Abstract
During schistosomiasis, interleukin-5 (IL-5)-dependent eosinophil responses have been implicated in immunopathology, resistance to superinfection, synergistic interactions with chemotherapeutic agents, and the inductive phase of the egg-induced Th2 response. We examined these issues in IL-5-deficient (IL-5−/−) mice. IL-5−/− and wild-type (WT) mice were indistinguishable in terms of susceptibility to primary infections and the ability to resist secondary infections. Moreover, hepatic pathology was similar in both strains apart from a relative lack of eosinophils and, during chronic infection, a significantly larger mast cell component in the granulomas of IL-5−/− mice. Splenocyte cytokine production in response to soluble egg antigen (SEA) or anti-CD3 revealed no significant differences except for heightened tumor necrosis factor alpha production by cells from chronically infected IL-5−/− mice compared to WT animals. In contrast, ionomycin-stimulated non-B, non-T (NBNT) cells from IL-5−/− mice produced significantly smaller IL-4 amounts than did NBNT cells from WT animals. This difference was not apparent following plate-bound anti-immunoglobulin E or SEA stimulation. The absence of IL-5 failed to affect the induction of Th2 responses in naive mice. Peritoneal exudate cells recovered from egg-injected IL-5−/− or WT mice produced equivalent levels of IL-4 following restimulation with SEA or anti-CD3.
Schistosomiasis is a helminthic infection affecting over 200 million people and causing severe disease in tens of millions (26). During infection, parasite eggs stimulate a strong type 2 response (17, 32, 43) which is essential for host survival (35). A characteristic feature of infection is the development of blood eosinophilia, mediated by interleukin-5 (IL-5), the principal eosinophil differentiation factor (7, 39), which is produced in quantity by Th2 cells and other cells. Parasite eggs are the major stimulus for this type 2 response (17, 32, 43), and eosinophils are a major component (50%) of the granulomas which form around tissue-trapped eggs (9, 28, 33). Early investigators have reported smaller granulomas, increased egg burdens, and more extensive tissue damage in mice depleted of eosinophils, leading to a proposed role for these cells both in the successful sequestration of egg-derived hepatotoxins and in the destruction of eggs (4, 20, 24, 29). IL-5-induced eosinophilia has also been correlated with protective immunity, as eosinophil-depleted mice showed increased susceptibility to superinfection (25). In contrast, more recent studies found no significant role for IL-5 or eosinophilia in immunity or pathogenesis. Mice treated with an anti-IL-5 monoclonal antibody (MAb) showed tissue damage and concomitant immunity levels comparable to those of control animals (41, 42).
In an attempt to address these conflicting findings, we compared disease progression and resistance to superinfection in wild-type (WT) versus IL-5-deficient (IL-5−/−) mice, which are unable to develop eosinophilia (22). We also evaluated immune response development in these mice, as our previous data indicated a role for eosinophils in producing IL-4 early in the response to schistosome eggs, and we have postulated that this IL-4 plays a role in allowing Th2 response development (37). Lastly, we used infected IL-5−/− mice to address the issue, raised by others previously, of whether eosinophils cooperate with antibodies in an antibody-dependent cellular cytotoxicity reaction to kill drug (praziquantel)-damaged schistosomes (2, 3, 14, 36). Remarkably, given their prevalence during infection, we could find little evidence that eosinophils play any essential role during murine schistosomiasis.
MATERIALS AND METHODS
Infections and immunizations.
Schistosoma mansoni (Puerto Rican strain NMRI)-infected Biomphalaria glabrata snails obtained from F. Lewis (Biomedical Research Institute, Rockville, Md.) were maintained in our laboratory. IL-5−/− C57BL/6 mice (22) and WT C57BL/6 mice (Taconic, Germantown, N.Y.) were infected percutaneously with ∼50 or ∼75 cercariae, and disease progression was monitored. At the times of acute (8 weeks postinfection) and chronic (16 to 24 weeks postinfection) disease, parasitological and immunological analyses were performed (35). For immunizations, eggs were isolated from the hepatic tissues of infected mice, washed extensively into sterile phosphate-buffered saline (PBS) and stored at −70°C. Mice were injected intraperitoneally via a 23-gauge needle with 5 × 103 eggs in 100 μl of PBS or with 100 μl of PBS alone (38).
Parasite and cell recovery.
Adult schistosomes were recovered by perfusion (46). Liver samples were collected to quantitate hepatic egg burdens (6), and additional samples were fixed for histological evaluation of tissue damage and granuloma volumes. Spleens and peritoneal exudate cells (PEC) were prepared as previously described (35, 38). Splenocytes (2 × 106 cells/well) and PEC (106 cells/well) were stimulated in 96-well flat-bottom plates with soluble egg antigen (SEA; 50 μg/ml) to induce antigen-specific responses or with a plate-bound anti-CD3 MAb (0.5 μg/well plate bound) to polyclonally stimulate T cells. In some experiments, splenocytes were depleted of specific cell populations by incubation with a cocktail of antibodies and then complement treated (49). We depleted B and T cells by incubation with RA3-6B2 (anti-B220/CD45R MAb), 53-2.1 (anti-CD90.2 MAb), 3.155 (anti-CD8 MAb), and GK1.5 (anti-CD4 MAb). Following 1 h of incubation on ice, cells were treated with a 1:10 dilution of low-toxicity M-rabbit complement (Accurate Chemicals, Westbury, N.Y.) for 45 min at 37°C. To ensure depletion, antibody incubations and complement treatments were repeated once. The extent of depletion was determined by flow cytometry, and total contaminating CD4+ was limited to 3 to 5% in three separate experiments. Non-B, non-T (NBNT) cells were stimulated in vitro with SEA, plate-bound anti-CD3 MAb, plate-bound anti-immunoglobulin E (IgE) (EM-95; 1 μg/well plate bound), or ionomycin (1 μg/well; Sigma, St. Louis, Mo.).
Cytokine and NO analyses.
Twenty-four- and 72-h cell culture supernatants were used to quantitate cytokine levels by enzyme-linked immunosorbent assay. Enzyme-linked immunosorbent assay reagents were prepared in our laboratory (IL-2, IL-4, and gamma interferon [IFN-γ]) as previously described (50) or obtained commercially (TNF-α from Genzyme, Boston, Mass., and IL-10 from PharMingen, San Diego, Calif.). Nitrite accumulation, an indicator of nitric oxide (NO) production, was measured with Greiss reagents (35).
Resistance to superinfection.
To determine a role for IL-5 in protection from superinfection, we separated IL-5−/− and WT mice into three groups and infected them as follows: mice in group A were exposed through the shaved abdomen to a low-dose (∼40 cercariae) primary infection, mice in group B received the primary infection and 8 weeks later were exposed to a high-dose (∼120 cercariae) challenge infection through the shaved flank, and mice in group C received the challenge infection alone. Eight weeks following the challenge infection, mice were euthanized and parasites were carefully recovered by perfusion. Resistance to secondary challenge was calculated as previously described (44).
Chemotherapy.
To establish a requirement for IL-5 in chemotherapy, we treated acutely infected WT and IL-5−/− mice on alternate days with three subcutaneous injections of praziquantel (250 mg/kg; Sigma) in the carrier cremophor EL (Sigma); control infected mice were treated with cremophor EL alone (2). Efficacy was evaluated in terms of percent reduction of the worm burden following treatment (2).
Statistical analyses.
Differences in worm and egg burdens, granuloma volume and cellularity, and cytokine and NO levels were compared by using Student’s t test. Probability values of ≤0.05 were considered significant.
RESULTS AND DISCUSSION
Infection and disease.
We found that compared with WT mice carrying infections similar in intensity, IL-5−/− mice failed to develop more severe morbidity, as measured by weight loss, and did not suffer increased mortality (data not shown). Moreover, during both acute and chronic disease, the lack of IL-5 had no effect on either worm or egg burdens (Table 1). We detected no significant differences in granuloma volumes of acutely infected IL-5−/− versus WT mice (Table 1). During chronic disease, in all but one of four experiments, IL-5−/− mice downregulated their granulomas as efficiently as did WT mice (Table 1). In IL-5−/− mice, the granulomatous eosinophilic infiltrate was significantly reduced but not completely absent (Table 1). This may be explained by the effects that other chemotactic factors, such as eotaxin, may have on eosinophil recruitment (10). Surprisingly, the number of mast cells in the granuloma was dramatically increased during chronic infection in IL-5−/− mice (Table 1); the mechanism underlying this mast cell elevation remains to be defined.
TABLE 1.
Comparison of disease progression in IL-5−/− and WT micea
| Stage of disease (wk) | Mice | Worm burden | Hepatic egg burden/g | Granuloma vol (mm3) | Cellular infiltrate in granuloma
|
|
|---|---|---|---|---|---|---|
| % Eosinophils | % Mast cells | |||||
| Acute (8) | IL-5−/− | 22.7 ± 2.2 | 12,141 ± 2,866 | 28.9 ± 3.0 | 7.5 ± 1.8b | NDc |
| WT | 20.0 ± 6.4 | 15,840 ± 2,344 | 26 ± 4.8 | 55 ± 2.4 | ND | |
| Chronic (>16) | IL-5−/− | 11.7 ± 2.2 | 8,500 ± 884 | 15.1 ± 1.3 | 0.7 ± 0.2b | 8.8 ± 0.8b |
| WT | 12.0 ± 0.5 | 6,400 ± 1,415 | 18.6 ± 4.7 | 38.3 ± 5.9 | 2.3 ± 0.2 | |
Results are means ± standard errors and are representative of at least three separate experiments.
P < 0.05 compared with WT mice.
ND, none detected.
Th2 response development.
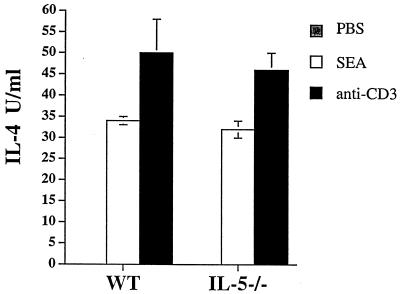
Schistosome eggs, injected into naive mice, induce a T-cell-independent, IL-5-dependent, early eosinophilia (12 to 24 h postinjection) at the site of antigen deposition (37, 38). Eosinophils in the infiltrate were found to produce IL-4, leading us to hypothesize that this cell type plays a role in promoting Th2 cell differentiation (37). However, we found that during infection, the lack of IL-5 and of eosinophilia had only a minimal effect on the development of the Th2 response (Table 2). Acutely and chronically infected WT and IL-5−/− animals produced comparable levels of type 1 (IFN-γ and IL-2) and type 2 (IL-4 and IL-10) cytokines (Table 2). To further examine whether the absence of IL-5 affects Th2 response development, we injected isolated eggs intraperitoneally into naive WT and IL-5−/− animals in a manner known to induce IL-5-dependent early IL-4 production followed by a Th2 response (37). Ten days later, we measured in vitro IL-4 production by SEA- or anti-CD3-stimulated PEC. We found no difference in the IL-4 levels produced in response to either of these stimuli by PEC from WT and IL-5−/− mice (Fig. 1). We concluded that the absence of IL-5 does not preclude the development of the Th2 response.
TABLE 2.
Comparison of immune responses developed during acute (week 8) and chronic (>week 16) infections by IL-5−/− and WT micea
| Infection and mice | IL-4 concn (U/ml)
|
IL-10 concn (U/ml)
|
IL-2 concn (U/ml)
|
IFN-γ concn (pg/ml)
|
TNF-α concn (pg/ml)
|
NO concn (μM)
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SEA | Anti-CD3 | SEA | Anti-CD3 | SEA | Anti-CD3 | SEA | Anti-CD3 | SEA | Anti-CD3 | SEA | Anti-CD3 | |
| Acute | ||||||||||||
| IL-5−/− | 36.2 ± 1.7 | 256 ± 19.4 | 38.6 ± 5.7 | 215 ± 32 | 0.85 ± 0.2 | 5 ± 0.9 | ND | 19.5 ± 1.8 | 106 ± 34 | 402 ± 130 | 5.2 ± 1.8 | 42.5 ± 8.3 |
| WT | 39.7 ± 15.2 | 235 ± 62 | 24.9 ± 4.8 | 244 ± 37 | 0.26 ± 0.3 | 3.1 ± 0.7 | ND | 18.3 ± 2.5 | 150 ± 46 | 358 ± 29 | 9.4 ± 1.7 | 32.1 ± 7.1 |
| Chronic | ||||||||||||
| IL-5−/− | 2.2 ± 0.5 | 54.2 ± 17 | 53.6 ± 6.3 | 254.4 ± 46 | 1.9 ± 0.1 | 3.1 ± 0.36 | ND | 75.8 ± 16.5 | 250 ± 29b | 673b ± 32 | 5.7 ± 1.8 | 25.2 ± 1.8 |
| WT | 0.53 ± 0.33 | 20.4 ± 5.5 | 28 ± 3.4 | 290.8 ± 34 | 1.0 ± 0.56 | 2.1 ± 0.5 | ND | 47.6 ± 7.7 | 47.4 ± 24 | 370 ± 43 | 4.4 ± 1.8 | 19.4 ± 4 |
Results are means ± standard errors and are representative of at least three separate experiments. ND, not detected.
P < 0.05 compared with WT mice.
FIG. 1.
Comparison of IL-4 cytokine levels from PEC of WT and IL-5−/− mice (three animals per group) injected 10 days earlier with either S. mansoni eggs or PBS. PEC were pooled and cultured for 72 h in duplicate wells in the presence of anti-IL-4R either alone, with SEA, or with plate-bound anti-CD3. Data are from one experiment; the experiment was repeated three times with similar results. Data are expressed as means ± standard errors.
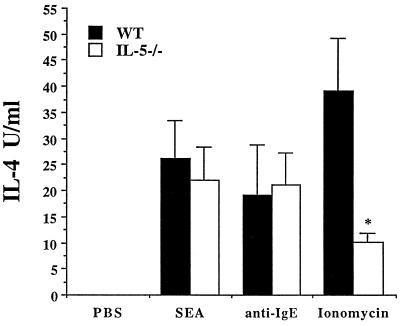
In addition to Th2 cells and eosinophils, splenic NBNT cells are a source of significant levels of IL-4 in schistosome-infected mice (8, 51). The expansion of this population of IL-4-producing cells is IL-4 dependent (1, 31, 40). Among these cells is a subset of FcɛR+ basophils that can respond to plate-bound anti-IgE, antigen (via FcR-bound antibody), and ionomycin, by making IL-4 (8). We investigated the role of IL-5 in the development of the NBNT-cell population by comparing IL-4 levels following SEA and anti-IgE stimulation of NBNT cells from WT and IL-5−/− mice (Fig. 2). FcɛR cross-linkage by plate-bound anti-IgE induced the production of comparable levels of IL-4 by both WT and IL-5−/− NBNT-cell populations. In contrast, following stimulation with ionomycin, NBNT cells from infected IL-5−/− mice made significantly less IL-4 than did NBNT cells from infected WT animals. An attractive explanation for the latter result is that among the whole NBNT-cell population, eosinophils, which in the mouse are FcɛR− (21), contribute to IL-4 production, and thus, NBNT-cell-derived IL-4 is reduced in eosinophil-deficient, infected IL-5−/− mice. Since plate-bound anti-IgE, which targets mouse basophils, stimulates similar levels of IL-4 production by NBNT cells from WT and IL-5−/− mice, we can conclude that the basophil response is unaffected by the absence of IL-5.
FIG. 2.
Comparison of IL-4 levels produced by NBNT cells of WT and IL-5−/− mice (at least three animals per group) infected with S. mansoni (8 weeks postinfection). NBNT cells were cultured for 24 h with PBS, SEA, plate-bound anti-IgE, or ionomycin. Results are from one experiment and are representative of data from three separate experiments. Data are expressed as means ± standard errors. An asterisk indicates P < 0.05 compared with WT animals.
Since type 2 cytokines can suppress the production of proinflammatory mediators secreted by macrophages activated by type 1 cytokines (12, 15, 23, 30), we also assessed whether there was a difference in the levels of tumor necrosis factor alpha (TNF-α) and NO made by spleen cells from infected IL-5−/− and WT mice. Whereas NO levels produced by cells from infected knockout and WT mice were similar during chronic, but not acute, infection, TNF-α production by cells from IL-5−/− mice was significantly elevated compared to that by cells from WT animals (Table 2). Based on the increased mast cell infiltrate levels in the granulomas of infected IL-5−/− mice (Table 1), we are currently testing the possibility that heightened TNF-α production is the result of an increased number of mast cells (16) in the spleens of chronically infected IL-5−/− versus WT mice.
Resistance to superinfection.
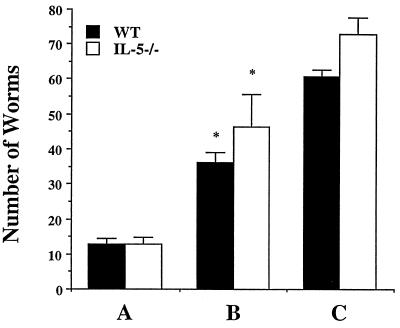
Epidemiological studies in areas where schistosomes are endemic have correlated type 2 responses with resistance to reinfection (13, 18, 34), and it is believed that prior to treatment, infected hosts exhibit concomitant immunity, to which adult worms are refractory but newly invading larval worms are susceptible (11, 47). Work on concomitant immunity in the mouse has indicated that both nonspecific mechanisms, including changes in the hepatic vasculature due to increased portal pressure resulting from granulomatous lesions (19, 52), and specific components of the immune response (45) mediate this process. Eosinophils have been heavily implicated in concomitant immunity as effector cells working in antibody-dependent cytotoxicity reactions against larval worms (4, 5, 25, 27). To experimentally address the role of eosinophils in immunity, we exposed IL-5−/− and WT mice to a primary infection and 8 weeks later attempted to superinfect them. Compared to mice receiving the challenge infection alone, mice exposed to primary and challenge infections had significantly reduced worm burdens (Fig. 3). Resistance to superinfection was statistically significant in both IL-5−/− (P < 0.05) and WT mice (P < 0.01), with both strains exhibiting similar levels of protection (Fig. 3, P = 0.64). Our data support previous findings reporting resistance to superinfection in anti-IL-5 MAb-treated, infected mice (41).
FIG. 3.
Comparison of resistance to superinfection in WT and IL-5−/− mice. Worm burdens in infected WT and IL-5−/− mice were determined after perfusion. Mice received a primary infection (group A; four WT and six IL-5−/− mice) primary and secondary infections (group B; five WT and five IL-5−/− mice), or a secondary infection (group C; six WT and six IL-5−/− mice). Resistance to reinfection was observed in both WT (61.4% ± 4.1%) and IL-5−/− (53.6% ± 10.2%) mice in group B. Data are expressed as means ± standard errors. An asterisk indicates P < 0.05 compared with groups receiving a challenge infection alone.
Chemotherapy.
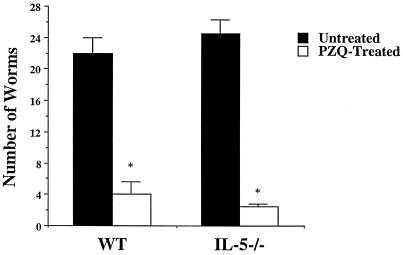
Effective treatment with praziquantel is dependent on the host immune response, as treatment of T-cell-deprived and antibody-deficient mice fails to clear infections (2, 36). It has been postulated that antibody serves to target drug-damaged worms for destruction by FcR+ leukocytes, with the eosinophil being a candidate effector cell (2, 3, 14, 36). To assess whether the absence of IL-5 and eosinophilia affects the efficacy of chemotherapy, we compared worm burdens in treated and untreated acutely infected IL-5−/− and WT mice. We found that treatment significantly reduced worm burdens in both IL-5−/− (89.8% ± 1.4%) and WT (81.8% ± 7.4%) mice (Fig. 4). The effects of praziquantel are therefore independent of IL-5.
FIG. 4.
Effect of praziquantel on worm burden in IL-5−/− and WT mice. Mice (three animals per group) were treated with praziquantel in carrier or with carrier alone. Data are expressed as means ± standard errors. Results are representative of two separate experiments. An asterisk indicates P < 0.05 compared with untreated groups.
Concluding remarks.
The magnitude of the Th2 response and associated eosinophilia during schistosomiasis can reasonably be seen as an indication that eosinophils play some important role in infection. Nevertheless, the absence of IL-5, which prevents the expansion of the eosinophil population, appears to have little effect on the ability of mice to support and tolerate a schistosome infection, on the development of resistance to superinfection, on the capacity of the immune response to participate in chemotherapy, or on the development of the Th2 response per se. The one exception to the latter point is that there is an effect on the ability of NBNT cells from infected mice to make IL-4 in response to ionomycin in the absence of IL-5. Our data, which in part support previous results obtained by using MAb-mediated neutralization of IL-5 during infection (41, 42), can be interpreted in several ways. First, previously identified roles for eosinophils during schistosomiasis are nonessential or redundant. Second, because in contrast to those in many other species, mouse eosinophils are FcɛR− (21), they cannot interact with IgE, the isotype most likely to be important for many of the functions in which eosinophils have been implicated (IgE levels in infected IL-5−/− mice are not significantly lower than those in infected WT mice; 35a). Third, the eosinophils which remain in an IL-5−/− animal are sufficient to perform all of the functions normally performed by the IL-5-expanded population. Given the higher-than-expected percentage of eosinophils in the granulomas of infected IL-5−/− mice, we suspect that the latter may be the most plausible explanation for our results. This issue is currently being tested experimentally by attempting to deplete eosinophils in IL-5−/− mice by using RB6-8C5 (anti-GR-1 MAb) (48).
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grant AI32573 to E.J.P. The Basel Institute was founded by and is supported by Hoffmann-La Roche. L.R.B. is supported by NRSA AI-09512. E.A.S. was supported by NRSA AI-09227 while at Cornell University.
We thank Padraic Fallon and Esther Racoosin for helpful discussions.
REFERENCES
- 1.Ben-Sasson S Z, Le Gros G, Conrad D H, Finkelman F F, Paul W E. Cross-linking Fc receptors stimulates splenic non-B, non-T cells to secrete interleukin 4 and other lymphokines. Proc Natl Acad Sci USA. 1990;87:1421–1425. doi: 10.1073/pnas.87.4.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brindley P, Sher A. The chemotherapeutic effect of praziquantel against Schistosoma mansoni is dependent on host antibody response. J Immunol. 1987;139:215–220. [PubMed] [Google Scholar]
- 3.Brindley P, Sher A. Immunological involvement in the efficacy of praziquantel. Exp Parasitol. 1990;71:245–248. doi: 10.1016/0014-4894(90)90028-b. [DOI] [PubMed] [Google Scholar]
- 4.Brito P D, Kazura J, Mahmoud A. Host granulomatous response in Schistosomiasis mansoni. Antibody and cell-mediated damage of parasite eggs in vitro. J Clin Investig. 1984;74:1715–1723. doi: 10.1172/JCI111589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Butterworth A E, Sturrock R F, Houba V, Mahmoud A A F, Sher A, Rees P H. Eosinophils as mediators of antibody dependent damage to schistosomula. Nature. 1975;256:727–729. doi: 10.1038/256727a0. [DOI] [PubMed] [Google Scholar]
- 6.Cheever A W. Relative resistance of the eggs of the human schistosome to digestion in potassium hydroxide. Bull WHO. 1970;43:601. [PMC free article] [PubMed] [Google Scholar]
- 7.Coffman R L, Seymour B W P, Haduk S, Jackson J, Rennick D. Antibody to IL-5 inhibits helminth-induced eosinophilia in mice. Science. 1989;245:308–310. doi: 10.1126/science.2787531. [DOI] [PubMed] [Google Scholar]
- 8.Cohn R G, Williams M, Sher A, Caulfield J P. Schistosoma mansoni: characterization of an FcɛR+ population of granule-containing splenocytes isolated from infected mice. Exp Parasitol. 1995;80:339–341. doi: 10.1006/expr.1995.1042. [DOI] [PubMed] [Google Scholar]
- 9.Colley D G. Schistosoma mansoni: eosinophilia and the development of lymphocyte blastogenesis in response to soluble egg antigen in inbred mice. Exp Parasitol. 1972;32:520–526. doi: 10.1016/0014-4894(72)90070-7. [DOI] [PubMed] [Google Scholar]
- 10.Collins P, Marleau S, Griffiths-Johnson D, Jose P, Williams T. Cooperation between IL-5 and the chemokine eotaxin to induce eosinophil accumulation in vivo. J Exp Med. 1995;182:1169–1174. doi: 10.1084/jem.182.4.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dean D A. Schistosoma and related genera: acquired resistance in mice. Exp Parasitol. 1983;55:1–104. doi: 10.1016/0014-4894(83)90002-4. [DOI] [PubMed] [Google Scholar]
- 12.Doherty T M, Kastelein R, Menon S, Andrade S, Coffman R L. Modulation of murine macrophage function by IL-13. J Immunol. 1993;151:7151–7160. [PubMed] [Google Scholar]
- 13.Dunne D W, Butterworth A E, Fulford A J C, Kariuki H C, Langley J G, Ouma J H, Capron A, Pierce R, Sturrock R. Immunity after treatment of human schistosomiasis: association between IgE antibodies to adult worm antigens and resistance to reinfection. Eur J Immunol. 1992;22:1483–1494. doi: 10.1002/eji.1830220622. [DOI] [PubMed] [Google Scholar]
- 14.Fallon P, Cooper R, Probert A, Doenhoff M. Immune dependent chemotherapy of schistosomiasis. Parasitology. 1992;105:s41–48. doi: 10.1017/s003118200007534x. [DOI] [PubMed] [Google Scholar]
- 15.Fiorentino D F, Zlotnik A, Mosmann T R, Howard M, O’Garra A. IL-10 inhibits cytokine production by activated macrophages. J Immunol. 1991;147:3815–3822. [PubMed] [Google Scholar]
- 16.Gordon J, Galli S. Mast cells as a source of both preformed and immunologically inducible TNF-alpha/cachectin. Nature. 1990;346:274–276. doi: 10.1038/346274a0. [DOI] [PubMed] [Google Scholar]
- 17.Gryzch J M, Pearce E J, Cheever A, Caulada Z A, Caspar P, Hieny S, Lewis F A, Sher A. Egg deposition is the major stimulus for the production of Th2 cytokines in murine schistosomiasis mansoni. J Immunol. 1991;146:1322–1327. [PubMed] [Google Scholar]
- 18.Hagan P, Wilkin H, Blumenthal U, Hayes R, Greenwood B. Eosinophilia and resistance to Schistosoma haematobium in man. Parasite Immunol. 1985;7:625–632. doi: 10.1111/j.1365-3024.1985.tb00106.x. [DOI] [PubMed] [Google Scholar]
- 19.Harrison R A, Bickle Q D, Doenhoff M J. Factors affecting the acquisition of resistance against Schistosoma mansoni in the mouse. IX. Evidence that the mechanisms which mediate resistance during early patent infections may lack immunological specificity. Parasitology. 1982;84:93–110. doi: 10.1017/s0031182000051696. [DOI] [PubMed] [Google Scholar]
- 20.James S L, Colley D G. Eosinophil-mediated destruction of Schistosoma mansoni eggs. J Reticuloendothel Soc. 1976;20:359–374. [PubMed] [Google Scholar]
- 21.Jones R E, Finkelman F D, Hester R B, Keyes S G. Toxocara canis: failure to find IgE receptors on eosinophils from infected mice suggests that murine eosinophils do not kill helminth larvae by an IgE-dependent mechanism. Exp Parasitol. 1994;78:64–75. doi: 10.1006/expr.1994.1006. [DOI] [PubMed] [Google Scholar]
- 22.Kopf M, Brombacher F, Hodgkin P D, Ramsay A J, Milbourne E A, Dai W J, Ovington K S, Behm C A, Kohler G, Young I G, Matthaei K I. IL-5 deficient mice have a developmental defect in CD5+ B-1 cells and lack eosinophilia but have normal antibody and cytotoxic T cell responses. Immunity. 1996;4:15–24. doi: 10.1016/s1074-7613(00)80294-0. [DOI] [PubMed] [Google Scholar]
- 23.Liew F Y, Li Y, Severn A, Millott S, Schmidt J, Salter M, Moncada S. A possible novel pathway of regulation by murine T helper type-2 (Th2) cells of a Th1 cell activity via the modulation of the induction of nitric oxide synthase on macrophages. Eur J Immunol. 1991;21:2489–2494. doi: 10.1002/eji.1830211027. [DOI] [PubMed] [Google Scholar]
- 24.Mahmoud A, Warren K, Graham R G., Jr Antieosinophilic serum and the kinetics of eosinophilia in schistosomiasis mansoni. J Exp Med. 1975;142:560–574. doi: 10.1084/jem.142.3.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mahmoud A A F, Warren K S, Peters P A. A role for the eosinophil in acquired resistance to Schistosoma mansoni infection as determined by anti-eosinophil serum. J Exp Med. 1975;142:805–813. doi: 10.1084/jem.142.4.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mahmoud A A F, Wahab M F A. Schistosomiasis. In: Warren K S, Mahmoud A A F, editors. Tropical and geographical medicine. New York, N.Y: McGraw Hill; 1990. pp. 458–473. [Google Scholar]
- 27.McLaren D, Ramalho-Pinto F. Eosinophil-mediates killing of schistosomula of Schistosoma mansoni in vitro: synergistic effect of antibody and complement. J Immunol. 1979;123:1431–1438. [PubMed] [Google Scholar]
- 28.Moore D, Grove D, Warren K. The Schistosoma mansoni egg granuloma: quantitation of cell populations. J Pathol. 1977;121:41–50. doi: 10.1002/path.1711210107. [DOI] [PubMed] [Google Scholar]
- 29.Olds G, Mahmoud A. Role of host granulomatous responses in murine schistosomiasis: eosinophil mediated destruction of eggs. J Clin Investig. 1980;66:1191–1199. doi: 10.1172/JCI109970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oswald I P, Gazzinelli R G, Sher A, James S L. IL-10 synergizes with IL-4 and TGF-β to inhibit macrophage cytotoxic activity. J Immunol. 1992;148:3578–3582. [PubMed] [Google Scholar]
- 31.Paul W E, Seder R A, Plaut M. Lymphokine and cytokine production by FcɛRI+ cells. Adv Immunol. 1993;53:1–29. [PubMed] [Google Scholar]
- 32.Pearce E J, Caspar P, Gryzch J M, Lewis F A, Sher A. Downregulation of Th1 cytokine production accompanies induction of Th2 responses by a helminth, Schistosoma mansoni. J Exp Med. 1991;173:159–165. doi: 10.1084/jem.173.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Phillips S M, Lammie P J. Immunopathology of granuloma formation and fibrosis in schistosomiasis. Parasitol Today. 1986;2:296–302. doi: 10.1016/0169-4758(86)90123-7. [DOI] [PubMed] [Google Scholar]
- 34.Rihet P, Demeure D E, Bourgois A, Prata A, Dessein A J. Evidence for an association between human resistance to Schistosoma mansoni and high anti-larval IgE levels. Eur J Immunol. 1991;21:2679–2686. doi: 10.1002/eji.1830211106. [DOI] [PubMed] [Google Scholar]
- 35.Rosa Brunet L, Finkelman F D, Cheever A W, Kopf M A, Pearce E J. IL-4 protects against TNF-α mediated cachexia and death during acute schistosomiasis. J Immunol. 1997;159:777–785. [PubMed] [Google Scholar]
- 35a.Rosa Brunet, L., and E. J. Pearce. Unpublished data.
- 36.Sabah A, Fletcher C, Webbe G, Doenhoff M. Schistosoma mansoni: reduced efficacy of chemotherapy in infected T-cell-deprived mice. Exp Parasitol. 1985;60:348–354. doi: 10.1016/0014-4894(85)90041-4. [DOI] [PubMed] [Google Scholar]
- 37.Sabin E A, Kopf M A, Pearce E J. Schistosoma mansoni egg induced early IL-4 production is dependent upon IL-5 and eosinophils. J Exp Med. 1996;184:1871–1878. doi: 10.1084/jem.184.5.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sabin E A, Pearce E J. Early IL-4 production by non-CD4+ cells at the site of antigen deposition predicts the development of a T helper 2 cell response to Schistosoma mansoni eggs. J Immunol. 1995;155:4844–4853. [PubMed] [Google Scholar]
- 39.Sanderson C, Campbell H, Young I. Molecular and cellular biology of eosinophil differentiation factor (IL-5) and its effect on human and mouse B cells. Immunol Rev. 1988;102:29–50. doi: 10.1111/j.1600-065x.1988.tb00740.x. [DOI] [PubMed] [Google Scholar]
- 40.Seder R A, Plaut M, Barbier S, Urban J, Finkelman F D, Paul W E. Purified FcɛR+ bone marrow and splenic non-B, non-T cells are highly enriched in the capacity to produce IL-4 in response to immobilized IgE, IgG2a, or ionomycin. J Immunol. 1991;147:903–909. [PubMed] [Google Scholar]
- 41.Sher A, Coffman R L, Hieny S, Cheever A W. Ablation of eosinophil and IgE responses with anti-IL-5 or anti-IL-4 antibodies fails to affect immunity against Schistosoma mansoni in the mouse. J Immunol. 1990;145:3911–3916. [PubMed] [Google Scholar]
- 42.Sher A, Coffman R L, Hieny S, Scott P, Cheever A W. Interleukin 5 (IL-5) is required for the blood and tissue eosinophilia but not granuloma formation induced by infection with Schistosoma mansoni. Proc Natl Acad Sci USA. 1990;87:61–66. doi: 10.1073/pnas.87.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sher A, Fiorentino D, Casper P, Pearce E J, Mosmann T R. Production of IL-10 by CD4+ T lymphocytes correlates with down regulation of Th1 cytokine synthesis. J Immunol. 1991;147:2713–2718. [PubMed] [Google Scholar]
- 44.Sher A, Mackenzie P, Smithers S R. Decreased recovery of invading parasites from the lungs as a parameter of acquired immunity to schistosomiasis in the laboratory mouse. J Infect Dis. 1974;130:626–633. doi: 10.1093/infdis/130.6.626. [DOI] [PubMed] [Google Scholar]
- 45.Sher A, Smithers S R, Mackenzie P. Passive transfer of acquired resistance to Schistosoma mansoni in laboratory mice. Parasitology. 1975;70:347–357. doi: 10.1017/s0031182000052124. [DOI] [PubMed] [Google Scholar]
- 46.Smithers D V, Terry R J. The infection of laboratory hosts with cercariae of Schistosoma mansoni and the recovery of adult worms. Parasitology. 1965;55:695–700. doi: 10.1017/s0031182000086248. [DOI] [PubMed] [Google Scholar]
- 47.Smithers S, Terry R. Immunity in schistosomiasis. Ann N Y Acad Sci. 1969;160:826–840. doi: 10.1111/j.1749-6632.1969.tb15904.x. [DOI] [PubMed] [Google Scholar]
- 48.Tepper R, Coffman R, Leder P. An eosinophil-dependent mechanism for the antitumor effect of interleukin-4. Science. 1992;257:548–551. doi: 10.1126/science.1636093. [DOI] [PubMed] [Google Scholar]
- 49.Vasconcelos J P, Pearce E J. Type 1 CD8+ T cell responses during infection with the helminth Schistosoma mansoni. J Immunol. 1996;157:3046–3053. [PubMed] [Google Scholar]
- 50.Vella A T, Pearce E J. CD4+ Th2 response induced by Schistosoma mansoni eggs develops rapidly, through an early, transient, Th0-like stage. J Immunol. 1992;148:2283–2290. [PubMed] [Google Scholar]
- 51.Williams M E, Kullberg M C, Barbieri S, Caspar P, Berzofsky J A, Seder R A, Sher A. Fc epsilon receptor-positive cells are a major source of antigen-induced interleukin-4 in spleens of mice infected with Schistosoma mansoni. Eur J Immunol. 1993;23:1910–1916. doi: 10.1002/eji.1830230827. [DOI] [PubMed] [Google Scholar]
- 52.Wilson R A, Coulson P S, McHugh S M. A significant part of the concomitant immunity of mice to Schistosoma mansoni is the consequence of a leaky hepatic portal system not immune killing. Parasite Immunol. 1983;5:595–605. doi: 10.1111/j.1365-3024.1983.tb00776.x. [DOI] [PubMed] [Google Scholar]