Abstract
Novel feed ingredients may improve poultry health, but functionality of these ingredients may vary across basal diet formulations. This study evaluated a proprietary algae ingredient's effects on broiler performance, intestinal health, systemic immunity, and metabolic/immune kinotypes between corn- or wheat-based diets. Ross 308 broilers were housed in 80 floor pens (14 birds/pen) and assigned to 1 of 4 corn or wheat-based diets ± 0.175% algae ingredient for 42 d. At the end of each 14 d starter, grower, and finisher period, 10 birds/treatment were euthanized for tissue collection to assess intestinal histomorphology, systemic immune cell populations by flow cytometry and kinotypes by peptide arrays. On d 28 and 29, forty-three birds/treatment underwent a 12 h feed restriction challenge followed by a fluorescein isothiocyanate-dextran intestinal permeability assay. For the entire 42 d study, wheat-based diets improved feed conversion rate (FCR) by 5 points compared to corn-based diets (P < 0.0001). Performance benefits related to algae inclusion were diet dependent, with algae inclusion improving 42 d FCR by 6 points only in corn-based diets (P = 0.006). Birds fed wheat-based diets had reduced splenic monocyte/macrophage, CD1.1+, and T cell populations in the first 14 d (P < 0.0001) and reduced serum fluorescence on d 28/29 (P = 0.0002). Algae inclusion in the corn-based diet increased villus height in the duodenum on d 28 and jejunum on d 42, while reducing splenic CD3+CD8α+ cytotoxic T cells 13.4 to 27.5% compared to the corn-based control at the same timepoints (P < 0.0001). Kinome results showed a significant innate immune toll-like receptor (TLR) response via MyD88 at d 14 in the small intestine of birds fed corn-based diets with algae that shifted to a more growth factor and adaptive immune-oriented response by d 42. Concurrent with immune changes, signaling changes indicative of lipid metabolism in the small intestine, ceca, and liver were seen in birds fed the corn-based diet with algae. The observed differential responses to basal diet composition and algae inclusion emphasize the need to comparatively evaluate feed ingredients in various diet formulations.
Key words: broiler; algae; intestinal integrity; immunity, wheat
INTRODUCTION
Algae-derived feed ingredients are a natural source of bioactive compounds, including n-3 polyunsaturated fatty acids (PUFA), carotenoids, B vitamins, and non-starch polysaccharides (NSP; Christaki et al., 2011; Światkiewicz et al., 2015). Carotenoids and PUFA are associated with health-promoting antioxidant and anti-inflammatory properties, respectively, and have been linked to the beneficial effects of algae on broiler health and performance at inclusion levels below 2% (Calder, 2006; Christaki et al., 2011; Eggersdorfer and Wyss, 2018). The nutritional components present in algae can vary between sources, strains, and genera, resulting in varying host health outcomes related to immunity and intestinal health, thereby supporting investigation into the mechanisms of action for novel algae products (Yaakob et al., 2014; Światkiewicz et al., 2015; Madeira et al., 2017).
Much of the reported immunological and intestinal health improvements in broiler chickens are often observed in response to microalgal strains from Spirulina or Chlorella genera. Serological outcomes have shown increased antibody titers in response to sheep red blood cells or Newcastle disease vaccine and unchallenged broilers fed ≤1.0% Spirulina or Chlorella (Qureshi et al., 1996; An et al., 2016; Kang et al., 2017; Mirzaie et al., 2018; Khan et al., 2020). Similarly, both Spirulina (0.01–0.8%) and fresh Chlorella (1.0%) increased white blood cell counts and lymphocytes in addition to reducing heterophils in broiler peripheral blood (Kang et al., 2013; Jamil et al., 2015; Khan et al., 2020). Algae-specific effects on intestinal integrity are sparse; however, dietary inclusion of 1.0 to 2.5% and 0.2% Chlorella or Spirulina, respectively, have been shown to increase villus height (VH) and crypt depth (CrD) in the broiler ileum and jejunum (Kang et al., 2017; Khan et al., 2020; Mirzaie et al., 2020). While these reports indicate some algae-associated benefits, they provide limited functional insight into intestinal morphology changes (e.g., intestinal integrity) or identify specific immune cell populations affected beyond the broad designations determined by complete blood counts.
Broiler performance responses to dietary algae vary, but improved body weight (BW) or BW gain (BWG) is most consistently reported at inclusion levels ranging from 0.15 to 7.5% Spirulina and Chlorella; however, improved feed conversion rate (FCR) in response to these ingredients at inclusions ≤1.0% is rarely described (Kang et al., 2013; An et al., 2016; Kang et al., 2017; Mirzaie et al., 2018; Khan et al., 2020). While previous outcomes suggest that immunological and intestinal changes in response to algae may improve broiler performance, these studies primarily document outcomes using corn-based diets. Wheat is also incorporated into broiler diets as a protein and energy source and is used worldwide to replace corn in least-cost diet formulations (Amerah, 2015; Liu and Kim, 2017). Unlike corn, wheat contains high levels of NSP that reduce energy availability through increased intestinal digesta viscosity, as such, wheat-based diets often require the use of exogenous enzymes to counteract associated detrimental performance outcomes (Choct and Annison, 1992; Amerah, 2015). Additionally, wheat germ agglutinin, a lectin present in wheat, can bind to the chicken intestinal epithelium with documented in vitro cytotoxic effects on broiler enterocytes and increased transport of fluorescein isothiocyanate- dextran (FITC-D) across CACO-2 epithelium layers (Dalla Pellegrina et al., 2005; Pohlmeyer et al., 2005; Babot et al., 2017).
Differing responses to corn and wheat-based diets in broilers emphasize the importance of assessing feed ingredients in both diet formulations for worldwide markets. Studies comparing performance and physiological responses to feed ingredients across different diet compositions are scarce. Some reports demonstrated differential performance and intestinal responses to mannanoligosaccharide, essential oil blend, and plant extracts in broilers fed corn and wheat-based diets, while another by Pirgozliev et al. (2019) showed no difference in essential oil responses between basal diet type (Jamroz et al., 2006; Yang et al., 2008; Bozkurt et al., 2012). To the best of the authors’ knowledge, similar comparative evaluations in algae-based ingredients have not been conducted and whether bioactive properties are maintained between varying practical diet formulations used in different regions of the United States and across the world is unknown. Hence, the study objective was to evaluate performance, immune, and intestinal responses to a proprietary algae-based ingredient in broiler chickens fed corn- or wheat-based diets.
MATERIALS AND METHODS
Birds and Experimental Diets
The Iowa State University Institutional Animal Care and Use Committee approved all animal protocols in this study. In total, 1,120 straight-run Ross 308 broilers were housed in 1 m × 1 m floor pens (80 pens; 14 birds/ pen) for 42 d on reused litter from a previous Eimeria vaccine challenge study, divided into 14 d starter, grower, and finisher periods. On d 0, birds were assigned to 1 of 4 dietary treatments consisting of either a corn or wheat basal diet ± 0.175% whole dried algal biomass from a freshwater proprietary strain (20 pens per treatment; Zivo Biosciences, Keego Harbor, MI). Prior to formulation, CP analysis was performed on 4 wheat subsamples in duplicate and found to contain 14.2 ± 0.2% CP. Diets were formulated using this CP value to be isocaloric/isonitrogenous and meet NRC and breed standard requirements for each period (Table 1, Table 2, Table 3; NRC, 1994; Aviagen, 2019). High wheat inclusion (>55%) necessitated inclusion of exogenous enzymes (Ronozyme WX and Ronozyme Multigrain; DSM, Netherlands) in all dietary treatments regardless of basal diet composition. Body weights were recorded weekly and feed disappearance was monitored throughout the study.
Table 1.
Composition of starter diets fed to Ross 308 broilers from d 0 to d 14.
| Experimental diet |
||||
|---|---|---|---|---|
| Ingredient, % | Corn control | Corn +Algae | Wheat control | Wheat +Algae |
| Corn | 54.26 | 54.15 | 0.00 | 0.00 |
| Wheat | 0.00 | 0.00 | 57.58 | 57.40 |
| Soybean meal 48 | 38.93 | 38.79 | 34.39 | 34.35 |
| Soy Oil | 1.39 | 1.46 | 2.87 | 2.95 |
| Salt | 0.40 | 0.40 | 0.40 | 0.40 |
| DL Methionine | 0.31 | 0.31 | 0.32 | 0.32 |
| Lysine HCl | 0.20 | 0.20 | 0.25 | 0.25 |
| Threonine | 0.20 | 0.20 | 0.20 | 0.15 |
| Limestone | 1.25 | 1.25 | 1.25 | 1.25 |
| Dicalcium phosphate | 2.02 | 2.02 | 1.70 | 1.71 |
| Choline chloride 60 | 0.40 | 0.40 | 0.40 | 0.40 |
| Vitamin-mineral premix1 | 0.63 | 0.63 | 0.63 | 0.63 |
| Algae | 0.00 | 0.175 | 0.00 | 0.175 |
| Ronozyme WX | 0.002 | 0.002 | 0.002 | 0.002 |
| Ronozyme MG | 0.01 | 0.01 | 0.01 | 0.01 |
| Calculated values, % | ||||
| Fat | 3.96 | 4.04 | 4.24 | 4.34 |
| Crude protein | 23.79 | 23.79 | 23.79 | 23.79 |
| Digestible Lys | 1.31 | 1.31 | 1.28 | 1.28 |
| Digestible Met | 0.61 | 0.60 | 0.59 | 0.59 |
| Digestible Arg | 1.44 | 1.44 | 1.38 | 1.38 |
| Digestible Thr | 1.00 | 0.99 | 0.94 | 0.90 |
| ME, kcal/kg | 3,000.00 | 3,000.00 | 3,000.00 | 3,000.00 |
| Analyzed values, % as fed | ||||
| Dry matter | 89.66 | 89.74 | 90.92 | 91.13 |
| Crude fat | 4.58 | 5.02 | 5.37 | 5.31 |
| Crude protein | 23.84 | 23.02 | 28.1 | 28.42 |
| Gross energy, kcal/kg | 4,313.00 | 4,291.68 | 4,377.94 | 4,348.02 |
Vitamin and mineral premix provided per kg of diet: selenium 250 μg; Vitamin A (retinyl acetate) 8,250 IU; cholecalciferol (vitamin D3) 2,750 IU; α-tocopherol acetate (vitamin E) 17.9 IU; menadione 1.1 mg; vitamin B12 12μg; biotin 41μg; choline 447 mg; folic acid 1.4 mg; niacin 41.3 mg; pantothenic acid 11 mg; pyridoxine 1.1 mg; riboflavin 5.5 mg; thiamine 1.4 mg; iron 282 mg; magnesium 125 mg; manganese 275 mg; zinc 275 mg; copper 27.5 mg; iodine 844 μg.
Table 2.
Composition of grower diets fed to Ross 308 broilers from d 14 to d 28.
| Experimental Diet |
||||
|---|---|---|---|---|
| Ingredient, % | Corn control | Corn + Algae | Wheat control | Wheat +Algae |
| Corn | 58.81 | 58.79 | 0.00 | 0.00 |
| Wheat | 0.00 | 0.00 | 66.86 | 66.83 |
| Soybean meal 48 | 33.72 | 33.71 | 24.50 | 24.50 |
| Soy Oil | 2.27 | 2.28 | 3.58 | 3.59 |
| Salt | 0.40 | 0.40 | 0.40 | 0.40 |
| DL Methionine | 0.30 | 0.30 | 0.30 | 0.30 |
| Lysine HCl | 0.40 | 0.40 | 0.40 | 0.40 |
| Threonine | 0.20 | 0.20 | 0.12 | 0.12 |
| Limestone | 1.00 | 1.00 | 1.00 | 1.00 |
| Dicalcium phosphate | 1.81 | 1.81 | 1.75 | 1.75 |
| Choline chloride 60 | 0.45 | 0.45 | 0.45 | 0.45 |
| Vitamin-mineral premix1 | 0.63 | 0.63 | 0.63 | 0.63 |
| Algae | 0.00 | 0.175 | 0.00 | 0.175 |
| Ronozyme WX | 0.002 | 0.002 | 0.002 | 0.002 |
| Ronozyme MG | 0.01 | 0.01 | 0.01 | 0.01 |
| Calculated values, % | ||||
| Fat | 4.93 | 4.93 | 4.98 | 4.99 |
| Crude protein | 21.85 | 21.85 | 21.85 | 21.85 |
| Digestible Lys | 1.32 | 1.32 | 1.15 | 1.15 |
| Digestible Met | 0.57 | 0.57 | 0.54 | 0.54 |
| Digestible Arg | 1.29 | 1.29 | 1.19 | 1.19 |
| Digestible Thr | 0.92 | 0.92 | 0.77 | 0.77 |
| ME, kcal/kg | 3,100.00 | 3,100.00 | 3,100.00 | 3,100.00 |
| Analyzed values, % As fed | ||||
| Dry matter | 90.02 | 90.17 | 91.16 | 91.32 |
| Crude fat | 6.19 | 6.33 | 6.33 | 5.84 |
| Crude protein | 21.08 | 21.31 | 24.61 | 23.89 |
| Gross energy, kcal/kg | 4,332.53 | 4,330.98 | 4,411.73 | 4,382.06 |
Vitamin and mineral premix provided per kg of diet: selenium 250 μg; Vitamin A (retinyl acetate) 8,250 IU; cholecalciferol (vitamin D3) 2,750 IU; α-tocopherol acetate (vitamin E) 17.9 IU; menadione 1.1 mg; vitamin B12 12 μg; biotin 4 μg; choline 447 mg; folic acid 1.4 mg; niacin 41.3 mg; pantothenic acid 11 mg; pyridoxine 1.1 mg; riboflavin 5.5 mg; thiamine 1.4 mg; iron 282 mg; magnesium 125 mg; manganese 275 mg; zinc 275 mg; copper 27.5 mg; iodine 844 μg.
Table 3.
Composition of finisher diets fed to Ross 308 broilers from d 28 to 42.
| Experimental diets |
||||
|---|---|---|---|---|
| Ingredients, % | Corn control | Corn +Algae | Wheat control | Wheat +Algae |
| Corn | 60.98 | 60.95 | 0.00 | 0.00 |
| Wheat | 0.00 | 0.00 | 69.46 | 69.44 |
| Soybean meal 48 | 30.9 | 30.9 | 21.00 | 21.00 |
| Soy oil | 3.48 | 3.49 | 4.85 | 4.85 |
| Salt | 0.40 | 0.40 | 0.40 | 0.40 |
| DL Methionine | 0.25 | 0.25 | 0.25 | 0.25 |
| Lysine HCl | 0.25 | 0.25 | 0.35 | 0.35 |
| Threonine | 0.10 | 0.10 | 0.10 | 0.10 |
| Limestone | 1.00 | 1.00 | 1.00 | 1.00 |
| Dicalcium phosphate | 1.57 | 1.57 | 1.5 | 1.5 |
| Choline chloride 60 | 0.42 | 0.42 | 0.45 | 0.45 |
| Vitamin-mineral premix1 | 0.63 | 0.63 | 0.63 | 0.63 |
| Algae | 0.00 | 0.175 | 0.00 | 0.175 |
| Ronozyme WX | 0.002 | 0.002 | 0.002 | 0.002 |
| Ronozyme MG | 0.01 | 0.01 | 0.01 | 0.01 |
| Calculated values, % | ||||
| Fat | 6.13 | 6.14 | 6.19 | 6.20 |
| Crude protein | 20.45 | 20.45 | 20.45 | 20.45 |
| Digestible Lys | 1.14 | 1.14 | 1.03 | 1.03 |
| Digestible Met | 0.51 | 0.51 | 0.48 | 0.48 |
| Digestible Arg | 1.21 | 1.21 | 1.10 | 1.10 |
| Digestible Thr | 0.79 | 0.79 | 0.70 | 0.70 |
| ME, kcal/kg | 3,200.00 | 3,200.00 | 3,200.00 | 3,200.00 |
| Analyzed values, % As fed | ||||
| Dry matter | 90.29 | 90.19 | 91.34 | 91.39 |
| Crude fat | 7.63 | 7.51 | 8.66 | 8.30 |
| Crude protein | 19.88 | 20.37 | 22.77 | 22.87 |
| Gross energy, kcal/kg | 4,432.38 | 4,459.98 | 4,548.82 | 4,526.41 |
Vitamin and mineral premix provided per kg of diet: selenium 250 μg; Vitamin A (retinyl acetate) 8,250 IU; cholecalciferol (vitamin D3) 2,750 IU; α-tocopherol acetate (vitamin E) 17.9 IU; menadione 1.1 mg; vitamin B12 12 μg; biotin 41 μg; choline 447 mg; folic acid 1.4 mg; niacin 41.3 mg; pantothenic acid 11 mg; pyridoxine 1.1mg; riboflavin 5. 5mg; thiamine 1.4 mg; iron 282 mg; magnesium 125 mg; manganese 275 mg; zinc 275 mg; copper 27.5 mg; iodine 844 μg.
Histomorphology
Ten birds per treatment were euthanized for tissue collection at the end of each 14 d performance period (d 14, 28, and 42). The entire duodenal loop and an 8 cm distal jejunum segment collected approximately 2 cm away from Meckel's diverticulum were fixed in 10% neutral-buffered formalin for 24 h and then transferred to 70% ethanol. Tissues were paraffin-embedded, mounted on microscope slides (3 cross-sections/ slide), and hematoxylin and eosin stained. Slides were imaged using an Olympus BX 54/43 microscope with DP80 Olympus camera, VH and CrD measurements were taken using the Olympus Cell Sens Dimension software, and the VH: CrD ratio was calculated (version 1.16; Olympus Corporation, Tokyo, Japan). Villus height was defined as the distance from villus-crypt junction to villus tip in sections with intact lamina propria and CrD was the invagination depth between adjacent villi. A total of 10 measurements for both VH and CrD were taken from 10 birds/treatment (100 measurements/ treatment).
FITC-D Intestinal Permeability
Five birds per pen were wing-banded at placement for the FITC-D intestinal permeability assay on d 28 and 29. This assay was conducted using 23 birds/ dietary treatment each day (46 birds/ diet) with half the birds in each treatment subjected to a 12-h feed restriction challenge. Prior to the assay, birds were weighed to calculate FITC-D dosage (8.32 mg/kg of BW) and feeders were removed from pens randomly assigned to the feed restriction challenge. On d 28 or 29, birds were orally gavaged with FITC-D and 2 to 3 mL of blood was collected from the brachial vein into serum separation tubes 1 h post-gavage. Blood from an additional 5 birds/ feed status (fed vs. restricted) and basal diet (corn vs. wheat) not given FITC-D was collected for serum blanks. Blood was centrifuged for 15 min at 1,000 × g and serum stored at −20°C in amber vials until analysis.
To determine FITC-D fluorescence, standards were prepared by dissolving FITC-D in blank serum at 6,400 ng/mL with a 2X serial dilution to 100 ng/mL and serum-only blank as the minimum value (0 ng/mL). Thawed serum was diluted 1:5 in saline and samples and standards were plated in duplicate on black 96-well plates that were read at 485 nm excitation and 528 nm emission wavelengths. The average reading from the serum blank (0 ng/mL FITC-D) was subtracted from all sample readings and the standard curve intercept set at 0. Serum concentration of FITC-D (ng/mL) was calculated using the resultant standard curve equation and results obtained on d 28 and d 29 were compiled for analysis.
Spleen Immune Cell Profiles
Spleens from 10 birds/ treatment were collected on d 14, 28, and 42, gently homogenized in PBS, and passed through a sterile 70-μm filter. Cells were washed twice in PBS and frozen in RPMI media with 42.5% chicken serum and 7.5% DMSO and stored at −80°C until flow cytometric analysis. Prior to staining, cells were thawed, enumerated by hemocytometer, and aliquoted into polystyrene tubes. Extracellular staining was accomplished by diluting 0.5 μL of fluorochrome-conjugated antibodies in 50 μL of PBS and implementing fluorescence-minus-one controls with associated isotype controls (0.2/50 μL PBS) for each marker. The panel consisted of mouse anti-chicken cluster of differentiation (CD) 1.1 FITC (clone CB3; mouse IgG1κ), CD3 Pacific Blue (clone CT-3; mouse IgG1κ), CD4 Alexa Fluor 700 (clone CT-4; mouse IgG1κ), CD8α SPRD (clone CT-8; mouse IgG1κ), TCRγδ PE (clone TCR-1; mouse IgG1κ), and monocyte/macrophage biotin (clone KUL01; mouse IgG1κ; Southern Biotech, Birmingham, AL). Cells were incubated in the dark for 30 min at 4°C before being washed in PBS. Brilliant Violet 785-conjugated streptavidin (BioLegend, San Diego, CA; 0.3 μL in 50 μL PBS) to label biotin-conjugated monocyte/macrophage antibody was added to each tube and incubated for an additional 30 min at 4°C in the dark. Cells were washed and resuspended in PBS before being analyzed by flow cytometry using a BD FACSCanto cytometer (BD Biosciences, San Jose, CA). Individual cell populations were identified and gated using FlowJo software (version 10.5.0; BD Biosciences).
Kinome Signal Transduction
On d 14 and 42, tissues were collected from 5 birds/ treatment from the corn-based diet ± algae, snap frozen in liquid nitrogen, shipped on dry ice to the University of Delaware, and stored at −80°C until analysis. Intestinal tissue comprised an approximately 4 cm section containing Meckel's diverticulum and adjacent jejunal and ileal segments, while an entire cecum without content was collected per bird. Approximately 3 cm2 sections were taken from the cranial region of the left breast for muscle samples and from the left lobe for liver samples.
The kinome peptide array protocol performed on each tissue is previously described (Arsenault, et al., 2017). Briefly, cells were lysed in 100 µL of lysis buffer containing protease inhibitors using a Bead Rupter and 1.5 mm ceramic beads (Omni International, Kennesaw, GA). Lysates were centrifuged and 70 µL of supernatant was mixed with 10 µL of activation mixture containing 500 µM ATP (New England Biolabs, Ipswich, MA). Approximately 80 µL of each sample was applied to a glass peptide array (JPT Peptide Technologies, Berlin, Germany) and incubated in a sealed container placed in a 5% CO2 incubator at 37°C for 2 h. After incubation, arrays were washed and stained in phospho-specific fluorescent ProQ Diamond Phosphoprotein Stain (Life Technologies, Carlsbad, CA) for 1 h, then submerged in destain solution containing 20% acetonitrile (Sigma-Aldrich, St. Louis, MO) and 50 mM sodium acetate (Sigma-Aldrich) to remove non-phospho-specific binding. Arrays were scanned in a Tecan PowerScanner microarray scanner (Tecan Systems, San Jose, CA) at 532 to 560 nm with a 580-nm filter to detect dye fluorescence.
Images of the scanned array were gridded manually to fit the phospho-specific spots and extract signal intensity using GenePix Pro software (version 7.2.29 1, Molecular Devices, CA). Microsoft Excel 2016 (Redmond, WA) files containing kinomic data were generated and analyzed using Platform for Intelligent, Integrated Kinome Analysis (PIIKA2) software (Trost, et al., 2013). Within this software, clustering is generated using the PVCLUST package in R 4.2 wherein similarities between groups are examined within the entire kinome dataset (Suzuki at al., 2006). Briefly, 2 confidence intervals are generated per cluster with an approximately unbiased P-value and bootstrap probability to indicate likelihood of true clustering agnostic to the biological groups. Values approaching 1 suggest high confidence and a threshold value ≥0.97 was used to determine reported clustering herein. Kinome peptide array data generated from PIIKA2 were analyzed using online databases, including STRING (Szklarczyk, et al., 2017), Reactome (Jassal, et al., 2020), UniProt (UniProt, 2021), and PhosphosSitePlus (Hornbeck, et al., 2015).
Statistical Analysis
Treatments were assigned using a completely randomized design and the following statistical model was used to analyze performance data:
In this model, yijk is the dependent variable, μ is the overall mean, Di is the effect of diet at the ith level (corn or wheat; i = 2), Aj is the effect of algae inclusion at the jth level (± algae; j = 2), (D x A)ij is the interaction effect of diet at the ith level and algae inclusion at the jth level, iBWijk is the initial BW covariate, and eijk denotes random error. Histomorphology and immune cell population data analysis used this same model without the initial BW covariate. The model used to analyze serum fluorescence contained additional terms for the effect of feed restriction:
In addition to the previously defined terms, in this model Rk is the effect of feed restriction at the kth level (fed or feed-restricted; k = 2), (D × R)ik is the interaction of diet at the ith level and feed restriction at the kth level, (A × R)ik is the interaction of algae inclusion at the jth level and feed restriction at the kth level, and (D × A × R)ijk is the interaction of diet at the ith level, algae inclusion at the jth level, and feed restriction at the kth level. Outliers 3 standard deviations from each treatment mean were identified and excluded using the UNIVARIATE procedure prior to being analyzed using the MIXED procedure in SAS 9.4 with significance at P ≤ 0.05 (SAS Institute, Cary, NC).
RESULTS
Performance
Performance results are presented in Table 4. In the starter period, birds fed the wheat-based control diet ate 4.8% more than those fed the corn-based control and gained 9.4% more BW (P = 0.02). The main effect of feeding wheat-based diets resulted in a 6-point more efficient FCR (P < 0.0001) compared to feeding corn-based diets (Table 4). Similarly, during the grower period, birds fed wheat-based diets ate 3.7% more, gained 8.8% more BW (P = 0.002), and had an 8-point more efficient FCR (P < 0.0001) than birds fed corn-based diets, regardless of algae inclusion (Table 4). When examining interaction effects, feeding corn-based diets with algae did not alter FI but numerically increased BWG during the finisher period, resulting in an 11-point more efficient FCR (P = 0.02) compared to the corn-based control (Table 4). For the entire 42 d study, birds fed the corn-based diet with algae gained 6.8% more BW (P = 0.01) and had a 5.4-point (3.2%; P = 0.006) more efficient FCR than those fed the corn-based control. While the main effect of feeding wheat-based diets increased BWG 4.8% and improved FCR by 5 points (P < 0.0001) compared to corn-based diets, algae inclusion did not alter performance in birds fed these diets (Table 4).
Table 4.
Per bird performance of Ross 308 broilers fed corn- or wheat-based diets ± 0.175% algae ingredient1 for 42 d divided into 14 d starter, grower, and finisher periods.
| Measure | Treatment |
SEM |
P-values2 |
|||||
|---|---|---|---|---|---|---|---|---|
| Corn control | Corn + Algae | Wheat control | Wheat + Algae | Diet | Algae | Diet × Algae | ||
| D 0 BW3, kg | 0.04 | 0.04 | 0.04 | 0.04 | 0.0004 | 0.84 | 0.24 | 0.75 |
| Starter (d 0–d 14) | ||||||||
| BWG, kg | 0.31b | 0.31b | 0.34a | 0.32ab | 0.009 | 0.007 | 0.27 | 0.02 |
| FI, kg | 0.40b | 0.41ab | 0.42a | 0.41ab | 0.008 | 0.27 | 0.76 | 0.02 |
| FCR | 1.33 | 1.33 | 1.25 | 1.29 | 0.01 | <0.0001 | 0.04 | 0.11 |
| Grower (d 14–d 28) | ||||||||
| BWG, kg | 0.90 | 0.92 | 1.01 | 0.98 | 0.02 | <0.0001 | 0.78 | 0.11 |
| FI, kg | 1.45 | 1.45 | 1.53 | 1.48 | 0.02 | 0.002 | 0.18 | 0.13 |
| FCR | 1.62 | 1.59 | 1.52 | 1.51 | 0.02 | <0.0001 | 0.16 | 0.64 |
| Finisher (d28-d42) | ||||||||
| BWG, kg | 1.43 | 1.51 | 1.49 | 1.49 | 0.04 | 0.44 | 0.12 | 0.09 |
| FI, kg | 2.56 | 2.57 | 2.58 | 2.54 | 0.03 | 0.75 | 0.66 | 0.27 |
| FCR | 1.80a | 1.69b | 1.71b | 1.71b | 0.03 | 0.10 | 0.02 | 0.01 |
| Overall (d 0–d 42) | ||||||||
| BWG, kg | 2.72c | 2.81bc | 2.94a | 2.87ab | 0.05 | <0.0001 | 0.66 | 0.02 |
| FI, kg | 4.54 | 4.54 | 4.67 | 4.55 | 0.06 | 0.12 | 0.19 | 0.19 |
| FCR | 1.66a | 1.60b | 1.58b | 1.58b | 0.02 | <0.0001 | 0.02 | 0.006 |
Data represent the per bird average calculated from 20 pens/ diet.
Data in the same row with different letter (abc) superscripts are significantly different, P ≤ 0.05.
Abbreviations: BW, body weight; BWG, body weight gain; FI, feed intake; FCR, feed conversion rate.
Intestinal Histomorphology
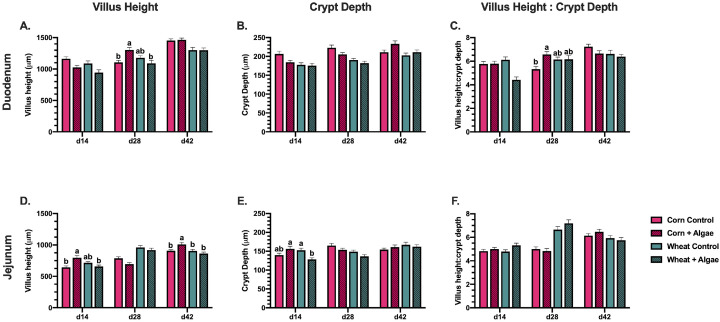
In the 14-day-old broiler duodenum, the algae main effect reduced VH by 12.5% (P = 0.002; Figure 1A). In 28-day-old broilers fed corn-based diets, algae inclusion increased average duodenal VH by 15.2% (P = 0.0002) compared to the corn-based control. As a result, algae inclusion in the corn-based diet increased the VH:CrD ratio by 19.1% (P = 0.01) compared to the corn-based control but similar VH and VH:CrD responses were not observed in birds fed either wheat-based diet (Figures 1A–1C). By the end of the study (d 42), the main effect of feeding corn-based diets increased duodenal VH and CrD by 11.0 and 6.7% (P < 0.05), respectively compared to feeding wheat-based diets; however, no differences in VH:CrD were observed at this timepoint (Figures 1A–1C).
Figure 1.
Intestinal histomorphology in the (A–C) duodenum and (D–F) jejunum of 14, 28, and 42-day-old Ross 308 broilers fed corn- or wheat-based diets ± 0.17% algae ingredient. Bars represent the mean measurement taken from 10 measures/bird from 10 birds/ treatment ± SEM. Different letter labels indicate significantly different outcomes, P ≤ 0.05.
While changes to duodenal histomorphology occurred at d 28 and d 42, changes in the distal jejunum due to algae inclusion were observed earlier. On d 14, birds fed the corn-based diet with algae had 20.0% increased jejunal VH (P < 0.0001) compared to birds fed the corn-based control. In contrast, algae inclusion in the wheat-based diet reduced d 14 jejunal CrD by 15.8% (P = 0.0002) compared to the wheat-based control (Figures 1D and 1E). On d 28 the significant diet main effect showed that birds fed wheat-based diets had a 21.0% greater average VH, culminating in a 29.0% increased VH:CrD ratio (P < 0.0001) compared to birds fed corn-based diets (Figures 1D and 1F). On d 42, algae inclusion in the corn-based diet increased VH 10% (P = 0.004) compared to the corresponding control but had no effect when included in either wheat-based diet.
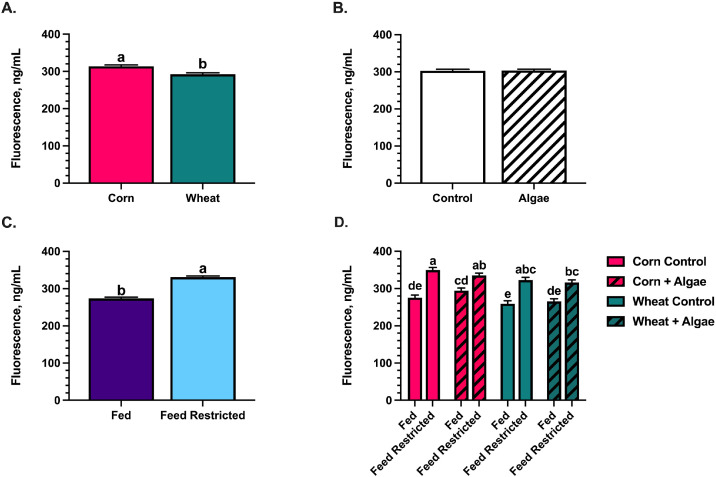
Intestinal Permeability
The serum fluorescence of birds fed wheat-based diets was reduced 6.8% (P = 0.0002) compared to those fed corn-based diets, regardless of algae inclusion, indicating that wheat-based diets contributed to improved intestinal integrity (Figure 2A). The feed restriction main effect increased serum fluorescence 17.3% (P < 0.001) compared to fed birds, which was observed among all dietary treatments (Figures 2C and 2D). While the overall interaction between dietary treatment and feed restriction was not significant (P = 0.09), serum fluorescence in feed-restricted birds assigned to the corn-based control diet was increased 9.5% compared to feed-restricted birds assigned to the wheat-based diet with algae. Feed-restricted birds assigned to the corn + algae and wheat control diets displayed intermediate serum fluorescence (Figure 2D).
Figure 2.
The main effects of (A) basal diet, (B) algae inclusion, (C) feed restriction, and (D) the diet × feed restriction interaction on the serum fluorescence of birds at d 28 and 29 fed a corn- or wheat-based diet ± 0.175% algae additive and orally gavaged with fluorescein isothiocyanate-dextran (FITC-D; 8.32 mg/kg of body weight). Values represent the mean serum fluorescence of 46 birds/ treatment ± SEM. Bars with differing superscripts are significant at P ≤ 0.05.
Spleen Immune Cell Profiles
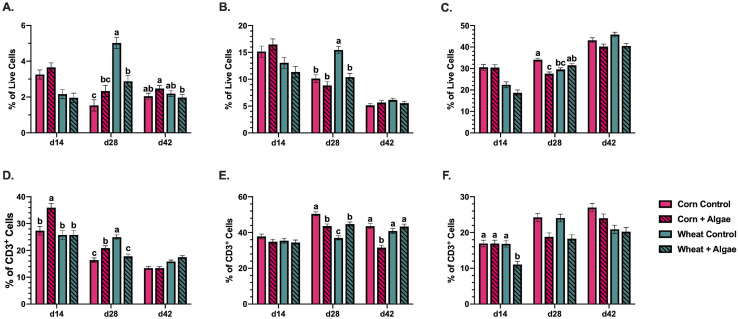
Two immune cell populations with antigen presentation capabilities were assessed in the broiler spleen: monocyte/macrophage+ cells involved in tissue-level innate immune responses and CD1.1+ cells associated with lipid-antigen presentation by avian dendritic and B cells (Pickel et al., 1990; Fearon and Locksley, 1996; Dvir et al., 2010; Wu et al., 2010; Epelman et al., 2014; Taebipour et al., 2017). In 14-day-old broilers the main effect of feeding wheat-based diets reduced monocyte/macrophage+ and CD1.1+ cells 40.4 and 22.8%, respectively, compared to corn-based diets (P < 0.0001). Interaction effects were observed at the end of the grower period (d 28) when birds fed the wheat control diet had 42.6% more splenic monocyte/macrophage+ (P < 0.0001) cells than birds fed the wheat-based diet with algae (Figure 3A). Similarly, splenic CD1.1+ cells in birds fed the wheat control were increased 32.7% (P = 0.007) compared to the wheat diet with algae (Figure 3B).
Figure 3.
Populations of (A) monocytes/macrophages, (B) CD1.1+ antigen presenting cells, (C) CD3+ T cells, (D) CD3+CD4+ helper T cells, (E) CD3+CD8α+ cytotoxic T cells, and (F) CD3+TCRγδ+ cells in the spleen of 14, 28, and 42-day-old Ross 308 broilers fed corn- or wheat-based diets ± 0.175% algae ingredient. Data represent the mean cell population with each marker identified within the (A–C) live cell gate or (D–F) CD3+ T cell gate from 10 birds/ treatment/ timepoint ± SEM. Bars with different letter labels are significantly different, P ≤ 0.05.
Additional cell types analyzed were CD3+ T cells, a predominant lymphocyte population in the broiler spleen, and subpopulations including CD3+CD4+ helper T cells (TH), CD3+CD8α+ cytotoxic T cells (TC), and CD3+TCRγδ+ (γδ) T cells. Among these T cell subtypes, TH activate other effector T cells and induce antibody production by B cells, TC function in cytotoxic pathogen clearance, and γδ T cells are generally regarded as immunoregulatory (Arstila et al., 1994; Vantourout and Hayday, 2013). The main effect of feeding wheat-based diets showed a 32.7% reduction in overall CD3+ T cell populations (P < 0.0001) on d 14 compared to corn-based diets. Interaction effects at d 14 showed that algae inclusion increased TH cells by 23.9% (P = 0.009) in the corn-based diet and reduced γδ T cells by 34.4% (P = 0.002) in the wheat-based diet on d 14.
In 28-day-old birds, splenic CD3+ T cells were reduced 18.9% by the corn-based diet with algae compared to the corresponding control (P < 0.0001; Figure 3C). The corn-based diets with algae increased TH cells 21.6% while reducing TC 13.4% compared to the corresponding control (P < 0.0001). In contrast, the wheat-based diet with algae decreased d28 TH cells 28.6% and increased TC 17.2% compared to the wheat-based control (P < 0.0001). Regardless of basal diet type, γδ T cells were reduced 23.2% by the algae inclusion main effect compared to control diets on d 28 (P < 0.0001; Figures 3D–3F). On d 42, birds fed the corn-based diet with algae had 27.5% fewer TC cells (P < 0.0001) compared to the corn-based control (Figures 3C–3F).
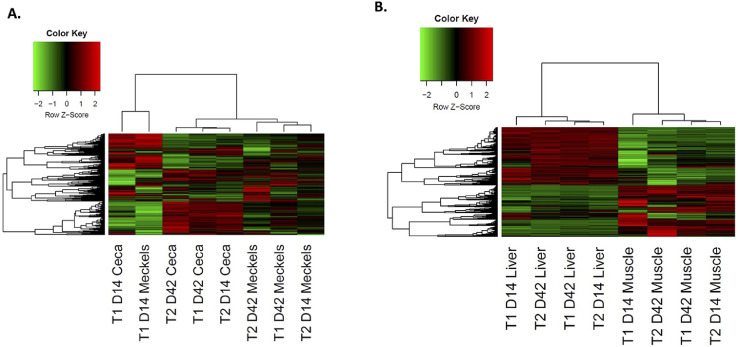
Broiler Tissue Kinome
Phosphorylation-dependent signal transduction occurring within the tissue can be determined by measuring protein kinase activity. This analysis focused on outcomes in birds fed corn-based diets ± algae due to more robust algae-related performance benefits compared to wheat-based diets. Figure 4 shows a heatmap and dendrogram of kinome data collected from the small intestine (Meckel's area and cecum), liver, and muscle tissue. Each column in the figure represents the total phosphorylation signal of the tissue, known as the kinome profile or tissue kinotype. Connecting lines at the top of the figure represent clustering of kinotypes between each tissue with strong similarity between samples from the same tissue regardless of treatment or bird age. The control (T1) d 14 small intestine, ceca, liver, and muscle samples clustered outside of their respective tissue clusters, indicating that algae shifted d 14 algae-fed groups into clusters with d 42 groups (Figure 4).
Figure 4.
Heatmap of (A) small intestine (Meckel's) and ceca and (B) liver and muscle tissue kinotype data post PIIKA2 analysis. Each colored line within each column represents a peptide on the array. Red indicates relative increase in phosphorylation, green is relative decrease. Each column represents the total phosphorylation signal or kinotype for each sample. Connecting lines at the top represent tissue clustering of kinotypes between each tissue. T1 is the corn control diet and T2 is corn + algae diet.
Analyzing protein changes compared at d 14 vs. d 42 provided insight into specific signaling changes underlying observed clustering patterns. Using the STRING database (Szklarczyk et al., 2017), proteins that displayed significant differences between control and algae-containing diets generated the top 20 tissue pathways altered by dietary algae inclusion at d 14 and d 42 (Table 5, Table 6, Table 7, Table 8). Pathways in bold text were unique to the specific day. At d 14, the small intestine kinotype of algae-fed birds showed substantial changes in toll-like receptor (TLR) and myeloid differentiation primary response 88 (MyD88) signaling as indicators of increased innate immunity. At d 42, dietary algae inclusion increased the small intestine adaptive immune response (Table 5). In the ceca, liver, and breast muscle, few unique signaling pathways between d 14 and 42 were observed (Table 6, Table 7, Table 8).
Table 5.
Small intestine (Meckel's adjacent) reactome pathways1 enriched in 14 d and 42-day-old Ross 308 broilers fed corn-based diets + 0.175% algae ingredient compared to a corn-based control.
| Reactome pathway | Proteins2 | Background3 | FDR4 |
|---|---|---|---|
| d 14 | |||
| Immune system | 122 | 1,925 | 9.21E-36 |
| Signal transduction | 136 | 2,605 | 1.88E-32 |
| Innate immune system | 84 | 1,012 | 7.99E-31 |
| Cytokine signaling in immune system | 68 | 654 | 9.75E-30 |
| Signaling by receptor tyrosine kinases | 56 | 437 | 2.77E-28 |
| Signaling by interleukins | 56 | 439 | 2.86E-28 |
| Toll-like receptor cascades | 35 | 151 | 1.15E-24 |
| Diseases of signal transduction | 47 | 360 | 6.48E-24 |
| Disease | 71 | 1,018 | 1.04E-21 |
| Toll-like receptor 4 (TLR4) cascade | 30 | 126 | 2.06E-21 |
| MAPK family signaling cascades | 37 | 273 | 3.74E-19 |
| MyD88:MAL(TIRAP) cascade initiated on plasma membrane | 25 | 94 | 9.17E-19 |
| Toll-like receptor 3 (TLR3) cascade | 25 | 95 | 9.78E-19 |
| Toll-like receptor 9 (TLR9) cascade | 25 | 96 | 1.08E-18 |
| TRIF(TICAM1)-mediated TLR4 signaling | 25 | 96 | 1.08E-18 |
| MyD88 cascade initiated on plasma membrane | 24 | 84 | 1.08E-18 |
| Intracellular signaling by second messengers | 36 | 274 | 1.59E-18 |
| TRAF6 mediated induction of NFκB and MAPK upon TLR7/8 or 9 activation | 24 | 91 | 3.28E-18 |
| PI3K/AKT signaling in cancer | 23 | 85 | 1.07E-17 |
| Fc Epsilon receptor (FCERI) signaling | 26 | 126 | 1.56E-17 |
| d 42 | |||
| Immune System | 115 | 1,925 | 6.05E-35 |
| Signaling by Receptor Tyrosine Kinases | 58 | 437 | 8.58E-32 |
| Signal transduction | 126 | 2,605 | 1.13E-30 |
| Signaling by interleukins | 53 | 439 | 2.93E-27 |
| Innate immune system | 75 | 1,012 | 6.87E-27 |
| Disease | 73 | 1,018 | 2.56E-25 |
| Cytokine signaling in immune system | 59 | 654 | 1.06E-24 |
| Diseases of signal transduction | 43 | 360 | 7.70E-22 |
| Intracellular signaling by second messengers | 37 | 274 | 2.76E-20 |
| Signaling by VEGF | 25 | 104 | 1.17E-18 |
| VEGFA-VEGFR2 pathway | 24 | 95 | 2.39E-18 |
| Signaling by NTRK | 24 | 97 | 3.32E-18 |
| Axon guidance | 46 | 541 | 4.10E-18 |
| MAPK family signaling cascades | 33 | 273 | 7.93E-17 |
| Signaling by NTRK1 (TRKA) | 21 | 76 | 9.14E-17 |
| PIP3 activates AKT signaling | 31 | 242 | 1.76E-16 |
| Toll-like receptor cascades | 26 | 151 | 1.76E-16 |
| Adaptive immune system | 49 | 733 | 1.73E-15 |
| Fc Epsilon receptor (FCERI) signaling | 23 | 126 | 4.58E-15 |
| Developmental biology | 57 | 1,023 | 6.71E-15 |
Abbreviations: AKT, protein kinase B; MAPK, mitogen-activated protein kinase; MyD88, myeloid differentiation primary response 88; MAL, MyD88 adaptor-like protein; NF, nuclear factor; PI3K, Phosphatidylinositol-3-kinsae; PIP, plasma membrane-intrinsic protein; NTRK, neurotrophic tyrosine receptor kinase; TIR, Toll/Interleukin 1 receptor homology; TIRAP, TIR-containing adaptor protein; TRIF, TIR-domain-containing adapter-inducing interferon-β; TICAM, TIR domain-containing adaptor molecule; TRAF, tumor necrosis factor receptor-associated factors; TLR, Toll-like receptor; TRK, tyrosine receptor kinase; VEGF, vascular endothelial growth factor.
Significant differentially phosphorylated proteins from the small intestine at d 14 and d 42 (n = 10 birds/ diet) were input into the STRING database to generate a list of the 20 most enriched Reactome Pathways. Bolded pathways are those unique between d 14 and d 42.
Proteins refers to the number of proteins within the pathway differentially phosphorylated on the array.
Background is the number of proteins within the pathway.
FDR is false discovery rate significance value of the pathway (significance ≤ 0.05).
Table 6.
Cecal reactome pathways1 enriched in 14 d and 42-day-old Ross 308 broilers fed corn-based diets + 0.175% algae ingredient compared to a corn-based control.
| Reactome pathway | Protein2 | Background3 | FDR4 |
|---|---|---|---|
| d 14 | |||
| Immune system | 154 | 1,925 | 9.14E-47 |
| Signal transduction | 176 | 2,605 | 1.35E-45 |
| Signaling by receptor tyrosine kinases | 75 | 437 | 5.60E-40 |
| Innate immune system | 104 | 1,012 | 1.99E-38 |
| Diseases of signal transduction | 61 | 360 | 6.74E-32 |
| Cytokine signaling in immune system | 77 | 654 | 3.53E-31 |
| Signaling by interleukins | 65 | 439 | 3.53E-31 |
| Disease | 94 | 1,018 | 3.92E-31 |
| Toll-like receptor cascades | 42 | 151 | 4.96E-29 |
| MAPK family signaling cascades | 48 | 273 | 1.34E-25 |
| Toll-like receptor 4 (TLR4) cascade | 35 | 126 | 4.63E-24 |
| Intracellular signaling by second messengers | 46 | 274 | 8.44E-24 |
| Signaling by NTRKs | 30 | 97 | 1.08E-21 |
| TRAF6 mediated induction of NFκB and MAP kinases upon TLR7/8 or 9 activation | 29 | 91 | 2.80E-21 |
| Signaling by VEGF | 30 | 104 | 4.48E-21 |
| MyD88:MAL(TIRAP) cascade initiated on plasma membrane | 29 | 94 | 4.65E-21 |
| Toll-like receptor 3 (TLR3) cascade | 29 | 95 | 5.35E-21 |
| PI3K/AKT signaling in cancer | 28 | 85 | 5.35E-21 |
| VEGFA-VEGFR2 pathway | 29 | 95 | 5.35E-21 |
| Signaling by NTRK1 (TRKA) | 27 | 76 | 5.36E-21 |
| d 42 | |||
| Immune system | 113 | 1,925 | 1.28E-36 |
| Signaling by receptor tyrosine kinases | 60 | 437 | 1.98E-35 |
| Signal transduction | 125 | 2,605 | 2.18E-33 |
| Innate immune system | 78 | 1,012 | 3.48E-31 |
| Signaling by interleukins | 53 | 439 | 8.52E-29 |
| Cytokine signaling in immune system | 62 | 654 | 8.84E-29 |
| Diseases of signal transduction | 46 | 360 | 8.65E-26 |
| Intracellular signaling by second messengers | 39 | 274 | 3.36E-23 |
| Disease | 66 | 1,018 | 3.89E-22 |
| Signaling by NTRK1 (TRKA) | 24 | 76 | 6.78E-21 |
| Toll-like receptor cascades | 29 | 151 | 2.99E-20 |
| VEGFA-VEGFR2 pathway | 25 | 95 | 3.05E-20 |
| PI3K/AKT signaling in cancer | 24 | 85 | 4.82E-20 |
| Toll-like receptor 4 (TLR4) cascade | 27 | 126 | 5.63E-20 |
| PIP3 activates AKT signaling | 33 | 242 | 3.37E-19 |
| MyD88 cascade initiated on plasma membrane | 23 | 84 | 4.90E-19 |
| MAPK family signaling cascades | 34 | 273 | 8.33E-19 |
| TRAF6 mediated induction of NFκB and MAP kinases upon TLR7/8 or 9 activation | 23 | 91 | 1.76E-18 |
| MyD88:MAL(TIRAP) cascade initiated on plasma membrane | 23 | 94 | 2.89E-18 |
| Toll-like receptor 3 (TLR3) cascade | 23 | 95 | 3.29E-18 |
Abbreviations: AKT, protein kinase B; MAPK, mitogen-activated protein kinase; MyD88, myeloid differentiation primary response 88; MAL, MyD88 adaptor-like protein; NF, nuclear factor; PI3K, Phosphatidylinositol-3-kinsae; PIP, plasma membrane-intrinsic protein; NTRK, neurotrophic tyrosine receptor kinase; TIR, Toll/Interleukin 1 receptor homology; TIRAP, TIR-containing adaptor protein; TRIF, TIR-domain-containing adapter-inducing interferon-β; TICAM, TIR domain-containing adaptor molecule; TRAF, tumor necrosis factor receptor-associated factors; TLR, Toll-like receptor; TRK, tyrosine receptor kinase; VEGF, vascular endothelial growth factor.
Significant differentially phosphorylated proteins from the small intestine at d 14 and d 42 (n = 10 birds/diet) were input into the STRING database to generate a list of the 20 most enriched Reactome Pathways. Bolded pathways are those unique between d 14 and d 42.
Proteins refers to the number of proteins within the pathway differentially phosphorylated on the array.
Background is the number of proteins within the pathway.
FDR is false discovery rate significance value of the pathway (significance ≤ 0.05).
Table 7.
Liver reactome pathways1 enriched in 14 d and 42-day-old Ross 308 broilers fed corn-based diets + 0.175% algae ingredient compared to a corn-based control.
| Reactome pathway | Protein2 | Background3 | FDR4 |
|---|---|---|---|
| d 14 | |||
| Immune system | 152 | 1927 | 4.53E-48 |
| Signaling by receptor tyrosine kinases | 78 | 437 | 4.69E-44 |
| Signal transduction | 168 | 2605 | 1.91E-43 |
| Cytokine sgnaling in immune system | 84 | 654 | 3.60E-38 |
| Innate immune system | 101 | 1012 | 5.56E-38 |
| Signaling by interleukins | 71 | 439 | 1.02E-37 |
| Disease | 94 | 1018 | 1.22E-32 |
| Diseases of signal transduction | 60 | 360 | 3.12E-32 |
| Toll-like receptor cascades | 40 | 151 | 1.34E-27 |
| Intracellular signaling by second messengers | 46 | 274 | 1.67E-24 |
| Toll-like receptor 4 (TLR4) cascade | 34 | 126 | 1.25E-23 |
| Signaling by VEGF | 32 | 104 | 1.25E-23 |
| VEGFA-VEGFR2 Pathway | 31 | 95 | 1.42E-23 |
| Signaling by NTRKs | 31 | 97 | 2.23E-23 |
| Signaling by NTRK1 (TRKA) | 28 | 76 | 1.86E-22 |
| Toll-like receptor 9 (TLR9) cascade | 30 | 96 | 2.03E-22 |
| PIP3 activates AKT signaling | 41 | 242 | 4.64E-22 |
| TRAF6 mediated induction of NFκB and MAP kinases upon TLR7/8 or 9 activation | 29 | 91 | 6.76E-22 |
| MyD88:MAL(TIRAP) cascade initiated on plasma membrane | 29 | 94 | 1.24E-21 |
| PI3K/AKT signaling in cancer | 28 | 85 | 1.50E-21 |
| d 42 | |||
| Signaling by receptor tyrosine kinases | 63 | 437 | 6.25E-41 |
| Signal transduction | 124 | 2,605 | 9.10E-38 |
| Immune system | 102 | 1,925 | 3.39E-33 |
| Diseases of signal transduction | 48 | 360 | 1.77E-29 |
| Signaling by interleukins | 50 | 439 | 5.51E-28 |
| Disease | 69 | 1,018 | 7.42E-27 |
| Intracellular signaling by second messengers | 41 | 274 | 7.62E-27 |
| Cytokine signaling in immune system | 56 | 654 | 5.24E-26 |
| Innate immune system | 67 | 1,012 | 1.39E-25 |
| VEGFA-VEGFR2 pathway | 28 | 95 | 5.17E-25 |
| PIP3 activates AKT signaling | 36 | 242 | 1.62E-23 |
| PI3K/AKT signaling in cancer | 25 | 85 | 2.58E-22 |
| Signaling by NTRKs | 25 | 97 | 3.81E-21 |
| Signaling by NTRK1 (TRKA) | 23 | 76 | 9.28E-21 |
| Negative regulation of the PI3K/AKT Network | 24 | 92 | 1.98E-20 |
| Toll-like receptor cascades | 27 | 151 | 2.36E-19 |
| MAPK family signaling cascades | 33 | 273 | 4.31E-19 |
| Toll-like receptor 4 (TLR4) cascade | 25 | 126 | 7.14E-19 |
| TRAF6 mediated induction of NFκB and MAP kinases Upon TLR7/8 or 9 activation | 22 | 91 | 3.76E-18 |
| Axon guidance | 42 | 541 | 5.95E-18 |
Abbreviations: AKT, protein kinase B; MAPK, mitogen-activated protein kinase; MyD88, myeloid differentiation primary response 88; MAL, MyD88 adaptor-like protein; NF, nuclear factor; PI3K, Phosphatidylinositol-3-kinsae; PIP, plasma membrane-intrinsic protein; NTRK, neurotrophic tyrosine receptor kinase; TIR, Toll/Interleukin 1 receptor homology; TIRAP, TIR-containing adaptor protein; TRIF, TIR-domain-containing adapter-inducing interferon-β; TICAM, TIR domain-containing adaptor molecule; TRAF, tumor necrosis factor receptor-associated factors; TLR, Toll-like receptor; TRK, tyrosine receptor kinase; VEGF, vascular endothelial growth factor.
Significant differentially phosphorylated proteins from the small intestine at d 14 and d 42 (n = 10 birds/ diet) were input into the STRING database to generate a list of the 20 most enriched Reactome Pathways. Bolded pathways are those unique between d 14 and d 42.
Proteins refers to the number of proteins within the pathway differentially phosphorylated on the array.
Background is the number of proteins within the pathway.
FDR is false discovery rate significance value of the pathway (significance ≤ 0.05).
Table 8.
Breast muscle reactome pathways1 enriched in 14 d and 42-day-old Ross 308 broilers fed corn-based diets + 0.175% algae ingredient compared to a corn-based control.
| Reactome pathway | Protein2 | Background3 | FDR4 |
|---|---|---|---|
| d 14 | |||
| Signal transduction | 130 | 2,605 | 1.65E-32 |
| Immune system | 111 | 1,925 | 4.34E-32 |
| Signaling by receptor tyrosine kinases | 57 | 437 | 7.32E-31 |
| Innate immune system | 78 | 1,012 | 6.60E-29 |
| Diseases of signal transduction | 49 | 360 | 3.81E-27 |
| Cytokine signaling in immune system | 61 | 654 | 3.87E-26 |
| Signaling by interleukins | 51 | 439 | 1.50E-25 |
| Disease | 71 | 1,018 | 8.23E-24 |
| Signaling by NTRKs | 27 | 97 | 1.49E-21 |
| Toll-like Receptor Cascades | 31 | 151 | 1.60E-21 |
| Signaling by NTRK1 (TRKA) | 25 | 76 | 1.82E-21 |
| MAPK family signaling cascades | 36 | 273 | 1.94E-19 |
| TRAF6 mediated induction of NFκB and MAP kinases upon TLR7/8 or 9 activation | 24 | 91 | 9.58E-19 |
| MyD88:MAL(TIRAP) cascade initiated on plasma membrane | 24 | 94 | 1.49E-18 |
| VEGFA-VEGFR2 pathway | 24 | 95 | 1.63E-18 |
| Toll-like receptor 4 (TLR4) cascade | 26 | 126 | 2.77E-18 |
| MyD88 cascade initiated on plasma membrane | 22 | 84 | 2.48E-17 |
| PI3K/AKT signaling in cancer | 22 | 85 | 2.74E-17 |
| Intracellular signaling by second messengers | 33 | 274 | 5.14E-17 |
| Toll-like receptor 3 (TLR3) cascade | 22 | 95 | 1.96E-16 |
| d 42 | |||
| Immune system | 134 | 1,925 | 6.30E-46 |
| Innate immune system | 91 | 1,012 | 5.36E-37 |
| Signaling by receptor tyrosine kinases | 62 | 437 | 3.31E-34 |
| Signal transduction | 136 | 2,605 | 6.38E-34 |
| Signaling by interleukins | 58 | 439 | 1.60E-30 |
| Diseases of signal transduction | 52 | 360 | 7.08E-29 |
| Cytokine signaling in immune system | 66 | 654 | 7.21E-29 |
| Disease | 80 | 1018 | 1.01E-38 |
| VEGFA-VEGFR2 pathway | 28 | 95 | 2.95E-22 |
| MAPK family signaling cascades | 40 | 273 | 3.23E-22 |
| Toll-like receptor cascades | 32 | 151 | 7.21E-22 |
| Intracellular signaling by second messengers | 37 | 274 | 1.78E-19 |
| PI3K/AKT signaling in cancer | 24 | 85 | 9.25E-19 |
| Toll-like Receptor 4 (TLR4) Cascade | 27 | 126 | 1.52E-18 |
| Toll-like Receptor 3 (TLR3) Cascade | 24 | 95 | 7.37E-18 |
| TRIF(TICAM1)-mediated TLR4 signaling | 24 | 96 | 8.54E-18 |
| MAPK1/MAPK3 signaling | 32 | 234 | 5.09E-17 |
| MyD88:MAL(TIRAP) cascade initiated on plasma membrane | 23 | 94 | 6.42E-17 |
| Toll-like receptor (TLR9) cascade | 23 | 96 | 8.72E-17 |
| PIP3 activates AKT signaling | 32 | 242 | 9.27E-17 |
Abbreviations: AKT, protein kinase B; MAPK, mitogen-activated protein kinase; MyD88, myeloid differentiation primary response 88; MAL, MyD88 adaptor-like protein; NF, nuclear factor; PI3K, Phosphatidylinositol-3-kinsae; NTRK, neurotrophic tyrosine receptor kinase; PIP, plasma membrane-intrinsic protein; TLR, Toll-like receptor; TRIF, TIR-domain-containing adapter-inducing interferon-β; TIR, Toll/Interleukin 1 receptor homology; TIRAP, TIR-containing adaptor protein; TICAM, TIR domain-containing adaptor molecule; TRAF, tumor necrosis factor receptor-associated factors; TRK, tyrosine receptor kinase; VEGF, vascular endothelial growth factor.
Significant differentially phosphorylated proteins from the small intestine at d 14 and d 42 (n = 10 birds/ diet) were input into the STRING database to generate a list of the 20 most enriched Reactome Pathways. Bolded pathways are those unique between d 14 and d 42.
Proteins refers to the number of proteins within the pathway differentially phosphorylated on the array.
Background is the number of proteins within the pathway.
FDR is false discovery rate significance value of the pathway (significance ≤ 0.05).
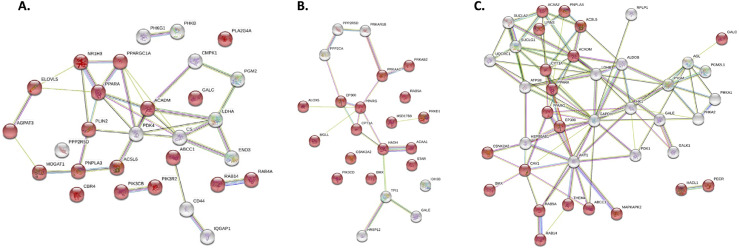
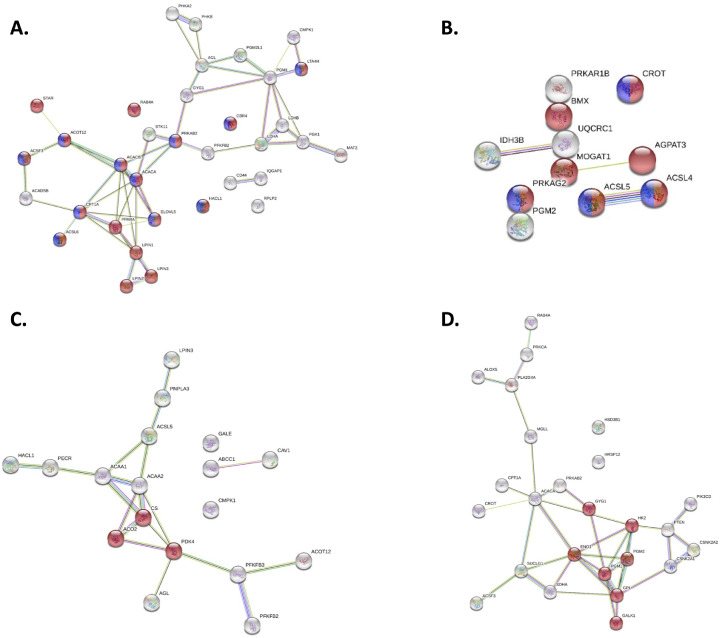
While not present in the top 20 Reactome Pathways, in all tissues and timepoints a broad pathway called “Metabolism” showed significant changes between control and algae-fed birds. The STRING database (Szklarczyk et al., 2017) compared differentially phosphorylated proteins to generate a protein interaction network. Within this network, certain macronutrient metabolic processes were enriched in algae-fed birds (Figure 5, Figure 6) and generated overwhelmingly contiguous protein-protein interactions indicating these proteins were part of an interacting metabolic process rather than disparate phosphorylation changes. In the small intestine and liver at d 14 and d 42, “metabolism of lipids” were enriched (Figures 5A-B and 6A-B, red circles), whereas these same interactions groups were enriched in the ceca only at d14 (Figure 5C). Differential metabolic pathways in the muscle were enriched for “Pyruvate metabolism and TCA cycle” and “Metabolism of carbohydrates” at d14 and d 42, respectively (Figures 6C and 6D).
Figure 5.
Unique “Metabolism” reactome pathway members in the small intestine (Meckel's adjacent) at (A) d 14 and (B) d 42 and (C) ceca at d 14 of Ross 308 broilers fed corn-based diets ± 0.175% algae. Proteins that were unique to each timepoint and tissue within the “Metabolism” reactome pathway were input into STRING to generate protein-protein interaction groups. Red proteins are members of the “Metabolism of Lipids” pathway.
Figure 6.
Unique “Metabolism” reactome pathway members in the d 14 and d 42 (A and B) liver and (C and D) breast muscle of Ross 308 broilers fed corn-based diets with 0.175% algae. Proteins that were unique to each timepoint and tissue within the “Metabolism” reactome pathway were input into STRING to generate protein-protein interaction groups. Red proteins represent members of the “Metabolism of Lipids” pathway while purple proteins represent members of the “Fatty acid metabolism” pathway in the liver at both (A) d14 and (B) d 42. In panel C, red proteins represent members of the “Pyruvate metabolism and TCA cycle” pathway in the breast muscle at d 14. In Panel D, red proteins represent members of the “metabolism of carbohydrates” in the breast muscle at d 42.
DISCUSSION
Outcomes in this study emphasize 2 key findings: algae provided a benefit to the broilers in a basal diet-specific manner, and corn vs. wheat-based diets themselves resulted in different benefits. While wheat-based diets generally altered broiler performance, intestinal morphology/integrity, and immunity, changes in these measures due to the algae ingredient were primarily observed in corn-based diets. Generally, performance improvements related to wheat-based diets were observed in the first 28 d of the grow-out period, whereas algae-related performance improvements in broilers fed corn-based diets were observed in the grower period and most pronounced in the finisher period (Table 4). It is important to note that while diets were formulated to be isonitrogenous, diet analysis showed a consistent CP increase between corn- and wheat-based diets despite measuring wheat CP prior to formulation (Table 1, Table 2, Table 3).
When comparing calculated AA profiles for the 14.2% CP wheat used in this study with analyzed values for 14.3% CP wheat reported by Matuz et al. (2000), Lys (2.7 vs. 2.4%), Val (4.4 vs. 4.4%), and Thr (3.0 vs. 3.6%) were consistent while Met was overestimated (1.6 vs. 0.6%). As performance was generally improved by wheat-based diets, it is not likely that this overestimation contributed to Met deficiency. Additionally, when increasing nitrogen content associated with 14.2% CP to 20.0% CP (N = CP / 6.25) in equations from Mossé et al. (1985), only essential AA Ile and Phe increased by 0.02 and 0.2 percentage points, respectively. With increasing wheat CP the remaining essential AA decreased 0.02 to 0.3 percentage points. Of the altered AA, Glu showed the greatest change increasing 2.2 percentage points when increasing wheat CP from 14.2 to 20.0%. In published literature comparing corn and wheat AA profiles, corn consistently had less Glu (20.2 vs. 32.3%) than wheat with Glu in both ingredients being similarly digestible in broiler chickens (Matuz et al., 2000; Huang et al., 2006). In broilers, 4 AA were more digestible in wheat compared to corn: Thr, Ser, Gly, and Tyr (Huang et al., 2006). Of these, only Thr is an essential AA and while more digestible, its concentration in wheat was expected to decrease with increasing CP. Combined, this suggests that changes to essential AA with increased CP from wheat do not favor those with increased digestibility in wheat vs. corn. Rather, the greatest difference would be increased Glu in wheat- vs. corn-based diets; however, as a non-essential AA, the health effects of Glu when supplemented to excess in broiler diets has not been well-studied. While AA profiles may have been minimally affected, the potential confounding effects of increased CP in wheat- vs. corn-based diets cannot be disregarded and outcomes observed at disparate growth stages may be explained by variations in underlying physiological responses.
The avian intestinal epithelium is a dynamic environment, with cell turnover occurring every 2 to 5 d (Imondi and Bird, 1966; Fernando and McCraw, 1973; Ghiselli et al., 2021). While intestinal histomorphology is not a specific indicator of broiler health, increased VH is associated with improved absorptive and digestive function, while increased CrD measurements can indicate greater capacity for epithelial cell turnover (Amat et al., 1996; Li et al., 2018; Bogucka et al., 2019). To functionally assess d 28 intestinal changes, a FITC-D uptake assay was implemented in conjunction with a 12 h feed restriction challenge. This assay, which has been validated in broiler chickens using similar feed restriction conditions, assesses the translocation of large molecules across the intestinal epithelium by measuring the presence of fluorescently tagged dextran in the serum (Kuttappan et al., 2015; Baxter et al., 2017). As such, increased serum fluorescence indicates increased intestinal permeability.
By the end of the grower period (d 28), birds consuming the corn-based diet with algae had increased duodenal VH with corresponding VH:CrD ratio increase. In contrast, feeding wheat-based diets increased distal jejunum VH and VH:CrD ratio regardless of algae inclusion (Figure 1). At the same time, feeding wheat-based diets generally reduced serum fluorescence compared to corn diets, suggesting improved intestinal integrity corresponding with performance improvements observed herein (Figure 2A). Consistent with the present study, previous research has reported increased ileal tight junction protein expression in wheat-fed broilers (Paraskeuas and Mountzouris, 2019). While the main effect of algae did not significantly alter intestinal permeability (Figure 2B), algae inclusion in the corn-based diet reduced the magnitude of feed restriction-induced intestinal permeability, but not to the same extent as seen in the wheat-based diet (Figure 1D). Feeding 0.175% algae in corn-based diets may have a protective effect on broiler intestinal integrity during acute off-feed events, but will require further evaluation to optimize these outcomes in commercial settings utilizing corn- and wheat-based rations. Combined, these outcomes point to 2 dietary formulations, corn-based diets with algae and wheat-based diets, that promote general phenotypes associated with improved digestive and absorptive function in disparate segments of the small intestine, which may explain improved grower and overall FCR in birds fed these diets.
In addition to feed restriction, feeding high NSP diets without exogenous enzymes has been a validated method for challenging the broiler intestine, culminating in increased serum fluorescence and bacterial translocation, while reducing BW in both broilers and turkey poults fed rye- vs. corn-based diets (Tellez et al., 2014, 2015). The results observed in birds fed wheat-based diets herein contradict these responses to high-NSP diets, likely due to the implementation of exogenous enzymes in all diets to mitigate anticipated performance reductions caused by diets containing >55% wheat and to align with industry practices. In this study, feeding a wheat-based diet with exogenous enzymes significantly improved performance in terms of BWG and FCR compared to enzyme supplementation in corn-based diets, a finding similar to those by Ghayour-Najafabadi et al. (2018) and is likely due to the relative amount of available dietary enzyme substrate. Future research into the effects of this algae product in broilers fed high-NSP diets could benefit from a lower inclusion level that does not require exogenous enzymes and mitigates any potential confounding effects linked to variations in CP between corn- and wheat-based rations.
Immunologically, wheat-based diets consistently reduced splenic monocytes and macrophages, CD1.1+ lipid antigen-presenting cells (APC), and T cells in 14-day-old birds (Figures 3 A–3C). As monocytes and macrophages are innate immune cells associated with innate inflammatory responses and APC contribute to T and natural killer T cell activation, these outcomes suggest general anti-inflammatory effects associated with wheat-based diets (Brigl and Brenner, 2004; Dvir et al., 2010; Yang et al., 2014). The percentage of n-6 linoleic acid and n-3 linolenic acid in corn and wheat is similar, suggesting that other nutritional factors including but not limited to increased CP may have contributed to the generalized anti-inflammatory effect of wheat-based diets or a different growth response in general (Price and Parsons, 1975; Ryan et al., 2007; Liu, 2011). Despite wheat-based diets reducing general populations of inflammatory cells, algae inclusion in both diets reduced γδ T cells, which are considered immunoregulatory (Vantourout and Hayday, 2013). Broilers in this study were raised on 3X composted and reused bedding from a previous coccidiosis trial utilizing Eimeria vaccine strains (Fries-Craft et al., 2021). Oocyst counts and lesion scores indicated no remaining oocysts or active cycling, suggesting that vaccine-strain Eimeria do not maintain viability in composted bedding (data not shown). In the absence of a health or other biological environmental challenge, it is difficult to ascertain the functional implications of γδ T cell reductions in a research setting. Regardless, inflammatory immune responses are energetically expensive and allocate energy away from growth (Rauw, 2012). In the context of these results, reduction of potentially inflammatory splenic immune cell populations by wheat-based diets may improve feed efficiency in the starter period by reducing immune system energetic demands and leaving available energy for growth; however, further research is needed to elucidate connections between splenic immune cell populations, their energy demands, and the effect on broiler FCR.
In addition to general responses to feeding wheat-based diets, differential responses to algae between different basal diet compositions were apparent in T cell subpopulations on d 28. Algae in wheat-based diets reduced underlying TH populations in favor of TC, while the inverse response was observed when the algae ingredient was fed in corn-based diets. Broiler chickens normally have as much as 88% more TC than TH cells within splenic T cell subpopulations, a phenotype generally considered to be a negative consequence of genetic selection for growth performance (Erf et al., 1998). In corn-based diets, algae-induced expansion of TH over TC subtypes could indicate alleviation of selection-induced changes to splenic T cell subpopulations or improved B cell activation for antibody production. By the end of the study, algae-inclusion in corn-based diets also continued to reduce TC populations in the broiler spleen at d 42, a continuation from outcomes at d 28 that may correspond with improved FCR in birds fed the corn-based control vs. corn-based diets with algae.
Overall, performance improvements due to the algae ingredient were observed only in corn-based diets. As a result, kinome peptide array analysis was conducted on tissues from birds fed corn-based diets ± algae to identify tissue-level signal transduction changes underlying performance outcomes. A consistent feature observed across all tissues was the relative similarity between d 14 and d 42 kinotypes in birds fed corn-based diets with algae that was not observed in birds fed the corn-based control diet (Figure 4). This suggests that algae matured local (small intestine around Meckel's diverticulum and ceca) and systemic (liver and muscle) tissues earlier than the control. Morphologically, jejunal CrD in birds fed corn-based diets with algae showed minimal changes from d 14 to d 42 (Figure 1E). This suggests a transition toward a structurally more mature jejunal phenotype as early as d 14 that roughly corresponds with intestinal kinome profiles within the same tissue.
When examining specific protein changes, kinome peptide array analysis showed signal transduction shifts between d 14 and d 42 in the corn-based diet with algae. Within the small intestine, this shift went from being innate and predominantly TLR-mediated at d 14 to adaptive and growth oriented at d 42 (Table 5). The ceca also showed significant induction of innate immune response signaling at d 14 that were not unique to this timepoint, suggesting that these kinome shifts were more robust in the small intestine where tissue had the most contact with the algae ingredient (Table 6). Within the spleen, significant changes to cell populations associated with innate immunity (monocytes/macrophages and APC) were not observed in birds fed corn-based diets with algae at d 14 (Figures 3A and 3B). However, minimal protein changes related to innate immunity were observed in the liver and muscle at d 14 (Tables 7 and 8), further suggesting that increased innate immune signaling at d 14 was localized to the small intestine and ceca. Intestinal outcomes additionally suggest algae inclusion in corn-based diets may have a priming effect on immunity, starting with the innate system then shifting the response to the adaptive system. This signaling pattern could indicate a shift toward a TH cell response that is roughly supported by the reduced disparity between TC and TH splenic subtypes observed in algae-fed birds at d 42 (Figures 3D and 3E).
In addition to immunological changes, metabolic profile shifts within the different tissues were observed. Within the intestine, ceca, and liver, unique protein-interaction groups were enriched for lipid and fatty acid metabolism at d 14 and d 42, suggesting that algae altered lipid metabolism for much of the grow-out period (Figures 5 and 6A–B). Previous work has shown that broiler chicken selection for reduced residual feed intake upregulated genes associated with lipid metabolism (Lee et al., 2015). In the current study, the muscle tissue profile was distinct in that metabolic protein-interaction groups affected by algae inclusion were associated with the TCA cycle at d 14 and carbohydrate metabolism at d 42 (Figures 6C and 6D). As the muscle is a major consumer of glucose, this would be consistent with increased muscle deposition and consequent growth. Combined, lipid metabolism outcomes in the gut and carbohydrate metabolism in the muscle are consistent with a more efficient FCR. While changes in metabolism observed at d 14 did not translate to improve FCR in the starter period, it may be that early and sustained metabolic shifts locally and systemically translated to improve FCR at later performance periods culminating in a more efficient FCR for the entire 42 d grow-out period (Table 4).
Findings from this study in algae-supplemented corn-based diets are in agreement with other published outcomes of improved BWG and FCR in healthy 42-day-old broilers fed Spirulina (Jamil et al., 2015; Khan et al., 2020). Algae-induced changes in histomorphology, intestinal integrity, systemic immunity, and tissue kinotypes in corn-fed broilers translated to improved performance in these birds, but similar outcomes did not translate to improved performance in birds fed wheat-based diets. The differential response to algae in corn vs. wheat-based diets herein emphasizes the importance of evaluating novel feed ingredients in different basal diets. Despite the need for additional study in wheat-based diets, the results herein provide support for the use of algae-based ingredients in broilers fed corn-based diets.
ACKNOWLEDGMENTS
The authors would like to thank DSM for donating Ronozyme WX and MG enzymes. Funding Source: Zivo Biosciences Inc. funded this work.
DISCLOSURES
The authors declare no conflict of interest.
REFERENCES
- Amat C., Planas J.M., Moreto M. Kinetics of hexose uptake by the small and large intestine of the chicken. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1996;271:1085–1089. doi: 10.1152/ajpregu.1996.271.4.R1085. [DOI] [PubMed] [Google Scholar]
- Amerah A.M. Interactions between wheat characteristics and feed enzyme supplementation in broiler diets. Anim. Feed Sci. Technol. 2015;199:1–9. [Google Scholar]
- An B.-K., Kim K.-E., Jeon J.-Y., Lee K.W. Effect of dried Chlorella vulgaris and Chlorella growth factor on growth performance, meat qualities and humoral immune responses in broiler chickens. SpringerPlus. 2016;5:1–7. doi: 10.1186/s40064-016-2373-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arsenault R.J., Lee J.T., Latham R., Carter B., Kogut M.H. Changes in immune and metabolic gut response in broilers fed β-mannanase in β-mannan-containing diets. Poult. Sci. 2017;96:4307–4316. doi: 10.3382/ps/pex246. [DOI] [PubMed] [Google Scholar]
- Arstila T.P., Vainio O., Lassila O. Central role of CD4+ T cells in avian immune response. Poult. Sci. 1994;73:1019–1026. doi: 10.3382/ps.0731019. [DOI] [PubMed] [Google Scholar]
- Aviagen. 2019. Ross broiler: nutrition specifications. Accessed Mar. 2021.https://en.aviagen.com/assets/Tech_Center/Ross_Broiler/RossBroilerNutritionSpecs2019-EN.pdf.
- Babot J.D., Argañaraz Martínez E., Lorenzo-Pisarello M.J., Apella M.C., Perez Chaia A. Lactic acid bacteria isolated from poultry protect the intestinal epithelial cells of chickens from in vitro wheat germ agglutinin-induced cytotoxicity. Br. Poult. Sci. 2017;58:76–82. doi: 10.1080/00071668.2016.1251574. [DOI] [PubMed] [Google Scholar]
- Baxter M.F.A., Merino-Guzman R., Latorre J.D., Mahaffey B.D., Yang Y., Teague K.D., Graham L.E., Wolfenden A.D., Hernandez-Velasco X., Bielke L.R., Hargis B.M., Tellez G. Optimizing fluorescein isothiocyanate dextran measurement as a biomarker in a 24-h feed restriction model to induce gut permeability in broiler chickens. Front. Vet. Sci. 2017;4 doi: 10.3389/fvets.2017.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogucka J., Ribeiro D.M., Bogusławska-Tryk M., Dankowiakowska A., Costa R.P.R., Bednarczyk M. Microstructure of the small intestine in broiler chickens fed a diet with probiotic or synbiotic supplementation. J. Anim. Physiol. Anim. Nutr. 2019;103:1785–1791. doi: 10.1111/jpn.13182. [DOI] [PubMed] [Google Scholar]
- Bozkurt M., Kucukyilmaz K., Catli A.U., Ozyildiz Z., Cinar M., Cabuk M., Coven F. Influences of an essential oil mixture supplementation to corn versus wheat-based practical diets on growth, organ size, intestinal morphology and immune response of male and female broilers. Ital. J. Anim. Sci. 2012;11 e54-e54. [Google Scholar]
- Brigl M., Brenner M.B. CD1: antigen presentation and T cell function. Ann. Rev. Immunol. 2004;22:817–890. doi: 10.1146/annurev.immunol.22.012703.104608. [DOI] [PubMed] [Google Scholar]
- Calder P.C. N−3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am. J. Clin. Nutr. 2006;83:1505S–1519S. doi: 10.1093/ajcn/83.6.1505S. [DOI] [PubMed] [Google Scholar]
- Choct M., Annison G. Anti-nutritive effect of wheat pentosans in broiler chickens: roles of viscosity and gut microflora. Br. Poult. Sci. 1992;33:821–834. doi: 10.1080/00071669208417524. [DOI] [PubMed] [Google Scholar]
- Christaki E., Florou-Paneri P., Bonos E. Microalgae: a novel ingredient in nutrition. Int. J. Food Sci. Nutr. 2011;62:794. doi: 10.3109/09637486.2011.582460. [DOI] [PubMed] [Google Scholar]
- Dalla Pellegrina C., Rizzi C., Mosconi S., Zoccatelli G., Peruffo A., Chignola R. Plant lectins as carriers for oral drugs: is wheat germ agglutinin a suitable candidate? Toxicol. Appl. Pharmacol. 2005;207:170–178. doi: 10.1016/j.taap.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Dvir H., Wang J., Ly N., Dascher C.C., Zajonc D.M. Structural basis for lipid-antigen recognition in avian immunity. J. Immunol. 2010;184:2504–2511. doi: 10.4049/jimmunol.0903509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggersdorfer M., Wyss A. Carotenoids in human nutrition and health. Arch. Biochem. Biophys. 2018;652:18–26. doi: 10.1016/j.abb.2018.06.001. [DOI] [PubMed] [Google Scholar]
- Epelman S., Lavine Kory j., Randolph Gwendalyn j. Origin and functions of tissue macrophages. Immunity. 2014;41:21–35. doi: 10.1016/j.immuni.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erf G.F., Bottje W.G., Bersi T.K. CD4, CD8 and TCR defined T-cell subsets in thymus and spleen of 2- and 7-week old commercial broiler chickens. Vet. Immunol. Immunopathol. 1998;62:339–348. doi: 10.1016/s0165-2427(97)00070-6. [DOI] [PubMed] [Google Scholar]
- Fearon D.T., Locksley R.M. The instructive role of innate immunity in the acquired immune response. Science. 1996;272:50–54. doi: 10.1126/science.272.5258.50. [DOI] [PubMed] [Google Scholar]
- Fernando M.A., McCraw B.M. Mucosal morphology and cellular renewal in the intestine of chickens following a single infection of Eimeria acervulina. J. Parasitol. 1973;59:493–501. [PubMed] [Google Scholar]
- Fries-Craft K., Meyer M.M., Bobeck E.A. Algae-based feed ingredient protects intestinal health during Eimeria challenge and alters systemic immune responses with differential outcomes observed during acute feed restriction. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101369. 101369-101369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghayour-Najafabadi P., Khosravinia H., Gheisari A., Azarfar A., Khanahmadi M. Productive performance, nutrient digestibility and intestinal morphometry in broiler chickens fed corn or wheat-based diets supplemented with bacterial- or fungal-originated xylanase. Ital. J. Anim. Sci. 2018;17:165–174. [Google Scholar]
- Ghiselli F., Rossi B., Felici M., Parigi M., Tosi G., Fiorentini L., Massi P., Piva A., Grilli E. Isolation, culture, and characterization of chicken intestinal epithelial cells. BMC Mol. Cell. Biol. 2021;22 doi: 10.1186/s12860-021-00349-7. 12-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornbeck P.V., Zhang B., Murray B., Kornhauser J.M., Latham V., Skrzypek E. PhosphoSitePlus, 2014: mutations, PTMs and recalibrations. Nucleic Acids Res. 2015;43:D512–D520. doi: 10.1093/nar/gku1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang K.H., Li X., Ravindran V., Bryden W.L. Comparison of apparent ileal amino acid digestibility of feed ingredients measured with broilers, layers, and roosters. Poult. Sci. 2006;85:625–634. doi: 10.1093/ps/85.4.625. [DOI] [PubMed] [Google Scholar]
- Imondi A.R., Bird F.H. The turnover of intestinal epithelium in the chick. Poult. Sci. 1966;45:142–147. doi: 10.3382/ps.0450142. [DOI] [PubMed] [Google Scholar]
- Jamil A.B.M., Akanda M., Rahman M., Hossain M., Islam M. Prebiotic competence of Spirulina on the production performance of broiler chickens. J. Adv. Vet. Anim. Res. 2015;2:304–309. [Google Scholar]
- Jamroz D., Wertelecki T., Houszka M., Kamel C. Influence of diet type on the inclusion of plant origin active substances on morphological and histochemical characteristics of the stomach and jejunum walls in chicken. J. Anim. Physiol. Anim. Nutr. 2006;90:255–268. doi: 10.1111/j.1439-0396.2005.00603.x. [DOI] [PubMed] [Google Scholar]
- Jassal B., Matthews L., Viteri G., Gong C., Lorente P., Fabregat A., Sidiropoulos K., Cook J., Gillespie M., Haw R., Loney F., May B., Milacic M., Rothfels K., Sevilla C., Shamovsky V., Shorser S., Varusai T., Weiser J., Wu G., Stein L., Hermjakob H., D'Eustachio P. The reactome pathway knowledgebase. Nucleic Acids Res. 2020;48:D498–D503. doi: 10.1093/nar/gkz1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang H.K., Park S.B., Kim C.H. Effects of dietary supplementation with a Chlorella by-product on the growth performance, immune response, intestinal microflora and intestinal mucosal morphology in broiler chickens. J. Anim. Pysiol. Anim. Nutr. 2017;101:208. doi: 10.1111/jpn.12566. [DOI] [PubMed] [Google Scholar]
- Kang H.K., Salim H.M., Akter N., Kim D.W., Kim J.H., Bang H.T., Kim M.J., Na J.C., Hwangbo J., Choi H.C., Suh O.S. Effect of various forms of dietary Chlorella supplementation on growth performance, immune characteristics, and intestinal microflora population of broiler chickens. J. Appl. Poult. Res. 2013;22:100–108. [Google Scholar]
- Khan S., Mobashar M., Mahsood F.K., Javaid S., Abdel-Wareth A.A., Ammanullah H., Mahmood A. Spirulina inclusion levels in a broiler ration: evaluation of growth performance, gut integrity, and immunity. Trop. Anim. Health Prod. 2020;52:3233–3240. doi: 10.1007/s11250-020-02349-9. [DOI] [PubMed] [Google Scholar]
- Kuttappan V.A., Vicuña E.A., Latorre J.D., Wolfenden A.D., Téllez G.I., Hargis B.M., Bielke L.R. Evaluation of gastrointestinal leakage in multiple enteric inflammation models in chickens. Front. Vet. Sci. 2015;2 doi: 10.3389/fvets.2015.00066. 66-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Karnuah A.B., Rekaya R., Anthony N.B., Aggrey S.E. Transcriptomic analysis to elucidate the molecular mechanisms that underlie feed efficiency in meat-type chickens. Mol. Genet. Genom. 2015;290:1673–1682. doi: 10.1007/s00438-015-1025-7. [DOI] [PubMed] [Google Scholar]
- Li J., Zhang S.Y., Li R.X., Lin X., Mi Y.L., Zhang C.Q. Culture and characterization of chicken small intestinal crypts. Poult. Sci. 2018;97:1536–1543. doi: 10.3382/ps/pey010. [DOI] [PubMed] [Google Scholar]
- Liu K. Comparison of lipid content and fatty acid composition and their distribution within seeds of 5 small grain species. J. Food Sci. 2011;76:C334–C342. doi: 10.1111/j.1750-3841.2010.02038.x. [DOI] [PubMed] [Google Scholar]
- Liu W.-C., Kim I.-H. Effects of dietary xylanase supplementation on performance and functional digestive parameters in broilers fed wheat-based diets. Poult. Sci. 2017;96:566–573. doi: 10.3382/ps/pew258. [DOI] [PubMed] [Google Scholar]
- Madeira M.S., Cardoso C., Lopes P.A., Coelho D., Afonso C., Bandarra N.M., Prates J.A.M. Microalgae as feed ingredients for livestock production and meat quality: a review. Livest. Sci. 2017;205:111–121. [Google Scholar]
- Matuz J., Tibor B., Katalin M.-S., Lajos B. Structure and potential allergenic character of cereal proteins: protein content and amino acid composition. Cereal Res Commun. 2000;28:263–270. [Google Scholar]
- Mirzaie S., Sharifi S.D., Zirak-Khattab F. The effect of a Chlorella by-product dietary supplement on immune response, antioxidant status, and intestinal mucosal morphology of broiler chickens. J. Appl. Phycol. 2020;32:1771–1777. [Google Scholar]
- Mirzaie S., Zirak-Khattab F., Abdollah H., Seyed. Donyaei-Darian H. Effects of dietary on antioxidant status, lipid profile, immune response and performance characteristics of broiler chickens reared under high ambient temperature. Asian-Australas. J. Anim. Sci. 2018;31:556–563. doi: 10.5713/ajas.17.0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossé J., Huet J.C., Baudet J. The amino acid composition of wheat grain as a function of nitrogen content. J. Cereal Sci. 1985;3:115–130. [Google Scholar]
- National Research Council . The National Academies Press; Washington, DC: 1994. Nutrient Requirements of Poultry: Ninth Revised Edition, 1994. [Google Scholar]
- Paraskeuas V., Mountzouris K.C. Broiler gut microbiota and expressions of gut barrier genes affected by cereal type and phytogenic inclusion. Anim. Nutr. 2019;5:22–31. doi: 10.1016/j.aninu.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickel J.M., Chen C.L., Cooper M.D. An avian B-lymphocyte protein associated with beta 2-microglobulin. Immunogenetics. 1990;32:1–7. doi: 10.1007/BF01787321. [DOI] [PubMed] [Google Scholar]
- Pirgozliev V., Mansbridge S.C., Rose S.P., Mackenzie A.M., Beccaccia A., Karadas F., Ivanova S.G., Staykova G.P., Oluwatosin O.O., Bravo D. Dietary essential oils improve feed efficiency and hepatic antioxidant content of broiler chickens. Animal. 2019;13:502–508. doi: 10.1017/S1751731118001520. [DOI] [PubMed] [Google Scholar]
- Pohlmeyer I., Jörns J., Schumacher U., Van Damme E.J.M., Peumans W.J., Pfüller U., Neumann U. Lectin histochemical investigations of the distal gut of chicks with special emphasis on the follicle-associated epithelium. J. Vet. Med. A Physiol. Pathol. Clin. Med. 2005;52:138–146. doi: 10.1111/j.1439-0442.2005.00696.x. [DOI] [PubMed] [Google Scholar]
- Price P.B., Parsons J.G. Lipids of seven cereal grains. J. Am. Oil Chem. Soc. 1975;52:490–493. doi: 10.1007/BF02640738. [DOI] [PubMed] [Google Scholar]
- Qureshi M.A., Garlich J.D., Kidd M.T. Dietary Spirulina platensis enhances humoral and cell-mediated immune functions in chickens. Immunopharmacol. Immunotoxicol. 1996;18:465–476. doi: 10.3109/08923979609052748. [DOI] [PubMed] [Google Scholar]
- Rauw W.M. Immune response from a resource allocation perspective. Front. Genet. 2012;3:267. doi: 10.3389/fgene.2012.00267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan E., Galvin K., O'Connor T.P., Maguire A.R., O'Brien N.M. Phytosterol, squalene, tocopherol content and fatty acid profile of selected seeds, grains, and legumes. Plant Foods Hum. Nutr. 2007;62:85–91. doi: 10.1007/s11130-007-0046-8. [DOI] [PubMed] [Google Scholar]
- Suzuki R., Shimodaira H. Pvclust: an R package for assessing the uncertainty in hierarchical clustering. Bioinformatics. 2006;22:1540–1542. doi: 10.1093/bioinformatics/btl117. [DOI] [PubMed] [Google Scholar]
- Szklarczyk D., Morris J.H., Cook H., Kuhn M., Wyder S., Simonovic M., Santos A., Doncheva N.T., Roth A., Bork P., Jensen L.J., von Mering C. The STRING database in 2017: quality-controlled protein-protein association networks, made broadly accessible. Nucleic Acids Res. 2017;45:D362–D368. doi: 10.1093/nar/gkw937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taebipour M.J., Dadras H., Nazifi S., Afsar M., Ansari-Lari M. Evaluation of blood monocyte and lymphocyte population in broiler chicken after vaccination and experimental challenge with Newcastle disease virus. Vet. Immunol. Immunopathol. 2017;190:31–38. doi: 10.1016/j.vetimm.2017.07.002. [DOI] [PubMed] [Google Scholar]
- Tellez G., Latorre J.D., Kuttappan V.A., Hargis B.M., Hernandez-Velasco X. Rye affects bacterial translocation, intestinal viscosity, microbiota composition and bone mineralization in Turkey poults. Plos One. 2015;10 doi: 10.1371/journal.pone.0122390. e0122390-e0122390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellez G., Latorre J.D., Kuttappan V.A., Kogut M.H., Wolfenden A., Hernandez-Velasco X., Hargis B.M., Bottje W.G., Bielke L.R., Faulkner O.B. Utilization of rye as energy source affects bacterial translocation, intestinal viscosity, microbiota composition, and bone mineralization in broiler chickens. Front. Genet. 2014;5 doi: 10.3389/fgene.2014.00339. 339-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trost B., Kindrachuk J., Maeaettaenen P., Napper S., Kusalik A. PIIKA 2: an expanded, web-based platform for analysis of kinome microarray data. Plos One. 2013;8 doi: 10.1371/journal.pone.0080837. e80837-e80837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UniProt C. UniProt: the universal protein knowledgebase in 2021. Nucleic Acids Res. 2021;49:D480–D489. doi: 10.1093/nar/gkaa1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vantourout P., Hayday A. Six-of-the-best: unique contributions of γδ T cells to immunology. Nat Rev. Immunol. 2013;13:88. doi: 10.1038/nri3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z., Rothwell L., Young J.R., Kaufman J., Butter C., Kaiser P. Generation and characterization of chicken bone marrow-derived dendritic cells. Immunology. 2010;129:133–145. doi: 10.1111/j.1365-2567.2009.03129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaakob Z., Ali E., Zainal A., Mohamad M., Takriff M. An overview: biomolecules from microalgae for animal feed and aquaculture. J. Biol. Res. (Thessalon). 2014;21:6. doi: 10.1186/2241-5793-21-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Zhang L., Yu C., Yang X.-F., Wang H. Monocyte and macrophage differentiation: circulation inflammatory monocyte as biomarker for inflammatory diseases. Biomark. Res. 2014;2:1. doi: 10.1186/2050-7771-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Iji P.A., Kocher A., Mikkelsen L.L., Choct M. Effects of dietary mannanoligosaccharide on growth performance, nutrient digestibility and gut development of broilers given different cereal-based diets. J. Anim. Physiol. Anim. Nutr. 2008;92:650–659. doi: 10.1111/j.1439-0396.2007.00761.x. [DOI] [PubMed] [Google Scholar]
- Światkiewicz S., Arczewska-Wlosek A., Józefiak D. Application of microalgae biomass in poultry nutrition. Worlds Poult. Sci. J. 2015;71:663–672. [Google Scholar]