Abstract
T-cell-mediated immunity is an important determinant in protection against primary infection with Coccidioides immitis, a dimorphic fungal pathogen that causes the disease coccidioidomycosis. To determine if interleukin-12 (IL-12) gene therapy could potentiate host response against C. immitis, we constructed a single-chain cDNA encoding the p40 and p35 subunits linked by a polylinker and, using a retroviral vector, transfected J774 macrophages with the construct. The transduced J774 cells expressed IL-12 in vitro, with a mean concentration of 28,440 pg from 106 cells in 48 h as measured by an IL-12 (p75)-specific enzyme-linked immunosorbent assay. The secreted IL-12 was biologically active, as judged by its ability to induce the production of gamma interferon (IFN-γ) by spleen cells from BALB/c mice. Treatment of the highly susceptible BALB/c mouse strain with the IL-12-transduced J774 cells inhibited C. immitis growth in tissues from mice challenged by a pulmonary route, as evidenced by 1.37-, 2.59-, and 1.22-log reductions in the number of CFU in the lungs, spleens, and livers, respectively, compared to the fungal load in mice given vector-transduced J774 cells. The protective effect of IL-12 gene therapy was accompanied by increased levels of IFN-γ in the lungs and sera of mice treated with IL-12-transduced J774 cells and the constitutive production of IFN-γ by their spleen cells cultured in vitro. These results suggest that IL-12 gene therapy could be used as adjunct therapy for coccidioidomycosis.
Coccidioidomycosis is a mycotic disease caused by the dimorphic fungus Coccidioides immitis. The disease is endemic in the semiarid areas of Texas, Arizona, New Mexico, and southern California. Primary infection is acquired by inhalation of mycelial-phase arthroconidia, which enter the alveoli and undergo a morphologic conversion into endosporulating spherules (35). Coccidioidomycosis presents a diverse clinical spectrum, ranging from benign, self-limited pulmonary infection to a severe, progressive, and often lethal extrapulmonary dissemination. Investigations in humans and experimentally infected animals have shown strong T-cell reactivity to coccidioidal antigens in subjects with controlled infection, whereas T-cell responses are depressed or nondemonstrable in subjects with progressive, multifocal disease (4, 6, 12–15, 26, 27). Recovery from primary asymptomatic or benign infection with C. immitis confers lifelong immunity to exogenous reinfection. The acquired resistance is associated with the acquisition of a delayed-type hypersensitivity response and the production of T helper-1 (Th1)-associated cytokines, such as gamma interferon (IFN-γ) and interleukin-2 (IL-2), to coccidioidal antigens (4, 6, 12–15, 26, 27).
IL-12, a heterodimeric Th1-promoting cytokine consisting of two disulfide-bonded subunits of 35 and 40 kDa, has been shown to have potent immunotherapeutic effects against tumor cells and a wide range of microbial pathogens (7, 11, 16, 20, 28, 29, 40, 42, 43). This cytokine has pleiotropic effects, including activation of macrophages, augmentation of the cytolytic activity of NK and T cells, and induction of the Th1-associated cytokines, notably IFN-γ (38). In a previous study, we showed that IL-12 plays a critical role in host defense against C. immitis (27). Treatment of the highly susceptible BALB/c mouse strain with recombinant murine IL-12 (mIL-12) ameliorated the course of the disease and enhanced production of IFN-γ. Daily injections of 0.1 μg of the recombinant cytokine were required, however, to achieve optimal therapeutic effects. Since gene therapy offers a means for constitutive production of a protein in vivo, we engineered a retroviral construct containing the cDNA encoding the p40 and p35 subunits of IL-12 as a single chain. Treatment of BALB/c mice with J774 macrophages that had been transduced with the single-chain IL-12 retroviral construct afforded a significant level of protection against lethal pulmonary challenge with C. immitis and was accompanied by increased production of IFN-γ.
MATERIALS AND METHODS
Animal model.
Pathogen-free female BALB/c mice, 5 to 7 weeks of age, were purchased from Jackson Laboratory (Bar Harbor, Maine) and used throughout this study. These animals arrived in filtered cages and were maintained for 1 more week before use.
Cell lines.
The J774A.1 (J774) and PA317 cell lines were obtained from the American Type Culture Collection, Rockville, Md. (ATCC TIB-67 and CRL-9078, respectively). The J774 cell line is a BALB/c-derived reticulum cell sarcoma having macrophage-like properties (30). The PA317 cell line was derived from NIH 3T3 TK fibroblast cells. The cell lines were maintained at 37°C under 5% CO2 in Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Grand Island, N.Y.), supplemented with 2 mM glutamine, 100 U of penicillin/ml, 100 μg of streptomycin/ml, 4,500 mg of glucose/ml, and 10% fetal bovine serum (Atlanta Biological, Inc., Norcross, Ga.).
Cloning of single-chain murine IL-12 into retroviral vector.
The cDNAs for murine IL-12 p35 and p40 subunits were generously provided by Ueli Gubler (Hoffmann-La Roche, Nutley, N.J.) (32). We introduced an XhoI site at the 5′ end of p40 and a BglII site at the 3′ end of p35 by PCR amplification, using synthetic oligonucleotide primers for p40 and p35, with a polylinker on the 3′ end of the p40 primer and the 5′ end for the p35 primer. The primers contained the following sequences (5′ to 3′): for p40, upstream TCTAGAGGCTCGAGCCCCACCAT and downstream TGGAATGACCCTAGATCCGCCGCCACCCGACCCACCACCGCCCGAGCCACCGCCACCGGATCGGACCCT; and for p35, upstream AGGGTCCGATCCGGTGGCGGTGGCTCGGGCGGTGGTGGGTCGGGTGGCGGCGGATCTAGGGTCATTCCA and downstream CGCTCTAGATCTAGTCGACCAATG. A 35-cycle PCR was performed to amplify the p40 and p35 fragments by high-fidelity PCR (Boehringer, Mannheim, Germany) under the following conditions: 94°C for 5 min; 94°C for 1 min, 55°C for 1.5 min, and 68°C for 1.5 min; and 72°C for 7 min. The single-chain PCR-amplified fragment for mIL-12 was constructed by linkage of the p35 and p40 cDNA fragments with the upstream primer of p40 and the downstream primer of p35 in a second round of PCR (22). The 1.66-kb PCR fragment of mIL-12 was directly cloned into PCR 2.1 vector (TA cloning kit; Invitrogen, San Diego, Calif.) and sequenced to confirm the nucleotide sequence of the adjacent subunit regions, linker, and the p40 and p35 coding regions. Thereafter, the single-chain cDNA of mIL-12 was ligated into the pLXSN vector (Clontech, Palo Alto, Calif.). The pLXSN/p40.LΔp35 construct, hereafter referred to as pLXSN/mIL-12, contains the gene encoding neomycin resistance as a selectable marker. The immunopotentiating capacity of the pLXSN/mIL-12 construct was compared with that of the pLXSN vector alone.
Cell transfection.
The pLXSN/mIL-12 construct was transduced into the amphotropic packaging cell line PA317 by calcium phosphate precipitation (Gibco). After a 72-h incubation, supernatants were collected from the transduced cells and assayed for IL-12 by using the p75 enzyme-linked immunosorbent assay (ELISA) procedures as described below. Transfectants were selected by culturing the cells for 2 weeks in complete DMEM containing G418 (Gibco) at a concentration of 800 μg/ml. IL-12 viral particle-producing PA317 clones were identified by reverse transcription (RT)-PCR (for both neomycin resistance gene and IL-12 mRNAs) and by a p75 ELISA as described below. IL-12-producing PA317 clones were expanded in DMEM, and virus-containing supernatant was harvested and used to infect J774 macrophages.
Expression of bioactive IL-12 by transduced J774 cells.
To test the ability of the pLXSN/mIL-12 constructs to induce the expression of mIL-12, total RNA was isolated from 5 × 106 transduced J774 cells and assayed for IL-12 mRNA by RT-PCR. PCR amplification was performed with primer pairs for β-actin (Clontech), the neomycin resistance gene (41), and IL-12 (p40Δp35 as described above) mRNA transcripts. Expression of IL-12 at the protein level was determined by a sandwich ELISA devised specifically to detect IL-12 p75. The capture antibody was a hamster immunoglobulin G (IgG) anti-mIL-12 monoclonal antibody (Red-T; PharMingen, San Diego, Calif.), which reacts with mIL-12 p35 and the p75 heterodimer but not the p40 monomer. Captured IL-12 was detected by the addition of biotinylated rat IgG2a anti-mIL-12 p40 (clone C17.8). Recombinant mIL-12 (PharMingen) was used to prepare a standard curve.
The bioactivity of the secreted IL-12 was assayed by measuring its ability to induce IFN-γ production by spleen cells from nonimmune mice. For these experiments, spleens were collected from normal BALB/c mice and gently teased into single-cell suspensions. The spleen cell suspension was treated with isotonic ammonium chloride to lyse erythrocytes and, after being washed by centrifugation, the splenocytes were resuspended in DMEM containing 10% fetal bovine serum. The cells were dispensed into wells on a microtiter plate at a concentration of 2 × 106 mononuclear cells per well. The cell cultures were incubated in medium alone or in medium containing a 1:10 dilution of the supernatant from the IL-12-transduced J774 cells. After a 48-h incubation at 37°C under 5% CO2, supernatants were collected for assays of IFN-γ protein by a two-site sandwich ELISA by using rat IgG1 anti-mouse IFN-γ monoclonal antibodies from clones R4-6A2 for capture and biotinylated XMG1.2 for detection (PharMingen) as previously reported (19). Recombinant mouse IFN-γ (PharMingen) was used to establish a standard curve.
Infection of mice.
The procedure for infecting mice via a pulmonary route has been detailed in an earlier report (13). In brief, arthroconidia were harvested from 6- to 8-week-old mycelial-phase cultures of C. immitis Silveira (ATCC 28868). The arthroconidial suspension was passed over a nylon column to remove hyphal elements, and the cells were enumerated by hemacytometer counts. Pulmonary challenge was performed by intranasal instillation of 60 arthroconidia in 30 μl of physiologic saline.
Gene therapy.
J774 cells, transduced with pLXSN/mIL-12 or with pLXSN alone, were administered via an intraperitoneal (i.p.) route 6 h after pulmonary challenge and again on days 1, 4, and 7. Control mice were treated in the same manner with saline alone. Mice were sacrificed at day 12 after challenge, and the lungs, livers, and spleens were collected, weighed, and homogenized. Serial dilutions of the homogenates were plated on mycobiotic medium (Difco Laboratories, Detroit, Mich.) for enumeration of fungal CFU.
To assess the effect of IL-12 gene therapy on the Th1 response in vivo, mice treated with the pLXSN/mIL-12-transduced J774 cells, vector-transduced J774 cells, or saline alone were sacrificed at 12 days postinfection, and their serum, lungs, and spleens were collected for assays of IFN-γ. Prior to assay, the lungs were suspended in sterile saline, homogenized in sterile Whirl-Pak bags (American Scientific Products, Dallas, Tex.), and filtered through a 22-μm-pore-size membrane. The filtered lung homogenates and the serum samples were maintained at −70°C until assayed by the ELISA described above. For assays of IFN-γ production by spleen cells, splenocytes (2 × 106) were incubated in tissue culture medium alone or medium containing concanavalin A (ConA, 2 μg; Sigma Chemical Co., St. Louis, Mo.). Forty-eight hours later, the spleen cell supernatants were collected and assayed for IFN-γ by ELISA.
Statistical analysis.
The statistical significance of differential findings between experimental groups of animals was determined by the nonparametric Mann-Whitney rank sum test. Findings were regarded as significant if two-tailed P values were <0.05.
RESULTS
Expression of bioactive mIL-12 in transduced cells.
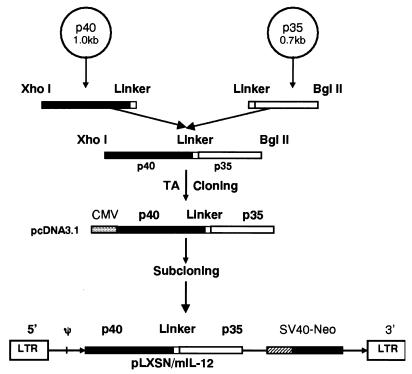
Generation of the functionally active IL-12 heterodimer requires the expression of both the p40 and the p35 genes (21, 22, 32). Although this can be achieved by simultaneously transfecting cells with two separate plasmids encoding the p40 and p35 genes, excessive p40 expression has been shown to lead to the inhibition of the bioactivities of IL-12 in mice (9, 17). To avoid this potential problem, we generated a single-chain construct containing both the p40 and the p35 genes (32), connected by a 45-bp linker encoding 15 amino acids (22), with the neomycin resistance gene as a selectable marker. This polycistronic construct is depicted in Fig. 1.
FIG. 1.
Schematic representation of the retroviral construct that carries both the p40 and p35 genes and the neomycin resistance gene selectable marker. The p40 and p35 cDNAs were linked by a (Gly4Ser)3 polylinker and were regulated by the long terminal repeat (LTR) promoter. This plasmid vector, designated pLXSN/mIL-12, was capable of coordinately expressing the p40, p35, and neomycin resistance genes. CMV, cytomegalovirus; SV40, simian virus 40.
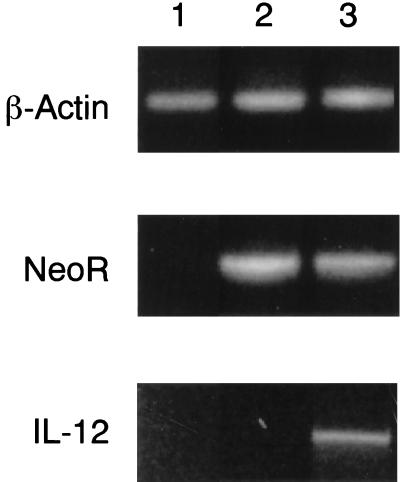
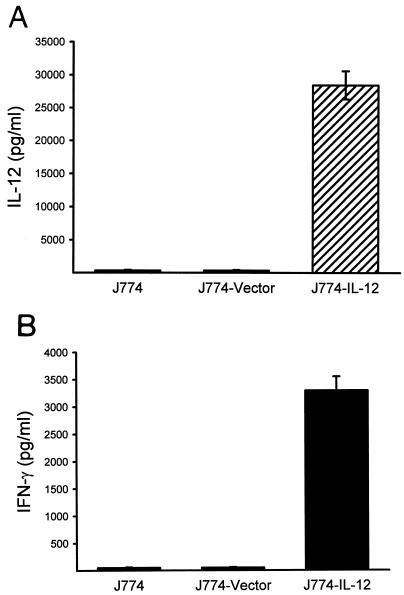
J774 cells were infected by coculture with viral particle-containing supernatant from pLXSN/mIL-12-transduced PA317 cells or PA317 cells that had been transduced with the pLXSN vector alone. The transduced J774 cell clones were obtained after a 2-week selection with G418 and examined for expression of IL-12 at both the molecular and protein levels by RT-PCR and IL-12 ELISA, respectively. The results obtained by the RT-PCR assays are shown in Fig. 2. J774 cells transduced with pLXSN/mIL-12 expressed mRNAs for both full-length 1.66-kb IL-12 and the 0.68-kb neomycin resistance gene, while vector-transduced J774 cells expressed mRNA only for the neomycin resistance gene. IL-12 was also detected when the supernatants from transduced J774 cells were assayed by ELISA, with a mean level of 28,440 pg from 106 cells in 48 h (Fig. 3A). The secreted IL-12 was bioactive, as evidenced by the induction of 3,300 pg of IFN-γ in resting spleen cells (Fig. 3B). These IL-12-transduced J774 cells have constitutively produced bioactive IL-12 for over 1 year and have retained their original morphology, growth pattern, and expression of the cell surface markers Mac-3, major histocompatibility complex class I (MHC-I), and MHC-II, as measured by flow cytometry (data not shown).
FIG. 2.
Expression of mRNA transcripts for β-actin, the neomycin resistance gene, and mIL-12 in pLXSN/mIL-12-J774 cells. Lanes 1, 2, and 3 depict the results obtained with cellular RNA obtained from nontransduced J774 cells, J774 cells transduced with the pLXSN plasmid vector alone, and J774 cells transduced with the pLXSN/mIL-12 construct, respectively.
FIG. 3.
Secretion of bioactive IL-12 from pLXSN/mIL-12-transduced J774 cells. Supernatants were collected at 48 h from in vitro cultures of 106 pLXSN/mIL-12-transduced J774 cells and, for negative controls, nontransduced and vector-transduced J774 cells. The supernatants were assayed for IL-12 by ELISA (A) and for bioactive IL-12 (B) as measured by the induction of IFN-γ production in (2 × 106) spleen cells from normal BALB/c mice. Results are representative of those obtained in at least two separate experiments.
Treatment of mice with IL-12-transduced J774 cells protects against pulmonary challenge with C. immitis.
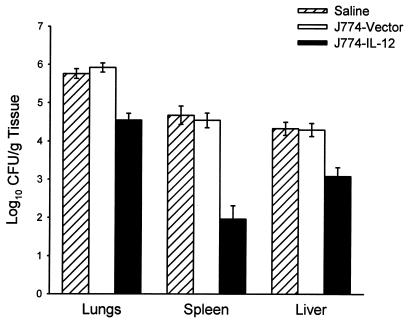
To examine the capacity of IL-12-transduced J774 cells to protect mice against challenge, BALB/c mice were infected with 60 arthroconidia via a pulmonary route and then treated with 2 × 106 pLXSN/mIL-12-transduced J774 cells or vector-transduced J774 cells. Twelve days after challenge, the mice were sacrificed and examined for fungal CFU. The results, depicted in Fig. 4, established that mice treated with pLXSN/mIL-12-transduced J774 macrophages showed a significant decrease in the number of C. immitis CFU in the lungs compared to the vector (P < 0.001) and saline control groups (P < 0.001). Recipients of the IL-12-transduced J774 cells also showed significant reductions in the fungal load in their spleens (P < 0.0001) and livers (P < 0.001).
FIG. 4.
Protection in BALB/c mice treated with pLXSN/mIL-12-transfected J774 cells. Mice were infected with 60 arthroconidia via a pulmonary route and then treated with 2 × 106 IL-12-transduced or vector-transduced J774 cells via the i.p. route. A third group of mice received saline alone. Treatments were begun 6 h after pulmonary challenge and repeated on days 1, 4, and 7 after challenge. Twelve days postchallenge, the mice were sacrificed and evaluated for C. immitis CFU in tissues. Bars depict means ± standard errors obtained in two experiments involving a total of 22 mice given saline alone, 23 mice treated with vector-transduced J774 cells, and 21 mice given IL-12-transduced J774 cells.
It is perhaps noteworthy that mice given pLXSN/mIL-12-transduced J774 cells, but not those receiving vector-transduced cells, showed marked splenomegaly, with a greater-than-twofold increase in weight compared to spleens from mice treated with the vector-transduced J774 cells. This finding is consistent with a recent report by Kim et al. (21) that mice given an IL-12 cDNA expression vector showed a level of splenomegaly that was comparable to that observed in mice given recombinant IL-12.
In vivo induction of Th1 response in mice given IL-12 gene therapy.
The preceding results established that retroviral vector-mediated IL-12 gene therapy effected a significant decrease in the fungal load in the lungs, livers, and spleens of mice. To determine if this protection was accompanied by induction of IFN-γ, mice treated with the IL-12-transduced J774 or nontransduced cells were sacrificed 12 days after challenge, and their sera and lung homogenates were assayed for IFN-γ. As shown in Table 1, sera from mice treated with the pLXSN/mIL-12-transduced J774 cells showed 1,580 pg of IFN-γ per ml, whereas no IFN-γ was detected in sera from mice treated with the vector-transduced J774 cells or saline. Likewise, the mean IFN-γ level in lung homogenates from mice treated with the pLXSN/mIL-12-transduced J774 cells was 1,300 pg/100 mg of tissue, compared to 200 pg and <15 pg in lung tissue from mice treated with the pLXSN vector or saline alone, respectively. Further proof that the transduced J774 cells induced IFN-γ production in vivo was evidenced by our finding that spleen cells from mice treated with the pLXSN/mIL-12-transduced J774 cells secreted 290 pg of IFN-γ when cultured in vitro in medium alone (Table 2). No IFN-γ was detected when splenocytes from mice given saline alone or vector-transduced J774 cells were incubated in medium alone. When the spleen cells were assayed for IFN-γ production in response to stimulation with ConA, cells from recipients of IL-12-transduced J774 cells secreted 3,900 pg compared to 60 and 160 pg by splenocytes from recipients of saline alone or vector-transduced J774 cells, respectively. The decreased production of IFN-γ by ConA-stimulated spleen cells from the latter two groups of mice is consistent with the immunosuppressive effect of active coccidioidomycosis on Th1-associated responses (12, 13, 15, 26).
TABLE 1.
IFN-γ levels in sera and homogenates of lung tissues from infected mice
| Source | IFN-γ level (pg) for indicated treatment groupa
|
||
|---|---|---|---|
| Saline | J774-Vector | J774-IL-12 | |
| Serum | <15b | <15 | 1,580 |
| Lung homogenates | <15 | 200 | 1,300 |
IFN-γ levels per milliliter of serum and per 100 mg of lung tissue obtained from groups of 10 mice at 12 days postinfection.
Lower limit of sensitivity of the assay.
TABLE 2.
Production of IFN-γ in vitro by spleen cells from infected mice
| Stimulant | IFN-γ secretion (pg) by splenocytes from BALB/c mice treated witha:
|
||
|---|---|---|---|
| Saline | J774-Vector | J774-IL-12 | |
| Medium | <15b | <15 | 290 |
| ConA (2 μg) | 60 | 160 | 3,900 |
IFN-γ levels per milliliter of supernatant from 2 × 106 spleen cells from a pool of at least 11 mice at 12 days postinfection.
Lower limit of assay sensitivity.
DISCUSSION
The results of this investigation demonstrate the efficacy of IL-12 gene therapy with a genetically engineered single-chain IL-12 fusion construct created by linkage of the p35 and p40 genes with a (Gly4Ser)3 polylinker. The monomeric nature of this single-chain IL-12 fusion protein ensures equimolar expression of each subunit, thereby avoiding the formation of p40 dimers which have been shown to antagonize the activity of IL-12 (9, 17). The bioactivity and efficacy of gene therapy with this construct were examined by treating BALB/c mice, which are highly susceptible to C. immitis (13), with J774 cells transduced with the single-chain IL-12 construct. Recipients of the IL-12-transduced J774 cells showed a reduced fungal load in their lungs, livers, and spleens after pulmonary challenge and an increased production of the Th1-associated cytokine IFN-γ.
It is now clearly established that IL-12 plays a pivotal role in orchestrating the immune response by amplifying cytokine networks involved in the induction of Th1 cells while suppressing Th2 responses (38). In murine studies, recombinant IL-12 has been shown to prevent growth of a wide spectrum of tumors (7, 22, 29) and to augment host resistance to several pathogens, including Mycobacterium tuberculosis (11), Listeria monocytogenes (40), Toxoplasma gondii (20), Leishmania major (28), Schistosoma mansoni (42), Histoplasma capsulatum (43), Cryptococcus neoformans (16), and C. immitis (27). The limitations of recombinant cytokine therapy include the need for daily administrations, often with significant systemic toxicity (27, 45), and the inability to target the cytokine to a specific organ or tissue site. To address these limitations, investigators have focused on using gene transfer therapy for the in vivo production of bioactive IL-12. This approach has proved to be highly effective as evaluated in experimental tumor models (8, 9, 17, 21–23, 30, 36, 45) and infectious diseases (1, 10, 18, 37, 39, 44) and appears to be without toxicity.
We have previously reported that IL-12 has an essential role in host defense against C. immitis (27). Administration of 0.1 μg of recombinant IL-12 to susceptible BALB/c mice on the day before pulmonary challenge with C. immitis and then daily for 12 days afterward resulted in a significant reduction in the fungal load in the spleens and livers but not the lungs. Protection at the lung level was not achieved even with the administration of a 10-fold-higher dose of the recombinant protein. These results and the finding that mice treated with the higher dose showed toxic manifestations, evidenced by ruffled fur, lethargy, and a marked reduction in total body weight by 8 days postinfection (27), led us to examine the efficacy of IL-12 gene therapy. In this investigation, we used a single-chain IL-12 retroviral construct expressed in J774 cells to provide a potent and stable delivery system for bioactive IL-12. Treatment of BALB/c mice with the IL-12-expressing J774 cells effected a reduction in the fungal load in tissues and induced IFN-γ production, as evidenced by increased levels of IFN-γ in serum and lungs from treated mice 12 days after challenge with C. immitis. The induction of IFN-γ is an important consequence of IL-12 gene therapy, since this cytokine has been shown to activate macrophages to an anticoccidioidal level, both in vitro and in vivo (5, 14).
The protective effect of IL-12 gene therapy has in other models been shown to be attributable to the induction of the IFN-γ by NK cells and T lymphocytes and to the subsequent development of Th1 responses (10, 11, 16, 36, 38–40, 42, 43). The in vivo elaboration of IFN-γ in the mice given IL-12-transduced J774 cells is consistent with a role of IL-12 in the activation of antifungal host defense via the induction of this Th1-associated cytokine. It is also possible that the IL-12-transduced J774 macrophages themselves were activated to an anticoccidioidal level via in situ expression of the IL-12 gene. We chose to use the J774 cell line for delivery of the pLXSN/mIL-12 retroviral construct because investigators have shown that J774 cells are highly effective for expressing retroviral vectors containing mycobacterial genes (25, 33, 34). Although we did not initially consider that IL-12 transduction of the J774 cells might enhance their antimicrobial activity, this possibility should be explored by comparing the anticoccidioidal effect of IL-12-transduced J774 cells with that of IL-12-transduced cells of a nonmacrophage lineage. Studies should also be done to examine the therapeutic efficacy of IL-12-transduced dendritic cells, since dendritic cells have been reported to be highly effective for expressing cytokine genes in the therapy of cancer and infectious diseases (2, 3, 24, 31).
Cytokine gene therapy is a promising approach for inducing efficient immune responses against infectious diseases. We have demonstrated the feasibility of IL-12 gene therapy for the treatment of coccidioidomycosis by retrovirally-transduced J774 cells. To our knowledge, this study is the first to show that gene therapy with a single-chain IL-12 fusion construct will induce protective immunity and increase IFN-γ production in a fungal disease. The results are extremely encouraging and indicate that IL-12 gene therapy has potential as adjunct therapy for coccidioidomycosis.
ACKNOWLEDGMENTS
This work was supported by grant AI32134 from the National Institutes of Health.
We gratefully acknowledge Yiqiang Zhang and Teresa Quitugua for their valuable assistance and advice in this study. We also thank Ueli Gubler for graciously providing the plasmid for the mIL-12 p35 and p40 subunits.
REFERENCES
- 1.Ahlers J D, Dunlop N, Alling D W, Nara P L, Berzofsky J A. Cytokine-in-adjuvant steering of the immune response phenotype to HIV-1 vaccine constructs. Granulocyte-macrophage colony-stimulating factor and TNF-α synergize with IL-12 to enhance induction of cytotoxic T lymphocytes. J Immunol. 1997;158:3947–3958. [PubMed] [Google Scholar]
- 2.Ahuja S S, Brown M R, Fleisher T A, Ahuja S K, Malech H L. Autocrine activation of hemopoietic progenitor-derived myelo-monocytic cells by IFN-γ gene transfer. J Immunol. 1996;156:4345–4353. [PubMed] [Google Scholar]
- 3.Ahuja S S, Mummidi S, Malech H L, Ahuja S K. Human dendritic cells (CD)-based anti-infective therapy: engineering dendritic cells to secrete functional IFN-γ and IL-12. J Immunol. 1998;161:868–876. [PubMed] [Google Scholar]
- 4.Ampel N M, Bejarano G C, Salas S D, Galgiani J N. In vitro assessment of cellular immunity in human coccidioidomycosis: relationship between dermal hypersensitivity, lymphocyte transformation, and lymphokine production by peripheral blood mononuclear cells from healthy adults. J Infect Dis. 1992;165:710–715. doi: 10.1093/infdis/165.4.710. [DOI] [PubMed] [Google Scholar]
- 5.Beaman L. Fungicidal activation of murine macrophages by recombinant gamma interferon. Infect Immun. 1987;55:2951–2955. doi: 10.1128/iai.55.12.2951-2955.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beaman L, Pappagianis D, Benjamini E. Significance of T cells in resistance to experimental murine coccidioidomycosis. Infect Immun. 1977;17:580–585. doi: 10.1128/iai.17.3.580-585.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brunda M J, Luistro L, Warrier R R, Wright R B, Hubbard B R, Murphy M, Wolf S F, Gately M K. Antitumor and antimetastatic activity of interleukin 12 against murine tumors. J Exp Med. 1993;178:1223–1230. doi: 10.1084/jem.178.4.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Car B D, Eng V M, Schnyder B, LeHir M, Shakhov A N, Woerly G, Huang S, Aguet M, Anderson T D, Ryffel B. Role of interferon-γ in interleukin 12-induced pathology in mice. Am J Pathol. 1995;147:1693–1707. [PMC free article] [PubMed] [Google Scholar]
- 9.Chen L, Chen D, Block E, O’Donnell M, Kufe D W, Clinton S K. Eradication of murine bladder carcinoma by intratumor injection of a bicistronic adenoviral vector carrying cDNAs for the IL-12 heterodimer and its inhibition by the IL-12 p40 subunit homodimer. J Immunol. 1997;159:351–359. [PubMed] [Google Scholar]
- 10.Chow Y-H, Chiang B-L, Lee Y-L, Chi W-K, Lin W-C, Cheu Y-T, Tao M-H. Development of Th1 and Th2 populations and the nature of the immune responses to hepatitis B virus DNA vaccines can be modulated by codelivery of various cytokine genes. J Immunol. 1998;160:1320–1329. [PubMed] [Google Scholar]
- 11.Cooper A M, Roberts A D, Rhoades E R, Callahan J E, Getzy D M, Orme I M. The role of interleukin-12 in acquired immunity to Mycobacterium tuberculosis. Immunology. 1995;84:423–432. [PMC free article] [PubMed] [Google Scholar]
- 12.Corry D B, Ampel N M, Christian L, Locksley R M, Galgiani J N. Cytokine production by peripheral blood mononuclear cells in human coccidioidomycosis. J Infect Dis. 1996;174:440–443. doi: 10.1093/infdis/174.2.440. [DOI] [PubMed] [Google Scholar]
- 13.Cox R A, Kennell W, Boncyk L, Murphy J W. Induction and expression of cell-mediated immune responses in inbred mice infected with Coccidioides immitis. Infect Immun. 1988;56:13–17. doi: 10.1128/iai.56.1.13-17.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cox R A, Magee D M. Protective immunity in coccidioidomycosis. Res Immunol. 1998;149:417–428. doi: 10.1016/s0923-2494(98)80765-7. [DOI] [PubMed] [Google Scholar]
- 15.Cox R A, Vivas J R. Spectrum of in vivo and in vitro immune responses in coccidioidomycosis. Cell Immunol. 1977;31:130–141. doi: 10.1016/0008-8749(77)90012-0. [DOI] [PubMed] [Google Scholar]
- 16.Decken K, Kohler G, Palmer-Lehmann K, Wunderlin A, Mattner F, Magram J, Gately M K, Alber G. Interleukin-12 is essential for a protective Th1 response in mice infected with Cryptococcus neoformans. Infect Immun. 1998;55:4994–5000. doi: 10.1128/iai.66.10.4994-5000.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gillessen S, Carvajal D, Ling P, Podlaski F J, Stremlo D L, Familletti P C, Gubler U, Presky D H, Stern A S, Gately M K. Mouse interleukin-12 (IL-12) p40 homodimer: a potent IL-12 antagonist. Eur J Immunol. 1995;23:200–206. doi: 10.1002/eji.1830250133. [DOI] [PubMed] [Google Scholar]
- 18.Greenberger M J, Kunkel S, Strieter R M, Lukacs N W, Bramson J, Gauldie J, Graham F L, Hitt M, Danforth J M, Standiford T J. IL-12 gene therapy protects mice in lethal Klebsiella pneumoniae. J Immunol. 1996;157:3006–3012. [PubMed] [Google Scholar]
- 19.Jiang C, Magee D M, Quitugua T N, Cox R A. Genetic vaccination against Coccidioides immitis: comparison of vaccine efficacy of recombinant antigen 2 and antigen 2 cDNA. Infect Immun. 1999;67:630–635. doi: 10.1128/iai.67.2.630-635.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson L L, Sayles P C. Interleukin-12, dendritic cells, and the initiation of host-protective mechanisms against Toxoplasma gondii. J Exp Med. 1997;186:1799–1802. doi: 10.1084/jem.186.11.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim J J, Ayyavoo V, Bagarazzi M L, Chaattergoon M A, Dang K, Wang B, Boyer J D, Weiner D B. In vivo engineering of a cellular immune response by coadministration of IL-12 expression vector with a DNA immunogen. J Immunol. 1997;158:816–826. [PubMed] [Google Scholar]
- 22.Lieschke G J, Rao P K, Gately M K, Mulligan R C. Bioactive murine and human interleukin-12 fusion proteins which retain antitumor activity in vivo. Nat Biotechnol. 1997;15:35–40. doi: 10.1038/nbt0197-35. [DOI] [PubMed] [Google Scholar]
- 23.Lotze M T, Zitvogel L, Campbell R, Robbins P D, Elder E, Haluszczak C, Martin D, Whiteside T L, Storkus W J, Tahara H. Cytokine gene therapy of cancer using interleukin-12: murine and clinical trials. Ann N Y Acad Sci. 1996;795:440–454. doi: 10.1111/j.1749-6632.1996.tb52715.x. [DOI] [PubMed] [Google Scholar]
- 24.Lotze M T, Hellerstedt B, Stolinski L, Tueting T, Wilson C, Kinzler D, Vu H, Rubin J T, Storkus W, Tahara H, Elder E, Whiteside T. The role of interleukin-2, interleukin-12, and dendritic cells in cancer therapy. Cancer J Sci Am. 1993;3(Suppl.):S109–S114. [PubMed] [Google Scholar]
- 25.Lowrie D B, Tascon R E, Colston M J, Silva C L. Towards a DNA vaccine against tuberculosis. Vaccine. 1994;12:1537–1540. doi: 10.1016/0264-410x(94)90080-9. [DOI] [PubMed] [Google Scholar]
- 26.Magee D M, Cox R A. Roles of gamma interferon and interleukin-4 in genetically determined resistance to Coccidioides immitis. Infect Immun. 1995;63:3514–3519. doi: 10.1128/iai.63.9.3514-3519.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Magee D M, Cox R A. Interleukin-12 regulation of host defenses against Coccidioides immitis. Infect Immun. 1996;64:3609–3613. doi: 10.1128/iai.64.9.3609-3613.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murray H W, Harisprashad J. Interleukin-12 is effective treatment for an established systemic intracellular infection: experimental visceral leishmaniasis. J Exp Med. 1995;181:387–391. doi: 10.1084/jem.181.1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nastala C L, Edington H D, McKinney T G, Tahara H, Nalesnik M A, Brunda M J, Gately M K, Wolf S F, Schreiber R D, Storkus W J. Recombinant IL-12 administration induces tumor regression in association with IFN-γ. J Immunol. 1994;153:1697–1705. [PubMed] [Google Scholar]
- 30.Ralph P, Prichard J, Cohn M. Reticulum cell sarcoma: an effector cell in antibody-dependent cell-mediated immunity. J Immunol. 1975;114:898–904. [PubMed] [Google Scholar]
- 31.Reid C D. The biology and clinical applications of dendritic cells. Transfusion Med. 1998;8:77–86. doi: 10.1046/j.1365-3148.1998.00144.x. [DOI] [PubMed] [Google Scholar]
- 32.Schoenhaut D S, Chua A O, Wolitzky A G, Phyllis M, Quinn P M, Dwyer C M, McComas W, Familletti P C, Gately M K, Gubler U. Cloning and expression of murine IL-12. J Immunol. 1992;148:3433–3440. [PubMed] [Google Scholar]
- 33.Silva C L, Lowrie D B. A single mycobacterial protein (hsp 65) expressed by a transgenic antigen-presenting cell vaccinates mice against tuberculosis. Immunology. 1994;82:244–248. [PMC free article] [PubMed] [Google Scholar]
- 34.Silva C L, Palacios A, Colston M J, Lowrie D B. Mycobacterium leprae 65 hsp antigen expressed from a retroviral vector in a macrophage cell line is presented to T cells in association with MHC class II in addition to MHC class I. Microb Pathog. 1992;12:27–38. doi: 10.1016/0882-4010(92)90063-t. [DOI] [PubMed] [Google Scholar]
- 35.Stevens D A. Current concepts: coccidioidomycosis. N Engl J Med. 1995;332:1077–1082. doi: 10.1056/NEJM199504203321607. [DOI] [PubMed] [Google Scholar]
- 36.Tahara H, Zitvogel L, Storkus W J, Zeh III H J, McKinney T G, Schreiber R D, Gubler U, Robbins P D, Lotze M T. Effective eradication of established murine tumors with IL-12 gene therapy using a polycistronic retroviral vector. J Immunol. 1995;154:6466–6474. [PubMed] [Google Scholar]
- 37.Tascon R E, Colston M J, Ragno S, Stavropoulos E, Gregory D, Lowrie D B. Vaccination against tuberculosis by DNA injection. Nat Med. 1996;2:888–898. doi: 10.1038/nm0896-888. [DOI] [PubMed] [Google Scholar]
- 38.Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol. 1995;152:1883–1887. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- 39.Tsuji T, Hamajima K, Fukushima J, Xin K Q, Ishii N, Aoki I, Ishigatsubo Y, Tani K, Kawamoto S, Nitta Y, Miyazaki J, Koff W C, Okubo T, Okuda K. Enhancement of cell-mediated immunity against HIV-1 induced by coinoculation of plasmid-encoded HIV-1 antigen with plasmid expressing IL-12. J Immunol. 1997;158:4008–4013. [PubMed] [Google Scholar]
- 40.Wagner R D, Steinberg H, Brown J F, Czuprynski C J. Recombinant interleukin 12 enhances resistance to mice to Listeria monocytogenes infection. Microb Pathog. 1994;17:175–186. doi: 10.1006/mpat.1994.1064. [DOI] [PubMed] [Google Scholar]
- 41.Westermann J, Aicher A, Qin Z, Cayeux Z, Daemen K, Blankenstein T, Dorken B, Pezzutto A. Retroviral interleukin-7 gene transfer into human dendritic cells enhances T cell activation. Gene Ther. 1998;5:264–271. doi: 10.1038/sj.gt.3300568. [DOI] [PubMed] [Google Scholar]
- 42.Wynn T A, Jankovic D, Hieny S, Cheever A W, Sher A. IL-12 enhances vaccine-induced immunity to Schistosoma mansoni in mice and decreases T helper 2 cytokine expression, IgE production, and tissue eosinophilia. J Immunol. 1995;154:4701–4709. [PubMed] [Google Scholar]
- 43.Zhou P, Sieve M C, Bennett J, Kwon-Chung K J, Tewari R P, Gazzinelli R T, Sher A, Seder R A. IL-12 prevents mortality in mice infected with Histoplasma capsulatum through induction of IFN-γ. J Immunol. 1995;155:785–795. [PubMed] [Google Scholar]
- 44.Zhu Z, Venkataprasad N, Thangaraj H S, Hill M, Singh M, Ivanyi J, Vordermeier H M. Functions and specificity of T cells following nucleic acid vaccination of mice against Mycobacterium tuberculosis infection. J Immunol. 1997;158:5921–5926. [PubMed] [Google Scholar]
- 45.Zitvogel L, Tahara H, Robbins P D, Storkes W J, Clarke M R, Nalesnik M A, Lotze M T. Cancer immunotherapy of established tumors with IL-12. Effective delivery by genetically engineered fibroblasts. J Immunol. 1995;155:1393–1403. [PubMed] [Google Scholar]