Abstract
Background:
Dementia, vascular disease, and cancer increase with age, enabling complex comorbid interactions. Understanding vascular and cancer contributions to dementia risk and neuropathology in oldest-old may improve risk modification and outcomes.
Objective:
Investigate the contributions of vascular factors and cancer to dementia and neuropathology.
Methods:
Longitudinal clinicopathologic study of prospectively followed Mayo Clinic participants dying≥95 years-old who underwent autopsy. Participants were stratified by dementia status and compared according to demographics, vascular risk factors, cancer, and neuropathology.
Results:
Participants (n = 161; 83% female; 99% non-Hispanic whites)≥95 years (95–106 years-old) with/without dementia did not differ based on demographics. APOE ɛ2 frequency was higher in no dementia (20/72 [28%]) versus dementia (11/88 [12%]; p = 0.03), but APOE ɛ4 frequency did not differ. Coronary artery disease was more frequent in no dementia (31/72 [43%]) versus dementia (23/89 [26%]; p = 0.03) associated with 56% lower dementia odds (odds ratio [OR] = 0.44 [confidence interval (CI) = 0.19–0.98]; p = 0.04) and fewer neuritic/diffuse plaques. Diabetes had an 8-fold increase in dementia odds (OR = 8.42 [CI = 1.39–163]; p = 0.02). Diabetes associated with higher cerebrovascular disease (Dickson score; p = 0.05). Cancer associated with 63% lower dementia odds (OR = 0.37 [CI = 0.17–0.78]; p < 0.01) and lower Braak stage (p = 0.01).
Conclusion:
Cancer exposure in the oldest-old was associated with lower odds of dementia and tangle pathology, whereas history of coronary artery disease was associated with lower odds of dementia and amyloid-β plaque pathology. History of diabetes mellitus was associated with increased odds of dementia and cerebrovascular disease pathology. Cancer-related mechanisms and vascular risk factor reduction strategies may alter dementia risk and neuropathology in oldest-old.
Keywords: Aging, Alzheimer’s disease, cancer, dementia, neuropathology, vascular disease
INTRODUCTION
The oldest-old are the fastest growing segment of the US population, projected to grow from 2.0% in 2020 to 4.8% by 2050 [1]. Aging is a major risk factor for dementia [2], vascular disease [3, 4], and cancer [5] that enables complex disease interactions. An inverse association between dementia and cancer [6–11] was initially described in an autopsy series in 1962 [6]. Since then, a 45–68% lower risk of cancer in patients with an amnestic dementia and a 35–50% lower risk of an amnestic dementia in patients with cancer were reported [7, 8, 11, 12]. Furthermore, slower memory decline was reported in patients prior to and after incident cancer diagnosis compared to non-cancer patients [13]. The nature of this association is complex [9] and may be biased by the presence of resilience factors (i.e., survival bias), diagnostic delays [14], and vascular risk factors known to decrease resilience to neurodegenerative pathology [15, 16].
Understanding the contributions of these factors to the association between cancer, cognition, and neuropathology may inform future strategies for dementia risk modification and improve outcomes. We studied a prospectively followed Mayo Clinic series of participants who lived≥95 years-old and donated their brains for neuropathologic evaluation. By limiting inclusion to autopsied participants from this age group, we sought to optimize the expression of potential comorbidities affecting neurodegeneration, permitting the association between risk-resilience factors and neurodegeneration to be studied cross-sectionally. We hypothesized that the presence of vascular comorbidities would be associated with worse cognitive outcomes and related neuropathologic changes, while a history of cancer would associate with lower odds of dementia and related neuropathology.
MATERIALS AND METHODS
Standard protocol approvals, registrations, and patient consents
The Mayo Clinic brain bank of neurodegenerative diseases was queried to identify participants who met the following inclusion criteria: study participants from the Alzheimer’s Disease Research Center (ADRC) or Mayo Clinic Study of Aging (MCSA) who came to autopsy at≥95 years-old (95+ series) from July 14, 1998 to January 24, 2020. A flowchart detailing participant inclusion is provided in Supplementary Figure 1. Relevant clinical and neuropathologic data were extracted from clinical and research records. The ADRC is a dementia clinic referral-based study and the MCSA is a population-based prospective study randomly sampled from Olmsted County, MN in an age- and sex-stratified manner [17]; both follow study participants until autopsy. Written informed consent was obtained from prospectively enrolled individuals or their legally authorized representatives, permitting collection of clinical details and post-mortem neuropathologic assessment. The Mayo Clinic Institutional Review Board approved the ADRC (IRB 712-98) and MCSA (IRB 14-004401) study protocols.
Participants and clinical features
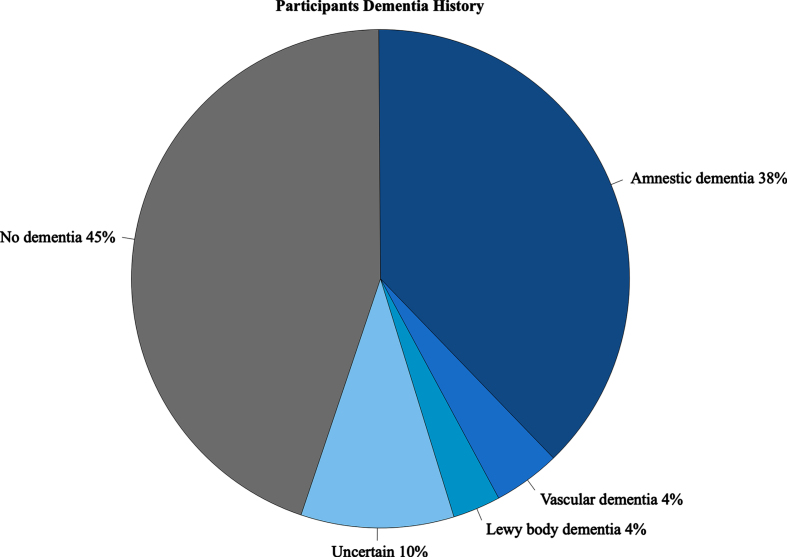
Databased consensus diagnosis of 164 study participants meeting inclusion criteria were reviewed and classified according to dementia status (present/absent). Consensus diagnoses for ADRC and MCSA were reached after comprehensive evaluation by an expert panel including physicians, nurses, and neuropsychologists per published criteria [17]. Study participants with dementia were classified as having an amnestic dementia (n = 61), vascular dementia (n = 7), Lewy body dementia (n = 5, including Parkinson’s disease dementia or dementia with Lewy bodies), or Uncertain dementia (n = 16) when dementia etiology could not be confidently determined. Participants without dementia included those who were cognitively unimpaired or with mild cognitive impairment and lacked evidence of dementia documented in clinic or research records within 3 years of death (Fig. 1). Three participants without dementia were excluded as their final clinical evaluation was > 3 years prior to death. The final 95+ series contained n = 161 participants (95–106 years-old; 134/161 females, 83%). Participants with dementia (n = 89) and without dementia (n = 72) were stratified and compared according to demographic variables, including age at death, sex, race, and years of education. We investigated APOE allele status focusing on presence of APOE ɛ2 (risk lowering [18]) and APOE ɛ4 (risk increasing [19]) allele frequency.
Fig. 1.
Participant Dementia History. Presence of dementia based on consensus diagnoses classified as No dementia (cognitively normal or having mild cognitive impairment with no evidence of dementia on medical records≤3 years prior to date of death), amnestic, Lewy body dementia (Parkinson’s disease dementia and dementia with Lewy bodies), and uncertain (dementia type could not be ascribed to known forms).
Clinical variables were retrospectively extracted from available records, including cognition (last Mini-Mental Status Examination [MMSE]), history of vascular risk factors or comorbidities (cerebrovascular disease, coronary artery disease, hypertension, heart failure, peripheral vascular disease, diabetes mellitus, smoking status [1-never smoker, 2-former smoker, or 3-current smoker; for further risk analysis, categories 2 and 3 were combined as presence of smoking exposure]), and cancer. Cancer history was dichotomized into skin cancer and non-skin cancer [20]. Skin cancers included melanoma and nonmelanoma skin cancer (basal cell carcinoma, squamous cell carcinoma). Non-skin cancers comprised hematological cancers (leukemia) or solid tumors, including carcinoma (bladder, breast, biliary, colon, esophagus, gastric, kidney, lung, ovary, pancreas, prostate, rectum, uroepithelial, and uterine), sarcoma, and metastatic disease. If a participant had multiple cancers, further sub-analyses prioritized skin cancer when binning cancer history.
Neuropathologic features
Neuropathologic examination and brain sampling were conducted per CERAD protocol [21]. Fixed brain weight was recorded or calculated from doubling the hemibrain weight. Standardized evaluation of Alzheimer’s Disease (AD)-related neuropathology was examined per recommended guidelines [22]. Braak tangle stage [23] was evaluated using Bielschowsky silver stain and phospho-tau immunohistochemistry (AT8, 1:7500, Ser202/Thr205, Thermo Fisher Scientific, Waltham, MA). Neuritic and diffuse plaques were evaluated in the cortex using Bielschowsky silver stain and amyloid-β immunohistochemistry (6F/3D, 1:250, human Aβ8-17, Dako, Carpinteria, CA). A semi-quantitative score for diffuse and neuritic plaques was recorded using a 4-point system: none, sparse, moderate, and frequent. A subset of AD cases (n = 18) previously underwent thioflavin-S staining to enable neurofibrillary tangle counts in hippocampal subsectors (CA1, subiculum) and association cortices (superior temporal, inferior parietal, middle frontal). The AD subtyping algorithm was applied to classify cases as hippocampal sparing, typical, or limbic predominant [24].
Lewy body pathology was evaluated according to consensus recommendations [25] using α-synuclein immunohistochemistry (LB509, 1:100, amino acid 115–122 on alpha-synuclein, Thermo Fisher Scientific, Waltham, MA). TAR DNA-binding protein of 43 kDa (TDP-43) pathology was evaluated for positivity in the amygdala using the MC2085 antibody (1:3000, amino acid 220–227 in C-terminal fragment of cleaved TDP-43, kind gift from Leonard Petrucelli) [26]. Cases lacking TDP-43 inclusions were considered negative. Cases with TDP-43-immunopositive neuronal cytoplasmic inclusions or perivascular bi-lobed Lin bodies [27] were considered positive. Cases with TDP-43-immunopositive structures that did not meet criteria for positivity were given the designation of “0.5” to indicate pathology was present, but insufficient for TDP-43 positivity [28]. Limbic-predominant age-related TDP-43 encephalopathy (LATE) neuropathologic change (LATE-NC) in amygdala is thus reported as negative, rare, or positive [29].
Cerebrovascular disease scales were retrospectively evaluated through abstraction of neuropathologic reports using a score sheet as shown in Supplementary Tables 1–3. The modified Kalaria cerebrovascular disease scale (Supplementary Table 1) evaluates both large and small vessel disease using hematoxylin and eosin-stained (H&E) sections of cerebral cortex and basal ganglia [30–32], as previously described [33]. Observations of vessel wall modifications, dilated perivascular spaces, white matter loss or infarction, microinfarction, cystic infarction and/or large infarction were used to inform the Kalaria score for a total of 10 points [30, 32]. The Strozyk cerebrovascular disease scale (Supplementary Table 2) evaluates number of large infarcts and lacunar/cystic infarcts, as well as severity of white matter disease (including white matter infarctions) observed on H&E-stained sections for a total of 6 points [34]. The Dickson Gestalt is traditionally used at the time of neuropathologic evaluation by D.W.D. to assess significance of cerebrovascular disease on H&E-stained sections for inclusion in the neuropathologic diagnosis. The number of large infarcts, lacunar infarcts, and microinfarcts are counted for a maximum of 2 points each. An additional point can be added for moderate-to-severe white matter loss or white matter infarction observed. The presence of significant neuronal loss characterized as hippocampal sclerosis of a vascular etiology counts for an additional point. The presence of severe cerebral amyloid angiopathy evaluated using Bielschowsky silver stain and/or amyloid-β immunohistochemistry qualifies for an additional point. The Dickson score (Supplementary Table 3) sums each of the above-mentioned pathologies for a total cumulative score of up to 9 points. Although not applied in this study,≥2 points was historically used for inclusion of significant cerebrovascular disease in the neuropathologic diagnosis. The arteriolosclerosis score was retrospectively applied based upon reported severity: 0 = none, 1 = mild, 2 = moderate, 3 = severe.
Statistical analyses
Statistical analyses and modeling were performed using R v4.0.3. Non-parametric pair-wise comparisons of continuous variables (e.g., age at death, brain weight) and ordered variables (e.g., Braak stage) were performed using the Kruskal-Wallis Rank Sum Test to evaluate differences in median values. The Pearson’s chi-squared test was used to evaluate differences in the proportion of categorical variables (e.g., sex, APOE genotype). Antemortem variables implicated as potential contributors to dementia (p < 0.10 on univariate analyses) were incorporated within a multivariable logistic regression model (forced entry: APOE ɛ2, coronary artery disease, hypertension, diabetes mellitus, smoking, cancer), quantifying the association between the clinical variables of interest and the odds of a dementia diagnosis using likelihood-ratio derived test statistic. Postmortem findings were then stratified by presence of dementia and according to significant variables obtained from the multivariable logistic regression model (i.e., cancer, diabetes mellitus, and coronary artery disease). To evaluate contribution of postmortem neurodegenerative pathologies and cerebrovascular disease to odds of developing dementia or amnestic dementia, the likelihood-ratio derived test statistic was used. Neurodegenerative variables included Braak stage (0-VI), neuritic plaque score (0–3), Lewy body disease (0 = none, 1 = brainstem, 2 = transitional, 3 = diffuse), LATE-NC positivity in amygdala (0 = none, 0.5 = insufficient for positivity, 1 = positive), and Dickson Gestalt score (0–5). Statistical significance was considered for 2-sided p < 0.05.
RESULTS
Participants from the 95+ series were stratified as having no dementia (72/161 [45%]) versus dementia (89/161 [55%]) (Table 1). Age at death (98 versus 98; p = 0.81), sex distribution (female 79% versus 86%; p = 0.30), and race (99% white versus 99% white; p = 0.58) did not differ in this primarily non-Hispanic white series. Years of education did not differ in those without dementia (median = 15 [interquartile range 12, 16]) compared to those with dementia (14 [12, 16]) (p = 0.08). The presence of APOE ɛ2 allele was more frequent in participants without dementia (28%) compared to those with dementia (12%) (p = 0.03), yet no differences were observed in the frequency of APOE ɛ4 (22% versus 27%; p = 0.58). Higher MMSE scores (27 points [24, 28]) were observed in participants without dementia compared to those with dementia (17 [11, 24] points) (p < 0.001).
Table 1.
Demographics and clinical variables
| Variable | No Dementia n = 72 | Dementia n = 89 | p |
| Demographics | |||
| Age at death, y | 98 (96,100) | 98 (96,99) | 0.81 |
| Females, % | 57/72 (79%) | 77/89 (86%) | 0.30 |
| Race, % | 0.58 | ||
| Non-Hispanic white decedent | 71/72 (99%) | 88/89 (99%) | |
| Non-Hispanic black decedent | 1/72 (1%) | 1/89 (1%) | |
| Education, years | 15 (12,16) | 14 (12,16) | 0.08 |
| APOE ɛ2 presence, % | 20/72 (28%) | 11/88 (12%) | 0.03 |
| APOE ɛ4 presence, % | 16/72 (22%) | 24/88 (27%) | 0.58 |
| Last MMSE | 27 (24,28) | 17 (11,24) | <0.001 |
| Vascular risk factors or comorbidities | |||
| Cerebrovascular Disease, % | 22/72 (31%) | 37/89 (42%) | 0.20 |
| Coronary Artery Disease, % | 31/72 (43%) | 23/89 (26%) | 0.03 |
| Hypertension, % | 59/72 (82%) | 63/89 (71%) | 0.15 |
| Heart Failure, % | 30/72 (42%) | 29/89 (33%) | 0.31 |
| Peripheral Vascular Disease, % | 15/72 (21%) | 17/89 (19%) | 0.94 |
| Diabetes Mellitus, % | 2/72 (3%) | 10/89 (11%) | 0.08 |
| Smoking status | 0.07 | ||
| Never-smoker, % | 43/64 (67%) | 59/72 (82%) | |
| Former smoker, % | 10/64 (16%) | 9/72 (12%) | |
| Current smoker, % | 11/64 (17%) | 4/72 (6%) | |
| Cancer, % | 41/72 (57%) | 27/89 (30%) | 0.001 |
Data are presented as median (25th percentile, 75th percentile) or as a proportion. APOE, Apolipoprotein E; MMSE, Mini-Mental State Examination.
Vascular risk factors and conditions including cerebrovascular disease (31% versus 42%; p = 0.20), hypertension (82% versus 71%; p = 0.15), heart failure (42% versus 33%; p = 0.31), and peripheral vascular disease (21% versus 19%; p = 0.94) did not differ in participants without dementia compared to those with dementia. Coronary artery disease was more frequent in participants without dementia compared to those with dementia (43% versus 26%; p = 0.03). Diabetes mellitus was less frequent in the no dementia group compared to the dementia group (3% versus 11%; p = 0.08). Never-smoker participants were less frequent in the no dementia group compared to the dementia group (67% versus 82%; p = 0.07).
As shown in Table 1 and Supplementary Table 4, cancer was more frequent in participants without dementia compared to those with dementia (57% versus 30%; p = 0.001). This observation persisted when a chi-square analysis was performed comparing participants without dementia to those with dementia, but further dichotomizing between no cancer history (43% versus 70%), skin cancer (29% versus 12%), and non-skin cancer (28% versus 18%) (p = 0.002). The percentage of participants with a history of cancer did not differ by dementia type (Supplementary Table 5). When stratifying the dementia group by smoking exposure, cancer was more frequent in smokers (9/13 [69%]) than non-smokers (15/59 [25%]; p = 0.007). Among participants without dementia, there was no association between smoking and cancer.
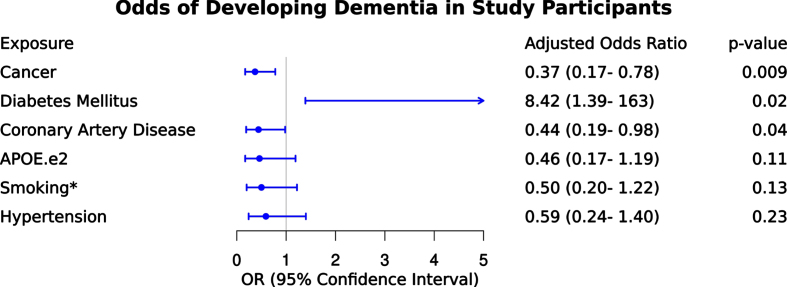
We next examined the odds of dementia for the following risk factors and conditions as illustrated in Fig. 2 – APOE ɛ2, coronary artery disease, hypertension, diabetes mellitus, smoking, cancer. After controlling for all variables in the model, APOE ɛ2, smoking, and hypertension did not remain significantly associated with dementia. After adjustment, cancer history remained associated with 63% lower odds of dementia (odds ratio [OR] = 0.37 [confidence interval (CI) = 0.17–0.78]; p = 0.009). Participants with diabetes mellitus remained significant and had a more than 8-fold higher odds of dementia compared to those without diabetes mellitus (OR = 8.42 [CI = 1.39–162]; p = 0.02). Participants with coronary artery disease also remained significant and had 56% lower odds of dementia compared to those without coronary artery disease (OR = 0.44 [CI = 0.19–0.98]; p = 0.04). We did not observe an independent association of dementia with APOE ɛ2, smoking, or hypertension.
Fig. 2.
Odds of dementia given exposure to clinical variables. Results of multivariable logistic regression depicting odds of dementia given exposure to clinical variables including cancer, diabetes mellitus, coronary artery disease, APOE ɛ2, smoking* (including former and current smoker categories as described in methods), and hypertension. The adjusted odds ratio is provided for each variable to contextualize independent association after controlling for other variables in the model.
Postmortem findings confirmed that participants without dementia had higher brain weights (1200 g [1120,1280]) than those with dementia (1115 g [1040,1215]) (p < 0.001) (Table 2). Braak staging was lower in the no dementia group (IV [II,IV]) compared to the dementia group (V [IV,V]; p < 0.001). Moderate-to-frequent neuritic plaques were observed less commonly in the no dementia group (50%) compared to those with dementia (79%; p < 0.001). Moderate-to-frequent diffuse plaques were also less commonly observed in the no dementia group (67%) compared with the dementia group (93%; p < 0.001). Lewy body pathology, including amygdala predominant Lewy bodies, was observed at similar proportions in those without dementia compared to participants with dementia. The presence of TDP-43 pathology in the amygdala was less frequently observed in the no dementia group (41%) compared to the dementia group (76%; p < 0.001). Cases without dementia were observed to have more frequent rare TDP-43 immunopositive neurites or glial pathology that lacked neuronal cytoplasmic inclusions (13%) compared to those with dementia (2%). Cerebrovascular disease including Kalaria score, Strozyk score Dickson score, and arteriolosclerosis severity did not differ between groups.
Table 2.
Neuropathologic findings stratified by dementia presence
| Variable | No Dementia n = 72 | Dementia n = 89 | p |
| Brain weight, g | 1200 (1120,1280) | 1115 (1040,1215) | <0.001 |
| Braak stage | IV (II,IV) | V (IV,V) | <0.001 |
| Neuritic plaque score | <0.001 | ||
| None, % | 25/72 (35%) | 6/89 (7%) | |
| Sparse, % | 11/72 (15%) | 13/89 (15%) | |
| Moderate, % | 21/72 (29%) | 37/89 (42%) | |
| Frequent, % | 15/72 (21%) | 33/89 (37%) | |
| Diffuse plaque score | <0.001 | ||
| None, % | 13/64 (20%) | 1/85 (1%) | |
| Sparse, % | 8/64 (13%) | 5/85 (6%) | |
| Moderate, % | 14/64 (22%) | 18/85 (21%) | |
| Frequent, % | 29/64 (45%) | 61/85 (72%) | |
| Lewy body pathology | 0.73 | ||
| None, % | 55/72 (76%) | 65/89 (73%) | |
| Lewy body disease, % | 12/72 (17%) | 19/89 (21%) | |
| Amygdala predominant Lewy bodies, % | 5/72 (7%) | 5/89 (6%) | |
| LATE-NC in amygdala | <0.001 | ||
| TDP-43 negative, % | 25/54 (46%) | 14/63 (22%) | |
| Rare, % | 7/54 (13%) | 1/63 (2%) | |
| TDP-43 positive, % | 22/54 (41%) | 48/63 (76%) | |
| Cerebrovascular disease scales | |||
| Kalaria score | 4 (3, 7) | 5 (3, 7) | 0.71 |
| Strozyk score | 2 (1, 2) | 2 (1, 3) | 0.51 |
| Dickson score | 1 (0, 2) | 1 (0, 2) | 0.69 |
| Arteriolosclerosis severity | 2 (2, 3) | 2 (2, 3) | 0.748 |
Data are presented as median (25th percentile, 75th percentile) or proportion. Kruskal-Wallis rank sum test for numerical and ordered variables, Pearson’s Chi-squared test for categorical variables. g, grams; TDP-43, TAR DNA-binding protein of 43 kDa; LATE-NC, Limbic predominant age-related TDP-43 neuropathologic change in amygdala.
Neuropathologic variables were further modeled to examine their contribution to the odds of a dementia diagnosis. As expected, Braak stage had a more than 3-fold higher odds of dementia (OR = 3.07 [CI = 1.80–5.86]; p < 0.001) or an amnestic dementia diagnosis (OR = 3.52 [CI = 1.90,7.66]; p < 0.001) compared to participants without dementia (Supplementary Figures 1 and 2, respectively). LATE-NC positivity had a more than 2-fold higher odds of dementia that approached significance (OR = 2.50 [CI = 0.94–6.73]; p = 0.065) but did not significantly increase odds of amnestic dementia. Neuritic plaque score, Lewy body disease, or Dickson Gestalt score were not found associated with higher odds of dementia or amnestic dementia. Of the cases with available AD neuropathologic subtyping, there were no (0%) hippocampal sparing AD, 10/18 (56%) typical AD, and 8/18 (44%) limbic predominant AD.
Postmortem findings were stratified according to the presence or absence of cancer, diabetes mellitus, and coronary artery disease (Table 3). Brain weight was lower in participants without a history of cancer (1120 [1040,1200]) compared to those with cancer (1210 [1100,1300]; p = 0.002). Braak stage was also higher in participants without a history of cancer (IV [IV,V] versus IV [III,V]; p = 0.01), as suggested by the interquartile range. The severity of amyloid-β plaques, Lewy body pathology, LATE-NC, and arteriolosclerosis did not differ regarding cancer history; however, cerebrovascular disease was higher in those without history of cancer using the Dickson scoring method (1 point [0,3] versus 0 [0,2]; p = 0.009). Study participants stratified by history of diabetes mellitus did not differ in brain weight, Braak stage, amyloid-β plaque severity, Lewy body pathology, LATE-NC, or arteriolosclerosis severity. Cerebrovascular disease measured using Dickson scoring method was lower in participants without diabetes mellitus compared to those with a history (1 point [0,2] versus 2 [1,3]; p = 0.05). Study participants stratified by history of coronary artery disease did not differ in brain weight, Braak stage, Lewy body pathology, LATE-NC, cerebrovascular disease, or arteriolosclerosis severity. Moderate-to-frequent neuritic plaques (70% versus 60%; p = 0.03) and moderate-to-frequent diffuse plaques (86 versus 75%; p = 0.008) were lower in participants with coronary artery disease.
Table 3.
Neuropathologic findings stratified according to history of cancer, diabetes mellitus and coronary artery disease
| Variable | No Cancer n = 93 | Cancer n = 68 | p | No Diabetes Mellitus n = 149 | Diabetes mellitus n = 12 | p | No Coronary Disease Arteryn = 107 | Coronary Artery Diseasen = 54 | p |
| Brain weight, g | 1120 (1040,1200) | 1210 (1100,1300) | 0.002 | 1140 (1060,1260) | 1174 (1021,1260) | 0.88 | 1140 (1052,1278) | 1140 (1100,1235) | 0.70 |
| Braak stage | IV (IV,V) | IV (III,V) | 0.01 | IV (III,V) | V (IV,V) | 0.11 | IV (III,V) | IV (III,V) | 0.22 |
| Neuritic plaques | 0.80 | 0.81 | 0.03 | ||||||
| None,% | 16 (17%) | 15 (22%) | 29 (19%) | 2 (17%) | 17 (16%) | 14 (26%) | |||
| Sparse,% | 17 (18%) | 7 (10%) | 22 (15%) | 2 (17%) | 16 (15%) | 8 (15%) | |||
| Moderate,% | 31 (33%) | 27 (40%) | 54 (36%) | 4 (33%) | 36 (34%) | 22 (41%) | |||
| Frequent,% | 29 (31%) | 19 (28%) | 44 (30%) | 4 (33%) | 38 (35%) | 10 (18%) | |||
| Diffuse plaques | 0.25 | 0.24 | 0.01 | ||||||
| None,% | 9 (10%) | 5 (8.3%) | 14 (10%) | 0 (0.0%) | 5 (5%) | 9 (17%) | |||
| Sparse,% | 4 (4.5%) | 9 (15.0%) | 12 (9.0%) | 1 (8.0%) | 9 (9%) | 4 (8%) | |||
| Moderate,% | 19 (21%) | 13 (22%) | 30 (22%) | 2 (17%) | 17 (18%) | 15 (29%) | |||
| Frequent,% | 57 (64%) | 33 (55%) | 81 (59%) | 9 (75%) | 66 (68%) | 24 (46%) | |||
| Lewy body | 0.50 | 0.21 | 0.57 | ||||||
| None,% | 71 (76%) | 49 (72%) | 111 (74%) | 9 (75%) | 78 (73%) | 42 (78%) | |||
| LBD,% | 18 (19%) | 13 (19%) | 30 (20%) | 1 (8%) | 23 (21%) | 8 (15%) | |||
| ALB,% | 4 (4.3%) | 6 (8.8%) | 8 (5%) | 2 (17%) | 6 (6%) | 4 (7%) | |||
| LATE-NC | 0.10 | >0.99 | 0.08 | ||||||
| Negative,% | 20 (29%) | 19 (40%) | 36 (33%) | 3 (38%) | 24 (30%) | 15 (41%) | |||
| Rare,% | 3 (4%) | 5 (10%) | 8 (7.0%) | 0 (0.0%) | 3 (4%) | 5 (14%) | |||
| Positive,% | 46 (67%) | 24 (50%) | 65 (60%) | 5 (62%) | 53 (66%) | 17 (46%) | |||
| Cerebrovascular | |||||||||
| Kalaria score | 5 (3, 7) | 4 (3, 6) | 0.11 | 5 (3, 7) | 6 (3, 8) | 0.26 | 4 (3, 7) | 5 (3, 7) | 0.31 |
| Strozyk score | 2 (1, 3) | 2 (1, 2) | 0.08 | 2 (1, 3) | 3 (2, 3) | 0.12 | 2 (1, 3) | 2 (1, 3) | 0.16 |
| Dickson score | 1 (0, 3) | 0 (0, 2) | 0.009 | 1 (0, 2) | 2 (1, 3) | 0.05 | 1 (0, 2) | 1 (0, 2) | 0.05 |
| Arteriolosclerosis | 2 (2, 3) | 2 (2, 3) | 0.685 | 2 (2, 3) | 2 (2, 3) | 0.376 | 2 (2, 3) | 2 (2, 3) | 0.427 |
Data are presented as median (25th percentile, 75th percentile) using Kruskal-Wallis rank sum test or proportion using Pearson’s Chi-squared test. g, grams; LBD, Lewy body disease; ALB, Amygdala predominant Lewy bodies; LATE-NC, Limbic predominant age-related TDP-43 neuropathologic change in amygdala.
DISCUSSION
Cancer, coronary artery disease, and diabetes mellitus were associated with odds of dementia in this 95+ series, while APOE ɛ4 allele and other vascular risk factors or comorbidities known to negatively affect cognition [16] were not associated with dementia. History of cancer or coronary artery disease was associated with lower odds of dementia, whereas the presence of diabetes mellitus was associated with 8-fold higher odds of dementia. Cancer was more frequent in participants without dementia, supporting the findings of a recent neuropathology cohort study, where participants with cancer had lower prevalence of AD neuropathology burden [35]. In our study, this association was independent of cancer type (non-melanoma skin cancer versus others) replicating previously reported findings [36, 37]. The association between cancer and dementia persisted after controlling for vascular risk factors and APOE ɛ2.
Underlying causes for the inverse association between cancer and dementia are not fully understood. Potential mechanisms include shared genes, dysregulation of common biological pathway systems involved in growth, cell maintenance and DNA repair (Supplementary Table 6), metabolic, mitochondrial, or immune dysfunction, and aging effects [38, 39]. Genetic correlations from GWAS studies between the most common form of dementia and cancer suggest gene expression regulators may have a shared a role in the genetic cause of these diseases [40]. The effects of aging on the immune system, innate immune activation, as well as neuroinflammation were also implicated in neurodegeneration [41, 42]. Interestingly, upregulation of immune response genes in deceased oldest-old brains of cognitively normal compared to those with dementia was reported [43], suggesting that an enhanced immune response may also contribute to cancer survival in these patients.
Coronary artery disease was associated with 56% reduced odds of dementia in this 95+ series, as well as with a lower burden of neuritic and diffuse plaques. Our findings differ from previous studies that reported an association between coronary artery disease and AD neuropathology [44]. Interestingly the reported positive association by Beeri and colleagues was diminished within the cases lacking an APOE ɛ4 allele [44]. This may partly explain our finding, as our 95+ oldest-old had a much lower frequency of APOE ɛ4 carriers (27% in with dementia group) than the 49% identified in a meta-analysis of amnestic dementia patients [44, 45]. Other possible explanations may include that coronary artery disease may behave as an “inactive” risk factor, where increased clinical surveillance and treatment efficacy (secondary prevention) decreases coronary and extracoronary atherosclerosis, decreasing dementia risk. We also acknowledge that other unmeasured factors such as resilience to vascular risks or protective factors [46] in this 95+ series are also possible. Although hypertension is a major risk factor for cognitive impairment and dementia [3], we surprisingly did not observe an association with dementia in this oldest-old series. Hypertension was previously shown to associate with cerebrovascular disease and executive dysfunction [47]; however, differences in contribution based upon mid-life and late-life onset of hypertension may alter risk. As the most common vascular comorbidity in the 95+ series, it is possible that we are observing survival bias in these highly resilient individuals or observing those who survived into late-life with well-controlled and treated hypertension possibly reducing brain-negative side-effects.
Diabetes mellitus, another major vascular risk factor [47], was associated with 8-fold higher odds of dementia and higher cerebrovascular disease using Dickson scoring method. These results support neuroimaging studies demonstrating an association of diabetes mellitus with cognitive impairment and subcortical infarctions [47] and a lack of association with amyloid-β and tau biomarkers [48]; a finding endorsed by neuropathology studies associating diabetes with vascular disease but not AD pathology [49, 50]. Our findings may emphasize the role of diabetes mellitus as an “active“ risk factor, especially when blood sugar regulation is suboptimal, and metabolic factors contributing to cerebrovascular disease or other AD neuropathology-independent factors increasing dementia risk or decreasing resilience [48, 51]. Independent of cause, diabetes mellitus remains a modifiable risk factor that, if adequately treated, may reduce dementia risk in the oldest-old.
APOE ɛ4, a known genetic risk factor [52], was not associated with dementia in this 95+ series suggesting a less substantial genetic influence, earlier age of death in APOE ɛ4 carriers with cognitive impairment [53], or other possible protective epigenetic factors lowering the risk of dementia. Our findings support the notion of APOE as a timing gene, as APOE ɛ4 is not thought to affect risk beyond the age of 85 [54]. Possession of the neuroprotective allele, APOE ɛ2 [18], was more frequent in the no dementia group, consistent with the 90+ Study findings [55]. Interestingly, smoking history, an important risk factor for cancer [56], was associated with cancer in participants with dementia, but not in those without dementia. Although smoking may increase the risk of cancer enabling proliferative pathways, the association does not hold in the no dementia group. This finding suggests that other potential tumor factors may contribute to cancer in participants without dementia or possible resiliency factors decrease the risk in those without dementia.
Neuropathologic findings in our study support previously reported lower brain weight, higher Braak stage, and higher frequency of neuritic and diffuse plaques observed in deceased participants with dementia [57–60]. We found a higher frequency of AD neuropathologic changes and LATE-NC (i.e., TDP-43 associated with aging) in the dementia group, even though, a high frequency was also observed in the no dementia group. Interestingly, the no dementia group was observed to have rare TDP-43 positive neurites that were insufficient to judge them as TDP-43 positive in the amygdala [28]. Although we cannot exclude the possibility of neuronal cytoplasmic inclusions in sections not reviewed by immunohistochemistry, neuropathologists evaluating TDP-43 pathology may consider noting rare immunopositive lesions [28, 57, 60, 61]. Several hypotheses emerge from these findings: 1) a passive role may exist for the abnormal accumulation of these proteins in normal aging, 2) we are capturing these participants in early stages of neurodegeneration, or 3) we are observing the influence of dementia resilience factors on neuropathology in the 95+ group without dementia. Interestingly, we found a lower Braak stage in participants with history of cancer and a lower burden of neuritic and diffuse plaques in participants with coronary artery disease. Even though these findings support the lower odds for neurodegeneration seen with consensus clinical dementia diagnoses in participants with cancer and coronary artery disease, the differences in neuropathology are difficult to explain and may warrant further research. Despite the robust increase in dementia odds associated with diabetes mellitus, neuropathologic findings did not differ apart from Dickson score for cerebrovascular disease pathology. Possible explanations include lower resilience towards neuropathology and dementia, or different metabolic or vascular expressions of systemic vs. cerebral disease mechanisms.
Several limitations should be acknowledged, including the inherent bias of autopsy studies that rely upon willingness of study participants to self-select to consent to autopsy [62]. Moreover, educational attainment, marital status, and social determinants of health may affect who elects to enroll in autopsy programs and thus may skew generalizability [62–64]. The mechanisms involved in the inverse association between cancer and lower risk of neurodegeneration are not well understood. It was postulated that cancer patients or survivors may have a shorter lifespan decreasing their risk of dementia (survival bias/competing risk), or that patients with dementia or cancer may not be appropriately screened for cancer or dementia respectively, compared to patients without these conditions (surveillance bias). In our study, cancer survivors lived to age 95+, therefore, their risk of dementia would not be expected to be lower compared to non-cancer patients. It is also unlikely that cancer presence was not detected in this series prior to their death at 95+ or at autopsy. A long-term prospective study [36] found a lower risk of incident amnestic dementia in cancer survivors, and a more recent study [65] explored the risk of dementia prior to, and following a cancer diagnosis, finding that dementia risk was lower in cancer patients, even prior to their cancer diagnosis. Such studies, suggest that bias is unlikely driving this inverse association. We acknowledge that survival bias is possible in our study, as this is a highly resilient elder cohort. Moreover, the focused nature of the 95+ series likely limits generalizability of results to younger populations. This limitation is counterbalanced by the optimized expression of risk and resilience factors in individuals who experienced a 95+ year lifespan. The oldest-old are unique, as participants are assumed to express sufficient resilience factors to endure genetic, environmental, behavioral, and illness-related risks allowing them to exceed their life expectancy at birth (i.e., 47.3 years) [66] by 100%, as well as current life expectancy (i.e., 78.7 years) [67] by at least 20%. Although we did not observe sex differences between dementia and no dementia groups, it is worth noting more than 75% of the overall 95+ autopsy series were women. Our data supports observations from the 90+ population study where 78% of participants were women [58]. We also acknowledge the possibility that more women may have participated in brain donation studies.
Inclusion of oldest-old brain donor participants provides a unique opportunity to examine the effects of aging, vascular risk factors and conditions, as well as cancer on neurodegeneration. We recognize possible surveillance bias, as patients with dementia may not be screened for cancer (even though active cancer was not documented on autopsy reports), or cancer patients may not be screened for dementia as much as the general population. We appreciate the importance different cancer types or their respective treatment effects may have on the risk of dementia, yet limitations in the data available did not allow for expanded analyses. The evolving literature in this area should encourage further studies to better address these interactions, which may lead to potential treatment strategies. We recognize the small sample size in some of our analyses, warranting caution in their interpretation due to low statistical power; including the presence of cancer in different dementia types, the high odds of dementia given diabetes, and the neuropathologic findings stratified according to diabetes. We also recognize the exploratory nature of our study with multiple analysis and acknowledge that trend statistical significance may therefore not be significant. Future studies should consider age of onset to examine mid-life and late-life contribution of these risk factors and comorbidities toward optimizing personalized medicine. The present study further extends observations that older age strongly influences a limbic predominant distribution of neurofibrillary tangle pathology. Although only a subset of AD cases was examined, the limbic predominant AD subtype was observed in 44% of the 95+ AD series compared to only 13% (age range 50–102) or 14% (age range 37–103) previously reported in individuals spanning a much younger age range [24, 68].
Conclusion
In this prospectively followed series of participants who died≥95 years-old and underwent autopsy, cancer, and coronary artery disease were associated with lower odds of dementia and lower burden of tangle and plaque pathology. Diabetes mellitus remained an important modifiable risk factor associated with dementia odds and a cumulative neuropathologic measure of cerebrovascular disease, despite the absence of neurodegenerative differences in the oldest-old. Genetic and disease-related mechanisms associated with cancer (proliferative pathways) and survival (resilience factors) may protect against neurodegeneration, warranting further study. Improving our understanding of these risk-resilience mechanisms may lead to novel strategies in the prevention and treatment of cancer, vascular disease, and neurodegeneration.
Supplementary Material
ACKNOWLEDGMENTS
We thank the patients and their families for their generous brain donations to help further our knowledge of Alzheimer’s disease.
Dr. Murray had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The investigators are supported by grants from the Alzheimer’s Association (AARG-17-533458); National Institute on Aging (R01-AG054449, R01-AG075802, U01-AG57195, P30-AG062677, U01-AG006786, R01-AG034676, U19-AG069701, RF1-AG069052, R01-AG037491, K23-AG064029, UF1-NS125417, AG061796, U01-AG046139, P01-AG003949); National Institute of Neurological Disorders and Stroke (R01-NS89757, P50-NS072187); National Institute on Deafness and Other Communication Disorders (R01-DC014942); the Florida Department of Health, and the Ed and Ethel Moore Alzheimer’s Disease Research Program (8AZ06, 20a22); David Eisenberg Professorship, Mayo Foundation; and a kind gift from David and Frances Strawn.
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/22-0440r1).
SUPPLEMENTARY MATERIAL
The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JAD-220440.
REFERENCES
- [1]. Bureau USC (2017), https://www.census.gove/data/tables/2017/demo/popproj/2017-summary-tables.html. [Google Scholar]
- [2]. Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP (2013) The global prevalence of dementia: A systematicreview and metaanalysis. Alzheimers Dement 9, 63–75 e62. [DOI] [PubMed] [Google Scholar]
- [3]. Wiesmann M, Kiliaan AJ, Claassen JA (2013) Vascular aspects of cognitive impairment and dementia. J Cereb Blood Flow Metab 33, 1696–1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4]. Fowkes R, Byrne M, Sinclair H, Tang E, Kunadian V (2016) Coronary artery disease in patients with dementia. Coron Artery Dis 27, 511–520. [DOI] [PubMed] [Google Scholar]
- [5]. Institute NC, Age and Cancer Risk,National Cancer Institute at the National Institutes of Health, https://www.cancer.gov/about-cancer/causes-prevention/risk/age, Accessed 05/12/2021.
- [6]. Corsellis JAN (1962), Mental Illness and the Ageing Brain. Oxford University Press, London. [Google Scholar]
- [7]. Shi HB, Tang B, Liu YW, Wang XF, Chen GJ (2015) Alzheimer disease and cancer risk: A meta-analysis. J Cancer Res Clin Oncol 141, 485–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8]. Catala-Lopez F, Suarez-Pinilla M, Suarez-Pinilla P, Valderas JM, Gomez-Beneyto M, Martinez S, Balanza-Martinez V, Climent J, Valencia A, McGrath J, Crespo-Facorro B, Sanchez-Moreno J, Vieta E, Tabares-Seisdedos R (2014) Inverseand direct cancer comorbidity in people with central nervous system disorders: A meta-analysis of cancerincidence in 577,013 participants of 50 observational studies.. Psychother Psychosom 83, 89–105. [DOI] [PubMed] [Google Scholar]
- [9]. Ganguli M (2015) Cancer and dementia: It’s complicated. Alzheimer Dis Assoc Disord 29, 177–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10]. Roe CM, Fitzpatrick AL, Xiong C, Sieh W, Kuller L, Miller JP, Williams MM, Kopan R, Behrens MI, Morris JC (2010) Cancer linked to Alzheimer disease but not vascular dementia. Neurology 74, 106–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11]. Musicco M, Adorni F, Di Santo S, Prinelli F, Pettenati C, Caltagirone C, Palmer K, Russo A (2013) Inverse occurrence of cancer and Alzheimer disease: A population-based incidence study. Neurology 81, 322–328. [DOI] [PubMed] [Google Scholar]
- [12]. Bowles EJA, Walker RL, Anderson ML, Dublin S, Crane PK, Larson EB (2017) Risk of Alzheimer’s disease or dementia following a cancer diagnosis. PLoS One 12, e0179857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13]. Ospina-Romero M, Abdiwahab E, Kobayashi L, Filshtein T, Brenowitz WD, Mayeda ER, Glymour MM (2019) Rate of memory change before and after cancer diagnosis. JAMA Netw Open 2, e196160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14]. Hayes-Larson E, Shaw C, Ackley SF, Zimmerman SC, Glymour MM, Graff RE, Witte JS, Kobayashi L, Mayeda ER (2022) The role of dementia diagnostic delay in the inverse cancer-dementia association. J Gerontol A Biol Sci Med Sci 77, 1254–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15]. Newman AB, Fitzpatrick AL, Lopez O, Jackson S, Lyketsos C, Jagust W, Ives D, Dekosky ST, Kuller LH (2005) Dementia and Alzheimer’s disease incidence in relationship to cardiovascular disease in the Cardiovascular Health Study cohort. J Am Geriatr Soc 53, 1101–1107. [DOI] [PubMed] [Google Scholar]
- [16]. Viswanathan A, Rocca WA, Tzourio C (2009) Vascular risk factors and dementia: How to move forward? Neurology 72, 368–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17]. Roberts RO, Geda YE, Knopman DS, Cha RH, Pankratz VS, Boeve BF, Ivnik RJ, Tangalos EG, Petersen RC, Rocca WA (2008) The Mayo Clinic Study of Aging: Design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology 30, 58–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18]. Corder EH, Saunders AM, Risch NJ, Strittmatter WJ, Schmechel DE, Gaskell PC Jr., Rimmler JB, Locke PA, Conneally PM, Schmader KE, et al. (1994) Protective effect of apolipoprotein E type 2 allele for late onset Alzheimer disease. Nat Genet 7, 180–184. [DOI] [PubMed] [Google Scholar]
- [19]. Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA (1993) Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science 261, 921–923. [DOI] [PubMed] [Google Scholar]
- [20]. Carbone A (2020) Cancer classification at the crossroads. Cancers (Basel) 12, 980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21]. Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, Vogel FS, Hughes JP, van Belle G, Berg L (1991) The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology 41, 479–486. [DOI] [PubMed] [Google Scholar]
- [22]. Montine TJ, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Dickson DW, Duyckaerts C, Frosch MP, Masliah E, Mirra SS, Nelson PT, Schneider JA, Thal DR, Trojanowski JQ, Vinters HV, Hyman BT, National Institute on Aging; Alzheimer’s Association (2012) National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: A practical approach. Acta Neuropathol 123, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23]. Braak H, Braak E (1991) Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 82, 239–259. [DOI] [PubMed] [Google Scholar]
- [24]. Murray ME, Graff-Radford NR, Ross OA, Petersen RC, Duara R, Dickson DW (2011) Neuropathologically defined subtypes of Alzheimer’s disease with distinct clinical characteristics: A retrospective study. Lancet Neurol 10, 785–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25]. McKeith IG, Boeve BF, Dickson DW, Halliday G, Taylor JP, Weintraub D, Aarsland D, Galvin J, Attems J, Ballard CG, Bayston A, Beach TG, Blanc F, Bohnen N, Bonanni L, Bras J, Brundin P, Burn D, Chen-Plotkin A, Duda JE, El-Agnaf O, Feldman H, Ferman TJ, Ffytche D, Fujishiro H, Galasko D, Goldman JG, Gomperts SN, Graff-Radford NR, Honig LS, Iranzo A, Kantarci K, Kaufer D, Kukull W, Lee VMY, Leverenz JB, Lewis S, Lippa C, Lunde A, Masellis M, Masliah E, McLean P, Mollenhauer B, Montine TJ, Moreno E, Mori E, Murray M, O’Brien JT, Orimo S, Postuma RB, Ramaswamy S, Ross OA, Salmon DP, Singleton A, Taylor A, Thomas A, Tiraboschi P, Toledo JB, Trojanowski JQ, Tsuang D, Walker Z, Yamada M, Kosaka K (2017) Diagnosis and management of dementia with Lewy bodies: Fourth consensus report of the DLB Consortium. Neurology 89, 88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26]. Zhang YJ, Xu YF, Cook C, Gendron TF, Roettges P, Link CD, Lin WL, Tong J, Castanedes-Casey M, Ash P, Gass J, Rangachari V, Buratti E, Baralle F, Golde TE, Dickson DW, Petrucelli L (2009) Aberrant cleavage of TDP-43 enhances aggregation and cellular toxicity. Proc Natl Acad Sci U S A 106, 7607–7612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27]. Lin WL, Castanedes-Casey M, Dickson DW (2009) Transactivation response DNA-binding protein 43 microvasculopathy in frontotemporal degeneration and familial Lewy body disease. J Neuropathol Exp Neurol 68, 1167–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28]. Josephs KA, Murray ME, Tosakulwong N, Weigand SD, Knopman DS, Petersen RC, Jack CR Jr., Whitwell JL, Dickson DW (2019) Brain atrophy in primary age-related tauopathy is linked to transactive response DNA-binding protein of 43 kDa. Alzheimers Dement 15, 799–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29]. Nelson PT, Dickson DW, Trojanowski JQ, Jack CR, Boyle PA, Arfanakis K, Rademakers R, Alafuzoff I, Attems J, Brayne C, Coyle-Gilchrist ITS, Chui HC, Fardo DW, Flanagan ME, Halliday G, Hokkanen SRK, Hunter S, Jicha GA, Katsumata Y, Kawas CH, Keene CD, Kovacs GG, Kukull WA, Levey AI, Makkinejad N, Montine TJ, Murayama S, Murray ME, Nag S, Rissman RA, Seeley WW, Sperling RA, White III CL, Yu L, Schneider JA (2019) Limbic-predominant age-related TDP-43 encephalopathy (LATE): Consensus working group report. Brain 142, 1503–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30]. Craggs LJ, Hagel C, Kuhlenbaeumer G, Borjesson-Hanson A, Andersen O, Viitanen M, Kalimo H, McLean CA, Slade JY, Hall RA, Oakley AE, Yamamoto Y, Deramecourt V, Kalaria RN (2013) Quantitative vascular pathology and phenotyping familial and sporadic cerebral small vessel diseases. Brain Pathol 23, 547–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31]. Deramecourt V, Slade JY, Oakley AE, Perry RH, Ince PG, Maurage CA, Kalaria RN (2012) Staging and natural history of cerebrovascular pathology in dementia. Neurology 78, 1043–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32]. Yamazaki Y, Shinohara M, Shinohara M, Yamazaki A, Murray ME, Liesinger AM, Heckman MG, Lesser ER, Parisi JE, Petersen RC, Dickson DW, Kanekiyo T, Bu G (2019) Selective loss of cortical endothelial tight junction proteins during Alzheimer’s disease progression. Brain 142, 1077–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33]. Nguyen AT, Kouri N, Labuzan SA, Przybelski SA, Lesnick TG, Raghavan S, Reid RI, Reichard RR, Knopman DS, Petersen RC, Jack CR Jr, Mielke MM, Dickson DW, Graff-Radford J, Murray ME, Vemuri P (2022) Neuropathologic scales of cerebrovascular disease associated with diffusion changes on MRI. Acta Neuropathol, doi: 10.1007/s00401-022-02465-w. [DOI] [PMC free article] [PubMed]
- [34]. Strozyk D, Dickson DW, Lipton RB, Katz M, Derby CA, Lee S, Wang C, Verghese J (2010) Contribution of vascular pathology to the clinical expression of dementia. Neurobiol Aging 31, 1710–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35]. Karanth SD, Katsumata Y, Nelson PT, Fardo DW, McDowell JK, Schmitt FA, Kryscio RJ, Browning SR, Braithwaite D, Arnold SM, Abner EL (2022) Cancer diagnosis is associated with a lower burden of dementia and less Alzheimer’s-type neuropathology. Brain 145, 2518–2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36]. Driver JA, Beiser A, Au R, Kreger BE, Splansky GL, Kurth T, Kiel DP, Lu KP, Seshadri S, Wolf PA (2012) Inverse association between cancer and Alzheimer’s disease: Results from the Framingham Heart Study. BMJ 344, e1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37]. White RS, Lipton RB, Hall CB, Steinerman JR (2013) Nonmelanoma skin cancer is associated with reduced Alzheimer disease risk. Neurology 80, 1966–1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38]. Snyder HM, Ahles T, Calderwood S, Carrillo MC, Chen H, Chang CH, Craft S, De Jager P, Driver JA, Fillit H, Knopman D, Lotze M, Tierney MC, Petanceska S, Saykin A, Seshadri S, Shineman D, Ganguli M (2017) Exploring the nexus of Alzheimer’s disease and related dementias with cancer and cancer therapies: A convening of the Alzheimer’s Association & Alzheimer’s Drug Discovery Foundation. Alzheimers Dement 13, 267–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39]. Driver JA (2014) Inverse association between cancer and neurodegenerative disease: Review of the epidemiologic and biological evidence. Biogerontology 15, 547–557. [DOI] [PubMed] [Google Scholar]
- [40]. Feng YA, Cho K, Lindstrom S, Kraft P, Cormack J, IGAP Consortium, Colorectal Transdisciplinary Study (CORECT); Discovery, Biology, and Risk of InheritedVariants in Breast Cancer (DRIVE); Elucidating Loci Involved in Prostate Cancer Susceptibility (ELLIPSE); Transdisciplinary Research in Cancer of the Lung (TRICL), Liang L, Driver JA (2017) Investigating the genetic relationship between Alzheimer’s disease and cancer using GWAS summary statistics. Hum Genet 136, 1341–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41]. Heneka MT, Kummer MP, Latz E (2014) Innate immune activation in neurodegenerative disease. Nat Rev Immunol 14, 463–477. [DOI] [PubMed] [Google Scholar]
- [42]. Singhal G, Jaehne EJ, Corrigan F, Toben C, Baune BT (2014) Inflammasomes in neuroinflammation and changes in brain function: a focused review. Front Neurosci 8, 315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43]. Katsel P, Tan W, Haroutunian V (2009) Gain in brain immunity in the oldest-old differentiates cognitively normal from demented individuals. PLoS One 4, e7642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44]. Beeri MS, Rapp M, Silverman JM, Schmeidler J, Grossman HT, Fallon JT, Purohit DP, Perl DP, Siddiqui A, Lesser G, Rosendorff C, Haroutunian V (2006) Coronary artery disease is associated with Alzheimer disease neuropathology in APOE4 carriers. Neurology 66, 1399–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45]. Ward A, Crean S, Mercaldi CJ, Collins JM, Boyd D, Cook MN, Arrighi HM (2012) Prevalence of apolipoprotein E4 genotype and homozygotes (APOE e4/4) among patients diagnosed with Alzheimer’s disease: A systematic review and meta-analysis. Neuroepidemiology 38, 1–17. [DOI] [PubMed] [Google Scholar]
- [46]. Silverman JM, Schmeidler J (2018) The protected survivor model: Using resistant successful cognitive aging to identify protection in the very old. Med Hypotheses 110, 9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47]. Roberts RO, Knopman DS, Przybelski SA, Mielke MM, Kantarci K, Preboske GM, Senjem ML, Pankratz VS, Geda YE, Boeve BF, Ivnik RJ, Rocca WA, Petersen RC, Jack CR Jr., (2014) Association of type 2 diabetes with brain atrophy and cognitive impairment. Neurology 82, 1132–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48]. Frison E, Proust-Lima C, Mangin JF, Habert MO, Bombois S, Ousset PJ, Pasquier F, Hanon O, Paquet C, Gabelle A, Ceccaldi M, Annweiler C, Krolak-Salmon P, Béjot Y, Belin C, Wallon D, Sauvee M, Beaufils E, Bourdel-Marchasson I, Jalenques I, Chupin M, Chêne G, Dufouil C ; MEMENTO Cohort Study Group (2021) Diabetesmellitus and cognition: Pathway analysis in the MEMENTO Cohort. Neurology 97, e836–e848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49]. Abner EL, Nelson PT, Kryscio RJ, Schmitt FA, Fardo DW, Woltjer RL, Cairns NJ, Yu L, Dodge HH, Xiong C, Masaki K, Tyas SL, Bennett DA, Schneider JA, Arvanitakis Z (2016) Diabetes is associated with cerebrovascular but not Alzheimer’s disease neuropathology. Alzheimers Dement 12, 882–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50]. Pruzin JJ, Nelson PT, Abner EL, Arvanitakis Z (2018) Review: Relationship of type 2 diabetes to human brain pathology. Neuropathol Appl Neurobiol 44, 347–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51]. Biessels GJ, Despa F (2018) Cognitive decline and dementia in diabetes mellitus: Mechanisms and clinical implications. Nat Rev Endocrinol 14, 591–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52]. Strittmatter WJ, Saunders AM, Schmechel D, Pericak-Vance M, Enghild J, Salvesen GS, Roses AD (1993) Apolipoprotein E: High-aviditybinding to beta-amyloid and increased frequency of type 4 allele inlate-onset familial Alzheimer disease. Proc Natl Acad Sci U SA 90, 1977–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53]. Robinson AC, Davidson YS, Roncaroli F, Minshull J, Tinkler P, Horan MA, Payton A, Pendleton N, Mann DMA (2020) Influence of APOE genotype on mortality and cognitive impairment. J Alzheimers Dis Rep 4, 281–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54]. Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, Myers RH, Pericak-Vance MA, Risch N, van Duijn CM (1997) Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA 278, 1349–1356. [PubMed] [Google Scholar]
- [55]. Berlau DJ, Corrada MM, Head E, Kawas CH (2009) APOE epsilon2 is associated with intact cognition but increasedAlzheimer pathology in the oldest old. Neurology 72, 829–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56]. Sasco AJ, Secretan MB, Straif K (2004) Tobacco smoking and cancer: A brief review of recent epidemiological evidence.S. Lung Cancer 45(Suppl 2), 3–9. [DOI] [PubMed] [Google Scholar]
- [57]. Tanprasertsuk J, Johnson EJ, Johnson MA, Poon LW, Nelson PT, Davey A, Martin P, Barbey AK, Barger K, Wang XD, Scott TM (2019) Clinico-neuropathological findings in the oldest old from the Georgia Centenarian Study. J Alzheimers Dis 70, 35–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58]. Corrada MM, Berlau DJ, Kawas CH (2012) A population-based clinicopathological study in the oldest-old: The 90+ study. Curr Alzheimer Res 9, 709–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59]. Gold G, Bouras C, Kövari E, Canuto A, Glaría BG, Malky A, Hof PR, Michel JP, Giannakopoulos P (2000) Clinical validity of Braak neuropathological staging in the oldest-old.579-582; discussion. Acta Neuropathol 99, 583–574. [DOI] [PubMed] [Google Scholar]
- [60]. Sajjadi SA, Corrada M, Phelan M, Kawas C (2020) TDP-43 is as important as Alzheimer’s disease neuropathology in the oldest old. Neurology 94, 1407. [Google Scholar]
- [61]. Wilson RS, Yu L, Trojanowski JQ, Chen E-Y, Boyle PA, Bennett DA, Schneider JA (2013) TDP-43 pathology, cognitive decline, and dementia in old age. JAMA Neurol 70, 1418–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62]. Tsuang D, Simpson KL, Li G, Barnhart RL, Edland SD, Bowen J, McCormick W, Teri L, Nochlin D, Larson EB, Thompson ML, Leverenz JB (2005) Evaluation of selection bias in an incident-based dementia autopsy case series. Alzheimer Dis Assoc Disord 19, 67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63]. Ighodaro ET, Nelson PT, Kukull WA, Schmitt FA, Abner EL, Caban-Holt A, Bardach SH, Hord DC, Glover CM, Jicha GA, Van Eldik LJ, Byrd AX, Fernander A (2017) Challenges and considerations related to studying dementia in Blacks/African Americans. J Alzheimers Dis 60, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64]. Barnes LL, Shah RC, Aggarwal NT, Bennett DA, Schneider JA (2012) The Minority Aging Research Study: Ongoing efforts to obtain brain donation in African Americans without dementia. Curr Alzheimer Res 9, 734–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65]. Sun M, Wang Y, Sundquist J, Sundquist K, Ji J (2020) The association between cancer and dementia: A national cohort study in Sweden. Front Oncol 10, 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66]. Centers for Disease Control and Prevention, Life expectancy at birth, at 65 years of age, and at 75 years of age, by race and sex: United States, selected years 1900–2007, https://www.cdc.gov/nchs/data/hus/2010/022.pdf, Accessed May 12, 2021.
- [67]. Centers for Disease Control and Prevention, Life Expectancy 2018, https://www.cdc.gov/nchs/fastats/life-expectancy.htm.
- [68]. Hanna Al-Shaikh FS, Duara R, Crook JE, Lesser ER, Schaeverbeke J, Hinkle KM, Ross OA, Ertekin-Taner N, Pedraza O, Dickson DW, Graff-Radford NR, Murray ME (2020) Selective vulnerability of the nucleus basalis of meynert among neuropathologic subtypes of Alzheimer disease. JAMA Neurol 77, 225–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.