Abstract
Acute exacerbations are common in children and potentially preventable. Currently, a past exacerbation is the best predictor of a future exacerbation. We undertook a systematic review of the literature describing the relationship between past and future exacerbations. Our analysis considered whether the odds ratios for one exacerbation to predict a recurrence were different across different categories of exacerbation.
Four databases were searched systematically (MEDLINE, Embase, the Cumulative Index to Nursing and Allied Health and PsycInfo). Exacerbations were categorised by severity as: presentation to emergency department (ED); hospital admission; paediatric intensive care unit (PICU) admission; and “unspecified severity” (i.e. no distinction between severity categories was made). Meta-analysis was performed for studies where sufficient data were provided for inclusion.
There were 26 eligible articles from 9185 identified. There was significant heterogeneity in duration of follow-up, healthcare system and exacerbation definition between studies. For the unspecified severity definition, the odds ratio for an exacerbation after a previous exacerbation was 9.87 (95% CI 5.02–19.39; six studies, 162 583 individuals). PICU admission was also associated with increased risk of future admission (OR 5.87, 95% CI 2.96–11.64; two studies, 730 individuals). Meta-analysis was not possible for ED visits or hospitalisation. The median odds ratio (range) for past ED visit predicting future ED visit was 6.27 (3.3–8.26) and for past hospitalisation predicting future hospitalisation was 3.37 (1.89–5.36).
The odds for a second asthma exacerbation do not necessarily increase with increasing severity of an initial exacerbation.
Short abstract
Asthma exacerbation in children is associated with increased odds for future exacerbation across all exacerbation severities. There is no evidence that severe exacerbations are associated with increased risk of future exacerbations. https://bit.ly/3n1O1Tv
Introduction
Asthma affects >1 million children in the United Kingdom (UK) [1] and >5 million in the United States of America [2], making it the most common long-term medical condition in young people. Patients with asthma can experience acute exacerbations, defined by the International Consensus on Pediatric Asthma as an acute or subacute episode of progressive increase in asthma symptoms, associated with airflow obstruction [3]. Exacerbations result in significant morbidity and socioeducational cost for the child through hospital admissions, interruption to education and social development, adverse effects from treatment and a decline in lung function [4, 5]. Moreover, there is economic impact on caregivers through working day and productivity loss [6].
A key goal of asthma treatment is to reduce risk of exacerbations. There has been limited systematic appraisal of the literature describing whether an exacerbation is followed by a subsequent exacerbation. The Global Initiative for Asthma, the British Thoracic Society/Scottish Intercollegiate Guidelines Network and the National Institute for Health and Care Excellence guidelines highlight the importance of identifying children who are at increased risk of exacerbations, and all cite a previous exacerbation as the major risk factor [7–9]. One systematic review which considered all risk factors for exacerbations in children with asthma identified 11 studies exploring previous exacerbation as a risk factor, and concluded that the odds ratio for an exacerbation being followed by a second exacerbation varied between 2.1 and 4.1 [10].
Here we describe a systematic review which was designed with a focus on exacerbation as a predictor of future exacerbation in children. Our hypothesis was the odds ratio (or a positive predictive value (PPV) for case-only populations) for an exacerbation would be greater following a more severe “index” exacerbation. Meta-analysis was carried out for studies where sufficient data were provided for inclusion.
Methods
Eligibility criteria and information sources
Full papers published in English from 2000 onwards describing asthma exacerbations in children where the mean age was between 5 and 18 years were eligible. Studies published before 2000 were ineligible, since they were considered less relevant to modern asthma care. Children aged <5 years were ineligible due to the potential to confuse an asthma exacerbation with lower respiratory tract infection in younger children. In articles where participants were of a wider age range than the desired population, if the mean age was between 5 and 18 years, or the desired age group of this review was reported separately, then the paper was included. Observational studies (including those using routinely acquired healthcare data and case–control and case-only studies), retrospective case–control studies and randomised controlled trials (RCT) were eligible. Data from RCTs were included regardless of the presentation of the results and whether the intervention may have influenced these. Letters and abstracts were ineligible. The outcome was exacerbation and was reported as odds ratio, since this is the best indicator of performance [11]. To make the best use of the data available (i.e. include data from case-only studies) and acknowledging odds ratio may have limitations [12], PPV was also reported for all studies with available data.
Literature was searched on 11 January 2021 using the databases MEDLINE, Embase, the Cumulative Index to Nursing and Allied Health and PsycInfo. Additional studies were identified from reviewing the references of the full papers assessed after the database search, including a previous review [10].
Search strategy
The search strategy was centred around the terms “asthma”, “asthma exacerbation/attack”, “child” and “risk factor” and appropriate derivatives and synonyms were included. These terms were decided upon after a review of terms used in another systematic review published in this research area [10] as well as discussion with an information assistant. The full search strategy can be found in the supplementary material.
Duplicate titles were removed. Papers were screened independently, initially by title and abstract and then by full paper by one researcher (R. Lowden). Decision making was reviewed at regular meetings with the second author.
Relevant data were extracted using a pre-designed template (table 1), and included publication date, study design, nation, study setting, data collection period, population/inclusion criteria, sample size, definition of acute exacerbation and results. For articles that did not report the required data for meta-analysis, authors were contacted and asked to provide additional data.
TABLE 1.
Summary of key study characteristics of included studies
| First author, year [reference] | Study design | Study setting | Data collection period | Population/inclusion criteria | Sample size (n) | Definition of acute exacerbation | Results |
| Emerman, 2001 [13]# | Prospective cohort combining two studies with identical protocols | 44 EDs in USA and Canada | Studies performed 1997–1998 Follow-up 2 weeks after index ED visit |
Children aged 2–17 years with ED visit for acute asthma Mean age 7.99 years 59% male 19% White, 55% Black, 24% Hispanic, 2% other |
1184 recruited; follow-up data available for 762 | ED visit with physician diagnosed acute asthma | Factors associated with acute asthma relapse: ED visits for asthma in past year (per 5 visits) OR 1.2 (1.0–1.5) Urgent clinic visits for asthma in past year (per 5 visits) OR 1.1 (0.9–1.3) |
| Lafata, 2002 [14] | Retrospective cohort study using routinely acquired data | Michigan, USA | 1992–1996 2-year observation: 1 baseline year and 1 follow-up year |
Children aged 5–14 years, with 1 hospitalisation or 2 outpatient encounters for asthma and ≥1 paediatrician office visit for each year of inclusion Mean age 8.7 years 63% male 49% White, 44% African American, 7% other |
452 | ED visit or hospitalisation for asthma | Factors associated with ED use: ED visit for asthma in prior year OR 8.26 (4.79–14.25) Hospital admission for asthma in prior year OR 0.85 (0.32–2.22) Factors associated with ED or hospital admission for asthma: Prior ED visit for asthma OR 7.97 (4.64–13.71) |
| Chen, 2003 [15] | Prospective cohort | Children's Hospital, St Louis, MO, USA | Admissions between June and December 1999 1 year follow-up |
Children aged 4–18 years, hospitalised for asthma Mean age 8.22 years 65% male 77% African American, 21% White, 2% other |
115 | Hospitalisation for asthma | Lifetime history of hospitalisations as a predictor of future hospitalisation: OR 5.36 (1.90–15.14) |
| Schatz, 2003 [16] | Retrospective cohort study using routinely acquired data | California, USA | 1998–1999 2-year observation: 1 baseline year and 1 follow-up year |
Individuals aged 3–64 years with asthma Children 62.2% male |
11 101 in total; 6904 children aged 3–17 years | Hospitalisation or ED visit for asthma | In children aged 3–17 years, 1998 hospitalisations as a predictor for asthma hospitalisation in 1999 OR 3.37 (1.61–7.04) |
| McCoy, 2006 [17] | Data from RCT used as an observational study | 19 American Lung Association clinical research centres, USA | Recruitment from 15 September to 30 November 2000 Follow-up for 14 days after each injection (28 days total) |
Volunteers aged 3–64 years with physician-diagnosed asthma Of children originally enrolled, 60% male 60.5% White, 28.7% Black, 5.6% Hispanic, 4.4% other |
2032 enrolled, 1949 completed trial; 353 children aged 3–10 years | New or increased OCS or an unscheduled healthcare encounter for asthma | History of intubation for asthma, hospitalisation ≥2 times for asthma, ≥3 courses of OCS for asthma in past year, or ≥2 unscheduled health contacts for asthma in past year in children aged 3–10 years as a predictor of exacerbations OR 2.19 (1.18–4.06) |
| Reznik, 2006 [18] | Retrospective case–control | Children's hospital, New York, USA | Admissions between January 1998–December 2004 30-day follow-up |
Cases: children aged 0–21 years hospitalised for asthma and readmitted within 30 days of discharge for same reason Controls: children aged 0–21 years hospitalised for asthma, but not readmitted within 30 days of discharge Mean age 5.99 years 61% male 62.2% Hispanic, 34.2% African American, 3.6% other |
445 Cases: 152 Controls: 293 |
Hospitalisation for asthma | Predictors of early asthma readmission: ED visit for asthma in past year OR 3.28 (1.55–6.94) Multivariate analysis of predictors of early asthma readmission: Hospital admission for asthma in past year OR 1.89 (1.10–3.25) Prior ICU admission for asthma OR 1.99 (0.93–4.27) |
| Covar, 2008 [19]# | Data from PACT RCT used as an observational study | USA | Recruited between October 2002 and January 2004 Trial period: 48 weeks |
Children aged 6–14 years with documented mild–moderate persistent asthma, screening FEV1 ≥80% predicted and methacholine reactivity 61.4% male 44.9% from a minority ethnic group |
285 | Systemic corticosteroids or emergency care (ED visit or hospitalisation) for acute asthma | Logistic regression analysis of factors at baseline predictive of exacerbation: History of exacerbation requiring corticosteroid course in past year OR 2.28 (1.59–3.26) Multivariable model of factors associated with exacerbations: Prednisone course in year prior to study OR 2.10 (1.42–3.09) |
| Miller, 2008 [20] | Prospective cohort | Michigan, USA | Enrolment over 1-year period Follow-up at 2 weeks and 6 months post-ED visit |
Children aged 2–17 years presenting to ED for acute asthma Mean age 8.1 years 61.5% male 71.7% White, 50% Black, 26% Hispanic, 7% American Indian or Alaska native, 2% Asian, 2% other |
197 enrolled; follow-up data available for 166 | Signs/symptoms compatible with asthma exacerbation (shortness of breath, coughing, wheezing, chest tightness) in a diagnosed asthmatic | Previous severe disease (e.g. systemic corticosteroids, ED visit or hospitalisation for asthma) as a predictor of 6-month morbidity (urgent care, ED or hospital admissions for asthma) Pearson correlation coefficient 0.17 |
| To, 2008 [21] | Prospective cohort | Children's hospital, Toronto, Canada | ED visit between January 2003 and June 2004 Follow-up at 1 and 6 months post-ED visit |
Children aged 2–17 years visiting ED for acute asthma 70% aged <7 years 59% male |
269 enrolled; 247 completed 1-month follow-up, 220 completed 6-month follow-up |
Sudden worsening of symptoms resulting in difficulty breathing often requiring extra medicine to relieve symptoms, with/without unscheduled ED/doctor visit | Predictors of acute asthma episode at 6-month follow-up: Acute asthma episode 6 months prior to baseline OR 4.73 (2.25–9.97) Predictors of ED visit at 6-month follow-up: ED visits in 12 months prior to baseline OR 6.27 (1.54–7.12) |
| Haselkorn, 2009 [22] | Prospective cohort Data from TENOR study |
USA | TENOR conducted from 2001 to 2004 Follow-up with semi-annual visits for 3 years |
Children with severe asthma or mild/moderate asthma considered difficult to treat Had ≥2 OCS bursts in past year; ≥2 unscheduled clinic or hospital visits for asthma in past year; requirement for chronic, daily high doses of ICS; or ≥5 mg oral prednisone or current use of ≥3 medications to control asthma 69% male 62% White, 38% other |
4756 637 children aged 6–11 years Data available for 563 children |
Use of a corticosteroid burst | Multivariate model including 6- and 12-month events Recent exacerbation as a predictor of future exacerbation OR 1.99 (1.51–2.61) Multivariate model including only 6-month events: Recent exacerbation as a predictor of future exacerbation OR 3.08 (2.21–4.28) |
| Tolomeo, 2009 [23] | Retrospective cohort study using routinely acquired data | Children's hospital, New England, USA | Hospitalisation between January and December 2006 Data for 1 year before and after hospitalisation |
Children aged 2–15 years admitted to hospital for asthma Mean age 6.35 years 66% male 36% White, 35% Black, 24% Hispanic, 5% other |
298 | Hospital admission with primary diagnosis of asthma | Previous asthma-related ED visit as a predictor of: Subsequent ED visit OR 3.3 (1.39–7.96) Subsequent hospitalisation for asthma OR 3.1 (1.17–8.33) |
| Triasih, 2011 [24]# | Retrospective cohort | Children's hospital, Melbourne, Australia | ICU admission between January 1990 and December 2004 Mean follow-up 10.3 years |
Children aged 2–18 years with asthma admitted to ICU Median age at admission 7.0 years 59% male |
410 | ICU admission for asthma | Risk factors for readmission to hospital: Previous hospital admission OR 3.3 (2.1–5.3) Admission in year prior to index admission aOR 4.5 (2.5–8.4) Multiple previous hospital admissions OR 2.4 (1.3–4.2) Risk factors for readmission to ICU: Previous hospital admission OR 16.9 (4.1–70.4) Admission in year prior to index admission aOR 4.7 (2.4–9.3) Multiple previous hospital admissions OR 3.2 (1.6–6.7) Previous ICU admission aOR 2.4 (0.8–6.7) |
| Wu, 2011 [25]# | Data from CAMP RCT used as an observational study | USA | Enrolment between December 1993 and September 1995 Follow-up over 4 years |
Children aged 5–12 years with mild/moderate persistent asthma 60% male 68% White, 14% Black, 9% Hispanic, 9% other |
1041 enrolled 1019 completed daily diary cards |
Episode requiring ≥3 days use of OCS, hospitalisation, or ED visit due to asthma (ATS/ERS statement) | History of ED visits or hospitalisations in prior year as a predictor of having ≥1 severe exacerbations regression coefficient 0.73 (0.50–0.96) History of ≥3 days of treatment with OCS in prior 3 months as a predictor of having ≥1 severe exacerbations regression coefficient 0.40 (0.17–0.62) |
| Li, 2012 [26] | Retrospective cohort study using routinely acquired data |
Ontario, Canada | ED visit between 14 April 2006 and 28 February 2009 Follow-up for 1 year |
Children aged 2–17 years with prevalent asthma with unplanned visit to ED for asthma 38.4% aged 2–5 years 63.1% male |
29 391 | ED visit for asthma | Adjusted hazard ratios for ED re-visits: Asthma admission(s) in prior 2 years HR 1.45 (1.35–1.55) Asthma ED visit(s) in prior 2 years HR 2.03 (1.91–2.14) Adjusted hazard ratios for hospital admissions: Asthma admission(s) in prior 2 years HR 2.87 (2.43–3.39) Asthma ED visit(s) in prior 2 years HR 1.85 (1.57–2.19) |
| van den Bosch, 2012 [27]# | Retrospective case–control | 4 hospitals in the Netherlands | January 1994–October 2006 | Cases: children with doctor-diagnosed asthma admitted to PICU for acute asthma Controls: patients with asthma who never needed PICU admission for any reason Median age at PICU admission 5.2 years 77% White |
230 Cases: 66 Controls: 164 |
PICU admission for acute asthma | Earlier hospitalisation for asthma (non-PICU) as a risk factor for PICU admission OR 5.4 (1.34–21.45) |
| Visitsunthorn, 2013 [28] | Retrospective case–control | Children's hospital, Bangkok, Thailand | January 2006–December 2007 1 year follow-up |
Children aged ≤14 years admitted to hospital for acute asthma 50% aged >6 years 64.5% male |
76 1 admission: 56 Readmission: 20 |
Acute asthma that was severe or did not improve after 3 doses of bronchodilator nebulisation | ICU admission at first admission as a risk factor for readmission OR 29.62 (3.35–262.18) |
| Kenyon, 2014 [29] | Retrospective cohort study using routinely acquired data | USA | Discharges between 1 July 2008 and 30 June 2010 1 year follow-up |
Children aged ≥2 years discharged from hospital after admission for asthma 61% aged 5–18 years 61.1% male 46.6% Black, 27.3% White, 16.8% Hispanic, 7.9% other |
36 601, contributing 44 203 hospitalisations | Hospital admission for asthma | Prior-year admission as a risk factor for asthma rehospitalisation 7 days OR 2.0 (1.4–2.7) 15 days OR 2.7 (2.1–3.3) 30 days OR 2.9 (2.5–3.4) 60 days OR 3.5 (3.1–3.9) 180 days OR 3.5 (3.3–3.8) 365 days OR 3.6 (3.4–3.8) |
| Zeiger, 2015 [30] | Retrospective cohort study using routinely acquired data | California, USA | 2010–2011: 1 baseline year and 1 outcome year | Children aged 5–11 years who met HEDIS criteria for persistent asthma Blood eosinophil level determined in 2010 With eosinophil level: Mean age 7.7 years 61.9% male 45.3% Hispanic, 24.6% White, 17.4% Black |
2451 With eosinophil count: 333 |
Asthma outpatient visits requiring systemic corticosteroids within ±7 days or asthma ED visits or hospitalisation | History of exacerbation as a risk factor for exacerbation: Adjusted rate ratio 2.35 (1.61–3.44) Adjusted risk ratio 1.94 (1.37–2.73) |
| Engelkes, 2016 [31]# | Retrospective cohort study using routinely acquired data | The Netherlands | 1 January 2000 to 1 January 2012 Mean follow-up 2.46 years |
Children with asthma aged 5–18 years Mean age: 10.5 years 58.7% male |
14 303 | Hospitalisation, ED visit or prescription of systemic corticosteroids for ≥3 days for asthma | Prior exacerbations as a risk factor for exacerbation: Total cohort model 1: relative rate 1.99 (1.40–2.83), model 2: relative rate 2.17 (1.30–3.60) Children with ≥1 exacerbation ever model 1: relative rate 1.60 (1.37–1.88), model 2: relative rate 1.52 (1.19–1.94) Exacerbations as a risk factor for nonfrequent exacerbations compared to frequent exacerbations <2 versus ≥2 OR 2.11 (1.66–2.68) versus OR 1.93 (1.42–2.63) <3 versus ≥3 OR 2.43 (1.84–3.23) versus OR 1.99 (1.35–2.94) |
| Quezada, 2016 [32]# | Data from SARCA and SARA RCTs used for an observational study | USA | 2007–2011 24-week treatment period |
SARCA trial: children aged 6–17 years, with poor asthma control, being treated with inhaled glucocorticoids 62% male 39% White, 49% Black, 12% other |
718 enrolled Data for 295 from SARCA |
Requirement for OCS or urgent healthcare visit for asthma symptoms | 83% of children with an exacerbation had an unscheduled visit for asthma in the previous year and 80% had been treated with OCS 69% of children without an exacerbation had an unscheduled visit for asthma in the previous year and 61% had been treated with OCS |
| Costa, 2018 [33]# | Retrospective case–control | Goiania, Brazil | June 2012–August 2013 Data collected for 1 year prior to ED visit |
Cases: children aged 4–14 years, admitted to ED for asthma who had ≥3 previous episodes of bronchospasm Controls: asthmatic children without exacerbation recruited during outpatient appointment Cases: Median age 7 years 56% male 62% White Controls: Median age 8 years 42% male 28% White |
153 Cases: 92 Controls: 61 |
Increased symptoms requiring change in medication, judged by physician according to ATS/ERS statement Severe exacerbation: hospital admission or course of OCS for asthma |
≥3 ED visits in past year for asthma as a risk factor for asthma exacerbation incidence risk ratio 1.40 (1.01–1.95) |
| Grunwell, 2018 [34] | Retrospective case–control Data from outpatient asthma clinical research studies at Emory University |
Georgia, USA | January 2004–December 2015 | Cases: children aged 6–18 years with historical admission to PICU for acute asthma Controls: asthmatic children without prior PICU admission Cases: Median age 10 years 56.7% male 52.3% Black, 35.2% White, 12.5% other Controls: Median age 12 years 61.8% male 71.8% Black, 17.1% White, 11.2% other |
579 Cases: 170 Controls: 409 |
PICU admission, hospitalisation or ED visit for asthma | Hospitalisation for asthma in year prior to data collection was associated with increased odds of PICU admission OR 8.19 (4.83–13.89) |
| To, 2018 [35] | Retrospective cohort study using routinely acquired data | Toronto, Canada | April 2008–March 2014 1 year follow-up |
Individuals aged 5–99 years with ED visit for asthma Aged 5–19 years Mean age 12.15 years 57% male 31% in most marginalised ethnic concentration quintile |
58 366 18 352 aged 5–19 years |
ED visit for asthma | Adjusted relative risk of asthma ED return visit within 1 year of ED discharge in children aged 5–19 years 1.13 (1.03–1.25) |
| Turner, 2018 [36]# | Retrospective cohort study using routinely acquired data | UK | January 1999–December 2012 1 baseline year and 1 outcome year |
Children aged 5–12 years diagnosed with asthma Mean age 9 years 57% male |
3776 | Hospitalisation, ED admission or OCS for asthma (ATS/ERS) | Previous asthma attack as a risk factor for ≥1 attack 1 OR 3.74 (2.92–4.80) ≥2 OR 7.72 (5.55–10.74) |
| Alsheri, 2020 [37]# | Retrospective case–control | Abha Hospital, Saudi Arabia | January 2014–December 2018 | Cases: children aged 2–14 years admitted to PICU for acute asthma Controls: children admitted to the ward for acute asthma Cases Mean age 6.3 years 41.7% male Controls Mean age 4.6 years 42.7% male |
320 Cases: 72 Controls: 248 |
PICU or hospital admission for asthma | Previous admission to PICU as a risk factor for PICU admission OR 7.83 (2.58–23.76) |
| Engelkes, 2020 [38]# | Retrospective cohort study using routinely acquired data from the Netherlands, Italy, UK, Denmark and Spain | The Netherlands | January 2008–December 2013 | Patients aged 5–17 years with asthma Subcohort with severe asthma (requiring high dose ICS+second controller and/or systematic corticosteroids for ≥120 consecutive days) Mean age 10.4 years (7.2–14.8 years across databases) Male preponderance across all databases |
212 060 Severe asthma 14 283 |
Use of systemic corticosteroids, ED visit and/or hospitalisation, for worsening asthma | Relative rates of exacerbation in those with history of exacerbation CPRD 5.76 (5.25–6.33) SIDIAP 2.53 (2.27–2.81) IPCI 20.04 (12.91–31.10) AUH 45.71 (31.2–66.92) PEDIANET 29.36 (16.25–53.05) HSD 10.07 (4.56–22.20) |
Data are presented with 95% confidence intervals in brackets where provided. ED: emergency department; RCT: randomised controlled trial; OCS: oral corticosteroids; ICU: intensive care unit; PACT: Pediatric Asthma Controller Trial; FEV1: forced expiratory volume in 1 s; TENOR: The Epidemiology and Natural History of Asthma; ICS: inhaled corticosteroids; aOR: adjusted odds ratio; CAMP: Childhood Asthma Management Program; ATS: American Thoracic Society; ERS: European Respiratory Society; HR: hazard ratio; PICU: paediatric intensive care unit; HEDIS: Healthcare Effectiveness Data and Information Set; SARCA: Study of Acid Reflux in Children with Asthma; SARA: Study of Acid Reflux in Adults with Asthma; CPRD: Clinical Practice Research Datalink; SIDIAP: Sistema d'Informació per al Desenvolupament de la Investigació en Atenció Primària; IPCI: Integrated Primary Care Information; AUH: Aarhus University prescription database; HSD: Health Search Database. #: included in meta-analysis.
Study risk-of-bias assessment
The Effective Public Health Practice Project (EPHPP) quality assessment tool for quantitative studies [39] was used to assess each individual study in terms of potential biases and global study quality. Studies are given a global rating of either strong, moderate or weak, based on their scoring in the first six domains. The tool was adapted to remove the domains blinding, intervention integrity and analyses, as these were not relevant to the design of studies evaluated in this review. This left the following domains: selection bias, study design, confounders, data collection methods and withdrawals and dropouts. This assessment was performed by one author (R. Lowden) with discussion undertaken with the second author. The supplementary material includes explanations of how the tool was applied.
Effect measures
The primary outcome was presence of an acute exacerbation, as measured by binary (yes/no) response.
Meta-analysis
When odds ratios were available, meta-analysis was performed using Review Manager v5.4 software (https://training.cochrane.org/online-learning/core-software/revman).
Other synthesis methods
Publication bias was explored using funnel plots. Exacerbations were categorised into three severity categories: presentation to emergency department (ED); admitted to hospital; admitted to paediatric intensive care unit (PICU). A fourth category of “unspecified severity” was used for remaining studies that did not distinguish between severity of exacerbation. The index exacerbation was defined as the first exacerbation in all studies. The subsequent exacerbation was defined as the exacerbation that occurred after the index exacerbation. PPVs were collectively described as a median and range. Subgroups based on study design and location of publication were created post hoc and analyses performed to explore potential reasons for heterogeneity between the study results. The review methodology was not registered. A protocol is available on request from the corresponding author.
Results
Study selection and study characteristics
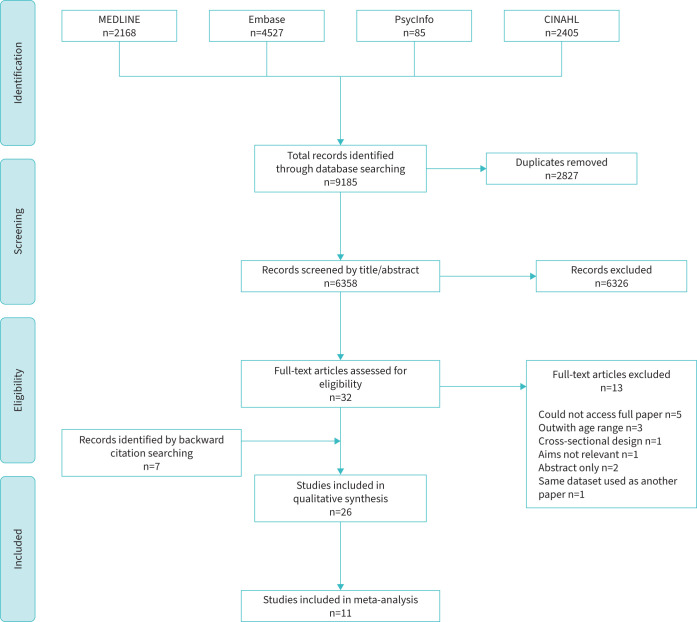
The initial database search identified 9185 potential titles. Figure 1 summarises the full selection process. After deletion of duplicates and screening by title and abstract, 32 full papers were reviewed, of which 19 were eligible. An additional seven papers were identified after reading citations, including four in the previous review [10], meaning that 26 papers were finally included. Four of the 11 papers in the previous review [10] were excluded due to their cross-sectional nature (n=2), using the same dataset used in another included paper (n=1) and the full paper not being accessible (n=1).
FIGURE 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram, showing details of the search and study inclusion process, including reasons for exclusion of full-text articles reviewed. CINAHL: Cumulative Index to Nursing and Allied Health.
Key characteristics are presented in table 1. 11 studies were published between 2000 and 2010 [13–23] and 15 between 2011 and 2021 [24–38]. 18 studies were from North America [13–23, 25, 26 29, 30, 32, 34, 35], three were from the Netherlands [31, 38, 27], and one each were from the UK [36], Saudi Arabia [37], Brazil [33], Thailand [28] and Australia [24].
Five studies had a prospective cohort design [13, 15, 20–22], with follow-up of 2 weeks [13], 6 months [20, 21], 12 months [15] and 3 years [22]. 10 studies used routinely acquired data [14, 16, 23, 26, 29–31, 35, 36, 38]; six had a retrospective case–control design [18, 27, 28, 33, 34, 37]; and one other [24]. Data from four RCTs [17, 19, 25, 32] were used, with follow-up of 28 days [17], 24 weeks [32], 48 weeks [17, 19, 25, 32] and 4 years [25]. In two RCTs, the intervention had no effect on exacerbation outcomes and data from both arms of the trial were pooled [17, 32]. The intervention in two RCTs may have influenced exacerbation outcome [19, 25].
The age range for populations varied: four included children aged 2–17 years [13, 20, 21, 26]; two included children aged 5–12 years [25, 36]; 19 had different age ranges spanning from 0 to 21 years; and one did not specify the age of the paediatric participants [27]. Unpublished data were provided by authors of two studies [19, 38], with one of these providing data from six populations [38]. The sample size varied from 76 [28] to 212 060 participants [38].
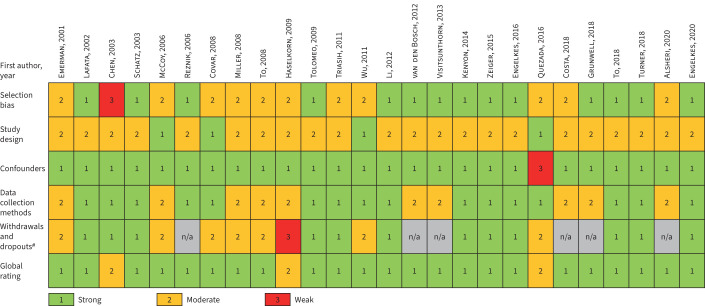
Risk of bias in studies
A summary of the quality assessment undertaken using the EPHPP quality assessment tool is presented in figure 2. 23 studies received a strong [13, 14, 16–21, 23–31, 33–38], three received a moderate [15, 22, 32] and none received a weak global rating.
FIGURE 2.
Summary of quality assessment using the Effective Public Health Practice Project quality assessment tool for quantitative studies. The domains blinding, intervention integrity and analyses were not applicable for any of the studies and were therefore removed. n/a: not applicable. #: retrospective case–control studies were given a n/a score in the withdrawals and dropouts domain in accordance with the tool recommendations.
Definition of exacerbation used
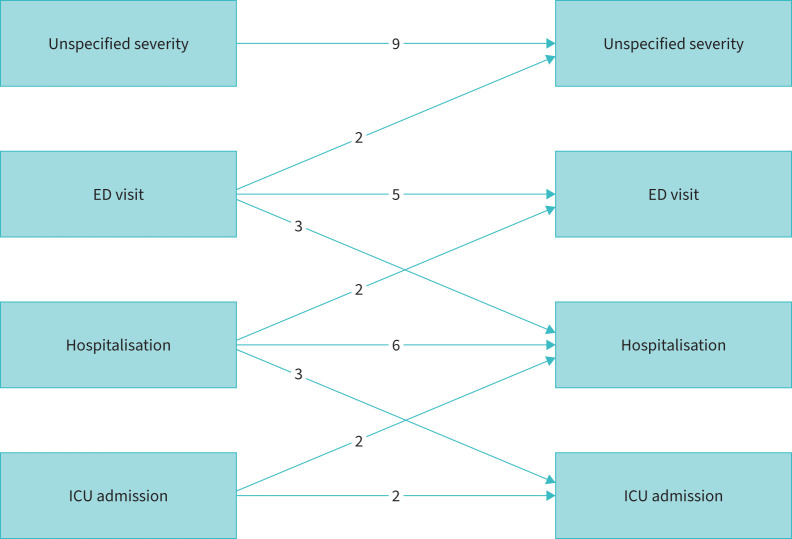
Nine studies had an unspecified severity for the index exacerbation [19, 21, 22, 25, 31, 31, 32, 26, 38], three used ED visit [26, 34, 39], four used hospital admission [15, 18, 23, 29], and two used PICU for the index exacerbation [24, 27]. Figure 3 shows the number of studies that relate these different categories of exacerbations at baseline to risk of future exacerbations. Additionally, two studies used either ED visit or hospital admission [14, 16], one used PICU or hospital admission [37] and one looked at PICU, hospital admission or ED visit [34]. Other studies used definitions of exacerbation as “appropriate signs and symptoms in a known asthmatic” [20], “sudden worsening of symptoms resulting in difficulty breathing often requiring extra medicine to relieve symptoms, with/without unscheduled ED/doctor visit” [21] and “acute asthma that was severe or did not improve after three doses of bronchodilator nebulisation” [28].
FIGURE 3.
Exacerbation outcomes, showing the number of studies that relate different categories of exacerbations at baseline to risk of future exacerbations. ED: emergency department; ICU: intensive care unit.
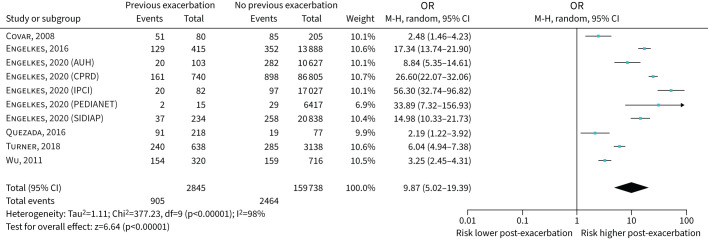
Nine studies were included where the severity of the index exacerbation was not specified [19, 21, 22, 25, 30, 31, 32, 35, 38] and odds ratios for meta-analysis were reported in six (including 10 populations and 162 583 individuals). The pooled OR (95% CI) for an index exacerbation having a subsequent exacerbation was 9.87 (5.02–19.39) (figure 4). A funnel plot was asymmetric (supplementary figure S1).
FIGURE 4.
Forest plot of studies assessing past exacerbation (unspecified severity) predicting future exacerbation. Data presented separately for five of the databases used in Engelkes et al. [38]. AUH: Aarhus University prescription database; CPRD: Clinical Practice Research Datalink; IPCI: Integrated Primary Care Information; SIDIAP: Sistema d'Informació per al Desenvolupament de la Investigació en Atenció Primària.
Eight studies were identified where the index exacerbation required an ED visit [13, 14, 18, 21, 23, 26, 33, 35]. Two studies identified an association between an ED visit for asthma and a future exacerbation, using an unspecified severity definition [13, 33] and three studies reported that children with an ED visit for asthma were at risk of a future hospitalisation for asthma [18, 23, 26]. Five studies assessed the link between a past ED visit for asthma and the risk of a future visit to the ED [14, 21, 23, 26, 35]. Raw data were not available to allow meta-analysis (e.g. only odds ratios were reported). The odds ratios for the three studies with available data (970 individuals) were 3.30, 6.27 and 8.26 [14, 21, 23] (table 2). The PPV for the two studies with available data were 0.12 and 0.22 [21, 35].
TABLE 2.
Key results shown by index and subsequent exacerbation type
| Category of subsequent exacerbation | |||
| ED | Hospital | PICU | |
| Category of index exacerbation | |||
| ED | 5 studies ORs 3.3 [23], 6.27 [21], 8.26 [14] HR 2.03 [26] Relative risk 1.13 [35] |
3 studies ORs 3.1 [23], 3.28 [18] HR 1.85 [26] |
No studies |
| Hospital | 2 studies OR 0.85 [14] HR 1.45 [26] |
6 studies ORs 1.89 [18], 3.3 [24], 3.37 [16], 3.6 [29], 5.36 [15] HR 2.87 [26] |
3 studies ORs 5.4 [27], 8.19 [34], 16.9 [24] |
| PICU | No studies | 2 studies ORs 2.18 [18], 29.62 [28] |
2 studies ORs 2.4 [24], 7.83 [37] |
ED: emergency department; PICU: paediatric intensive care unit; HR: hazard ratio.
Nine studies were identified where the index exacerbation was a hospital admission [14–16, 18, 24, 27, 29, 34, 36], including six that described the relationship between an index and subsequent hospital admission for asthma [15, 18, 16, 29, 26, 24]. The median (range) odds ratio was 3.60 (1.89–5.36) for the five studies with available data (41 475 individuals) [15, 16, 18, 24, 29] (table 2). The PPVs for the two case-only studies with available data were 0.17 and 0.69 [15, 29].
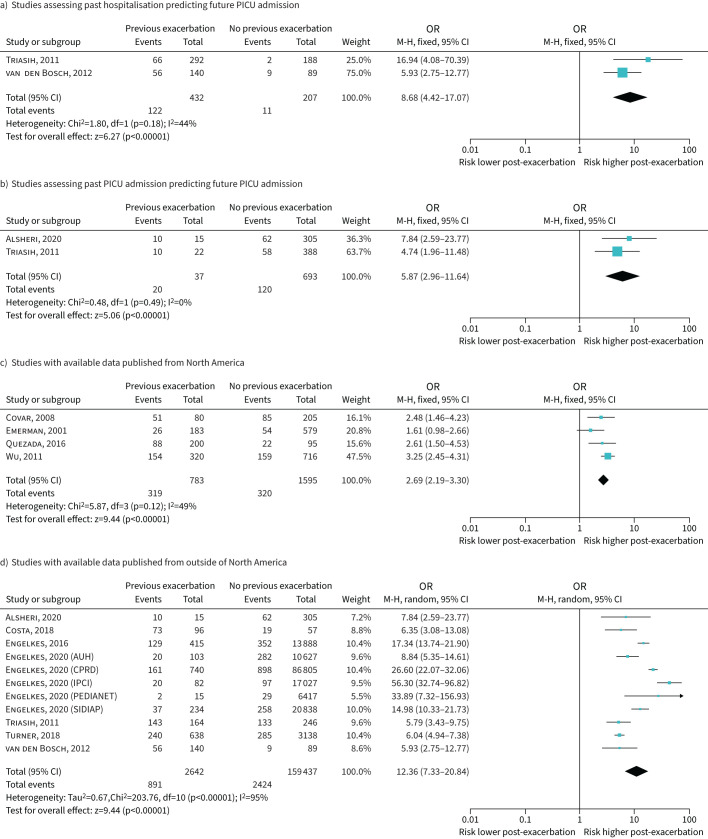
Table 2 shows the results of two studies that looked at prior hospitalisation for asthma predicting future ED use for asthma. Three studies found an association between hospitalisation for asthma and future PICU admission with data from two studies (639 individuals) available for meta-analysis with OR 8.68 (95% CI 4.42–17.07) (figure 5a).
FIGURE 5.
Forest plots based on exacerbation severity definition and location of study publication. In d), data are presented separately for five of the databases used in Engelkes et al. [38]. PICU: paediatric intensive care unit.
Four studies were identified where the index exacerbation was a PICU admission [18, 37, 24, 28]. Two studies assessed the link between index and subsequent PICU admissions and the odds ratio from meta-analysis was 5.87 (95% CI 2.96–11.64) [37, 24] (figure 5a). Two other studies reported a link between a history of PICU admission for asthma and subsequent hospitalisation with the reported odds ratios being 2.18 [18] and 29.62 [28] (table 2).
Factors associated with heterogeneity
Recognising the heterogeneity in results within different exacerbation definitions we explored post hoc whether heterogeneity was reduced when subgrouping by healthcare system (i.e. North American versus other). Of the 11 studies with available data for inclusion in a meta-analysis, the odds ratio for an index exacerbation to have a subsequent exacerbation was 2.69 (95% CI 2.19–3.30, I2 49%) in the four published from North America [13, 19, 32, 25] (figure 5c), and 12.36 (95% CI 7.33–20.84, I2 95%) for the seven published outside North America [24, 27, 31, 33, 36–38] (figure 5d). The asthma severity was not specified in three of the four studies [19, 32, 25] from North America and four of the seven studies from other regions [31, 33, 36, 38].
In addition, we explored whether results differed by study methodology (i.e. RCTs, prospective cohort, routinely acquired data and retrospective case–control). Where more than one exacerbation category was reported we deferred to the one with highest incidence. The median (range) of PPV differed by study design as follows: for three RCTs [19, 25, 32] 0.58 (0.42–0.77); for four prospective cohort studies [13, 15, 20, 21] 0.31 (0.14–0.54); for six studies analysing routinely acquired data [23, 29, 31, 35, 36, 38] 0.16 (0.06–0.43); and for four retrospective case–control studies [27, 28, 33, 37] 0.50 (0.25–0.78) (supplementary table S1). Across different study design there was an even distribution of exacerbation severity definitions.
Two papers reported how the risk of experiencing a future exacerbation increased over time after the index exacerbation; however, this increase plateaued after 3 months in a study of exacerbations requiring hospitalisation [29] and after 6 months in another study using the unspecified severity definition [31].
Discussion
Our main finding was that although as previously reported an asthma exacerbation is a risk factor for a further exacerbation, there was no evidence of a relationship between increasing severity of an index asthma exacerbation and increasing odds ratio for a subsequent exacerbation. Additional findings were that odds ratios for a second exacerbation differed by geography (i.e. North America compared to other continents), study methodology (i.e. RCT and retrospective case-only study compared to cohort and routinely acquired data) and duration since index exacerbation. These findings give insight into the complexity of the relationship between index and subsequent asthma exacerbations. When speaking to parents and patients, clinicians should be aware that “mild” and “severe” initial exacerbations may both have equal odds ratios for a subsequent exacerbation.
A previous review with a broader remit than ours has reported how an exacerbation is associated with increased odds ratio of a future exacerbation in children [10]. In our review we included a larger number of studies and in addition, to confirm the earlier report [10], we find a greater magnitude of association than the earlier review for an index exacerbation being followed by a subsequent exacerbation.
In adults, the best predictor of a future exacerbation is also a past exacerbation [40], but in adults (unlike our findings in children) the odds ratios of future exacerbations are highest in those with history of a severe exacerbation [40]. We were able to confirm in children the observation made in adults, that the risk of further exacerbation is highest in the period following the index exacerbation, although in adults this appears to remain over a period of years, whereas in children the risk may reach a plateau after a period of months. Differences between asthma in children and adults are well described [41], so discordant findings between exacerbation risk in children and adults is expected. These findings highlight the importance of inclusion of previous exacerbation in risk stratification models for future exacerbation.
There are some limitations to the literature, which should be considered. First, there was little standardisation of the definition of exacerbation between studies, e.g. criteria for ED presentation or hospital/PICU admission were not pre-specified, and this will have introduced variability into the relationship between past and future exacerbations. The odds ratios and PPVs for a second ED presentation or hospital admission were not different and this may be due to children with similar exacerbation severities entering different pathways of care in different healthcare systems, for example those in North America compared to other countries.
A second limitation was that data from 15 of the studies could not be included in the meta-analysis due to either raw data not being available or insufficient details given. An additional limitation was that the meta-analysis used raw data and could not adjust for factors that may have differed between groups with and without an exacerbation (e.g. gender, age, race, severity of asthma, month of exacerbation) and the magnitude of the actual odds ratio may differ from that reported herein. A further limitation is that none of the studies considered the cause of the exacerbation nor described different outcomes for different triggers. Therefore, we cannot comment whether different triggers are associated with different risk for future exacerbations and therefore risk assessment should consider whether causes of exacerbation could be avoided. Additionally, details of the treatment that children received, both for acute symptoms and asthma prevention, were not provided. Furthermore, adherence to preventer therapy was not considered. Lack of adequate asthma preventer treatment and/or lack of adherence to asthma preventer treatment are both important risk factors for future exacerbations and should be considered as part of comprehensive future risk assessment.
A further limitation is that 18 of the 26 studies identified in this review were from North American populations and we demonstrated that the odds ratio for an exacerbation following an index exacerbation were lower for these studies compared to others, possibly due to differences in healthcare systems in North America and other continents. The results from five different European countries [38] were heterogeneous, and this may highlight differences in how asthma exacerbations are managed between countries. The reason for these differences could come from variations in asthma management, including medications, follow-up and asthma action plans, as well as varying compliance with medications, environmental factors and cultural differences [38]. There was also evidence of publication bias in the included studies, although these had minimal effect on the overall odds ratio of the meta-analyses performed.
A limitation of the methodology used in this review is that the literature search, data extraction and quality assessment processes were performed by one individual, leading to potential bias. The effects of this were mitigated by detailed discussion and involvement of an experienced paediatric clinician at every stage of the review. A second limitation to our methodology is that our results cannot be generalised to children aged <5 years, whose results are not included in this review.
The odds ratios and PPVs of an index exacerbation being followed by a further exacerbation were higher in the unspecified severity category studies when compared with studies that applied any of the three specified exacerbation severities. This initially seems counterintuitive, as it would be expected that children requiring hospitalisation for asthma, especially requiring PICU admission, would have more severe disease and are therefore more likely to experience a future exacerbation. However, the experience of having symptoms which necessitate a visit to the ED or an admission to hospital may improve treatment compliance and avoidance of triggers for exacerbation and thus reduce the likelihood of further exacerbations.
In summary, an index asthma exacerbation in children aged 5–16 years is a predictor of future acute exacerbation, and this relationship is not necessarily affected by exacerbation severity but is related to the period of follow-up, healthcare system and study methodology.
Conclusion
Our review of the literature supports asthma guideline advice that a past exacerbation is predictive of a future exacerbation. Additionally, our study places an estimated magnitude on the odds ratio and PPV for an exacerbation predictive of further relapse. Finally, our work gives insight into the complexity of the relationship between successive asthma exacerbations in children.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00174-2022.SUPPLEMENT (328.8KB, pdf)
Footnotes
Provenance: Submitted article, peer reviewed.
Author contributions: R. Lowden and S. Turner contributed to the conception, design, data collection, analysis of the results, and to the writing and reviewing of the manuscript. S. Turner is the guarantor of the paper.
This article has an editorial commentary: https://doi.org/10.1183/23120541.00322-2022
Conflict of interest: The authors declare that there are no conflicts of interest to disclose.
References
- 1.Royal College of Paediatrics and Child Health . State of Child Health in the UK. 2020. https://stateofchildhealth.rcpch.ac.uk Date last accessed: 12 January 2021. Date last updated: May 2020.
- 2.Centers for Disease Control and Prevention . Asthma, National Data. 2019. www.cdc.gov/asthma/most_recent_national_asthma_data.htm Date last accessed: 31 May 2021. Date last updated: 30 March 2021.
- 3.Papadopoulos NG, Arawaka H, Carlsen KH, et al. International Consensus On (ICON) Pediatric Asthma. Allergy 2012; 67: 976–997. doi: 10.1111/j.1398-9995.2012.02865.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belgrave DCM, Buchan I, Bishop C, et al. Trajectories of lung function during childhood. Am J Respir Care Med 2014; 189: 1101–1109. doi: 10.1164/rccm.201309-1700OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fleming L. Asthma exacerbation prediction: recent insights. Curr Opin Allergy Clin Immunol 2018; 18: 117–123. doi: 10.1097/ACI.0000000000000428 [DOI] [PubMed] [Google Scholar]
- 6.Ferrante G, La Grutta S. The burden of pediatric asthma. Front Pediatr 2018; 6: 186. doi: 10.3389/fped.2018.00186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Global Initiative for Asthma (GINA) . Global Strategy for Asthma Management and Prevention. 2021. Available from: www.ginasthma.org/ Date last accessed: 31 May 2021.
- 8.Scottish Intercollegiate Guidelines Network (SIGN) . British Guideline on the Management of Asthma. (SIGN Guideline 158). 2019. Available from: www.sign.ac.uk. Date last accessed: 9 January 2021.
- 9.National Institute for Health and Care Excellence (NICE) . Asthma: Diagnosis, Monitoring and Chronic Asthma Management (NG80). 2017. www.nice.org.uk/guidance/ng80. Date last accessed: 10 January 2021. Date last updated: 22 March 2021.
- 10.Buelo A, McLean S, Julious S, et al. At-risk children with asthma (ARC): a systematic review. Thorax 2018; 73: 813–824. doi: 10.1136/thoraxjnl-2017-210939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glas AS, Lijmer JG, Prins MH, et al. The diagnostic odds ratio: a single indicator of test performance. J Clin Epidemiol 2003; 56: 1129–1135. doi: 10.1016/S0895-4356(03)00177-X [DOI] [PubMed] [Google Scholar]
- 12.Pepe MS, Janes H, Longton G, et al. Limitations of the odds ratio in gauging the performance of a diagnostic, prognostic, or screening marker. Am J Epidemiol 2004; 159: 882–890. doi: 10.1093/aje/kwh101 [DOI] [PubMed] [Google Scholar]
- 13.Emerman CL, Cydulka RK, Crain EF, et al. Prospective multicenter study of relapse after treatment for acute asthma among children presenting to the emergency department. J Pediatr 2001; 138: 318–324. doi: 10.1067/mpd.2001.111320 [DOI] [PubMed] [Google Scholar]
- 14.Lafata JE, Xi H, Divine G. Risk factors for emergency department use among children with asthma using primary care in a managed care environment. Ambul Pediatr 2002; 2: 268–275. doi: [DOI] [PubMed] [Google Scholar]
- 15.Chen E, Bloomberg GR, Fisher EB Jr, et al. Predictors of repeat hospitalizations in children with asthma: the role of psychosocial and socioenvironmental factors. Health Psychol 2003; 22: 12–18. doi: 10.1037/0278-6133.22.1.12 [DOI] [PubMed] [Google Scholar]
- 16.Schatz M, Cook EF, Joshua A, et al. Risk factors for asthma hospitalizations in a managed care organization: development of a clinical prediction rule. Am J Manag Care 2003; 9: 538–547. [PubMed] [Google Scholar]
- 17.McCoy K, Shade DM, Irvin CG, et al. Predicting episodes of poor asthma control in treated patients with asthma. J Allergy Clin Immunol 2006; 118: 1226–1233. doi: 10.1016/j.jaci.2006.09.006 [DOI] [PubMed] [Google Scholar]
- 18.Reznik M, Halipern SM, Ozuah PO. Predictors of early readmission for asthma among inner-city children. J Asthma 2006; 43: 37–40. doi: 10.1080/02770900500446997 [DOI] [PubMed] [Google Scholar]
- 19.Covar RA, Szefler SJ, Zeiger RS, et al. Factors associated with asthma exacerbations during a long-term clinical trial of controller medications in children. J Allergy Clin Immunol 2008; 122: 741–747. doi: 10.1016/j.jaci.2008.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller TP, Oswald FL, Reeves MJ. An exploration of factors underlying asthma care and morbidity: a factor analysis of clinical variables. J Allergy Clin Immunol 2008; 122: 328–334. doi: 10.1016/j.jaci.2008.05.021 [DOI] [PubMed] [Google Scholar]
- 21.To T, Wang C, Dell S, et al. Risk factors for repeat adverse asthma events in children after visiting an emergency department. Ambul Pediatr 2008; 8: 281–287. doi: 10.1016/j.ambp.2008.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haselkorn T, Zeiger RS, Chipps BE, et al. Recent asthma exacerbations predict future exacerbations in children with severe or difficult-to-treat asthma. J Allergy Clin Immunol 2009; 124: 921–927. doi: 10.1016/j.jaci.2009.09.006 [DOI] [PubMed] [Google Scholar]
- 23.Tolomeo C, Savrin C, Heinzer M, et al. Predictors of asthma-related pediatric emergency department visits and hospitalizations. J Asthma 2009; 46: 829–834. doi: 10.1080/02770900903162753 [DOI] [PubMed] [Google Scholar]
- 24.Triasih R, Duke T, Robertson CF. Outcomes following admission to intensive care for asthma. Arch Dis Child 2011; 96: 729–734. doi: 10.1136/adc.2010.205062 [DOI] [PubMed] [Google Scholar]
- 25.Wu AC, Tantisira K, Li L, et al. Predictors of symptoms are different from predictors of severe exacerbations from asthma in children. Chest 2011; 140: 100–107. doi: 10.1378/chest.10-2794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li P, To T, Guttmann A. Follow-up care after an emergency department visit for asthma and subsequent healthcare utilization in a universal-access healthcare system. J Pediatr 2012; 161: 208–213. doi: 10.1016/j.jpeds.2012.02.038 [DOI] [PubMed] [Google Scholar]
- 27.van den Bosch GE, Merkus PJFM, Buysse CMP, et al. Risk factors for pediatric intensive care admission in children with acute asthma. Respir Care 2012; 57: 1391–1397. doi: 10.4187/respcare.01325 [DOI] [PubMed] [Google Scholar]
- 28.Visitsunthorn N, Lilitwat W, Jirapongsananuruk O, et al. Factors affecting readmission for acute asthmatic attacks in children. Asian Pac J Allergy Immunol 2013; 31: 138–141. doi: 10.12932/AP0247.31.2.2013 [DOI] [PubMed] [Google Scholar]
- 29.Kenyon CC, Melvin PR, Chiang VW, et al. Rehospitalization for childhood asthma: timing, variation, and opportunities for intervention. J Paediatr 2014; 164: 300–305. doi: 10.1016/j.jpeds.2013.10.003 [DOI] [PubMed] [Google Scholar]
- 30.Zeiger RS, Schatz M, Li Q, et al. The association of blood eosinophil counts to future asthma exacerbations in children with persistent asthma. J Allergy Clin Immunol Pract 2015; 3: 283–287. doi: 10.1016/j.jaip.2014.10.009 [DOI] [PubMed] [Google Scholar]
- 31.Engelkes M, Janssens HM, de Ridder MAJ, et al. Real life data on incidence and risk factors of severe asthma exacerbations in children in primary care. Respir Med 2016; 119: 48–54. doi: 10.1016/j.rmed.2016.08.016 [DOI] [PubMed] [Google Scholar]
- 32.Quezada W, Kwak ES, Reibman J, et al. Predictors of asthma exacerbation among patients with poorly controlled asthma despite inhaled corticosteroid treatment. Ann Allergy Asthma Immunol 2016; 116: 112–117. doi: 10.1016/j.anai.2015.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Costa LDC, Camargos PAM, Brand PLP, et al. Asthma exacerbations in a subtropical area and the role of respiratory viruses: a cross-sectional study. BMC Pulm Med 2018; 18: 109. doi: 10.1186/s12890-018-0669-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grunwell JR, Travers C, Fitzpatrick AM. Inflammatory and comorbid features of children admitted to a PICU for status asthmaticus. Pediatr Crit Care Med 2018; 19: e585–e594. doi: 10.1097/PCC.0000000000001695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.To T, Zhu J, Ryckman Ket al. Risk factors for return to the emergency department for asthma: a population-based study. J Allergy Clin Immunol Pract 2018; 6: 1907–1913. doi: 10.1016/j.jaip.2018.04.016 [DOI] [PubMed] [Google Scholar]
- 36.Turner SW, Murray C, Thomas M, et al. Applying UK real-world care data to predict asthma attacks in 3776 well-characterised children: a retrospective cohort study. NPJ Prim Care Respir Med 2018; 28: 28. doi: 10.1038/s41533-018-0095-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alsheri MA, Rodgers SE, Lyons RA, et al. Risk factors for pediatric intensive care admission among asthmatic children in Aseer, South-West Saudi Arabia. Indian J Public Health Res Dev 2020; 11: 1684–1689. doi: 10.37506/v11/i2/2020/ijphrd/195069 [DOI] [Google Scholar]
- 38.Engelkes M, Baan EJ, de Ridder MAJ, et al. Incidence, risk factors and re-exacerbation rate of severe asthma exacerbations in a multinational, multidatabase pediatric cohort study. Pediatr Allergy Immunol 2020; 31: 496–505. doi: 10.1111/pai.13237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Effective Public Health Practice Project (EPHPP) . Quality Assessment Tool for Quantitative Studies. 2009. www.ephpp.ca/quality-assessment-tool-for-quantitative-studies/ Date last accessed: 2 February 2021.
- 40.Bloom CI, Palmer T, Feary J, et al. Exacerbation patterns in adults with asthma in England. A population-based study. Am J Respir Crit Care Med 2019; 199: 446–453. doi: 10.1164/rccm.201808-1516OC [DOI] [PubMed] [Google Scholar]
- 41.Turner SW, Upham J. Asthma in children and adults – what are the differences and what can they tell us about asthma? Front Pediatr 2020; 8: 141. doi: 10.3389/fped.2020.00141 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00174-2022.SUPPLEMENT (328.8KB, pdf)