Abstract
IMPORTANCE
Cardiogenic shock affects between 40 000 and 50 000 people in the US per year and is the leading cause of in-hospital mortality following acute myocardial infarction.
OBSERVATIONS
Thirty-day mortality for patients with cardiogenic shock due to myocardial infarction is approximately 40%, and 1-year mortality approaches 50%. Immediate revascularization of the infarct-related coronary artery remains the only treatment for cardiogenic shock associated with acute myocardial infarction supported by randomized clinical trials. The Percutaneous Coronary Intervention Strategies with Acute Myocardial Infarction and Cardiogenic Shock (CULPRIT-SHOCK) clinical trial demonstrated a reduction in the primary outcome of 30-day death or kidney replacement therapy; 158 of 344 patients (45.9%) in the culprit lesion revascularization-only group compared with 189 of 341 patients (55.4%) in the multivessel percutaneous coronary intervention group (relative risk, 0.83 [95% CI, 0.71-0.96]; P = .01). Despite a lack of randomized trials demonstrating benefit, percutaneous mechanical circulatory support devices are frequently used to manage cardiogenic shock following acute myocardial infarction.
CONCLUSIONS AND RELEVANCE
Cardiogenic shock occurs in up to 10% of patients immediately following acute myocardial infarction and is associated with mortality rates of nearly 40% at 30 days and 50% at 1 year. Current evidence and clinical practice guidelines support immediate revascularization of the infarct-related coronary artery as the primary therapy for cardiogenic shock following acute myocardial infarction.
Cardiogenic shock (CS) is defined by systemic hypoperfusion and tissue hypoxia due to cardiac dysfunction. The most common etiology of CS is acute myocardial ischemia due to occlusion of an epicardial coronary artery, resulting in regional cardiac myocyte necrosis (acute myocardial infarction [AMI]) and loss of ventricular function.1 CS is the leading cause of in-hospital death in patients with AMI. Between 40 000 and 50 000 patients in the US have CS associated with AMI each year, which correlates to an incidence of approximately 5% to 10% of all patients with AMI.2-5 Thirty-day mortality is nearly 40% and approaches approximately 50% at 1 year (Box).5-8
Box. Commonly Asked Questions About Cardiogenic Shock.
What Is Cardiogenic Shock?
A clinical condition of inadequate tissue (end-organ) perfusion due to the inability of the heart to pump an adequate amount of blood. The reduction in tissue perfusion results in decreased oxygen and nutrient delivery to the tissues and, if prolonged, potentially end-organ damage and multisystem failure.
When Does Cardiogenic Shock Occur?
The most common cause of cardiogenic shock is acute myocardial infarction. Cardiogenic shock occurs in 5% to 10% of people with acute myocardial infarction.
What Is the Prognosis for Patients With Cardiogenic Shock After Acute Myocardial Infarction?
Thirty-day mortality is nearly 40% and approaches approximately 50% at 1 year.
What Treatments Have Been Shown to Reduce Mortality for Patients With Cardiogenic Shock?
Based on the results of the CULPRIT-SHOCK trial, coronary angiography and revascularization of the infarct related artery reduced 30-day mortality from 51.6% to 43.3%.
Severe left ventricular (LV) dysfunction is the most common presentation of CS in the setting of AMI, most frequently occurring after anterior MI. Of the 686 patients included in the Percutaneous Coronary Intervention Strategies with Acute Myocardial Infarction and Cardiogenic Shock (CULPRIT-SHOCK) trial, 288 (42.0%) had a left anterior descending MI and 53 (7.7%) had a left main coronary artery MI.7 Few treatment approaches reduce short- or long-term morbidity and mortality in patients with CS. This review describes the pathophysiology, diagnosis, and management of CS in the setting of AMI.
Methods
A literature search was performed that applied the Cochrane Highly Sensitive Search Strategy for randomized clinical trials (RCTs), a string for meta-analyses and systematic reviews, and established Medical Subject Headings for “cardiogenic shock” and “treatment” to the PubMed and Cochrane databases for articles published from January 1, 1995, through August 5, 2021. The literature search identified 1552 articles. The authors prioritized RCTs, meta-analyses, and larger observational studies. A total of 46 papers were included, including 12 randomized trials, 2 meta-analyses, 1 systematic review, and 31 observational studies.
Pathophysiology
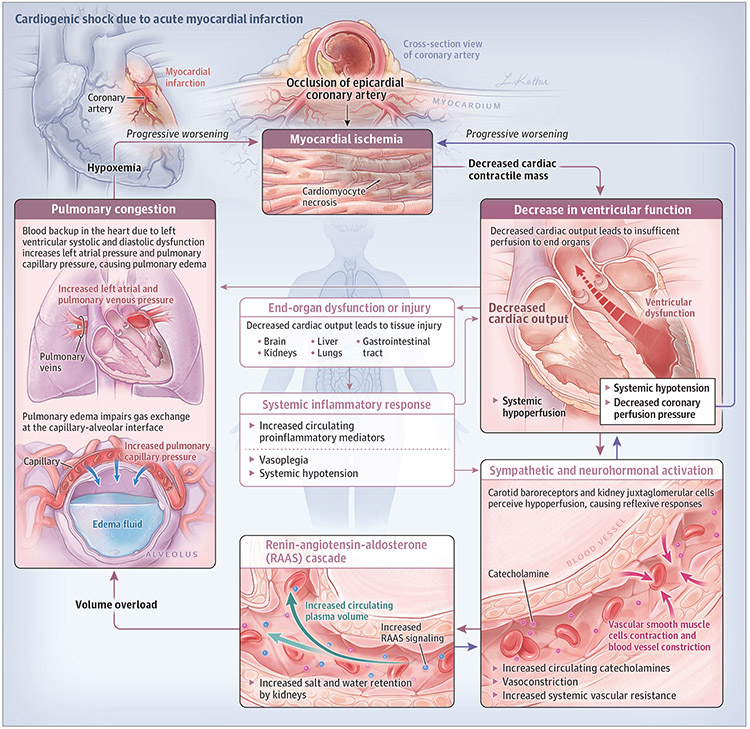
The “classic” pathophysiological paradigm of CS associated with AMI consists of a myocardial ischemic insult resulting in regional necrosis and a decrease in cardiac contractile mass. A consequent decrease in ventricular function with associated decrease in cardiac output and systemic hypoperfusion is perceived by carotid baroreceptors and juxtaglomerular cells in the kidney. The decreased perfusion leads to reflexive sympathetic/neurohormonal activation and increased circulating catecholamines. Vascular endothelial cells typically constrict to maintain systemic perfusion and the renin-angiotensin-aldosterone cascade is activated to increase salt and water retention. Together, these reflexive responses increase myocardial afterload and circulating plasma volume (ie, cardiac preload), which can reduce cardiac performance and lead to pulmonary edema. If ventricular function cannot be restored, or rapid decongestion does not occur, a self-perpetuating cycle of decreasing cardiac output and progressive volume overload ensues. Ultimately, this cycle leads to a reduction in coronary artery perfusion pressure, myocardial ischemia, worsening cardiac function, and circulatory collapse (Figure).
Figure.
Cardiogenic Shock Associated With Acute Myocardial Infarction
The Should We Emergently Revascularize Occluded Coronaries In Cardiogenic Shock? (SHOCK) trial and registry provided some findings that challenge this pathophysiological paradigm. The SHOCK trial and registry were designed to study the effect or association of early coronary artery revascularization for patients with CS associated with AMI. The clinical trial included 302 patients with CS associated with AMI randomized to receive either coronary revascularization within 12 hours of CS diagnosis or initial medical stabilization including fibrinolysis and implantation of an intra-aortic balloon pump (IABP). Patients with suspected CS within 36 hours of AMI were included if they had clinical hypotension (defined as systolic blood pressure <90 mm Hg for at least 30 minutes or requirement of supportive measures to maintain the systolic blood pressure at 90 mm Hg). Patients also met hemodynamic criteria of a cardiac index of less than or equal to 2.2 L/min/m2 and a pulmonary capillary wedge pressure greater than or equal to 15 mm Hg. Results of the trial showed no statistically significant difference in the primary outcome of 30-day mortality (71 of 152 patients [46.7%] in the revascularization group vs 84 of 150 [56%] in the medical therapy group; between-group difference, 9.3% [95% CI, −20.5% to 1.9%]).9 However, early revascularization significantly reduced mortality at the 6-month follow-up (50.3% vs 63.1%) and the 1-year follow-up (53.3% vs 66.4%).9,10 The SHOCK registry included patients with suspected CS who did not meet all SHOCK trial inclusion criteria or specified time windows, met a trial exclusion criterion, or were unable or refused to give consent.11 Of the 1190 patients included in the SHOCK registry, 256 had invasive hemodynamic assessment. Of these patients, 245 (95%) had persistently low systemic vascular resistance, despite continuous use of infused catecholamines.12 This associated systemic vasodilation, unresponsive to continuously infused catecholamines, may be due to a systemic inflammatory response syndrome characterized by hyperthermia, leukocytosis, and increased levels of proinflammatory mediators. These proinflammatory pathways can promote hypotension through direct inhibition of cardiac contractility, suppression of mitochondrial respiration throughout the body, reduced catecholamine responsiveness, and, occasionally, systemic vasodilation.13
Clinical Presentation
In patients with CS associated with AMI due to LV infarction, the inability to efficiently eject blood leads to an increase in LV end-diastolic pressure. The increased pressure is associated with elevated pulmonary capillary wedge pressure. Patients with increased LV end-diastolic pressure typically present with an S3 gallop, tachypnea, and hypoxemia due to pulmonary edema that may be manifest with lung rales. When pulmonary edema develops rapidly due to LV systolic and diastolic dysfunction, patients can present with respiratory distress and failure.
CS can be present at the time of hospital arrival after AMI or can develop later after an initial ischemic myocardial injury. A secondary analysis from the SHOCK trial and registry reported a median (IQR) time from AMI symptom onset to CS onset of 6.2 (1.7-20.1) hours.14 The SHOCK registry reported a median (IQR) time from AMI symptom onset to CS onset of 5.5 (2.3-14.1) hours.14 Very early shock (onset <6 h after AMI) occurred in 46.6% of SHOCK registry patients, early shock (onset <24 h) occurred in 74.1% of SHOCK registry patients, and late shock (onset ≥24 h) occurred in 25.9% of SHOCK registry patients. Shock was diagnosed at presentation in 9% of registry patients and 14% of the trial patients.14
Patients with CS after an acute LV infarction can present with hypotension; signs of hypoperfusion, such as altered mentation or cool/mottled extremities; signs of increased intracardiac filling pressures (due to ventricular systolic and diastolic dysfunction), such as pulmonary edema, orthopnea, or elevated jugular venous pressure; or a combination of all. Hypotension is generally defined as systolic blood pressure less than 90 mm Hg or mean arterial pressure 30 mm Hg less than the patient’s baseline. An arterial pulse pressure (systolic blood pressure – diastolic blood pressure) that is less than 25% of the systolic pressure indicates reduced cardiac output.
Hypoperfusion can manifest as decreased or altered mentation, cool extremities with decreased intensity of distal pulses, or oliguria (urine output <30 mL/h).7-9 Elevated serum lactate greater than 2.0 mmol/L at presentation is a sensitive laboratory marker of hypoperfusion, and is among the diagnostic criteria for CS after AMI.
A subgroup of patients with CS after AMI due to LV failure exhibit findings of systemic hypoperfusion despite maintaining blood pressure greater than 90 mm Hg without vasopressor use.12 This entity is referred to as nonhypotensive cardiogenic shock and is associated with increased rates of adverse events.12 In a secondary analysis of 1068 patients eligible for the SHOCK registry, 49 (4.6%) had nonhypotensive CS, defined as evidence of oliguria (urine output <30 mL/h) or extremities that were cold to touch on physical examination; 76 (7.1%) had hypotension, defined as a systolic blood pressure less than 90 mm Hg without a therapeutic intervention to maintain blood pressure, without hypoperfusion; and 943 of 1068 (88.3%) had classic CS, defined as hypotension plus hypoperfusion. The mean blood pressure values for the groups were 104/62 for the nonhypotensive CS group, 86/51 for the classic CS group, and 98/57 for the hypotension group (3-way P values <.001 each for systolic and diastolic comparisons). The mean cardiac index was 1.9 L/min/m2 for the nonhypotensive CS group, 2.0 L/min/m2 for the classic CS group, and 2.5 L/min/m2 for the hypotension group (3-way P value = .48). In-hospital mortality rates were 43% for patients with nonhypotensive shock, 66% for patients with classic shock (P = .001), and 26% for patients with isolated hypotension (P = .08 compared with nonhypotensive shock). These findings underscore the importance of clinical assessment for hypoperfusion, because it may be a more important indicator of adverse outcomes than hypotension, especially in the presence of a “normal” arterial pulse pressure.12 Moreover, a strictly defined blood pressure threshold may not adequately define relatively reduced perfusion pressure.
CS following isolated right ventricular infarction is less common than LV infarction, and occurred in 49 of 893 patients (5.5%) in the SHOCK registry.15 Compared with patients with CS following LV infarction, patients with right ventricular infarction and CS were younger (mean [SD] age of 64.5 [12.0] vs 68.5 [12.1] years; P = .031), had lower prevalence of previous AMI (25.5% vs 40.1%; P = .047) and multivessel coronary artery disease (34.8% vs 77.8%; P < .001), and had a shorter median time between the index MI and the diagnosis of shock (2.9 h vs 6.2 h; P = .003).15 Patients with CS following right ventricular infarction and failure present with a classical triad of hypotension, elevated jugular venous pressure, and normal oxygen saturation.
Assessment and Diagnosis
The Society for Cardiovascular Angiography and Intervention (SCAI) has proposed a classification schema for CS, which characterizes the spectrum of CS from “at risk” to “extremis.”16 However, the SCAI shock classification does not give specific, objective criteria to define a shock state or occurrence of transitioning between shock classifications, making this schema challenging for clinical use.
The cystatin C (kidney function), lactate (hypoperfusion), interleukin-6 (inflammation), and brain natriuretic peptide (heart failure) (CLIP) score was developed and validated as a biomarker-based risk score to predict 30-day mortality for patients with CS following AMI. The CLIP score was derived and internally validated from the CULPRIT-SHOCK trial and externally validated using the Intra-aortic Balloon Pump in Cardiogenic Shock II (IABP-SHOCK II) trial. The CLIP score yielded C statistics of 0.82 (95% CI, 0.78-0.86) in internal validation, 0.82 (95% CI, 0.75-0.89) in temporal internal validation (based on randomization date), and 0.73 (95% CI, 0.65-0.81) in external validation.17 This score yielded a higher C statistic than the Simplified Acute Physiology Score II (0.83 vs 0.62; P < .001) and IABP-SHOCK II risk score in prognostication (0.83 vs 0.76; P = .03), both of which are clinically based risk models.17
In addition to a directed physical examination, a detailed clinical assessment of a patient with presumed CS associated with AMI should include an electrocardiogram to assess for myocardial ischemia or infarction; laboratory assessment for metabolic acidosis (serum pH <7.3) and markers of end-organ function, such as acute kidney or liver injury; and an echocardiogram to assess biventricular and valvular function and identify mechanical complications of AMI. Invasive hemodynamic assessment may be appropriate for the initial evaluation of patients with AMI who present with hypotension or signs suggestive of hypoperfusion. Based on observational evidence, the use of pulmonary artery catheterization in patients with AMI and hypotension or signs of hypoperfusion may lead to earlier and more accurate diagnosis of CS.18-21 An observational study using the Nationwide Inpatient Sample identified 5925 patients between 2008 and 2014 who were treated with a percutaneous mechanical circulatory support device following a diagnosis of CS associated with AMI. From 2008 to 2014, there was a decrease in use of invasive hemodynamic assessment in patients receiving percutaneous mechanical circulatory support from 40.4% to 29.8% (P for trend = .0005). Invasive hemodynamic assessment was associated with a decrease in mortality (56.0% to 42.6%; P for trend = .005), whereas a lack of invasive hemodynamic assessment was associated with increased mortality (44.4% to 48.4%; P for trend = .001).22 Importantly, these data are based on observational evidence and are limited by potential confounding. Use of invasive hemodynamic assessment has been designated a class IIb, level of evidence B recommendation by the European Society of Cardiology given the absence of prospective randomized data.23 A consensus statement by the American Heart Association (AHA) supports invasive hemodynamic assessment in select circumstances, although it should not delay primary revascularization.24
Mechanical Complications of AMI
Interventricular septum rupture, papillary muscle rupture with acute mitral regurgitation, and LV free wall rupture are complications of AMI that can result in CS. Patients with these conditions are at an increased risk of developing CS and associated mortality and morbidity, including acute kidney injury and respiratory failure.25 An observational study using data from the National Inpatient Sample identified 3 951 861 ST-elevation MI (STEMI) hospitalizations and 5 114 270 non–ST-elevation MI (NSTEMI) hospitalizations between January 2003 and September 2015.25 LV free wall rupture occurred in 10 726 (0.27%) STEMI hospitalizations and 3041 (0.06%) NSTEMI hospitalizations. Interventricular septal rupture occurred in 8401 (0.21%) STEMI hospitalizations and 1943 (0.04%) NSTEMI hospitalizations. Papillary muscle rupture with mitral regurgitation occurred in 2024 (0.05%) STEMI hospitalizations and 628 (0.01%) NSTEMI hospitalizations. Free wall rupture occurred in 301 (0.01%) STEMI and 470 (0.01%) NSTEMI hospitalizations.25 Although rare, these complications are associated with an in-hospital mortality of approximately 40%.25 Due to the association with increased mortality, all patients with CS associated with AMI should be immediately assessed for mechanical complications. Bedside echocardiography or left ventriculogram in patients undergoing emergency cardiac catheterization can confirm a complication associated with rupture of the interventricular septum, papillary muscle, or free wall, and is recommended by international professional society practice guidelines.23,26
Management
Coronary Artery Revascularization
CS associated with AMI can occur after STEMI or NSTEMI. Emergency revascularization of the infarct-related artery remains the mainstay of treatment and is the only therapy that has significantly reduced mortality in CS in a randomized trial. Emergency revascularization has a class I recommendation (indicating that the procedure should be performed) for management of CS in international professional society practice guidelines (Table 1). These recommendations are supported by data from longer-term follow-up from the SHOCK trial as well as positive primary results of the CULPRIT-SHOCK trial.7,10 Despite the lack of significant difference in mortality in the SHOCK trial at 30-day follow-up, immediate revascularization reduced mortality at the 6-month follow-up, compared with initial medical stabilization (50.3% vs 63.1%; [95% CI for the difference, 23.2%-0.9%]; P = .027).9 The benefit of early revascularization persisted at 1 year (53.3% vs 66.4%; [95% CI for the difference, 24.1%-2.2%]; P < .03).10
Table 1.
Professional Society Guidelines for Management of Cardiogenic Shock (CS) Associated With Acute Myocardial Infarction (AMI)
| Recommendation | Recommendation class |
Level of evidence |
Year | Society |
|---|---|---|---|---|
| Non-ST-elevation myocardial infarction (NSTEMI) with CS | ||||
| Emergency coronary angiography | I | B | 202027 | ESC |
| Revascularization for cardiogenic shock | I | B | 201428 | ACCF/AHA |
| Emergency PCI of the culprit lesion is recommended for patients with CS due to NSTEMI, independent of the time delay from symptom onset, if the coronary anatomy is amenable to PCI | I | B | 202027 | ESC |
| Emergency CABG is recommended for patients with CS if the coronary anatomy is not amenable to PCI | I | B | 202027 | ESC |
| Routine immediate revascularization of nonculprit lesions in patients with NSTEMI with multivessel disease presenting with CS is not recommended | III | B | 202027 | ESC |
| ST-elevation myocardial infarction (STEMI) in CS | ||||
| Immediate transfer to a PCI-capable hospital for coronary angiography is recommended for suitable patients with STEMI who develop CS irrespective of the time delay from MI onset | I | B | 201326 | ACCF/AHA |
| Cardiac catheterization and coronary angiography with intent to perform revascularization should be performed after STEMI in patients with CS | I | B | 201326 | ACCF/AHA |
| I | B | 201729 | ESC | |
| Primary PCI should be performed in patients with STEMI and CS irrespective of time delay from MI onset | I | B | 201326 | ACCF/AHA |
| PCI of an anatomically significant stenosis in the infarct artery should be performed in patients with suitable anatomy and CS | I | B | 201326 | ACCF/AHA |
| Patients who were treated with fibrinolytic therapy or who did not receive reperfusion therapy who develop CS associated with AMI should undergo coronary angiography | I | B | 201326 | ACCF/AHA |
| PCI of an infarct artery in patients who were treated with fibrinolytic therapy or who did not receive reperfusion therapy | I | B | 201326 | ACCF/AHA |
| Emergent CABG is indicated in patients with STEMI and coronary anatomy not amenable to PCI who have CS | I | B | 201326 | ACCF/AHA |
| Emergency revascularization with either PCI or CABG is recommended in suitable patients with CS after STEMI irrespective of the time delay from MI onset | I | B | 201326 | ACCF/AHA |
| In the absence of contraindications, fibrinolytic therapy should be administered to patients with STEMI and CS who are unsuitable candidates for either PCI or CABG | I | B | 201326 | ACCF/AHA |
| PCI of a noninfarct artery may be considered in select patients with STEMI and multivessel disease who are hemodynamically stable, either at the time of primary PCI or as a planned staged procedure | IIb | B | 201326 | ACCF/AHA |
| IIa | C | 201729 | ESC | |
| Pharmacotherapies | ||||
| Inotropic/vasopressor agents may be considered for hemodynamic stabilization | IIb | C | 201729 | ESC |
| No specific recommendation | 201326 | ACCF/AHA | ||
| Temporary percutaneous mechanical circulatory support | ||||
| IABP can be useful for patients with CS after STEMI who do not quickly stabilize with pharmacological therapy | IIa | B | 201428 | ACCF/AHA |
| Alternative LV assist devices for circulatory support may be considered in patients with refractory CS | IIb | C | 201428 | ACCF/AHA |
| In select patients with MI and CS, short-term mechanical circulatory support may be considered, depending on patient age, comorbidities, neurological function, and the prospects for long-term survival and predicted quality of life | IIa | C | 201730 | ESC |
| Routine use of IABP in patients with CS and no mechanical complications due to MI is not recommended | III | B | 201729 | ESC |
| Echocardiography | ||||
| Immediate Doppler echocardiography is indicated to assess ventricular and valvular functions and loading conditions and to detect mechanical complications | I | C | 201729 | ESC |
Abbreviations: ACCF, American College of Cardiology Foundation; AHA, American Heart Association; CABG, coronary artery bypass grafting; CS, cardiogenic shock; ESC, European Society of Cardiology; IABP, intra-aortic balloon pump; LV, left ventricle; PCI, percutaneous coronary intervention.
Multivessel coronary artery disease is common in patients with CS associated with AMI; for example, in the SHOCK trial, 53.4% of patients who underwent angiography had 3-vessel coronary artery disease.31 The question of whether to perform multivessel PCI in CS associated with AMI was studied in the CULPRIT-SHOCK trial, which randomized 706 patients with CS associated with AMI who had multivessel coronary artery disease to one of 2 initial revascularization strategies: immediate PCI of the culprit lesion only with the option of staged revascularization for nonculprit lesions (n = 344) vs immediate multivessel PCI (n = 341).7 For the primary composite end point of 30-day death or kidney replacement therapy, 158 patients (45.9%) in the culprit lesion–only group experienced an event, compared with 189 patients (55.4%) in the multivessel PCI group (relative risk [RR], 0.83 [95% CI, 0.71-0.96]; P = .01). The RR of 30-day death from any cause with the culprit lesion–only PCI strategy (149/344 [43.3%]) vs the multivessel PCI strategy (176/341 [51.6%]) was 0.84 ([95% CI, 0.72-0.98]; P = .03). At 1 year, 172 patients (50.0%) in the culprit lesion–only PCI group died compared with 194 (56.9%) in the multivessel PCI group (RR, 0.88 [95% CI, 0.76-1.01]).7
Clinical practice guidelines from the American College of Cardiology Foundation (ACC)/AHA, European Society of Cardiology (ESC), and SCAI recommend immediate invasive coronary angiography for patients presenting with CS associated with AMI to define coronary anatomy (class I recommendation [high-quality evidence shows that benefit exceeds potential risk and the therapy should be provided]). In patients with multivessel coronary artery disease, guidelines recommend revascularization of the infarct-related artery (class I recommendation).32 However, ESC recommendations designate multivessel revascularization for CS associated with AMI as a class III recommendation, suggesting that there is no benefit and may be associated harm (Table 1; eFigure in the Supplement).27
Pharmacologic Therapies
Vasoactive medications are prescribed to nearly 90% of patients with CS following AMI to manage hypoperfusion and/or hypotension.8,24 Inotropic agents, such as dobutamine or milrinone, are used to manage hypoperfusion when their vasodilatory effect is not anticipated to cause severe hypotension. Dobutamine stimulates β-receptors to increase cardiac contractility (inotropy) and relaxes vascular smooth muscle to reduce afterload (vasodilation), and is administered via continuous infusion. Milrinone is a phosphodiesterase-3 inhibitor. Within myocardial cells, phosphodiesterase-3 inhibitors decrease rates of intracellular cyclic adenosine monophosphate breakdown, which increases intracellular calcium, myocardial contractility, and cardiomyocyte relaxation (lusitropy). Phosphodiesterase-3 inhibitors cause arterial and venous vasodilation through effects on vascular endothelium. Together, these effects increase myocardial contractility and reduce afterload.
Vasopressors that promote myocardial contractility, such as high-dose dopamine, epinephrine, or norepinephrine, have α-receptor–vasoconstricting properties and may be used to manage CS associated with AMI with refractory hypotension. An RCT randomized 1679 patients to receive either dopamine or norepinephrine as the first-line vasopressor to manage shock. Participants had mean arterial blood pressure less than 70 mm Hg or systolic blood pressure less than 100 mm Hgdespite adequate fluid resuscitation (1000 mL of crystalloids or 500 mL of colloids, unless there was an elevation in the central venous pressure to >12 mm Hg or in pulmonary-artery occlusion pressure to >14 mm Hg). There was no difference in the primary outcome of death at 28 days between patients randomized to receive dopamine (n = 858) vs norepinephrine (n = 821): 52.5% vs 48.5% (odds ratio, 1.17 [95% CI, 0.97-1.42]; P = .10). A prespecified subanalysis of patients with CS (not necessarily dueto AMI) (N = 280) showed that dopamine, compared with norepinephrine, was associated with increased mortality at 28 days (P = .03). More arrhythmic events occurred among patients treated with dopamine than among those treated with norepinephrine (207 events [24.1%] vs 102 events [12.4%]; P < .001).33 A small RCT of 57 patients with CS after AMI compared epinephrine (n = 27) with norepinephrine (n = 30) and found no difference in the primary outcome of change in cardiac index at 72 hours (P = .43; absolute values not available). However, refractory CS after AMI was more common in patients treated with epinephrine compared with norepinephrine (10 of 27 [37%] vs 2 of 30 [7%]; P = .01).34
Percutaneous Mechanical Circulatory Support Devices
Observational data from a US national registry demonstrated an increasing use of percutaneous mechanical circulatory support devices for treating patients with CS associated with AMI.35 The most frequently used percutaneous mechanical circulatory support devices were the IABP and the microaxial LV assist device (LVAD). Both are intravascular catheter-mounted devices that are inserted percutaneously via the femoral (or axillary) artery. The IABP increases coronary artery blood flow and reduces LV afterload via timed diastolic inflation and systolic deflation.36 The microaxial LVAD is an axial-flow pump that is placed across the aortic valve into the LV and continuously draws blood from the LV, delivering it directly to the proximal aorta.37-41 In contrast to an IABP, which enhances cardiac output indirectly through a reduction in afterload and corresponding increase in LV stroke volume, the microaxial LVAD directly pumps blood from the LV into the aorta. Hemodynamic studies have shown that the microaxial LVAD provides more hemodynamic support (2.5-5.5 L/min), as measured by cardiac output, compared with an IABP (0.8-1.0 L/min).42,43
Since 1993, only 3 RCTs of CS associated with AMI have been published, including the SHOCK and CULPRIT-SHOCK trials, that were adequately powered to detect meaningful differences in clinical outcomes.7-9 The third trial was the Intra-aortic Balloon Pump in Cardiogenic Shock II (IABP-SHOCK II) trial, which was an open-label RCT of 600 patients with CS associated with AMI undergoing coronary artery revascularization. Patients with CS associated with AMI were randomized to receive an IABP (n = 301) or no IABP (control; n = 299). There was no significant difference in 30-day all-cause mortality (primary end point): 119 patients (39.7%) in the IABP group and 123 patients (41.3%) in the control group died (RR with IABP, 0.96 [95% CI, 0.79-1.17]; P = .69).
Other RCTs of percutaneous mechanical circulatory support in CS associated with AMI have had small sample sizes.5 The Impella Versus IABP Reduces Mortality in STEMI Patients Treated With Primary PCI in Severe Cardiogenic Shock (IMPRESS Severe Shock) trial randomized 48 patients with CS associated with AMI who required mechanical ventilation to receive either IABP (n = 24) or microaxial LVAD (n = 24). Patients treated with either IABP or microaxial LVAD had no significant difference in the primary outcome of 30-day mortality (12/24 [50%] vs 11/24 [46%]; hazard ratio with microaxial LVAD, 0.96 [95% CI, 0.42-2.18]; P = .92).44 However, a high proportion of patients in both treatment groups died due to anoxic brain injury, perhaps related to cardiac arrest that preceded randomization. The trial likely lacked statistical power to demonstrate an effect on mortality.
Most treatment data regarding percutaneous mechanical circulatory support other than IABPs in CS associated with AMI are from observational studies. The National Cardiogenic Shock Initiative (N = 171)and catheter-based ventricular-assist device (N = 287) registries were uncontrolled studies that assessed outcomes associated with microaxial LVAD use in patients with CS associated with AMI who were treated with percutaneous revascularization. Of the 171 patients in the National Cardiogenic Shock Initiative registry, 123 (71.9%) survived to hospital discharge.24 Most patients included in the catheter-based ventricular-assist device registry would not be considered for clinical trials due to presence of characteristics such as anoxic brain injury (51/287), cardiac arrest prior to presentation (58/287), and transfers from other health care facilities (123/286), which are common exclusion criteria for RCTs. Overall, 127 of 287 patients (44.2%) from the catheter-based ventricular-assist device registry survived to hospital discharge.28 The survival rates in both studies were improved compared with rates reported in previously conducted RCTs and registries. In the catheter-based ventricular-assist device registry study, microaxial LVAD placement prior to percutaneous revascularization was associated with decreased in-hospital mortality (odds ratio, 0.485 [95% CI, 0.24-0.98]; P = .44) and improved rates of survival to hospital discharge.45 Data from other registries of all percutaneous mechanical circulatory support use suggest significant variation in deployment and selection of percutaneous mechanical circulatory support devices. From 2004 through 2016, a US claims registry that included patients with CS associated with AMI (N = 4782) demonstrated a proportional increase in microaxial LVAD use ranging from 0% to 100% across 432 US hospitals, without a significant change in IABP use. Over the same period, it was estimated that propensity-matched patients had a mean 5.77-fold differing likelihood of receiving a microaxial LVAD at one randomly selected hospital compared with another.37 Two other observational studies that used propensity-adjusted association reported that the microaxial LVAD was associated with a higher risk for death, stroke, acute kidney injury, vascular injury, and bleeding complications.37,46,47 A propensity-matched comparison of the microaxial LVAD (n = 237) vs patients from the IABP-SHOCK II trial (n = 237) reported that microaxial LVAD use was not associated with any difference in the primary outcome of 30-day all-cause mortality compared with IABP-SHOCK II (115/237 [48.5%] vs 110/237 [46.4%]; P = .64). Severe or life-threatening bleeding (20/237 [8.5%] for microaxial LVAD vs 7/237 [3.0%] for IABP-SHOCK II; P < .01)and peripheral vascular complications (23/237 [9.8%] for microaxial LVAD vs 9/237 [3.8%] for IABP-SHOCK II; P = .01) were more common in the microaxial LVAD than the IABP-SHOCK II group.48 Until further data from RCTs are available, the use of percutaneous mechanical circulatory support should be guided by professional society practice guidelines, which are based on expert consensus.24,26,27
The ACCF/AHA clinical practice guidelines for the management of STEMI and a consensus statement from the AHA recommend a stepwise strategy of treatment for patients with CS associated with AMI, beginning with vasoactive medications, such as dopamine, followed by insertion of percutaneous mechanical circulatory support devices if vasoactive medications do not improve hemodynamics.24,26 Early revascularization and early treatment with vasoactive medications may prevent the need for percutaneous mechanical circulatory support and the attendant risks.26 However, vasoactive medications, such as dopamine, have not been shown to reduce mortality and may not provide adequate hemodynamic support for some patients. An alternative strategy is immediate insertion of a percutaneous mechanical circulatory support device.24 This strategy may provide more hemodynamic support than initial treatment with pharmacotherapies, but evidence from RCTs is lacking. Importantly, there are no adequately powered RCTs that demonstrate mortality benefit of percutaneous mechanical circulatory support devices for patients with CS associated with AMI. Current practice guidelines acknowledge the absence of data supporting percutaneous mechanical circulatory support use as represented by the class of recommendations given (II or III) and associated levels of evidence (B or C) (Table 1 and Table 2).
Table 2.
Summary of Studies of Therapeutic Interventions for Patients With Cardiogenic Shock (CS) Associated With Acute Myocardial Infarctiona
| Source | Intervention | Study design | No. of participants |
Primary outcome | Adverse effects |
|---|---|---|---|---|---|
| Coronary artery revascularization | |||||
| SHOCK,9 1999 | Emergency revascularization vs initial medical stabilization with delayed revascularization at least 54 h after randomization | RCT | 302 | 30-d all-cause mortality: 46.7% vs 56.0%; RR, 0.83 (95% CI, 0.67-1.04); P = .11 | Acute kidney failure (defined as serum creatinine >3.0 mg/dL): 13% vs 24%; P = .03 |
| SMASH,49 1999 | Emergency revascularization vs initial medical stabilization | RCT | 55 | 30-d all-cause mortality: 22/32 (69%) vs 18/23 (78%)b | Recurrent myocardial infarction: 1/32 (3.1%) vs 1/23 (4.3%) |
| CULPRIT-SHOCK,7 2017 | Culprit lesion-only PCI, with option of staged PCI of nonculprit lesions vs immediate multivessel PCI | RCT | 706 | All-cause death or kidney replacement therapy at 30-d follow up: 158 (45.9%) vs 189 (55.4%); RR, 0.83 (95% CI, 0.71-0.96); P = .01 | Recurrent myocardial infarction: 4 (1.2%) vs 3 (0.9%); RR, 1.32 (95% CI, 0.30-5.86); P = 1.00 Stroke: 12 (3.5%) vs 10 (2.9%); RR, 1.19 (95% CI, 0.52-2.72); P = .68 |
| Percutaneous mechanical circulatory support | |||||
| IABP-SHOCK I,50 2010 | IABP compared with no IABP | RCT | 45 | Change in APACHE II score at 4 d: 2.4 vs 2.8 pointsc; difference not significant | |
| IABP-SHOCK II,8 2012 | IABP compared with no IABP | RCT | 600 | 30-d mortality: 39.7% vs 41.3%; RR, 0.96 (95% CI, 0.79-1.17); P = .69 | Life-threatening bleeding: 4.3% vs 3.4%; RR, 1.29 (95% CI, 0.58-2.90); P = .53 Stroke in hospital |
| IMPRESS,44 2017 | Microaxial LVAD vs IABP | RCT | 48 | 30-d mortality: 11/24 (45.8%) vs 12/24 (50%); HR with microaxial LVAD, 0.96 (95% CI, 0.42-2.18); P = .92 | Ischemic stroke: 1/24 (4.2%) vs 1/24 (4.2%) Major vascular complication: 1/24 (4%) vs 0/24 Life threatening bleeding: 8/24 (33.3%) vs 2/24 (8.3%) |
| National Cardiogenic Shock Initiative,19 2019 | Standardized implantation of microaxial LVAD before PCI compared with no receipt of microaxial LVAD | Observational | 171 | Survival to hospital discharge: 123/171 (71.9%) | Life-threatening bleeding: 17/171 (9.9%) Ischemic limb requiring intervention: 7/171 (4.1%) Thrombus formation on device: 2/171 (1.2%) Refractory CS requiring escalation of hemodynamic support: 15/171 (8.8%) |
| Catheter-based Ventricular Assist Device Registry,45 2017 | Comparison of receipt of standardized implantation of microaxial LVAD before PCI with no receipt of microaxial LVAD | Observational | 287 | Survival to hospital discharge: 127/287 (44.2%) | Not reported |
| Dhruva et al,46 2020 | Propensity-matched microaxial LVAD compared with IABP using US National Registry Data | Observational | 1680 matched pairs | In-hospital mortality: 756/1680 (45%) vs 573/1680 (34.1%); absolute risk difference, 10.9% (95% CI, 7.6%-14.2%); P <.001 | Life-threatening bleeding: 526/1680 (31.3%) vs 268/1680 (16.0%); absolute risk difference, 15.4% (95% CI, 12.5%-18.2%); P <.001 |
| Schrage et al,48 2019 | Propensity-matched microaxial LVAD (from US National Registry Data) compared with IABP (from IABP-SHOCK II) | Observational | 237 matched pairs | 30-d mortality: 115/237 (48.5%) vs 110/237 (46.4%); P = .64 | In-hospital recurrent MI: 7/237 (3.5%) vs 6/237 (2.5%); P = .56 Stroke in hospital: 6/237 (2.5%) vs 5/237 (2.5%); P = .76 Peripheral ischemic complications requiring intervention: 23/237 (9.8%) vs 40/237 (16.9%); P = .05 Life-threatening bleeding: 20/237 (8.5%) vs 7/237 (3.0%); P <.01 |
| Medications | |||||
| TRIUMPH,51 2007 | Ilarginine (L-NG-monomethylarginine), 1-mg/kg bolus and 1-mg/kg per hour 5-h infusion vs matching placebo | RCT | 398 | 30-d mortality: 97/201 (48%) vs placebo 76/180 (42%); HR, 1.14 (95% CI, 0.92-1.41); P = .24 | Recurrent MI: 8/198 (4.0) vs 7/179 (3.9); HR, 1.02 (95% CI, 0.59-1.77); P = .95 |
| PRAGUE-7,52 2011 | Abciximab prior to PCI vs periprocedural PCI in patients with CS associated with AMI | RCT | 80 | 30-d death, recurrent MI, stroke, new kidney failure: 17/40 (42.5%) vs 11/40 (27.5%); P = .24 | Life-threatening bleeding: 7/40 (17.5%) vs 3/40 (7.5%); P = .31 |
| Levy et al,34 2018 | Norepinephrine compared with dopamine for CS following AMI | RCT | 57 | Change in cardiac index at 72-h: no significant difference | Refractory cardiogenic shock: 10/27 (37.0%) norepinephrine vs 2/30 (6.7%) dopamine; P = .01 |
Abbreviations: AMI, acute myocardial infarction; HR, hazard ratio; IABP, intra-aortic balloon pump; LVAD, left ventricular assist device; PCI, percutaneous coronary intervention; RCT, randomized clinical trial; RR, relative risk.
Studies included are limited to those that specifically included patients with CS associated with AMI.
P value reported as nonsignificant.
Acute Physiology and Chronic Health Evaluation (APACHE) II score provides an estimate of intensive care unit mortality; range, 0-74; higher scores are associated with increased mortality.53
Extracorporeal Life Support
Venoarterial extracorporeal membrane oxygenation (VA-ECMO) is a mechanical circulatory support system that can be inserted percutaneously and provides complete cardiopulmonary hemodynamic support. De-oxygenated blood is drained from a central vein via a large bore cannula and cycled through an external oxygenator and centrifugal or rotational blood pump. Oxygenated blood is returned to a central artery via large bore cannula. VA-ECMO can rapidly stabilize hemodynamics by increasing aortic blood flow and organ perfusion pressure, which facilitates recovery of end-organ function. However, VA-ECMO can increase LV afterload and worsen pulmonary edema. To reduce LV end-diastolic pressure and pulmonary edema, concomitant unloading of the LV can be done using either an IABP or microaxial LVAD, although these strategies have yet to be compared via RCT.54 Adverse effects of VA-ECMO include acute kidney injury (55.6%), clinically significant bleeding (40.8%), lower extremity ischemia (16.9%), lower extremity amputation (4.7%), and stroke (5.9%).55
Management of Mechanical Complications
Immediate management of mechanical complications of AMI, such as interventricular septum rupture, papillary muscle rupture with acute mitral regurgitation, and LV free wall rupture, should involve management of CS as well as intervention to correct the structural abnormality. Both American and European practice guidelines state that IABP can be considered to reduce LV afterload and attempt hemodynamic stabilization in patients with mechanical complications of AMI, including interventricular septal rupture and papillary muscle rupture.26,27 For patients with ventricular septal rupture, emergency surgical repair is necessary, and the surgical mortality rate ranges from 20% to 87%, especially among patients with CS.56-59 For patients with papillary muscle rupture, definitive mitral valve surgery should be considered. Although emergency mitral valve replacement is associated with a mortality rate of approximately 20%, observational data suggest surgery improves survival and ventricular function compared with medical therapy alone.26,60 Delay to operation is associated with an increased risk of further myocardial injury, organ failure, and death.26,60 For patients who are not candidates for surgery, observational data suggest that percutaneous repair of ventricular septal defects and acute mitral regurgitation provide mortality benefit that is comparable to surgery.61-63
Limitations
This review has some limitations. First, relatively few randomized trials of CS after AMI have been performed. Observational studies are associated with selection bias and confounding by treatment indication. For example, the association between an exposure (percutaneous mechanical circulatory support) and the outcome (mortality) can be distorted by the presence of an indication for the exposure that is the true cause of the outcome. Second, this review was not a systematic review and quality of included evidence was not formally evaluated. Third, it is possible that this review missed some relevant published papers.
Conclusions
Cardiogenic shock occurs in up to 10% of patients immediately after AMI and is associated with mortality rates of nearly 40% at 30 days and 50% mortality at 1 year. Current evidence and clinical practice guidelines support immediate revascularization of the infarct-related coronary artery as the primary therapy for CS following acute myocardial infarction.27,28
Supplementary Material
Footnotes
Conflict of Interest Disclosures: Dr Morrow reported receiving research grants to Brigham and Women’s Hospital from Abbott Laboratories, Amgen, Anthos, Arca Biopharma, AstraZeneca, Bayer Healthcare, Daiichi Sankyo, Eisai, and Merck; grants from Novartis, Pfizer, Quark, Regeneron, Roche Diagnostics, and Siemens; personal fees for consulting unrelated to shock from Bayer Healthcare, Merck, Novartis, and Roche Diagnostics; and personal fees from InCarda for data and safety monitoring board membership outside the submitted work and being a member of the TIMI Study Group, which has received institutional research grant support through Brigham and Women's Hospital from Abbott, Amgen, Anthos Therapeutics, AstraZeneca, Bayer HealthCare Pharmaceuticals, Daiichi-Sankyo, Eisai, Intarcia, MedImmune, Merck, Novartis, Pfizer, Quark Pharmaceuticals, Regeneron Pharmaceuticals, Roche, Siemens Healthcare Diagnostics, The Medicines Company, and Zora Biosciences. Dr Proudfoot reported receiving grants from Abbott Vascular during the conduct of the study. Dr Rao reported receiving institutional research funding from Bayer and the National Heart, Lung, and Blood Institute outside the submitted work. No other disclosures were reported.
Contributor Information
Marc D. Samsky, Duke Clinical Research Institute, Duke University School of Medicine, Durham, North Carolina.
David A. Morrow, TIMI Study Group, Cardiovascular Division, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts.
Alastair G. Proudfoot, Perioperative Medicine Department, Barts Heart Centre, St Bartholomew’s Hospital, London, United Kingdom; Clinic For Anesthesiology & Intensive Care, Charité-Universitätsmedizin Berlin corporate member of Free University Berlin and Humboldt University Berlin, Germany; Department of Anaesthesiology & Intensive Care, German Heart Centre Berlin, Germany.
Judith S. Hochman, Cardiovascular Clinical Research Center, Division of Cardiology, Department of Medicine, NYU Grossman School of Medicine, New York, New York.
Holger Thiele, Department of Internal Medicine/Cardiology, Leipzig Heart Institute, Heart Center Leipzig at University of Leipzig, Leipzig, Germany.
Sunil V. Rao, Duke Clinical Research Institute, Duke University School of Medicine, Durham, North Carolina.
REFERENCES
- 1.Harjola VP, Lassus J, Sionis A, et al. ; CardShock Study Investigators; GREAT network. Clinical picture and risk prediction of short-term mortality in cardiogenic shock. Eur J Heart Fail. 2015;17(5):501–509. doi: 10.1002/ejhf.260 [DOI] [PubMed] [Google Scholar]
- 2.Fox KA, Steg PG, Eagle KA, et al. ; GRACE Investigators. Decline in rates of death and heart failure in acute coronary syndromes, 1999-2006. JAMA. 2007;297(17):1892–1900. doi: 10.1001/jama.297.17.1892 [DOI] [PubMed] [Google Scholar]
- 3.Samsky M, Krucoff M,Althouse AD, et al. Clinical and regulatory landscape for cardiogenic shock: a report from the Cardiac Safety Research Consortium ThinkTank on cardiogenic shock. Am Heart J. 2020;219:1–8. doi: 10.1016/j.ahj.2019.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Samsky MD, Krucoff MW, Morrow DA, et al. Cardiac safety research consortium “shock II” think tank report: Advancing practical approaches to generating evidence for the treatment of cardiogenic shock. Am Heart J. 2020;230:93–97. doi: 10.1016/j.ahj.2020.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thiele H, Ohman EM, de Waha-Thiele S, Zeymer U, Desch S. Management of cardiogenic shock complicating myocardial infarction: an update 2019. Eur Heart J. 2019;40(32):2671–2683. doi: 10.1093/eurheartj/ehz363 [DOI] [PubMed] [Google Scholar]
- 6.Thiele H, Akin I, Sandri M, et al. ; CULPRIT-SHOCK Investigators. One-year outcomes after PCI strategies in cardiogenic shock. N Engl J Med. 2018;379(18):1699–1710. doi: 10.1056/NEJMoa1808788 [DOI] [PubMed] [Google Scholar]
- 7.Thiele H, Akin I, Sandri M, et al. ; CULPRIT-SHOCK Investigators. PCI strategies in patients with acute myocardial infarction and cardiogenic shock. N Engl J Med. 2017;377(25):2419–2432. doi: 10.1056/NEJMoa1710261 [DOI] [PubMed] [Google Scholar]
- 8.Thiele H,Zeymer U, Neumann FJ, et al. ; IABP-SHOCK II Trial Investigators. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med. 2012;367(14):1287–1296. doi: 10.1056/NEJMoa1208410 [DOI] [PubMed] [Google Scholar]
- 9.Hochman JS, Sleeper LA, Webb JG, et al. ; SHOCK Investigators. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. N Engl J Med. 1999;341(9):625–634. doi: 10.1056/NEJM199908263410901 [DOI] [PubMed] [Google Scholar]
- 10.Hochman JS, Sleeper LA, White HD, et al. ; SHOCK Investigators. One-year survival following early revascularization for cardiogenic shock. JAMA. 2001;285(2):190–192. doi: 10.1001/jama.285.2.190 [DOI] [PubMed] [Google Scholar]
- 11.Hochman JS, Buller CE, Sleeper LA, et al. Cardiogenic shock complicating acute myocardial infarction—etiologies, management and outcome: a report from the SHOCK Trial Registry. J Am Coll Cardiol. 2000;36(3)(suppl A):1063–1070. doi: 10.1016/S0735-1097(00)00879-2 [DOI] [PubMed] [Google Scholar]
- 12.Menon V, Slater JN, White HD, Sleeper LA, Cocke T, Hochman JS. Acute myocardial infarction complicated by systemic hypoperfusion without hypotension: report of the SHOCK trial registry. Am J Med. 2000;108(5):374–380. doi: 10.1016/S0002-9343(00)00310-7 [DOI] [PubMed] [Google Scholar]
- 13.Hochman JS. Cardiogenic shock complicating acute myocardial infarction: expanding the paradigm. Circulation. 2003;107(24):2998–3002. doi: 10.1161/01.CIR.0000075927.67673.F2 [DOI] [PubMed] [Google Scholar]
- 14.Webb JG, Sleeper LA, Buller CE, et al. Implications of the timing of onset of cardiogenic shock after acute myocardial infarction: a report from the SHOCK Trial Registry. J Am Coll Cardiol. 2000;36(3)(suppl A):1084–1090. doi: 10.1016/S0735-1097(00)00876-7 [DOI] [PubMed] [Google Scholar]
- 15.Jacobs AK, Leopold JA, Bates E, et al. Cardiogenic shock caused by right ventricular infarction: a report from the SHOCK registry. J Am Coll Cardiol. 2003;41(8):1273–1279. doi: 10.1016/S0735-1097(03)00120-7 [DOI] [PubMed] [Google Scholar]
- 16.Baran DA, Grines CL, Bailey S, et al. SCAI clinical expert consensus statement on the classification of cardiogenic shock. Catheter Cardiovasc Interv. 2019;94(1):29–37. doi: 10.1002/ccd.28329 [DOI] [PubMed] [Google Scholar]
- 17.Ceglarek U, Schellong P, Rosolowski M, et al. The novel cystatin C, lactate, interleukin-6, and N-terminal pro-B-type natriuretic peptide (CLIP)-based mortality risk score in cardiogenic shock after acute myocardial infarction. Eur Heart J. 2021;42(24):2344–2352. doi: 10.1093/eurheartj/ehab110 [DOI] [PubMed] [Google Scholar]
- 18.Tehrani BN, Truesdell AG, Sherwood MW, et al. Standardized team-based care for cardiogenic shock. J Am Coll Cardiol. 2019;73(13):1659–1669. doi: 10.1016/j.jacc.2018.12.084 [DOI] [PubMed] [Google Scholar]
- 19.Basir MB, Kapur NK, Patel K, et al. ; National Cardiogenic Shock Initiative Investigators. Improved outcomes associated with the use of shock protocols: updates from the National Cardiogenic Shock Initiative. Catheter Cardiovasc Interv. 2019;93(7):1173–1183. doi: 10.1002/ccd.28307 [DOI] [PubMed] [Google Scholar]
- 20.Taleb I, Koliopoulou AG,Tandar A, et al. Shock team approach in refractory cardiogenic shock requiring short-term mechanical circulatory support: a proof of concept. Circulation. 2019;140(1):98–100. doi: 10.1161/CIRCULATIONAHA.119.040654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Diepen S, Hochman JS, Stebbins A, Alviar CL, Alexander JH, Lopes RD. Association between delays in mechanical ventilation initiation and mortality in patients with refractory cardiogenic shock. JAMA Cardiol. 2020;5(8):965–967. doi: 10.1001/jamacardio.2020.1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nalluri N, Patel NJ, Atti V, Kumar V, Basir MB, O’Neill WW. Temporal trends in utilization of right-sided heart catheterization among percutaneous ventricular assist device recipients in acute myocardial infarction complicated by cardiogenic shock. Am J Cardiol. 2018;122(12):2014–2017. doi: 10.1016/j.amjcard.2018.08.065 [DOI] [PubMed] [Google Scholar]
- 23.Ibanez B, James S, Agewall S, et al. ; ESC Scientific Document Group. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the task force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society ofCardiology (ESC). Eur Heart J. 2018;39(2):119–177 doi: 10.1093/eurheartj/ehx393 [DOI] [PubMed] [Google Scholar]
- 24.Henry TD, Tomey MI, Tamis-Holland JE, et al. ; American Heart Association Interventional Cardiovascular Care Committee of the Council on Clinical Cardiology; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular and Stroke Nursing. Invasive management of acute myocardial infarction complicated by cardiogenic shock: a scientific statement from the American Heart Association. Circulation. 2021;143(15):e815–e829. doi: 10.1161/CIR.0000000000000959 [DOI] [PubMed] [Google Scholar]
- 25.Elbadawi A, Elgendy IY, Mahmoud K, et al. Temporal trends and outcomes of mechanical complications in patients with acute myocardial infarction. JACC Cardiovasc interv. 2019;12(18):1825–1836. doi: 10.1016/j.jcin.2019.04.039 [DOI] [PubMed] [Google Scholar]
- 26.O’Gara PT, Kushner FG, Ascheim DD, et al. ; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines.2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127(4):e362–e425. doi: 10.1161/CIR.0b013e3182742c84 [DOI] [PubMed] [Google Scholar]
- 27.Collet J-P, Thiele H, Barbato E, et al. 2020 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: the task force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2020;42(14):1289–1367. doi: 10.1093/eurheartj/ehaa575 [DOI] [PubMed] [Google Scholar]
- 28.Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC guideline for the management of patients with non-st-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;64(24):e139–e228. doi: 10.1016/j.jacc.2014.09.017 [DOI] [PubMed] [Google Scholar]
- 29.Ibanez B, James S, Agewall S, et al. ; ESC Scientific Document Group. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the task force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39(2):119–177. doi: 10.1093/eurheartj/ehx393 [DOI] [PubMed] [Google Scholar]
- 30.McDonagh TA, Metra M, Adamo M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42(36):3599–3726. doi: 10.1093/eurheartj/ehab368 [DOI] [PubMed] [Google Scholar]
- 31.Wong SC, Sanborn T, Sleeper LA, et al. Angiographic findings and clinical correlates in patients with cardiogenic shock complicating acute myocardial infarction: a report from the SHOCK Trial Registry. J Am Coll Cardiol. 2000;36(3)(suppl A):1077–1083. doi: 10.1016/S0735-1097(00)00873-1 [DOI] [PubMed] [Google Scholar]
- 32.Levine GN, Bates ER, Blankenship JC, et al. 2015 ACC/AHA/SCAI focused update on primary percutaneous coronary intervention for patients with ST-elevation myocardial infarction: an update of the 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention and the 2013 ACCF/AHA guideline for the management of ST-Elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation. 2016;133(11):1135–1147. doi: 10.1161/CIR.0000000000000336 [DOI] [PubMed] [Google Scholar]
- 33.De Backer D, Biston P, Devriendt J, et al. ; SOAP II Investigators. Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med. 2010;362(9):779–789. doi: 10.1056/NEJMoa0907118 [DOI] [PubMed] [Google Scholar]
- 34.Levy B, Clere-Jehl R, Legras A, et al. Epinephrine versus norepinephrine for cardiogenic shock after acute myocardial infarction. J Am Coll Cardiol. 2018;72(2):173–182. doi: 10.1016/j.jacc.2018.04.051 [DOI] [PubMed] [Google Scholar]
- 35.Wayangankar SA, Bangalore S, McCoy LA, et al. Temporal trends and outcomes of patients undergoing percutaneous coronary interventions for cardiogenic shock in the setting of acute myocardial infarction: a report from the Cath PCI Registry. JACC Cardiovasc Interv. 2016;9(4):341–351. doi: 10.1016/j.jcin.2015.10.039 [DOI] [PubMed] [Google Scholar]
- 36.Bregman D, Nichols AB, Weiss MB, Powers ER, Martin EC, Casarella WJ. Percutaneous intraaortic balloon insertion. Am J Cardiol. 1980;46(2):261–264. doi: 10.1016/0002-9149(80)90067-3 [DOI] [PubMed] [Google Scholar]
- 37.Amin AP, Spertus JA, Curtis JP, et al. The evolving landscape of impella use in the united states among patients undergoing percutaneous coronary intervention with mechanical circulatory support. Circulation. 2020;141(4):273–284. doi: 10.1161/CIRCULATIONAHA.119.044007 [DOI] [PubMed] [Google Scholar]
- 38.Helgestad OKL, Josiassen J, Hassager C, et al. Contemporary trends in use of mechanical circulatory support in patients with acute MI and cardiogenic shock. Open Heart. 2020;7(1):e001214. doi: 10.1136/openhrt-2019-001214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brener M, Rosenblum H, Ranard LS, et al. Temporal trends in mechanical circulatory support use and door-to-unloading time in a stemi population over nearly a decade. J Am Coll Cardiol. 2020;75(11)(suppl 1):137. doi: 10.1016/S0735-1097(20)30764-6 [DOI] [Google Scholar]
- 40.Scholz KH, Maier SKG, Maier LS, et al. Impact of treatment delay on mortality in ST-segment elevation myocardial infarction (STEMI) patients presenting with and without haemodynamic instability: results from the German prospective, multicentre FITT-STEMI trial. Eur Heart J. 2018;39(13):1065–1074. doi: 10.1093/eurheartj/ehy004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shah M, Patnaik S, Patel B, et al. Trends in mechanical circulatory support use and hospital mortality among patients with acute myocardial infarction and non-infarction related cardiogenic shock in the United States. Clin Res Cardiol. 2018;107(4):287–303. doi: 10.1007/s00392-017-1182-2 [DOI] [PubMed] [Google Scholar]
- 42.Burkhoff D, Sayer G, Doshi D, Uriel N. Hemodynamics of mechanical circulatory support. J Am Coll Cardiol. 2015;66(23):2663–2674. doi: 10.1016/j.jacc.2015.10.017 [DOI] [PubMed] [Google Scholar]
- 43.Tehrani BN, Basir MB, Kapur NK. Acute myocardial infarction and cardiogenic shock: should we unload the ventricle before percutaneous coronary intervention? Prog Cardiovasc Dis. 2020;63(5):607–622. doi: 10.1016/j.pcad.2020.09.001 [DOI] [PubMed] [Google Scholar]
- 44.Ouweneel DM, Eriksen E, Sjauw KD, et al. Percutaneous mechanical circulatory support versus intra-aortic balloon pump in cardiogenic shock after acute myocardial infarction. J Am Col Cardiol. 2017;69(3):278–287. doi: 10.1016/j.jacc.2016.10.022 [DOI] [PubMed] [Google Scholar]
- 45.Basir MB, Schreiber TL, Grines CL, et al. Effect of early initiation of mechanical circulatory support on survival in cardiogenic shock. Am J Cardiol. 2017;119(6):845–851. doi: 10.1016/j.amjcard.2016.11.037 [DOI] [PubMed] [Google Scholar]
- 46.Dhruva SS, Ross JS, Mortazavi BJ, et al. association of use of an intravascular microaxial left ventricular assist device vs intra-aortic balloon pump with in-hospital mortality and major bleeding among patients with acute myocardial infarction complicated by cardiogenic shock. JAMA. 2020;323(8):734–745.doi: 10.1001/jama.2020.0254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Khera R, Cram P, Lu X, et al. Trends in the use of percutaneous ventricular assist devices: analysis of national inpatient sample data, 2007 through 2012. JAMA Intern Med. 2015;175(6):941–950. doi: 10.1001/jamainternmed.2014.7856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schrage B, Ibrahim K, Loehn T, et al. Impella support for acute myocardial infarction complicated by cardiogenic shock. Circulation. 2019;139(10):1249–1258. doi: 10.1161/CIRCULATIONAHA.118.036614 [DOI] [PubMed] [Google Scholar]
- 49.Urban P, Stauffer JC, Bleed D, et al. A randomized evaluation of early revascularization to treat shock complicating acute myocardial infarction: the (Swiss) Multicenter Trial of Angioplasty for Shock-(S)MASH. Eur Heart J. 1999;20(14):1030–1038. doi: 10.1053/euhj.1998.1353 [DOI] [PubMed] [Google Scholar]
- 50.Prondzinsky R, Lemm H, Swyter M, et al. Intra-aortic balloon counterpulsation in patients with acute myocardial infarction complicated by cardiogenic shock: the prospective, randomized IABP SHOCK Trial for attenuation of multiorgan dysfunction syndrome. Crit Care Med. 2010;38(1):152–160. doi: 10.1097/CCM.0b013e3181b78671 [DOI] [PubMed] [Google Scholar]
- 51.Alexander JH, Reynolds HR, Stebbins AL, et al. ; TRIUMPH Investigators. Effect of tilarginine acetate in patients with acute myocardial infarction and cardiogenic shock: the TRIUMPH randomized controlled trial. JAMA. 2007;297(15):1657–1666. doi: 10.1001/jama.297.15.joc70035 [DOI] [PubMed] [Google Scholar]
- 52.Tousek P, Rokyta R, Tesarova J, et al. Routine upfront abciximab versus standard periprocedural therapy in patients undergoing primary percutaneous coronary intervention for cardiogenic shock: the PRAGUE-7 study: an open randomized multicentre study. Acute Card Care. 2011;13(3):116–122. doi: 10.3109/17482941.2011.567282 [DOI] [PubMed] [Google Scholar]
- 53.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818–829. doi: 10.1097/00003246-198510000-00009 [DOI] [PubMed] [Google Scholar]
- 54.Vallabhajosyula S, O'Horo JC, Antharam P, et al. Venoarterial extracorporeal membrane oxygenation with concomitant impella versus venoarterial extracorporeal membrane oxygenation for cardiogenic shock. ASAIO J. 2020;66(5):497–503. doi: 10.1097/MAT.0000000000001039 [DOI] [PubMed] [Google Scholar]
- 55.Cheng R, Hachamovitch R, Kittleson M, et al. Complications of extracorporeal membrane oxygenation for treatment of cardiogenic shock and cardiac arrest: a meta-analysis of 1866 adult patients. Ann Thorac Surg. 2014;97(2):610–616. doi: 10.1016/j.athoracsur.2013.09.008 [DOI] [PubMed] [Google Scholar]
- 56.Crenshaw BS, Granger CB, Birnbaum Y, et al. ; GUSTO-I (Global Utilization of Streptokinase and TPA for Occluded Coronary Arteries) Trial Investigators. Risk factors, angiographic patterns, and outcomes in patients with ventricular septal defect complicating acute myocardial infarction. Circulation. 2000;101(1):27–32. doi: 10.1161/01.CIR.101.1.27 [DOI] [PubMed] [Google Scholar]
- 57.Menon V, Webb JG, Hillis LD, et al. Outcome and profile of ventricular septal rupture with cardiogenic shock after myocardial infarction: a report from the SHOCK Trial Registry. J Am Coll Cardiol. 2000;36(3)(suppl A):1110–1116. doi: 10.1016/S0735-1097(00)00878-0 [DOI] [PubMed] [Google Scholar]
- 58.Prêetre R, Ye Q, Grünenfelder J, Lachat M, Vogt PR, Turina MI. Operative results of “repair” of ventricular septal rupture after acute myocardial infraction. Am J Cardiol. 1999;84(7):785–788. doi: 10.1016/S0002-9149(99)00438-5 [DOI] [PubMed] [Google Scholar]
- 59.Topaz O, Taylor AL. Interventricular septal rupture complicating acute myocardial infarction: from pathophysiologic features to the role of invasive and noninvasive diagnostic modalities in current management. Am J Med. 1992;93(6):683–688. doi: 10.1016/0002-9343(92)90203-N [DOI] [PubMed] [Google Scholar]
- 60.Tepe NA, Edmunds LH Jr. Operation for acute postinfarction mitral insufficiency and cardiogenic shock. J Thorac Cardiovasc Surg. 1985;89(4):525–530. doi: 10.1016/S0022-5223(19)38756-2 [DOI] [PubMed] [Google Scholar]
- 61.Jung RG, Simard T, Kovach C, et al. Transcatheter mitral valve repair in cardiogenic shock and mitral regurgitation: a patient-level, multicenter analysis. JACC Cardiovasc Interv. 2021;14(1):1–11. doi: 10.1016/j.jcin.2020.08.037 [DOI] [PubMed] [Google Scholar]
- 62.Thiele H, Kaulfersch C, Daehnert I, et al. Immediate primary transcatheter closure of postinfarction ventricular septal defects. Eur Heart J. 2009;30(1):81–88. doi: 10.1093/eurheartj/ehn524 [DOI] [PubMed] [Google Scholar]
- 63.Schlotter F, de Waha S, Eitel I, Desch S, Fuernau G, Thiele H. Interventional post-myocardial infarction ventricular septal defect closure: a systematic review of current evidence. EuroIntervention. 2016;12(1):94–102. doi: 10.4244/EIJV12I1A17 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.