity of macrophages. Of note, studies in our laboratory have demonstrated the functional anergy of macrophages in advanced periodontitis lesions (6).
The IFN-γs from different sources show similarity in sensitivity to P. gingivalis cysteine proteinases. The glycosylated form from human lymphocytes and the unglycosylated IFN-γ from E. coli were both degraded by the gingipains. Human IFN-γ is a well-characterized secretory glycoprotein that has two potential glycosylation sites at asparagine 25 (Asn25) and asparagine 97 (Asn97) at the consensus sequences Asn-X-Thr and Asn-X-Ser, respectively. Natural IFN-γ has three forms that are different in glycosylation: diglycosylated at Asn25 and Asn97 (2N), monoglycosylated at Asn25 (1N), and nonglycosylated (22). Results indicate that the sugar side chains are ineffective in protecting the gingipain cleavage sites of the proteins on the IFN-γ molecule, although the glycan residues constitute 15 to 25% of the molecular mass of the protein.
From the immunoblot analysis with polyclonal antibody to detect the human IFN-γ molecule, one major fragment corresponding to 15 kDa was observed after incubation at 37°C, whereas with the polyclonal antibody specific for the COOH terminus of the IFN-γ molecule, this band was not observed, indicating that the 15-kDa fragment lacks the COOH-terminal epitope. Based on the molecular size difference between the intact rIFN-γ and the 15-kDa protein, we suggest the latter lacks 13 to 14 amino acids at the COOH terminus. Since the COOH terminus-directed antibody was made against a sequence of 19 COOH-terminal residues of intact rIFN-γ, removal of the 13 or 14 COOH-terminal residues could destroy the immunoreactivity of the protein. Thus, based on the above data, cleavage of the rIFN-γ with P. gingivalis cysteine proteinases occurred probably at the carboxylic side of Arg-129 or Lys-130.
From the flow cytometric analysis, it is significant that the pretreatment of rIFN-γ or nIFN-γ with gingipain correlated with the loss of induction of HLA-DR. It is therefore likely that the loss of inducing activity of MHC-II expression with the digested IFN-γ molecule is due to the removal of the 13 to 14 amino acids at the COOH-terminal end of IFN-γ. These data are consistent with other studies which have indicated that enzymatic removal of residues 129 to 143 of the protein with endopeptidases such as clostripain (30) or trypsin (1) results in a 10- to 100-fold reduction in IFN-γ’s specific antiviral activity.
In chronic inflammatory periodontal disease, the predominant lymphocyte in the stable lesion of gingivitis is the Th1 phenotype cell, while increased proportions of B cells and plasma cells can be demonstrated in the progressive lesion (42, 45). IFN-γ may be essential for the induction of Th1 phenotype with an inhibitory effect on Th2 cell profile (15, 39, 40). Inactivation of IFN-γ by gingipains from P. gingivalis could lead to proliferation of Th2 phenotype cells and locally to mediate progression of the periodontal lesion.
We are beginning clinical studies to investigate the status of IFN-γ present in the gingival fluid and tissues, and preliminary evidence indicates that IFN-γ hydrolysis is present in diseased but not in healthy samples. Chronic inflammatory periodontal disease, however, must be seen to be multifactorial in etiology and pathogenesis, and it is difficult to link these findings to the situation in vivo. These data do, however, suggest another, potentially important mechanism for virulence of P. gingivalis and the gingipains. To conclude, proteolysis of IFN-γ and other cytokines by P. gingivalis may disturb the complex cytokine network responsible for maintaining a protective response to bacterial challenge.
ACKNOWLEDGMENT
This study was supported by a grant from the National Health and Medical Research Council of Australia.
REFERENCES
- 1.Arakawa T, Hsu Y R, Parker C G, Lai P H. Role of polycationic C-terminal portion in the structure and activity of recombinant human interferon-gamma. J Biol Chem. 1986;261:8334–8339. [PubMed] [Google Scholar]
- 2.Benacerraf B. Role of MHC gene products in immune regulation. Science. 1981;212:1229–1238. doi: 10.1126/science.6165083. [DOI] [PubMed] [Google Scholar]
- 3.Boehm U, Klamp T, Groot M, Howard J C. Cellular responses to interferon-γ. Annu Rev Immunol. 1997;15:749–795. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- 4.Calkins C C, Platt K, Potempa J, Travis J. Inactivation of tumor necrosis factor-α by proteinases (gingipains) from the periodontal pathogen, Porphyromonas gingivalis: implications of immune evasion. J Biol Chem. 1998;273:6611–6614. doi: 10.1074/jbc.273.12.6611. [DOI] [PubMed] [Google Scholar]
- 5.Carlsson J, Herrmann B F, Höfling J F, Sundqvist G K. Degradation of the human proteinase inhibitors alpha-1-antitrypsin and alpha-2-macroglobulin by Bacteroides gingivalis. Infect Immun. 1984;43:644–648. doi: 10.1128/iai.43.2.644-648.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chapple C C, Srivastava M, Hunter N. Failure of macrophage activation in destructive periodontal disease. J Pathol. 1998;186:281–286. doi: 10.1002/(SICI)1096-9896(1998110)186:3<281::AID-PATH200>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 7.Chen Z, Potempa J, Polanowski A, Wikström M, Travis J. Purification and characterization of a 50 kDa cysteine proteinase (gingipain) of Porphyromonas gingivalis. J Biol Chem. 1992;267:18896–18901. [PubMed] [Google Scholar]
- 8.Ciborowski P, Nishikata M, Allen R D, Lantz M S. Purification characterization of two forms of a high-molecular-weight cysteine proteinase (Porphypain) from Porphyromonas gingivalis. J Bacteriol. 1994;176:4549–4557. doi: 10.1128/jb.176.15.4549-4557.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeCarlo A A, Windsor L J, Bodden M K, Harber G J, Birkedal-Hansen B, Birkedal-Hansen H. Activation and novel processing of matrix metalloproteinases by a thiol-proteinase from the oral anaerobe Porphyromonas gingivalis. J Dent Res. 1997;76:1260–1270. doi: 10.1177/00220345970760060501. [DOI] [PubMed] [Google Scholar]
- 10.DiScripio R G, Daffern P J, Kawahara M, Pike R, Potempa J, Travis J, Hugli T E. Cleavage of human complement component C5 by cysteine proteinases from Porphyromonas (Bacteroides) gingivalis. Prior oxidation of C5 augments neutrophil activating capacity in Arg-gingipain and Lys-gingipain digests of C5. Immunology. 1996;87:660–667. doi: 10.1046/j.1365-2567.1996.478594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farrar M A, Schreiber R D. The molecular cell biology of interferon-γ and its receptor. Annu Rev Immunol. 1993;11:571–611. doi: 10.1146/annurev.iy.11.040193.003035. [DOI] [PubMed] [Google Scholar]
- 12.Fletcher J, Reddi K, Poole S, Nair S, Henderson B, Tabona P, Wilson M. Interactions between periodontopathogenic bacteria and cytokines. J Periodont Res. 1997;32:200–205. doi: 10.1111/j.1600-0765.1997.tb01406.x. [DOI] [PubMed] [Google Scholar]
- 13.Fletcher J, Nair S, Poole S, Henderson B, Wilson M. Cytokine degradation by biofilms of Porphyromonas gingivalis. Curr Microbiol. 1998;36:216–219. doi: 10.1007/s002849900297. [DOI] [PubMed] [Google Scholar]
- 14.Fujihashi K, Kono Y, Beagley K W, Yamamoto M, McGhee J R, Mestecky J, Kiyono H. Cytokine and periodontal disease: immunopathological role of interleukins for B cell responses in chronic inflamed gingival tissues. J Periodontol. 1993;64:400–406. [PubMed] [Google Scholar]
- 15.Gajewski T F, Fitch F W. Antiproliferative effect of IFN-gamma in immune regulation. I. IFN-gamma inhibits the proliferation of Th2 but not Th1 murine helper T lymphocyte clones. J Immunol. 1988;140:4245–4252. [PubMed] [Google Scholar]
- 16.Gemmell E, Seymour G J. Modulation of immune responses to periodontal bacteria. Curr Opin Periodontol. 1994;1994:28–38. [PubMed] [Google Scholar]
- 17.Gemmell E, Kjeldsen M, Yamazaki K, Nakajima T, Aldred M J, Seymour G J. Cytokine profiles of Porphyromonas gingivalis-reactive T lymphocyte lines and clones derived from P. gingivalis-infected subjects. Oral Dis. 1995;1:139–146. doi: 10.1111/j.1601-0825.1995.tb00176.x. [DOI] [PubMed] [Google Scholar]
- 18.Gemmell E, Roderick I M, Seymour G J. Cytokines and prostaglandins in immune homeostasis and tissue destruction in periodontal disease. Periodontol 2000. 1997;14:112–143. doi: 10.1111/j.1600-0757.1997.tb00194.x. [DOI] [PubMed] [Google Scholar]
- 19.Gemmell E, Seymour G J. Cytokine profiles of cells extracted from humans with periodontal diseases. J Dent Res. 1998;77:16–26. doi: 10.1177/00220345980770010101. [DOI] [PubMed] [Google Scholar]
- 20.Grøn H, Pike R, Potempa J, Travis J, Thøgersen I B, Enghild J J, Pizzo S V. The potential role of α2-macroglobulin in the control of cysteine proteinases (gingipains) from Porphyromonas gingivalis. J Periodontol Res. 1997;32:61–68. doi: 10.1111/j.1600-0765.1997.tb01383.x. [DOI] [PubMed] [Google Scholar]
- 21.Holt S C, Ebersole J, Felton J, Brunsvold M, Kornmann K S. Implantation of Bacteroides gingivalis in nonhuman primates initiates progression of periodontitis. Science. 1988;239:55–57. doi: 10.1126/science.3336774. [DOI] [PubMed] [Google Scholar]
- 22.Hooker A, James D. The glycosylation heterogeneity of recombinant human IFN-γ. J Interferon Cytokine Res. 1998;18:287–295. doi: 10.1089/jir.1998.18.287. [DOI] [PubMed] [Google Scholar]
- 23.Imamura T, Potempa J, Pike R N, Travis J. Pathogenesis of periodontitis: a major arginine-specific cysteine proteinase from Porphyromonas gingivalis induces vascular permeability enhancement through activation of the kallikrein/kinin pathway. J Clin Invest. 1994;94:361–369. doi: 10.1172/JCI117330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Imamura T, Potempa J, Pike R N, Travis J. Dependence of vascular permeability enhancement on cysteine proteinases in vesicles of Porphyromonas gingivalis. Infect Immun. 1995;63:1999–2003. doi: 10.1128/iai.63.5.1999-2003.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kilian M. Degradation of immunoglobulins A1, A2, and G by suspected principal periodontal pathogens. Infect Immun. 1981;34:757–765. doi: 10.1128/iai.34.3.757-765.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kjeldsen M, Holmstrup P, Bendtzen K. Marginal periodontitis and cytokines: a review of the literature. J Oral Pathol. 1993;14:833–843. doi: 10.1902/jop.1993.64.11.1013. [DOI] [PubMed] [Google Scholar]
- 27.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 28.Lantz M S, Rowland R W, Switalski L M, Höök M. Interactions of Bacteroides gingivalis with fibrinogen. Infect Immun. 1986;54:654–658. doi: 10.1128/iai.54.3.654-658.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lantz M S, Allen R D, Duck L W, Blume J L, Switalski L M, Höök M. Identification of Porphyromonas gingivalis components that mediate its interactions with fibronectin. J Bacteriol. 1991;173:4263–4270. doi: 10.1128/jb.173.14.4263-4270.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leinikki P O, Calderon J, Luquette M H, Schreiber R D. Reduced receptor binding by a human interferon-γ fragment lacking 11 carboxyl-terminal amino acids. J Immunol. 1987;139:3360–3366. [PubMed] [Google Scholar]
- 31.Mach B, Steimle V, Reith W. MHC class II-deficient combined immunodeficiency: a disease of gene regulation. Immunol Rev. 1994;138:207–221. doi: 10.1111/j.1600-065x.1994.tb00853.x. [DOI] [PubMed] [Google Scholar]
- 32.Nilsson T, Carlsson J, Sundqvist G. Inactivation of key factors of the plasma proteinase cascade systems by Bacteroides gingivalis. Infect Immun. 1985;50:467–471. doi: 10.1128/iai.50.2.467-471.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Page R C. The role of inflammatory mediators in the pathogenesis of periodontal disease. J Periodontol Res. 1991;26:230–242. doi: 10.1111/j.1600-0765.1991.tb01649.x. [DOI] [PubMed] [Google Scholar]
- 34.Pavloff N, Potempa J, Pike R N, Prochazka V, Kiefer M C, Travis J, Barr P J. Molecular cloning and structural characterization of the Arg-gingipain proteinase of Porphyromonas gingivalis: biosynthesis as a proteinase-adhesin polyprotein. J Biol Chem. 1995;270:1007–1010. doi: 10.1074/jbc.270.3.1007. [DOI] [PubMed] [Google Scholar]
- 35.Pavloff N, Pemberton P A, Potempa J, Chen W C A, Pike N R, Prochazka V, Kiefer M C, Travis J, Barr P. Molecular cloning and characterization of Porphyromonas gingivalis Lys-gingipain. A new member of an emerging family of pathogenic bacterial cysteine proteinases. J Biol Chem. 1997;272:1595–1600. doi: 10.1074/jbc.272.3.1595. [DOI] [PubMed] [Google Scholar]
- 36.Pike R, McGraw W, Potempa J, Travis J. Lysine- and arginine-specific proteinases from Porphyromonas gingivalis: isolation and evidence for the existence of complexes with hemagglutinins. J Biol Chem. 1994;269:406–411. [PubMed] [Google Scholar]
- 37.Potempa J, Mikolajczyk-Pawlinska J, Brassell D, Nelson D, Thøgersen I B, Enghild J J, Travis J. Comparative properties of two cysteine proteinases (gingipains R), the products of two related but individual genes of Porphyromonas gingivalis. J Biol Chem. 1998;273:21648–21657. doi: 10.1074/jbc.273.34.21648. [DOI] [PubMed] [Google Scholar]
- 38.Scholl P R, Geha R S. MHC class II signaling in B cell activation. Immunol Today. 1994;15:418–422. doi: 10.1016/0167-5699(94)90271-2. [DOI] [PubMed] [Google Scholar]
- 39.Scott P. Selective differentiation of CD4+ T helper cell subsets. Curr Opin Immunol. 1993;5:391–397. doi: 10.1016/0952-7915(93)90058-z. [DOI] [PubMed] [Google Scholar]
- 40.Sedar R A, Paul W E. Acquisition of lymphokine-producing phenotype by CD4+ T cells. Annu Rev Immunol. 1994;12:635–673. doi: 10.1146/annurev.iy.12.040194.003223. [DOI] [PubMed] [Google Scholar]
- 41.Seymour G J, Gemmell E, Walsh L J, Powell R N. Immunohistological analysis of experimental gingivitis in humans. Clin Exp Immunol. 1988;71:132–137. [PMC free article] [PubMed] [Google Scholar]
- 42.Seymour G J. Importance of the host response in the periodontium. J Clin Periodontol. 1991;18:421–426. doi: 10.1111/j.1600-051x.1991.tb02310.x. [DOI] [PubMed] [Google Scholar]
- 43.Slots J, Listgarten M A. Bacteroides gingivalis, Bacteroides intermedius and Actinobacillus actinomycetemcomitans in human periodontal diseases. J Clin Periodontol. 1988;15:85–93. doi: 10.1111/j.1600-051x.1988.tb00999.x. [DOI] [PubMed] [Google Scholar]
- 44.Sundqvist G, Carlsson J, Herrmann B, Tarnvik A. Degradation of human immunoglobulins G and M and complement factors C3 and C5 by black pigmented Bacteroides. J Med Microbiol. 1985;19:85–94. doi: 10.1099/00222615-19-1-85. [DOI] [PubMed] [Google Scholar]
- 45.Taubman M A, Stoufi E D, Seymour G J, Smith D J, Ebersole J L. Immunoregulatory aspects of periodontal disease. Adv Dent Res. 1988;2:328–333. doi: 10.1177/08959374880020022201. [DOI] [PubMed] [Google Scholar]
- 46.Thornton S C, Mueller S N, Levine E M. Human endothelial cells: use of heparin in cloning and long-term serial cultivation. Science. 1983;222:623–625. doi: 10.1126/science.6635659. [DOI] [PubMed] [Google Scholar]
- 47.Trowsdale J. Genomic structure and function in the MHC. Trends Genet. 1993;9:117–122. doi: 10.1016/0168-9525(93)90205-v. [DOI] [PubMed] [Google Scholar]
- 48.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Whitney C, Ant J, Moncla B, Johnson B, Page R C, Engel P. Serum IgG antibody to Porphyromonas gingivalis in rapidly progressive periodontitis: titer, avidity, and subclass distribution. Infect Immun. 1992;60:2194–2200. doi: 10.1128/iai.60.6.2194-2200.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wihaskoro S, Herminajeng E. The immunopathology of chronic inflammatory periodontal disease. FEMS Immunol Med Microbiol. 1995;10:171–180. doi: 10.1111/j.1574-695X.1995.tb00030.x. [DOI] [PubMed] [Google Scholar]
- 51.Wikström M, Linde A. Ability of oral bacteria to degrade fibronectin. Infect Immun. 1986;51:707–711. doi: 10.1128/iai.51.2.707-711.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wingrove J A, DiScripio R G, Hugli T E, Chen Z, Potempa J, Travis J. Activation of complement components C3 and C5 by a cysteine proteinase (gingipain) from Porphyromonas (Bacteroides) gingivalis. J Biol Chem. 1992;267:18902–18907. [PubMed] [Google Scholar]
- 53.Young H A, Hardy K J. Role of interferon-γ in immune cell regulation. J Leukoc Biol. 1995;58:373–381. [PubMed] [Google Scholar]