Abstract
Macitentan is an oral endothelin receptor antagonist for the management of pulmonary arterial hypertension (PAH). The OPsumit® USers Registry (OPUS) and the OPsumit® Historical USers cohort (OrPHeUS) medical chart review provide real‐world data for patients newly initiating macitentan. This study aims to describe the characteristics, safety profile, and clinical outcomes of PAH patients newly treated with macitentan in the combined OPUS/OrPHeUS data set. OPUS was a prospective, multicenter, long‐term, observational drug registry from April 2014 to June 2020. OrPHeUS was a retrospective, US, multicenter chart review: observation period October 2013 to March 2017. All analyses were descriptive. At registry closure in June 2020, the combined population consisted of 5654 patients, of whom 81.9% were diagnosed with PAH. For these 4626 patients, median duration of macitentan exposure observed was 14.5 (Q1 = 5.2, Q3 = 29.0) months; idiopathic PAH (54.8%) was the most common form of PAH; macitentan was initiated as monotherapy (37.9%), or as part of double (48.0%) or triple therapy (14.1%); discontinuation due to nonhepatic/hepatic adverse events occurred in 17.1%/0.3% of patients; 9.9% of patients experienced ≥1 hepatic adverse events; Kaplan–Meier estimates showed that at 1 year 59.9% (95% confidence interval: 58.3, 61.5) of patients were free from hospitalization and survival was 90.4% (89.3, 91.3). This analysis of real‐world data from the combined OPUS and OrPHeUS populations demonstrated that macitentan is well tolerated in a large, diverse population of PAH patients, with overall and hepatic safety profiles consistent with previous macitentan clinical trials.
Keywords: cardiopulmonary disease, epidemiology, hepatic safety, pulmonary arterial hypertension, real‐world evidence
Abbreviations
- 6MWD
six‐minute walk distance
- AE(s)
adverse event(s)
- AESI(s)
adverse event(s) of special interest
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- BMI
body mass index
- BNP
brain natriuretic peptide
- CI
confidence interval
- CTD
connective tissue disease
- CTEPH
chronic thromboembolic pulmonary hypertension
- eCRF
electronic case report form
- ERA
endothelin receptor antagonist
- FC
functional class
- FDA
Food and Drug Administration
- HAE(s)
hepatic adverse event(s)
- HAESI(s)
hepatic adverse event(s) of special interest
- IPAH
idiopathic pulmonary arterial hypertension
- ILSDRB
Independent Liver Safety Data Review Board
- I/HPAH
idiopathic/heritable pulmonary arterial hypertension
- KM
Kaplan–Meier
- LHD
left heart disease
- NT‐proBNP
N‐terminal pro‐brain natriuretic peptide
- OPUS
OPsumit® Users Registry
- OrPHeUS
OPsumit® Historical Users cohort
- PAH
pulmonary arterial hypertension
- PDE‐5i
phosphodiesterase type‐5 inhibitor
- PH
pulmonary hypertension
- PT
preferred term
- sGC
soluble guanylate cyclase
- TBIL
total billirubin
- ULN
upper limit of normal
- US
United States
- WHO
World Health Organization
INTRODUCTION
Opsumit® (macitentan), an oral endothelin receptor antagonist (ERA), was approved for the long‐term treatment of pulmonary arterial hypertension (PAH) in the United States (US) in October 2013 1 and as of September 2021, an estimated 106,691 patients had been exposed to commercial macitentan worldwide (Janssen data on file). In the pivotal randomized controlled phase 3 SERAPHIN trial, macitentan 10 mg significantly reduced the risk of a composite morbidity/mortality event by 45% (p < 0.001) and was well‐tolerated. 2
Risk of liver toxicity has previously been associated with the ERA drug class, 1 , 3 , 4 , 5 , 6 and safety data from postmarketing surveillance programs were required by Health Authorities for the previously‐approved ERAs bosentan and ambrisentan. 7 , 8 Such requirements are common, particularly in rare diseases where the sample size of clinical trials may be limited. 9 , 10 Therefore, despite the favorable hepatic safety profile in SERAPHIN, 2 the U.S. Food and Drug Administration (FDA) granted marketing authorization for macitentan with the postmarketing requirement to further evaluate its potential for serious hepatic risks.
The OPsumit® USers Registry (OPUS) and the OPsumit® Historical USers cohort (OrPHeUS) medical chart review aimed to characterize the safety profile of macitentan and to describe the clinical characteristics, treatment patterns, and outcomes of patients newly treated with the drug in the United States. This article presents findings from the combined OPUS and OrPHeUS database, describing these parameters in a large pulmonary hypertension (PH) population newly treated with macitentan in a postmarketing, real‐world clinical setting.
METHODS
Although these data are not currently publicly available for sharing, requests for sharing can be sent to the Corresponding Author and will be evaluated on an individual basis.
Study design
OPUS was a prospective, multicenter, long‐term, observational drug registry (NCT02126943). All patients newly initiated on macitentan ≤30 days before signing informed consent were eligible for enrollment. Information describing patient characteristics, clinical assessments, treatment patterns, hospitalizations, and survival generated via routine clinical practice was collected in an electronic case report form (eCRF). The first patient was enrolled in OPUS in April 2014, and the study ended in June 2020. To achieve the FDA‐requested sample size of 5000 patients newly treated with macitentan, the retrospective, multicenter medical chart review OrPHeUS (NCT03197688) was undertaken and included patients initiating macitentan for the first time between October 2013 and December 2016 (inclusive). OrPHeUS captured individual patient data up to March 2017 and its eCRF was designed to be similar to that of OPUS. Patients enrolled in OPUS were not allowed to participate in OrPHeUS. Patients were excluded from OPUS if they were enrolled in an ongoing clinical trial, and from OrPHeUS if they were enrolled in a clinical trial involving macitentan.
Ethical approval
The OPUS and OrPHeUS studies were conducted in compliance with Good Pharmacoepidemiology Practices, 11 the 2008 Declaration of Helsinki ethical principles, the ISHLT ethics statement, and the protocols were reviewed by the FDA with written informed consent obtained from all patients in OPUS (informed consent was not required in OrPHeUS; Institutional Review Board [IRB] waiver was obtained). IRB approvals were provided by WIRB and Quorum (now Advarra) (OPUS registry; WIRB approval number 2014‐0816, Quorum Review File number 29120/Advarra Pro00035124) and WCG‐IRB (OrPHeUS study; IRB numbers 2017‐8051 and 2017‐2348).
Observations and assessments
Data in OPUS were collected at macitentan initiation and throughout the observation period, that is, from macitentan initiation to study end, or until death, loss to follow‐up, withdrawal of consent, or date of macitentan discontinuation +30 days. For OrPHeUS, data were entered in the eCRF from medical charts during the observation period, that is, from the date of macitentan initiation until the earliest of the following: macitentan discontinuation, last patient data available in the medical chart, patients’ last day under the center's care, death, or March 31, 2017.
In OPUS and OrPHeUS, information was collected per routine clinical practice and no assessments were mandated. Data recorded at macitentan initiation could include, but were not limited to, patient demographics and clinical characteristics: age; race; gender; ethnicity; World Health Organization (WHO) Functional Class (FC); six‐minute walk distance (6MWD); brain natriuretic peptide (BNP)/N‐terminal pro‐BNP (NT‐proBNP); reason for macitentan prescription and date of diagnosis; previous and concomitant PAH and select non‐PAH therapies; laboratory data including alanine aminotransferase (ALT), aspartate aminotransferase (AST) and total bilirubin (TBIL); comorbidities and hepatobiliary events. During macitentan exposure, data collected could include, but were not limited to, WHO FC, 6MWD, concomitant PAH and select non‐PAH therapies, laboratory data, all hepatic adverse events (HAEs), any other adverse events (AEs) (OPUS only), discontinuation of macitentan and reason for stopping therapy, hospitalizations and death. Information on hospitalizations, death, HAEs, and other AEs (OPUS only) could also be identified from the Sponsor's pharmacovigilance database. In OrPHeUS, HAEs were identified from the clinical data collected; however, due to the retrospective design, no other AE reporting was conducted. HAEs were defined as any hepatobiliary event, including ALT or AST ≥ 3 × upper limit of normal (ULN), or TBIL ≥ 2 ×ULN, or any hepatic event identified using the Standardized MedDRA Query “Hepatic disorders” (code 20000005).
Further analyses were performed to assess hepatic adverse events of special interest (HAESI) and other AEs known to be associated with ERAs, such as edema and anemia/hemoglobin decrease. HAESIs were defined as events meeting any of the following criteria: ALT or AST ≥ 5 × ULN; ALT or AST ≥ 3 × ULN and TBIL ≥ 2 × ULN; TBIL ≥ 2 × ULN; any event with Preferred Term (PT) under MedDRA System Organ Class “Hepatobiliary Disorders” and having fatal outcome, or any event under MedDRA High‐level Group Term “Hepatobiliary Investigations” and having fatal outcome; any PT denoting hepatic failure, hepatic encephalopathy, hepatic cirrhosis or fibrosis, liver injury or liver transplant, noninfectious hepatitis or jaundice. An Independent Liver Safety Data Review Board (ILSDRB) was involved in establishing the HAESI definition and reviewed and assessed all reported HAESIs in OPUS. The ILSDRB also reviewed all HAESIs identified in OrPHeUS that met the biochemical criteria of a potential Hy's law case (i.e., concurrent elevation of ALT or AST ≥ 3 × ULN and TBIL ≥ 2 × ULN). The AEs of special interest (AESIs) of edema and anemia/hemoglobin decrease comprised all PTs relating to those conditions (PT lists for HAESIs and AESIs are included in the Supporting Information Methods).
Statistical and other analyses
Exploratory heterogeneity analyses indicated that the OPUS and OrPHeUS datasets had similar patient and treatment characteristics at baseline and could be combined for this analysis (see Supporting Information Methods). 12 For the combined OPUS/OrPHeUS analysis, three analysis sets were defined: (1) Enrolled set; the combined population, (2) Overall follow‐up set; the combined population with follow‐up data, and (3) PAH follow‐up set; the combined population from the follow‐up set who had PAH as one of the investigator‐assessed reasons for macitentan prescription.
Reasons for macitentan prescription were classified according to WHO Groups of PH as defined in the ESC/ERS Guidelines and other conditions. 3 , 4 Multiple PH groups, as well as conditions other than PH, could be entered as a reason for macitentan prescription.
All analyses were descriptive and no formal statistical comparisons were made between populations. Event rates (for HAEs, HAESIs, AEs [OPUS only], discontinuation of macitentan, hospitalization, and death) were calculated using time to first event. Patients without an event were included in the analysis and censored at 30 days after discontinuation of macitentan or at date of last information if that was earlier. All Poisson models included log (exposure time) as an offset to account for varying length of patients’ time on treatment. Confidence intervals (CI) (95%) for rates per person‐year were estimated using an unadjusted Poisson model. Time to first event (for hospitalization and death) was presented using Kaplan–Meier (KM) estimates of the percentage of patients who were event‐free during the exposure period for the outcome measures, along with 95% CIs. Imputation rules are described in the Supporting Information Methods.
Here, we present results from these analyses in the overall and PAH follow‐up sets from the combined OPUS/OrPHeUS data set.
RESULTS
Study population and characteristics
As of June 2020, the combined OPUS/OrPHeUS population consisted of 5654 new users of macitentan (enrolled set), including 2670 participants from OPUS and 2984 participants from OrPHeUS. There were 5650/5654 (99.9%) patients with follow‐up data (overall follow‐up set), of whom 4626/5650 (81.9%) were diagnosed with PAH (PAH follow‐up set) (Figure 1). The reason(s) for macitentan prescription for the remaining 18.1% of enrolled patients are listed in Table 1. Patient characteristics at macitentan initiation were similar between both follow‐up sets (Table 1).
Figure 1.
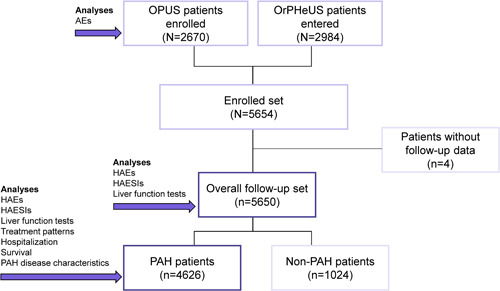
Patient disposition. The combined patient database is equivalent to the enrolled set. AE, adverse event; HAE, hepatic adverse event; HAESI, hepatic adverse event of special interest; OPUS, OPsumit USers (Registry); OrPHeUS, OPsumit Historical USers cohort (study); PAH, pulmonary arterial hypertension.
Table 1.
Patient demographics and characteristics at macitentan initiation
| OPUS and OrPHeUS | ||
|---|---|---|
| Characteristic | Overall follow‐up set | PAH follow‐up seta |
| N = 5650 | N = 4626 | |
| Demographics | ||
| Age—years; median (Q1, Q3) | 62 (51, 71) | 62 (51, 71) |
| Female sex—n (%) | 4148 (73.4) | 3493 (75.5) |
| Raceb—n | 5595 | 4589 |
| White—n (%) | 4172 (74.6) | 3484 (75.9) |
| Black or African American—n (%) | 1002 (17.9) | 752 (16.4) |
| American Indian or Alaska Native—n (%) | 66 (1.2) | 53 (1.2) |
| Native Hawaiian or Other Pacific Islander—n (%) | 28 (0.5) | 26 (0.6) |
| Other—n (%) | 327 (5.8) | 274 (6.0) |
| Missing—n (%) | 55 (1.0) | 37 (0.8) |
| Time from diagnosis—n | 5444 | 4496 |
| Months; median (Q1, Q3) | 8.5 (1.5, 40.5) | 7.7 (1.3, 40.2) |
| ≤6 months before macitentan initiation—n (%) | 2431 (44.7) | 2081 (46.3) |
| >6 months before macitentan initiation—n (%) | 3013 (55.3) | 2415 (53.7) |
| Reason for macitentan prescription—n | 5643 | 4626 |
| Group 1 – PAH—n (%) | 4459 (79.0) | 4459 (96.4) |
| Group 2 – PH due to LHD—n (%) | 139 (2.5) | 0 |
| Group 3 – PH due to lung disease and/or hypoxia—n (%) | 373 (6.6) | 0 |
| Group 4 – CTEPH—n (%) | 192 (3.4) | 0 |
| Group 5 – PH with unclear multifactorial mechanisms—n (%) | 257 (4.6) | 0 |
| Otherc—n (%) | 26 (0.5) | 0 |
| Multiple reasons including PAH—n (%) | 167 (3.0) | 167 (3.6) |
| Multiple reasons not including PAH—n (%) | 30 (0.5) | 0 |
| Hepatic comorbidities—n (%) | 654 (11.6) | 559 (12.1) |
| Non‐hepatic comorbidities and symptoms of interest—n (%) | ||
| Hypertension | 2069 (36.6) | 1621 (35.0) |
| Obesity (BMI ≥ 30 kg/m2) | 1661 (29.4) | 1349 (29.2) |
| Diabetes mellitus | 1340 (23.7) | 1048 (22.7) |
| History of edema | 726 (12.8) | 593 (12.8) |
| History of anemia | 575 (10.2) | 465 (10.1) |
| Renal insufficiency | 452 (8.0) | 339 (7.3) |
Abbreviations: BMI, body mass index; CTEPH, chronic thromboembolic pulmonary hypertension; LHD, left heart disease; PAH, pulmonary arterial hypertension; PH, pulmonary hypertension; Q1, Q3, interquartile range.
The PAH follow‐up set is part of the overall follow‐up set.
Percentages may not add up to 100% due to rounding.
Included 17 due to PH.
The most common forms of PAH were idiopathic (IPAH; 54.8%) and associated with connective tissue disease (CTD‐PAH; 26.8%), and most patients were female (75.5%). At the time of macitentan initiation and registry enrollment, median age of the PAH follow‐up cohort was 62 (Q1 = 51, Q3 = 71), most patients were WHO FC III (57%), median 6MWD was 293 (Q1 = 195, Q3 = 378) meters and the most common risk category by BNP/NT‐proBNP plasma levels 3 , 4 was intermediate (37.7%) (Table 2).
Table 2.
PAH disease characteristics at macitentan initiation
| OPUS and OrPHeUS | |
|---|---|
| Characteristic | PAH follow‐up seta |
| N = 4626 | |
| PAH etiology—n | 4626 |
| Idiopathic PAH—n (%) | 2533 (54.8) |
| Heritable PAH—n (%) | 65 (1.4) |
| Drug‐ and toxin‐induced PAH—n (%) | 232 (5.0) |
| Associated PAH: | |
| Connective tissue disease—n (%) | 1239 (26.8) |
| HIV infection—n (%) | 38 (0.8) |
| Portal hypertension—n (%) | 201 (4.3) |
| Congenital heart disease—n (%) | 281 (6.1) |
| Unknown—n (%) | 2 (<0.1) |
| Multiple PAH etiologiesb—n (%) | 5 (0.1) |
| Otherc—n (%) | 21 (0.5) |
| Missing—n (%) | 9 (0.2) |
| WHO functional class—n | 2513 |
| I—n (%) | 201 (8.0) |
| II—n (%) | 723 (28.8) |
| III—n (%) | 1425 (56.7) |
| IV—n (%) | 164 (6.5) |
| Missing—n/N (%) | 2113/4626 (45.7) |
| 6‐minute walk distance—n | 1670 |
| Meters; median (Q1, Q3) | 293 (195, 378) |
| Missing—n/N (%) | 2956/4626 (63.9) |
| BNP/NT‐proBNP biomarker categoryd—n | 1706 |
| Low risk—n (%) | 461 (27.0) |
| Intermediate risk—n (%) | 644 (37.7) |
| High risk—n (%) | 601 (35.2) |
| Missing—n/N (%) | 2920/4626 (63.1) |
| No prior PAH therapy—n | 1606 |
| ≥1 prior PAH therapy—n | 3020 |
| PDE‐5 inhibitor—n (%) | 2422 (52.4) |
| Endothelin receptor antagonist—n (%) | 797 (17.2) |
| i.v./s.c. prostanoid—n (%) | 561 (12.1) |
| Inhaled prostanoid—n (%) | 305 (6.6) |
| Oral prostanoid—n (%) | 112 (2.4) |
| Soluble guanylate cyclase stimulator—n (%) | 186 (4.0) |
| Investigational druge—n (%) | 7 (0.2) |
| ≥1 commonly prescribed therapy—n | 3579 |
| Diuretic—n (%) | 2675 (57.8) |
| Oxygen therapy—n (%) | 1655 (35.8) |
| Anticoagulation agent—n (%) | 1271 (27.5) |
| Calcium channel blocker—n (%) | 745 (16.1) |
| HIV treatment—n (%) | 34 (0.7) |
Note: Percentages may not add to 100% due to rounding or because patients could be receiving more than one therapy.
Abbreviations: BNP, brain natriuretic peptide; HIV, human immunodeficiency virus; i.v., intravenous; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; PAH, pulmonary arterial hypertension; PDE‐5, phosphodiesterase type‐5; Q1, Q3, interquartile range; s.c., subcutaneous; WHO, World Health Organization.
The PAH follow‐up set is part of the overall follow‐up set.
Some patients in OrPHeUS had multiple etiologies at macitentan initiation.
Includes pulmonary veno‐occlusive disease and/or pulmonary capillary hemangiomatosis or persistent pulmonary hypertension of the newborn.
Combinations with investigational drug are only in OrPHeUS.
Patient characteristics at macitentan initiation were similar between the OPUS and OrPHeUS populations (Supporting Information: Tables S1 and S2); however, one notable exception was the time from diagnosis to macitentan initiation, which was shorter in the OPUS population compared to the OrPHeUS population (median of 4.6 [Q1 = 1.1, Q3 = 31.0] months in OPUS and 13.1 [Q1 = 2.0, Q3 = 47.7] months in OrPHeUS) (Supporting Information: Table S1). This difference is likely due to the timing of the studies relative to the availability of macitentan in the US.
All 155 enrolling sites were in the United States; details and specific study participation (OPUS, OrPHeUS, or both studies) are shown in Supporting Information: Figure S1.
Safety
The median length of macitentan exposure observed was 13.6 (Q1 = 4.8, Q3 = 28.0) months in the overall follow‐up set and 14.5 (Q1 = 5.2, Q3 = 29.0) months in the PAH follow‐up set, and the total exposure was 8322 and 7044 person‐years, respectively. Safety information presented here is for patients in the overall follow‐up set; similar results were observed for the PAH follow‐up set (Tables 3 and 4). Liver function tests were performed at a rate (95% CI) of 2.0 (2.0, 2.1) tests per person‐year across the overall macitentan exposure period, and at a rate (95% CI) of 2.6 (2.5, 2.7) tests per person year during the first 6 months after macitentan initiation (Table 3). During the observation period, 561/5650 (9.9%) patients experienced ≥1 HAE and 352/5650 (6.2%) experienced ≥1 HAESI (Table 3, Supporting Information: Tables S3 and 4). ALT or AST levels ≥3 × ULN were observed in 186/5650 (3.3%) of patients, and a combined AST or ALT ≥ 3 × ULN and TBIL ≥ 2 × ULN was experienced by 49/5650 (0.9%) of patients (Table 3). The ILSDRB reviewed patient‐level data for all 165/2667 (6.2%) patients reporting HAESIs in OPUS and aggregate data for the 187/2983 (6.3%) patients reporting HAESIs in OrPHeUS. They additionally performed an individual review of the OrPHeUS cases with information reported in the Sponsor's pharmacovigilance database (n = 11) and those of liver test elevations that met the biochemical criteria of a potential Hy's law case (n = 36). Among the reviewed cases, 20/2667 (0.7%) patients in OPUS and 1 patient in OrPHeUS were judged as difficult to assess, and 2/2667 (0.1%) patients in OPUS and none in OrPHeUS had events that were deemed by the ILSDRB to be possibly related to macitentan treatment.
Table 3.
Liver function tests, enzyme elevations, hepatic adverse events, and hepatic adverse events of special interest
| OPUS and OrPHeUS | ||
|---|---|---|
| Overall follow‐up set | PAH follow‐up seta | |
| N = 5650 | N = 4626 | |
| Liver function tests | ||
| Rate—per person‐year (95% CI) (entire macitentan exposure period) | 2.0 (2.0, 2.1) | 2.0 (1.9, 2.1) |
| Rate—per person‐year (95% CI) (first 6 months after macitentan initiation) | 2.6 (2.5, 2.7) | 2.5 (2.4, 2.6) |
| Patients with abnormal liver function testsb—KM estimates, % (95% CI) | ||
| At 3 months | 2.7 (2.3, 3.2) | 2.6 (2.2, 3.2) |
| Number at risk | 4484 | 3736 |
| At 6 months | 3.7 (3.2, 4.3) | 3.6 (3.1, 4.2) |
| Number at risk | 3865 | 3238 |
| Patients with ≥1 HAE—n (%) | 561 (9.9) | 457 (9.9) |
| Incidence rate—per person‐year (95% CI) | 0.07 (0.07, 0.08) | 0.07 (0.06, 0.08) |
| Most common HAEs (≥1% in either group)c | ||
| By Preferred Term—n (%) | ||
| Increased blood bilirubin | 113 (2.0) | 92 (2.0) |
| Increased AST | 104 (1.8) | 83 (1.8) |
| Increased ALT | 67 (1.2) | 57 (1.2) |
| Increased blood alkaline phosphatase | 66 (1.2) | 53 (1.1) |
| Laboratory values—n (%) | ||
| Patients with ALT/AST ≥ 3 × ULN | 186 (3.3) | 153 (3.3) |
| Patients with TBIL ≥ 2 × ULN | 227 (4.0) | 177 (3.8) |
| Patients with ≥1 HAESId— n (%) | 352 (6.2) | 281 (6.1) |
| Incidence rate—per person‐year (95% CI) | 0.04 (0.04, 0.05) | 0.04 (0.04, 0.05) |
| Most common HAESIs (≥1% in either group)c | ||
| By Preferred Term—n (%) | ||
| Increased blood bilirubin | 76 (1.3) | 64 (1.4) |
| Laboratory values—n (%) | ||
| Patients with TBIL ≥ 2 × ULN | 227 (4.0) | 177 (3.8) |
| Patients with ALT/AST ≥ 5 × ULN | 86 (1.5) | 67 (1.4) |
| Patients with ALT/AST ≥ 3 × ULN and TBIL ≥ 2 × ULN | 49 (0.9) | 36 (0.8) |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; CI, confidence interval; HAE, hepatic adverse event; HAESI, hepatic adverse event of special interest; ILSDRB, Independent Liver Safety Data Review Board; KM, Kaplan–Meier; PAH, pulmonary arterial hypertension; TBIL, total bilirubin; ULN, upper limit of normal.
The PAH follow‐up set is part of the overall follow‐up set.
Defined as ALT ≥ 3 × ULN, AST ≥ 3 × ULN, and/or TBIL ≥ 2 × ULN.
Patients may appear in more than one row.
Of the 352 patients reporting HAESIs in the Overall follow‐up set, only two cases of increased liver enzymes were deemed by the ILSDRB to be possibly related to macitentan treatment.
Table 4.
Study discontinuations
| OPUS and OrPHeUS | ||
|---|---|---|
| Overall follow‐up set | PAH follow‐up seta | |
| N = 5650 | N = 4626 | |
| Patients discontinuing macitentan treatment—n (%) | 2487 (44.0) | 1974 (42.7) |
| Due to a non‐hepatic AE | 1026 (18.2) | 790 (17.1) |
| Due to an HAE | 17 (0.3) | 15 (0.3) |
| Not due to an AE/HAE | 1045 (18.5) | 838 (18.1) |
| Missing reason | 399 (7.1) | 331 (7.2) |
| Macitentan discontinuation at 1 year — KM estimates, % (95% CI) | 32.1 (30.9, 33.4) | 30.2 (28.9, 31.6) |
| Number at risk | 3065 | 2577 |
Abbreviations: AE, adverse event; CI, confidence interval; HAE, hepatic adverse event; KM, Kaplan–Meier.
The PAH follow‐up set is part of the overall follow‐up set.
In OPUS, 2141/2667 (80.3%) of patients experienced ≥1 AE during the observation period, the most common AEs being dyspnea (617/2667 [23.1%]), headache (309/2667 [11.6%]) and peripheral edema (283/2667 [10.6%]) (Supporting Information: Table S5). With respect to AESIs (grouped terms), 747/2667 (28.0%) and 270/2667 (10.1%) of patients experienced ≥1 event of edema and anemia/hemoglobin decrease, respectively (Supporting Information: Table S5).
Macitentan treatment was discontinued during follow‐up in 2487/5650 (44.0%) patients: 1026/5650 (18.2%) due to a non‐hepatic AE, 17/5650 (0.3%) due to an HAE, 1045/5650 (18.5%) not due to a hepatic AE/HAE and 399/5650 (7.1%) had a missing reason (Table 4). The KM estimate of macitentan discontinuation at 1 year was 32.1% (95% CI: 30.9, 33.4). There were no major differences in exposure and discontinuations between the OPUS and OrPHeUS populations (Supporting Information: Table S6).
PAH treatment patterns
For the PAH follow‐up set, macitentan was initiated as monotherapy in 38% (1752/4626) of patients, double therapy in 48% (2222/4626) of patients, and triple therapy in 14% (652/4626) of patients (Figure 2). Among patients initiating macitentan as double or triple combination therapy, macitentan was used in combination with a phosphodiesterase type‐5 inhibitor (PDE‐5i) in 81% (1802/2222) and 95% (617/650) of patients, respectively. Of the 667/4626 (14%) patients who switched from another ERA to macitentan, 482 (10%) switched from bosentan and 194 (4%) from ambrisentan.
Figure 2.
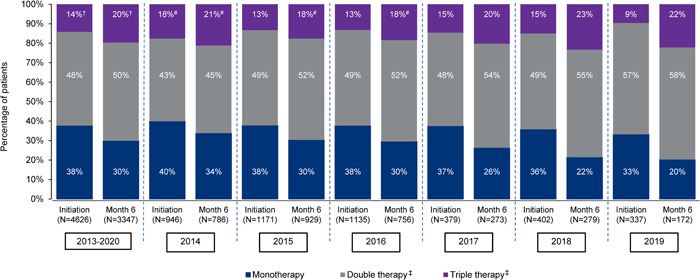
PAH therapy by class at macitentan initiation and 6 months after initiation, overall and by year* of macitentan initiation, in the PAH follow‐up set. Percentages may not add to 100% due to rounding. *For those years with 12 months of data available; †Includes patients receiving >3 classes of PAH therapy: two patients at macitentan initiation and four patients at 6 months; #Includes patients receiving >3 classes of PAH therapy: two patients at macitentan initiation and one patient at 6 months in 2014, and one patient at 6 months in 2015 and two at 6 months in 2016; ‡Includes combinations with phosphodiesterase type‐5 inhibitors, prostanoids (oral, intravenous/subcutaneous or inhaled), soluble guanylate cyclase stimulators, and investigational drug (OrPHeUS only). Double therapy group includes patients taking macitentan plus 1 other class of PAH therapy and triple therapy includes patients taking macitentan plus 2 other classes of PAH therapy. PAH therapy class combinations are shown in Supporting Information: Table S7 . PAH, pulmonary arterial hypertension.
At 6 months following macitentan initiation, the percentage of patients receiving monotherapy decreased to 30% (1003/3347), and the percentages of patients receiving double and triple therapy increased to 50% (1688/3347) and 20% (656/3347) (Figure 2). PAH treatment combinations at macitentan initiation and 6 months after macitentan initiation are shown in Supporting Information: Table S7.
By year of enrollment, the percentage of patients receiving any combination therapy at macitentan initiation/6 months after initiation increased from 60%/66% in 2014 to 66%/80% in 2019 (the first and last years with complete data) (Figure 2).
Hospitalization and survival
For the PAH follow‐up set, KM estimates showed that 60% (95% CI: 58, 62) of patients were free from hospitalization at 1 year (Figure 3a, Supporting Information: Table 8). Similar 1‐year KM estimates were observed for patients with idiopathic/heritable pulmonary arterial hypertension (I/HPAH; 60% [58, 62]) and for CTD patients (59% [56, 62]) (Figure 3b, Supporting Information: Figure 2C). At 3 years, for the PAH follow‐up set, 36% (95% CI; 34, 38) of patients were free from hospitalization; the 3‐year KM estimates were 38% (35, 40) for I/HPAH patients and 33% (29, 37) for CTD patients.
Figure 3.
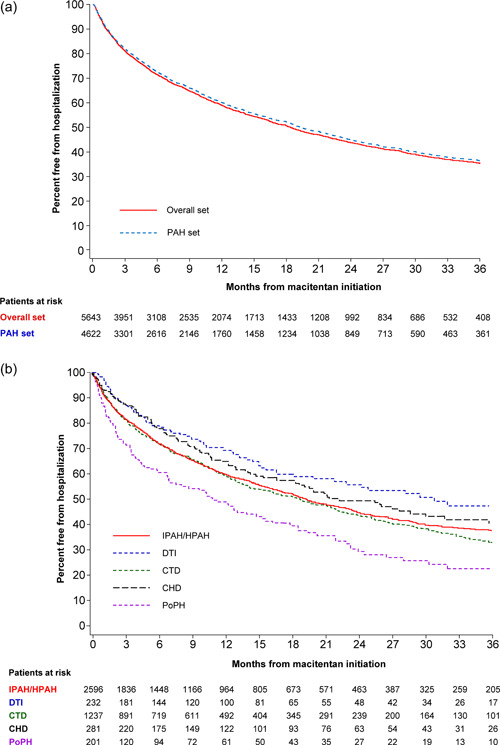
Kaplan–Meier estimates of hospitalization overall (a) and by etiology (b) from macitentan initiation in the OPUS and OrPHeUS PAH follow‐up set*. *Four patients that took macitentan for 1 day only are not included in the analysis of hospitalizations. CHD, congenital heart disease PAH; CTD, connective tissue disease PAH; DTI, drug‐ and toxin‐induced PAH; IPAH/HPAH, idiopathic PAH/heritable PAH; PAH, pulmonary arterial hypertension; PoPH, PAH associated with portal hypertension.
KM estimates of survival were 90% (95% CI; 89, 91) at 1 year and 75% (73, 77) at 3 years for patients in the PAH follow‐up set (Figure 4a; Supporting Information: Table S8). During the observation period, there were 697/4626 (15.1%) deaths resulting in a mortality rate of 0.10 (95% CI; 0.09, 0.11) per person‐year. KM survival estimates at 1 and 3 years were similar for I/HPAH (90% [95% CI; 89, 92] and 75% [73, 78]) and CTD patients (91% [95% CI; 89, 92] and 74% [70, 77]) (Figure 4b, Supporting Information: Figure S3C). Hospitalization and survival overall and by age, etiology and race in the PAH follow‐up set are shown in Supporting Information: Table S8 and Supporting Information: Figures 2 and 3.
Figure 4.
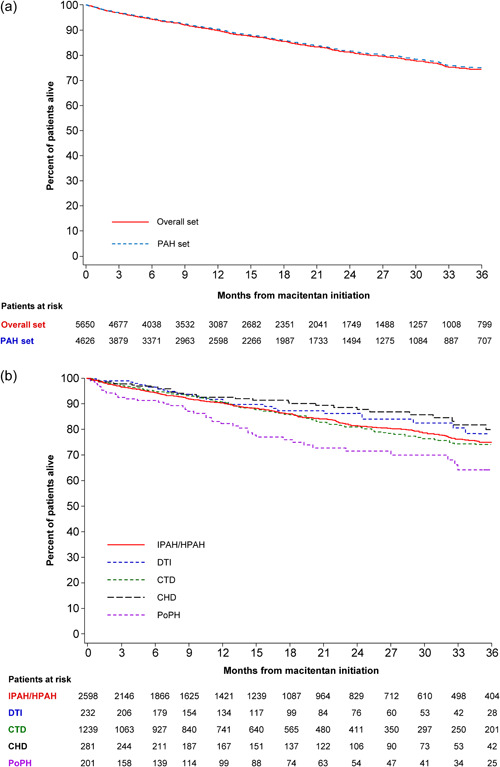
Kaplan–Meier estimates of survival overall (a) and by etiology (b) from macitentan initiation in the OPUS and OrPHeUS PAH follow‐up set. CHD, congenital heart disease PAH; CTD, connective tissue disease PAH; DTI, drug‐ and toxin‐induced PAH; IPAH/HPAH, idiopathic PAH/heritable PAH; OPUS, OPsumit® USers Registry; OrPHeUS, OPsumit® Historical USers cohort; PAH, pulmonary arterial hypertension; PoPH, PAH associated with portal hypertension.
DISCUSSION
The combined OPUS/OrPHeUS data set is the largest new‐users database for macitentan, describing a large population of patients who newly initiated macitentan in real‐world clinical practice in the United States. Importantly, this patient population reflects the full heterogeneity of the PAH disease state, including patients with different disease severities, etiologies, and times from diagnosis. The data set included patients typically excluded from clinical trials due to stringent eligibility criteria, such as those with portopulmonary hypertension or elevated baseline liver function tests. OPUS/OrPHeUS, therefore, provides clinically‐relevant real‐world data and important evidence to supplement findings from clinical studies.
The demographics of the combined OPUS/OrPHeUS PAH population are comparable with those of other real‐world PAH registries. The racial composition of the OPUS/OrPHeUS PAH population was similar to that of other US registries, REVEAL 13 and the Pulmonary Hypertension Association Registry (PHAR), 14 with white (75% in our study vs. 72% in REVEAL and 77% in PHAR) and black patients (18% vs. 13% and 14%, respectively) representing the two most common races. IPAH was the most frequent etiology in OPUS/OrPHeUS (55%); this was also the case in other real‐world databases (REVEAL 46%, 15 PHAR 41%, 14 VOLT 45%, 7 French registry 39%, 16 Tracleer postmarketing surveillance study [TRAX] 34% 8 ). As observed for OPUS/OrPHeUS, CTD‐PAH was the second most frequent etiology in all of these studies. 7 , 8 , 14 , 15 , 16 Patients in the OPUS/OrPHeUS data set (2013–2020) were older (median 62 years at macitentan initiation) than those enrolled in earlier analyses from the French registry (2002–2004; mean age 50 years), 16 REVEAL (2006–2007; mean age 53 years), 15 TRAX (2002–2004; mean age 54.5 years), 8 and VOLT (2008–2011; mean age 59.5 years). 7 This is consistent with the changing demographics of PAH. 17
No new safety signals were identified during the study. The results of the study confirm the overall and hepatic safety profiles of macitentan and are consistent with data reported from SERAPHIN, the largest macitentan clinical trial to date. 2 As a result of these safety data, which were supported by the ILSDRB, the FDA announced in September 2019 that the postmarketing requirement to further evaluate macitentan's potential for serious hepatic risks had been fulfilled.
Discontinuations due to an AE in OPUS/OrPHeUS (PAH follow‐up set, 17.4%) were similar to those reported for ambrisentan in the VOLT registry 7 (overall safety population, 17%; mean exposure of 2.2 years), but were higher than in the SERAPHIN study 2 (macitentan 10 mg arm, 10.7%; mean exposure of 2.0 years). Many non safety‐related barriers to PAH therapy adherence have been identified, including factors related to the healthcare system, complexity and duration of therapeutic regimens, lack of disease education, and patients’ personal circumstances. 18 , 19 , 20 That 18.1% of patients in this analysis discontinued macitentan for reasons other than an AE suggests the need to address these factors to ensure optimal outcomes for patients, as low adherence is associated with suboptimal clinical benefits. 19 , 20 , 21 It is important to note that the phenomenon of non‐adherence is observed across the PAH population, encompassing a broad spectrum of patients and PAH etiologies, rather than being restricted to specific subgroups. A recent analysis of real‐world data demonstrated that among PAH patients, adherence to ERAs is higher than to PDE‐5is, albeit with the caveat that the baseline characteristics of patients receiving PDE‐5is were different to those receiving ERAs. 21 In contrast, randomized controlled trial data from TRITON show that PAH patients receiving ERAs have a higher discontinuation rate than those receiving PDE‐5is. 22 Furthermore, ERAs are also associated with higher drug costs, 21 which could impact adherence and thereby health disparities.
Hospitalizations and survival in the OPUS/OrPHeUS PAH follow‐up set are consistent with observations with macitentan in the randomized controlled trial SERAPHIN. 2 Importantly, these real‐world data affirm the improvement in PAH patient survival over the past few decades, with 1‐ and 3‐year overall survival rates after study entry of 90% and 75% in the PAH follow‐up set. By comparison, 1‐ and 3‐year overall survival rates after study entry were 87% and 67% for patients enrolled in the French Registry from 2002 to 2003, despite the French registry containing a greater proportion of prevalent patients, who typically have a better prognosis versus newly diagnosed patients. 23 Survival and time to first hospitalization were similar in I/HPAH and CTD‐PAH patients, in contrast to prior reports suggesting worse prognosis in patients with CTD‐PAH. 24 When stratified by race, survival was similar between subgroups, aligning with findings from REVEAL reporting that race/ethnicity are not significant predictors of mortality. 13 However, in our study, time to first hospitalization was shorter in the Black/African American subgroup compared with the White and Other race subgroups.
Results from the OPUS/OrPHeUS combined data set indicate that a large proportion of PAH patients initiate macitentan as monotherapy, despite most being WHO FC III at initiation, and a substantial number of patients remained on monotherapy at 6 months after initiation. Since 2007, PAH guidelines and expert consensuses have recommended combination therapy in patients with inadequate response to monotherapy. 25 Strong recommendations for use of upfront or early sequential combination therapy in the majority of patients were first made in the 2015 ESC/ERS Guidelines. 3 , 4 As enrollment of patients in OPUS/OrPHeUS encompasses the period of time before and after the publication of the ESC/ERS 2015 Guidelines, 3 , 4 this combined data set enabled assessment of changes in treatment patterns during this period. We anticipated that over time, more patients in OPUS/OrPHeUS would initiate PAH therapy as a combination regimen, or would be moved to combination therapy within 6 months. Although the proportions of patients on combination therapy at macitentan initiation and 6 months thereafter increased slightly year‐on‐year, there was little overall change in treatment patterns during this time. This is in line with data from other registries, which show that although the percentage of incident patients receiving monotherapy decreased over time, it remains high. The Swiss PH Registry reported that 40% patients received monotherapy in 2001–2005 versus 34% in 2016–2019, 26 and the COMPERA registry observed that 64.7% patients were receiving monotherapy 1 year after diagnosis in 2010 versus 52.4% in 2019. 27 The contemporary PHAR registry reported that of patients enrolled in 2015–2020, 30% were on monotherapy at enrollment. 14 These findings may denote a lack of urgency regarding use of combination therapy. Of the PAH patients in the combined data set receiving combination therapy, macitentan with a PDE‐5i was the most frequently used treatment regimen, consistent with other findings on ERA + PDE‐5i use in clinical practice. 28 , 29 , 30
The observational nature of OPUS/OrPHeUS is associated with known limitations. Patient follow‐up was via routine clinical practice, rather than scheduled visits with protocol‐mandated assessments. Incomplete patient follow‐up and assessments not being performed led to incomplete data, with a greater impact in the retrospective OrPHeUS study than the prospective OPUS study. Many parameters reported in our analysis (e.g., PAH diagnosis and deaths) were investigator‐assessed and were not adjudicated. In the OPUS/OrPHeUS PAH follow‐up set, baseline 6MWD, WHO FC, and BNP/NT‐proBNP assessments were only conducted in 36.1%, 54.3%, and 36.9% of patients, respectively. The lack of data for these variables precluded risk assessment analyses and the ability to perform baseline adjustments that would have permitted direct comparison of outcomes between subgroups. Notably, this degree of non‐reported data may indicate that for a large proportion of patients accurate risk assessment was not performed as recommended in the ESC/ERS 2015 Guidelines. 3 , 4
Combining data from two studies with different designs may have led to some degree of heterogeneity in our results. However, substantial efforts were made to ensure the studies and their data collection were as similar as possible, and heterogeneity analyses support this approach. 12 Moreover, combining study designs increases the representativeness of the data, mitigates bias related to a single study design, and increases sample size allowing important clinical questions to be answered in a shorter time frame.
In summary, the combined OPUS/OrPHeUS analysis provided an opportunity to evaluate treatment patterns and the hepatic safety profile of macitentan in a large, diverse population of patients, providing data that go beyond the setting of clinical trials and capture real‐world use of this medication. Macitentan was well tolerated in clinical practice with overall and hepatic safety profiles consistent with previous macitentan clinical trials. 2 Further investigation into why combination therapy is not more frequently used in this contemporary era is necessary.
AUTHOR CONTRIBUTIONS
All authors made a substantial contribution to the manuscript, as detailed. Vallerie V. McLaughlin, Richard Channick, Nick H. Kim, Kelly M. Chin, and Monika Brand contributed to the conception and design of the study in collaboration with the funders. Vallerie V. McLaughlin, Richard Channick, Nick H. Kim, Robert P. Frantz, John W. McConnell, Lana Melendres‐Groves, Chad Miller, Ashwin Ravichandran, Josanna Rodriguez‐Lopez, and Kelly M. Chin were involved in the collection of the data. Graham Wetherill was responsible for the statistical analyses. All authors were involved in interpretation of the data, development of the manuscript, and approval of the final version of the manuscript.
CONFLICTS OF INTEREST
V. V. M. reports serving as a steering committee member, as well as receiving consultant fees, and research grants/support from Janssen Pharmaceutical Companies of Johnson & Johnson; consultant fees and research grants/support from United Therapeutics, Bayer, and Acceleron Pharma, Inc.; consultant fees from Altavant, Caremark, CiVi Biopharma Inc., and Gossamer Bio; and research grants/support from Reata Pharmaceuticals and SoniVie. R. C. serves as a steering committee member, served on an advisory board, and received research grants/support, and speaker fees from Janssen Pharmaceutical Companies of Johnson & Johnson; has served on an advisory board for Bayer; has received consultancy and speaker fees from Bayer and Arena Pharmaceuticals; and has received research grants from United Therapeutics. N. H. K. has served a steering committee member and received research grants/support and speaker fees from Janssen Pharmaceutical Companies of Johnson & Johnson; has received research grants/support from Bellerophon, Eiger, Gossamer Bio, Lung Biotechnology, and SoniVie; consultant fees from Bayer, Merck, and United Therapeutics; and speaker fees from Bayer. R. P. F has served as a steering committee and advisory board member for Janssen Pharmaceutical Companies of Johnson & Johnson, Gossamer Bio, Liquidia, and Tenax; and as an advisory board member for Altavant Sciences and ShoTi; and received royalties from UptoDate. J.W. M. received financial support, research grants and personal fees from Janssen Pharmaceutical Companies of Johnson & Johnson; personal fees and financial support from Bayer Pharmaceuticals, United Therapeutics, Reata Pharmaceuticals and Arena Pharmaceuticals; and financial support from Eiger Pharmaceuticals, and Bellerophon. L. M. G. has served on advisory boards and received speaker fees from Janssen Pharmaceutical Companies of Johnson & Johnson, has received speaker fees from United Therapeutics, Gilead, and Bayer Pharmaceuticals; and served on advisory boards for United Therapeutics and Bayer Pharmaceuticals. C. M. reports personal fees from Janssen Pharmaceutical Companies of Johnson & Johnson, United Therapeutics, Gilead, and Bayer. A. R. reports speaker fees from Janssen Pharmaceutical Companies of Johnson & Johnson, Bayer Pharmaceuticals, United Therapeutics. J. R. L. has served on an advisory board for and received research support from Janssen Pharmaceutical Companies of Johnson & Johnson; has received research support from United Therapeutics and Bayer, and has served on an advisory board for Bayer. M. B. and S. L. are employees of Janssen Pharmaceutical Companies of Johnson & Johnson and in the past held stock/stock options for Actelion Pharmaceuticals Ltd. and currently hold stock/stock options in the parent company Johnson & Johnson. G. W. was an employee of Janssen Pharmaceutical Companies of Johnson & Johnson at the time that the analyses and this publication were developed. K. C. has served as a steering committee member, on an advisory board, and has received research grants/support, and consultancy fees from Janssen Pharmaceutical Companies of Johnson & Johnson; has received research grants from United Therapeutics and Ironwood Pharmaceuticals; has served on an advisory board for Bayer Healthcare; and has received consultancy fees from United Therapeutics.
ETHICS STATEMENT
The OPUS and OrPHeUS studies were conducted in compliance with Good Pharmacoepidemiology Practices11, the 2008 Declaration of Helsinki ethical principles, the ISHLT ethics statement, and the protocols were reviewed by the FDA with written informed consent to participate and to publish the study data obtained from all patients in OPUS (informed consent was not required in OrPHeUS; Institutional Review Board [IRB] waiver was obtained). IRB approvals were provided by WIRB and Quorum (now Advarra) (OPUS registry; WIRB approval number 2014‐0816, Quorum Review File number 29120/Advarra Pro00035124) and WCG‐IRB (OrPHeUS study; IRB numbers 2017‐8051 and 2017‐2348).
Supporting information
Supplementary information.
ACKNOWLEDGMENTS
The OPUS Registry and the OrPHeUS medical chart review were sponsored by Actelion Pharmaceuticals Ltd., a Janssen Pharmaceutical Company of Johnson & Johnson. The Sponsor participated in the conception and design of the analysis and interpretation of the data, drafting and critical revision of the report, and approved submission of the manuscript. Medical writing assistance was provided by Caroline Wadsworth (eluSCIdate Ltd., Meggen, Switzerland) and was funded by Actelion Pharmaceuticals Ltd, a Janssen Pharmaceutical Company of Johnson & Johnson. Clinical evaluation of hepatic safety was performed by the ILSDRB: Willis Maddrey MD (chairperson), Paul Watkins, MD, and James Freston, MD. Megan Flynn, PhD contributed to the analysis of data and development of the manuscript. OPUS and OrPHeUS were funded by Actelion Pharmaceuticals Ltd, a Janssen Pharmaceutical Company of Johnson & Johnson.
McLaughlin VV, Channick R, Kim NH, Frantz RP, McConnell JW, Melendres‐Groves L, Miller C, Ravichandran A, Rodriguez‐Lopez J, Brand M, Leroy S, Wetherill G, Chin KM. Safety of macitentan for the treatment of pulmonary hypertension: real‐world experience from the OPsumit® USers Registry (OPUS) and OPsumit® historical USers cohort (OrPHeUS). Pulmonary Circulation. 2022;12:e12150. 10.1002/pul2.12150
Clinical Trial Registration Numbers: https://clinicaltrials.gov/NCT02126943; https://clinicaltrials.gov/NCT03197688
REFERENCES
- 1. Opsumit® (macitentan) . Summary of Product Characteristics. Janssen Pharmaceuticals Ltd., April 2021.
- 2. Pulido T, Adzerikho I, Channick RN, Delcroix M, Galiè N, Ghofrani HA, et al. Macitentan and morbidity and mortality in pulmonary arterial hypertension. N Engl J Med. 2013;369:809–18. [DOI] [PubMed] [Google Scholar]
- 3. Galiè N, Humbert M, Vachiery J‐L, Gibbs S, Lang I, Torbicki A, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the joint task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS) endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J. 2015;46:903–75. [DOI] [PubMed] [Google Scholar]
- 4. Galiè N, Humbert M, Vachiéry JL, Gibbs S, Lang I, Torbicki A, et al. ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2015;2016:67–119. [DOI] [PubMed] [Google Scholar]
- 5. Tracleer® . Summary of Product Characteristics, Janssen Pharmaceuticals Ltd. October 2021.
- 6. Volibris® (ambrisentan) . Summary of Product Characteristics. GlaxoSmithKline UK. January 2021.
- 7. Vachiéry J‐L, Hoeper MM, Peacock AJ, Sitbon O, Cheli M, Church C, et al. Ambrisentan use for pulmonary arterial hypertension in a post‐authorization drug registry: the VOLibris Tracking Study. J Heart Lung Transplant. 2017;36:399–406. [DOI] [PubMed] [Google Scholar]
- 8. Humbert M, Segal ES, Kiely DG, Carlsen J, Schwierin B, Hoeper MM. Results of European post‐marketing surveillance of bosentan in pulmonary hypertension. Eur Respir J. 2007;30:338–44. [DOI] [PubMed] [Google Scholar]
- 9. Sardella M, Belcher G. Pharmacovigilance of medicines for rare and ultrarare diseases. Ther Adv Drug Saf. 2018;9:631–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Maier WC, Christensen RA, Anderson P. Post‐approval studies for rare disease treatments and orphan drugs. Adv Exp Med Biol. 2017;1031:197–205. [DOI] [PubMed] [Google Scholar]
- 11. International Society for Pharmacoepidemiology ISPE . Guidelines for good pharmacoepidemiology practices (GPP). Pharmacoepidemiol Drug Saf. 2008;17:200–8. [DOI] [PubMed] [Google Scholar]
- 12. Leroy S, Brand M, Rosenberg D, et al. Combining a prospective registry with a retrospective medical chart review. Pharmacoepidemiol Drug Saf. 2019;28(S2):118. [Google Scholar]
- 13. Medrek S, Sahay S, Zhao C, Selej M, Frost A. Impact of race on survival in pulmonary arterial hypertension: results from the REVEAL registry. J Heart Lung Transplant. 2020;39:321–30. [DOI] [PubMed] [Google Scholar]
- 14. Chang KY, Duval S, Badesch DB, Bull TM, Chakinala MM, De Marco T, et al. Mortality in pulmonary arterial hypertension in the modern era: early insights from the pulmonary hypertension association registry. J Am Heart Assoc. 2022;11:e024969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Badesch DB, Raskob GE, Elliott CG, Krichman AM, Farber HW, Frost AE, et al. Pulmonary arterial hypertension. Chest. 2010;137:376–87. [DOI] [PubMed] [Google Scholar]
- 16. Humbert M, Sitbon O, Chaouat A, Bertocchi M, Habib G, Gressin V, et al. Pulmonary arterial hypertension in France: results from a national registry. Am J Respir Crit Care Med. 2006;173:1023–30. [DOI] [PubMed] [Google Scholar]
- 17. Hoeper MM, Simon R. Gibbs J. The changing landscape of pulmonary arterial hypertension and implications for patient care. Eur Respir Rev. 2014;23:450–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Narechania S, Torbic H, Tonelli AR. Treatment discontinuation or interruption in pulmonary arterial hypertension. J Cardiovasc Pharmacol Ther. 2020;25:131–41. [DOI] [PubMed] [Google Scholar]
- 19. Grady D, Weiss M, Hernandez‐Sanchez J, Pepke‐Zaba J. Medication and patient factors associated with adherence to pulmonary hypertension targeted therapies. Pulm Circ. 2018;8:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kjellström B, Sandqvist A, Hjalmarsson C, Nisell M, Näsman P, Ivarsson B. Adherence to disease‐specific drug treatment among patients with pulmonary arterial hypertension or chronic thromboembolic pulmonary hypertension. ERJ Open Res. 2020;6:00299‐2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Frantz RP, Hill JW, Lickert CA, Wade RL, Cole MR, Tsang Y, Drake W. Medication adherence, hospitalization, and healthcare resource utilization and costs in patients with pulmonary arterial hypertension treated with endothelin receptor antagonists or phosphodiesterase type‐5 inhibitors. Pulm Circ. 2020;10:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chin KM, Sitbon O, Doelberg M, Feldman J, Gibbs JSR, Grünig E, et al. Three‐ versus two‐drug therapy for patients with newly diagnosed pulmonary arterial hypertension. J Am Coll Cardiol. 2021;78:1393–403. [DOI] [PubMed] [Google Scholar]
- 23. Humbert M, Sitbon O, Yaici A, Montani D, O'Callaghan DS, Jais X, et al. Survival in incident and prevalent cohorts of patients with pulmonary arterial hypertension. Eur Respir J. 2010;36:549–55. [DOI] [PubMed] [Google Scholar]
- 24. Chung L, Liu J, Parsons L, Hassoun PM, McGoon M, Badesch DB, et al. Characterization of connective tissue disease‐associated pulmonary arterial hypertension from REVEAL. Chest. 2010;138:1383–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Badesch DB, Abman SH, Simonneau G, Rubin LJ, McLaughlin VV. Medical therapy for pulmonary arterial hypertension. Chest. 2007;131:1917–28. [DOI] [PubMed] [Google Scholar]
- 26. Appenzeller P, Lichtblau M, Berlier C, Aubert JD, Azzola A, Fellrath JM, Geiser T, Lador F, Pohle S, Opitz I, Schwerzmann M, Stricker H, Tamm M, Saxer S, Ulrich S. Disease characteristics and clinical outcome over two decades from the Swiss pulmonary hypertension registry. Pulm Circ. 2022;12:e12001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hoeper MM, Pausch C, Grünig E, Staehler G, Huscher D, Pittrow D, et al. Temporal trends in pulmonary arterial hypertension: results from the COMPERA registry. Eur Respir J. 2021;59:2102024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hoeper MM, Huscher D, Ghofrani HA, Delcroix M, Distler O, Schweiger C, et al. Elderly patients diagnosed with idiopathic pulmonary arterial hypertension: results from the COMPERA registry. Int J Cardiol. 2013;168:871–80. [DOI] [PubMed] [Google Scholar]
- 29. Boucly A, Weatherald J, Savale L, Jaïs X, Cottin V, Prevot G, et al. Risk assessment, prognosis and guideline implementation in pulmonary arterial hypertension. Eur Respir J. 2017;50:1700889. [DOI] [PubMed] [Google Scholar]
- 30. Rådegran G, Kjellström B, Ekmehag B, Larsen F, Rundqvist B, Blomquist SB, et al. Characteristics and survival of adult Swedish PAH and CTEPH patients 2000‐2014. Scand Cardiovasc J. 2016;50:243–50. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information.


