Summary
Background
Recognising the importance of clinical outcomes assessments (COAs), the Response Assessment in Neuro-Oncology-Patient Reported Outcome (RANO-PRO) Working Group recommended inclusion of core symptoms and functions in clinical care or research for malignant glioma patients. This study evaluated the association of the recommended symptoms (pain, perceived cognition, seizures, aphasia, symptomatic adverse events) and functions (weakness, walking, work, usual activities) with disease progression in these patients.
Methods
In this retrospective cohort study, patients with malignant glioma were included from the US National Cancer Institute Neuro-Oncology Branch Natural History Study (NOB–NHS) which follows primary central nervous system tumour patients aged 18 years and older throughout their disease trajectory. The M.D. Anderson Symptom Inventory-Brain Tumor (MDASI-BT), EQ-5D-3L, Karnofsky Performance Status (KPS), and Neurologic Function scores (NFS) were evaluated in relation to disease progression by chi-square tests, independent- and paired-samples t-tests, adjusted for multiple comparisons at first assessment and over time to a second assessment. Radiographic disease progression was determined on the interpretation of the imaging study by a radiologist and neuro-oncologist using standard criteria as part of clinical trial participation or routine standard of care. The priority constructs were evaluated to provide initial evidence of their relevance, relationship to disease status over time, and sensitivity to change in a diverse group of patients with malignant glioma.
Findings
Seven hundred and sixty-five patients had enrolled into the NOB–NHS between September 1, 2016 and January 31, 2020. Three hundred and thirty-six patients had a diagnosis of a malignant glioma (anaplastic astrocytoma, anaplastic oligodendroglioma, glioblastoma, and gliosarcoma) and were included in the current study. The sample was 64% male (n = 215), 36% female (n = 121), median age of 52 years (IQR = 18.75), 82% White (n = 276), and 65% had tumour recurrence (n = 219). One hundred and fifty-four (46%) had radiographic disease progression. Difficulty remembering, fatigue, and weakness were worse in the group whose imaging was interpreted as radiographic disease progression versus stable disease, as well as the functions of walking, work, activity, and self-care (1.1 < difference < 1.8). Patients with disease progression were four times more likely to have a poor KPS (≤80) and worse NFS. Among patients with disease progression at a second assessment (n = 112), all symptoms, except seizures, worsened between first assessment and disease progression and up to 22% of patients (n = 25) reported worsening mobility, self-care, and usual activity; 46% (n = 51) and 35% (n = 30) had worsened KPS and NFS, respectively. On average, 4 symptoms or functions (SD = 3) were reported as moderate-to-severe and 30% (n = 33) and 23% (n = 26) had a change to moderate-to-severe fatigue and walking, respectively, at time of disease progression. Over 7% of patients with worsening (n = 7 of 100) reported every symptom and function as having changed the most severely including seizures with fatigue and activity reported as the top symptom and function, respectively.
Interpretation
The identified core symptoms and functions worsened at the time of progression, supporting the relevance and sensitivity of the priority constructs identified by the RANO-PRO Working Group for clinical care and clinical trials for malignant glioma patients.
Funding
The Natural History Study is supported by Intramural Project 1ZIABC011786-03.
Research in context.
Evidence before this study
From past research, no single symptom has emerged that encompasses how a patient feels and functions. Core sets of symptoms have been proposed both for use in general oncology and in neuro-oncology, specifically. The Response Assessment in Neuro-Oncology-Patient Reported Outcome (RANO-PRO) Working Group and subsequent Fast Track group published recommended priority constructs for standardized use in clinical trials. However, evidence of real-life application of the priority constructs is limited. Authors searched prominent neuro-oncology journals, such as Lancet Oncology, Neuro Oncology, Neuro Oncology Practice, and Journal of Neuro-Oncology, for studies that described 1) clinical outcome assessments, in particular patient-reported outcomes (PROs), 2) their use in clinical trials, 3) suggested guidelines from agencies and consortiums, and 4) core symptom sets. Search terms included: patient-reported outcomes, MDASI-BT, RANO, clinical outcomes assessments, net clinical benefit. Our search was between February and December of 2021. We expected the studies to be of good quality due to their publication in journals with relevance in the neuro-oncology field and high impact scores. However, we recognized the sphere of PRO research is small.
Added value of this study
The current study evaluated a set of priority constructs that are recommended for inclusion in clinical trials by a multidisciplinary team of subject matter experts. It provides evidence that existing validated clinical outcomes assessments can be used in a standardized manner to detect changes in disease status.
Implications of all the available evidence
Clinical outcome assessments can be used in clinical trials and clinical care of patients with central nervous system tumors. Together, research studies have shown them to be valid and reliable, feasible in collecting, sensitive to disease status, and additive to the determination of an intervention's effectiveness.
Introduction
Patients with a primary brain tumour (PBT), either due to the disease, its treatment, or a combination of both, are highly symptomatic and exhibit multifocal neurological dysfunction, throughout the disease trajectory, across ages, in all tumour grades, and in the context of clinical trials.1 Historically, evaluation of symptoms and functions were often excluded from clinical trials or were included as optional secondary objectives, thereby limiting the knowledge gained regarding treatment efficacy.2
In the last decade,1,3,4 the neuro-oncology community has increasingly recognised the need for clinical outcomes assessments (COAs), “an outcome that describes or reflects how an individual feels, functions, or survives”5 in both clinical care and research, noting that survival data is not enough to gauge the “net clinical benefit” of a tumour-directed therapy.2 PBT patients pose a unique challenge in that their disease outcomes are measured using standard oncology metrics, such as tumour measurements, progression-free survival, and overall survival. However, their neurologic functioning is also considered when determining disease status.6,7 Therefore, incorporation of COAs into neuro-oncology clinical trials provide an additional metric of the treatment's effectiveness for neuro-oncology patients. Numerous guidelines now exist for minimum standards of measurement, analysis, and reporting, particularly for patient-reported outcomes.8, 9, 10, 11 Currently in the U.S., objective trial evidence should be accompanied by evidence of improvement in symptoms and functions if regulatory approval of treatment regimens is being sought.12 As such, the research and discussion regarding COA inclusion has now moved from general guidelines to specific constructs.
There are four types of COAs: 1) patient-reported outcomes (PROs), 2) clinician-reported outcomes (ClinROs), 3) observer-reported outcomes (ObsROs), and 4) performance outcomes (PerfROs).13 Recognising the wide variety each type represents, the Response Assessment in Neuro-Oncology-Patient Reported Outcomes (RANO-PRO) Working Group narrowed their focus to PROs beginning in 2015.2 By 2018, representatives from the U.S. Food and Drug Administration (FDA), RANO, European Medical Agency, and U.S. National Cancer Institute (NCI) recommended a list of possible symptom and functional constructs for standardised use in clinical trials to limit patient burden and improve compliance.14 In 2020, the Fast Track COA Group, comprised of members similar to the RANO-PRO Working Group, further refined these recommendations and published a core set of five priority symptom constructs and two priority functional constructs for evaluation in clinical trials and clinical care through a variety of validated COAs.15
The five priority symptom constructs are 1) pain, 2) difficulty communicating (the ability to express or understand speech), 3) perceived cognition (including executive function, memory, or concentration), 4) seizures (severity and frequency), and 5) symptomatic adverse events (or treatment-specific symptoms chosen among expected adverse events of the treatment being evaluated). The two priority functional constructs are 1) physical functioning (ability to do activities that require physical effort, and includes weakness and walking), and 2) role functioning (ability to do work or participate in social activities).
In this study, the seven priority constructs were evaluated in a large cohort to provide initial evidence of their relevance, relationship to disease status over time, and sensitivity to change in a diverse group of patients with malignant glioma.
Methods
Study design and participants
The study cohort was sampled from the NCI Neuro-Oncology Branch Natural History Study (NOB–NHS, NCT02851706), a US National Institutes of Health Institutional Review Board-approved study that follows patients with central nervous system tumours throughout their disease trajectory. All patients aged 18 years or older with a primary central nervous system tumour who undergo clinical evaluation at the US National Institutes of Health Neuro-Oncology clinic are eligible for the NOB–NHS after giving written informed consent, regardless of disease status or geography. Clinical status, treatment information, and PROs are routinely collected at every clinical evaluation. A subset of patients was identified for the current analysis based on the following criteria: 1) a diagnosis of malignant glioma (defined as anaplastic astrocytoma, anaplastic oligodendroglioma, glioblastoma, gliosarcoma); and 2) available PROs and ClinROs corresponding to an imaging visit. Diagnosis of a malignant glioma was based on the diagnostic criteria at the time of the patient's initial diagnosis, which pre-date the current 2021 World Health Organization classification. The current report adheres to the STROBE reporting guidelines for observational studies.
Procedures
PROs were completed prior to the participant's visit with their healthcare team and before magnetic resonance imaging findings were discussed with the patient. ClinROs were determined by the clinician at the time of clinical evaluation. All assessments were collected from September 21, 2016 to January 31, 2020 via an electronic data capture system.
To evaluate the difference in symptom severity in terms of disease progression, patients were categorised as having stable disease or radiographic disease progression based on the interpretation of their imaging study by their radiologist and neuro-oncologist using standard criteria as part of their clinical trial participation or routine standard of care. For patients who had disease progression, their earliest assessment where imaging study showed radiographic disease progression was used. For patients who did not have radiographic disease progression, the latest assessment was used.
To evaluate change associated with disease progression, a set of two PROs and ClinROs consisting of the patient's first assessment on study entry and the earliest subsequent assessment where imaging study showed disease progression were compared. For patients who did not have subsequent disease progression, the latest assessment was used.
The largest magnitude of worsening was found for each patient. The symptoms and/or functions corresponding to the most severe worsening was tallied and percentages were reported.
Patient-reported outcomes (PROs)
The FAST Track COA Group reviewed PROs commonly used in neuro-oncology as part of their work to identify the priority constructs and listed recommended PROs based on their validity and availability, rather than recommending a specific PRO. The MD Anderson Symptom Inventory-Brain Tumor Module (MDASI-BT) and the EQ-5D-3L were administered as part of the NOB–NHS and are listed as recommended PROs. Therefore, items were selected from these two PROs based on their correspondence with the priority constructs (Supplementary Table S1).
The MDASI-BT is a reliable, validated self-report measure of symptom burden and symptom interference within the past 24 h.16 It consists of 22 symptom items rated 0 (Not present) to 10 (As bad as you can imagine) and six interference items rated 0 (Did not interfere) to 10 (Interfered completely). The MDASI-BT items used to correspond to the priority constructs were pain (symptom: pain), difficulty remembering (symptom: perceived cognition), difficulty concentrating (symptom: perceived cognition), seizures (symptom: seizures), difficulty understanding (symptom: difficulty communicating), difficulty speaking (symptom: difficulty communicating), fatigue (symptom: symptomatic adverse event), weakness on one side of body (function: physical), interference with walking (function: physical), interference with work, including around household (function: role), and interference with general activity (function: role). Since the present study does not evaluate a specific treatment, fatigue (MDASI-BT: fatigue) was chosen as a symptomatic adverse event due to its high prevalence among PBT patients on various treatments. Symptoms rated five or higher are considered moderate-to-severe (pain, difficulty remembering, difficulty concentrating, seizures, difficulty understanding, difficulty speaking, fatigue, weakness on one side of body).17,18 Shi et al.19 demonstrated a mean of two or greater on the interference subscales can be considered moderate-to-severe. This cut-off of two or greater on the interference items was used to evaluate severity (interference with walking, interference with work, interference with general activity). A decrease or increase of one-point was used to categorise change in MDASI-BT items.
The EQ-5D-3L is a validated self-report of general health status.20 It assesses five dimensions: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. Patients choose a level for each dimension that reflects their “own health state today”. The possible levels represent “no problems”, “some problems” and “extreme problems”. An EQ-5D-3L index score of the health state is calculated using a scoring algorithm based on U.S. population-based preference weights,21 and ranges from −0.11 to 1.0 based on the US population. A score of 1.0 describes health as perfect, a score of 0.0 describes health as death-like and negative scores describe health as worse than death. The EQ-5D-3L dimensions used to correspond to the RANO-PRO Working Group standardised priority constructs were mobility (function: physical), self-care (function: role), usual activities (function: role), and pain/discomfort (symptom: pain). A decrease or increase of one level was used to categorise change in EQ-5D-3L dimensions.
Clinician reported outcomes (ClinROs)
Two ClinROs are collected through the NOB–NHS: the Karnofsky Performance Status and the Neurologic Function score. The Karnofsky Performance Status (KPS)22 provides a standard way to measure a cancer patient's ability to perform usual tasks. It is a measure of functional impairment with 11 categories ranging from “Dead” (0) to “Normal; no complaints; no evidence of disease” (100). Based on previous work,1,23 KPS was dichotomized as good (≥90) and poor (≤80). Changes in KPS were categorised as no change, improved (up at least one status), or worsened (down at least one status).
The Neurologic Function Score (NFS)24 provides a grading of neurologic function and is another measure of functional impairment with five categories ranging from “No neurologic symptoms; fully active at home/work without assistance” (0) to “Severe neurologic symptoms; totally inactive requiring complete assistance; unable to work” (4). Changes in NFS were categorised as no change, improved (down at least one status), or worsened (up at least one status).
Statistical analysis
Cases with missing ClinROs were considered missing at random (physicians not documenting their assessment as part of their routine progress note and not related to disease status) and excluded from their respective univariate analysis. The difference in symptom and function severity between disease states was evaluated via independent samples t-tests. Associations among severity levels and disease states were evaluated via chi-square tests. To evaluate change associated with disease progression, the change in severity was calculated between the two assessments. Each change was categorised as no change, improved, or worsened as described above. Paired-samples t-tests also were used to evaluate the mean difference in symptom and function severity between the two assessments. Changes in proportions were evaluated with McNemar tests.25 Effect sizes (Hedge's g, Glass' Δ, odds ratios (OR) or Cramer's V) and 95% confidence intervals (CI) for mean differences are reported. Since more than one symptom or function was evaluated, Holm-Bonferroni's adjustment for multiple comparison was applied. Distributional assumptions were checked to evaluate the appropriateness of the parametric tests. On review and using the smaller sample of patients with disease progression, with a sample of 112, we have 88% power to detect an effect size difference of 0.3 between two assessment timepoints via paired-sample test at 5% significance level. All analyses were conducted via IBM SPSS Statistics Version 26.26
Role of the funding source
The Natural History Study is supported by Intramural Project 1ZIABC011786-03 (Principal Investigator: T.S. Armstrong) and hence the funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. Authors E.V., T.R.M., and T.S.A. had access to the dataset and had final responsibility for the decision to submit for publication.
Results
Patient sample
Seven hundred and sixty-five patients had enrolled into the NOB–NHS between September 1, 2016 and January 31, 2020. Three hundred thirty-six patients met the criteria for inclusion in the current study (Table 1). The majority of patients were White (n = 276, 82%), males (n = 215, 64%) with a median age of 52 years (IQR = 18.75). Glioblastoma was the most common diagnosis (n = 214, 64%). Thirty-six percent were undergoing treatment at time of assessment (n = 122) and 65% had a prior recurrence (n = 219). The median time from diagnosis to assessment was 24 months (IQR = 78.5).
Table 1.
Patient characteristics, N = 336.
| n | % | ||
|---|---|---|---|
| Sex | Female | 121 | 36 |
| Male | 215 | 64 | |
| Race/Ethnicity | Asian | 19 | 6 |
| Black/African American | 22 | 7 | |
| Native Hawaiian/Pacific Islander | 1 | 1 | |
| White | 276 | 82 | |
| Other race | 3 | 1 | |
| Missing race | 15 | 5 | |
| Hispanic ethnicity | 27 | 8 | |
| Current diagnosis | Anaplastic astrocytoma | 66 | 20 |
| Anaplastic oligodendroglioma | 44 | 13 | |
| Glioblastoma | 214 | 64 | |
| Gliosarcoma | 12 | 4 | |
| Prior progression | Yes | 219 | 65 |
| No | 117 | 35 | |
| Treatment phase | Active treatment | 122 | 36 |
| Surveillance | 214 | 64 | |
| KPS | 100 | 59 | 18 |
| 90 | 110 | 33 | |
| 80 | 70 | 21 | |
| 70 | 39 | 12 | |
| 60 | 33 | 10 | |
| 50 | 17 | 5 | |
| 40 | 5 | 2 | |
| With a second assessment | Yes, with disease progression | 112 | 33 |
| Yes, with stable disease | 145 | 43 | |
| No | 79 | 24 |
Abbreviation: KPS = Karnofsky performance status.
Between group differences in symptom and function severity
Patient-reported MDASI-BT
For 154 patients (46%), their imaging study at first assessment was interpreted as radiographic disease progression. Patients whose imaging showed disease progression reported worse pain (difference = 0.8, 95% CI [0.2, 1.4], Hedge's g = 0.31, p = 0.0054), difficulty remembering (difference = 1.1, 95% CI [0.4, 1.7], Hedge's g = 0.35, p = 0.0016), difficulty speaking (difference = 0.8, 95% CI [0.2, 1.4], Hedge's g = 0.28, p = 0.012), difficulty understanding (difference = 1.1, 95% CI [0.5, 1.7], Hedge's g = 0.40, p < 0.0004), and fatigue (difference = 1.3, 95% CI [0.6, 1.9], Hedge's g = 0.42, p = 0.0002) compared to patients whose imaging showed stable disease. Patients whose imaging showed disease progression also reported worse weakness on one side of body (difference = 1.6, 95% CI [0.8, 2.4], Hedge's g = 0.47, p < 0.0001), interference with walking (difference = 1.8, 95% CI [1.1, 2.5], Hedge's g = 0.54, p < 0.0001), interference with work (difference = 1.7, 95% CI [1.0, 2.5], Hedge's g = 0.50, p < 0.0001), and interference in general activity (difference = 1.7, 95% CI [1.0, 2.4], Hedge's g = 0.52, p < 0.0001) (Fig. 1).
Fig. 1.
MD Anderson Symptom Inventory-Brain Tumor symptom and function ratings by disease status. Patients with disease progression on imaging rated symptoms and functions more severely compared to patients with stable disease in all symptoms and functions except difficulty concentrating and seizures.
Compared to patients with stable disease, patients with disease progression were more likely to have moderate-to-severe difficulty remembering [n = 61 (40%) v n = 43 (24%), X2 (1) = 9.8, p = 0.0018], difficulty understanding [n = 43 (28%) v n = 27 (15%), X2 (1) = 8.5, p = 0.0035], fatigue [n = 81 (53%) v n = 66 (37%), X2 (1) = 8.8, p = 0.0030], weakness on one side of body [n = 57 (37%) v n = 34 (19%), X2 (1) = 14.0, p < 0.0001], interference with walking [n = 99 (64%) v n = 61 (34%), X2 (1) = 31.2, p < 0.0001], interference with work [n = 110 (71%) v n = 82 (46%), X2 (1) = 23.2, p < 0.0001], and general activity [n = 110 (71%) v n = 83 (46%), X2 (1) = 22.3, p < 0.0001 (Supplementary Fig. S1).
Patient-reported EQ-5D-3L
On the EQ-5D-3L, more patients with disease progression reported some problems walking (n = 84, 55%) than patients with stable disease (n = 56, 31%, Cramer's V = 0.30); some problems washing or dressing (n = 55, 36%) than stable disease (n = 33, 18%, Cramer's V = 0.26); and inability to perform usual activities (n = 32, 21%) than stable disease (n = 14, 8%, Cramer's V = 0.24). Thirty-eight percent of patients with disease progression (n = 59) reported moderate or extreme pain/discomfort compared to 28% of patients with stable disease (n = 51, p = 0.13) (Table 2). Thirty-four percent of patients with stable disease (n = 61) reported no problems in any dimension (11111 health state) compared to 14% of patients with disease progression (n = 22). Patients with disease progression also perceived their own health to be worse compared to patients with stable disease, as evidenced by their EQ-5D-3L index score (progression = 0.70 vs stable = 0.81, 95% CI [0.07, 0.16], Hedges' g = 0.53, p < 0.0001).
Table 2.
EQ-5D-3L dimension, Karnofsky Performance Status, and Neurologic Function Score severity levels by disease status.
| Measure | Severity level | Stable disease |
Disease progression |
||
|---|---|---|---|---|---|
| n | % | n | % | ||
| EQ-5D-3L | n | 182 | ·· | 154 | ·· |
| Mobility | No problems walking | 124 | 68 | 60 | 39 |
| Some problems walking | 56 | 31 | 84 | 55 | |
| Confined to bed | 2 | 1 | 10 | 6 | |
| Self-care | No problems in self-care | 143 | 79 | 84 | 55 |
| Some problems in self-care | 33 | 18 | 55 | 36 | |
| Unable to self-care | 6 | 10 | 15 | 10 | |
| Usual activities | No problems with usual activities | 104 | 57 | 55 | 36 |
| Some problems with usual activities | 64 | 35 | 67 | 44 | |
| Unable to perform usual activities | 14 | 8 | 32 | 21 | |
| Pain/Discomfort | None | 131 | 72 | 95 | 62 |
| Moderate | 47 | 26 | 54 | 35 | |
| Extreme | 4 | 2 | 5 | 3 | |
| KPS | n | 181 | ·· | 152 | ·· |
| 100 | 50 | 28 | 9 | 6 | |
| 90 | 70 | 39 | 40 | 26 | |
| 80 | 30 | 17 | 40 | 26 | |
| 70 | 18 | 10 | 21 | 14 | |
| 60 | 8 | 4 | 25 | 16 | |
| 50 | 5 | 3 | 12 | 8 | |
| 40 | 0 | 0 | 5 | 3 | |
| NFS | n | 170 | ·· | 143 | ·· |
| 0 | 76 | 45 | 21 | 15 | |
| 1 | 56 | 33 | 56 | 39 | |
| 2 | 18 | 11 | 25 | 18 | |
| 3 | 20 | 12 | 34 | 24 | |
| 4 | 0 | 0 | 7 | 5 | |
Abbreviations: KPS = Karnofsky performance status; NFS = Neurologic function score.
Clinician-reported KPS and NFS
Clinicians reported KPS scores of 40–100 and NFS of 0–4 (Table 2). Patients with disease progression were four times more likely to have a poor KPS than patients with stable disease [n = 103 (68%) v n = 61 (34%), OR = 4.1]. A smaller proportion of patients with disease progression had a NFS = 0 (n = 21, 15%) compared to patients with stable disease (n = 76, 45%) and no patients with stable disease had a NFS = 4 (Cramer's V = 0.36).
Within group change in symptom and function severity
Patient-reported: MDASI-BT
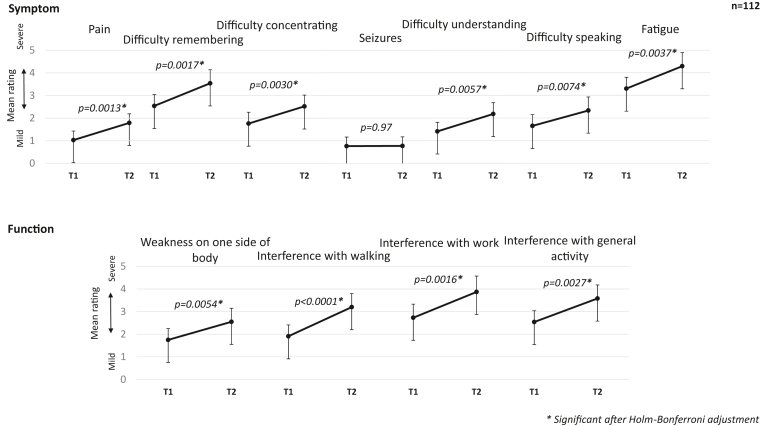
One hundred and twelve patients had a second assessment that corresponded to an imaging study interpreted as radiographic disease progression, a median of four months (IQR = 6.75) from first assessment. From time of first assessment to time of disease progression, symptom severity worsened in pain (difference = 0.8, 95% CI [0.3, 1.2], Glass' Δ = 0.38, p = 0.0013), difficulty remembering (difference = 1.0, 95% CI [0.4, 1.6], Glass' Δ = 0.35, p = 0.0017), difficulty concentrating (difference = 0.8, 95% CI [0.3, 1.3], Glass' Δ = 0.31, p = 0.0030), difficulty speaking (difference = 0.7, 95% CI [0.2, 1.3], Glass' Δ = 0.27, p = 0.0074), difficulty understanding (difference = 0.8, 95% CI [0.2, 1.3], Glass' Δ = 0.34, p = 0.0057), and fatigue (difference = 1.0, 95% CI [0.3, 1.7], Glass' Δ = 0.36, p = 0.0037). Seizure severity was similar between the two timepoints. Function severity also worsened among patients with disease progression for weakness on one side of body (difference = 0.8, 95% CI [0.2, 1.4], Glass' Δ = 0.28, p = 0.0054), interference with walking (difference = 1.3, 95% CI [0.7, 1.9], Glass' Δ = 0.49, p < 0.0001), interference with activity (difference = 1.0, 95% CI [0.4, 1.7], Glass' Δ = 0.36, p = 0.0027), and interference with work (difference = 1.1, 95% CI [0.4, 1.8], Glass’ Δ = 0.37, p = 0.0016) (Fig. 2). Patients reported, on average, four (SD = 3) symptoms and functions as moderate-to-severe at time of disease progression and 26% of patients (n = 29) reported six or more of the 11 items as moderate-severe (data not shown). There was a change in proportions of moderate-to-severe fatigue and interference with walking at time of disease progression (Supplementary Table S2). Thirty percent of patients (n = 33) reported none-to-mild fatigue at the first assessment then moderate-to-severe fatigue at time of disease progression (p = 0.0045). Twenty-three percent (n = 26) reported none-to-mild interference with walking at the first assessment then moderate-severe interference with walking at time of disease progression (p = 0.0013).
Fig. 2.
Change in MD Anderson Symptom Inventory-Brain Tumor symptom and function ratings from first assessment to disease progression. Patients with disease progression showed worsening pain, difficulty remembering, difficulty concentrating, difficulty understanding, difficulty speaking, fatigue, walking, work, and activity from first assessment (T1) to time of disease progression (T2).
When viewed as strictly improvement or worsening, irrespective of magnitude, at least 30% of patients (at least n = 35) reported worsening in every symptom and function, except seizures. More than 50% reported worsening at the time of disease progression in fatigue (n = 59, 53%), interference with general activity (n = 59, 53%), and interference with work (n = 56, 50%). For most symptoms, the magnitude of worsening was greater than the magnitude of no change or improvement (Table 3).
Table 3.
Percentage of patients with worsening and improvement in symptoms and functions at second assessment by patient report and clinician report.
| No change |
Improvement |
Worsening |
||
|---|---|---|---|---|
| n (%) | n (%) | n (%) | ||
| MDASI-BT, n = 112 | Pain | 54 (48) | 20 (18) | 38 (34) |
| Difficulty remembering | 29 (26) | 29 (26) | 54 (48) | |
| Difficulty concentrating | 43 (38) | 23 (21) | 46 (41) | |
| Seizures | 84 (75) | 14 (13) | 14 (13) | |
| Difficulty understanding | 43 (38) | 24 (21) | 45 (40) | |
| Difficulty speaking | 44 (39) | 23 (21) | 45 (40) | |
| Fatigue | 15 (13) | 38 (34) | 59 (53) | |
| Weakness on one side of body | 60 (54) | 17 (15) | 35 (31) | |
| Interference with walking | 38 (34) | 20 (18) | 54 (48) | |
| Interference with work | 30 (27) | 26 (23) | 56 (50) | |
| Interference with general activity | 25 (22) | 28 (25) | 59 (53) | |
| EQ-5D-3L, n = 112 | Mobility | 82 (73) | 5 (5) | 25 (22) |
| Self-care | 87 (78) | 2 (2) | 23 (21) | |
| Usual activities | 75 (67) | 12 (11) | 25 (22) | |
| Pain/Discomfort | 77 (69) | 17 (15) | 18 (16) | |
| KPS, n = 111 | 48 (43) | 12 (11) | 51 (46) | |
| NFS, n = 87 | 50 (58) | 7 (8) | 30 (35) | |
Abbreviations: KPS = Karnofsky performance status; MDASI-BT = MD Anderson symptom inventory brain tumor module; NFS = Neurologic function score.
Change based on: MDASI-BT, 1 point change; EQ-5D-3L, 1-level change; KPS, 1-status change; NFS, 1-status change.
Patient-reported: EQ-5D-3L
Patients with disease progression had worsening mobility (n = 25, 22%), self-care (n = 23, 21%), and usual activities (n = 25, 22%). Pain/Discomfort also worsened among 16% of patients with disease progression (n = 18) (Table 3). Patients' perception of their own health worsened at time of disease progression (difference = −0.06, 95% CI [−0.10, −0.02], Glass’ Δ = 0.43, p = 0.0024).
Clinician-reported: KPS and NFS
Among patients with disease progression, 46% (n = 51) had a worsened KPS and 35% (n = 30) had a worsened NFS, compared to 23% (n = 32) and 20% (n = 23) of patients with stable disease, respectively (Table 3).
Symptom with most worsening
One hundred patients had worsening symptoms and functions at time of disease progression. Each of the priority symptom and function was reported by at least 7% (at least n = 7) as having worsened the most. When considering magnitude, fatigue was the symptom most frequently reported as having worsened the most with 19% (n = 19), followed by difficulty remembering with 17% (n = 17). Among functions, interference with general activity was most frequently reported as having worsened the most (19%, n = 19) followed by interference with walking (18%, n = 18) (Supplementary Fig. S2).
Discussion
While efficient for evaluation in a clinical trial, past and present studies have not identified a single symptom or construct that adequately reflects the symptom burden a patient experiences while on a clinical trial or receiving standard treatment. Reeve et al.4 identified 12 symptoms that occur most frequently among oncology patients. Armstrong et al. 1 further confirmed the presence of a core set of symptoms reported by PBT patients, where patients reported, on average, three moderate-severe symptoms simultaneously. The RANO-PRO Working Group broadened the idea to priority constructs that can encompass several symptoms and functions through available COAs. The present study provided initial support for the priority constructs in terms of their relevance, relationship with disease status and treatment, sensitivity to change over time, and evaluation via COAs.
The priority constructs were shown to be related to the disease and/or treatment, as measured by disease progression. Symptoms and functions among patients with disease progression were more severe compared to patients with stable disease, particularly in the functions of walking, work, and usual activities. Interference with work, activities, and walking has been shown to be predictive of disease progression in PBT patients.23 Both clinicians and patients themselves perceived the patient's functional and health status as worse, as reflected in the lower proportion of patients with a 11111 health state on the EQ-5D-3L (no problems in any dimension) and in the worsened KPS and NFS.
Changes in priority constructs was evident over time. Symptoms and functions, related to both disease and treatment, worsened to time of disease progression. The change is observed in symptoms related to the tumour, such as weakness, and symptoms related to the treatment, such as fatigue. Study patients reported all possible directions of change, yet only patients with radiographic disease progression reported significant worsening, with the magnitude of worsening greater than that of stability or improvement (a median of three points worse, data not shown). Also, when compared to patients with stable disease at the second assessment (n = 145), only difficulty understanding worsened over time and less than 16% (less than n = 23) reported worsening mobility, self-care, usual activities, or pain/discomfort (Supplementary Table S3).
The priority constructs were demonstrated to be relevant to patients in that they were reported as important by the patient. Patients were reporting each symptom as worsening the most for them individually. There was no single predominate symptom among all study patients based on their reporting. The priority constructs also were consistent with the top priorities of brain tumour patients and caregivers, as reported in a 2014 survey from the National Brain Tumor Society.27 Patients reported retaining brain functioning and ability to walk and perform basic physical tasks as most important. Our results mirror these findings and show that patients recognise and report changes in their cognition and physical abilities.
Previous discussions have considered the distinction between statistical significance and clinical relevance.2 The minimally important difference (MID) for the MDASI-BT is estimated at one point on a 0–10 scale and for the EQ-5D-3L Index score at 0.06 on a −0.33 to 1.0 scale.28,29 Using these MIDs, difficulty remembering, difficulty understanding, fatigue, weakness on one side of body, interference with walking, interference with work, interference with general activity, and the patient's perception of their health (EQ-3D-3L Index score) met the criteria for clinical relevance as well as statistical significance.
Regardless of COA, the pattern of clinical worsening associated with radiographic disease progression was consistent. Symptoms and functions were reported as more severe among patients with disease progression using PROs and a concomitant functional decline was noted using ClinROs by clinicians. The implications for patient care cannot be overstated. Recognition of core symptoms that worsen with disease progression is useful in planning patient and caregiver education for monitoring and reporting and in planning symptom management approaches. Exploring changes in symptoms prior to imaging studies is also necessary to establish and evaluate a standardised assessment and its ability to recognise clinical and radiographic disease progression.
While the Fast Track COA Group's recommended list of priority constructs included 11 items, the MDASI-BT measures an additional 17 symptoms and functions. A preliminary look at these non-priority items revealed that feeling drowsy, interference with enjoyment of life, and interference with mood were also significantly worse among patients with disease progression compared to stable disease (Supplementary Table S4). Feeling distressed, feeling drowsy, numbness/tingling in arms or legs, and interference with enjoyment of life also worsened over time to disease progression (Supplementary Table S5). Feeling drowsy may be offered as a symptomatic adverse event if it is a common or expected event of the treatment regimen. Enjoyment of life can be placed under role functioning as it can describe leisure or social activities in general. Mood changes, which could include feeling distressed, was recommended by the RANO-PRO Working Group but was later decided to be a confounding construct and was therefore not included in the Fast Track COA Group's final recommended list.
While the recommended guidelines are for both clinical research and clinical care, there are limitations to the present study that influence its current generalisability to clinical trials. There is variability in the treatments received by the study's patient sample, with not all having participated in a clinical trial. Cumulative effect of prior treatments can impact both disease status and symptom reports. Further work will consider how number and type of prior treatments can influence the observed relationship. The sample also includes patients across the disease phases. Future work will focus on the use of the prior constructs in clinical trial populations specifically. With that goal in mind, the current statistical analysis has been proposed for future use by the FDA's Oncology Center of Excellence. Another limitation of this study is that we chose to focus on evaluating the relationship of priority constructs with disease progression. Future work could include examining the relationship of priority constructs with progression free survival or overall survival that is currently beyond the scope of the present paper.
In summary, this is the first study to evaluate the clinical impact of the RANO-PRO Working Group's recommended priority constructs in a large cohort of patients with malignant glioma. A pattern of worsening symptoms and functions was observed using patient-reported outcomes and clinician-reported outcomes among patients with disease progression, including over time. The present study provides initial evidence to the relevance, sensitivity, feasibility, and relationship to disease of the seven recommended priority constructs for use in clinical research and clinical care for primary brain tumour patients.
Contributors
E.V.: Conceptualization, Data curation, Formal analysis, Methodology, Project administration, Software, Validation, Visualization, Writing-Original draft; Accessed and verified underlying data. A.C.: Data curation, Project administration, Writing-Review & editing. E.G.: Data curation, Writing-Review & editing. N.B.: Data curation, Investigation, Writing-Review & editing. A.C.: Project administration, Writing-Review & editing. S.P.C.: Investigation, Project administration, Writing-Review & editing. K.W.: Investigation, Project administration, Writing-Review & editing. M.L.: Investigation, Project administration, Writing-Review & editing. H.L.: Investigation, Resources, Writing-Review & editing. J.L.: Data curation, Resources, Software, Writing-Review & editing. J.R.: Project administration, Writing-Review & editing. A.A.: Investigation, Writing-Review & editing. A.L.K.: Investigation, Writing-Review & editing. V.J.: Investigation, Writing-Review & editing. K.R.: Investigation, Writing-Review & editing. J.L.R.: Investigation, Writing-Review & editing. M.T.: Investigation, Writing-Review & editing. L.B.: Investigation, Writing-Review & editing. N.L.: Investigation, Writing-Review & editing. M.P.: Investigation, Writing-Review & editing. L.P.: Investigation, Writing-Review & editing. T.P.: Investigation, Writing-Review & editing. E.B.: Investigation, Resources, Writing-Review & editing. M.P.P.: Investigation, Resources, Writing-Review & editing. B.T.: Investigation, Resources, Writing-Review & editing. J.W.: Investigation, Resources, Writing-Review & editing. M.R.G.: Conceptualization, Investigation, Resources, Supervision, Writing-Review & editing. T.S.A.: Conceptualization, Data curation, Investigation, Methodology, Project administration, Supervision, Validation, Writing-Original draft; Accessed and verified underlying data. T.R.M.: Conceptualization, Formal analysis, Writing-Original draft; Accessed and verified underlying data.
Data sharing statement
Individual participant data that underlie the results reported in this article (text, tables, figures, and appendices) and NOB–NHS protocol will be shared after de-identification beginning 3 months and ending 36 months following article publication with researchers who provide a methodologically sound proposal for the purpose of achieving aims in approved proposal. Proposals should be directed to terri.armstrong@nih.gov. To gain access, data requestors may need to sign a data access agreement.
Declaration of interests
All authors declare no competing interests.
Acknowledgments
The Natural History Study is supported by Intramural Project 1ZIABC011786-03 (Principal Investigator: T.S. Armstrong). The manuscript reports work done by TRM while at the Department of Symptom Research, UT MD Anderson Cancer Center, Houston, Texas, United States of America.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2022.101718.
Appendix A. Supplementary data
References
- 1.Armstrong T.S., Vera-Bolanos E., Acquaye A., et al. The symptom burden of primary brain tumors: evidence for a core set of tumor- and treatment-related symptoms. Neuro Oncol. 2016;18(2):252–260. doi: 10.1093/neuonc/nov166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dirven L., Armstrong T.S., Taphoorn M.J. Health-related quality of life and other clinical outcome assessments in brain tumor patients: challenges in the design, conduct and interpretation of clinical trials. Neurooncol Pract. 2015;2(1):2–5. doi: 10.1093/nop/npv002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dirven L., Taphoorn M.J., Reijneveld J.C., et al. The level of patient-reported outcome reporting in randomized controlled trials of brain tumour patients: a systematic review. Eur J Cancer. 2014;50(14):2432–2448. doi: 10.1016/j.ejca.2014.06.016. [DOI] [PubMed] [Google Scholar]
- 4.Reeve B.B., Mitchell S.A., Dueck A.C., et al. Recommended patient-reported core set of symptoms to measure in adult cancer treatment trials. J Natl Cancer Inst. 2014;106(7):1–7. doi: 10.1093/jnci/dju129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Basch E., Geoghegan C., Coons S.J., et al. Patient-reported outcomes in cancer drug development and US regulatory review: perspectives from industry, the Food and Drug Administration, and the patient. JAMA Oncol. 2015;1(3):375–379. doi: 10.1001/jamaoncol.2015.0530. [DOI] [PubMed] [Google Scholar]
- 6.Sul J., Kluetz P.G., Papadopoulos E.J., et al. Clinical outcome assessments in neuro-oncology: a regulatory perspective. Neurooncol Pract. 2016;3(1):4–9. doi: 10.1093/nop/npv062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Molinari E., Mendoza T.R., Gilbert M.R. Opportunities and challenges of incorporating clinical outcome assessments in brain tumor clinical trials. Neurooncol Pract. 2019;6(2):81–92. doi: 10.1093/nop/npy032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calvert M., Blazeby J., Altman D.G., et al. Reporting of patient-reported outcomes in randomized trials; the CONSORT PRO extension. JAMA. 2013;309(8):814–822. doi: 10.1001/jama.2013.879. [DOI] [PubMed] [Google Scholar]
- 9.Reeve B.B., Wyrwich K.W., Wu A.W., et al. ISOQOL recommends minimum standards for patient-reported outcome measures used in patient-centered outcomes and comparative effectiveness research. Qual Life Res. 2013;22(8):1889–1905. doi: 10.1007/s11136-012-0344-y. [DOI] [PubMed] [Google Scholar]
- 10.Calvert M., Kyte D., Mercieca-Bebber R., et al. Guidelines for inclusion of patient-reported outcomes in clinical trial protocols: the SPIRIT-PRO extension. JAMA. 2018;319(5):483–494. doi: 10.1001/jama.2017.21903. [DOI] [PubMed] [Google Scholar]
- 11.Coens C., Pe M., Dueck A.C., et al. International standards for the analysis of quality-of-life and patient-reported outcome endpoints in cancer randomized controlled trials: recommendations of the SISAQOL Consortium. Lancet Oncol. 2020;21(2):e83–e96. doi: 10.1016/S1470-2045(19)30790-9. [DOI] [PubMed] [Google Scholar]
- 12.U.S. Food and Drug Administration Guidance for industry patient-reported outcome measures: use in medical product development to support labeling claims. 2009. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/patient-reported-outcome-measures-use-medical-product-development-support-labeling-claims Available at: [DOI] [PMC free article] [PubMed]
- 13.BEST. (Biomarkers, EndpointS, and other Tools) resource. US Food and Drug Administration, National Institutes of Health; Silver Spring, MD: 2016. [PubMed] [Google Scholar]
- 14.Dirven L., Armstrong T.S., Blakeley J.O., et al. Working plan for the use of patient-reported outcome measures in adults with brain tumors: a response assessment in neuro-oncology (RANO) initiative. Lancet Oncol. 2018;19(3):e173–e180. doi: 10.1016/S1470-2045(18)30004-4. [DOI] [PubMed] [Google Scholar]
- 15.Armstrong T.S., Dirven L., Arons D., et al. Glioma patient-reported outcome assessment in clinical care and research: a response assessment in neuro-oncology collaborative report. Lancet Oncol. 2020;21(2):e97–e103. doi: 10.1016/S1470-2045(19)30796-X. [DOI] [PubMed] [Google Scholar]
- 16.Armstrong T.S., Mendoza T., Gning I., et al. Validation of the M.D. Anderson Symptom Inventory Brain Tumor Module (MDASI-BT) J Neuro Oncol. 2006;80(1):27–35. doi: 10.1007/s11060-006-9135-z. [DOI] [PubMed] [Google Scholar]
- 17.Serlin R.C., Mendoza T.R., Nakamura Y., Edwards K.R., Cleeland C.S. When is cancer pain mild, moderate or severe? Grading pain severity by its interference with function. Pain. 1995;61(2):277–284. doi: 10.1016/0304-3959(94)00178-H. [DOI] [PubMed] [Google Scholar]
- 18.Mendoza T.R., Wang X.S., Cleeland C.S., et al. The rapid assessment of fatigue severity in cancer patients: use of the Brief Fatigue Inventory. Cancer. 1999;85(5):1186–1196. doi: 10.1002/(sici)1097-0142(19990301)85:5<1186::aid-cncr24>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 19.Shi Q., Mendoza T.R., Dueck A.C., et al. Determination of mild, moderate, and severe pain interference in patients with cancer. Pain. 2017;158:1108–1112. doi: 10.1097/j.pain.0000000000000890. [DOI] [PubMed] [Google Scholar]
- 20.Group T.E. EuroQol-a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 21.Shaw J.W., Johnson J.A., Coons S.J. US valuation of the EQ-5D health states: development and testing of the D1 valuation model. Med Care. 2005;43(3):203–220. doi: 10.1097/00005650-200503000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Karnofsky D.A., Burchenal J.H. In: Evaluation of chemotherapeutic agents. MacLeod C.M., editor. Columbia Unversity Press; New York: 1949. The clinical evaluation of chemotherapeutic agents in cancer; pp. 191–205. [Google Scholar]
- 23.Armstrong T.S., Vera-Bolanos E., Gning I., et al. The impact of symptom interference using the MD Anderson Symptom Inventory-Brain Tumor Module (MDASI-BT) on prediction of recurrence in primary brain tumor patients. Cancer. 2011;117(14):3222–3228. doi: 10.1002/cncr.25892. [DOI] [PubMed] [Google Scholar]
- 24.Levin V.A., Crafts D.C., Norman D.M., Hoffer P.B., Spire J.P., Wilson C.B. Criteria for evaluating patients undergoing chemotherapy for malignant brain tumors. J Neurosurg. 1977;47:329–335. doi: 10.3171/jns.1977.47.3.0329. [DOI] [PubMed] [Google Scholar]
- 25.McNemer Q. Note on the sampling error of the difference between correlated proportions or percentages. Psychometrika. 1947;12(2):153–157. doi: 10.1007/BF02295996. [DOI] [PubMed] [Google Scholar]
- 26.IBM Corp . IBM Corp; Armonk, NY: 2019. IBM SPSS statistics for windows, version 26.0. [Google Scholar]
- 27.National Brain Tumor Society Brain tumor patient & caregiver survey. https://braintumor.org/our-research/funded-research-and-accomplishments/brain-tumor-patient-caregiver-survey
- 28.UT MD Anderson Cancer Center The MD Anderson Symptom Inventory user guide. https://www.mdanderson.org/documents/Departments-and-Divisions/Symptom-Research/MDASI_userguide.pdf
- 29.Pickard A.S., Neary M.P., Cella D. Estimation of minimally important differences in EQ-5D utility and VAS scores in cancer. Health Qual Life Outcomes. 2007;5:70. doi: 10.1186/1477-7525-5-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.