Abstract
Background
The aim of the trial is to evaluate the effectiveness of interventions provided by online support program apps, adopting health-related quality of life (HR-QOL) scores as indicators.
Methods
The design is as an open, randomized, parallel-group trial with longitudinal data collection. The subjects will be female cancer patients receiving treatment in a Japanese National Cancer Hospital. Patients assigned to the experimental group will use three apps: an app for them to monitor their own health (monitoring app), an app to assess their understanding of their diagnosis and treatment and their readiness to receive treatment (confirmation app), and an app to address mental health issues (writing app); patients assigned to the control group will use only the monitoring app. At baseline (before patients undergo cancer treatment) and three other times during the study, evaluation indicators will be obtained from three different standardized HR-QOL scales that are incorporated in the monitoring app. The study hypothesis is that at 6 months after patients’ baseline health monitoring, patients in the experimental group will have improved HR-QOL as compared with patients in the control group.
Conclusion
This study is based on self-regulation theory, so it is important that the online support program works in an efficient way with respect to patients finding and setting their own health-related goals and adapting their behaviors to achieve those goals. Verifying the effectiveness of the combination of the three apps will show that it is a scientifically valid approach to maintaining or improving the HR-QOL of cancer patients.
Keywords: eHealth, Female cancer patients, Health-related quality of life, Randomized controlled trial
1. Introduction
For patients diagnosed with cancer, coping with the experience and keeping themselves in good health requires that they successfully manage various health-related issues. Information and communications technology (ICT) is being proactively introduced into the healthcare management of cancer patients. Several systematic reviews have corroborated the positive effects of e-health–based self-management [1], web-based interventions in managing symptoms [2], and mobile phone apps for cancer-related information needs [3]. In response to such a context, we have developed an online support program designed to maintain or improve the quality of life in female cancer patients by empowering them to manage their own health. To verify the effectiveness of our program, we have planned a randomized controlled trial.
1.1. Theoretical basis of the online support program
This study protocol and the online support program were developed from within the context of self-regulation theory, in which goals form an important part of human behavior and energize and direct one's activities [4]. Many patients diagnosed with cancer struggle with how they should manage their health and life, because their real life is filled with experiences that threaten their goals, standards, and reference values for health. According to self-regulation theory, however, if one is adequately aware of a discrepancy between existing goals and reality, one can take self-regulatory actions in a variety of ways to reduce that discrepancy.
Our online support program consists of three different apps: a monitoring app, a confirmation app, and a writing app. These apps are designed to stimulate cancer patients to find and set their own health-related goals and adapt their behavior to achieve those goals through providing opportunities to monitor health conditions (monitoring app), assess their own understanding of the disease and its treatment (confirmation app), and address mental health issues (writing app). When cancer patients use these apps, they may develop a clearer awareness about discrepancies between their own reality and their goals, standards, and reference values related to their health and illness. At that time, if they want to maintain or improve health-related quality of life (HR-QOL), their desire for reducing discrepancies can elicit self-regulatory actions.
In our program, patient-reported outcomes (PROs) about HR-QOL are collected electronically as patients monitor their health using the monitoring app. Quality of life is a term that represents “an overall sense of well-being, including aspects of happiness and satisfaction with life as a whole” [5], and the HR-QOL concept covers aspects of overall quality of life that affect physical and mental health [5]. PROs refer to “health experiences and evaluations that are assessed by patient report, such as symptoms, assessments of functioning, well-being, health perceptions, and satisfaction with care” [6]. In this study we will measure PROs by using three different HR-QOL scales incorporated in the monitoring app: the Ferrans and Powers Quality of Life Index (QLI) [7,8], the M.D. Anderson Symptom Inventory (MDASI) [9], and the Hospital Anxiety and Depression Scale (HADS) [10,11]. The validity and effectiveness of measuring PROs via electronic media (ePROs) with HR-QOL scales in clinical cancer settings is supported by the results of several studies [[12], [13], [14], [15]]. In our current program, patients can use the monitoring app to compare their current and previous HR-QOL ePROs to track their health progress. Such comparisons seem to work in favor of setting and striving for realistic goals with respect to their health without having too high expectations.
Meanwhile, cancer patients’ understanding of their disease and treatment has a high impact on their goals, standards, and values regarding their health and illness. We think that if using the confirmation app can promote a correct understanding of their disease and treatment and adequate preparation for their illness, it will function effectively to improve HR-QOL by increasing self-regulation to reduce the discrepancies between their goals and reality.
The third component—the writing app—is used to provide an expressive writing intervention, which is one type of expressive psychotherapy. The effectiveness of expressive writing interventions was first tested in 1986 [16,17], and their effectiveness in terms of both psychological and biological health has been subsequently supported by many studies, including a meta-analysis [18]. Patients are asked to write down their deepest thoughts and feelings about a stressful event, and such reflection on the cancer experience is expected to reactivate patients’ self-regulatory actions to manage their disturbed mood and ruminations.
1.2. Theoretical framework of this study
The apps constituting our online support program currently only target patients who have breast cancer, colon cancer, uterine cancer, ovarian cancer, and thyroid cancer. These cancers are particularly common among Japanese women; for example, according to 2016–2018 data [19], breast cancer and colon cancer rank the first and second in incidence/morbidity among Japanese women, and the incidence rate of thyroid cancer in women is 2.7 times that in men. Since there is a wide range of cancer types and treatments, the scope of cancers covered by the confirmation app is restricted to those that are most typical among Japanese women. The age range of the target population is broadly set from 18 to 75 years old; this is based on a study of Japanese breast cancer patients that found that acceptability of using tablet computers to record ePROs was not necessarily age dependent [20].
Because of the several types of cancers in the targeted patients, it is necessary to take into consideration the influence that variations in recovery course, experience, and environmental characteristics have on patients’ HR-QOL. For example, in one study of breast cancer patients, several factors such as older age, economic burden, and higher levels of depression were found to be predictive of the trajectory of their HR-QOL [21]. Therefore, we have chosen to conduct a randomized controlled trial (RCT) to control the influence of such factors.
1.2.1. Study hypothesis
The study hypothesis is that female cancer patients who use all three of the apps composing this online support program will better maintain and improve their HR-QOL measured at 6 months after their first health monitoring compared with those who only monitor their health. We are assuming that compared to the ePROs measured before patients undergo cancer treatment (baselines), the ePROs at 1 month after the first health monitoring will be lower, and that the ePROs at 3 months after the first health monitoring will be higher than those at 1 month after the first health monitoring. It is said that the existential plight that results from being diagnosed with cancer diminishes after the first 100 days [22]. Therefore, the discrepancy that patients notice between their goals and the reality of having cancer might also decrease around that time which will have the effect of reducing their self-regulatory behaviors. However, we thought that if patients who had experienced using the confirmation app to think about their disease and treatment could use the writing app to reactivate their self-regulation activities, they might begin to take more action to improve their HR-QOL. And we expect these reactivated self-regulation activities to continue for 3 months. Meanwhile, we thought that the ePROs of patients who use only the monitoring app (the control group) would probably not improve by 6 months after the first health monitoring compared to those who use all three apps. Additionally, the type of cancer is predicted to make a difference in the average scores of ePROs measured at each time point but to make no difference in the longitudinal recuperative process with respect to HR-QOL.
2. Materials and methods
2.1. Design
The aim of this RCT is to test the effectiveness of intervention using the three apps of the online support program, adopting HR-QOL scores as an indicator to assess the outcome of the intervention. The study is designed as an open, randomized parallel-group trial, with a longitudinal data-collecting method. Patients assigned to the experimental group will use all three apps: the monitoring app, confirmation app, and writing app. Patients assigned to the control group will use only the monitoring app. Additionally, stratified permuted block randomization will be adopted, with the stratification factor being the type of cancer: breast, colon, uterine (two sites: corpus of the uterus and endocervix), ovarian, and thyroid.
2.2. Sampling and participants
This study's subjects are to be female cancer patients receiving treatment at the National Cancer Center Hospital East (NCCHE) in Japan. Participants will be recruited at the outpatient units until the target sample size is achieved. The planned sample size was set to a total of 210 subjects in two groups, and the sampling period is slated to last 1 year.
2.2.1. Inclusion criteria
Patients will be included if they meet the following criteria: (a) have a diagnosis of cancer (breast cancer, colon cancer, uterine cancer, ovarian cancer, or thyroid cancer) and have had their medical condition and treatment courses explained by their physician; (b) are receiving treatment for the first time in the NCCHE; (c) are waiting to be treated in hospital or in an outpatient setting; (d) are from 18 to 75 years old at the time of obtaining informed consent; (e) can go through the online support program using a tablet computer, personal computer, or smartphone in an outpatient setting or in their homes; and (f) are required to receive treatment at either the Department of Breast Surgery, Department of Medical Oncology, Department of Gynecology, Department of Gastrointestinal Oncology, or Department of Head and Neck Medical Oncology.
2.2.2. Exclusion criteria
To ensure safety and efficacy, patients will be excluded if (a) the physician or nurse in charge determines that there is a high possibility of a detrimental health effect occurring if the patient participates in the online support program; (b) the physician or nurse in charge determines that the patient is mentally unstable and will have difficulty participating in the online support program; or (c) a physician in charge or a nurse in charge knows that the patient needs to receive hospital treatment for at least 1 month or longer.
2.2.3. Withdrawal of participants
A participant will be regarded as withdrawn from the study if (a) the clinical diagnosis of cancer is reversed by subsequent histopathological diagnosis; (b) the participant requests to be withdrawn or withdraws consent; (c) hospitalization for more than 1 month is required; (d) the physician or nurse in charge judges that it is appropriate that participation be withdrawn; or (e) the participant discontinues and does not resume using the apps.
2.3. Procedures
A member of this research team will obtain informed consent from each participant after careful explanation of the study, by both oral and written means. The participants will then be registered for this clinical study through the Electronic Data Capture (EDC) system and be randomly allocated to one of two groups: the experimental group or the control group.
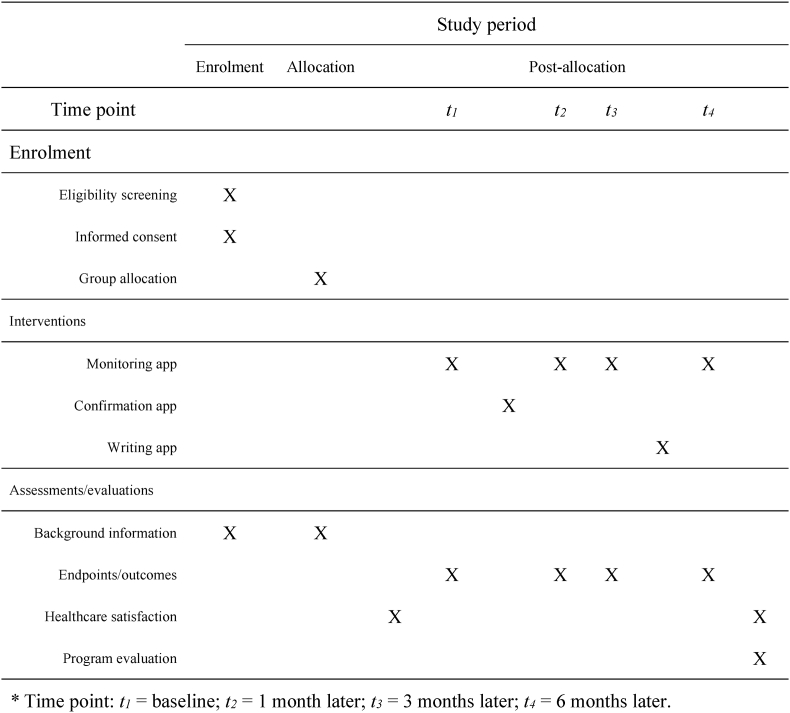
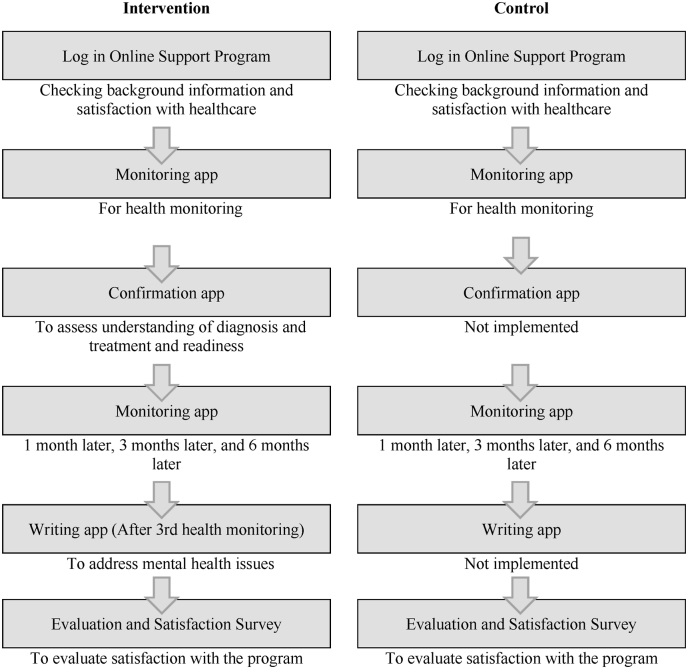
The flowchart of participation and the participant flow through study in this longitudinal study is shown in Fig. 1, Fig. 2, respectively. Participants will access the apps for this online support program via a dedicated URL. After completion of registration and before initiation of treatment, participants of both groups will use the monitoring app for health monitoring; the ePROs generated at that time become the baseline of each group. Subsequently, the confirmation app will be used by participants in the experimental group to assess their understanding of their diagnosis and treatment and their readiness. In addition to the first health monitoring (baseline), participants of both groups will use the monitoring app another three times (four times in all): 1 month later, 3 months later, and 6 months later. After their third health monitoring, participants of the experimental group will use the writing app to address mental health issues. Additionally, participants of both groups will answer a questionnaire at the beginning of the study and again at the end to assess their satisfaction with respect to healthcare, and they will answer an additional questionnaire at the end evaluating their satisfaction with the program. Participants will receive an email reminder shortly before each app needs to be run.
Fig. 1.
Flowchart of participation.
Fig. 2.
Participant flow through the study.
2.3.1. Data storage/management
Both the EDC and online support program systems will be login controlled (ID and password authentication) for each user. Two research members authorized to access the clinical information will anonymize participants’ personal information at the time of case registration. Therefore, names and other identifiable information will not be stored on the online sites. After the RCT is completed, the electronic data will be downloaded onto a portable hard drive in the form of CSV files, and the Principal Investigator will securely store them in a locked filing cabinet for 10 years. Personally identifiable information such as written informed consent will be securely stored in the NCCHE Ladies Center for 10 years.
2.3.2. Ethical considerations and declarations
Approval was obtained in March 2022 from the institutional review board (IRB) of the university hospital with which the Principal Investigator is affiliated (Ethics Committee University of Tsukuba Hospital, R03-243), and implementation of the study was then approved by the IRB of NCCHE, the hospital in which recruitment of study subjects is to be conducted. This study protocol complies with the Japanese government's Ethical Guidelines for Medical and Biological Research Involving Human Subjects, which came into effect in March 2021 [23], and is conducted so as to protect the participants' rights in terms of privacy and confidentiality. The ethical guidelines used have been repeatedly revised to comply with the Act on the Protection of Personal Information in Japan. This clinical trial is registered in the Japan Registry of Clinical Trials (Registration Number: jRCT1030210638).
2.4. Support program and apps
2.4.1. Monitoring app
The monitoring app incorporates Japanese-language versions of three different HR-QOL scales (QLI, MDASI, and HADS) that have been proven to be of high reliability and validity [[7], [8], [9], [10], [11]]. Users of the monitoring app answer the questions of these HR-QOL scales and receive a personalized HR-QOL report in the form of average scores and visual line plots representing changes in their HR-QOL scores over time. The app is set up so that users must answer all of the questions required for one scale before opening the next scale.
The QLI consists of two parts that respectively measure satisfaction with and importance of various aspects of daily life using a 1–6 scale across 33 paired items [24]. Once the QLI questions are answered, users are provided, in the form of average scores, with information about their own subjective well-being and overall QOL (total QLI score) and QOL in four domains (subscale scores): health and functioning, psychological and spiritual, social and economic, and family. The possible range of final QLI scores is from 0 to 30, with a lower score indicating a worse QOL. Studies have shown that the Japanese version of the QLI is culturally appropriate [7] and has sufficient reliability and validity to be able to evaluate the impact of cancer and treatment in research and clinical practice [8].
The MDASI consists of two parts that respectively assess the extent of symptoms experienced during the last 24 h and how much those symptoms interfere with various aspects of the patient's life [25]. Users get to know outcomes of their own assessment through answering questions of a 6-item symptom scale and a 13-item interference scale. Each question of both scales is measured using a 0–10 scale, with higher scores indicating more severe symptoms and interference. The reliability and validity of the Japanese version of the MDASI have been verified [9], and it has been applied to a wide range of research on cancer treatment and clinical practice.
The HADS consists of 14 questions that measure depression and anxiety in patients treated in a clinical setting [26]. Through answering the questions, users can detect their own state of anxiety and depression as either ‘negative’, ‘doubtful’, or ‘definite’, with cutoff points of both scores within the total range of 0–21 for each state. It has been verified that the Japanese version of the HADS is a sensitive and specific screening tool to detect psychological distress in Japanese cancer patients [10,11].
2.4.2. Confirmation app
By answering each online question, users of the confirmation app are able to assess their comprehension of their disease and treatment and their readiness to receive treatment. The confirmation app provides users with an opportunity to think of the impact of the disease and the treatment on themselves and their significant others, life with the illness, and the rest of their life, and to ascertain whether they want to consult someone else (doctor, nurse, other health professional, or family) to help them understand aspects of their disease and treatment that they do not fully comprehend.
The composition of questions used to assess comprehension and readiness was framed around those on the website from the National Cancer Center Japan [19], which offers a ‘cancer information providing service’ for the general population. A nursing representative on this research team prepared a draft version of questions for the confirmation app. Physicians on the research team who conduct diagnosis and treatment and explain those aspects to patients then adjusted the draft questions to better fit their clinical practice, and nurses of the research team added improvements to the content and form of the questions to better fit the patients' health care needs. The Principal Investigator made the final decision on the contents of the app.
2.4.3. Writing app
The writing app provides the expressive writing intervention. The format of this intervention follows that of Pennebaker's guidebook [27], taking into consideration findings from studies that tested the effects of expressive writing interventions on Asian breast cancer patients [[28], [29], [30], [31]]. After the health monitoring at 3 months—a point by which the impact of a cancer diagnosis generally seems to have become less intense—users of the writing app are asked to recall a shocking episode or emotionally stirring story. For 3 days a week over 3 weeks, they write or type for 20 min a day their account of that stressful event to express their deepest thoughts and feelings. Their writing is stored on the dedicated server so that users can go back over it at any time they choose.
2.5. Evaluation indicators
For each subject in this study, ID code, birth month, date of first visit, provisional name of the disease, treatment planning, medical history, departments providing medical care, group allocation (experimental group or control group), and information on their pathological diagnosis, staging, functional disorders, treatment status, and departments providing medical care will be collected through the EDC system. Information on subjects' education, cohabitant(s), and occupation will be collected from participants’ responses in the online support program. The ePROs that are collected through the apps will be used as outcome measures of this trial.
2.5.1. Primary endpoint
The primary endpoint will be the difference between total QLI scores measured at baseline and those measured 6 months later.
2.5.2. Secondary endpoints
The indicators used as the secondary endpoints will be: (a) the total QLI scores measured at baseline, 1 month later, and 3 months later; and (b) four QLI subscale scores (health/functioning, social/economic, psychological/spiritual, family), two MDASI subscale scores (symptoms, symptom-related interference), and two HADS subscale scores (anxiety, depression) measured at baseline, 1 month later, 3 months later, and 6 months later.
2.5.3. Other indicators
Other indicators will be (a) nominal variables drawn from participants’ responses to questions in the confirmation app, (b) descriptions stored when participants engage in expressive writing, (c) level of satisfaction with healthcare, and (d) evaluation of the functioning of each app of the online support program.
2.6. Statistical analyses
2.6.1. Sample size
Based on previously published data [8,[32], [33], [34], [35]], we assume that the mean difference between the experimental and control groups will be 2 points in the QLI scale and the standard deviation will be 5 points in the QLI scale. The possible range of total QLI score is from 0 to 30. A sample size of 100 subjects per treatment group will be required to detect a difference of this magnitude with 80% power for the primary analysis. In consideration of differences between our study and the previous study [8] in the methods of handling missing data, the planned sample size is conservatively set to 105 subjects per group.
2.6.2. Analyses
Three statistical data sets will be used in the analyses: (a) a full analysis set (FAS) including all subjects who used the apps at least twice and underwent assessment of the primary endpoint after randomization; (b) a per-protocol set (PPS) including all subjects without violations of the eligibility criteria (selection and exclusion criteria) and who used the apps at the prescribed frequency; and (c) a safety analysis set (SAS) including all subjects who were randomized. The efficacy analysis will be performed with the FAS and PPS. The safety analysis will be performed with the SAS.
To evaluate the mean treatment effect, we will conduct a mixed model for repeated measures (MMRM) analysis [36] to detect changes in the total QLI score from baseline to 6 months. The MMRM analysis model will include the fixed effects of group, time point (1 month later, 3 months later, and 6 months later), group × time interaction, and baseline measurements. As the primary statistical test, a t-test for the difference of the adjusted means of the total QLI score between the groups at 6 months will be conducted. For the secondary analysis, a subgroup analysis will be conducted by each cancer type. Furthermore, we will evaluate the demographics of the patients and use this data to provide descriptive characteristics of the population. We will evaluate whether any differences in the baseline characteristics between the two groups is found by a two-sample t-test or Fisher's exact test after calculating the mean, standard deviation, and frequency of each baseline characteristic. The safety assessment will calculate the frequencies and rates of adverse events. The difference between groups will be analyzed by using Fisher's exact test. We will use Stata 16 (StataCorp LP, College Station, TX, USA) and SAS version 9.4 (SAS Institute Inc., Cary, NC, USA) to conduct the statistical analyses. Results will be deemed significant at P < 0.05, and statistical tests will be conducted for two-tailed hypotheses.
3. Discussion
A study of Japanese cancer patients found that simply providing them with the opportunity to measure their HR-QOL using an electronic device and providing them a report of their own ePRO was insufficient to improve or maintain their HR-QOL [20]. In this study, we regard the confirmation app and writing app as necessary to support the function of the monitoring app, which provides patients the opportunity to assess the state that their own health condition is in. The writing app is expected to give patients the opportunity to process suppressed thoughts and to address illness-related tasks and cope with them.
3.1. Limitations
With regard to the effectiveness of patients conducting a dialogue with physicians and nurses about the ePRO profiles elicited through these apps, although this matter is beyond the scope of the intervention of our online support program and this study protocol, it has been reported that a physician's communication style with patients holds the key to the benefit of assessing HR-QOL using an electronic tool when patients initiate discussions with the physician about their HR-QOL profile [37]. Future studies will be needed to identify what approaches patients need to take to make most efficient use of their own ePRO profiles.
3.2. Conclusions
If our study's hypothesis is correct, female cancer patients who use all three apps will have better HR-QOL than those who use only the monitoring app. Because our program is based on self-regulation theory, it is important that the program works in an efficient way to help female cancer patients find and set goals relevant to their experience of health and illness and change their behavior to meet their goals through using each app. That is, it is expected that the experimental group will find it easier to find and set goals and to refer to those goals to improve their health-related behaviors than will the control group.
Funding
Development of this study protocol was supported by a grant from Relay for Life Japan's Project MIRAI 2018, through the Japan Cancer Society and a JSPS KAKENHI grant (Grant Number 20H03975). Implementation of the RTC will be supported by a JSPS KAKENHI grant (Grant Number 20H03975) and a grant from the Pfizer Health Research Foundation.
Authors’ contributions
Conceptualization and Funding acquisition: Michiyo Mizuno.
App development: Michiyo Mizuno, Ikuko Chiba, Toru Mukohara, Miki Kondo, Miki Naruo, Yoshihiro Asano, Tatsuya Onishi, Hiroshi Tanabe, Rieko Muta, Saori Mishima, Susumu Okano, Masami Yuda, Ako Hosono, Yuri Ueda, Hiroko Bando, Hiroya Itagaki, Tetsuo Akimono.
Statistical analyses: Kazushi Maruo, Tomohiro Ohigashi.
Investigation: Ikuko Chiba.
Project administration: Tetsuo Akimono.
Supervisor/advisor: Carol Estwing Ferrans.
Writing – original draft: Michiyo Mizuno.
Writing – review & editing: All members.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Xu A., Wang Y., Wu X. Effectiveness of e-health based self-management to improve cancer-related fatigue, self-efficacy and quality of life in cancer patients: systematic review and meta-analysis. J. Adv. Nurs. 2019;75(12):3434–3447. doi: 10.1111/jan.14197. Epub 2019/10/01. PubMed PMID: 31566769. [DOI] [PubMed] [Google Scholar]
- 2.Fridriksdottir N., Gunnarsdottir S., Zoega S., Ingadottir B., Hafsteinsdottir E.J.G. Effects of web-based interventions on cancer patients' symptoms: review of randomized trials. Support. Care Cancer. 2018;26(2):337–351. doi: 10.1007/s00520-017-3882-6. Epub 2017/09/19. PubMed PMID: 28921391. [DOI] [PubMed] [Google Scholar]
- 3.Richards R., Kinnersley P., Brain K., McCutchan G., Staffurth J., Wood F. Use of mobile devices to help cancer patients meet their information needs in non-inpatient settings: systematic review. JMIR Mhealth Uhealth. 2018;6(12) doi: 10.2196/10026. Epub 2018/12/16. PubMed PMID: 30552082; PubMed Central PMCID: PMCPMC6315262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scheier M.F., Carver C.S. In: Goals and Confidence as Self-Regulatory Elements Underlying Health and Illness Behavior. Cameron L.D., Leventhal H., editors. Routledge; New York, NY, US: 2003. pp. 17–41. [Google Scholar]
- 5.Centers for Disease Control and Prevention . CDC; Atlanta, Georgia: November 2000. Measuring Healthy Days. [Google Scholar]
- 6.Broderick J.E., DeWitt E.M., Rothrock N., Crane P.K., Forrest C.B. Advances in patient-reported outcomes: the NIH PROMIS(®) measures. EGEMS (Wash DC) 2013;1(1):1015. doi: 10.13063/2327-9214.1015. Epub 2013/01/01. PubMed PMID: 25848562; PubMed Central PMCID: PMCPMC4371419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mizuno M., Sugimoto K., Mayers T., Ferrans C.E. Ensuring cultural and cognitive integrity in instrument translation: quality of life index for Japanese cancer patients. Asia-Pacific journal of oncology nursing. 2019;6(1):64–71. doi: 10.4103/apjon.apjon_57_18. Epub 2019/01/02. PubMed PMID: 30599018; PubMed Central PMCID: PMCPMC6287387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mizuno M., Munezawa N., Yamashita M., Sasahara T., Mayers T., Park C., et al. Reliability and validity of the Japanese version of the Quality of Life Index for patients with cancer. Res. Nurs. Health. 2020;43(2):176–185. doi: 10.1002/nur.22011. Epub 2020/01/28. PubMed PMID: 31985085. [DOI] [PubMed] [Google Scholar]
- 9.Okuyama T., Wang X.S., Akechi T., Mendoza T.R., Hosaka T., Cleeland C.S., et al. Japanese version of the M.D. Anderson Symptom Inventory: a validation study. J. Pain Symptom Manag. 2003;26(6):1093–1104. doi: 10.1016/j.jpainsymman.2003.05.003. [DOI] [PubMed] [Google Scholar]
- 10.Kugaya A., Akechi T., Okuyama T., Okamura H., Uchitomi Y. Screening for psychological distress in Japanese cancer patients. Jpn. J. Clin. Oncol. 1998;28(5):333–338. doi: 10.1093/jjco/28.5.333. Epub 1998/08/15. PubMed PMID: 9703862. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki N., Ninomiya M., Maruta S., Hosonuma S., Nishigaya Y., Kobayashi Y., et al. Psychological characteristics of Japanese gynecologic cancer patients after learning the diagnosis according to the hospital anxiety and depression scale. J. Obstet. Gynaecol. Res. 2011;37(7):800–808. doi: 10.1111/j.1447-0756.2010.01437.x. Epub 2011/04/01. PubMed PMID: 21450027. [DOI] [PubMed] [Google Scholar]
- 12.Abernethy A.P., Herndon J.E., 2nd, Wheeler J.L., Patwardhan M., Shaw H., Lyerly H.K., et al. Improving health care efficiency and quality using tablet personal computers to collect research-quality, patient-reported data. Health Serv. Res. 2008;43(6):1975–1991. doi: 10.1111/j.1475-6773.2008.00887.x. Epub 2008/09/03. PubMed PMID: 18761678; PubMed Central PMCID: PMCPMC2613994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berry D.L., Blonquist T.M., Patel R.A., Halpenny B., McReynolds J. Exposure to a patient-centered, Web-based intervention for managing cancer symptom and quality of life issues: impact on symptom distress. J. Med. Internet Res. 2015;17(6):e136. doi: 10.2196/jmir.4190. Epub 2015/06/05. PubMed PMID: 26041682; PubMed Central PMCID: PMCPMC4526904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Egbring M., Far E., Roos M., Dietrich M., Brauchbar M., Kullak-Ublick G.A., et al. A mobile app to stabilize daily functional activity of breast cancer patients in collaboration with the physician: a randomized controlled clinical trial. J. Med. Internet Res. 2016;18(9):e238. doi: 10.2196/jmir.6414. Epub 2016/09/08. PubMed PMID: 27601354; PubMed Central PMCID: PMCPMC5030453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang F.W.K., Chan C.W.H., Choy Y.P., Loong H.H.F., Chow K.M., So W.K.W. A feasibility study on using tablet personal computers for self-reported symptom assessment in newly diagnosed lung cancer patients. Int. J. Nurs. Pract. 2018;24(4) doi: 10.1111/ijn.12658. Epub 2018/04/12. PubMed PMID: 29642280. [DOI] [PubMed] [Google Scholar]
- 16.Pennebaker J.W. Writing about emotional experiences as a therapeutic process. Psychol. Sci. 1997;8:162–166. [Google Scholar]
- 17.Pennebaker J.W. Expressive writing in psychological science. Perspect. Psychol. Sci. 2018;13(2):226–229. doi: 10.1177/1745691617707315. Epub 2017/10/11. PubMed PMID: 28992443. [DOI] [PubMed] [Google Scholar]
- 18.Frattaroli J. Experimental disclosure and its moderators: a meta-analysis. Psychol. Bull. 2006;132(6):823–865. doi: 10.1037/0033-2909.132.6.823. [DOI] [PubMed] [Google Scholar]
- 19.National Cancer Center Japan ganjoho.jp. 2022. https://ganjoho.jp/public/index.html%202022.0204 [cited 2022 March 30]. Available from:
- 20.Kanakubo A., Mizuno M., Asano Y., Inoue Y. Acceptability to making a self-assessment using a tablet computer and health-related quality of life in ambulatory breast cancer patients. Asia-Pacific Journal of Oncology Nursing. 2021 doi: 10.1016/j.apjon.2021.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park J.H., Jung Y.S., Kim J.Y., Jo Y., Bae S.H. Trajectories of health-related quality of life in breast cancer patients. Support. Care Cancer. 2020;28(7):3381–3389. doi: 10.1007/s00520-019-05184-3. Epub 2019/11/27. PubMed PMID: 31768734. [DOI] [PubMed] [Google Scholar]
- 22.Weisman A.D., Worden J.W. The existential plight in cancer: significance of the first 100 days. Int. J. Psychiatr. Med. 1976;7(1):1–15. doi: 10.2190/uq2g-ugv1-3ppc-6387. Epub 1976/01/01. PubMed PMID: 1052080. [DOI] [PubMed] [Google Scholar]
- 23.Ministry of Health, Labour and Welfare Hito wo taisho tosuru seimeikagaku-igakukei kenkyu ni kansuru rinri shisin [ethical guidelines for medical and biological research involving human subjects] 2021. https://www.lifescience.mext.go.jp/files/pdf/n2262_01.pdf [cited 2022 March 30]. Available from:
- 24.Ferrans C.E., Powers M.J. Quality of life index: development and psychometric properties. ANS Advances in nursing science. 1985;8(1):15–24. doi: 10.1097/00012272-198510000-00005. Epub 1985/10/01. PubMed PMID: 3933411. [DOI] [PubMed] [Google Scholar]
- 25.National Cancer Center Japan M.D. Anderson symptom inventory in Japanese version. https://www.ncc.go.jp/jp/epoc/division/psycho_oncology/kashiwa/020/030/MDASI.pdf n.d. [cited 2022 March 30]. Available from:
- 26.Zigmond A.S., Snaith R.P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. Epub 1983/06/01. PubMed PMID: 6880820. [DOI] [PubMed] [Google Scholar]
- 27.Pennebaker J.W. New Harbinger Publications; Oakland, CA: 2004. Writing to Heal: A Guided Journal for Recovering from Trauma & Emotional Upheaval; p. 164. [Google Scholar]
- 28.Chu Q., Wu I.H.C., Lu Q. Expressive writing intervention for posttraumatic stress disorder among Chinese American breast cancer survivors: the moderating role of social constraints. Qual. Life Res. 2020;29(4):891–899. doi: 10.1007/s11136-019-02385-5. Epub 2020/01/05. PubMed PMID: 31900761. [DOI] [PubMed] [Google Scholar]
- 29.Ji L.L., Lu Q., Wang L.J., Sun X.L., Wang H.D., Han B.X., et al. The benefits of expressive writing among newly diagnosed mainland Chinese breast cancer patients. J. Behav. Med. 2020;43(3):468–478. doi: 10.1007/s10865-019-00127-z. Epub 2019/12/22. PubMed PMID: 31863269. [DOI] [PubMed] [Google Scholar]
- 30.Lu Q., Dong L., Wu I.H.C., You J., Huang J., Hu Y. The impact of an expressive writing intervention on quality of life among Chinese breast cancer patients undergoing chemotherapy. Support. Care Cancer. 2019;27(1):165–173. doi: 10.1007/s00520-018-4308-9. Epub 2018/06/20. PubMed PMID: 29915994. [DOI] [PubMed] [Google Scholar]
- 31.Lu Q., Wong C.C., Gallagher M.W., Tou R.Y., Young L., Loh A. Expressive writing among Chinese American breast cancer survivors: a randomized controlled trial. Health Psychol. 2017;36(4):370–379. doi: 10.1037/hea0000449. Epub 2016/12/09. PubMed PMID: 27929333. [DOI] [PubMed] [Google Scholar]
- 32.Ferrans C.E. Development of a quality of life index for patients with cancer. Oncol. Nurs. Forum. 1990;17(3 Suppl):15–19. discussion 20-1. Epub 1990/05/01. PubMed PMID: 2342979. [PubMed] [Google Scholar]
- 33.Bliley A.V., Ferrans C.E. Quality of life after coronary angioplasty. Heart Lung. 1993;22(3):193–199. Epub 1993/05/01. PubMed PMID: 8491654. [PubMed] [Google Scholar]
- 34.Hathaway D.K., Hartwig M.S., Milstead J., Elmer D., Evans S., Gaber A.O. Improvement in quality of life reported by diabetic recipients of kidney-only and pancreas-kidney allografts. Transplant. Proc. Apr 1994;26(2):512–514. [PubMed] [Google Scholar]
- 35.Johnson C.D., Wicks M.N., Milstead J., Hartwig M., Hathaway D.K. Racial and gender differences in quality of life following kidney transplantation. Image - J. Nurs. Scholarsh. 1998;30(2):125–130. doi: 10.1111/j.1547-5069.1998.tb01266.x. Epub 1998/10/17. PubMed PMID: 9775552. [DOI] [PubMed] [Google Scholar]
- 36.Mallinckrodt C.H., Clark W.S., David S.R. Accounting for dropout bias using mixed-effects models. J. Biopharm. Stat. 2001;11(1–2):9–21. doi: 10.1081/bip-100104194. Epub 2001/07/19. PubMed PMID: 11459446. [DOI] [PubMed] [Google Scholar]
- 37.Lindberg-Scharf P., Steinger B., Koller M., Hofstadter A., Ortmann O., Kurz J., et al. Long-term improvement of quality of life in patients with breast cancer: supporting patient-physician communication by an electronic tool for inpatient and outpatient care. Support. Care Cancer. 2021;29(12):7865–7875. doi: 10.1007/s00520-021-06270-1. Epub 2021/06/28. PubMed PMID: 34176020; PubMed Central PMCID: PMCPMC8550515. [DOI] [PMC free article] [PubMed] [Google Scholar]