Background
Regulations have been proposed to limit e-cigarette flavours, but limited research has examined potential impacts of such policies. This study examined adult e-cigarette users’ reactions to a hypothetical e-cigarette flavour ban.
Methods
In 2019, a convenience sample of current e-cigarette users in the USA (n=81, 53.1% women, mean age=37.6, 59.3% dual users of cigarettes) completed an online concept mapping study. Participants provided statements describing anticipated reactions to a hypothetical policy in which only tobacco, menthol or unflavoured e-cigarettes were available for purchase. Seventy-one unique statements were generated. Participants sorted statements into thematic groups and rated statements on how likely they would be to have each reaction. Multidimensional scaling was used to identify thematic clusters of statements.
Results
Twelve clusters were identified: negative reaction, take action against flavour limitation, youth prevention effectiveness perception, tolerance, acceptance, willingness to try new flavours, maintain vaping, reduce vaping, new flavours as vaping cessation transition, alternative sources for banned flavours, do-it-yourself mixing behaviours and alternative tobacco products. The highest rated cluster (negative reaction) described being angry or upset that flavours were banned, while the lowest rated clusters related to quitting/reducing e-cigarette use or switching to other tobacco products. Non-tobacco or non-menthol/mint flavoured e-cigarette users had higher ratings for clusters describing negative sentiment for the hypothetical policy.
Conclusions
Some e-cigarette users may dislike an e-cigarette flavour ban; however, some e-cigarette users would likely be willing to use tobacco, menthol or unflavoured e-cigarette liquids with lower likelihood of quitting vaping or switching to other tobacco products.
Keywords: Electronic nicotine delivery devices, Non-cigarette tobacco products, Public policy
WHAT IS ALREADY KNOWN ON THIS TOPIC
Flavoured tobacco products appeal to youth and young adults. Many jurisdictions have banned flavoured combustible cigarettes (excluding menthol), and policies have been proposed and implemented in jurisdictions that limit flavours in e-cigarette products. Flavour-limiting policies have been associated with decreased tobacco product use previously, but little is known about the reactions and responses e-cigarette users may have if flavours were limited in e-cigarette products.
WHAT THIS STUDY ADDS
This study identified adult e-cigarette users’ anticipated reactions to a hypothetical policy that limited e-cigarette flavours to tobacco, menthol or unflavoured only. Some e-cigarette users may view a flavour-limiting policy with negative sentiment; however, some may support flavour-limiting policies and be willing to use tobacco or menthol e-cigarette flavours. Potential unintended consequences of e-cigarette flavour-limiting policies may include users attempting to obtain prohibited flavours from alternative sources that may increase risk to users, such as from black markets or making their own e-cigarette liquids. E-cigarette users rated switching to combustible tobacco products as less likely than other responses.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
This study provides data that suggest e-cigarette flavour-limiting policies would likely have a positive public health impact and also identifies potential unintended consequences of flavour-limiting policies. The data may allow regulators to anticipate and prevent unintended consequences of an e-cigarette flavour-limiting policy, such as informing surveillance efforts or supporting affected populations.
Electronic cigarettes (e-cigarettes) use an electric heater to aerosolise a liquid often containing propylene glycol, vegetable glycerin, nicotine and chemical flavourants for user inhalation1 and are available in a great variety of flavours. E-cigarette use has increased since e-cigarettes were introduced to the US market in 2006. In the USA, prevalence of current e-cigarette use (ie, use in the past 30 days) among adults increased from 1.9% in 20122 to 4.5% in 2019.3 E-cigarette use among youth and young adults has increased more rapidly. In 2011, current e-cigarette use was reported by 1.5% of high school students and 0.6% among middle school students.4 In 2020, current e-cigarette use was reported by 19.6% of high school students and 4.7% of middle school students in the USA.5
There were more than 7700 unique e-cigarette flavours in 20136 and over 15 000 flavours in 2016.7 Flavour is a less endorsed reason for use among adults 25 years and older compared with youth and young adults under the age of 25 years, and more adults 25+ years old prefer tobacco flavoured e-cigarettes.8 Conversely, flavoured e-cigarette products are appealing to youth,9 10 who use non-tobacco sweet flavours most commonly.11 Among youth, the availability of appealing flavours is the most important factor when trying e-cigarettes12 and is a frequently reported reason for use.13 Data from 2020 show that 84.7% of high school students and 73.9% of middle school students who currently use e-cigarettes in the USA use non-tobacco flavoured e-cigarettes.14
Policies have been proposed to prevent youth e-cigarette use. Some countries, such as India, have banned all e-cigarette products.15 Others, including Canada, have proposed a ban on non-tobacco and non-menthol/mint flavoured e-cigarette products,16 while the USA announced an enforcement policy to remove non-tobacco and non-menthol flavoured e-cigarettes in ‘cartridge-based’ e-cigarette products from the market in January of 2020 and began removing products from the market after allowing manufacturers time to comply.17 However, policy implementation and change in available products on the market may result in unintended consequences. Data are needed to inform possible outcomes of flavour-limiting e-cigarette policies.
One approach for examining the effect of policies before they are implemented is to ask consumers to report anticipated responses to a hypothetical policy, such as a ban of flavoured tobacco products. Surveys of tobacco users have been used to examine anticipated impacts of hypothetical menthol cigarette bans18–22 and responses can be predictive of actual behaviours after policy implementation.23 While surveys allow researchers to gather data from many participants, the closed ended nature of surveys may not assess all the possible reactions to proposed policies. Concept mapping,24 a mixed methods participatory approach, is ideally suited to examine potential impacts of policies restricting e-cigarette flavours and has been used previously to examine users’ responses to implemented policies limiting flavoured tobacco products.25 This study’s purpose was to examine e-cigarette users’ reactions to a hypothetical policy limiting the availability of e-cigarette flavours to only tobacco, menthol or unflavoured.
Methods
Participants
In 2019 (prior to the implementation of the US policy prohibiting non-tobacco and non-menthol flavoured cartridge-based e-cigarette devices), current (past 30 days) e-cigarette users were recruited by posting advertisements at 24 randomly selected Craigslist locations from each of the four US census regions (eight per region; as in refs 26 27). Advertisements invited current e-cigarette users to complete a screening questionnaire. Research staff sent email invitations to eligible participants. Participants were eligible if they reported past 30-day e-cigarette use and were over the age of 18 years at the time of screening. Eligible individuals were invited to participate (n=249) and 82 participants consented to participate in the study (response rate=32.9% among those who completed the screening questionnaire and were invited to participate). However, one participant did not complete any study tasks after consenting, resulting in a final sample of 81.
Brainstorming
At the study website (The Concept System Global MAX), participants completed a demographic questionnaire including items assessing e-cigarette/tobacco use and product preferences. Preferred e-cigarette flavour was assessed using the question, ‘Which of the following best categorizes the e-liquid/e-juice flavors (ie, smell and taste) you used the most in the past 30 days?’. After the questionnaire, participants brainstormed statements that completed the prompt, ‘If e-liquid flavors were limited so that the only e-liquid flavors available for purchase in the U.S. were tobacco only flavor, menthol only flavor, or unflavored (and no other flavors), what is a specific action you would take or a specific reaction you would have?’. To increase understanding, detailed instructions provided examples of flavours that would be prohibited, including fruit, sweet, chocolate, flavours with spices, as well as ‘ice’ flavours (such as ‘strawberry-ice’). Participants were encouraged to provide multiple statements. Each statement was added to an ongoing list that was visible to other participants such that participants who completed the brainstorming task after others were able to see statements generated previously. Participants were asked to review previous statements and attempt to avoid duplicating previous content. Having participants contribute to a shared statement list but complete the task individually prevents interference due to having to wait one’s turn to speak (as in a focus group setting)28 and also can generate more29 30 unique31 32 ideas. Statements were monitored by the study team, and when content saturation was reached (ie, additional participants no longer provided unique content), a final reminder email was sent to the invited participants to complete the brainstorming task. After 3 days, the brainstorming task was closed. A total of 81 participants generated 153 statements, and each participants received $10 for completing brainstorming.
After brainstorming, three reviewers examined each statement independently to identify statements that did not relate to the prompt or did not describe a specific reaction or response (eg, ‘WHO DOES THAT???’) as well as statements that represented duplicate content (eg, ‘I would try to quit’ and ‘I would work on quitting’). Statements were candidates for removal if two or three reviewers identified statements as not relating to the prompt. These statements were removed based on group discussion to reach consensus among the reviewers that the statement did not relate to the prompt. Similarly, when multiple statements were identified by two or three reviewers as representing redundant content, the statements were identified as candidates for removal. Based on group discussion and consensus, the statement that was the simplest (ie, fewest words) that best conveyed the idea was retained, and all others were removed. After review, 71 statements were retained. The study team reviewed and edited statements for consistency (eg, ‘vaping’ used throughout for e-cigarette use) and corrected grammar and spelling errors.
Sorting
All participants that completed the brainstorming task (n=81) were invited to return to the study website to complete a sorting and rating task. For sorting, participants grouped statements into ‘piles’ of similar content with the following instructions: piles were to represent a single idea or theme, there could not be an ‘other/miscellaneous’ pile or pile that grouped statements relating to multiple themes together, and all statements could not be in a single pile. This task was completed by each participant independently. The research team reviewed each participant’s sorting and contacted those who did not follow instructions. After review, 54 participants completed sorting; however, 15 did not follow instructions and did not return to the study website to complete sorting correctly. Thirty-nine participants completed the sorting task according to instructions, consistent with the number of participants needed to achieve good model fit based on a pooled analysis of concept mapping studies.33 Participants received $25 for completing sorting.
Rating
Participants were asked to rate each of the 71 statements based on the prompt, ‘This is an action I would take or a reaction I would have if the only e-liquid flavors available for purchase in the U.S. were tobacco only, menthol only, or unflavored (and no other flavors)’, with response options ranging from 1 (definitely not true for me) to 7 (definitely true for me). Research staff reviewed participants’ rating responses for straightlining or obvious patterns in responses. Fifty participants completed the rating task and received $10.
Representation
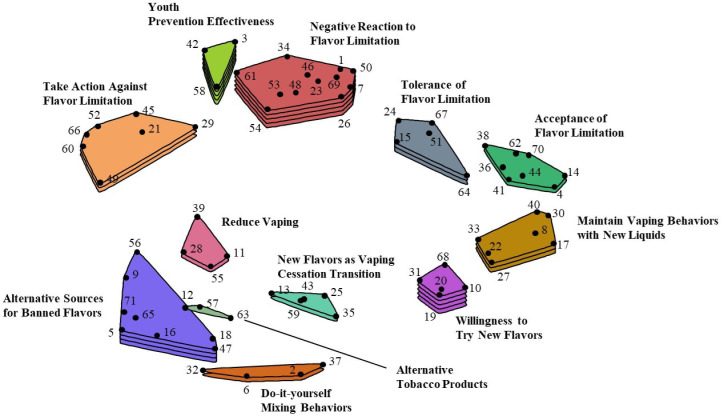
A 71×71 matrix of similarities was generated that identified the number of times statements were sorted together by participants. Using an algorithm,34 non-metric multidimensional scaling (MDS) of participant sorting data was used to create a point map (see figure 1). Each point on the map represented a statement with the location corresponding to sorting data, such that points that were closer together represented statements that were sorted together by more participants and thus represented similar content. The final stress of the model, an indicator of congruence between sorting data and the MDS,35 was 0.25, consistent with previous concept mapping studies indicating good model fit.33
Figure 1.
Concept map displaying 12 e-cigarette user-identified clusters and statements describing a specific action/reaction if the only e-liquid flavours available for purchase in the USA were tobacco-only flavour, menthol-only flavour or unflavoured (and no other flavours). Numbered points on the map that are closer to one another represent statements of more similar content, whereas points on the map that are further apart represent statements of less similar content. Greater number of layers in clusters indicate higher mean ratings of statements within each cluster based on the rating task.
Analysis and interpretation
To identify content themes, an algorithm36 was used to identify clusters of statements that limited the total distance between points on the point map to the centroid of the identified cluster of statements. First, a two-cluster model was examined. Subsequent models were examined in a hierarchical cluster analysis process by using the same algorithm to identify additional clusters of statements by separating one cluster from the previous model into two clusters. For example, a three-cluster model was created by separating one cluster from a two-cluster model into two clusters and leaving the other cluster the same. This procedure was repeated, and the research team examined each model until a final model was identified that was most parsimonious (ie, fewest clusters preferred) with each cluster only describing a single idea or theme. The final model was determined based on group consensus. Mean cluster ratings were calculated by averaging the ratings of each statement within each cluster by all participants who completed the rating task. Mean cluster ratings were compared between participants based on their preferred e-cigarette liquid flavours and e-cigarette device type using independent t-tests.
Results
Participant characteristics
As displayed in table 1, the mean age of participants was 37.6 (SD=11.4) and the majority identified as women (53.1%), non-Hispanic (85.2%), and white/European American (74.1%). Regular e-cigarette use for 1 year or more was reported by 60.5% of the participants. On the days participants used e-cigarettes, over half (54.4%) reported using e-cigarettes ‘almost always throughout most of the day’ or ‘fairly frequently throughout the day’. Most common regular e-cigarette devices included pod mods (39.5%), rebuildable/box mods (29.6%) or disposable e-cigarettes (13.6%). Just under one-third reported that their preferred e-cigarette liquid flavour was menthol/mint (30.9%), followed by fruit (24.7%) or other flavours (24.7%) including clove, spice, nut, alcoholic drink, coffee/tea, candy or dessert. No participants reported using unflavoured e-cigarettes. Most (86.4%) reported 100+ lifetime cigarettes and 59.3% reported current cigarette smoking.
Table 1.
Sample demographics and e-cigarette/tobacco use characteristics (n=81)
| Characteristic | n | % |
| Age (M, SD) | 37.6 | 11.4 |
| Gender | ||
| Women | 43 | 53.1 |
| Men | 33 | 40.7 |
| Transgender or other Did not respond |
2 3 |
2.5 3.7 |
| Ethnicity | ||
| Hispanic/Latino(a) | 12 | 14.8 |
| Race | ||
| American Indian/Alaskan Native | 0 | 0 |
| Asian | 4 | 4.9 |
| Native Hawaiian/Pacific Islander | 1 | 1.2 |
| Black/African-American | 11 | 13.6 |
| White/European American | 60 | 74.1 |
| More than one race Did not respond |
2 4 |
2.5 4.9 |
| Education | ||
| High school diploma or General Education Development Test | 13 | 16.0 |
| Some college credit but less than 1 year | 11 | 13.6 |
| 1 or more years of college, no degree | 18 | 22.2 |
| Associate’s degree | 10 | 12.3 |
| Bachelor’s degree | 20 | 24.7 |
| Higher than a bachelor’s degree Did not respond |
6 4 |
7.4 4.9 |
| Regular e-cigarette use history* | ||
| 0–3 months | 5 | 6.2 |
| 4–6 months | 10 | 12.3 |
| 7–12 months | 16 | 19.8 |
| Between 1 and 2 years | 16 | 19.8 |
| More than 2 years Did not respond |
33 2 |
40.7 2.5 |
| E-cigarette use frequency | ||
| At least once per day | 12 | 14.8 |
| Every once in a while throughout the day | 24 | 29.6 |
| Fairly frequently throughout the day | 22 | 27.2 |
| Almost always throughout most of the day Did not respond |
22 2 |
27.2 2.5 |
| Regular e-cigarette device | ||
| Prefilled disposable/cig-alike | 11 | 13.6 |
| E-hookah | 1 | 1.2 |
| Vape pen/eGo style device | 10 | 12.3 |
| Rebuildable/Mechanical Mod or Box Mod | 24 | 29.6 |
| E-cigar | 2 | 2.5 |
| Pod mod such as JUUL | 32 | 39.5 |
| E-cigarette liquid nicotine concentration | ||
| 0–4 mg/mL | 17 | 21.0 |
| 5–10 mg/mL | 21 | 25.9 |
| 11–20 mg/mL | 13 | 16.0 |
| 21–30 mg/mL | 8 | 9.9 |
| 31–40 mg/mL | 1 | 1.2 |
| 50–60 mg/mL | 14 | 17.1 |
| Don’t know | 5 | 6.1 |
| E-cigarette liquid flavour preference | ||
| Menthol or mint | 25 | 30.9 |
| Tobacco | 14 | 17.3 |
| Fruit | 20 | 24.7 |
| Other (including clove, spice, nut, alcoholic drink, coffee/tea, candy or dessert) | 20 | 24.7 |
| E-cigarette use after waking | ||
| After 60 min | 20 | 24.7 |
| 31–60 min | 16 | 19.8 |
| 6–30 min | 19 | 23.5 |
| Within 5 min | 24 | 29.6 |
| E-cigarette Dependence Scale – Reach for e-cigarette† | ||
| Never | 3 | 3.7 |
| Rarely | 8 | 9.9 |
| Sometimes | 30 | 37.0 |
| Often | 25 | 30.9 |
| Almost always | 13 | 16.0 |
| E-cigarette Dependence Scale – Vape more before not allowed† | ||
| Never | 9 | 11.1 |
| Rarely | 7 | 8.6 |
| Sometimes | 24 | 29.6 |
| Often | 21 | 25.9 |
| Almost always | 18 | 22.2 |
| E-cigarette Dependence Scale - Drop everything to buy e-cigarettes† | ||
| Never | 15 | 18.5 |
| Rarely | 20 | 24.7 |
| Sometimes | 28 | 34.6 |
| Often | 10 | 12.3 |
| Almost always | 5 | 6.2 |
| E-cigarette Dependence Scale – craving gets intolerable† | ||
| Never | 15 | 1853 |
| Rarely | 14 | 17.3 |
| Sometimes | 27 | 33.3 |
| Often | 17 | 21.0 |
| Almost always | 6 | 7.4 |
| Lifetime use of 100+ cigarettes | ||
| Yes | 70 | 86.4 |
| Current use of other tobacco products | ||
| Cigarettes | 48 | 59.3 |
| Cigar | 9 | 11.1 |
| Cigarillo or little cigar | 17 | 21.0 |
| Smokeless | 5 | 6.2 |
| Waterpipe | 15 | 18.5 |
Note: total n and percentages for sample characteristics is based on the 81 participants who completed the participant questions.
Reactions and responses to a hypothetical flavour restriction clusters.
*Regular use was defined as using an e-cigarette some days or most days.
†Items from the four-item E-cigarette Dependence Scale51 including ‘I find myself reaching for my e-cigarette without thinking about it’, ‘I drop everything to go out and buy e-cigarettes or e-juice’, I vape more before going into a situation where vaping is not allowed’, and ‘When I haven’t been able to vape for a few hours, the craving gets intolerable’.
A 12-cluster model was determined to be the best fit (see figure 1). Each cluster is described below in groups of related clusters (see table 2).
Table 2.
E-cigarette user-identified clusters and statements describing a specific action/reaction if the only e-liquid flavours available for purchase in the USA were tobacco-only flavour, menthol-only flavour or unflavoured (and no other flavours)
| Cluster | Statement | Average rating |
| Negative reaction to flavour limitation | 4.68 | |
| 34. I would think consumers have a right to choose what they want to buy. | 5.84 | |
| 69. I would think that limiting the flavours is silly. | 5.36 | |
| 26. I would feel bad for the people that liked different flavors. | 5.00 | |
| 1. I would hate it because I enjoy vaping with flavored e-liquid as an outlet. | 4.88 | |
| 50. I would not like the flavors being banned. | 4.82 | |
| 48. I would hate it since it did help me/others quit cigarettes. | 4.76 | |
| 46. I would be annoyed. | 4.62 | |
| 7. I think that using the same flavor would get boring after a while. | 4.47 | |
| 23. I would be upset. | 4.44 | |
| 53. I would panic. | 2.60 | |
| Willingness to try new flavours | 4.43 | |
| 19. Try the new e-liquids and if they were tolerable, I would keep vaping. | 4.70 | |
| 20. Vape/try the menthol flavored e-liquid to see if I liked it. | 4.52 | |
| 31. Vape/try the unflavored e-liquid to see if I liked it. | 4.50 | |
| 10. Vape/try the tobacco flavored e-liquid to see if I liked it. | 4.48 | |
| 68. I would be curious about the unflavored e-liquid and be interested in trying it. | 3.94 | |
| Perception of youth prevention effectiveness | 4.04 | |
| 3. I would think limiting flavors will not stop kids from vaping. | 4.84 | |
| 42. I would wonder if having less flavors would reduce vaping among youth. | 4.26 | |
| 58. I would be happy that kids are not being targeted with flavored e-liquids. | 3.88 | |
| 54. I would think that it might make vaping less tempting to youth who shouldn’t be using tobacco and/or nicotine products. | 3.86 | |
| 61. Lose all respect for the U.S. government. | 3.38 | |
| Alternative sources for banned flavours | 3.95 | |
| 9. Stock up on the e-liquid I currently use. | 5.44 | |
| 18. Try to find a place that still has the flavored e-liquid. | 5.28 | |
| 47. I would buy my current e-liquid flavors online. | 4.80 | |
| 56. Talk to friends to find another option. | 3.58 | |
| 5. Encourage others to make their own e-liquid. | 3.56 | |
| 71. Find a way to order flavored e-liquid from outside the U.S. | 3.56 | |
| 16. Look into buying flavored e-liquids off the dark web. | 2.82 | |
| 65. Buy ‘black market’ e-liquid. | 2.56 | |
| Maintain vaping behaviours with new liquids | 3.93 | |
| 17. Vape about the same as I do with my current e-liquid flavor(s). | 4.68 | |
| 33. I would eventually adapt to the limited flavors. | 4.50 | |
| 8. My e-liquid buying habits would stay the same. | 4.10 | |
| 30. I would be fine as long as menthol/mint flavored e-liquid is available. | 3.88 | |
| 27. I would vape only menthol or unflavored e-liquids. | 3.72 | |
| 40. I would enjoy the menthol flavored e-liquid – menthol is good. | 3.44 | |
| 22. Alternate between tobacco only and menthol only flavors. | 3.18 | |
| Tolerance of flavour limitation | 3.63 | |
| 67. It would bother me, but not enough to go out and actively do anything about it. | 3.88 | |
| 24. I like menthol flavored e-liquid, but it does get boring after a while. | 3.76 | |
| 51. I would be mad, but I would tolerate the flavor ban. | 3.60 | |
| 15. I would try these new flavors, but I do not think I would like them. | 3.28 | |
| Acceptance of flavour limitation | 3.61 | |
| 62. I would be grateful that all e-liquid was not banned. | 4.36 | |
| 36. I would not do anything about the flavors being limited. | 4.10 | |
| 64. Even though I mainly use the tobacco flavor, I like having the option to try something new. | 3.84 | |
| 44. I would remain content since I only use these flavors anyway. | 3.72 | |
| 41. I would not be bothered if e-liquid flavors were limited. | 3.60 | |
| 4. I would think menthol and non-menthol are how cigarettes come, so it makes sense for e-liquid too. | 3.56 | |
| 14. I like many different flavors, but I would not be affected by the flavor ban. | 3.46 | |
| 70. I would be fine with limiting e-liquid flavors to tobacco, menthol, and unflavored only. | 3.46 | |
| 38. I would think this is perfect. | 2.42 | |
| Take action against flavour limitation | 3.58 | |
| 29. I would be concerned about illegal or black market e-liquid flavors being put on the market. | 4.94 | |
| 66. Never vote for anyone who backed this flavor ban. | 3.62 | |
| 21. I would do whatever I could to fight the flavor ban. | 3.58 | |
| 60. I would protest the flavor limitation. | 3.54 | |
| 45. Post about the flavor limitation on social media. | 3.50 | |
| 52. I would write every representative I can to complain about the flavor ban. | 3.10 | |
| 49. Call customer service. | 2.78 | |
| Do-it-yourself mixing behaviours | 3.44 | |
| 37. Add different flavors to the new e-liquids. | 3.94 | |
| 6. I would make my own or learn how to make my own e-liquid. | 3.58 | |
| 2. Use/add CBD oil in my e-liquid. | 3.32 | |
| 32. Add essential oils to the new e-liquid. | 2.92 | |
| New flavours as vaping cessation transition | 3.23 | |
| 13. Change my habit to something else besides vaping. | 3.70 | |
| 43. Try the new e-liquids and if I didn’t like them, I would try to stop vaping. | 3.20 | |
| 35. I would use menthol flavored e-liquid and work on quitting vaping. | 3.18 | |
| 25. I would use the tobacco flavored e-liquid and work on quitting vaping. | 3.10 | |
| 59. I would use the unflavored e-liquid and work on quitting vaping. | 2.98 | |
| Reduce vaping | 3.23 | |
| 39. I would consider quitting vaping if my particular flavor was affected. | 3.46 | |
| 11. Slowly taper my vaping down. | 3.42 | |
| 55. I would vape less. | 3.20 | |
| 28. I would quit vaping. | 2.84 | |
| Alternate tobacco products | 2.73 | |
| 12. I would smoke cigarettes. | 3.76 | |
| 63. Buy a hookah pipe or hookah tower. | 2.34 | |
| 57. I would use smokeless tobacco (ie, dip, chew, snus, etc.). | 2.10 | |
Note: mean ratings are based on responses to the prompt ‘If e-liquid flavors were limited so that the only e-liquid flavors available for purchase in the U.S. were tobacco only flavor, menthol only flavor, or unflavored (and no other flavors), what is a specific action you would take or a specific reaction you would have?’ using a seven-point scale from 1 (definitely not true for me) to 7 (definitely true for me). Statement numbers refer to point numbers displayed in figure 1.
Cognitive, emotional and protesting reactions to policy
The first group of four clusters described perceptions that e-cigarette users would have negative reactions to a flavour-limiting policy. The first cluster, negative reaction to flavour, had the most statements of all clusters (n=11) and the highest mean cluster rating (M=4.68 out of 7, SD=0.80). The statements described disapproval and negative affect as anticipated responses, such as the highest rated of all statements in the study, ‘I would think consumers have a right to choose what they want to buy’. Other statements described that e-cigarette users would ‘hate’ or be upset by the policy because they enjoyed flavours and that flavours helped some quit smoking cigarettes. The next highest rated cluster in this group, youth prevention effectiveness perceptions (n=5 statements, M=4.04, SD=0.49), described anticipation of mixed beliefs regarding how the policy may be effective in preventing youth from using e-cigarettes or tobacco products. Statements were opposed, with some describing perceptions that a flavour ban would be ineffective and inappropriate, while others described how the ban may deter youth from tobacco use due to not being targeted with flavoured e-cigarette liquids. Despite describing positive and negative sentiment towards the flavour-limiting policy, all statements shared a common theme of perceptions related to the appropriateness or effectiveness of the hypothetical policy.
The statements in the take action against flavour limitation cluster (n=7 statements, M=3.58, SD=0.62) described plans to take political or other actions to directly affect or challenge the flavour-limiting policy. Actions included ‘never vote for anyone who backed the ban’, ‘protest the flavor limitation’, or ‘post about the flavor limitation on social media’. The last cluster in this group, tolerance of flavour limitation (n=4, M=3.63, SD=0.23), described similar disappointment or disapproval of a flavour-limiting policy; however, statements suggested that participants perceived they would tolerate the ban and would not take any actions to change the policy.
Adapting to and accepting flavour limitation
The next group of three clusters described more neutral to positive responses to a flavour-limiting policy. The cluster with the most statements in this group, acceptance of flavour limitation (n=9, M=3.61, SD=0.51) described being content and grateful that all flavours were not removed. One statement compared flavours in e-cigarette liquids to cigarettes: ‘I would think menthol and non-menthol are how cigarettes come, so it makes sense for e-liquid too’. Several statements also described how e-cigarette users may desire or miss non-tobacco or non-menthol flavours, but in general, e-cigarette users would not be affected by limiting e-cigarette flavours to only tobacco, menthol or unflavoured. The highest rated cluster in this group, willingness to try new flavours (n=5, M=4.43, SD=0.26) included statements describing how e-cigarette users would try menthol, tobacco and unflavoured liquids to see if they liked them. Similarly, the highest rated statement in this cluster indicated that e-cigarette users would try the new flavours that were available and continue to use them if they were ‘tolerable’. One statement also described curiosity about unflavoured liquids. The last cluster in this group, maintain vaping behaviours (n=7, M=3.93, SD=0.50) included statements that described how e-cigarette users would maintain their same e-cigarette use behaviours but with the new e-cigarette flavours. Several statements described how e-cigarette purchasing and use would not change after the flavour limitation. One statement suggested a strategy of alternating between tobacco and menthol flavours, and several statements described a preference for menthol flavoured liquid.
E-cigarette use reduction and cessation
Two related clusters included statements that described intentions to decrease e-cigarette use after a flavour limitation. The reduce vaping cluster (n=4, M=3.23, SD=0.25) included statements that described intentions to ‘consider quitting’ e-cigarette use if a preferred flavour was affected, ‘slowly taper down’ e-cigarette use, ‘vape less’ and ‘quit vaping’. The new flavours as vaping cessation transition cluster (n=5, M=3.23, SD=0.25) had the same mean rating as the reduce vaping cluster, but most statements in this cluster described how participants would use the new flavours as part of a transition away from e-cigarette use.
Alternative e-cigarette liquid sources and tobacco products
The final group of three clusters described attempts to continue e-cigarette use with flavours beyond tobacco, menthol or unflavoured liquid or use other tobacco products. The alternative sources for banned flavours cluster had the highest rating and most statements (n=8, M=3.95, SD=1.02) in this group; however, there was a large range in mean statement ratings. For example, higher rated statements in this cluster included ‘Stock up on the e-liquid I currently use’ (M=5.44), ‘Try to find a place that still has the flavoured e-liquid’ (M=5.28) and ‘I would buy my current e-liquid flavours online’ (M=4.80). Lower rated statements included ‘Look into buying flavoured e-liquids off the dark web’ (M=2.82) and ‘Buy “black market” e-liquid’ (M=2.56).
The do-it-yourself mixing behaviours cluster (n=4, M=3.44, SD=0.37) described e-cigarette users’ planned attempts to create their own liquids (ie, DIY liquids). Behaviours included adding flavours, learning how to make DIY liquids, adding flavours to the tobacco, menthol or unflavored liquids that were available or adding CBD or essential oils in e-cigarette liquids. The alternative tobacco products cluster had three statements, had the lowest mean cluster rating (M=2.73, SD=0.73) and identified smoking cigarettes, hookah or using smokeless tobacco as an alternative to e-cigarette use if flavours were limited.
Mean cluster comparisons
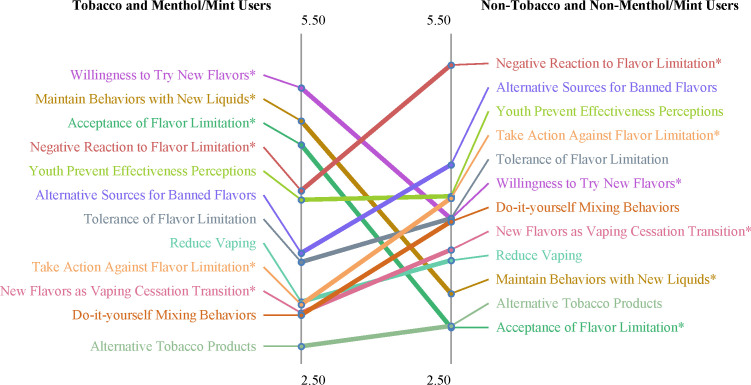
Mean cluster ratings were associated with preferred e-cigarette liquid flavour. As displayed in figure 2, compared with participants who reported tobacco or menthol/mint as their preferred e-cigarette liquid flavour, participants who reported using other flavours that would be prohibited in the hypothetical flavour limitation had higher ratings (ie, rated as more true) for the negative reaction (t=3.11, p<0.01), take action against flavour limitation (t=2.48, p<0.05) and new flavours as vaping cessation transition clusters (t=3.00, p<0.05). Non-tobacco and non-menthol/mint flavour users had lower ratings for acceptance of the flavour limitation (t=6.51, p<0.001), maintain behaviours with new flavours (t=5.46, p<0.001) and willingness to try new flavours (t=7.14, p<0.001) clusters.
Figure 2.
Pattern match of mean cluster ratings between e-cigarette users who report their preferred flavour as tobacco or menthol/mint (n=24) and e-cigarette users who report their preferred flavour as a non-tobacco or non-menthol/mint flavour (n=25). Asterisks (*) indicate significantly different mean cluster ratings between groups (p’s<0.05).
Mean cluster ratings also differed by e-cigarette device type. Compared with those who used pod mod or disposable devices, mechanical/box mod users had higher ratings for the negative reaction cluster (t=3.35, p<0.005) and lower ratings for the acceptance (t=6.81, p<0.001), maintain behaviours with new flavours (t=5.34, p<0.001) and willingness to try new flavours (t=8.31, p<0.001) clusters.
Discussion
This study identified 12 themes describing current e-cigarette users’ responses to a hypothetical policy prohibiting the sale of flavoured e-cigarette products besides tobacco, menthol and unflavoured products. Reported responses included those describing tolerance, acceptance or even support for an e-cigarette flavour-limiting policy. Other responses described negative emotions in response to the policy, with some statements describing planned actions in attempts to challenge or change the new policy. Finally, some responses described behaviours e-cigarette users might engage in to adapt to a flavour-limiting policy, including trying the new flavours, reducing or quitting e-cigarette use, or seeking alternative sources to access e-cigarette or other products with desired flavours.
Findings from this study are consistent with previous research examining bans on flavoured e-cigarettes. In the current study, non-tobacco or non-menthol/mint e-cigarette users had higher ratings for the new flavours as vaping cessation transition cluster compared with tobacco or menthol/mint flavoured e-cigarette users. This is similar to findings from a study examining responses to a hypothetical e-cigarette flavour ban among e-cigarette users recruited from vape shops in California in which non-tobacco flavoured e-cigarette users were less likely to report planning to continue e-cigarette use after a flavour ban.37 Additionally, those who reported e-cigarette use for smoking cessation reported they would be more likely to continue e-cigarette use with tobacco-only flavours if all non-tobacco flavoured e-cigarettes were banned.37 These data in combination with the findings displayed in figure 2 of the current study that demonstrate greater acceptability of a flavour-limiting policy among tobacco or menthol/mint flavoured e-cigarette users have important implications. If cigarette smokers are willing to use tobacco or menthol-only flavoured e-cigarettes, then a policy that limits e-cigarette flavours to only tobacco or menthol may prevent youth or tobacco naïve individuals from e-cigarette use while still maintaining e-cigarettes as an acceptable option for combustible cigarette smokers.
Other studies examining tobacco flavour bans have also described negative sentiment towards flavour bans but limited evidence for unintended consequences. Similar to the negative reaction to the flavour limitation cluster, analyses of Twitter posts related to a flavour ban in New York State found that a vast majority of posts before and after an imposed flavour ban were negative.38 While there is often mixed sentiment regarding support for a flavour ban,18 many tobacco users report intentions to quit following a flavour ban.20 Additionally, analyses of behaviours after tobacco product flavour ban implementation suggest tobacco use decreases after ban implementation23 39 40 with minimal unintended consequences, such as ‘black market’ purchasing behaviours.25
This study has important regulatory implications in the USA and globally. First, policies limiting e-cigarette flavours may be viewed unfavourably by e-cigarette users whose preferred flavours are eliminated. However, higher rated statements relating to trying new flavours suggest e-cigarette users may adapt to the reduced flavours and some e-cigarette users who prefer non-tobacco and non-menthol flavours may consider reducing or quitting vaping. At the same time, having flavours that are known to be appealing to youth removed from the market may prevent youth e-cigarette use. High ratings for statements in the alternative sources for banned flavours cluster suggest that flavour-limiting policies need to consider that, if available, e-cigarette users will likely seek out appealing flavours, and menthol may have higher appeal than tobacco flavour. One study of JUUL product sales found that after JUUL removed crème brulée, mango and other flavours from the market in 2019, mint-flavoured JUUL products became the most popular products among high school students,41 and menthol sales increased after mint was removed in 2020.42 Other research suggests that flavour-limiting policies that are not comprehensive across all e-cigarette products will likely be ineffective: after all non-tobacco or non-menthol flavoured cartridge-based e-cigarettes were removed from the US market beginning in 2020, there was a rapid increase in disposable e-cigarette products that resembled cartridge-based products but were available in flavours prohibited in cartridge-based products due to a regulatory loophole.43 44
Some statements indicated that e-cigarette users may attempt to obtain prohibited flavours through alternative sources, either the black market, make their own liquids or stock up from locations that currently or continue to sell prohibited flavours. Unlike other flavoured tobacco products, such as menthol cigarettes, flavoured e-cigarette liquids can be made relatively easily in large quantities. As long as open system e-cigarettes remain available, regulating e-cigarettes and their devices will present challenges.45 Because the primary ingredients in flavoured e-cigarette liquids can be purchased easily, and all but nicotine likely fall outside of tobacco regulatory authority, regulators may consider product standards that prevent or limit the ability of consumers from adding non-authorised e-cigarette liquids to e-cigarette devices, such as requiring that e-cigarette devices be ‘closed-systems’. Additionally, enforcement efforts following an e-cigarette flavour ban will likely need to ensure that e-cigarette retailers continue to sell only e-cigarette products in authorised flavours. Industry responses should also be monitored that would undermine the intended effects a flavour-limiting policy, such as the introduction of products that consumers can add to products to provide desired flavours/tastes.46
This study had several limitations. The sample size may limit generalisability to all e-cigarette users. However, participants included current e-cigarette users from 22 states across the USA, and the sample size was appropriate for generation of content themes and is consistent with recommended sample sizes for concept mapping studies.47 While menthol is derived from the mint plant, some policies may allow menthol flavours but prohibit mint flavours. The survey item in the current study assessing flavour preference did not distinguish between participants who preferred mint versus menthol. Therefore, while this study treated mint and menthol as being allowed in the hypothetical policy, responses may have differed if mint was not included as an authorised flavour. Finally, while reported responses to a hypothetical flavour-limiting policy may be predictive of future behaviours, future research is needed to evaluate the consistency between the predicted behaviours reported in the current study and actual behaviours that result if a similar policy is implemented in the USA.
A ban on flavoured e-cigarettes may be seen as unpopular by some e-cigarette users whose flavours are impacted, but the current study and others suggest that policies limiting flavours in tobacco products could have a positive public health impact (eg, tobacco use reduction and decreased youth tobacco use). Indeed, proponents of e-cigarette use for smoking cessation or harm reduction suggest that eliminating flavoured e-cigarettes ‘enhances their safety profiles and maintains the avenue as a possible smoking cessation aid’48 and clinical laboratory studies demonstrate that tobacco flavoured e-cigarettes can suppress withdrawal in current cigarette smokers (eg, refs 49 50). Results from the current study may provide insights into e-cigarette users’ behaviours after flavour-limiting policies are implemented, with several behaviours highlighted in the current study already being documented after a ban on non-tobacco or non-menthol flavoured cartridge-based e-cigarettes in the USA. With data from the current study suggesting tobacco-only flavoured e-cigarettes may be acceptable among current e-cigarette users and some cigarette smokers, implementing policies that limit e-cigarette flavours to tobacco and menthol only (or tobacco only) would likely have a positive impact on public health, especially if these flavours have limited appeal to youth and non-tobacco users.
Footnotes
Contributors: ES, AB, MCG and PF conceptualised the study. ES led planning and conduct of the study. ES, SM and WS contributed to data collection and analysis. WS and LT assisted with development of tables and figures. ES wrote the first draft of the manuscript, and all authors provided critical review of the manuscript. ES is the guarantor of the study and manuscript.
Funding: This work was supported by grant number U54DA036105 from the National Institute on Drug Abuse of the National Institutes of Health and the Center for Tobacco Products of the US Food and Drug Administration. ES’s effort is also supported by grant number grant number P50MD017319 of the National Institute on Minority Health and Health Disparities, grant number R15ES032138 of the National Institute of Environmental Health Sciences, grant number R21CA239188 of the National Cancer Institute of the National Institutes of Health.
Competing interests: ES is named on a patent application for a smartphone app that determines electronic cigarette device and liquid characteristics. All other authors have no conflicts to report.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available on reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by East Carolina University and Medical Center Institutional Review Board UMCIRB 19-002116. Participants gave informed consent to participate in the study before taking part.
References
- 1. Breland A, Soule E, Lopez A, et al. Electronic cigarettes: what are they and what do they do? Ann N Y Acad Sci 2017;1394:5–30. 10.1111/nyas.12977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Agaku IT, King BA, Husten CG, et al. Tobacco product use among adults--United States, 2012-2013. MMWR Morb Mortal Wkly Rep 2014;63:542–7. [PMC free article] [PubMed] [Google Scholar]
- 3. Cornelius ME, Wang TW, Jamal A, et al. Tobacco Product Use Among Adults - United States, 2019. MMWR Morb Mortal Wkly Rep 2020;69:1736–42. 10.15585/mmwr.mm6946a4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang TW. Tobacco product use among middle and high school students — United States, 2011–2017. MMWR Morb Mortal Wkly Rep 2018;67. 10.15585/mmwr.mm6722a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang TW, Neff LJ, Park-Lee E, et al. E-cigarette Use Among Middle and High School Students - United States, 2020. MMWR Morb Mortal Wkly Rep 2020;69:1310–2. 10.15585/mmwr.mm6937e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhu S-H, Sun JY, Bonnevie E. Four hundred and sixty brands of e-cigarettes and counting: implications for product regulation. Tob Control 2014;23 Suppl 3:3–9. 10.1136/tobaccocontrol-2014-051670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hsu G, Sun JY, Zhu S-H. Evolution of electronic cigarette brands from 2013-2014 to 2016-2017: analysis of brand websites. J Med Internet Res 2018;20:e80. 10.2196/jmir.8550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Soneji SS, Knutzen KE, Villanti AC. Use of flavored e-cigarettes among adolescents, young adults, and older adults: findings from the population assessment for tobacco and health study. Public Health Rep 2019;134:282–92. 10.1177/0033354919830967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cullen KA, Gentzke AS, Sawdey MD, et al. E-Cigarette use among youth in the United States, 2019. JAMA 2019;322:2095. 10.1001/jama.2019.18387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Villanti AC, Johnson AL, Ambrose BK, et al. Flavored tobacco product use in youth and adults: findings from the first wave of the PATH study (2013-2014). Am J Prev Med 2017;53:139–51. 10.1016/j.amepre.2017.01.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lanza HI, Leventhal AM, Cho J, et al. Young adult e-cigarette use: a latent class analysis of device and flavor use, 2018-2019. Drug Alcohol Depend 2020;216:108258. 10.1016/j.drugalcdep.2020.108258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zare S, Nemati M, Zheng Y. A systematic review of consumer preference for e-cigarette attributes: flavor, nicotine strength, and type. PLoS One 2018;13:e0194145. 10.1371/journal.pone.0194145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gupta PS, Kalagher KM. Where there is (NO) smoke, there is still fire: a review of trends, reasons for use, preferences and harm perceptions of adolescent and young adult electronic cigarette use. Curr Pediatr Rep 2021:1–5. 10.1007/s40124-021-00240-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang TW, Gentzke AS, Neff LJ, et al. Characteristics of e-cigarette use behaviors among US youth, 2020. JAMA Netw Open 2021;4:e2111336. 10.1001/jamanetworkopen.2021.11336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chakma JK, Kumar H, Bhargava S, et al. The e-cigarettes ban in India: an important public health decision. Lancet Public Health 2020;5:e426. 10.1016/S2468-2667(20)30063-3 [DOI] [PubMed] [Google Scholar]
- 16. Government of Canada PW and GSC . Canada Gazette, part 1, volume 155 number 25: order amending schedules 2 and 3 to the tobacco and Vaping products act (flavours), 2021. Available: https://gazette.gc.ca/rp-pr/p1/2021/2021-06-19/html/reg2-eng.html [Accessed 09 Jun 2022].
- 17. Office of the Commissioner . FDA finalizes enforcement policy on unauthorized flavored cartridge-based e-cigarettes that appeal to children, including fruit and MINT, 2020. Available: https://www.fda.gov/news-events/press-announcements/fda-finalizes-enforcement-policy-unauthorized-flavored-cartridge-based-e-cigarettes-appeal-children [Accessed 22 Feb 2022].
- 18. Pearson JL, Abrams DB, Niaura RS, et al. A ban on menthol cigarettes: impact on public opinion and smokers' intention to quit. Am J Public Health 2012;102:e107:14–114. 10.2105/AJPH.2012.300804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Winickoff JP, McMillen RC, Vallone DM, et al. Us attitudes about banning menthol in cigarettes: results from a nationally representative survey. Am J Public Health 2011;101:1234–6. 10.2105/AJPH.2011.300146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. O'Connor RJ, Bansal-Travers M, Carter LP, et al. What would menthol smokers do if menthol in cigarettes were banned? behavioral intentions and simulated demand. Addiction 2012;107:1330–8. 10.1111/j.1360-0443.2012.03822.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wackowski OA, Manderski MTB, Delnevo CD. Young adults' behavioral intentions surrounding a potential menthol cigarette ban. Nicotine Tob Res 2014;16:876–80. 10.1093/ntr/ntu003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wackowski OA, Delnevo CD, Pearson JL. Switching to e-cigarettes in the event of a menthol cigarette ban. Nicotine Tob Res 2015;17:1286–7. 10.1093/ntr/ntv021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chaiton MO, Nicolau I, Schwartz R, et al. Ban on menthol-flavoured tobacco products predicts cigarette cessation at 1 year: a population cohort study. Tob Control 2020;29:341–7. 10.1136/tobaccocontrol-2018-054841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Trochim WMK. An introduction to concept mapping for planning and evaluation. Eval Program Plann 1989;12:1–16. 10.1016/0149-7189(89)90016-5 [DOI] [Google Scholar]
- 25. Soule EK, Chaiton M, Zhang B, et al. Menthol cigarette smoker reactions to an implemented menthol cigarette ban. Tobacco Regulatory Science 2019;5:50–64. 10.18001/TRS.5.1.5 [DOI] [Google Scholar]
- 26. Soule EK, Lee JGL, Egan KL, et al. "I cannot live without my vape": Electronic cigarette user-identified indicators of vaping dependence. Drug Alcohol Depend 2020;209:107886. 10.1016/j.drugalcdep.2020.107886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Soule EK, Bode KM, Desrosiers AC, et al. User-Perceived negative respiratory symptoms associated with electronic cigarette use. Nicotine Tob Res 2020;22:S45–53. 10.1093/ntr/ntaa179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dennis AR, Valacich JS. Computer brainstorms: more heads are better than one. Journal of Applied Psychology 1993;78:531–7. 10.1037/0021-9010.78.4.531 [DOI] [Google Scholar]
- 29. Dennis AR, Williams ML, Theory Electronicbrainstorming. research, and future directions. In: Group creativity: Innovation through collaboration. New York, NY, US: Oxford University Press, 2003: 160–78. [Google Scholar]
- 30. DeRosa DM, Smith CL, Hantula DA. The medium matters: mining the long-promised merit of group interaction in creative idea generation tasks in a meta-analysis of the electronic group brainstorming literature. Comput Human Behav 2007;23:1549–81. 10.1016/j.chb.2005.07.003 [DOI] [Google Scholar]
- 31. Dugosh KL, Paulus PB, Roland EJ, et al. Cognitive stimulation in brainstorming. J Pers Soc Psychol 2000;79:722–35. 10.1037/0022-3514.79.5.722 [DOI] [PubMed] [Google Scholar]
- 32. Leggett Dugosh K, Paulus PB. Cognitive and social comparison processes in brainstorming. J Exp Soc Psychol 2005;41:313–20. 10.1016/j.jesp.2004.05.009 [DOI] [Google Scholar]
- 33. Rosas SR, Kane M. Quality and rigor of the concept mapping methodology: a pooled study analysis. Eval Program Plann 2012;35:236–45. 10.1016/j.evalprogplan.2011.10.003 [DOI] [PubMed] [Google Scholar]
- 34. Kruskal JB, Wish M. Multidimensional scaling. Beverly Hills, CA: Sage Publications, 1978. [Google Scholar]
- 35. Davidson M. Multidimensional scaling. New York, NY: John Wiley and Sons, 1983. [Google Scholar]
- 36. Ward JH. Hierarchical grouping to optimize an objective function. J Am Stat Assoc 1963;58:236–44. 10.1080/01621459.1963.10500845 [DOI] [Google Scholar]
- 37. Huh J, Yu S, Galimov A, et al. Hypothetical flavour ban and intention to vape among vape shop customers: the role of flavour preference and e-cigarette dependence. Tob Control 2021. doi: 10.1136/tobaccocontrol-2020-056321. [Epub ahead of print: 10 Jun 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sun L, Lu X, Xie Z, et al. Public reactions to the new York state policy on flavored electronic cigarettes on Twitter: observational study. JMIR Public Health Surveill 2022;8:e25216. 10.2196/25216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rossheim ME, Livingston MD, Krall JR, et al. Cigarette use before and after the 2009 flavored cigarette ban. J Adolesc Health 2020;67:432–7. 10.1016/j.jadohealth.2020.06.022 [DOI] [PubMed] [Google Scholar]
- 40. Chaiton M, Schwartz R, Cohen JE, et al. Association of Ontario's ban on menthol cigarettes with smoking behavior 1 month after implementation. JAMA Intern Med 2018;178:710-711. 10.1001/jamainternmed.2017.8650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Leventhal AM, Miech R, Barrington-Trimis J, et al. Flavors of e-cigarettes used by youths in the United States. JAMA 2019;322:2132–4. 10.1001/jama.2019.17968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Diaz MC, Donovan EM, Schillo BA, et al. Menthol e-cigarette sales rise following 2020 FDA guidance. Tob Control 2021;30:700–3. 10.1136/tobaccocontrol-2020-056053 [DOI] [PubMed] [Google Scholar]
- 43. Delnevo C, Giovenco DP, Hrywna M. Rapid proliferation of illegal pod-mod disposable e-cigarettes. Tob Control 2020;29:e150–1. 10.1136/tobaccocontrol-2019-055485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gaiha SM, Lempert LK, McKelvey K, et al. E-Cigarette devices, brands, and flavors attract youth: informing FDA's policies and priorities to close critical gaps. Addict Behav 2022;126:107179. 10.1016/j.addbeh.2021.107179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Eissenberg T, Soule E, Shihadeh A, et al. 'Open-System' electronic cigarettes cannot be regulated effectively. Tob Control 2021;30:234–5. 10.1136/tobaccocontrol-2019-055499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chaiton MO, Schwartz R, Cohen JE, et al. The use of flavour cards and other additives after a menthol ban in Canada. Tob Control 2021;30:601–2. 10.1136/tobaccocontrol-2020-055698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kane M, Trochim WMK. Concept mapping for planning and evaluation. Thousand Oaks, CA: Sage Publications, 2007. [Google Scholar]
- 48. Rambaran K, Sakhamuri S, Pereira LP. E-Cigarettes: banning flavours is better than an outright ban. Lancet Respir Med 2019;7:e37. 10.1016/S2213-2600(19)30359-5 [DOI] [PubMed] [Google Scholar]
- 49. Hiler M, Breland A, Spindle T, et al. Electronic cigarette user plasma nicotine concentration, puff topography, heart rate, and subjective effects: influence of liquid nicotine concentration and user experience. Exp Clin Psychopharmacol 2017;25:380–92. 10.1037/pha0000140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hiler M, Karaoghlanian N, Talih S. Effects of electronic cigarette heating coil resistance and liquid nicotine concentration on user nicotine delivery, heart rate, subjective effects, puff topography, and liquid consumption. Exp Clin Psychopharmacol. 10.1037/pha0000337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Morean ME, Krishnan-Sarin S, S O'Malley S, O’Malley S S. Assessing nicotine dependence in adolescent e-cigarette users: the 4-item patient-reported outcomes measurement information system (PROMIS) nicotine dependence item bank for electronic cigarettes. Drug Alcohol Depend 2018;188:60–3. 10.1016/j.drugalcdep.2018.03.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available on reasonable request.