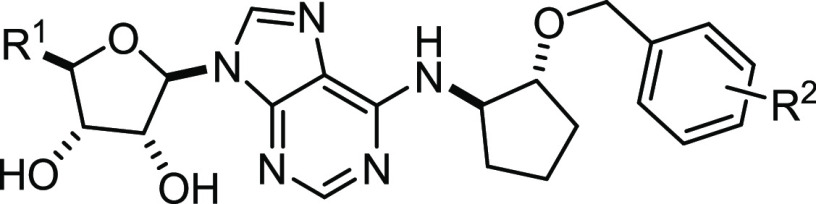
Abstract
A series of benzyloxy and phenoxy derivatives of the adenosine receptor agonists N6-cyclopentyl adenosine (CPA) and N6-cyclopentyl 5′-N-ethylcarboxamidoadenosine (CP-NECA) were synthesized, and their potency and selectivity were assessed. We observed that the most potent were the compounds with a halogen in the meta position on the aromatic ring of the benzyloxy- or phenoxycyclopentyl substituent. In general, the NECA-based compounds displayed greater A1R selectivity than the adenosine-based compounds, with N6-2-(3-bromobenzyloxy)cyclopentyl-NECA and N6-2-(3-methoxyphenoxy)cyclopentyl-NECA showing ∼1500-fold improved A1R selectivity compared to NECA. In addition, we quantified the compounds’ affinity and kinetics of binding at both human and rat A1R using a NanoBRET binding assay and found that the halogen substituent in the benzyloxy- or phenoxycyclopentyl moiety seems to confer high affinity for the A1R. Molecular modeling studies suggested a hydrophobic subpocket as contributing to the A1R selectivity displayed. We believe that the identified selective potent A1R agonists are valuable tool compounds for adenosine receptor research.
Introduction
The adenosine A1 receptor (A1R) is a G protein-coupled receptor (GPCR) that belongs to the adenosine receptor family consisting of four receptor subtypes (A1R, A2AR, A2BR, and A3R). All four receptor subtypes are nonselectively activated by the endogenous ligand adenosine, a naturally occurring purine nucleoside. The adenosine receptors are widely expressed in the body and therefore implicated in various pathological conditions including cancer; sleep regulation; and cardiovascular, neurodegenerative, and inflammatory diseases.1−9 The wide expression pattern has led to the reality that despite more than four decades of intense medicinal research, very few compounds have actually made it to the clinic due to unacceptable side effects based on insufficient subtype selectivity and/or low efficacy, leaving a big untapped need for subtype-selective compounds.10,11
The selective activation of the A1R, in particular, is a very promising strategy for the treatment of glaucoma, type 2 diabetes mellitus, pain, epilepsy, heart arrhythmias, and cerebral ischemia, in which there are clear unmet clinical needs that could be addressed with novel more selective therapeutics.11,12 Although all members of the adenosine receptor family are activated by endogenous adenosine, the A1R and A3R receptors are predominantly Gi/o-coupled, while the A2AR and A2BR are predominantly Gs-coupled. The classical pathway following Gi/o activation is the inhibition of adenylyl cyclase (AC) and subsequent inhibition of 3′,5′-cyclic adenosine monophosphate (cAMP) accumulation in the cell, while activation of Gs activates AC, resulting in the promotion of cAMP accumulation.
Several potent and A1R-selective agonists are based on the endogenous adenosine scaffold. Substitution at the purine C-2 position, e.g., with chloride, and at the N6 position with cycloalkyl- and bicycloalkyl groups has led to potent and A1R-selective agonists.11−15 In addition, the ribose moiety has been the focus of synthetic modifications in AR agonist development. The ribose C-5′ position tolerates certain substitutions, such as a 5′-carboxamido group in the prototypical, very potent, albeit not highly subtype-selective AR agonist 5′-N-ethylcarboxyamidoadenosine (NECA). Small 5′-chlorine substituents have also been used, and it was shown that they, together with N6-bicycloalkyl groups, lead to high-affinity and highly selective human A1R agonists, which have antinociceptive effects in mice without affecting motor or cardiovascular functions.16,17 More bulky pyrazole groups have also been employed at this C-5′ position and yielded potent and selective A1R agonists that showed analgesic effects in mice.15 Other successful selective and CNS active A1R agonist examples feature conformationally constrained ribose ring systems.14 Alternatively, non-nucleoside 3,5-dicyanopyridines have been synthetically optimized to yield potent and A1R-selective full agonists, which have also been developed into PET tracers very recently.18 Rather than modulating receptor activity by orthosteric exogenous agonists, Christopoulos and collaborators have recently presented MIPS521, a positive allosteric modulator of the A1R, with which they were able to show in vivo analgesic efficacy in rats.19 Their cryo-EM structural study of the human A1R bound to adenosine, MIPS521, and a Gi2 protein heterotrimer (PDB code 7LD3) revealed the allosteric binding pocket at the lipid interface that could spark structure-based drug design campaigns.
We have previously reported the adenosine-based potent and highly A1R-selective full agonist BnOCPA (Chart 1), which emerged from a structure–activity relationship (SAR) study with respect to cyclic and bicyclic purine N6 substituents.13 Our SAR study also showed that the synthetic NECA derivatives, such as BnOCP-NECA (Chart 1), were generally less subtype-selective than the adenosine ones. BnOCPA and BnOCP-NECA are extension derivatives of the prototypical, non-subtype-selective AR agonist N6-cyclopentyl adenosine (CPA). The N6-hydroxycyclopentyl moiety is present in the known A1R-selective partial agonist CVT-3619 (later renamed GS 9667) and full agonist GR79236X. It should be noted that the stereochemical configuration of the N6-hydroxycyclopentyl group in GR79236X is opposite to that in CVT-3619 and BnOCPA (Chart 1). Both GR79236X and CVT-3619 are able to increase insulin sensitivity and thus have been evaluated in clinical trials for the treatment of type II diabetes; however, their development was not successful and later discontinued.20,21 Appending a benzyl group to the N6-hydroxylcyclopentyl moiety, we have found previously that BnOCPA retained high potency at A1R and displayed very high A1R selectivity compared to the nonbenzylated congener.13
Chart 1. Known N6-Cyclopentyl Adenosine A1R Agonists, Previously Synthesized A1R Agonists, and Derivatives Presented in This Work.

Subsequently, we demonstrated that BnOCPA was able to specifically activate Gαob protein subtype-mediated signaling, which translated into potent in vivo analgesia without causing sedation, bradycardia, hypotension, or respiratory depression.22 Molecular dynamics (MD) simulations using the cryo-EM structure of the active adenosine-bound A1R-heterotrimeric Gi2 protein complex (PDB code 6D9H(23)) proposed four binding modes of BnOCPA22 due to the high flexibility of the N6-appended benzyloxy group.24
Based on these molecular modeling studies, we have designed a series of adenosine- and NECA-based compounds with extended N6-benzyloxy- and N6-phenoxycyclopentyl substituents (Chart 1) with the aim of improving the potency at A1R while maintaining or improving the subtype selectivity. To test the potency, selectivity, and affinity of the designed compounds at the adenosine receptors, we have employed the cAMP accumulation and NanoBRET binding assays previously validated and employed at ARs.25 We explored subtype selectivity at human A1R, A2AR, A2BR, and A3R in mammalian CHO-K1 cells and confirmed the binding of the compounds at both human and rat A1R in HEK293 cells. Kinetic studies were performed to dissect the binding properties of the compounds to hA1R and rA1R and outline their structure–kinetic relationship (SKR). Finally, we have also used MD modeling to evaluate the binding pose of some of the agonists and validated the findings using mutagenesis. Together, this approach has identified novel adenosine- and NECA-based derivatives with improved potency, selectivity, and affinity at the A1R receptor. Hence, these compounds constitute valuable tools for cellular studies of the A1R receptor and show interesting therapeutic promise.
Results and Discussion
Chemistry
Our initial synthetic strategy was designed for keeping the route as concise as possible and entailed a projected O-alkylation of ribose-protected N6-hydroxycyclopentyl adenosine and NECA precursors. After exploring different O-alkylation protocols and ribose hydroxyl protecting groups, we abandoned this synthetic plan, as our attempts resulted in low conversions, trace amounts of desired products, and many side products stemming from the loss or migration of protecting groups, elimination reactions, and N-alkylation of amine and amide groups (Scheme S1).
We therefore adapted our strategy and carried out the O-alkylation on N-protected (1R,2R)-2-aminocyclopentanol 1(26) (Scheme 1). Under optimized conditions, treating a mixture of 1 and benzyl bromide (1 equiv) in THF at 0 °C with sodium hydride (2 equiv) yielded 58% of desired 2a after 4 h. It was important to monitor this reaction carefully, as after a certain time (2–4 h), side products started to emerge that diminished the isolated yields. These optimized conditions were applied for the preparation of benzyloxycyclopentyl intermediates 2b–i, which were isolated in moderate to very good yields (30–91%).
Scheme 1. Synthesis of Benzyloxycyclopentyl and Phenoxycyclopentyl Amine Building Blocks.
Reagents and conditions: (a) R-BnBr (1 equiv), NaH (2 equiv), THF, 0 °C, 2–4 h, 30–91%; (b) HCl (4 M in dioxane), 1,4-dioxane, rt, 4–18 h, 50%-quant; and (c) R-PhOH, PPh3, DIAD, THF, 0 °C to rt, 18 h, 51–62%.
For the introduction of the phenoxy substituents on the cyclopentyl ring, we first tosylated epimeric N-protected (1S,2R)-2-aminocyclopentanol 4 (p-TsCl, pyridine, rt, 24 h, 57% yield), which was followed by SN2-type substitution with phenoxide (Figure S1). However, the latter reaction required forcing conditions (phenol, K2CO3, DMF, 70 °C, 3 days) to obtain 5a with the desired (1R,2R) stereochemistry in acceptable 61% yield. We therefore sought a milder, more efficient method and hypothesized that the Mitsunobu reaction27 might allow us to directly access protected phenoxycyclopentyl amines 5 from 4 (Scheme 1). The Mitsunobu reaction is commonly used to convert primary and secondary alcohols to a variety of functional groups with inversion at the alcohol stereogenic center and requires an acidic nucleophile (e.g. carboxylic acids). In rare cases, phenols (pKa ∼9–10) were employed as nucleophiles, but to the best of our knowledge, cyclic secondary alcohols were not reported as Mitsunobu substrates to date. We were therefore pleased to find that adding diisopropyl azodicarboxylate (DIAD) to a solution of 4, phenol, and triphenylphosphine at 0 °C and subsequent stirring at room temperature overnight delivered 5a in 60% yield. 1H NMR spectra of 5a obtained via Mitsunobu reaction and of 5a isolated after SN2 phenoxide substitution of tosylated 4 were identical, confirming full inversion at C-1 during the Mitsunobu reaction (Figure S1). For comparison, reaction with epimeric 1 under identical Mitsunobu conditions yielded the C-1 epimer of 5a with a distinctively different 1H NMR spectrum. Overall, our new synthetic strategy efficiently produced phenoxycyclopentyl building block 5 in moderate to good yields. Clean removal of the Boc-protecting group from 2 and 5 was achieved with HCl in dioxane to deliver ammonium salts 3 and 6 (Scheme 1).
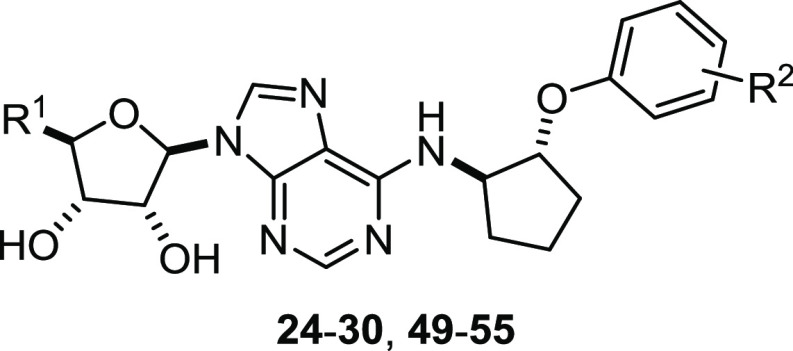
Nucleophilic aromatic substitution (SNAr) of 6-chloropurines 7 and 31 with amines 3 and 6 assembled the final agonist scaffolds (Scheme 2). Chloropurines 7 and 31 were synthesized starting from inosine using procedures adopted from Kotra et al.28 and Middleton et al.,29 with minor experimental modifications. Removal of the acetate groups was carried out with potassium carbonate in methanol at room temperature, while removal of the ribose acetonide group was accomplished with acetic acid in water at 80 °C, yielding final adenosine derivatives 15–30 and NECA derivatives 44–55 in high purity and sufficient quantity.
Scheme 2. Synthesis of Benzyloxy- and Phenoxycyclopentyl Adenosine and NECA Derivatives.
Reagents and conditions: (a) Ac2O, pyridine, rt 18 h, quant.; (b) SOCl2, DMF, CH2Cl2, 50 °C, 18 h, 86%; (c) 3b–i or 6a,c,e,f,h–j, NaHCO3, i-PrOH, 105 °C, 18 h, 36%-quant.; (d) K2CO3, MeOH, rt, 30 min, 36–93%; (e) K2CO3, H2O2 (aq. 30%), MeOH, 40 °C, 7 h, 83%; (f) 2,2-dimethoxypropane, p-TsOH, acetone, rt, 18 h, 32%; (g) TEMPO, DAIB, MeCN/H2O, rt, 18 h, 80%; (h) SOCl2, DMF, CH2Cl2, 50 °C, 18 h and then EtNH2 (2 M in THF), 0 °C to rt, 30 min, 41%; and (i) AcOH, H2O, 80 °C, 18 h, 43–73%.
In the adenosine series, we observed partial cleavage of the acetate groups during the SNAr reaction for some substrates, which led to complex but separable mixtures of desired nucleosides 8–14 and various deacetylated side products. We found that it was more convenient to take these crude mixtures into the subsequent deprotection step and isolate the fully deacetylated final products 15–30 (Scheme 2). Primary carboxamide derivative 18 was obtained from protected nitrile nucleoside 10 through hydrolysis with basic hydrogen peroxide in methanol at elevated temperatures.30
Biological Activity at the Human A1 Receptor
BnOCPA has previously been identified as a high-potency A1R-selective full agonist.13,22 Using insights from BnOCPA MD simulations22,24 and with the aim of further improving the A1R selectivity and potency, we designed extended BnOCPA derivatives 15–18. Their binding and activity at human A1R (hA1R) were then explored using both a NanoBRET binding assay and a cAMP accumulation assay, respectively (Table 1). To determine the A1R mediated Gi/o response, CHO-K1-A1R cells were co-stimulated for 30 min with 10 μM forskolin (which promotes cAMP production by activating adenylyl cyclase), and the test compounds 15–18 were added in a range of concentrations (10–13 to 10–4 M).
Table 1. Affinity (pKi) and Potency (pEC50) of Extended BnOCPA Derivatives at Human A1Ra.
| compd | R1 | R2 | pEC50 (hA1R)b | Emaxc | pKi (hA1R)d |
|---|---|---|---|---|---|
| BnOCPA | –CH2OH | H | 8.43 ± 0.09 | 51.49 ± 1.9 | 6.18 ± 0.09 |
| 15 | –CH2OH | p-i-Pr | 7.87 ± 0.16 | 51.95 ± 3.1 | 5.77 ± 0.08 |
| 16 | –CH2OH | p-t-Bu | 8.20 ± 0.13 | 54.59 ± 2.8 | 5.58 ± 0.10 |
| 17 | –CH2OH | p-CN | 7.40 ± 0.19 | 58.03 ± 4.2 | 5.85 ± 0.06 |
| 18 | –CH2OH | p-CONH2 | 7.71 ± 0.13 | 59.51 ± 4.1 | 5.98 ± 0.05 |
Data are the mean ± SEM of at least three independent repeats conducted in duplicate.
The negative logarithm of the agonist concentration required to produce a half-maximal inhibition response of the 10 μM forskolin-induced cAMP accumulation in CHO-K1-A1R cells.
The % maximal inhibition of cAMP accumulation for each agonist. Calculated as the % inhibition of the 10 μM forskolin response.
Binding affinity (pKi) determined through the NanoBRET binding assay in HEK293 cells stably expressing human Nluc-A1R. The resulting concentration-dependent decrease in NanoBRET ratio at 10 min was used to calculate pKi. Statistical significance (*p < 0.05) determined using one-way ANOVA and Dunnett’s post-test and presented as described by Curtis et al.31
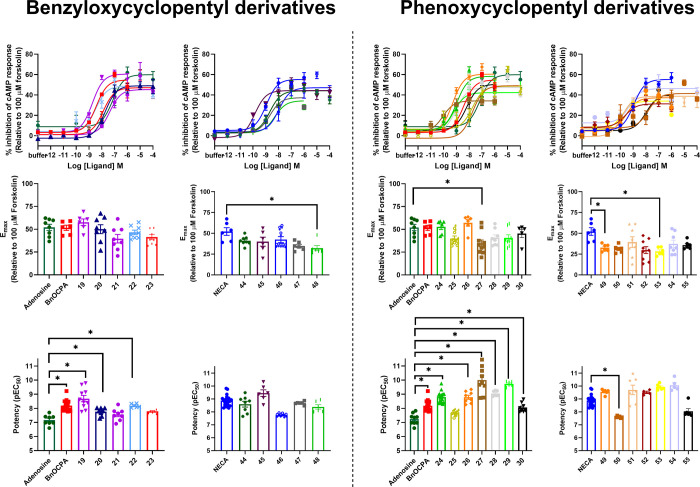
All four compounds were found to be agonists at the hA1R using the inhibiting forskolin-stimulated cAMP accumulation assay with equivalent Emax values to that of the full agonist BnOCPA. 16 showed the highest potency with pEC50 of 8.20 ± 0.13, which was similar to BnOCPA (pEC50 of 8.43 ± 0.09). 15–18 were further tested for their ability to displace the specific binding of CA200645, a fluorescent A1R/A3R antagonist, in HEK293 cells stably expressing an N-terminally tagged human Nanoluc-hA1R (Nluc-hA1R), as described previously.25 All four explored BnOCPA derivative compounds 15–18 displayed similar affinity for Nluc-hA1R with Ki in the range of 1–3 μM, which remained similar or lower than BnOCPA (Ki of 0.66 μM). Since none of derivatives 15–18 improved upon BnOCPA potency or affinity at the A1R, we have decided to not continue with these compounds further and instead designed a new series of compounds based on adenosine (19–30) and their structural analogs based on NECA (44–55). Full cAMP inhibition curves in the CHO-K1-hA1R cells were obtained as described above (Figure 1, Tables 2 and 3). Except for 27, 48, 49, and 53, which showed partial activity, all the tested compounds behaved as full agonists at the hA1R. 27 was the most potent (pEC50 of 10.0 ± 0.24), closely followed by 26, 45, 49, and 51–54. Furthermore, all these compounds displayed a higher potency than adenosine, NECA, or BnOCPA, making them very promising candidate compounds. It is interesting to note that, except for 49 and 53, the most potent compounds have a substituent in the meta position and, except for 26, 49 and 51, all have a halogen substituent. Therefore, it seems that a halogen in the meta position on the aromatic ring confers high potency at the hA1R. In addition, all most potent hA1R agonists except 45 feature a N6-phenoxycyclopentyl moiety.
Figure 1.
Efficacy and potency of synthetic benzyloxy- and phenoxycyclopentyl adenosine and NECA derivatives at A1R. cAMP response in CHO-K1 cells stably expressing human A1R in response to varying concentrations of AR ligands and 10 μM forskolin. Emax and pEC50 values for individual repeats are plotted at the bottom. Data are the mean ± SEM of at least three independent repeats conducted in duplicate. Statistical significance (*p < 0.05) determined using one-way ANOVA and Dunnett’s post-test, presented as described in ref (31).
Table 2. Affinity (pKi) and Potency (pEC50) of Synthetic Adenosine and NECA Benzyloxycyclopentyl Derivatives.
| compd | R1 | R2 | hA1R |
hA2AR |
hA2BR |
hA3R |
hA1R | rA1R | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pEC50a | Emaxb | pEC50a | Emaxc | pEC50a | Emaxc | pEC50a | Emaxb | pKid | pKid | |||
| adenosine | 7.16 ± 0.23 | 51.09 ± 4.9 | 7.60 ± 0.11 | 21.53 ± 0.9 | 7.28 ± 0.12 | 59.07 ± 2.9 | 7.87 ± 0.23 | 24.71 ± 2.5 | 6.09 ± 0.06 | 6.06 ± 0.05 | ||
| BnOCPA | –CH2OH | H | 8.43 ± 0.09* | 51.49 ± 1.9 | 4.95 ± 0.38* | 17.13 ± 4.6 | N.D.e | N.R.f | 6.18 ± 0.09 | 6.41 ± 0.06* | ||
| 19 | –CH2OH | m-OMe | 8.74 ± 0.10* | 57.41 ± 2.4 | N.D. | N.D. | 5.98 ± 0.47* | 9.52 ± 2.1* | 6.67 ± 0.10* | 6.55 ± 0.06* | ||
| 20 | –CH2OH | m-Br | 7.74 ± 0.13* | 51.44 ± 2.8 | N.R. | N.D. | 5.08 ± 0.26* | 12.59 ± 1.8* | 6.16 ± 0.10 | 6.13 ± 0.08 | ||
| 21 | –CH2OH | p-Br | 7.39 ± 0.16 | 44.25 ± 3.2 | N.D. | N.D. | N.R. | 5.94 ± 0.07 | 6.06 ± 0.07 | |||
| 22 | –CH2OH | o-Cl | 8.47 ± 0.24* | 44.66 ± 4.3 | 5.17 ± 0.33* | 18.55 ± 3.5 | 4.57 ± 0.12* | 96.93 ± 8.1* | N.R. | 6.56 ± 0.07* | 6.56 ± 0.04* | |
| 23 | –CH2OH | m-Cl | 7.88 ± 0.17 | 45.97 ± 3.3 | N.D. | N.D. | N.R. | 6.15 ± 0.07 | 6.43 ± 0.03* | |||
| NECA | 8.96 ± 0.11 | 50.11 ± 2.4 | 7.95 ± 0.26 | 22.03 ± 2.4 | 7.20 ± 0.07 | 68.12 ± 2.0 | 7.83 ± 0.26 | 34.34 ± 3.7 | 6.61 ± 0.06 | 6.38 ± 0.04 | ||
| 44 | –CONHEt | m-OMe | 8.67 ± 0.19 | 41.15 ± 3.3 | N.R. | 4.42 ± 0.16* | 73.07 ± 8.9 | 5.38 ± 0.09* | 46.56 ± 2.1 | 6.39 ± 0.08 | 6.11 ± 0.07* | |
| 45 | –CONHEt | m-Br | 9.85 ± 0.19 | 46.12 ± 5.2 | N.R. | N.D. | 5.56 ± 0.10* | 49.17 ± 2.4* | 6.54 ± 0.15 | 6.46 ± 0.07 | ||
| 46 | –CONHEt | p-Br | 7.97 ± 0.24 | 43.32 ± 4.5 | N.D. | N.D. | 5.82 ± 0.20* | 30.56 ± 2.8 | 6.15 ± 0.06* | 6.38 ± 0.03 | ||
| 47 | –CONHEt | o-Cl | 8.67 ± 0.15 | 34.67 ± 2.2 | 5.68 ± 0.32 | 20.34 ± 3.1 | 5.36 ± 0.08* | 87.58 ± 3.3 | 5.85 ± 0.13* | 43.92 ± 2.8 | 6.63 ± 0.07 | 6.85 ± 0.05* |
| 48 | –CONHEt | m-Cl | 8.29 ± 0.16 | 31.57 ± 2.1* | 5.04 ± 0.29* | 17.18 ± 2.9 | 4.72 ± 0.11* | 96.14 ± 6.8* | 5.83 ± 0.39* | 31.06 ± 5.7 | 6.49 ± 0.08 | 6.90 ± 0.05* |
The negative logarithm of the agonist concentration required to produce a half-maximal response in the cAMP accumulation assay in CHO-K1 cells stably expressing a human AR subtype. Forskolin is included in the assay (10 and 1 μM for A1R and A3R, respectively).
The % maximal inhibition of cAMP accumulation for each agonist. Forskolin is included in the assay (10 and 1 μM for A1R and A3R, respectively).
The % maximal accumulation of each agonist relative to 10 μM forskolin stimulation.
Binding affinity (pKi) determined through the NanoBRET binding assay in HEK293 cells stably expressing human or rat Nluc-A1R. The resulting concentration-dependent decrease in NanoBRET ratio at 10 min was used to calculate pKi.
N.D., not determined. Full dose–response curve was not feasible.
N.R., no response detected in the assay. All data are the mean ± SEM of at least three independent repeats conducted in duplicate. Statistical significance (*p < 0.05) determined using one-way ANOVA and Dunnett’s post-test, presented according to ref (31). Adenosine derivatives were compared to adenosine, while NECA derivatives were compared to NECA.
Table 3. Affinity (pKi) and Potency (pEC50) of Synthetic Adenosine and NECA Phenoxycyclopentyl Derivatives.
| compd | R1 | R2 | hA1R |
hA2AR |
hA2BR |
hA3R |
hA1R | rA1R | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pEC50a | Emaxb | pEC50a | Emaxc | pEC50a | Emaxc | pEC50a | Emaxb | pKid | pKid | |||
| adenosine | 7.16 ± 0.23 | 51.09 ± 4.9 | 7.60 ± 0.11 | 21.53 ± 0.9 | 7.28 ± 0.12 | 59.07 ± 2.9 | 7.87 ± 0.23 | 24.71 ± 2.5 | 6.09 ± 0.06 | 6.06 ± 0.05 | ||
| 24 | –CH2OH | H | 8.98 ± 0.14* | 52.71 ± 3.1 | N.D.e | 4.90 ± 0.14* | 64.19 ± 5.4 | 5.78 ± 0.42* | 17.83 ± 3.7 | 6.84 ± 0.06* | 6.60 ± 0.02* | |
| 25 | –CH2OH | p-t-Bu | 7.74 ± 0.28 | 42.14 ± 5.1 | N.D.f | N.D. | N.R. | 6.35 ± 0.08 | 6.70 ± 0.05* | |||
| 26 | –CH2OH | m-OMe | 9.28 ± 0.10* | 56.74 ± 2.4 | 5.24 ± 0.55* | 17.25 ± 5.0 | N.D. | N.R. | 6.61 ± 0.07* | 6.56 ± 0.06* | ||
| 27 | –CH2OH | m-Br | 10.0 ± 0.24* | 30.55 ± 3.3* | N.D. | 4.63 ± 0.12* | 82.53 ± 6.2 | N.R. | 7.55 ± 0.11* | 6.94 ± 0.08* | ||
| 28 | –CH2OH | o-Cl | 9.03 ± 0.19* | 44.15 ± 3.6 | 5.96 ± 0.28* | 17.01 ± 2.2 | 5.31 ± 0.09* | 96.77 ± 4.4* | 6.73 ± 0.42 | 13.42 ± 2.4* | 7.17 ± 0.06* | 7.28 ± 0.04* |
| 29 | –CH2OH | m-Cl | 9.21 ± 0.19* | 38.25 ± 3.0 | 6.26 ± 0.34 | 13.63 ± 2.1 | 5.29 ± 0.07* | 96.93 ± 8.1* | 6.81 ± 0.47 | 11.77 ± 2.3* | 7.19 ± 0.07* | 7.36 ± 0.03* |
| 30 | –CH2OH | p-Cl | 8.19 ± 0.18* | 45.06 ± 3.6 | 4.86 ± 0.58* | 15.15 ± 5.5 | N.D. | 6.88 ± 0.60 | 12.5 ± 3.2* | 6.23 ± 0.11 | 6.22 ± 0.06 | |
| NECA | 8.96 ± 0.11 | 50.11 ± 2.4 | 7.95 ± 0.26 | 22.03 ± 2.4 | 7.20 ± 0.07 | 68.12 ± 2.0 | 7.83 ± 0.26 | 34.34 ± 3.7 | 6.61 ± 0.06 | 6.38 ± 0.04 | ||
| 49 | –CONHEt | H | 9.53 ± 0.20 | 32.68 ± 2.5* | 5.48 ± 0.42* | 15.41 ± 3.0 | 6.04 ± 0.09* | 87.99 ± 3.7* | 7.17 ± 0.16 | 51.24 ± 3.4* | 7.30 ± 0.05* | 7.41 ± 0.03* |
| 50 | –CONHEt | p-t-Bu | 7.81 ± 0.41* | 33.66 ± 6.1 | 4.84 ± 0.30* | 20.11 ± 3.8 | 4.77 ± 0.08* | 96.35 ± 5.0* | 6.63 ± 0.19* | 39.85 ± 3.2 | 6.35 ± 0.07 | 6.85 ± 0.05* |
| 51 | –CONHEt | m-OMe | 9.88 ± 0.29 | 39.71 ± 5.9 | 5.20 ± 1.11* | 5.16 ± 3.1* | 5.10 ± 0.07* | 84.76 ± 3.3 | 5.56 ± 0.14* | 59.38 ± 4.0* | 7.26 ± 0.14* | 6.85 ± 0.06* |
| 52 | –CONHEt | m-Br | 9.62 ± 0.35 | 39.71 ± 5.9 | 4.58 ± 0.87* | 11.48 ± 6.7 | 5.37 ± 0.11* | 61.12 ± 3.3 | 5.52 ± 0.12* | 68.26 ± 3.8* | 7.05 ± 0.16* | 6.82 ± 0.10* |
| 53 | –CONHEt | o-Cl | 9.91 ± 0.23 | 28.65 ± 2.7* | 5.67 ± 0.46* | 14.69 ± 3.2 | 6.22 ± 0.09* | 80.91 ± 3.1 | 7.00 ± 0.19* | 41.6 ± 3.3 | 7.39 ± 0.04* | 7.60 ± 0.04* |
| 54 | –CONHEt | m-Cl | 9.28 ± 0.28 | 34.67 ± 2.1 | 5.86 ± 0.41 | 13.32 ± 2.6 | 6.01 ± 0.09* | 78.81 ± 3.4 | 6.79 ± 0.14* | 44.20 ± 2.6 | 7.43 ± 0.05* | 7.51 ± 0.06* |
| 55 | –CONHEt | p-Cl | 7.99 ± 0.15 | 35.07 ± 2.5 | 4.86 ± 0.32* | 25.01 ± 5.2 | 5.10 ± 0.09* | 83.73 ± 4.5 | 6.92 ± 0.17* | 47.16 ± 3.3 | 6.86 ± 0.10 | 7.08 ± 0.04* |
The negative logarithm of the agonist concentration required to produce a half-maximal response in the cAMP accumulation assay in CHO-K1 cells stably expressing a human AR subtype. Forskolin is included in the assay (10 and 1 μM for A1R and A3R, respectively).
The % maximal inhibition of cAMP accumulation for each agonist. Forskolin is included in the assay (10 and 1 μM for A1R and A3R, respectively).
The % maximal accumulation of each agonist relative to 10 μM forskolin stimulation.
Binding affinity (pKi) determined through the NanoBRET binding assay in HEK293 cells stably expressing human or rat Nluc-A1R. The resulting concentration-dependent decrease in NanoBRET ratio at 10 min was used to calculate pKi.
N.D., not determined. Full dose–response curve was not feasible.
N.R., no response detected in the assay. All data are the mean ± SEM of at least three independent repeats conducted in duplicate. Statistical significance (*p < 0.05) determined using one-way ANOVA and Dunnett’s post-test, presented according to ref (31). Adenosine derivatives were compared to adenosine, while NECA derivatives were compared to NECA.
Subtype Selectivity of Adenosine and NECA Derivatives
As the structural similarity between the orthosteric site of the four adenosine receptor subtypes often results in reduced selectivity of the compounds targeting them, we utilized CHO-K1 cells stably expressing human A2AR, A2BR, or A3R (hA2AR, hA2BR, or hA3R) and incubated them with increasing concentrations of the tested compounds (10–13 to 10–4 M) to measure the cAMP accumulation in the cells in response to the agonists. For the Gi/o-coupled hA3R, 1 μM forskolin was also included. This addition was not required for hA2AR or hA2BR since both are Gs-coupled and thus stimulate cAMP production. All the tested compounds displayed only weak efficacy at either the hA2AR or hA2BR, with many failing to generate full dose-dependent response curves at the concentrations tested, resulting in adenosine and NECA remaining as the only potent compounds at these two receptors (Tables 2 and 3). At the hA3R, the adenosine derivatives showed either a loss of efficacy or partial activity, while all the NECA-based compounds (44–55) behaved as full agonists, although with reduced potency compared to NECA alone. To further assess the compound selectivity, we have calculated the relative activity (RA) for all agonists at the different receptor subtypes (Figure 2).
Figure 2.
Adenosine and NECA derivatives show selectivity towards A1R subtype. Log(RA) values of AR ligands at human A1R, A2AR, A2BR, and A3R normalized to (A) NECA or (B) adenosine response at A1R.
Overall, all the compounds display at least partial selectivity for hA1R except adenosine that is close to being an equipotent agonist at all the receptors. From the adenosine-based derivatives, compounds 22, 23, 26, and 27 display the most hA1R selectivity, while compounds 28–30 also show activity at hA3R. However, with NECA itself being hA1R selective by ∼10-fold, it is the NECA-based compounds that display the highest hA1R selectivity, in particular compounds 44, 45, and 51–53. Compounds 45 and 51 are ∼1500-fold more hA1R selective than NECA itself, suggesting >10,000-fold selectivity overall.
Differences between Adenosine and NECA Derivatives
When comparing the adenosine and NECA analogs, the compounds based on NECA seem to be generally more potent at inhibiting cAMP accumulation at the hA1R receptor. Their potencies are either equivalent to or reduced compared to NECA at the other three AR subtypes. As a result, the NECA-based derivatives are more hA1R-selective than the adenosine derivatives. When we looked more closely at the adenosine and NECA-derived analog pairs, most of them displayed very similar selectivity across all AR subtypes (Tables 2 and 3). For example, adenosine-derived 29 and its NECA-derived analog 54 are both potent hA1R full agonists (pEC50 = 9.21 ± 0.19 and 9.28 ± 0.28, respectively), whereas 30 and 55 are relatively less potent dual hA1R and hA3R agonists (pEC50 = 8.19 ± 0.18, 7.99 ± 0.15 (hA1R) and 6.88 ± 0.60, 6.92 ± 0.17 (hA3R), respectively). Therefore, for these compounds, it seems to be the position of the substituent on the phenoxy group that has the most effect on compound selectivity. For some analog pairs, however, the patterns do not show such a close relationship. 27 and 52 are both potent agonists at hA1R (pEC50 = 10.0 ± 0.24 and 9.62 ± 0.35, respectively), but 52 also weakly activated hA3R (pEC50 = 5.52 ± 0.12), while 27 showed no response for this subtype. Consequently, in this case, the ribose C-5′ substituent group also affects the selectivity of the compounds, with the adenosine-derived compound being more hA1R selective.
Kinetics of Binding of Adenosine and NECA Derivatives at Human and Rat A1R
Since A1R agonists are promising compounds for the treatment of glaucoma, type 2 diabetes mellitus, pain, epilepsy, and cerebral ischemia, it is important to assess their binding properties at both human and rat A1R (rA1R), as the latter is commonly used as a model in preclinical studies.2,11,12 We have tested the compounds’ ability to displace the specific binding (at equilibrium) of CA200645 in HEK293 cells stably expressing human and rat Nluc-A1R (Figure 3, Tables 2 and 3).
Figure 3.
Binding affinity of AR ligands at human and rat A1R measured by NanoBRET. HEK293 cells stably expressing (A) human or (C) rat Nluc-A1R were treated with 20 nM CA200645 and increasing concentrations of unlabeled AR ligand. pKi values for individual repeats from (B) human A Nluc-A1R and (D) rat Nluc-A1R. Data are the mean ± SEM of at least three independent repeats conducted in duplicate. Statistical significance (*p < 0.05) determined using one-way ANOVA and Dunnett’s post-test, presented as described in ref (31).
The NanoBRET binding assay can also enable determination of real-time kinetics and affinities of the compound binding, as was previously described at the ARs.25,32−34 Values were derived using the ″kinetics of competitive binding″ model35 built into GraphPad Prism v9.1, enabling determinations of the compounds’ kon and koff values (Tables 4 and 5).
Table 4. Kinetics of Binding for Synthetic Adenosine and NECA Benzyloxycyclopentyl Derivatives to the Orthosteric Binding Site at Human and Rat A1R.
| compd | R1 | R2 | hA1R |
rA1R |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| kon (k3) ×105 (M–1 min–1)a | koff (k4) (min–1)b | pKdc | RT (min)d | kon (k3) ×105 (M–1 min–1)a | koff (k4) (min–1)b | pKdc | RT (min)d | |||
| adenosine | 1.65 ± 0.24 | 0.048 ± 0.002 | 6.53 ± 0.07 | 21.15 ± 0.84 | 0.52 ± 0.22 | 0.079 ± 0.007 | 5.71 ± 0.17 | 12.86 ± 1.10 | ||
| BnOCPA | –CH2OH | H | 1.47 ± 0.33 | 0.068 ± 0.002 | 6.30 ± 0.09 | 14.75 ± 0.56 | 1.57 ± 0.54 | 0.054 ± 0.007 | 6.40 ± 0.11 | 19.42 ± 2.21 |
| NECA | 2.04 ± 0.07 | 0.049 ± 0.005 | 6.63 ± 0.06 | 20.97 ± 1.98 | 2.29 ± 0.39 | 0.066 ± 0.008 | 6.53 ± 0.04 | 15.84 ± 2.05 | ||
| 19 | –CH2OH | m-OMe | 2.51 ± 0.36 | 0.041 ± 0.004 | 6.77 ± 0.02 | 24.64 ± 1.98 | 2.52 ± 0.31 | 0.083 ± 0.016 | 6.49 ± 0.03 | 13.22 ± 2.10 |
| 20 | –CH2OH | m-Br | 0.56 ± 0.12 | 0.080 ± 0.016 | 5.84 ± 0.02 | 14.84 ± 3.83 | 1.50 ± 0.29 | 0.097 ± 0.008 | 6.17 ± 0.09 | 10.56 ± 0.81 |
| 21 | –CH2OH | p-Br | 0.66 ± 0.02 | 0.065 ± 0.010 | 6.02 ± 0.06 | 16.72 ± 3.01 | 1.13 ± 0.09 | 0.066 ± 0.010 | 6.24 ± 0.03 | 16.05 ± 2.09 |
| 22 | –CH2OH | o-Cl | 2.75 ± 0.14 | 0.055 ± 0.012 | 6.74 ± 0.10 | 22.22 ± 6.21 | 5.07 ± 0.26 | 0.090 ± 0.015 | 6.77 ± 0.06 | 12.10 ± 2.06 |
| 23 | –CH2OH | m-Cl | 1.40 ± 0.08 | 0.051 ± 0.015 | 6.48 ± 0.09 | 24.12 ± 5.29 | 2.85 ± 0.22 | 0.060 ± 0.006 | 6.68 ± 0.06 | 17.13 ± 1.54 |
| 44 | –CONHEt | m-OMe | 1.12 ± 0.09 | 0.110 ± 0.024 | 6.04 ± 0.10 | 10.59 ± 2.34 | 1.96 ± 0.13 | 0.141 ± 0.018 | 6.15 ± 0.06 | 7.41 ± 0.82 |
| 45 | –CONHEt | m-Br | 0.83 ± 0.06 | 0.095 ± 0.011 | 5.94 ± 0.02 | 10.78 ± 1.28 | 2.95 ± 0.41 | 0.121 ± 0.012 | 6.38 ± 0.06 | 8.51 ± 0.82 |
| 46 | –CONHEt | p-Br | 1.01 ± 0.06 | 0.076 ± 0.007 | 6.13 ± 0.03 | 13.55 ± 1.36 | 2.14 ± 0.14 | 0.058 ± 0.003 | 6.56 ± 0.01 | 17.30 ± 1.02 |
| 47 | –CONHEt | o-Cl | 3.64 ± 0.33 | 0.044 ± 0.009 | 6.94 ± 0.08 | 25.70 ± 4.63 | 7.31 ± 0.61 | 0.046 ± 0.002 | 7.20 ± 0.03 | 21.98 ± 1.13 |
| 48 | –CONHEt | m-Cl | 1.80 ± 0.17 | 0.040 ± 0.006 | 6.67 ± 0.05 | 27.96 ± 5.57 | 7.54 ± 0.30 | 0.073 ± 0.004 | 7.02 ± 0.03 | 13.90 ± 0.72 |
kon (k3) for ligands as determined using NanoBRET binding assays using either human or rat Nluc-A1R expressing HEK 293 cells and determined through fitting with the ″kinetics of competitive binding″ model.35
koff (k4) for ligands determined as in footnote a.
Kinetic dissociation constant (pKd) for each ligand as determined from koff/kon.
Residence time of each ligand as determined by the reciprocal of the koff. All data are the mean ± SEM of at least three independent repeats conducted in duplicate.
Table 5. Kinetics of Binding for Synthetic Adenosine and NECA Phenoxycyclopentyl Derivatives to the Orthosteric Binding Site at Human and Rat A1R.
| compd | R1 | R2 | hA1R |
rA1R |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| kon (k3) ×105 (M–1 min–1)a | koff (k4) (min–1)b | pKdc | RT (min)d | kon (k3) ×105 (M–1 min–1)a | koff (k4) (min–1)b | pKdc | RT (min)d | |||
| 24 | –CH2OH | H | 4.78 ± 0.93 | 0.048 ± 0.004 | 6.97 ± 0.06 | 21.56 ± 2.13 | 2.70 ± 0.34 | 0.053 ± 0.001 | 6.70 ± 0.06 | 18.91 ± 0.30 |
| 25 | –CH2OH | p-t-Bu | 1.48 ± 0.04 | 0.054 ± 0.005 | 6.44 ± 0.03 | 19.12 ± 2.01 | 4.84 ± 0.37 | 0.484 ± 0.173 | 6.17 ± 0.30 | 5.53 ± 3.31 |
| 26 | –CH2OH | m-OMe | 2.97 ± 0.27 | 0.037 ± 0.003 | 6.90 ± 0.01 | 27.61 ± 2.09 | 3.32 ± 0.52 | 0.085 ± 0.007 | 6.58 ± 0.03 | 12.06 ± 1.03 |
| 27 | –CH2OH | m-Br | 10.12 ± 1.29 | 0.038 ± 0.012 | 7.47 ± 0.09 | 33.13 ± 10.40 | 13.30 ± 1.21 | 0.050 ± 0.005 | 7.42 ± 0.03 | 20.40 ± 1.86 |
| 28 | –CH2OH | o-Cl | 11.61 ± 1.31 | 0.052 ± 0.004 | 7.34 ± 0.04 | 19.55 ± 1.48 | 17.92 ± 1.30 | 0.048 ± 0.005 | 7.58 ± 0.01 | 21.46 ± 2.08 |
| 29 | –CH2OH | m-Cl | 25.90 ± 2.14 | 0.040 ± 0.003 | 7.81 ± 0.02 | 25.37 ± 1.70 | 30.07 ± 4.02 | 0.071 ± 0.010 | 7.63 ± 0.12 | 15.20 ± 2.50 |
| 30 | –CH2OH | p-Cl | 1.01 ± 0.31 | 0.056 ± 0.009 | 6.22 ± 0.13 | 19.46 ± 3.34 | 2.51 ± 0.90 | 0.056 ± 0.001 | 6.58 ± 0.15 | 17.90 ± 0.25 |
| 49 | –CONHEt | H | 16.43 ± 2.81 | 0.028 ± 0.002 | 7.63 ± 0.01 | 32.82 ± 1.02 | 25.12 ± 2.08 | 0.037 ± 0.002 | 7.83 ± 0.05 | 27.61 ± 1.66 |
| 50 | –CONHEt | p-t-Bu | 1.53 ± 0.11 | 0.091 ± 0.010 | 6.23 ± 0.06 | 11.47 ± 1.48 | 6.29 ± 0.62 | 0.070 ± 0.007 | 6.95 ± 0.01 | 14.76 ± 1.64 |
| 51 | –CONHEt | m-OMe | 4.27 ± 0.03 | 0.027 ± 0.007 | 7.23 ± 0.11 | 42.68 ± 10.88 | 11.91 ± 1.33 | 0.030 ± 0.006 | 7.62 ± 0.11 | 38.05 ± 8.74 |
| 52 | –CONHEt | m-Br | 5.57 ± 2.56 | 0.075 ± 0.038 | 6.91 ± 0.04 | 27.87 ± 14.23 | 4.46 ± 1.28 | 0.056 ± 0.008 | 6.85 ± 0.13 | 19.17 ± 2.68 |
| 53 | –CONHEt | o-Cl | 17.27 ± 1.12 | 0.041 ± 0.010 | 7.66 ± 0.11 | 29.44 ± 6.91 | 37.59 ± 1.91 | 0.048 ± 0.003 | 7.90 ± 0.00 | 21.23 ± 1.22 |
| 54 | –CONHEt | m-Cl | 20.28 ± 1.11 | 0.043 ± 0.013 | 7.75 ± 0.16 | 34.70 ± 13.43 | 35.85 ± 3.95 | 0.062 ± 0.007 | 7.76 ± 0.10 | 16.78 ± 2.22 |
| 55 | –CONHEt | p-Cl | 3.75 ± 1.04 | 0.076 ± 0.024 | 6.71 ± 0.03 | 17.48 ± 4.79 | 11.62 ± 0.26 | 0.064 ± 0.003 | 7.26 ± 0.01 | 15.65 ± 0.78 |
kon (k3) for ligands as determined using NanoBRET binding assays using either human or rat Nluc-A1R expressing HEK 293 cells and determined through fitting with the ″kinetics of competitive binding″ model.35
koff (k4) for ligands determined as in footnote a.
Kinetic dissociation constant (pKd) for each ligand as determined from koff/kon.
Residence time of each ligand as determined by the reciprocal of the koff. All data are the mean ± SEM of at least three independent repeats conducted in duplicate.
The reciprocal of the koff enables a determination of the residence time (RT) of a compound.25 RT is a quantification of the time a ligand spends bound to the receptor, and it is increasingly considered in drug design because of its correlation with pharmacodynamics.36 Beyond this, we also determined the pKd of the compounds (koff/kon) from the kinetics assays and compared these values to those determined from the saturation binding assays. The kinetic parameters for CA200645 binding at the human Nluc-A1R were determined as kon (k1) = 3.67 ± 0.34 × 106 M–1 min–1 and koff (k2) = 0.064 ± 0.0023 min–1 with a Kd = 18.29 ± 2.4 nM. For the rA1R, the kinetics of binding for CA200645 were determined as kon (k1) = 2.93 ± 0.24 × 106 M–1 min–1 and koff (k2) = 0.066 ± 0.0022 min–1 with a Kd = 32.96 ± 2.8 nM. With the help of these parameters, we were then able to provide estimates of the kinetics of binding for adenosine and NECA benzyloxycyclopentyl and phenoxycyclopentyl derivatives 19–30 and 44–55 at the human and rat A1R (Tables 4 and 5).
The adenosine and NECA benzyloxycyclopentyl derivatives (Table 4) displayed RT comparable to adenosine and NECA on hA1R (∼21 min), while the phenoxycyclopentyl analogs generally had RT > 20 min (Table 5). As a general trend, the compounds are faster binders at rA1R regardless of linker length. The reason for this could be the divergent amino acid composition of the extracellular loops between hA1R and rA1R, which would favor different binding paths to the orthosteric site.24
Overall, the compounds displayed a very similar binding profile across the human and rat A1R, suggesting that further studies in rats would be highly relevant for the potential use of the compounds in humans. The adenosine and NECA-derived analog pairs also display very similar affinities for both human and rat A1R, suggesting that it is the R2 substituent on the phenoxy or benzyloxy ring that is key in determining the compound affinity for A1R. At the hA1R, the compounds with the highest affinity are 27–29, 49, 51, 53, and 54. All of these have higher affinity at hA1R than adenosine and NECA alone and are all phenoxycyclopentyl derivatives. It is interesting to note that except for 49 and 51, all of these compounds have a halogen (chloride or bromide) substituent, mostly in the meta-position of the aromatic phenoxy ring. 27, 49, 51, 53, and 54 all also have RT = 29–43 min at the human A1R, while the RT for the rest of the compounds is lower. By comparison, the benzyloxycyclopentyl derivatives generally display weaker binding and lower RT. At the rA1R, compounds with the highest affinity are 28, 29, 49, 53, and 54. 27 and 52, which have the bromide substituent on the aromatic ring, display reduced affinity at the rA1R as well as the hA1R when compared to 29 and 54, respectively, which bear the chloride substituent at the same position. Considering the substitution position on the phenoxy ring, we observed the highest affinity with the chloride in the meta-position (29, 54) followed by ortho- (28, 53) and the para-position (30, 55). Overall, halogen substituents as the R2 group on the aromatic ring seem to confer high affinity for the A1R, with chloride being preferential over bromide for binding at both the human and rat versions of the receptor.
Finally, we performed a comparison of the affinity data obtained from the NanoBRET binding assay with the potency for inhibition of cAMP accumulation for the hA1R, which showed a clear positive correlation (r = 0.82) with compounds 27, 29, 49, and 51–54 identified as both the most potent and strongest binders (Figure 4A). A similar correlation was also observed between potency and compounds’ residence time (Figure 4B, r = 0.65). Overall, in this work, we have identified high-affinity, very selective potent hA1R agonists, namely, 27, 49, and 51–54.
Figure 4.
NECA and adenosine derivatives show correlation between potency and affinity or residence time at the hA1R. (A) Potency pEC50 values of individual compounds from cAMP inhibition experiments plotted against pKi values from NanoBRET experiments at hA1R. (B) Potency pEC50 values of individual compounds from cAMP inhibition experiments plotted against RT values from NanoBRET experiments at hA1R.
Molecular Dynamics Simulations
To retrieve insight into the possible binding mode of the studied agonists and rationalize the selectivity displayed, in silico experiments were performed on the phenoxycyclopentyl adenosine derivative 27, the most A1R-selective and potent agonist, and its benzyloxycyclopentyl congener 20. A1R and A2AR structures solved in complex with adenosine or NECA (or homology models obtained from them, see the Experimental Section) present a closed conformation of the extracellular vestibule due to the lack of induced fit by N6 substituents, not present on adenosine or NECA. This structural feature does not allow molecular docking of compounds bearing bulky N6 groups to reproduce the binding mode of AR full agonists (Figure S2), which is characterized by the fundamental hydrogen bonds between the purine scaffold and the conserved Asn residue in position 6.55 and between the ribose ring and the Ser/Thr7.42 or His7.43. Therefore, molecular dynamics (MD) simulations of the four ARs subtypes were performed in the absence of any orthosteric agonists to sample receptors’ conformations more open at the extracellular loop 2 (ECL2) and ECL3 levels. Molecular docking results for 20 and 27 on the MD-derived AR structures were remarkably enhanced in the case of experimental structures A1R and A2AR, were slightly improved for the structural A3R model, and produced very little improvement for the A2BR model (Figure S3).
The best pose (in terms of similarity to adenosine) of 20 within A1R and the best pose of 27 within A1R or A2AR were further evaluated during 6 μs of MD simulations. For a complete comparison (Movie S1) of all four ARs subtypes, the best docking pose of 27 obtained on A2AR was superimposed on both A2BR and A3R and subjected to MD simulations. During the MD trajectories, 27 remained stably bound to A1R and A2AR but displayed less stable binding modes within A3R and, in particular, the A2BR orthosteric site (Movie S1, Figure S4A). Compound 20 within A1R (Movie S2) was steady throughout the simulations (Movie S2), as indicated by RMSD values in line with 27 (Figure S4A). In terms of flexibility, N6 substituents explored divergent conformations in the different systems (Figure 5A, Movies S1 and S2): the 3-bromophenyl group of 27 was highly flexible in A3R or A2AR and more stable in A1R, while the 3-bromobenzyl group of 20 displayed intermediate flexibility.
Figure 5.
Molecular dynamics docking of 20 and 27. (A) Atomic root mean square fluctuation (RMSF) of 20 within A1R and 27 within A1R, A2AR, and A3R plotted on the agonists’ structure. (B) Compound 27 (salmon stick representation) binding mode within A1R (white ribbon and sticks); the key hydrogen bonds with N2546.55 are shown as red dotted lines, while the hydrophobic subpocket is shown as a cyan transparent surface (coordinates provided in the Supporting Information (A1R_cmpd27_binding_mode.pdb)). (C) Two views (view 1, side; view 2, top) comparing the structural water molecules detected in A1R (red spheres), A2AR (green), A2BR (cyan), and A3R (purple). The position of the stable water cluster only present in A2AR, A2BR, and A3R is highlighted. Binding mode of 27 (salmon sticks) within A1R is superimposed for reference. Presented data are based on PDB structures 6D9H (A1R) and 5G53 (A2AR) and AlphaFold2 models of A2BR and A3R.
Compound 27 bound to A1R formed key hydrogen bonds with N2546.55 and hydrophobic contacts with F171ECL2 and oriented the 3-bromophenyl moiety in a hydrophobic subpocket formed by I692.64, N702.65, Y2717.36, and T2707.35 (Figure 5B, Movies S1 and S2). The bulkier analog 20 was not able to completely accommodate the 3-bromobenzyl group within this pocket and therefore displayed higher flexibility at the N6 level (Figure 5A, Movie S2). It is plausible that this contributes to the reduced A1R affinity and potency of 20 (pKi = 6.16 ± 0.10, pEC50 = 7.74 ± 0.13) compared to 27 (pKi = 7.55 ± 0.11, pEC50 = 10.0 ± 0.24). On the other hand, the interaction fingerprints of 20 (bound to A1R) and 27 are unique for each simulated complex (Figure S4B) and do not allow a straightforward rationalization of the selectivity displayed by the agonists. We therefore focused on the water molecule network present in the apo forms of the four AR subtypes (Figure 5C, Figure S4C–F). Our data suggest the presence of structural water molecules (A2A/A2B/A3 water cluster in Figure 5C) in the proximity of positions 2.64 and 2.65 of A2AR, A2BR, and A3R but not A1R stabilized by the short polar side chain of Ser2.65 (Asn2.65 in A1R, Figure S4B). It follows that the hydrophobic subpocket is putatively present only in A1R; hence, 27 cannot be completely stabilized by the other AR subtypes.
Taken together, computational results suggest that a one-atom linker between the N6-cyclopentane and the phenyl rings is optimal for stable binding to the hydrophobic pocket in A1R. The absence of this pocket and the presence of stable water molecules competing with the ligands in A2AR, A2BR, and A3R are probably responsible for the loss in affinity and potency of the tested agonists. The better complementarity with hA1R could explain why adenosine and NECA benzyloxycyclopentyl derivatives (Table 4) displayed RT comparable to adenosine and NECA at hA1R (∼21 min), while the phenoxycyclopentyl analogs generally had RT > 20 min (Table 5). Indeed, the shorter linker, as present in 27, would stabilize the compounds and increase the energy required to produce dissociation.
Validating the Predictions from the In Silico Experiments for A1R Bound to 20 and 27
As described in Figure 5B, MD simulations suggested that the 3-bromophenyl moiety binds in a hydrophobic subpocket formed by I692.64, N702.65, Y2717.36, and T2707.35, while 20 was not able to completely accommodate the 3-bromobenzyl group within this pocket (Movie S2). To test these observations, we made use of previously described mutants (I692.64A, N702.65A, Y2717.36A) of the Nluc-A1R24 that enable comparison of ligand affinities with the wild-type receptor. We also included the mutant T2576.58A since we have previously shown that this residue is a good discriminator between different A1R agonists.24 We did not consider mutations of N2546.55 or F171ELC2 since these are known to prevent ligand binding to the A1R (including CA200645) and therefore cannot be studied.24,37 Furthermore, we did not consider mutating T2707.35 since, when we compared the sequences of the hA1R and the rA1R, we observed that, in the rA1R, the equivalent residue at position T2707.35 is an Ile. Comparison of the binding affinities (pKi) for 20 and 27 between the hA1R and the rA1R shows that 20 is equipotent between the two species while 27 has reduced affinity at the rA1R (pKi (hA1R) = 7.55 ± 0.11; pKi (rA1R) = 6.94 ± 0.08). Initially, we determined the Kd for CA200645 at each of the four A1R mutants (I692.64A, N702.65A, T2576.58A, and Y2717.36A) and found the values to show close agreement with those previously reported.24 We next performed a NanoBRET competition binding assay for the four mutants with BnOCPA (as a reference agonist), 20, and 27 (Table 6, Figure S5).
Table 6. NanoBRET Competition-Binding Assay at Human Wild-Type and Mutant Nluc-A1Ra.
| A1R | pKia (cmpd) |
|||||
|---|---|---|---|---|---|---|
| BnOCPA | n | 20 | n | 27 | n | |
| WT | 6.24 ± 0.04 | 3 | 6.38 ± 0.17 | 3 | 7.20 ± 0.16 | 3 |
| I692.64A | 5.03 ± 0.02* | 3 | 5.06 ± 0.04* | 3 | 6.37 ± 0.10* | 3 |
| N702.65A | 6.01 ± 0.05 | 3 | 5.48 ± 0.15* | 3 | 7.00 ± 0.24 | 3 |
| T2576.58A | 6.93 ± 0.10* | 3 | 6.23 ± 0.02 | 3 | 8.09 ± 0.18* | 3 |
| Y2717.36A | 5.40 ± 0.23* | 3 | 5.19 ± 0.13* | 3 | 6.33 ± 0.10* | 3 |
Compound affinity (pKi) determined through NanoBRET competition-binding assays with CA200645 in wild-type (WT) or mutant Nluc-A1R stably expressing HEK293 cells. The resulting concentration-dependent decrease in BRET ratio at 10 min was used to calculate pKi. Data are expressed as mean ± SEM obtained in n separate experiments. All individual experiments were conducted in duplicate. Statistical significance (*p < 0.05) compared to WT was determined by one-way ANOVA with Dunnett’s post-test and presented according to ref (31).
Consistent with MD simulation predictions, mutation of I692.64 and Y2717.36 reduced the affinity of BnOCPA, 20, and 27. Interestingly, while the affinity at the A1R of BnOCPA and 27 was not affected by the mutation of N702.65, 20 was significantly reduced. A closer analysis of the MD simulations suggested that the side chain of N702.65 orients differently between 20 and 27. For 20, simulations predicted N702.65 amidic side chain group interactions with the purine ring (Figure S6, Movie S2), which are lost in N702.65A. These interactions can comprise water bridges involving the first solvation shell around the purine scaffold of the ligand.38 Conversely, for 27, the N702.65 amidic side chain group does not interact with the purine ring, but instead, it forms hydrophobic interactions through its methylene group with the bromobenzene ring (Figure 5B, Movie S2), implying that the mutation to Ala does not play a significant role in binding. Finally, as we have previously reported,24 the mutation of T2576.58A did provide a clear discriminator between the three different agonists. 27 together with BnOCPA both showed increases in binding affinity, while 20 displayed no significant change. In our previous studies, we observed that both CPA and BnOCPA displayed increased affinities at the T2576.58A mutant, while NECA showed reduced affinity and there was no change for adenosine. We attributed these changes to an increase in the lipophilicity of the protein environment underneath extracellular loop 3 (ECL3), which surrounds the cyclopentyl groups of the molecules. It is therefore apparent that the small molecule 27 favors a more hydrophobic environment within the binding pocket that is already suitable for 20.
Conclusions
Herein, we report the synthesis of novel N6-benzyloxycyclopentyl and N6-phenoxycyclopentyl derivatives of adenosine and NECA. These compounds were evaluated using the cAMP accumulation assay in CHO-K1 cells and the NanoBRET binding assay in HEK293 cells for potency, selectivity, and binding at ARs. Our pharmacology data show that compounds including halogen substituents, chloride in particular, on the aromatic phenoxy and benzyloxy rings confer high affinity for the human and rat A1R. These compounds also have high potency at the A1R, particularly ones with a meta substituent on the aromatic rings. Furthermore, we also show that NECA-based derivatives have generally higher A1R selectivity over the other AR subtypes. Molecular modeling studies suggest that the selectivity is driven by a short linker and the absence of stable water molecules within a subpocket of the hA1R orthosteric site. It is worth noting that compounds 45 and 51 show approximately 1500 times improved A1R selectivity over NECA itself. Overall, we have identified very selective and very potent A1R agonists with high affinity for the receptor, namely, phenoxycyclopentyl compounds 27, 49, and 51–54, which have great therapeutic promise for overcoming insufficient receptor selectivity and potency that many current compounds face.
Experimental Section
General Chemistry
All reactions were performed in dry glassware under an inert argon atmosphere. Anhydrous solvents were purchased as dry over molecular sieves from Sigma-Aldrich (Merck). Solvents were evaporated under reduced pressure at approximately 45 °C using a Büchi Rotavapor or under high vacuum on a Schlenk line. Reagents were purchased from Sigma-Aldrich (Merck), Fluorochem, or Brunschwig and used without further purification. Reactions were monitored by thin layer chromatography (TLC) using aluminum sheets precoated with 0.2 mm silica (Macherey-Nagel ALUGRAM Xtra SII, G/UV254) or aluminum oxide (Macherey-Nagel POLYGRAM Alox N/UV254). Detection was under a UV light source (λmax 254 nm or 366 nm) or through staining with a vanillin solution, with subsequent heating. Flash column chromatography was carried out on a Teledyne ISCO CombiFlash using prepacked RediSep Normal-phase Silica Flash Columns.
Proton nuclear magnetic resonance (1H NMR) and carbon nuclear magnetic resonance (13C NMR) spectra were recorded at room temperature using a Bruker Avance IIIHD-400, II-400, or IIIHD-300 spectrometer operating at 400 or 300 MHz, respectively, for 1H and at 101 and 75 MHz, respectively, for 13C. Chemical shifts (δ) are reported in parts per million (ppm) and are referenced to the residual solvent peak (DMSO-d6: δH = 2.50 ppm, δC = 39.52 ppm; CDCl3: δH = 7.26 ppm, δC = 77.16 ppm; methanol-d4: δH = 3.31 ppm, δC = 49.00 ppm) or Me4Si (δH = 0.00 ppm). The order of citation in parentheses is (1) multiplicity: s (singlet), d (doublet), t (triplet), q (quartet), quint (quintet), m (multiplet), etc., and br (broad); (2) coupling constants (J) in hertz (Hz); and (3) number of equivalent nuclei (by integration). COSY, HSQC, and DEPT were routinely used to assign peaks in 1H and 13C NMR spectra. Addition of D2O was used to confirm the assignment of OH and NH peaks. High-resolution mass spectra (HRMS) were recorded on a Thermo-Scientific LTQ Orbitrap XL spectrometer consisting of a linear ion trap (LTQ) featuring an HCD collision cell, coupled to the Orbitrap mass analyzer, equipped with a nanoelectrospray ion source (NSI). HRMS spectra were determined by the Mass Spectrometry Group at the Department of Chemistry, Biochemistry, and Pharmaceutical Sciences, University of Bern, Switzerland (Prof. Dr. S. Schürch).
The purity of the compounds was determined with UPLC-MS on a Dionex UltiMate 3000 Rapid Separation LC system using a reversed-phase column (Acclaim RSLC, 120 C18, 3 × 50 mm, 2.2 μm, pore size 120 Å, flow rate 1.2 mL/min), which was coupled to a ESI-MS Micromass Platform (quadrupole mass spectrometer). The gradient used was 100% A to 100% D over 7 min, with A = MilliQ H2O + 0.1% TFA and D = 10% MilliQ H2O/90% HPLC-grade MeCN + 0.1% TFA. Compounds 24 and 29 were measured on a Thermo-Scientific UltiMate 3000 HPLC equipped with a reverse-phase column (Acclaim 120 C18, 4.6 × 150 mm, 5 μm, pore size 120 Å) and eluted with a gradient of MilliQ H2O/HPLC-grade MeCN + 0.1% TFA. Purity was determined by total absorbance at 254 nm. All tested compounds were >95% pure, except for 24 and 29 that were 95 and 94% pure, respectively (Tables S1 and S2).
Established Adenosine Receptor Agonists
Adenosine and 5′-N-ethylcarboxamidoadenosine (NECA) were purchased from R & D Systems (Bristol, UK). Where possible, compounds were prepared as 10 mM stocks in DMSO.
Chemical Synthesis
Intermediates 1, 7, and 31 and BnOCPA were synthesized as described previously.13,39
General Procedure A (O-Alkylation) for the Synthesis of Intermediates 2a–i
Boc-protected (1R,2R)-2-aminocyclopentanol 1 and the appropriate benzyl bromide were dissolved in dry THF (50–100 mM). The reaction mixture was cooled to 0 °C, and NaH (60% dispersion in mineral oil) was added. After stirring at 0 °C, the reaction was quenched with MeOH (0.1 mL) and sat. aq. NH4Cl. The reaction mixture was extracted with EtOAc, and the organic phase was dried over Na2SO4 and concentrated under reduced pressure. The crude material was purified by flash column chromatography.
General Procedure B (Mitsunobu) for the Synthesis of Intermediates 5a,c,e,f,h–j
Boc-protected (1S,2R)-2-aminocyclopentanol 4, the appropriate phenol, and PPh3 were dissolved in dry THF (50–100 mM) and cooled to 0 °C. DIAD was added dropwise. Cooling was removed, and the reaction mixture was left to warm to room temperature and stirred overnight. Water was added, and the aqueous phase was extracted with EtOAc. The organic phase was dried over Na2SO4 and concentrated under reduced pressure. The crude material was purified by flash column chromatography.
General Procedure C (Boc Deprotection) for the Synthesis of Intermediates 3b–i and 6a,c,e,f,h–j
Boc-protected precursors 2b–i and 5a,c,e,f,h–j were dissolved in dioxane (85–830 mM), and HCl (4 M in dioxane) was added. After stirring the reaction mixture at room temperature, the solvent was removed under reduced pressure. The residual ammonium chloride salt was co-evaporated with CH2Cl2 and dried.
General Procedure D (SNAr Reaction) for the Synthesis of Intermediates 8–14 and 32–43
The appropriate 6-chloropurine (7 or 31) and the appropriate benzyloxy- or phenoxycyclopentyl amine intermediate (3b–i or 6a,c,e,f,h–j) were dissolved in i-PrOH (11–36 mM). NaHCO3 was added, and the reaction mixture was heated at reflux (ca. 105 °C) overnight. After cooling, the solid was filtered off and washed with EtOH, and the solvents were removed under reduced pressure. The crude material was purified by flash column chromatography. In some examples, loss of acetate groups on the secondary alcohols was observed during the SNAr reaction with 7. In these cases, the crude material was subjected directly to the deprotection protocol (see general procedure G).
General Procedure E (Acetate Deprotection) for the Synthesis of Compounds 15–17, 19, 24, 26, and 30
Acetate-protected intermediates 8–14 were dissolved in MeOH (9–22 mM), and K2CO3 was added. The reaction mixture was stirred at room temperature, filtered, and concentrated under reduced pressure. The crude material was purified by flash column chromatography.
General Procedure F (Acetonide Deprotection) for the Synthesis of Compounds 44–55
Acetonide-protected intermediates 32–43 were dissolved in water (38–75 mM) and acetic acid and stirred at 80 °C overnight. The water and acetic acid were removed in vacuo, and the crude material was purified by flash column chromatography.
General Procedure G (SNAr Reaction and Subsequent Acetate Deprotection) for the Synthesis of Compounds 20–23, 25, and 27–29
6-Chloropurine 7 and the appropriate benzyloxy- or phenoxycyclopentyl amine intermediate (3f–i or 6c,f,h,i) were dissolved in i-PrOH (23–46 mM). NaHCO3 was added, and the reaction mixture was heated at reflux (ca. 105 °C) overnight. After cooling, the solid was filtered off and washed with EtOH, and the solvents were removed under reduced pressure. The crude material was dissolved in MeOH (11–17 mM), and K2CO3 was added. The reaction mixture was stirred at room temperature, filtered, and concentrated under reduced pressure. The crude material was purified by flash column chromatography.
tert-Butyl ((1R,2R)-2-(Benzyloxy)cyclopentyl)carbamate (2a)
2a was synthesized according to general procedure A using 1 (0.497 mmol), benzyl bromide (0.497 mmol), and NaH (0.994 mmol). The reaction was run for 2 h. After purification with flash column chromatography (EtOAc/cHex, 15%), 2a was obtained (83 mg, 0.285 mmol, 58%). 1H NMR: (300 MHz, DMSO-d6) δ 7.30 (m, 5H), 6.90 (d, J = 7.7, 1H), 4.53 (d, J = 12.2, 1H), 4.58–4.41 (m, 1H), 3.82–3.74 (m, 1H), 3.73–3.66 (m, 1H), 1.95–1.73 (m, 2H), 1.60 (m, 3H), 1.43–1.36 (m, 1H) 1.40 (s, 9H). 13C NMR: (75 MHz, DMSO-d6) δ 155.5, 139.4, 128.6, 127.8, 127.7, 85.0, 78.0, 70.3, 56.9, 30.6, 30.5, 28.8, 21.8. HRMS: (NSI+) m/z calcd for C17H26NO3 [M + H]+ 292.1902, found 292.1907.
tert-Butyl ((1R,2R)-2-((4-Isopropylbenzyl)oxy)cyclopentyl)carbamate (2b)
2b was synthesized according to general procedure A using 1 (1.242 mmol), 4-isopropylbenzyl bromide (1.242 mmol), and NaH (2.484 mmol). The reaction was run for 1 h 20 min. After purification with flash column chromatography (EtOAc/cHex, 15%), 2b was obtained (135 mg, 0.406 mmol, 33%). 1H NMR: (300 MHz, DMSO-d6) δ 7.24–7.18 (m, 4H), 6.89 (d, J = 7.8, 1H), 4.5–4.38 (m, 2H), 3.80–3.72 (m, 1H), 3.72–3.65 (m, 1H), 2.87 (sept, J = 6.9, 1H), 1.93–1.73 (m, 2H), 1.67–1.49 (m, 3H), 1.42–1.32 (m, 1H) 1.40 (s, 9H), 1.19 (d, J = 6.9, 6H). HRMS: (NSI+) m/z calcd for C20H32NO3 [M + H]+ 334.2369, found 334.2377.
tert-Butyl ((1R,2R)-2-((4-(tert-Butyl)benzyl)oxy)cyclopentyl)carbamate (2c)
2c was synthesized according to general procedure A using 1 (1.242 mmol), 4-tert-butylbenzyl bromide (1.242 mmol), and NaH (2.484 mmol). The reaction was run for 1 h 30 min. After purification with flash column chromatography (EtOAc/cHex, 20%), 2c was obtained as an oil (126 mg, 0.363 mmol, 30%). 1H NMR: (300 MHz, DMSO-d6) δ 7.35 (d, J = 8.2, 2H), 7.22 (d, J = 8.2, 2H), 6.89 (d, J = 7.8, 1H), 4.51–4.40 (m, 2H), 3.82–3.72 (m, 1H), 3.71–3.64 (m, 1H), 1.94–1.72 (m, 2H), 1.65–1.51 (m, 3H), 1.45–1.33 (m, 1H), 1.40 (s, 9H), 1.27 (s, 9H). 13C NMR: (101 MHz, DMSO-d6) δ 155.4, 150.1, 136.3, 127.8, 125.3, 84.8, 78.0, 70.1, 56.9, 34.7, 31.6, 30.7, 30.6, 28.8, 21.9. HRMS: (NSI+) m/z calcd for C21H34NO3 [M + H]+ 348.2533, found 348.2533.
tert-Butyl ((1R,2R)-2-((4-Cyanobenzyl)oxy)cyclopentyl)carbamate (2d)
2d was synthesized according to general procedure A using 1 (1 mmol), 4-(bromomethyl)benzonitrile (1 mmol), and NaH (2 mmol). The reaction was run for 6 h 30 min. After purification with flash column chromatography (EtOAc/cHex, 20%), 2d was obtained as an oil (287 mg, 0.91 mmol, 91%). 1H NMR: (300 MHz, methanol-d4) δ 7.69 (d, J = 8.3, 2H), 7.58–7.47 (m, 2H), 4.71–4.56 (m, 2H), 3.96–3.83 (m, 1H), 3.83–3.71 (m, 1H), 2.14–1.61 (m, 5H), 1.44 (s, 10H). 13C NMR: (101 MHz, methanol-d4) δ 151.2, 146.3, 133.2, 129.0, 118.9, 87.0, 71.0, 58.2, 31.3, 31.3, 28.8, 22.5. HRMS: (NSI+) m/z calcd for C18H25N2O3 [M + H]+ 317.1860, found 317.1858.
tert-Butyl ((1R,2R)-2-((3-Methoxybenzyl)oxy)cyclopentyl)carbamate (2e)
2e was synthesized according to general procedure A using 1 (0.497 mmol), 3-methoxybenzyl bromide (0.497 mmol), and NaH (0.994 mmol). The reaction was run for 3 h. After purification with flash column chromatography (EtOAc/cHex, 20%), 2e was obtained (76 mg, 0.235 mmol, 47%). 1H NMR: (300 MHz, DMSO-d6) δ 7.24 (t, J = 8.0, 1H), 6.93–6.80 (m, 3H), 4.53–4.42 (m, 2H), 3.82–3.72 (m, 1H), 3.75 (s, 3H), 3.72–3.65 (m, 1H), 1.95–1.73 (m, 2H), 1.69–1.49 (m, 3H), 1.44–1.32 (m, 1H),1.39 (s, 9H). 13C NMR: (75 MHz, DMSO-d6) δ 159.7, 155.5, 141.0, 129.7, 119.9, 113.2, 84.8, 78.0, 70.1, 56.9, 55.4, 30.6, 30.5, 28.7, 21.8. HRMS: (NSI+) m/z calcd for C18H28NO4 [M + H]+ 322.2021, found 322.2013.
tert-Butyl ((1R,2R)-2-((3-Bromobenzyl)oxy)cyclopentyl)carbamate (2f)
2f was synthesized according to general procedure A using 1 (1.242 mmol), 3-bromobenzyl bromide (1.242 mmol), and NaH (2.484 mmol). The reaction was run for 3 h. After purification with flash column chromatography (EtOAc/cHex, 15–20%), 2f was obtained (268 mg, 0.725 mmol, 58%). 1H NMR: (300 MHz, DMSO-d6) δ 7.51–7.46 (m, 1H), 7.38–7.31 (m, 1H), 7.27–7.13 (m, 2H), 6.91 (d, J = 7.7, 1H), 4.57–4.45 (m, 2H), 3.81–3.72 (m, 1H), 3.72–3.64 (m, 1H), 1.95–1.75 (m, 2H), 1.68–1.49 (m, 3H), 1.46–1.33 (m, 1H), 1.40 (s, 9H). 13C NMR: (75 MHz, methanol-d4) δ 156.5, 141.5, 130.1, 130.1, 129.7, 125.9, 121.9, 85.2, 78.6, 69.7, 56.7, 30.0, 27.5, 26.6, 21.1. HRMS: (NSI+) m/z calcd for C17H24BrNNaO3 [M + Na]+ 392.0830, found 392.0832.
tert-Butyl ((1R,2R)-2-((4-Bromobenzyl)oxy)cyclopentyl)carbamate (2g)
2g was synthesized according to general procedure A using 1 (0.8 mmol), 4-bromobenzyl bromide (0.8 mmol), and NaH (1.6 mmol). The reaction was run for 3 h. After purification with flash column chromatography (EtOAc/cHex, 20%), 2g was obtained (198 mg, 0.535 mmol, 67%). 1H NMR (300 MHz, DMSO-d6) δ 7.52 (d, J = 8.4, 2H), 7.27 (d, J = 8.4, 2H), 4.48 (m, 2H), 3.82–3.78 (m, 1H), 3.70–3.59 (m, 1H), 1.94–1.74 (m, 2H), 1.59 (m, 3H), 1.44–1.34 (m, 1H), 1.39 (s, 9H). 13C NMR: (101 MHz, methanol-d4) δ 156.5, 144.9, 131.8, 127.6, 118.4, 110.6, 85.6, 78.6, 69.6, 56.8, 29.9, 27.4, 21.1.
tert-Butyl ((1R,2R)-2-((2-Chlorobenzyl)oxy)cyclopentyl)carbamate (2h)
2h was synthesized according to general procedure A using 1 (0.994 mmol), 2-chlorobenzyl bromide (0.994 mmol), and NaH (1.987 mmol). The reaction was run for 1.5 h at 0 °C and then for another 1.5 h at room temperature. After purification with flash column chromatography (EtOAc/cHex, 20%), 2h was obtained (178 mg, 0.504 mmol, 51%). 1H NMR: (300 MHz, DMSO-d6) δ 7.53–7.40 (m, 2H), 7.37–7.29 (m, 2H), 6.94 (d, J = 7.7, 1H), 4.65–4.50 (m, 2H), 3.85–3.70 (m 2H), 1.99–1.78 (m, 2H), 1.68–1.53 (m, J = 9.3, 3H), 1.46–1.35 (m, 1H), 1.40 (s, 9H). 13C NMR: (75 MHz, methanol-d4) δ 156.5, 136.3, 132.6, 129.05, 128.8, 128.5, 126.5, 85.6, 78.6, 67.9, 56.8, 30.0, 29.8, 27.4, 21.0. HRMS: (NSI+) m/z calcd for C17H24ClNNaO3 [M + Na]+ 348.1336, found 348.1337.
tert-Butyl ((1R,2R)-2-((3-Chlorobenzyl)oxy)cyclopentyl)carbamate (2i)
2i was synthesized according to general procedure A using 1 (0.994 mmol), 3-chlorobenzyl bromide (0.994 mmol), and NaH (1.987 mmol). The reaction was run for 3 h. After purification with flash column chromatography (EtOAc/cHex, 20%), 2i was obtained (224 mg, 0.688 mmol, 70%). 1H NMR: (300 MHz, DMSO-d6) δ 7.42–7.23 (m, 4H), 6.91 (d, J = 7.7, 1H), 4.58–4.45 (m, 2H), 3.81–3.73 (m, 1H), 3.73–3.65 (m, 1H), 1.96–1.71 (m, 2H), 1.68–1.47 (m, 3H), 1.46–1.33 (m, 1H), 1.40 (s, 9H). 13C NMR: (75 MHz, methanol-d4) δ 156.5, 141.3, 133.8, 129.4, 127.2, 127.1, 125.5, 85.2, 78.6, 69.7, 56.7, 30.0, 29.9, 27.4, 21.1. HRMS: (NSI+) m/z calcd for C17H24ClNNaO3 [M + Na]+ 348.1338, found 348.1337.
(1R,2R)-2-((4-Isopropylbenzyl)oxy)cyclopentan-1-aminium Chloride (3b)
3b was synthesized according to general procedure C using 2b (0.405 mmol) and HCl (2.03 mmol). The reaction was run for 3 h. 3b was obtained as a solid (100 mg, 0.43 mmol, quant.). 1H NMR: (300 MHz, DMSO-d6) δ 8.05 (br s, 3H), 7.32–7.19 (m, 4H), 4.54–4.40 (m, 2H), 3.95–3.87 (m, 1H), 3.42 (br s, 1H), 2.88 (sept, J = 6.9, 1H), 2.09–1.90 (m, 2H), 1.76–1.45 (m, 4H), 1.20 (d, J = 6.9, 6H). HRMS: (NSI+) m/z calcd for C15H24NO [M]+ 234.1849, found 234.1852.
(1R,2R)-2-((4-(tert-Butyl)benzyl)oxy)cyclopentan-1-aminium Chloride (3c)
3c was synthesized according to general procedure C using 2c (0.329 mmol) and HCl (1.649 mmol). The reaction was run for 5 h. 3c was obtained as a colorless solid (93 mg, 0.328 mmol, 99%). 1H NMR: (300 MHz, DMSO-d6) δ 8.02 (br s, 3H), 7.38 (d, J = 8.4, 2H), 7.28 (d, J = 8.4, 2H), 4.54–4.39 (m, 2H), 3.93–3.86 (m, 1H), 3.47–3.36 (m, 1H), 2.11–1.92 (m, 2H), 1.75–1.48 (m, 4H), 1.28 (s, 9H). 13C NMR: (75 MHz, DMSO-d6) δ 150.4, 135.7, 128.0, 125.4, 82.8, 70.7, 56.3, 34.7, 31.6, 30.3, 28.9, 21.6. HRMS: (NSI+) m/z calcd for C16H26NO [M]+ 248.2005, found 248.2009.
(1R,2R)-2-((4-Cyanobenzyl)oxy)cyclopentan-1-aminium Chloride (3d)
3d was synthesized according to general procedure C using 2d (0.825 mmol) and HCl (8 mmol). After stirring for 1 h, a colorless precipitate formed. Drops of water were added until all the solid dissolved. The reaction mixture was then stirred for an additional 3 h. 3d was obtained as a colorless solid (209 mg, 0.825 mmol, quant.). 1H NMR: (300 MHz, DMSO-d6) δ 8.02 (s, 3H), 7.84 (d, J = 8.2, 2H), 7.58 (d, J = 8.3, 2H), 4.68–4.57 (m, 2H), 3.99–3.93 (m, 1H), 3.44 (s, 1H), 2.11–1.92 (m, 2H), 1.76–1.49 (m, 4H). HRMS: (NSI+) m/z calcd for C13H17N2O [M]+ 217.1335, found 217.1333.
(1R,2R)-2-((3-Methoxybenzyl)oxy)cyclopentan-1-aminium Chloride (3e)
3e was synthesized according to general procedure C using 2e (0.69 mmol) and HCl (3.49 mmol). The reaction was run for 4 h. 3e was obtained as a colorless solid (88 mg, 0.341 mmol, 50%). 1H NMR: (300 MHz, DMSO-d6) δ 8.20 (br s, 3H), 7.27 (t, J = 8.1, 1H), 6.98–6.82 (m, 3H), 4.57–4.42 (m, 2H), 3.98–3.88 (m, 1H), 3.76 (s, 3H), 3.47–3.37 (m, 1H), 1.76–1.49 (m, 2H), 1.76–1.49 (m, 4H). 13C NMR: (75 MHz, DMSO-d6) δ 159.7, 140.4, 129.7, 117.5, 113.5, 113.4, 82.9, 70.8, 56.3, 55.5, 30.2, 28.9, 21.6. HRMS: (NSI+) m/z calcd for C13H20NO2 [M]+ 222.1491, found 222.1489.
(1R,2R)-2-((3-Bromobenzyl)oxy)cyclopentan-1-aminium Chloride (3f)
3f was synthesized according to general procedure C using 2f (0.716 mmol) and HCl (14.3 mmol). The reaction was run for 1 h, after which a few drops of MeOH were added to dissolve precipitate. The reaction mixture was then stirred for another 1.5 h. 3f was obtained as a solid (224 mg, 0.732 mmol, quant.). 1H NMR: (300 MHz, methanol-d4) δ 7.48 (t, J = 1.9, 1H), 7.37–7.32 (m, 1H), 7.27–7.13 (m, 2H), 4.50 (d, J = 12.0, 1H), 4.42 (d, J = 12.0, 1H), 3.92–3.80 (m, 1H), 3.45–3.34 (m, 1H), 2.16–1.91 (m, 2H), 1.82–1.44 (m, 4H). 13C NMR: (75 MHz, methanol-d4) δ 140.7, 130.4, 130.3, 129.9, 126.1, 122.0, 82.8, 70.3, 56.6, 29.1, 27.6, 20.3. HRMS: (NSI+) m/z calcd for C12H17BrNO [M]+ 270.0489, found 270.0488.
(1R,2R)-2-((4-Bromobenzyl)oxy)cyclopentan-1-aminium Chloride (3g)
3g was synthesized according to general procedure C using 2g (0.481 mmol) and HCl (9.61 mmol). The reaction was run for 30 min, after which a few drops of MeOH were added to dissolve precipitate. The reaction mixture was then stirred for another 30 min. 3g was obtained as a solid (114 mg, 0.373 mmol, 77%). 1H NMR (300 MHz, methanol-d4) δ 7.40 (d, J = 8.4, 2H), 7.21 (d, J = 8.1, 2H), 4.51–4.37 (m, 2H), 3.89–3.80 (m, 1H), 3.44–3.34 (m, 1H), 2.17–1.90 (m, 2H), 1.79–1.41 (m, 4H). 13C NMR: (75 MHz, methanol-d4) δ 137.3, 131.1, 129.4, 121.1, 82.7, 70.4, 56.6, 29.1, 27.6, 20.3. HRMS: (NSI+) m/z calcd for C12H17BrNO [M]+ 270.0493, found 270.0488.
(1R,2R)-2-((2-Chlorobenzyl)oxy)cyclopentan-1-aminium Chloride (3h)
3h was synthesized according to general procedure C using 2h (0.467 mmol) and HCl (9.33 mmol). The reaction was run for 45 min, after which a few drops of MeOH were added to dissolve precipitate. The reaction mixture was then stirred for another 1.5 h. 3h was obtained as a solid (121 mg, 0.462 mmol, 99%). 1H NMR: (300 MHz, methanol-d4) δ 7.50–7.41 (m, 1H), 7.34–7.12 (m, 3H), 4.63–4.49 (m, 2H), 4.00–3.89 (m, 1H), 3.49–3.35 (m, 1H), 2.20–1.94 (m, 2H), 1.85–1.48 (m, 4H). 13C NMR: (75 MHz, methanol-d4) δ 132.8, 129.5, 128.93, 128.92, 126.7, 83.2, 68.5, 56.6, 29.2, 27.7, 20.4. HRMS: (NSI+) m/z calcd for C12H17ClNO [M]+ 226.0995, found 226.0993.
(1R,2R)-2-((3-Chlorobenzyl)oxy)cyclopentan-1-aminium Chloride (3i)
3i was synthesized according to general procedure C using 2i (0.687 mmol) and HCl (13.75 mmol). The reaction was run for 45 min, after which a few drops of MeOH were added to dissolve the precipitate. The reaction mixture was then stirred for another 1.5 h. 3i was obtained as a solid (176 mg, 0.671 mmol, 97%). 1H NMR: (300 MHz, DMSO-d6) δ 8.26 (br s, 3H), 7.48–7.29 (m, 4H), 4.60–4.46 (m, 2H), 4.00–3.92 (m, 1H), 3.49–3.37 (m, 1H), 2.09–1.93 (m, 2H), 1.80–1.50 (m, 4H). 13C NMR: (75 MHz, methanol-d4) δ 140.5, 133.9, 129.6, 127.4, 127.3, 125.7, 82.8, 66.8, 56.6, 29.1, 27.6, 20.3. HRMS: (NSI+) m/z calcd for C12H17ClNO [M]+ 226.1002, found 226.0993.
tert-Butyl ((1R,2R)-2-Phenoxycyclopentyl)carbamate (5a)
5a was synthesized according to general procedure B using 4 (0.994 mmol), phenol (1.242 mmol), PPh3 (1.242 mmol), and DIAD (1.242 mmol). The reaction was run for 21 h. After purification with flash column chromatography (EtOAc/cHex, 20%), 5a was obtained (165 mg, 0.594 mmol, 60%). 1H NMR: (300 MHz, DMSO-d6) δ 7.33–7.23 (m, 2H), 7.04 (d, J = 7.3, 1H), 6.99–6.86 (m, 3H), 4.57–4.48 (m, 1H), 3.89–3.79 (m, 1H), 2.09–1.87 (m, 2H), 1.79–1.42 (m, 4H), 1.39 (s, 9H). 13C NMR: (101 MHz, DMSO-d6) δ 157.6, 155.2, 129.4, 120.4, 115.3, 82.2, 77.7, 56.54, 29.8, 29.6, 28.2, 21.2. HRMS: (NSI+) m/z calcd for C16H24NO3 [M + H]+ 278.1756, found 278.1747.
tert-Butyl ((1R,2R)-2-(4-(tert-Butyl)phenoxy)cyclopentyl)carbamate (5c)
5c was synthesized according to general procedure B using 4 (0.994 mmol), 4-tert-butylphenol (1.242 mmol), PPh3 (1.242 mmol), and DIAD (1.242 mmol). The reaction was run for 19 h. After purification with flash column chromatography (EtOAc/cHex, 20%), 5c was obtained (189 mg, 0.567 mmol, 57%). 1H NMR: (300 MHz, DMSO-d6) δ 7.31–7.24 (m, 2H), 7.02 (d, J = 7.3, 1H), 6.86 (d, J = 8.5, 2H), 4.52–4.45 (m, 1H), 3.88–3.75 (m, 1H), 2.07–1.89 (m, 2H), 1.80–1.43 (m, 4H), 1.39 (s, 9H), 1.25 (s, 9H). 13C NMR: (75 MHz, methanol-d4) δ 156.5, 155.7, 143.0, 125.7, 114.8, 82.6, 78.7, 56.9, 33.5, 30.6, 29.8, 29.7, 27.4, 21.0. HRMS: (NSI+) m/z calcd for C20H32NO3 [M + H]+ 334.2364, found 334.2377.
tert-Butyl ((1R,2R)-2-(3-Methoxyphenoxy)cyclopentyl)carbamate (5e)
5e was synthesized according to general procedure B using 4 (1.987 mmol), 3-methoxyphenol (2.484 mmol), PPh3 (2.484 mmol), and DIAD (2.484 mmol). The reaction was run for 18 h. After purification with flash column chromatography (EtOAc/cHex, 20%), 5e was obtained (381 mg, 1.236 mmol, 62%). 1H NMR: (300 MHz, DMSO-d6) δ 7.15 (t, J = 8.2, 1H), 7.05 (d, J = 7.3, 1H), 6.58–6.44 (m, 3H), 4.54–4.47 (m, 1H), 3.88–3.78 (m, 1H), 3.73 (s, 3H), 2.05–1.87 (m, 2H), 1.77–1.54 (m, 3H), 1.53–1.42 (m, 1H), 1.39 (s, 9H). 13C NMR: (101 MHz, methanol-d4) δ 161.0, 159.2, 156.5, 129.4, 107.6, 106.1, 101.4, 82.7, 78.7, 56.9, 54.3, 29.8, 27.4, 21.1. HRMS: (NSI+) m/z calcd for C17H25NNaO4 [M + Na]+ 330.1683, found 330.1687.
tert-Butyl ((1R,2R)-2-(3-Bromophenoxy)cyclopentyl)carbamate (5f)
5f was synthesized according to general procedure B using 4 (0.497 mmol), 3-bromophenol (0.621 mmol), PPh3 (0.621 mmol), and DIAD (0.621 mmol). The reaction was run for 18 h. After purification with flash column chromatography (EtOAc/cHex, 20%), 5f was obtained (90 mg, 0.253 mmol, 51%). 1H NMR: (300 MHz, DMSO-d6) δ 7.26–6.91 (m, 4H), 4.58–4.51 (m, 1H), 3.88–3.76 (m, 1H), 2.09–1.86 (m, 2H), 1.80–1.42 (m, 4H), 1.39 (s, 9H). 13C NMR: (75 MHz, methanol-d4) δ 158.9, 156.4, 130.4, 123.33, 122.3, 118.6, 114.2, 83.2, 78.7, 56.9, 29.8, 29.7, 21.1, 20.4. HRMS: (NSI+) m/z calcd for C16H22BrNNaO3 [M + Na]+ 378.0686, found 378.0675.
tert-Butyl ((1R,2R)-2-(2-Chlorophenoxy)cyclopentyl)carbamate (5h)
5h was synthesized according to general procedure B using 4 (0.994 mmol), 2-chlorophenol (1.242 mmol), PPh3 (1.242 mmol), and DIAD (1.242 mmol). The reaction was run for 19 h. After purification with flash column chromatography (EtOAc/cHex, 10%), 5h was obtained (187 mg, 0.597 mmol, 60%). 1H NMR: (300 MHz, DMSO-d6) δ 7.44–7.38 (m, 1H), 7.32–7.20 (m, 2H), 7.06 (d, J = 7.2, 1H), 6.98–6.90 (m, 1H), 4.64–4.57 (m, 1H), 3.92–3.81 (br s, 1H), 2.08–1.91 (m, 2H), 1.82–1.44 (m, 4H), 1.39 (s, 9H). 13C NMR: (75 MHz, methanol-d4) δ 156.4, 153.5, 129.8, 127.5, 123.2, 121.2, 115.3, 83.9, 78.7, 56.8, 29.9, 29.6, 27.4, 21.1. HRMS: (NSI+) m/z calcd for C16H23ClNO3 [M + H]+ 312.1359, found 312.1361.
tert-Butyl ((1R,2R)-2-(3-Chlorophenoxy)cyclopentyl)carbamate (5i)
5i was synthesized according to general procedure B using 4 (0.994 mmol), 3-chlorophenol (1.242 mmol), PPh3 (1.242 mmol), and DIAD (1.242 mmol). The reaction was run for 18 h. After purification with flash column chromatography (EtOAc/cHex, 10%), 5i was obtained (170 mg, 0.544 mmol, 55%). 1H NMR: (300 MHz, DMSO-d6) δ 7.29 (t, J = 8.1, 1H), 7.11–6.90 (m, 4H), 4.59–4.52 (m, 1H), 3.89–3.77 (m, 1H), 2.12–1.86 (m, 2H), 1.81–1.42 (m, 4H), 1.39 (s, 9H). 13C NMR: (75 MHz, methanol-d4) δ 158.9, 156.5, 134.4, 130.1, 120.3, 115.59, 113.8, 83.2, 78.7, 56.9, 29.74, 29.65, 27.4, 21.1. HRMS: (NSI+) m/z calcd for C16H23ClNO3 [M + H]+ 312.1354, found 312.1361.
tert-Butyl ((1R,2R)-2-(4-Chlorophenoxy)cyclopentyl)carbamate (5j)
5j was synthesized according to general procedure B using 4 (0.994 mmol), 4-chlorophenol (1.242 mmol), PPh3 (1.242 mmol), and DIAD (1.242 mmol). The reaction was run for 20 h. After purification with flash column chromatography (EtOAc/cHex, 10%), 5j was obtained (191 mg, 0.611 mmol, 62%). 1H NMR: (300 MHz, DMSO-d6) δ 7.30 (d, J = 9.0, 2H), 7.05 (d, J = 7.3, 1H), 6.98 (d, J = 8.9, 2H), 4.56–4.47 (m, 1H), 3.87–3.77 (m, 1H), 2.08–1.86 (m, 2H), 1.78–1.43 (m, 4H), 1.38 (s, 9H). 13C NMR: (75 MHz, DMSO-d6) δ 157.0, 155.6, 129.7, 124.6, 117.6, 83.2, 78.3, 57.0, 30.2, 30.0, 28.7, 21.6. HRMS: (NSI+) m/z calcd for C16H22ClNNaO3 [M + Na]+ 334.1182, found 334.1180.
(1R,2R)-2-Phenoxycyclopentan-1-aminium chloride (6a)
6a was synthesized according to general procedure C using 5a (0.804 mmol) and HCl (16.09 mmol). The reaction was run for 18 h. 6a was obtained as a solid (173 mg, 0.809 mmol, quant.). 1H NMR: (300 MHz, DMSO-d6) δ 8.20 (s, 3H), 7.39–7.25 (m, 2H), 7.04–6.91 (m, 3H), 4.77–4.68 (m, 1H), 3.64–3.55 (m, 1H), 2.25–2.02 (m, 2H), 1.83–1.56 (m, 4H). 13C NMR: (75 MHz, methanol-d4) δ 157.3, 129.3, 121.1, 115.3, 80.6, 57.0, 29.4, 28.0, 20.7. HRMS: (NSI+) m/z calcd for C11H16NO [M]+ 178.1228, found 178.1226.
(1R,2R)-2-(4-(tert-Butyl)phenoxy)cyclopentan-1-aminium Chloride (6c)
6c was synthesized according to general procedure C using 5c (0.507 mmol) and HCl (10.14 mmol). The reaction was run for 20 h, after which a few drops of MeOH were added to dissolve the precipitate. Stirring was continued for another 21 h, and more 4 M HCl in dioxane (1 mL) was added to bring the reaction to completion. 6c was obtained as a solid (117 mg, 0.432 mmol, 85%). 1H NMR: (300 MHz, DMSO-d6) δ 8.11 (br s, 3H), 7.37–7.28 (m, 2H), 6.92–6.85 (m, 2H), 4.69–4.63 (m, 1H), 3.65–3.53 (m, 1H), 2.23–2.02 (m, 2H), 1.84–1.54 (m, 4H), 1.26 (s, 9H). 13C NMR: (75 MHz, methanol-d4) δ 155.0, 144.0, 126.0, 114.9, 80.7, 57.0, 33.6, 30.6, 29.5, 28.0, 20.7. HRMS: (NSI+) m/z calcd for C15H24NO [M]+ 234.1850, found 234.1852.
(1R,2R)-2-(3-Methoxyphenoxy)cyclopentan-1-aminium chloride (6e)
6e was synthesized according to general procedure C using 5e (1.236 mmol) and HCl (6.18 mmol). The reaction was run for 19 h, after which more 4 M HCl in dioxane (2 mL) was added to bring the reaction to completion. 6e was obtained as a solid (322 mg, 1.23 mmol, quant.). 1H NMR: (300 MHz, DMSO-d6) δ 8.33 (br s, 3H), 7.21 (t, J = 8.2, 1H), 6.61–6.47 (m, 3H), 4.80–4.71 (m, 1H), 3.74 (s, 3H), 3.61–3.51 (m, 1H), 2.25–2.04 (m, 2H), 1.86–1.59 (m, 4H). 13C NMR: (101 MHz, methanol-d4) δ 161.1, 158.5, 129.8, 107.4, 106.6, 102.0, 80.8, 57.0, 54.4, 29.5, 27.9, 20.7. HRMS: (NSI+) m/z calcd for C12H18NO2 [M]+ 208.1329, found 208.1332.
(1R,2R)-2-(3-Bromophenoxy)cyclopentan-1-aminium chloride (6f)
6f was synthesized according to general procedure C using 5f (0.253 mmol) and HCl (5.05 mmol). The reaction was run for 1 h, after which a few drops of MeOH were added to dissolve precipitate. Stirring was then continued for another 19 h. 6f was obtained as a solid (74 mg, 0.252 mmol, quant.). 1H NMR: (300 MHz, DMSO-d6) δ 8.20 (br s, 3H), 7.29 (t, J = 8.3, 1H), 7.20–7.15 (m, 2H), 7.03–6.96 (m, 1H), 4.77–4.71 (m,1H), 3.63–3.53 (m, 1H), 2.24–2.03 (m, 2H), 1.84–1.56 (m, 4H). 13C NMR: (101 MHz, methanol-d4) δ 158.2, 130.7, 124.2, 122.4, 118.7, 114.2, 81.1, 56.9, 29.3, 27.9, 20.7. HRMS: (NSI+) m/z calcd for C11H15BrNO [M]+ 256.0330, found 256.0332.
(1R,2R)-2-(2-Chlorophenoxy)cyclopentan-1-aminium chloride (6h)
6h was synthesized according to general procedure C using 5 h (0.6 mmol) and HCl (12 mmol). The reaction was run for 69 h. 6h was obtained as a solid (149 mg, 0.6 mmol, quant.). 1H NMR: (300 MHz, DMSO-d6) δ 8.30 (br s, 3H), 7.46 (dd, J = 7.9, 1.6, 1H), 7.36–7.30 (m, 1H), 7.23 (dd, J = 8.4, 1.5, 1H), 7.05–6.97 (m, 1H), 4.90–4.83 (m, 1H), 3.68–3.59 (m, 1H), 2.24–2.09 (m, 2H), 1.86–1.62 (m, 4H). 13C NMR: (75 MHz, methanol-d4) δ 152.7, 130.1, 127.8, 123.4, 122.2, 115.6, 82.1, 57.0, 29.6, 28.4, 21.0. HRMS: (NSI+) m/z calcd for C11H15ClNO [M]+ 212.0832, found 212.0837.
(1R,2R)-2-(3-Chlorophenoxy)cyclopentan-1-aminium chloride (6i)
6i was synthesized according to general procedure C using 5i (0.481 mmol) and HCl (4.81 mmol). The reaction was run for 48 h. 6i was obtained as a solid (118 mg, 0.476 mmol, 99%). 1H NMR: (300 MHz, methanol-d4) δ 7.29 (t, J = 8.1, 1H), 7.06–6.91 (m, 3H), 4.90–4.80 (m, 1H), 3.78–3.69 (m, 1H), 2.38–2.23 (m, 2H), 1.99–1.67 (m, 4H). 13C NMR: (75 MHz, methanol-d4) δ 158.2, 134.6, 130.5, 121.2, 115.85, 113.9, 81.0, 57.0, 29.4, 28.1, 20.9. HRMS: (NSI+) m/z calcd for C11H15ClNO [M]+ 212.0830, found 212.0837.
(1R,2R)-2-(4-Chlorophenoxy)cyclopentan-1-aminium chloride (6j)
6j was synthesized according to general procedure C using 5j (0.611 mmol) and HCl (12.22 mmol). The reaction was run for 1.5 h, after which a few drops of MeOH were added to dissolve the precipitate. Stirring was continued for another hour. 6j was obtained as a solid (150 mg, 0.604 mmol, 99%). 1H NMR: (300 MHz, DMSO-d6) δ 8.59 (br s, 3H), 7.38–7.29 (m, 2H), 7.07–6.93 (m, 2H), 4.85–4.75 (m, 1H), 3.59–3.50 (m, 1H), 2.29–2.01 (m, 2H), 1.87–1.57 (m, 4H). 13C NMR: (75 MHz, DMSO-d6) δ 156.4, 129.8, 125.2, 117.8, 81.5, 56.4, 30.0, 28.7, 21.6. HRMS: (NSI+) m/z calcd for C11H15ClNO [M]+ 212.0840, found 212.0837.
(2R,3R,4R,5R)-2-(Acetoxymethyl)-5-(6-(((1R,2R)-2-((4-isopropylbenzyl)oxy)cyclopentyl)amino)-9H-purin-9-yl)tetrahydrofuran-3,4-diyl Diacetate (8)
8 was synthesized according to general procedure D using 7 (0.286 mmol), 3b (0.428 mmol), and NaHCO3 (0.857 mmol). The reaction was heated at reflux overnight. After purification with flash column chromatography (MeOH/EtOAc, 2%), 8 was obtained as a ∼1:1 mixture with starting material 7 (determined by 1H NMR). This mixture was used in the next step without further purification to obtain 15.
(2R,3R,4R,5R)-2-(Acetoxymethyl)-5-(6-(((1R,2R)-2-((4-(tert-butyl)benzyl)oxy)cyclopentyl)amino)-9H-purin-9-yl)tetrahydrofuran-3,4-diyl Diacetate (9)
9 was synthesized according to general procedure D using 7 (0.235 mmol), 3c (0.352 mmol), and NaHCO3 (0.705 mmol). The reaction was heated at reflux for 15 h. After purification with flash column chromatography (EtOAc/cHex, 80%), 9 was obtained as a solid (53 mg, 0.0845 mmol, 36%). 1H NMR: (300 MHz, methanol-d4) δ 8.31 (s, 1H), 8.24 (s, 1H), 7.32 (d, J = 8.5, 2H), 7.23 (d, J = 8.4, 2H), 6.25 (d, J = 5.3, 1H), 6.03 (t, J = 5.5, 1H), 5.74–5.71 (m, 1H), 4.75–4.65 (m, 1H), 4.63 (s, 2H), 4.50–4.35 (m, 3H), 4.04–3.98 (m, 1H), 2.32–2.19 (m, 1H), 2.16 (s, 3H), 2.08 (s, 3H), 2.07 (s, 3H), 2.05–1.99 (m, 1H), 1.90–1.57 (m, 4H), 1.29 (s, 9H). 13C NMR: (101 MHz, methanol-d4) δ 170.8, 170.0, 169.7, 154.3, 152.9, 150.2, 148.5, 139.2, 135.5, 127.34, 124.7, 119.5, 86.4, 84.7, 80.2, 78.0, 73.0, 70.7, 70.6, 62.8, 33.9, 30.4, 30.3, 30.0, 21.1, 19.2, 19.0, 18.9. HRMS: (NSI+) m/z calcd for C32H42N5O8 [M + H]+ 624.3012, found 624.3028.
(2R,3R,4R,5R)-2-(Acetoxymethyl)-5-(6-(((1R,2R)-2-((4-cyanobenzyl)oxy)cyclopentyl)amino)-9H-purin-9-yl)tetrahydrofuran-3,4-diyl Diacetate (10)
10 was synthesized according to general procedure D using 7 (0.83 mmol), 3d (0.83 mmol), and NaHCO3 (2.5 mmol). The reaction was heated at reflux for 16 h. After purification with flash column chromatography (EtOAc/cHex, 80%), 10 was obtained as a solid (492 mg, 0.83 mmol, quant.). 1H NMR: (300 MHz, DMSO-d6) δ 8.37 (s, 1H), 8.27 (s, 1H), 7.99 (d, J = 6.0, 1H), 7.75 (d, J = 7.8, 2H), 7.47 (d, J = 7.8, 2H), 6.21 (d, J = 5.4, 1H), 6.04 (t, J = 5.6, 1H), 5.63 (t, J = 5.2, 1H), 4.70–4.52 (m, 1H), 4.66 (s, 2H), 4.45–4.32 (m, 2H), 4.28–4.19 (m, 1H), 4.02–3.87 (m, 1H), 2.15–1.88 (m, 2H), 2.12 (s, 3H), 2.04 (s, 3H), 2.01 (s, 3H), 1.81–1.54 (m, 4H). 13C NMR (101 MHz, CDCl3) δ 170.4, 169.7, 169.5, 153.8, 149.4, 144.5, 138.7, 132.2, 127.8, 120.3, 119.0, 111.2, 86.5, 85.69, 85.67, 80.5, 77.4, 73.4, 70.7, 70.5, 63.2, 31.02, 30.99, 30.5, 21.7, 20.9, 20.6, 20.5. HRMS: (NSI+) m/z calcd for C29H33N6O8 [M + H]+ 593.2360, found 593.2362.
(2R,3R,4R,5R)-2-(Acetoxymethyl)-5-(6-(((1R,2R)-2-((3-methoxybenzyl)oxy)cyclopentyl)amino)-9H-purin-9-yl)tetrahydrofuran-3,4-diyl Diacetate (11)
11 was synthesized according to general procedure D using 7 (0.18 mmol), 3e (0.271 mmol), and NaHCO3 (0.542 mmol). The reaction was heated at reflux for 23 h. After purification with flash column chromatography (EtOAc/cHex, 80%), 11 was obtained (68 mg, 0.114 mmol, 67%). 1H NMR: (300 MHz, methanol-d4) δ 8.37 (s, 1H), 8.26 (s, 1H), 7.98 (d, J = 7.9, 1H), 7.19 (t, J = 7.8, 1H), 6.89–6.75 (m, 3H), 6.22 (d, J = 5.4, 1H), 6.04 (t, J = 5.7, 1H), 5.64 (t, J = 5.3, 1H), 4.66–4.57 (m, 1H), 4.54 (s, 2H), 4.46–4.33 (m, 3H), 4.28–4.21 (m, 1H), 4.02–3.96 (m, 1H) 3.66 (s, 3H), 2.13 (s, 3H), 2.10–1.88 (m, 2H), 2.04 (s, 3H), 2.01 (s, 3H) 1.77–1.55 (m, 4H). 13C NMR: (101 MHz, methanol-d4) δ 170.8, 170.0, 169.8, 159.7, 154.3, 152.8, 148.5, 140.3, 139.2, 128.8, 119.7, 119.5, 112.62, 112.61, 86.4, 84.9, 80.2, 73.0, 70.9, 70.6, 62.8, 57.0, 54.1, 30.2, 30.0, 21.1, 19.2, 19.0, 18.9. HRMS: (NSI+) m/z calcd for C29H36N5O9 [M + H]+ 598.2506, found 598.2508.
(2R,3R,4R,5R)-2-(Acetoxymethyl)-5-(6-(((1R,2R)-2-phenoxycyclopentyl)amino)-9H-purin-9-yl)tetrahydrofuran-3,4-diyl Diacetate (12)
12 was synthesized according to general procedure D using 7 (0.137 mmol), 6a (0.206 mmol), and NaHCO3 (0.412 mmol). The reaction was heated at reflux for 18 h. After purification with flash column chromatography (EtOAc, 100%), 12 was obtained (50 mg, 0.09 mmol, 65%). 1H NMR: (300 MHz, methanol-d4) δ 8.30 (s, 1H), 8.22 (s, 1H), 7.22 (t, J = 7.7, 2H), 7.06–6.82 (m, 3H), 6.24 (d, J = 5.2, 1H), 6.02 (t, J = 5.6, 1H), 5.72 (t, J = 5.2, 1H), 4.85–4.73 (m, 2H), 4.50–4.33 (m, 3H), 2.38–2.18 (m, 2H), 2.15 (s, 3H), 2.07 (s, 6H), 1.98–1.69 (m, 4H). 13C NMR: (101 MHz, methanol-d4) δ 170.8, 170.0, 169.8, 158.0, 154.4, 152.8, 148.6, 139.4, 129.0, 120.3, 119.5, 115.4, 86.4, 82.5, 80.2, 73.0, 70.6, 62.8, 57.1, 30.0, 29.7, 21.1, 19.2, 19.0, 18.9. HRMS: (NSI+) m/z calcd for C27H32N5O8 [M + H]+ 554.2239, found 554.2245.
(2R,3R,4R,5R)-2-(Acetoxymethyl)-5-(6-(((1R,2R)-2-(3-methoxyphenoxy)cyclopentyl)amino)-9H-purin-9-yl)tetrahydrofuran-3,4-diyl Diacetate (13)
13 was synthesized according to general procedure D using 7 (0.196 mmol), 6e (0.197 mmol), and NaHCO3 (0.394 mmol). The reaction was heated at reflux for 23 h. After purification with flash column chromatography (EtOAc/cHex, 80%), 13 was obtained (63 mg, 0.108 mmol, 55%). 1H NMR: (300 MHz, DMSO-d6) δ 8.38 (s, 1H), 8.26 (s, 1H), 8.15 (d, J = 6.5, 1H), 7.14 (t, J = 8.1, 1H), 6.57–6.44 (m, 3H), 6.22 (d, J = 5.4, 1H), 6.07–6.01 (m, 1H), 5.66–5.61 (m, 1H), 4.89–4.83 (m, 1H), 4.70–4.60 (m, 1H), 4.46–4.34 (m, 2H), 4.28–4.20 (m, 1H), 3.67 (s, 3H), 2.23–2.13 (m, 2H), 2.12 (s, 3H), 2.04 (s, 3H), 2.02 (s, 3H), 1.89–1.62 (m, 4H). 13C NMR: (75 MHz, methanol-d4) δ 170.8, 170.0, 169.8, 160.9, 159.1, 154.3, 152.8, 139.4, 129.4, 119.5, 107.8, 106.0, 101.7, 86.4, 82.6, 80.2, 73.0, 70.6, 62.8, 57.0, 54.3, 30.0, 29.8, 21.1, 19.2, 19.1, 18.9. HRMS: (NSI+) m/z calcd for C28H34N5O9 [M + H]+ 584.2343, found 584.2351.
(2R,3R,4R,5R)-2-(Acetoxymethyl)-5-(6-(((1R,2R)-2-(4-chlorophenoxy)cyclopentyl)amino)-9H-purin-9-yl)tetrahydrofuran-3,4-diyl Diacetate (14)
14 was synthesized according to general procedure D using 7 (0.347 mmol), 6j (0.52 mmol), and NaHCO3 (1.04 mmol). The reaction was heated at reflux for 17 h. After purification with flash column chromatography (EtOAc/cHex, 80%), 14 was obtained (192 mg, 0.327 mmol, 82%). 1H NMR: (300 MHz, methanol-d4) δ 8.19 (s, 1H), 8.10 (s, 1H), 7.12–7.04 (m, 2H), 6.94–6.84 (m, 2H), 6.12 (d, J = 5.2, 1H), 5.91 (t, J = 5.5, 1H), 5.60 (t, J = 5.5, 1H), 4.68–4.63 (m, 1H), 4.63–4.55 (m, 1H), 4.35–4.22 (m, 3H), 2.23–2.05 (m, 2H), 2.03 (s, 3H), 1.95 (s, 6H), 1.87–1.55 (m, 4H). 13C NMR: (75 MHz, methanol-d4) δ 170.8, 170.0, 169.8, 156.7, 154.3, 152.8, 139.4, 128.8, 125.1, 119.5, 116.9, 86.4, 82.9, 80.2, 73.0, 70.6, 62.8, 56.9, 29.8, 29.6, 21.0, 19.3, 19.1, 18.9. HRMS: (NSI+) m/z calcd for C27H31ClN5O8 [M + H]+ 588.1854, found 588.1856.
(2R,3S,4R,5R)-2-(Hydroxymethyl)-5-(6-(((1R,2R)-2-((4-isopropylbenzyl)oxy)cyclopentyl)amino)-9H-purin-9-yl)tetrahydrofuran-3,4-diol (15)
15 was synthesized according to general procedure E using the ∼1:1 mixture of 7 and 8 from above and K2CO3 (0.137 mmol). The reaction was run for 1.5 h. After purification with flash column chromatography (MeOH/EtOAc, 2%), 15 was obtained as a colorless solid (27 mg, 0.055 mmol, 19% over two steps). 1H NMR: (300 MHz, DMSO-d6) δ 8.37 (s, 1H), 8.23 (br s, 1H), 7.93 (d, J = 5.6, 1H), 7.22–7.12 (m, 4H), 5.89 (d, J = 6.1, 1H), 5.46–5.37 (m, 2H), 5.18 (d, J = 4.6, 1H), 4.66–4.44 (m, 3H), 4.62 (q, J = 6.0, 1H), 4.18–4.12 (m, 1H), 4.04–3.94 (m, 2H), 3.72–3.63 range (m, 1H), 3.61–3.50 (m, 1H), 2.84 (sept, J = 6.7, 1H), 2.13–1.88 (m, 2H), 1.78–1.56 (m, 4H), 1.17 (d, J = 6.9, 6H). HRMS: (NSI+) m/z calcd for C25H34N5O5 [M + H]+ 484.2539, found 484.2554. UPLC analysis: tR = 2.87 min; peak area > 95% (detection at 254 nm).
(2R,3R,4S,5R)-2-(6-(((1R,2R)-2-((4-(tert-Butyl)benzyl)oxy)cyclopentyl)amino)-9H-purin-9-yl)-5-(hydroxymethyl)tetrahydrofuran-3,4-diol (16)
16 was synthesized according to general procedure E using 9 (0.083 mmol) and K2CO3 (0.05 mmol). The reaction was run for 30 min. After purification with flash column chromatography (MeOH/CH2Cl2, 5%), 16 was obtained as a colorless solid (30 mg, 0.06 mmol, 73%). 1H NMR: 300 MHz, methanol-d4) δ 8.16 (s, 2H), 7.21 (d, J = 8.4, 2H), 7.11 (d, J = 8.3, 2H), 5.85 (d, J = 6.5, 1H), 4.60–4.44 (m, 4H), 4.22 (dd, J = 5.1, 2.5, 1H), 4.06–4.09 (m, 1H), 3.87–3.91 (m, 1H), 3.79 (dd, J = 12.6, 2.5, 1H), 3.64 (dd, J = 12.5, 2.6, 1H), 2.20–2.09 (m, 1H), 1.99–1.85 (m, 1H), 1.80–1.45 (m, 3H), 1.58–1.18 (m, 1H) 1.18 (s, 9H). 13C NMR: (101 MHz, methanol-d4) δ 154.5, 152.2, 150.2, 147.8, 140.1, 135.5, 127.3, 124.7, 120.0, 90.0, 86.9, 84.6, 74.1, 71.3, 70.7, 62.1, 56.9, 33.9, 30.4, 30.2, 30.0, 21.1. HRMS: (NSI+) m/z calcd for C26H36N5O5 [M + H]+ 498.2690, found 498.2711. UPLC analysis: tR = 3.01 min; peak area > 95% (detection at 254 nm).
4-((((1R,2R)-2-((9-((2R,3R,4S,5R)-3,4-Dihydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)-9H-purin-6-yl)amino)cyclopentyl)oxy)methyl)benzonitrile (17)
17 was synthesized according to general procedure E using 10 (0.552 mmol) and K2CO3 (0.552 mmol). The reaction was run for 3 h. After purification with flash column chromatography (EtOAc, 100%), 17 was obtained as a colorless solid (183 mg, 0.392 mmol, 71%). 1H NMR: (300 MHz, DMSO-d6) δ 8.37 (s, 1H), 8.23 (s, 1H), 7.93 (d, J = 6.0, 1H), 7.76 (d, J = 8.3, 2H), 7.48 (d, J = 8.0, 2H), 5.89 (d, J = 6.2, 1H), 5.42 (d, J = 6.3, 1H), 5.38 (dd, J = 7.1, 4.5, 1H), 5.18 (d, J = 4.7, 1H), 4.69–4.50 (m, 1H), 4.67 (s, 2H), 4.61 (q, J = 5.9, 1H), 4.28–4.11 (m, 1H), 4.08–3.88 (m, 2H), 3.68 (dt, J = 12.0, 4.1, 1H), 3.55 (ddd, J = 11.7, 7.2, 3.7, 1H), 2.10–2.01 (m, 1H), 2.00–1.89 (m, 1H), 1.89–1.55 (m, 4H). HRMS: (NSI+) m/z calcd for C23H27N6O5 [M + H]+ 467.2037, found 467.2031. UPLC analysis: tR = 2.28 min; peak area > 95% (detection at 254 nm).
4-((((1R,2R)-2-((9-((2R,3R,4S,5R)-3,4-Dihydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)-9H-purin-6-yl)amino)cyclopentyl)oxy)methyl)benzamide (18)
10 (0.186 mmol) was dissolved in MeOH (15 mL) and K2CO3 (0.186 mmol) was added. The reaction mixture was stirred at room temperature for 4 h and concentrated under reduced pressure. The residue was dissolved in MeOH (1.5 mL), and aq. 30% H2O2 (0.15 mL) was added. The solution was heated to 40 °C and stirred for 3 h. The volatiles were removed under reduced pressure. After purification with flash column chromatography (MeOH/EtOAc, 20%), 18 was obtained as a colorless solid (75 mg, 0.155 mmol, 83%). 1H NMR: 400 MHz, methanol-d4) δ 8.24 (s, 2H), 7.76 (d, J = 8.3, 2H), 7.39 (d, J = 7.9, 2H), 5.95 (d, J = 6.5, 1H), 4.76 (dd, J = 6.5, 5.1, 1H), 4.75–4.56 (m, 1H), 4.71 (s, 2H), 4.33 (dd, J = 5.1, 2.5, 1H), 4.17 (q, J = 2.6, 1H), 4.00 (dt, J = 6.5, 4.1, 1H), 3.89 (dd, J = 12.6, 2.5, 1H), 3.75 (dd, J = 12.6, 2.7, 1H), 2.29–2.18 (m, 1H), 2.14–1.96 (m, 1H), 1.96–1.72 (m, 3H), 1.72–1.55 (m, 1H). 13C NMR: (101 MHz, methanol-d4) δ 172.1, 155.8, 153.5, 149.2, 144.5, 141.5, 133.8, 128.6, 128.4, 121.4, 91.4, 88.2, 86.6, 75.4, 72.7, 71.7, 63.5, 58.3, 31.5, 31.4, 22.5. HRMS: (NSI+) m/z calcd for C23H28N6NaO6 [M + Na]+ 485.2143, found 485.2156. UPLC analysis: tR = 1.79 min; peak area > 95% (detection at 254 nm).
(2R,3S,4R,5R)-2-(Hydroxymethyl)-5-(6-(((1R,2R)-2-((3-methoxybenzyl)oxy)cyclopentyl)amino)-9H-purin-9-yl)tetrahydrofuran-3,4-diol (19)
19 was synthesized according to general procedure E using 11 (0.084 mmol) and K2CO3 (0.05 mmol). The reaction was run for 30 min. After purification with flash column chromatography (MeOH/EtOAc, 5–10%), 19 was obtained as a colorless solid (36 mg, 0.076 mmol, 93%). 1H NMR: (300 MHz, DMSO-d6) δ 8.36 (s, 1H), 8.23 (s, 1H), 7.93 (d, J = 4.9, 1H), 7.20 (t, J = 7.7, 1H), 6.89–6.73 (m, 3H), 5.89 (d, J = 6.2, 1H), 5.46–5.37 (m, 2H), 5.18 (d, J = 4.6, 1H), 4.71–4.56 (m, 2H), 4.54 (s, 2H), 4.18–4.11 (m, 1H), 4.06–3.92 (m, 2H), 3.72–3.67 (m, 1H), 3.67 (s, 3H), 3.61–3.52 (m, 1H), 2.12–1.89 (m, 2H), 1.76–1.57 (m, 4H). 13C NMR: (101 MHz, methanol-d4) δ 159.8, 154.4, 152.1, 147.8, 140.2, 140.0, 128.8, 120.0, 119.5, 112.7, 112.6, 90.0, 86.9, 84.8, 74.1, 71.3, 70.8, 62.1, 56.7, 54.1, 30.1, 30.0, 21.1. HRMS: (NSI+) m/z calcd for C23H30N5O6 [M + H]+ 472.2186, found 472.2191. UPLC analysis: tR = 2.33 min; peak area > 95% (detection at 254 nm).
(2R,3R,4S,5R)-2-(6-(((1R,2R)-2-((3-Bromobenzyl)oxy)cyclopentyl)amino)-9H-purin-9-yl)-5-(hydroxymethyl)tetrahydrofuran-3,4-diol (20)
20 was synthesized according to general procedure G using 7 (0.296 mmol), 3f (0.326 mmol), and NaHCO3 (0.652 mmol). The reaction was heated at reflux for 18 h. After purification with flash column chromatography (EtOAc, 100%) a mixture of the substitution product and fully and semi-deacetylated products was isolated (53 mg). This mixture was dissolved in MeOH (5 mL), and K2CO3 (0.05 mmol) was added. After stirring for 30 min at room temperature, the reaction mixture was filtered and concentrated under reduced pressure. After purification with flash column chromatography (MeOH/EtOAc, 5%), 20 was obtained as a colorless solid (33 mg, 0.063 mmol, 66% over two steps). 1H NMR: (300 MHz, methanol-d4) δ 8.15 (s, 1H), 8.14 (s, 1H), 7.37–7.32 (m, 1H), 7.25–6.99 (m, 3H), 5.85 (d, J = 6.5, 1H), 4.69–4.62 (m, 1H), 4.62–4.45 (m, 3H), 4.25–4.20 (m, 1H), 4.07 (q, J = 2.5, 1H), 3.89–3.82 (m, 1H), 3.79 (dd, J = 12.6, 2.4, 1H), 3.64 (dd, J = 12.6, 2.6, 1H), 2.19–2.05 (m, 1H), 1.97–1.85 (m, 1H), 1.81–1.45 (m, 4H). 13C NMR: (75 MHz, methanol-d4) δ 154.4, 152.2, 141.5, 140.1, 130.1, 130.0, 129.6, 125.8, 121.9, 120.0, 90.0, 86.9, 85.1, 74.1, 71.3, 70.0, 62.2, 56.7, 30.1, 29.9, 21. HRMS: (NSI+) m/z calcd for C22H27BrN5O5 [M + H]+ 520.1189, found 520.1189. UPLC analysis: tR = 2.58 min; peak area > 95% (detection at 254 nm).
(2R,3R,4S,5R)-2-(6-(((1R,2R)-2-((4-Bromobenzyl)oxy)cyclopentyl)amino)-9H-purin-9-yl)-5-(hydroxymethyl)tetrahydrofuran-3,4-diol (21)
21 was synthesized according to general procedure G using 7 (0.209 mmol), 3g (0.209 mmol), and NaHCO3 (0.417 mmol). The reaction was heated at reflux for 18 h. The crude material was deacetylated using K2CO3 (0.125 mmol) in MeOH for 30 min at room temperature. After purification with flash column chromatography (MeOH/EtOAc, 5%), 21 was obtained (25 mg, 0.048 mmol, 23% over two steps). 1H NMR (300 MHz, methanol-d4) δ 8.15 (s, 1H), 8.14 (s, 1H), 7.29 (d, J = 8.4, 2H), 7.11 (d, J = 8.3, 2H), 5.85 (d, J = 6.4, 1H), 4.65 (dd, J = 6.4, 5.1, 1H), 4.62–4.51 (m, 1H), 4.49 (s, 2H), 4.23 (dd, J = 5.1, 2.5, 1H), 4.07 (q, J = 2.5, 1H), 3.90–3.84 (m, 1H), 3.79 (dd, J = 12.6, 2.5, 1H), 3.64 (dd, J = 12.6, 2.6, 1H), 2.19–2.07 (m, 1H), 1.97–1.84 (m, 1H), 1.80–1.44 (m, 4H). 13C NMR: (75 MHz, methanol-d4) δ 154.4, 152.1, 140.1, 138.1, 130.9, 129.1, 120.7, 120.0, 90.0, 86.9, 85.0, 74.1, 71.3, 70.1, 62.1, 56.9, 30.1, 30.0, 21. HRMS: (NSI+) m/z calcd for C22H27BrN5O5 [M + H]+ 520.1187, found 520.1190. UPLC analysis: tR = 2.64 min; peak area > 99% (detection at 254 nm).
(2R,3R,4S,5R)-2-(6-(((1R,2R)-2-((2-Chlorobenzyl)oxy)cyclopentyl)amino)-9H-purin-9-yl)-5-(hydroxymethyl)tetrahydrofuran-3,4-diol (22)
22 was synthesized according to general procedure G using 7 (0.229 mmol), 3h (0.229 mmol), and NaHCO3 (0.458 mmol). The reaction was heated at reflux for 19 h. The crude material was deacetylated using K2CO3 (0.22 mmol) in MeOH for 30 min at room temperature. After purification with flash column chromatography (MeOH/EtOAc, 5%), 22 was obtained (51 mg, 0.107 mmol, 47% over two steps). 1H NMR: (300 MHz, methanol-d4) δ 8.13 (br s, 2H), 7.39–7.32 (m, 1H), 7.22–7.02 (m, 3H), 5.84 (d, J = 6.5, 1H), 4.71–4.47 (m, 4H), 4.25–4.20 (m, 1H), 4.07 (q, J = 2.5, 1H), 3.94–3.87 (m, 1H), 3.84–3.72 (m, 1H), 3.67–3.59 (m, 1H), 2.20–2.06 (m, 1H), 2.00–1.85 (m, 1H), 1.82–1.62 (m, 3H), 1.61–1.45 (m, 1H). 13C NMR: (75 MHz, methanol-d4) δ 154.4, 152.1, 147.8, 140.0, 136.3, 132.5, 128.9, 128.7, 128.4, 126.5, 120.0, 90.0, 86.9, 85.5, 74.1, 71.3, 68.1, 62.2, 56.7, 30.1, 30.0, 21.2. HRMS: (NSI+) m/z calcd for C22H27ClN5O5 [M + H]+ 476.1673, found 476.1695. UPLC analysis: tR = 2.51 min; peak area > 99% (detection at 254 nm).
(2R,3R,4S,5R)-2-(6-(((1R,2R)-2-((3-Chlorobenzyl)oxy)cyclopentyl)amino)-9H-purin-9-yl)-5-(hydroxymethyl)tetrahydrofuran-3,4-diol (23)
23 was synthesized according to general procedure G using 7 (0.336 mmol), 3i (0.336 mmol), and NaHCO3 (0.732 mmol). The reaction was heated at reflux for 19 h. The crude material was deacetylated using K2CO3 (0.22 mmol) in MeOH for 30 min at room temperature. After purification with flash column chromatography (MeOH/EtOAc, 4%), 23 was obtained (45 mg, 0.095 mmol, 26% over two steps). 1H NMR: (300 MHz, methanol-d4) δ 8.14 (s, 1H), 8.13 (s, 1H), 7.19 (s, 1H), 7.04–7.03 (m, 3H), 5.85 (d, J = 6.4, 1H), 4.69–4.61 (m, 1H), 4.61–4.44 (m, 3H), 4.26–4.20 (m, 1H), 4.07 (q, J = 2.5, 1H), 3.89–3.82 (m, 1H), 3.82–3.74 (m, 1H), 3.69–3.60 (m, 1H), 2.18–2.05 (m, 1H), 1.96–1.79 (m, 2H), 1.78–1.45 (m, 4H). 13C NMR: (75 MHz, methanol-d4) δ 154.4, 152.2, 148.0, 141.3, 140.1, 133.8, 129.3, 127.1, 127.0, 125.4, 120.0, 90.0, 86.9, 85.1, 74.1, 71.3, 70.0, 62.15, 56.8, 30.1, 29.9, 21.1. HRMS: (NSI+) m/z calcd for C22H27ClN5O5 [M + H]+ 476.1699, found 476.1695. UPLC analysis: tR = 2.56 min; peak area > 99% (detection at 254 nm).
(2R,3S,4R,5R)-2-(Hydroxymethyl)-5-(6-(((1R,2R)-2-phenoxycyclopentyl)amino)-9H-purin-9-yl)tetrahydrofuran-3,4-diol (24)
24 was synthesized according to general procedure E using 12 (0.089 mmol) and K2CO3 (0.053 mmol). The reaction was run for 30 min. After purification with flash column chromatography (EtOAc, 100%), 24 was obtained as a colorless solid (16 mg, 0.038 mmol, 42%). 1H NMR (400 MHz, methanol-d4) δ 8.15 (s, 1H), 8.15 (s, 1H), 7.11 (t, J = 7.9, 2H), 6.94–6.87 (m, 2H), 6.77 (t, J = 7.3, 1H), 5.85 (d, J = 6.4, 1H), 4.72–4.67 (m, 1H), 4.66–4.54 (m, 2H), 4.22 (dd, J = 5.1, 2.5, 1H), 4.07 (q, J = 2.6, 1H), 3.79 (dd, J = 12.6, 2.5, 1H), 3.64 (dd, J = 12.5, 2.7, 1H), 2.26–2.05 (m, 2H), 1.60–1.56 (m, 4H). 13C NMR: (75 MHz, methanol-d4) δ 158.0, 154.5, 152.1, 148.0, 140.1, 129.0, 120.4, 120.0, 115.4, 89.9, 86.8, 82.5, 74.1, 71.3, 62.1, 57.2, 29.9, 29.7, 21.0. HRMS: (NSI+) m/z calcd for C21H26N5O5 [M + H]+ 428.1924, found 428.1928. HPLC analysis: tR = 7.22 min; peak area 95% (detection at 254 nm).
(2R,3R,4S,5R)-2-(6-(((1R,2R)-2-(4-(tert-Butyl)phenoxy)cyclopentyl)amino)-9H-purin-9-yl)-5-(hydroxymethyl)tetrahydrofuran-3,4-diol (25)
25 was synthesized according to general procedure G using 7 (0.241 mmol), 6c (0.241 mmol), and NaHCO3 (0.482 mmol). The reaction was heated at reflux for 19 h. The crude material was deacetylated using K2CO3 (0.144 mmol) in MeOH for 30 min at room temperature. After purification with flash column chromatography (MeOH/EtOAc, 3%), 25 was obtained (46 mg, 0.095 mmol, 40% over two steps). 1H NMR: (300 MHz, methanol-d4) δ 8.14 (s, 1H), 8.13 (s, 1H), 7.16–7.09 (m, 2H), 6.84–6.77 (m, 2H), 5.85 (d, J = 6.4, 1H), 4.68–4.51 (m, 3H), 4.25–4.20 (dd, J = 5.1, 2.5, 1H), 4.07 (q, J = 2.3, 1H), 3.78 (dd, J = 12.6, 2.5, 1H), 3.63 (dd, J = 12.6, 2.7, 1H), 2.24–1.98 (m, 2H), 1.85–1.54 (m, 4H), 1.14 (s, 9H). 13C NMR: (75 MHz, methanol-d4) δ 155.6, 154.4, 152.1, 147.9, 143.1, 140.1, 125.7, 120.0, 115.0, 90.0, 86.8, 82.5, 74.1, 71.3, 62.1, 57.0, 33.5, 30.6, 30.0, 29.7, 21.1. HRMS: (NSI+) m/z calcd for C25H34N5O5 [M + H]+ 484.2568, found 484.2554. UPLC analysis: tR = 3.21 min; peak area > 99% (detection at 254 nm).
(2R,3S,4R,5R)-2-(Hydroxymethyl)-5-(6-(((1R,2R)-2-(3-methoxyphenoxy)cyclopentyl)amino)-9H-purin-9-yl)tetrahydrofuran-3,4-Diol (26)
26 was synthesized according to general procedure E using 13 (0.12 mmol) and K2CO3 (0.072 mmol). The reaction was run for 30 min. After purification with flash column chromatography (EtOAc, 100%), 26 was obtained as a solid (36 mg, 0.079 mmol, 66%). 1H NMR: (300 MHz, DMSO-d6) δ 8.38 (s, 1H), 8.23 (s, 1H), 8.10 (d, J = 7.6, 1H), 7.14 (t, J = 8.1, 1H), 6.57–6.42 (m, 3H), 5.89 (d, J = 6.1, 1H), 5.44 (d, J = 6.2, 1H), 5.41–5.35 (m, 1H), 5.19 (d, J = 4.7, 1H), 4.90–4.82 (m, 1H), 4.74–4.64 (m, 1H), 4.64–4.56 (m, 1H), 4.15 (q, J = 4.5, 1H), 3.99–3.94 (m, 1H), 3.73–3.64 (m, 1H) 3.68 (s, 3H), 3.60–3.51 (m, 1H), 2.23–2.09 (m, 2H), 1.87–1.62 (m, 4H). 13C NMR: (75 MHz, methanol-d4) δ 160.9, 159.1, 154.4, 152.1, 140.1, 129.4, 120.0, 107.8, 105.9, 101.7, 90.0, 86.8, 82.6, 74.1, 71.3, 62.1, 57.1, 54.3, 29.9, 29.8, 21.1. HRMS: (NSI+) m/z calcd for C22H28N5O6 [M + H]+ 458.2030, found 458.2034. UPLC analysis: tR = 2.50 min; peak area > 95% (detection at 254 nm).
(2R,3R,4S,5R)-2-(6-(((1R,2R)-2-(3-Bromophenoxy)cyclopentyl)amino)-9H-purin-9-yl)-5-(hydroxymethyl)tetrahydrofuran-3,4-diol (27)
27 was synthesized according to general procedure G using 7 (0.12 mmol), 6f (0.12 mmol), and NaHCO3 (0.239 mmol). The reaction was heated at reflux for 18 h. After purification with flash column chromatography (EtOAc, 100%), a mixture of the substitution product and fully and semi-deacetylated products was isolated (40 mg). This mixture was dissolved in MeOH (5 mL), and K2CO3 (0.033 mmol) was added. After stirring for 30 min at room temperature, the reaction mixture was filtered and concentrated under reduced pressure. After purification with flash column chromatography (EtOAc, 100%), 27 was obtained as a solid (21 mg, 0.042 mmol, 35% over two steps). 1H NMR (300 MHz, methanol-d4) δ 8.24 (s, 1H), 8.16 (s, 1H), 7.49 (br s, 1H), 7.02 (t, J = 8.0, 1H), 6.96–6.82 (m, 2H), 5.86 (d, J = 6.4, 1H), 4.70–4.62 (m, 2H), 4.61–4.51 (m, 1H), 4.23 (dd, J = 5.1, 2.5, 1H), 4.09–4.05 (m, 1H), 3.79 (dd, J = 12.6, 2.5, 1H), 3.65 (dd, J = 12.5, 2.6, 1H), 2.23–1.97 (m, 2H), 1.90–1.58 (m, 4H). 13C NMR: (75 MHz, methanol-d4) δ 158.9, 154.3, 152.2, 140.2, 130.3, 123.3, 122.3, 120.0, 118.4, 114.8, 90.0, 86.9, 82.8, 74.1, 71.3, 62.1, 56.6, 29.6, 29.4, 21.0. HRMS: (NSI+) m/z calcd for C21H25BrN5O5 [M + H]+ 506.1025, found 506.1034. UPLC analysis: tR = 2.83 min; peak area > 95% (detection at 254 nm).
(2R,3R,4S,5R)-2-(6-(((1R,2R)-2-(2-Chlorophenoxy)cyclopentyl)amino)-9H-purin-9-yl)-5-(hydroxymethyl)tetrahydrofuran-3,4-diol (28)
28 was synthesized according to general procedure G using 7 (0.322 mmol), 6 h (0.322 mmol), and NaHCO3 (0.644 mmol). The reaction was heated at reflux for 18 h. The crude material was deacetylated using K2CO3 (0.193 mmol) in MeOH for 45 min at room temperature. After purification with flash column chromatography (EtOAc, 100%), 28 was obtained (29 mg, 0.063 mmol, 20% over two steps). 1H NMR (300 MHz, methanol-d4) δ 8.15 (s, 1H), 8.14 (s, 1H), 7.30–7.01 (m, 3H), 6.80–6.71 (m, 1H), 5.85 (d, J = 6.4, 1H), 4.76–4.72 (m, 1H), 4.70–4.60 (m, J = 6.4, 5.1, 2H), 4.22 (dd, J = 5.1, 2.5, 1H), 4.07 (q, J = 2.5, 1H), 3.79 (dd, J = 12.6, 2.5, 1H), 3.64 (dd, J = 12.6, 2.6, 1H), 2.31–2.19 (m, 1H), 2.13–2.00 (m, 1H), 1.93–1.75 (m, 3H), 1.70–1.57 (m, 1H). 13C NMR: (75 MHz, methanol-d4) δ 154.4, 153.5, 152.1, 140.2, 129.8, 127.4, 123.2, 121.3, 120.0, 115.6, 89.9, 86.8, 83.8, 74.1, 71.3, 62.1, 56.9, 29.7, 29.6, 21.1. HRMS: (NSI+) m/z calcd for C21H25ClN5O5 [M + H]+ 462.1529, found 462.1539. UPLC analysis: tR = 2.72 min; peak area > 99% (detection at 254 nm).
(2R,3R,4S,5R)-2-(6-(((1R,2R)-2-(3-Chlorophenoxy)cyclopentyl)amino)-9H-purin-9-yl)-5-(hydroxymethyl)tetrahydrofuran-3,4-diol (29)
29 was synthesized according to general procedure G using 7 (0.234 mmol), 6i (0.234 mmol), and NaHCO3 (0.467 mmol). The reaction was heated at reflux for 18 h. The crude material was deacetylated using K2CO3 (0.138 mmol) in MeOH for 2 h at room temperature. After purification with flash column chromatography (MeOH/EtOAc, 6%), 29 was obtained (36 mg, 0.078 mmol, 33% over two steps). 1H NMR: (300 MHz, DMSO-d6) δ 8.39 (s, 1H), 8.27 (s, 1H), 8.09 (d, J = 7.1, 1H), 7.28 (t, J = 8.2, 2H), 7.00–6.91 (m, 2H), 5.90 (d, J = 6.1, 1H), 5.43 (d, J = 6.2, 1H), 5.41–5.34 (m, 1H), 5.19 (d, J = 4.7, 1H), 4.91–4.83 (m, 1H), 4.71–4.57 (m, 2H), 4.15 (q, J = 4.7, 2H), 4.00–3.95 (m, 1H), 3.73–3.64 (m, 1H), 3.61–3.51 (m, 1H), 2.22–2.07 (m, 2H), 1.88–1.64 (m, 4H). 13C NMR: (101 MHz, methanol-d4) δ 158.9, 154.4, 152.1, 152.1, 140.2, 134.4, 130.0, 120.3, 120.0, 115.6, 114.3, 90.0, 86.8, 82.9, 74.1, 71.3, 62.1, 56.8, 29.6, 29.5, 21.0. HRMS: (NSI+) m/z calcd for C21H25ClN5O5 [M + H]+ 462.1543, found 462.1539. HPLC analysis: tR = 7.16 min; peak area 94% (detection at 254 nm).
(2R,3R,4S,5R)-2-(6-(((1R,2R)-2-(4-Chlorophenoxy)cyclopentyl)amino)-9H-purin-9-yl)-5-(hydroxymethyl)tetrahydrofuran-3,4-diol (30)
30 was synthesized according to general procedure E using 14 (0.325 mmol) and K2CO3 (0.195 mmol). The reaction was run for 45 min. After purification with flash column chromatography (MeOH/EtOAc, 10%), 30 was obtained as a solid (54 mg, 0.117 mmol, 36%). 1H NMR: (300 MHz, DMSO-d6) δ 8.38 (s, 1H), 8.25 (s, 1H), 8.08 (d, J = 7.5, 1H), 7.30 (d, J = 8.9, 2H), 7.03 (d, J = 8.3, 2H), 5.90 (d, J = 6.1, 1H), 5.43 (d, J = 6.0, 1H), 5.40–5.33 (m, 1H), 5.18 (d, J = 4.6, 1H), 4.87–4.80 (m, 1H), 4.74–4.56 (m, 2H), 4.15 (q, J = 4.2, 1H), 3.97 (dd, J = 9.9, 3.3, 2H), 3.72–3.63 (m, 1H), 3.59–3.50 (m, 1H), 2.24–2.06 (m, 2H), 1.88–1.60 (m, 4H). 13C NMR: (75 MHz, methanol-d4) δ 156.7, 154.4, 152.1, 148.0, 140.2, 128.8, 125.2, 120.0, 116.9, 89.9, 86.8, 82.9, 74.1, 71.3, 62.1, 57.0, 29.7, 29.6, 21.0. HRMS: (NSI+) m/z calcd for C21H25ClN5O5 [M + H]+ 462.1525, found 462.1539. UPLC analysis: tR = 2.81 min; peak area > 99% (detection at 254 nm).
(3aS,4S,6R,6aR)-N-Ethyl-6-(6-(((1R,2R)-2-((3-methoxybenzyl)oxy)cyclopentyl)amino)-9H-purin-9-yl)-2,2-dimethyltetrahydrofuro[3,4-d][1,3]dioxole-4-carboxamide (32)
32 was synthesized according to general procedure D using 31 (0.256 mmol), 3e (0.256 mmol), and NaHCO3 (0.698 mmol). The reaction was heated at reflux for 19 h. After purification with flash column chromatography (MeOH/EtOAc, 3%), 32 was obtained (92 mg, 0.166 mmol, 71%). 1H NMR: (300 MHz, methanol-d4) δ 8.25 (s, 1H), 8.17 (s, 1H), 7.16 (t, J = 8.1, 1H), 6.89–6.82 (m, 2H), 6.77–6.72 (m, 1H), 6.32 (d, J = 1.4, 1H), 5.60 (dd, J = 6.1, 1.9, 1H), 5.48 (dd, J = 6.1, 1.0, 1H), 4.72–4.57 (m, 1H), 4.63 (d, J = 1.9, 1H), 4.60 (s, 2H), 4.01–3.94 (m, 1H), 3.71 (s, 3H), 2.95–2.74 (m, 2H), 2.30–2.17 (m, 1H), 2.06–1.96 (m, 1H), 1.91–1.69 (m, 3H), 1.67–1.59 (m, 1H), 1.58 (s, 3H), 1.39 (s, 3H), 0.63 (t, J = 7.3, 3H). 13C NMR: (75 MHz, methanol-d4) δ 170.0, 159.7, 154.3, 152.7, 148.3, 140.4, 140.3, 128.9, 119.5, 119.3, 113.5, 112.7, 112.5, 91.0, 87.2, 84.8, 83.8, 83.7, 70.7, 56.8, 54.2, 33.4, 30.2, 30.0, 25.8, 24.0, 21.1, 12.8. HRMS: (NSI+) m/z calcd for C28H37N6O6 [M + H]+ 553.2774, found 553.2769.
(3aS,4S,6R,6aR)-6-(6-(((1R,2R)-2-((3-Bromobenzyl)oxy)cyclopentyl)amino)-9H-purin-9-yl)-N-ethyl-2,2-dimethyltetrahydrofuro[3,4-d][1,3]dioxole-4-carboxamide (33)
33 was synthesized according to general procedure D using 31 (0.359 mmol), 3f (0.326 mmol), and NaHCO3 (0.978 mmol). The reaction was heated at reflux for 18 h. After purification with flash column chromatography (EtOAc, 100%), 33 was obtained (119 mg, 0.198 mmol, 55%). 1H NMR: (300 MHz, methanol-d4) δ 8.14 (s, 1H), 8.07 (s, 1H), 7.32 (br s, 1H), 7.23–6.97 (m, 3H), 6.20 (d, J = 1.6, 1H), 5.47 (dd, J = 6.1, 1.9, 1H), 5.39–5.32 (m, 1H), 4.62–4.42 (m, 4H), 3.87–3.80 (m, 1H) 2.85–2.65 (m, 2H), 2.17–2.02 (m, 1H), 1.94–1.82 (m, 1H), 1.77–1.56 (m, 3H), 1.55–1.46 (m, 1H), 1.45 (s, 3H), 1.27 (s, 3H), 0.52 (t, J = 7.3, 3H). 13C NMR: (75 MHz, methanol-d4) δ 170.0, 154.2, 152.7, 148.2, 141.5, 140.5, 130.0, 130.0, 129.7, 125.8, 121.9, 119.3, 113.5, 91.0, 87.2, 85.0, 83.8, 83.7, 69.9, 56.7, 33.4, 30.2, 30.0, 25.8, 24.1, 21.1, 12.8. HRMS: (NSI+) m/z calcd for C27H34BrN6O5 [M + H]+ 601.1767, found 601.1796.
(3aS,4S,6R,6aR)-6-(6-(((1R,2R)-2-((4-Bromobenzyl)oxy)cyclopentyl)amino)-9H-purin-9-yl)-N-ethyl-2,2-dimethyltetrahydrofuro[3,4-d][1,3]dioxole-4-carboxamide (34)
34 was synthesized according to general procedure D using 31 (0.163 mmol), 3g (0.163 mmol), and NaHCO3 (0.489 mmol). The reaction was heated at reflux for 17 h. After purification with flash column chromatography (EtOAc, 100%), 34 was obtained (65 mg, 0.108 mmol, 66%). 1H NMR: (300 MHz, methanol-d4) δ 8.12 (s, 1H), 8.07 (s, 1H), 7.29–7.22 (m, 2H), 7.13–7.06 (m, 2H), 6.21 (d, J = 1.4, 1H), 5.49 (dd, J = 6.1, 1.9, 1H), 5.37 (dd, J = 6.1, 1.4, 1H), 4.60–4.42 (m, 4H), 3.90–3.82 (m, 1H), 2.84–2.63 (m, 2H), 2.18–2.04 (m, 1H), 1.98–1.83 (m, 1H), 1.76–1.56 (m, 3H), 1.54–1.42 (m, 1H), 1.46 (s, 3H), 1.28 (s, 3H), 0.51 (t, J = 7.3, 3H). 13C NMR: (75 MHz, methanol-d4) δ 170.1, 154.2, 152.7, 148.3, 140.5, 138.1, 130.9, 129.1, 120.7, 119.3, 113.5, 91.0, 87.3, 85.0, 83.9, 83.7, 70.1, 56.7, 33.4, 30.2, 30.0, 25.7, 24.0, 21.1, 12.8. HRMS: (NSI+) m/z calcd for C27H34BrN6O5 [M + H]+ 601.1789, found 601.1769.
(3aS,4S,6R,6aR)-6-(6-(((1R,2R)-2-((2-Chlorobenzyl)oxy)cyclopentyl)amino)-9H-purin-9-yl)-N-ethyl-2,2-dimethyltetrahydrofuro[3,4-d][1,3]dioxole-4-carboxamide (35)
35 was synthesized according to general procedure D using 31 (0.221 mmol), 3h (0.221 mmol), and NaHCO3 (0.663 mmol). The reaction was heated at reflux for 19 h. After purification with flash column chromatography (EtOAc/cHex, 90%), 35 was obtained (79 mg, 0.141 mmol, 64%). 1H NMR: (300 MHz, methanol-d4) δ 8.13 (s, 1H), 8.06 (s, 1H), 7.40–7.31 (m, 1H), 7.21–7.02 (m, 3H), 6.21 (d, J = 1.4, 1H), 5.48 (dd, J = 6.1, 1.9, 1H), 5.35 (dd, J = 6.1, 1.4, 1H), 4.69–4.52 (m, 3H), 4.51 (d, J = 1.9, 1H), 3.94–3.86 (m, 1H), 2.84–2.62 (m, 2H), 2.22–2.05 (m, 1H), 2.00–1.86 (m, 1H), 1.85–1.58 (m, 3H), 1.57–1.45 (m, 1H), 1.45 (s, 3H), 1.27 (s, 3H), 0.51 (t, J = 7.3, 3H). 13C NMR: (75 MHz, methanol-d4) δ 170.1, 154.2, 152.7, 148.3, 140.5, 136.3, 132.4, 128.9, 128.8, 128.4, 126.5, 119.3, 113.5, 91.0, 87.2, 85.4, 83.8, 83.7, 68.1, 56.9, 33.4, 30.2, 30.0, 25.7, 24.0, 21.2, 12.8. HRMS: (NSI+) m/z calcd for C27H34ClN6O5 [M + H]+ 557.2265, found 557.2274.
(3aS,4S,6R,6aR)-6-(6-(((1R,2R)-2-((3-Chlorobenzyl)oxy)cyclopentyl)amino)-9H-purin-9-yl)-N-ethyl-2,2-dimethyltetrahydrofuro[3,4-d][1,3]dioxole-4-carboxamide (36)
36 was synthesized according to general procedure D using 31 (0.305 mmol), 3i (0.305 mmol), and NaHCO3 (0.915 mmol). The reaction was heated at reflux for 18 h. After purification with flash column chromatography (EtOAc, 100%), 36 was obtained (115 mg, 0.206 mmol, 68%). 1H NMR: (300 MHz, methanol-d4) δ 8.26 (s, 1H), 8.19 (s, 1H), 7.30 (br s, 1H), 7.27–7.14 (m, 3H), 6.33 (d, J = 1.5, 1H), 5.60 (dd, J = 6.1, 1.9, 1H), 5.48 (dd, J = 6.1, 1.5, 1H), 4.72–4.57 (m, 4H), 4.00–3.93 (m, 1H), 2.98–2.75 (m, 2H), 2.30–2.17 (m, 1H), 2.08–1.96 (m, 1H), 1.92–1.59 (m, 4H), 1.57 (s, 3H), 1.39 (s, 3H), 0.64 (t, J = 7.3, 3H). 13C NMR: (75 MHz, methanol-d4) δ 170.0, 154.2, 152.7, 148.2, 141.3, 140.5, 133.7, 129.4, 127.1, 127.0, 125.4, 119.3, 113.5, 91.0, 87.2, 85.0, 83.8, 83.7, 70.0, 56.8, 33.4, 30.2, 29.9, 25.8, 24.0, 21.1, 12.8. HRMS: (NSI+) m/z calcd for C27H34ClN6O5 [M + H]+ 557.2277, found 557.2274.
(3aS,4S,6R,6aR)-N-Ethyl-2,2-dimethyl-6-(6-(((1R,2R)-2-phenoxycyclopentyl)amino)-9H-purin-9-yl)tetrahydrofuro[3,4-d][1,3]dioxole-4-arboxamide (37)
37 was synthesized according to general procedure D using 31 (0.234 mmol), 6a (0.234 mmol), and NaHCO3 (0.702 mmol). The reaction was heated at reflux for 18 h. After purification with flash column chromatography (EtOAc, 100%), 37 was obtained (90 mg, 0.176 mmol, 75%). 1H NMR: (300 MHz, methanol-d4) δ 8.25 (s, 1H), 8.18 (s, 1H), 7.26–7.17 (m, 2H), 7.05–6.94 (m, 2H), 6.86 (t, J = 7.3, 1H), 6.32 (d, J = 1.4, 1H), 5.60 (dd, J = 6.1, 2.0, 1H), 5.47 (dd, J = 6.0, 1.2, 1H), 4.82–4.68 (m, 2H), 4.63 (d, J = 1.9, 1H), 2.97–2.74 (m, 2H), 2.35–2.12 (m, 2H), 1.97–1.64 (m, 4H), 1.57 (s, 3H), 1.39 (s, 3H), 0.62 (t, J = 7.3, 3H). 13C NMR: (75 MHz, methanol-d4) δ 170.0, 157.9, 154.3, 152.6, 148.6, 140.6, 129.0, 120.3, 119.3, 115.4, 113.5, 91.0, 87.2, 83.8, 83.7, 82.4, 57.1, 33.4, 30.0, 29.7, 25.8, 24.1, 21.1, 12.8. HRMS: (NSI+) m/z calcd for C26H33N6O5 [M + H]+ 509.2505, found 509.2507.
(3aS,4S,6R,6aR)-6-(6-(((1R,2R)-2-(4-(tert-Butyl)phenoxy)cyclopentyl)amino)-9H-purin-9-yl)-N-ethyl-2,2-dimethyltetrahydrofuro[3,4-d][1,3]dioxole-4-carboxamide (38)
38 was synthesized according to general procedure D using 31 (0.185 mmol), 6c (0.185 mmol), and NaHCO3 (0.556 mmol). The reaction was heated at reflux for 18 h. After purification with flash column chromatography (EtOAc, 100%), 38 was obtained (58 mg, 0.103 mmol, 56%). 1H NMR: (300 MHz, methanol-d4) δ 8.12 (s, 1H), 8.07 (s, 1H), 7.12 (d, J = 8.8, 2H), 6.78 (d, J = 8.8, 2H), 6.21 (d, J = 1.4, 1H), 5.49 (dd, J = 6.1, 2.0, 1H), 5.36 (dd, J = 6.1, 1.4, 1H), 4.67–4.54 (m, 2H), 4.51 (d, J = 1.9, 1H), 2.82–2.62 (m, 2H), 2.22–2.02 (m, 2H), 1.87–1.51 (m, 4H), 1.46 (s, 3H), 1.28 (s, 3H), 1.14 (s, 9H), 0.50 (t, J = 7.3, 3H). 13C NMR: (75 MHz, methanol-d4) δ 170.1, 155.6, 154.3, 152.6, 148.4, 143.1, 140.6, 125.7, 119.3, 114.9, 113.5, 91.0, 87.3, 83.9, 83.71, 82.5, 57.2, 33.5, 33.4, 30.6, 30.1, 29.8, 25.7, 24.0, 21.1, 12.7.
(3aS,4S,6R,6aR)-N-Ethyl-6-(6-(((1R,2R)-2-(3-methoxyphenoxy)cyclopentyl)amino)-9H-purin-9-yl)-2,2-dimethyltetrahydrofuro[3,4-d][1,3]dioxole-4-carboxamide (39)
39 was synthesized according to general procedure D using 31 (0.27 mmol), 6e (0.27 mmol), and NaHCO3 (0.738 mmol). The reaction was heated at reflux for 18 h. After purification with flash column chromatography (EtOAc, 100%), 39 was obtained (92 mg, 0.166 mmol, 71%). 1H NMR: (300 MHz, methanol-d4) δ 8.25 (s, 1H), 8.18 (s, 1H), 7.09 (t, J = 8.2, 1H), 6.64–6.53 (m, 2H), 6.48–6.41 (m, 1H), 6.32 (d, J = 1.4, 1H), 5.60 (dd, J = 6.1, 1.9, 1H), 5.48 (dd, J = 6.1, 1.4, 1H), 4.80–4.66 (m, 2H), 4.63 (d, J = 1.9, 1H), 3.72 (s, 3H), 2.95–2.74 (m, 2H), 2.34–2.11 (m, 2H), 1.98–1.63 (m, 4H), 1.57 (s, 3H), 1.40 (s, 3H), 0.63 (t, J = 7.3, 3H). 13C NMR: (75 MHz, methanol-d4) δ 170.0, 160.9, 159.1, 154.3, 152.6, 148.4, 140.6, 129.4, 119.3, 113.5, 107.7, 105.9, 101.7, 91.0, 87.2, 83.8, 83.7, 82.5, 57.3, 54.3, 33.4, 30.0, 29.8, 25.7, 24.0, 21.1, 12.7. HRMS: (NSI+) m/z calcd for C27H35N6O6 [M + H]+ 539.2598, found 539.2613.
(3aS,4S,6R,6aR)-6-(6-(((1R,2R)-2-(3-Bromophenoxy)cyclopentyl)amino)-9H-purin-9-yl)-N-ethyl-2,2-dimethyltetrahydrofuro[3,4-d][1,3]dioxole-4-carboxamide (40)
40 was synthesized according to general procedure D using 31 (0.109 mmol), 6f (0.109 mmol), and NaHCO3 (0.218 mmol). The reaction was heated at reflux for 18 h. After purification with flash column chromatography (EtOAc, 100%), 40 was obtained (47 mg, 0.081 mmol, 73%). 1H NMR: (300 MHz, methanol-d4) δ 8.54 (s, 1H), 8.40 (s, 1H), 7.77 (br s, 1H), 7.32 (t, J = 8.0, 1H), 7.25–7.12 (m, 2H), 6.53 (d, J = 1.4, 1H), 5.81 (dd, J = 6.1, 2.0, 1H), 5.68 (dd, J = 6.1, 1.4, 1H), 4.99–4.93 (m, 1H), 4.92–4.80 (m, 1H), 4.83 (d, J = 1.9, 1H), 3.15–2.94 (m, 2H), 2.53–2.31 (m, 2H), 2.21–1.85 (m, 4H), 1.78 (s, 3H), 1.60 (s, 3H), 0.83 (t, J = 7.3, 3H). 13C NMR: (75 MHz, methanol-d4) δ 170.1, 158.9, 154.2, 152.7, 148.5, 140.7, 130.3, 123.3, 122.3, 119.3, 118.4, 114.76, 113.5, 91.1, 87.3, 83.9, 83.7, 82.8, 56.7, 33.4, 29.6, 29.5, 25.7, 24.0, 21.0, 12.7. HRMS: (NSI+) m/z calcd for C26H32BrN6O5 [M + H]+ 587.1595, found 587.1612.
(3aS,4S,6R,6aR)-6-(6-(((1R,2R)-2-(2-Chlorophenoxy)cyclopentyl)amino)-9H-purin-9-yl)-N-ethyl-2,2-dimethyltetrahydrofuro[3,4-d][1,3]dioxole-4-carboxamide (41)
41 was synthesized according to general procedure D using 31 (0.282 mmol), 6h (0.282 mmol), and NaHCO3 (0.846 mmol). The reaction was heated at reflux for 19 h. After purification with flash column chromatography (EtOAc, 100%), 41 was obtained (114 mg, 0.21 mmol, 74%). 1H NMR: (300 MHz, methanol-d4) δ 8.25 (s, 1H), 8.19 (s, 1H), 7.52 (t, J = 5.8, 1H), 7.35–7.26 (m, 2H), 7.23–7.16 (m, 1H), 6.91–6.81 (m, 1H), 6.33 (d, J = 1.4, 1H), 5.60 (dd, J = 6.1, 1.9, 1H), 5.48 (dd, J = 6.1, 1.4, 1H), 4.86–4.80 (m, 1H), 4.80–4.66 (m, 1H), 4.63 (d, J = 1.9, 1H), 2.96–2.75 (m, 2H), 2.40–2.27 (m, 1H), 2.23–2.11 (m, 1H), 2.00–1.83 (m, 3H), 1.80–1.65 (m, 1H), 1.57 (s, 3H), 1.39 (s, 3H), 0.63 (t, J = 7.3, 3H). 13C NMR: (75 MHz, methanol-d4) δ 170.0, 154.2, 153.5, 152.6, 148.4, 140.6, 129.8, 127.5, 123.2, 121.3, 119.3, 115.5, 113.5, 91.0, 87.2, 83.8, 83.8, 83.7, 57.0, 33.4, 29.8, 29.7, 25.8, 24.0, 21.1, 12.8. HRMS: (NSI+) m/z calcd for C26H32ClN6O5 [M + H]+ 543.2106, found 543.2117.
(3aS,4S,6R,6aR)-6-(6-(((1R,2R)-2-(3-Chlorophenoxy)cyclopentyl)amino)-9H-purin-9-yl)-N-ethyl-2,2-dimethyltetrahydrofuro[3,4-d][1,3]dioxole-4-carboxamide (42)
42 was synthesized according to general procedure D using 31 (0.243 mmol), 6i (0.243 mmol), and NaHCO3 (0.73 mmol). The reaction was heated at reflux for 19 h. After purification with flash column chromatography (EtOAc, 100%), 42 was obtained (101 mg, 0.186 mmol, 78%). 1H NMR: (300 MHz, methanol-d4) δ 8.31 (s, 1H), 8.20 (s, 1H), 7.38 (br s, 1H), 7.17 (t, J = 8.1, 1H), 6.99–6.84 (m, 2H), 6.33 (d, J = 1.5, 1H), 5.61 (dd, J = 6.1, 2.0, 1H), 5.48 (dd, J = 6.0, 1.5, 1H), 4.80–4.72 (m, 1H), 4.71–4.59 (m , 2H), 2.97–2.74 (m, 2H), 2.34–2.09 (m, 2H), 2.00–1.65 (m, 4H), 1.58 (s, 3H), 1.40 (s, 3H), 0.64 (t, J = 7.3, 3H). 13C NMR: (75 MHz, methanol-d4) δ 170.0, 158.9, 154.2, 152.6, 148.4, 140.6, 134.4, 130.0, 120.3, 119.3, 115.6, 114.2, 113.5, 91.1, 87.2, 83.8, 83.7, 82.8, 56.8, 33.4, 29.6, 29.5, 25.8, 24.0, 21.0, 12.8. HRMS: (NSI+) m/z calcd for C26H32ClN6O5 [M + H]+ 543.2123, found 543.2117.
(3aS,4S,6R,6aR)-6-(6-(((1R,2R)-2-(4-Chlorophenoxy)cyclopentyl)amino)-9H-purin-9-yl)-N-ethyl-2,2-dimethyltetrahydrofuro[3,4-d][1,3]dioxole-4-carboxamide (43)
43 was synthesized according to general procedure D using 31 (0.35 mmol), 6j (0.35 mmol), and NaHCO3 (1.052 mmol). The reaction was heated at reflux for 20 h. After purification with flash column chromatography (EtOAc, 100%), 43 was obtained (118 mg, 0.217 mmol, 65%). 1H NMR: (300 MHz, methanol-d4) δ 8.25 (s, 1H), 8.19 (s, 1H), 7.21–7.12 (m, 2H), 7.05–6.97 (m, 2H), 6.32 (d, J = 1.4, 1H), 5.60 (dd, J = 6.1, 2.0, 1H), 5.47 (dd, J = 6.1, 1.5, 1H), 4.77–4.65 (m, 2H), 4.63 (d, J = 1.9, 1H), 2.95–2.76 (m, 2H), 2.32–2.11 (m, 2H), 1.97–1.65 (m, 4H), 1.57 (s, 3H), 1.39 (s, 3H), 0.63 (t, J = 7.2, 3H). 13C NMR: (75 MHz, methanol-d4) δ 170.0, 156.7, 154.2, 152.6, 148.4, 140.6, 128.8, 125.1, 119.3, 116.9, 113.5, 91.0, 87.2, 83.8, 83.7, 82.8, 57.0, 33.39, 29.8, 29.6, 25.8, 24.1, 21.0, 12.8. HRMS: (NSI+) m/z calcd for C26H32ClN6O5 [M + H]+ 543.2109, found 543.2117.
(2S,3S,4R,5R)-N-Ethyl-3,4-dihydroxy-5-(6-(((1R,2R)-2-((3-methoxybenzyl)oxy)cyclopentyl)amino)-9H-purin-9-yl)tetrahydrofuran-2-carboxamide (44)
44 was synthesized according to general procedure F using 32 (0.075 mmol) and acetic acid (4 mL). The reaction was run for 23 h. After purification with flash column chromatography (MeOH/CH2Cl2, 6%), 44 was obtained (23 mg, 0.046 mmol, 60%). 1H NMR: (300 MHz, methanol-d4) δ 8.20 (s, 1H), 8.13 (s, 1H), 7.04 (t, J = 8.0, 1H), 6.79–6.72 (m, 2H), 6.68–6.62 (m, 1H), 5.90 (d, J = 7.7, 1H), 4.65 (dd, J = 7.7, 4.8, 1H), 4.62–4.52 (m, 1H), 4.51 (s, 2H), 4.37 (d, J = 1.5, 1H), 4.21 (dd, J = 4.8, 1.6, 1H), 3.92–3.85 (m, 1H), 3.60 (s, 3H), 3.32–3.23 (m, 2H), 2.20–2.07 (m, 1H), 1.98–1.82 (m, 1H), 1.79–1.46 (m, 4H), 1.10 (t, J = 7.3, 3H. 13C NMR: (75 MHz, methanol-d4) δ 170.7, 159.8, 154.4, 152.4, 148.1, 140.6, 140.3, 128.9, 120.1, 119.5, 112.7, 112.6, 89.2, 85.1, 84.8, 73.6, 72.0, 70.8, 58.8, 54.2, 33.7, 30.1, 30.0, 21.1, 13.7. HRMS: (NSI+) m/z calcd for C25H33N6O6 [M + H]+ 513.2441, found 513.2456. UPLC analysis: tR = 2.59 min; peak area > 99% (detection at 254 nm).
(2S,3S,4R,5R)-5-(6-(((1R,2R)-2-((3-Bromobenzyl)oxy)cyclopentyl)amino)-9H-purin-9-yl)-N-ethyl-3,4-dihydroxytetrahydrofuran-2-carboxamide (45)
45 was synthesized according to general procedure F using 33 (0.196 mmol) and acetic acid (16 mL). The reaction was run for 18 h. After purification with flash column chromatography (MeOH/CH2Cl2, 2%), 45 was obtained (69 mg, 0.123 mmol, 63%). 1H NMR: (300 MHz, methanol-d4) δ 8.33 (s, 1H), 8.25 (s, 1H), 7.46 (br s, 1H), 7.37–7.07 (m, 3H), 6.02 (d, J = 7.7, 1H), 4.77 (dd, J = 7.7, 4.8, 1H), 4.71–4.55 (m, 1H), 4.61 (s, 3H), 4.50 (d, J = 1.5, 1H), 4.38–4.28 (m, 1H), 3.42–3.28 (m, 2H), 2.29–2.10 (m, 1H), 1.87–1.59 (m, 4H), 1.21 (t, J = 7.3, 3H). 13C NMR: (75 MHz, methanol-d4) δ 174.0, 154.4, 152.4, 147.9, 141.5, 140.7, 130.1, 130.0, 129.7, 125.8, 121.9, 120.1, 113.6, 89.2, 85.1, 73.6, 72.1, 70.0, 56.8, 33.7, 30.1, 29.9, 19.5, 13.8. HRMS: (NSI+) m/z calcd for C24H30BrN6O5 [M + H]+ 561.1439, found 561.1456. UPLC analysis: tR = 2.97 min; peak area > 95% (detection at 254 nm).
(2S,3S,4R,5R)-5-(6-(((1R,2R)-2-((4-Bromobenzyl)oxy)cyclopentyl)amino)-9H-purin-9-yl)-N-ethyl-3,4-dihydroxytetrahydrofuran-2-carboxamide (46)
46 was synthesized according to general procedure F using 34 (0.108 mmol) and acetic acid (8 mL). The reaction was run for 20 h. After purification with flash column chromatography (MeOH/CH2Cl2, 10%), 46 was obtained (36 mg, 0.064 mmol, 64%). 1H NMR: (300 MHz, methanol-d4) δ 8.19 (s, 1H), 8.14 (s, 1H), 7.28 (d, J = 8.4, 2H), 7.10 (d, J = 8.3, 2H), 5.90 (d, J = 7.7, 1H), 4.65 (dd, J = 7.7, 4.8, 1H), 4.62–4.46 (m, 1H), 4.49 (s, 2H), 4.37 (d, J = 1.5, 1H), 4.21 (dd, J = 4.8, 1.6, 1H), 3.92–3.82 (m, 1H), 3.32–3.21 (m, 2H), 2.20–2.06 (m, 1H), 1.97–1.84 (m, 1H), 1.78–1.58 (m, 3H), 1.58–1.45 (m, 1H), 1.10 (t, J = 7.3, 3H). 13C NMR: (75 MHz, methanol-d4) δ 170.7, 154.4, 152.4, 148.1, 140.7, 138.1, 130.9, 129.1, 120.7, 120.1, 89.2, 85.1, 85.0, 73.6, 72.0, 70.1, 56.9, 33.7, 30.1, 30.0, 21.1, 13.7. HRMS: (NSI+) m/z calcd for C24H30BrN6O5 [M + H]+ calculated 561.1458, found 561.1456. UPLC analysis: tR = 2.94 min; peak area > 99% (detection at 254 nm).
(2S,3S,4R,5R)-5-(6-(((1R,2R)-2-((2-Chlorobenzyl)oxy)cyclopentyl)amino)-9H-purin-9-yl)-N-ethyl-3,4-dihydroxytetrahydrofuran-2-carboxamide (47)
47 was synthesized according to general procedure F using 35 (0.141 mmol) and acetic acid (15 mL). The reaction was run for 19 h. After purification with flash column chromatography (MeOH/CH2Cl2, 8%), 47 was obtained (33 mg, 0.069 mmol, 49%). 1H NMR: (300 MHz, DMSO-d6) δ 8.90 (t, J = 5.6, 1H), 8.42 (s, 1H), 8.29 (s, 1H), 8.07 (br s, 1H), 7.50–7.45 (m, 1H), 7.43–7.38 (m, 1H), 7.33–7.27 (m, 2H), 5.98 (d, J = 7.5, 1H), 5.75 (d, J = 4.3, 1H), 5.54 (d, J = 6.5, 1H), 4.75–4.58 (m, 4H), 4.32 (d, J = 1.5, 1H), 4.18–4.12 (m, 1H), 4.10–4.03 (m, 1H), 3.28–3.17 (m, 2H), 2.15–1.94 (m, 2H), 1.84–1.59 (m, 4H), 1.09 (t, J = 7.2, 3H). 13C NMR (101 MHz, DMSO-d6) δ 169.6, 154.7, 152.6, 148.8, 141.0, 136.8, 132.3, 129.6, 129.5, 129.4, 127.6, 120.5, 88.3, 85.3, 85.1, 73.6, 72.5, 68.0, 56.9, 33.8, 30.8, 30.7, 22.0, 15.2. HRMS: (NSI+) m/z calcd for C24H30ClN6O5 [M + H]+ calculated 517.1943, found 517.1961. UPLC analysis: tR = 2.82 min; peak area > 99% (detection at 254 nm).
(2S,3S,4R,5R)-5-(6-(((1R,2R)-2-((3-Chlorobenzyl)oxy)cyclopentyl)amino)-9H-purin-9-yl)-N-ethyl-3,4-dihydroxytetrahydrofuran-2-carboxamide (48)
48 was synthesized according to general procedure F using 36 (0.206 mmol) and acetic acid (16 mL). The reaction was run for 20 h. After purification with flash column chromatography (MeOH/CH2Cl2, 8%), 48 was obtained (64 mg, 0.123 mmol, 60%). 1H NMR: (300 MHz, methanol-d4) δ 8.32 (s, 1H), 8.24 (s, 1H), 7.32 (br s, 1H), 7.27–7.14 (m, 3H), 6.02 (d, J = 7.7, 1H), 4.77 (dd, J = 7.7, 4.8, 1H), 4.70–4.60 (m, 1H) 4.62 (s, 2H), 4.50 (d, J = 1.5, 1H), 4.34 (dd, J = 4.8, 1.6, 1H), 4.01–3.94 (m, 1H) 3.43–3.29 (m, 2H), 2.31–2.15 (m, 1H), 2.07–1.94 (m, 1H), 1.90–1.58 (m, 4H), 1.21 (t, J = 7.3, 3H). 13C NMR (75 MHz, methanol-d4) δ 170.6, 154.4, 152.4, 148.0, 141.3, 140.7, 133.7, 129.3, 127.1, 127.0, 125.3, 120.1, 89.2, 85.1, 73.6, 72.0, 70.0, 56.8, 33.7, 30.1, 29.9, 21.1, 13.8. HRMS: (NSI+) m/z calcd for C24H30ClN6O5 [M + H]+ 517.1956, found 517.1961. UPLC analysis: tR = 2.87 min; peak area > 99% (detection at 254 nm).
(2S,3S,4R,5R)-N-Ethyl-3,4-dihydroxy-5-(6-(((1R,2R)-2-phenoxycyclopentyl)amino)-9H-purin-9-yl)tetrahydrofuran-2-carboxamide (49)
49 was synthesized according to general procedure F using 37 (0.175 mmol) and acetic acid (16 mL). The reaction was run for 23 h. After purification with flash column chromatography (MeOH/CH2Cl2, 7%), 49 was obtained (56 mg, 0.12 mmol, 68%). 1H NMR: (300 MHz, methanol-d4) δ 8.31 (s, 1H), 8.25 (s, 1H), 7.26–7.16 (m, 2H), 7.05–6.96 (m, 2H), 6.87 (t, J = 7.3, 1H), 6.02 (d, J = 7.7, 1H), 4.83–4.66 (m, 3H), 4.49 (d, J = 1.6, 1H), 4.37–4.30 (m, 1H), 3.42–3.31 (m, 2H), 2.39–2.13 (m, 2H), 2.02–1.65 (m, 4H), 1.20 (t, J = 7.3, 3H). 13C NMR: (75 MHz, methanol-d4) δ 170.6, 157.9, 154.5, 152.4, 148.2, 140.8, 129.0, 120.3, 120.2, 115.4, 89.2, 85.1, 82.5, 73.6, 72.0, 57.1, 33.7, 29.9, 29.7, 21.1, 13.7. HRMS: (NSI+) m/z calcd for C23H29N6O5 [M + H]+ 469.2186, found 469.2194. UPLC analysis: tR = 2.77 min; peak area > 99% (detection at 254 nm).
(2S,3S,4R,5R)-5-(6-(((1R,2R)-2-(4-(tert-Butyl)phenoxy)cyclopentyl)amino)-9H-purin-9-yl)-N-ethyl-3,4-dihydroxytetrahydrofuran-2-carboxamide (50)
50 was synthesized according to general procedure F using 38 (0.103 mmol) and acetic acid (8 mL). The reaction was run for 19 h. After purification with flash column chromatography (MeOH/CH2Cl2, 7%), 50 was obtained (31 mg, 0.059 mmol, 57%). 1H NMR (300 MHz, DMSO-d6) δ 8.88 (t, J = 5.6, 1H), 8.43 (s, 1H), 8.30 (s, 1H), 8.17 (d, J = 6.5, 1H), 7.26 (d, J = 8.8, 2H), 6.87 (d, J = 8.3, 2H), 5.98 (d, J = 7.5, 1H), 5.75 (d, J = 4.3, 1H), 5.54 (d, J = 6.5, 1H), 4.88–4.77 (m, 1H), 4.70–4.57 (m, 2H), 4.32 (d, J = 1.6, 1H), 4.17–4.12 (m, 1H), 3.27–3.18 (m, 2H), 2.22–1.97 (m, 2H), 1.87–1.63 (m, 4H), 1.23 (s, 9H), 1.09 (t, J = 7.2, 3H); (300 MHz, methanol-d4) δ 8.20 (s, 1H), 8.14 (s, 1H), 7.19–7.10 (m, 2H), 6.85–6.73 (m, 2H), 5.90 (d, J = 7.7, 1H), 4.70–4.56 (m, 3H), 4.36 (d, J = 1.6, 1H), 4.21 (dd, J = 4.9, 1.6, 1H), 3.31–3.21 (m, 2H), 2.24–2.02 (m, 2H), 1.90–1.54 (m, 4H), 1.15 (s, 9H), 1.09 (t, J = 7.3, 3H). 13C NMR: (75 MHz, methanol-d4) δ 170.6, 155.6, 154.5, 152.4, 148.1, 143.1, 140.7, 125.7, 120.2, 115.0, 89.2, 85.1, 82.6, 73.6, 72.0, 57.1, 33.7, 33.5, 30.6, 30.0, 29.8, 21.1, 13.7. HRMS: (NSI+) m/z calcd for C27H37N6O5 [M + H]+ 525.2816, found 525.2820. UPLC analysis: tR = 3.44 min; peak area > 99% (detection at 254 nm).
(2S,3S,4R,5R)-N-Ethyl-3,4-dihydroxy-5-(6-(((1R,2R)-2-(3-methoxyphenoxy)cyclopentyl)amino)-9H-purin-9-yl)tetrahydrofuran-2-carboxamide (51)
51 was synthesized according to general procedure F using 39 (0.231 mmol) and acetic acid (16 mL). The reaction was run for 22 h. After purification with flash column chromatography (MeOH/CH2Cl2, 8%), 51 was obtained (49 mg, 0.099 mmol, 43%). 1H NMR: (300 MHz, DMSO-d6) δ 8.87 (t, J = 5.7, 1H), 8.43 (s, 1H), 8.29 (s, 1H), 8.20 (d, J = 7.6, 1H), 7.14 (t, J = 8.1, 1H), 6.59–6.43 (m, 3H), 5.98 (d, J = 7.5, 1H), 5.75 (d, J = 4.3, 1H), 5.54 (d, J = 6.4, 1H), 4.93–4.84 (m, 1H), 4.76–4.54 (m, 2H), 4.32 (d, J = 1.6, 1H), 4.18–4.13 (m, 1H), 3.28–3.20 (m, 2H), 2.25–2.06 (m, 2H), 1.88–1.60 (m, 4H), 1.09 (t, J = 7.2, 3H). 13C NMR: (75 MHz, methanol-d4) δ 170.6, 160.9, 159.1, 154.4, 152.4, 148.1, 140.7, 129.4, 120.2, 107.74, 105.9, 101.7, 89.2, 85.1, 82.6, 73.6, 72.0, 57.1, 54.3, 33.7, 29.9, 29.8, 21.1, 13.7. HRMS: (NSI+) m/z calcd for C24H31N6O6 [M + H]+ 499.2296, found 499.2300. UPLC analysis: tR = 2.78 min; peak area > 99% (detection at 254 nm).
(2S,3S,4R,5R)-5-(6-(((1R,2R)-2-(3-Bromophenoxy)cyclopentyl)amino)-9H-purin-9-yl)-N-ethyl-3,4-dihydroxytetrahydrofuran-2-carboxamide (52)
52 was synthesized according to general procedure F using 40 (0.07 mmol) and acetic acid (4 mL). The reaction was run for 18 h. After purification with flash column chromatography (MeOH/CH2Cl2, 8%), 52 was obtained (28 mg, 0.052 mmol, 74%). 1H NMR: (300 MHz, methanol-d4) δ 8.30 (s, 1H), 8.15 (s, 1H), 7.50 (br s, 1H), 7.01 (t, J = 8.0, 1H), 6.94–6.82 (m, 2H), 5.90 (d, J = 7.7, 1H), 4.70–4.61 (m, 2H), 4.60–4.48 (m, 1H), 4.36 (d, J = 1.5, 1H), 4.25–4.18 (m, 1H), 3.30–3.21 (m, 2H), 2.22–1.98 (m, 2H), 1.93–1.80 (m, 2H), 1.80–1.59 (m, 2H), 1.10 (t, J = 7.3, 3H). 13C NMR: (75 MHz, methanol-d4) δ 170.6, 158.9, 154.4, 152.4, 148.2, 140.8, 130.3, 123.3, 122.3, 118.4, 114.8, 89.2, 85.1, 82.8, 73.6, 72.1, 56.7, 33.7, 29.6 29.4, 21.0, 13.7. HRMS: (NSI+) m/z calcd for C23H28BrN6O5 [M + H]+ 547.1295, found 547.1299. UPLC analysis: tR = 3.17 min; peak area > 99% (detection at 254 nm).
(2S,3S,4R,5R)-5-(6-(((1R,2R)-2-(2-Chlorophenoxy)cyclopentyl)amino)-9H-purin-9-yl)-N-ethyl-3,4-dihydroxytetrahydrofuran-2-carboxamide (53)
53 was synthesized according to general procedure F using 41 (0.21 mmol) and acetic acid (16 mL). The reaction was run for 19 h. After purification with flash column chromatography (MeOH/CH2Cl2, 8%), 53 was obtained (55 mg, 0.109 mmol, 52%). 1H NMR: (300 MHz, DMSO-d6) δ 8.87 (t, J = 5.6, 1H), 8.43 (s, 1H), 8.30 (s, 1H), 8.20 (br s, 1H), 7.42–7.24 (m, 3H), 6.97–6.87 (m, 1H), 5.98 (d, J = 7.5, 1H), 5.75 (d, J = 4.3, 1H), 5.54 (d, J = 6.4, 1H), 4.97–4.90 (m, 1H), 4.77–4.66 (m, 1H), 4.66–4.57 (m, 1H), 4.32 (d, J = 1.6, 1H), 4.18–4.12 (m, 1H), 3.29–3.19 (m, 2H), 2.24–2.14 (m, 2H), 1.91–1.67 (m, 4H), 1.09 (t, J = 7.2, 3H). 13C NMR (75 MHz, methanol-d4) δ 170.6, 154.4, 153.4, 152.4, 148.2, 140.8, 129.8, 127.4, 123.2, 121.2, 120.2, 115.5, 89.2, 85.1, 83.8, 73.6, 72.0, 56.9, 33.71, 29.7, 29.6, 21.1, 13.7. HRMS: (NSI+) m/z calcd for C23H28ClN6O5 [M + H]+ 503.1810, found 503.1804. UPLC analysis: tR = 3.00 min; peak area > 95% (detection at 254 nm).
(2S,3S,4R,5R)-5-(6-(((1R,2R)-2-(3-Chlorophenoxy)cyclopentyl)amino)-9H-purin-9-yl)-N-ethyl-3,4-dihydroxytetrahydrofuran-2-carboxamide (54)
54 was synthesized according to general procedure F using 42 (0.184 mmol) and acetic acid (16 mL). The reaction was run for 20 h. After purification with flash column chromatography (MeOH/CH2Cl2, 8%), 54 was obtained (68 mg, 0.135 mmol, 73%). 1H NMR: (300 MHz, DMSO-d6) δ 8.86 (t, J = 5.6, 1H), 8.44 (s, 1H), 8.33 (s, 1H), 8.19 (d, J = 6.4, 1H), 7.32–7.23 (m, 2H), 6.99–6.92 (m, 2H), 5.98 (d, J = 7.5, 1H), 5.75 (d, J = 4.3, 1H), 5.54 (d, J = 6.4, 1H), 4.94–4.87 (m, 1H), 4.70–4.57 (m, 2H), 4.32 (d, J = 1.6, 1H), 4.18–4.13 (m, 1H), 3.29–3.17 (m, 2H), 2.23–2.08 (m, 2H), 1.90–1.64 (m, 4H), 1.09 (t, J = 7.2, 3H). 13C NMR (75 MHz, methanol-d4) δ 170.6, 158.8, 154.3, 152.3, 148.2, 140.8, 134.4, 129.9, 120.3, 120.1, 115.6, 114.3, 89.2, 85.1, 82.8, 73.6, 72.1, 56.7, 33.7, 29.6, 29.4, 21.0, 13.7. HRMS: (NSI+) m/z calcd for C23H28ClN6O5 [M + H]+ 503.1780, found 503.1804. UPLC analysis: tR = 3.10 min; peak area > 95% (detection at 254 nm).
(2S,3S,4R,5R)-5-(6-(((1R,2R)-2-(4-Chlorophenoxy)cyclopentyl)amino)-9H-purin-9-yl)-N-ethyl-3,4-dihydroxytetrahydrofuran-2-carboxamide (55)
55 was synthesized according to general procedure F using 43 (0.217 mmol) and acetic acid (8 mL). The reaction was run for 23 h. After purification with flash column chromatography (MeOH/CH2Cl2, 7%), 55 was obtained (74 mg, 0.146 mmol, 67%). 1H NMR: (300 MHz, DMSO-d6) δ 8.87 (t, J = 5.7, 1H), 8.43 (s, 1H), 8.32 (s, 1H), 8.19 (d, J = 6.5, 1H), 7.34–7.27 (m, 2H), 7.04 (d, J = 8.1, 2H), 5.98 (d, J = 7.5, 1H), 5.75 (d, J = 4.3, 1H), 5.54 (d, J = 6.4, 1H), 4.90–4.80 (m, 1H), 4.76–4.58 (m, 2H), 4.32 (d, J = 1.6, 1H), 4.18–4.12 (m, 1H), 3.28–3.17 (m, 2H), 2.26–2.10 (m, 2H), 1.91–1.57 (m, 4H), 1.09 (t, J = 7.2, 3H). 13C NMR: (75 MHz, methanol-d4) δ 170.6, 156.6, 154.4, 152.4, 148.2, 140.8, 128.8, 125.1, 120.1, 116.8, 89.2, 85.1, 82.8, 73.6, 72.1, 56.9, 33.7, 29.7, 29.6, 21.0, 13.7. HRMS: (NSI+) m/z calcd for C23H28ClN6O5 [M + H]+ 503.1789, found 503.1804. UPLC analysis: tR = 3.10 min; peak area > 99% (detection at 254 nm).
Cell Culture
CHO-K1-hA1R, CHO-K1-hA2AR, CHO-K1-hA2BR, and CHO-K1-hA3R cells were routinely cultured in Ham’s F-12 supplemented with 10% fetal bovine serum (FBS). HEK293 human Nluc-A1R and HEK293 rat Nluc-A1R were cultured in Dulbecco’s modified Eagle’s medium (DMEM)/Nutrient Mixture F12 supplemented with 10% FBS. All cells were maintained at 37 °C with 5% CO2 in humidified air.
cAMP Accumulation Assay
cAMP accumulation experiments were performed using a LANCE Ultra cAMP detection kit as described previously.25,40 Briefly, CHO-K1 cells stably expressing human WT A1R, A2AR, A2BR, and A3R were seeded at 2000 cells per well in a white 384-well OptiPlate. Cells were then incubated with adenosine receptor ligands (ranging between 100 μM and 1 pM) for 30 min at room temperature. For A1R and A3R expressing cells, 10 and 1 μM forskolin, respectively, was added at the same time as the addition of the adenosine receptor ligands, as we have described previously.13,24,25
NanoBRET Assay for Binding
To determine the affinity (pKi) of adenosine receptor ligands, a NanoBRET competition binding assay was performed as described previously.24,25 Briefly, CA200645 was used at 20 nM. Kinetic data were fitted with the ″kinetic of competitive binding″ model35 (built into Prism v9.1 (GraphPad Software, San Diego, CA)) to determine affinity (pKi) values and the association rate constant (kon) and dissociation rates (koff) for AR ligands. In agreement with our previous studies, we determined the Kd of CA200645 to be 18.29 ± 2.4 nM at the human A1R and 32.96 ± 2.8 nM at the rat A1R.25,40 The ″one-site–Ki model″ derived from the Cheng and Prusoff correction and available in Prism was fitted with the BRET ratio at 10 min post simulation, and affinity (pKi) constant values at equilibrium for adenosine receptor ligands were determined.
Data and Statistical Analysis
Data were analyzed using Prism v9.1 (GraphPad Software, San Diego, CA). Dose–response curves were fitted using a three-parameter logistic equation to calculate response range and pEC50, and normalized to forskolin stimulation (A2AR and A2BR) or forskolin inhibition (A1R and A3R), expressed as percentage of 100 μM forskolin. Adenosine and NECA stimulations were used as intrinsic controls across all experiments.
Receptor binding kinetics was determined as described previously25 using the Motulsky and Mahan method35 (built into Prism v9.1) to determine the test compound association rate constant and dissociation rate constant. The kon and koff values for binding of CA200645 were determined to be kon = 3.67 ± 0.34 × 106 M–1 min–1 and koff = 0.064 ± 0.0023 min–1 at the human A1R and kon = 2.93 ± 0.24 × 106 M–1 min–1 and koff = 0.066 ± 0.0022 min–1 at the rat A1R.
To calculate the relative activities (RA) of compounds (Figure 2), eq 1 was used:
| 1 |
where Emax is the maximal response and EC50 is the agonist concentration required to produce a half-maximal response, and web plot was plotted using Microsoft Excel. Since the receptors are expressed in the same cell background and adenosine and NECA are full potent agonists across all the adenosine subtypes, we reasoned that changes in log(RA) for a given ligand, relative to NECA or adenosine at A1R, would provide a quantitative means of comparing receptor selectivity of individual adenosine receptor ligands.
The statistical analysis was performed in Prism v9.1 using one-way ANOVA with a Dunnett’s post-test for multiple comparisons following the guidelines as described by Curtis et al.31 All experiments were performed in a minimum of three repeats conducted in duplicate, and data were reported as mean ± SEM.
Adenosine Receptor Structures
The active state A1R coordinates were retrieved from Protein Data Bank (PDB) database41 entry 7LD4.19 PDB ID 5G53(42) was used for A2AR in active conformation. A2BR in active conformation was modeled using 5G53 as a template through Modeller 9.19.43 The A2BR ECL2 (L142ECL2-K170ECL2) was retrieved from the inactive state model by AlphaFold244 (entry P29275) and inserted in the homology model by superposition. A3R in active conformation was modeled using 7LD4 as a template through Modeller 9.19.43 The A3R ECL2 (K152ECL2-S165ECL2) was retrieved from the inactive state model by AlphaFold2 (entry P0DMS8) and inserted in the homology model by superposition. All the ARs structures did not present either the ICL3 or the G protein bound to its intracellular binding site.
Force Field and Ligand Parameters for MD Simulations
The CHARMM3645,46/CGenFF 3.0.147−49 force field combination was employed in this work. The force field, topology, and parameter files for 20 and 27 were obtained from the ParamChem webserver.47
System Preparations for MD Simulations
For all systems, hydrogen atoms were added by means of the pdb2pqr50 and propka51 software (considering a simulated pH of 7.0). The protonation of titratable side chains was checked by visual inspection. The resulting receptors were separately inserted in a square 90 × 90 Å 1-palmitoyl-2-oleyl-sn-glycerol-3-phosphocholine (POPC) bilayer (previously built by using the VMD Membrane Builder plugin 1.1, Membrane Plugin, Version 1.1. at http://www.ks.uiuc.edu/Research/vmd/plugins/membrane/) through an insertion method,52 along with their co-crystallized ligand (and the crystallographic water molecules within 5 Å of the ligand). The receptor orientation was obtained by superposing the coordinates on the corresponding structure retrieved from the OPM database.53 Lipids overlapping the receptor transmembrane helical bundle were removed, and TIP3P water molecules54 were added to the simulation box by means of the VMD Solvate plugin 1.5 (Solvate Plugin, Version 1.5. at http://www.ks.uiuc.edu/Research/vmd/plugins/solvate/). Finally, overall charge neutrality was reached by adding Na+/Cl– counterions up to the final concentration of 0.150 M using the VMD Autoionize plugin 1.3 (Autoionize Plugin, Version 1.3. at http://www.ks.uiuc.edu/Research/vmd/plugins/autoionize/).
System Equilibration and MD Settings
The MD engine ACEMD55 was employed for both the equilibration and productive simulations. The equilibration was achieved in isothermal–isobaric conditions (NPT) using a Berendsen barostat56 (target pressure 1 atm) and Langevin thermostat57 (target temperature 300 K) with low damping of 1 ps–1. A multistage procedure was performed (integration time step of 2 fs): first, clashes between protein and lipid atoms were reduced through 1500 conjugate-gradient minimization steps, and then 1 kcal mol–1 Å–2 positional restraints on lipid phosphorus atoms, protein atoms other than Cα, and protein Cα atoms were gradually removed over 2, 60, and 80 ns, respectively. The last 20 ns of equilibration was performed without any positional restraints. Productive trajectories were computed with an integration time step of 4 fs in the canonical ensemble (NVT). The target temperature was set at 300 K using a thermostat damping of 0.1 ps–1. The M-SHAKE algorithm58,59 was employed to constrain the bond lengths involving hydrogen atoms. The cutoff distance for electrostatic interactions was set at 9 Å, with a switching function applied beyond 7.5 Å. Long-range Coulomb interactions were handled using the particle mesh Ewald summation method (PME)60 by setting the mesh spacing to 1.0 Å.
Molecular Docking
A first attempt to dock 20 and 27 into all the four ARs subtypes was performed on structures prepared as reported above using Vina61 in a 30 × 30 × 30 Å cube centered on the atom CZ of the ECL2 conserved phenylalanine residue (F171 in A1R, F168 in A2AR, F173 in A2BR, and F168 in A3R). Successive molecular docking simulations of 20 and 27, with the same settings, were performed into the AR structure extracted from MD simulations (see below). A1R, A2AR, and A2BR were extracted from adiabatic MD simulations, while the A3R structure was the equilibrated apo structure.
Adiabatic MD of the apo Adenosine Receptors
The apo AR structures were prepared and equilibrated as reported above. A1R, A2AR, and A2BR were subjected to 50 ns of adiabatic MD.62 A target distance of 13 Å and a force constant of 100 kJ mol–1 Å–2 were set to favor the opening of the salt bridge between ECL2 and ECL3: E172ECL2(Cδ)-K265ECL3(Nζ) on A1R, E169ECL2(Cδ)-H264(Hε2) on A2AR, and E174(Cδ)-K265(Nζ) and K267(Nζ) on A2BR.
MD Analysis
Root mean square deviations (RMSD) and fluctuation (RMSF) were computed using VMD.63 Interatomic contacts and hydrogen bonds were detected using the GetContacts scripts tool (https://getcontacts.github.io). Contacts and hydrogen bond persistency were quantified as the percentage of frames (over all the frames obtained by merging the different replicas) in which protein residues formed contacts or hydrogen bonds with the ligand. Structural water molecules were detected in the apo ARs using AquaMMapS.64 Short 10 ns simulations were performed with a time step 2 fs, restraining the Cα atoms and saving a frame every 20 ps of simulation.
Residue Numbering System
Throughout the manuscript, the Ballesteros–Weinstein residue numbering system for GPCRs is adopted.65
Acknowledgments
This study was supported by the Swiss National Science Foundation (B.P., M.Le. and M.Lo.), the Cambridge Trust (A.S.), the Leverhulme Trust (K.B. and G.L.), an AstraZeneca studentship (S.C.), a British Pharmacological Society Vacation Studentship (I.M.), a China Scholarship Council Cambridge International Scholarship (X.H.), and the BBSRC (G.L.). We thank Matt Ladds for his help with proof checking the statistical analysis and the Analytical Services from the Department of Chemistry, Biochemistry, and Pharmaceutical Sciences, University of Bern, Switzerland, for measuring NMR and MS spectra of synthetic intermediates and final compounds.
Glossary
Abbreviations Used
- AC
adenylyl cyclase
- AR
adenosine receptor
- A1R
adenosine A1 receptor
- A2AR
adenosine A2A receptor
- A2BR
adenosine A2B receptor
- A3R
adenosine A3 receptor
- BRET
bioluminescence resonance energy transfer
- DAIB
(diacetoxyiodo)benzene
- DIAD
diisopropyl azodicarboxylate
- NECA
5′-N-ethylcarboxamidoadenosine
- RA
relative activity
- RT
residence time
- TEMPO
(2,2,6,6-tetramethylpiperidin-1-yl)oxyl
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.jmedchem.2c01414.
Summary of O-alkylation studies (Scheme S1); synthetic confirmation of 2-aminocyclopentanol stereochemistry (Figure S1); molecular docking of 20 and 27 (Figures S2–S3); molecular dynamics simulations (Figures S4 and S6); mutagenesis study (Figure S5); purity assessment for all tested compounds (Tables S1 and S2); molecular formula strings of all final compounds (Table S3); and reproduction of 1H and 13C NMR spectra for all tested compounds (PDF)
MD simulations of 27 (orange stick representation) in complex with the four AR subtypes (MP4)
MD simulations of 27 and 20 in complex with A1R (white transparent ribbon) during three MD simulations of 2 μs each (MP4)
Coordinates of 27 bound to A1R (PDB)
Molecular formula strings of all final compounds (CSV)
Author Contributions
# B.P., A.S. and G.D. contributed equally to this work. M.Lo. and G.H. also contributed equally.
The authors declare no competing financial interest.
Supplementary Material
References
- Jacobson K. A.; Müller C. E. Medicinal chemistry of adenosine, P2Y and P2X receptors. Neuropharmacology 2016, 104, 31–49. 10.1016/j.neuropharm.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawynok J. Adenosine receptor targets for pain. Neuroscience 2016, 338, 1–18. 10.1016/j.neuroscience.2015.10.031. [DOI] [PubMed] [Google Scholar]
- Huang Z. L.; Qu W. M.; Eguchi N.; Chen J. F.; Schwarzschild M. A.; Fredholm B. B.; Urade Y.; Hayaishi O. Adenosine A2A, but not A1, receptors mediate the arousal effect of caffeine. Nat. Neurosci. 2005, 8, 858–859. 10.1038/nn1491. [DOI] [PubMed] [Google Scholar]
- Lazarus M.; Shen H. Y.; Cherasse Y.; Qu W. M.; Huang Z. L.; Bass C. E.; Winsky-Sommerer R.; Semba K.; Fredholm B. B.; Boison D.; Hayaishi O.; Urade Y.; Chen J. F. Arousal effect of caffeine depends on adenosine A2A receptors in the shell of the nucleus accumbens. J. Neurosci. 2011, 31, 10067–10075. 10.1523/JNEUROSCI.6730-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eltzschig H. K.; Sitkovsky M. V.; Robson S. C. Purinergic signaling during inflammation. N. Engl. J. Med. 2012, 367, 2322–2333. 10.1056/NEJMra1205750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eltzschig H. K.; Warner D. S.; Warner M. A. Adenosine: an old drug newly discovered. Anesthesiology 2009, 111, 904–915. 10.1097/ALN.0b013e3181b060f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredholm B. B. Adenosine, an endogenous distress signal, modulates tissue damage and repair. Cell Death Differ. 2007, 14, 1315–1323. 10.1038/sj.cdd.4402132. [DOI] [PubMed] [Google Scholar]
- Haskó G.; Linden J.; Cronstein B.; Pacher P. Adenosine receptors: therapeutic aspects for inflammatory and immune diseases. Nat. Rev. Drug Discov. 2008, 7, 759–770. 10.1038/nrd2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eltzschig H. K.; Carmeliet P. Hypoxia and inflammation. N. Engl. J. Med. 2011, 364, 656–665. 10.1056/NEJMra0910283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson K. A.; Gao Z. G. Adenosine receptors as therapeutic targets. Nat. Rev. Drug Discov. 2006, 5, 247–264. 10.1038/nrd1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. F.; Eltzschig H. K.; Fredholm B. B. Adenosine receptors as drug targets - what are the challenges?. Nat. Rev. Drug Discov. 2013, 12, 265–286. 10.1038/nrd3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson K. A.; Tosh D. K.; Jain S.; Gao Z. G. Historical and current adenosine receptor agonists in preclinical and clinical development. Front. Cell. Neurosci. 2019, 13, 124. 10.3389/fncel.2019.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight A.; Hemmings J. L.; Winfield I.; Leuenberger M.; Frattini E.; Frenguelli B. G.; Dowell S. J.; Lochner M.; Ladds G. Discovery of novel adenosine receptor agonists that exhibit subtype selectivity. J. Med. Chem. 2016, 59, 947–964. 10.1021/acs.jmedchem.5b01402. [DOI] [PubMed] [Google Scholar]
- Tosh D. K.; Rao H.; Bitant A.; Salmaso V.; Mannes P.; Lieberman D. I.; Vaughan K. L.; Mattison J. A.; Rothwell A. C.; Auchampach J. A.; Ciancetta A.; Liu N.; Cui Z.; Gao Z. G.; Reitman M. L.; Gavrilova O.; Jacobson K. A. Design and in vivo characterization of A1 adenosine receptor agonists in the native ribose and conformationally constrained (N)-methanocarba series. J. Med. Chem. 2019, 62, 1502–1522. 10.1021/acs.jmedchem.8b01662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrelli R.; Scortichini M.; Belardo C.; Boccella S.; Luongo L.; Capone F.; Kachler S.; Vita P.; Del Bello F.; Maione S.; Lavecchia A.; Klotz K. N.; Cappellacci L. Structure-based design, synthesis, and in vivo antinociceptive effects of selective A1 adenosine receptor agonists. J. Med. Chem. 2018, 61, 305–318. 10.1021/acs.jmedchem.7b01399. [DOI] [PubMed] [Google Scholar]
- Franchetti P.; Cappellacci L.; Vita P.; Petrelli R.; Lavecchia A.; Kachler S.; Klotz K.-N.; Marabese I.; Luongo L.; Maione S.; Grifantini M. N6-Cycloalkyl- and N6-bicycloalkyl-C5′(C2′)-modified adenosine derivatives as high-affinity and selective agonists at the human A1 adenosine receptor with antinociceptive effects in mice. J. Med. Chem. 2009, 52, 2393–2406. 10.1021/jm801456g. [DOI] [PubMed] [Google Scholar]
- Luongo L.; Petrelli R.; Gatta L.; Giordano C.; Guida F.; Vita P.; Franchetti P.; Grifantini M.; Novellis V. D.; Cappellacci L.; Maione S. 5’-Chloro-5′-deoxy-(±)-ENBA, a potent and selective adenosine A1 receptor agonist, alleviates neuropathic pain in mice through functional glial and microglial changes without affecting motor or cardiovascular functions. Molecules 2012, 17, 13712–13726. 10.3390/molecules171213712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo M.; Bakhoda A.; Gao Z. G.; Ramsey J. M.; Li Y.; O’Conor K. A.; Kelleher A. C.; Eisenberg S. M.; Kang Y.; Yan X.; Javdan C.; Fowler J. S.; Rice K. C.; Hooker J. M.; Jacobson K. A.; Kim S. W.; Volkow N. D. Discovery of highly potent adenosine A1 receptor agonists: targeting positron emission tomography probes. ACS Chem. Neurosci. 2021, 12, 3410–3417. 10.1021/acschemneuro.1c00397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper-Joyce C. J.; Bhola R.; Wang J.; Bhattarai A.; Nguyen A. T. N.; Cowie-Kent I.; O’Sullivan K.; Chia L. Y.; Venugopal H.; Valant C.; Thal D. M.; Wootten D.; Panel N.; Carlsson J.; Christie M. J.; White P. J.; Scammells P.; May L. T.; Sexton P. M.; Danev R.; Miao Y.; Glukhova A.; Imlach W. L.; Christopoulos A. Positive allosteric mechanisms of adenosine A1 receptor-mediated analgesia. Nature 2021, 597, 571–576. 10.1038/s41586-021-03897-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elzein E.; Zablocki J. A1 adenosine receptor agonists and their potential therapeutic applications. Expert Opin. Invest. Drugs 2008, 17, 1901–1910. 10.1517/13543780802497284. [DOI] [PubMed] [Google Scholar]
- Staehr P. M.; Dhalla A. K.; Zack J.; Wang X.; Ho Y. L.; Bingham J.; Belardinelli L. Reduction of free fatty acids, safety, and pharmacokinetics of oral GS-9667, an A1 adenosine receptor partial agonist. J. Clin. Pharmacol. 2013, 53, 385–392. 10.1002/jcph.9. [DOI] [PubMed] [Google Scholar]
- Wall M. J.; Hill E.; Huckstepp R.; Barkan K.; Deganutti G.; Leuenberger M.; Preti B.; Winfield I.; Carvalho S.; Suchankowa A.; Wei H.; Safitri D.; Huang X.; Imlach W.; La Mache C.; Dean E.; Hume C.; Hayward S.; Oliver J.; Zhao F.-Y.; Spanswick D.; Reynolds C. A.; Lochner M.; Ladds G.; Frenguelli B. G. Selective activation of Gαob by an adenosine A1 receptor agonist elicits analgesia without cardiorespiratory depression. Nat. Commun. 2022, 13, 4150. 10.1038/s41467-022-31652-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper-Joyce C. J.; Khoshouei M.; Thal D. M.; Liang Y.-L.; Nguyen A. T. N.; Furness S. G. B.; Venugopal H.; Baltos J.-A.; Plitzko J. M.; Danev R.; Baumeister W.; May L. T.; Wootten D.; Sexton P. M.; Glukhova A.; Christopoulos A. Structure of the adenosine-bound human adenosine A1 receptor–Gi complex. Nature 2018, 558, 559–563. 10.1038/s41586-018-0236-6. [DOI] [PubMed] [Google Scholar]
- Deganutti G.; Barkan K.; Preti B.; Leuenberger M.; Wall M.; Frenguelli B. G.; Lochner M.; Ladds G.; Reynolds C. A. Deciphering the agonist binding mechanism to the adenosine A1 receptor. ACS Pharmacol. Transl. Sci. 2021, 4, 314–326. 10.1021/acsptsci.0c00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkan K.; Lagarias P.; Stampelou M.; Stamatis D.; Hoare S.; Safitri D.; Klotz K. N.; Vrontaki E.; Kolocouris A.; Ladds G. Pharmacological characterisation of novel adenosine A3 receptor antagonists. Sci. Rep. 2020, 10, 20781. 10.1038/s41598-020-74521-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagtap P.Adenosine compounds and their use thereof. WO2011/119919 A1, 2011.
- Mitsunobu O.; Yamada M. Prepration of esters of carboxylic and phosphoric acid via quaternary phosphonium salts. Bull. Chem. Soc. Jpn. 1967, 40, 2380–2382. 10.1246/bcsj.40.2380. [DOI] [Google Scholar]
- Kotra L. P.; Manouilov K. K.; Cretton-Scott E.; Sommadossi J. P.; Boudinot F. D.; Schinazi R. F.; Chu C. K. Synthesis, biotransformation, and pharmacokinetic studies of 9-(β-D-arabinofuranosyl)-6-azidopurine: a prodrug for Ara-A designed to utilize the azide reduction pathway. J. Med. Chem. 1996, 39, 5202–5207. 10.1021/jm960339p. [DOI] [PubMed] [Google Scholar]
- Middleton R. J.; Briddon S. J.; Cordeaux Y.; Yates A. S.; Dale C. L.; George M. W.; Baker J. G.; Hill S. J.; Kellam B. New fluorescent adenosine A1-receptor agonists that allow quantification of ligand-receptor interactions in microdomains of single living cells. J. Med. Chem. 2007, 50, 782–793. 10.1021/jm061279i. [DOI] [PubMed] [Google Scholar]
- Murthy S.; Desantis J.; Verheugd P.; Maksimainen M. M.; Venkannagari H.; Massari S.; Ashok Y.; Obaji E.; Nkizinkinko Y.; Lüscher B.; Tabarrini O.; Lehtiö L. 4-(Phenoxy) and 4-(benzyloxy)benzamides as potent and selective inhibitors of mono-ADP-ribosyltransferase PARP10/ARTD10. Eur. J. Med. Chem. 2018, 156, 93–102. 10.1016/j.ejmech.2018.06.047. [DOI] [PubMed] [Google Scholar]
- Curtis M. J.; Alexander S.; Cirino G.; Docherty J. R.; George C. H.; Giembycz M. A.; Hoyer D.; Insel P. A.; Izzo A. A.; Ji Y.; MacEwan D. J.; Sobey C. G.; Stanford S. C.; Teixeira M. M.; Wonnacott S.; Ahluwalia A. Experimental design and analysis and their reporting II: updated and simplified guidance for authors and peer reviewers. Br. J. Pharmacol. 2018, 175, 987–993. 10.1111/bph.14153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo D.; Heitman L. H.; IJzerman A. P. Kinetic aspects of the interaction between ligand and G protein-coupled receptor: the case of the adenosine receptors. Chem. Rev. 2017, 117, 38–66. 10.1021/acs.chemrev.6b00025. [DOI] [PubMed] [Google Scholar]
- Sykes D. A.; Stoddart L. A.; Kilpatrick L. E.; Hill S. J. Binding kinetics of ligands acting at GPCRs. Mol. Cell. Endocrinol. 2019, 485, 9–19. 10.1016/j.mce.2019.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vauquelin G. Effects of target binding kinetics on in vivo drug efficacy: koff , kon and rebinding. Br. J. Pharmacol. 2016, 173, 2319–2334. 10.1111/bph.13504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motulsky H. J.; Mahan L. C. The kinetics of competitive radioligand binding predicted by the law of mass action. Mol. Pharmacol. 1984, 25, 1–9. [PubMed] [Google Scholar]
- Lu H.; Tonge P. J. Drug–target residence time: critical information for lead optimization. Curr. Opin. Chem. Biol. 2010, 14, 467–474. 10.1016/j.cbpa.2010.06.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchankova A.; Stampelou M.; Koutsouki K.; Pousias A.; Dhingra L.; Barkan K.; Pouli N.; Marakos P.; Tenta R.; Kolocouris A.; Lougiakis N.; Ladds G. Discovery of a high affinity adenosine A1/A3 receptor antagonist with a novel 7-amino-pyrazolo[3,4-d]pyridazine scaffold. ACS Med. Chem. Lett. 2022, 13, 923–934. 10.1021/acsmedchemlett.2c00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter B.; Lebon G. Human adenosine A2A receptor: molecular mechanism of ligand binding and activation. Front. Pharmacol. 2017, 8, 898. 10.3389/fphar.2017.00898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenguelli B. G.; Wall M. J.. Adenosine receptor agonists. WO2020/188288 A1, 2020.
- Deganutti G.; Barkan K.; Ladds G.; Reynolds C. A. Multisite model of allosterism for the adenosine A1 receptor. J. Chem. Inf. Model. 2021, 61, 2001–2015. 10.1021/acs.jcim.0c01331. [DOI] [PubMed] [Google Scholar]
- Berman H. M.; Westbrook J.; Feng Z.; Gilliland G.; Bhat T. N.; Weissig H.; Shindyalov I. N.; Bourne P. E. The protein data bank. Nucleic Acids Res. 2000, 28, 235–242. 10.1093/nar/28.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter B.; Nehmé R.; Warne T.; Leslie A. G. W.; Tate C. G. Structure of the adenosine A2A receptor bound to an engineered G protein. Nature 2016, 536, 104–107. 10.1038/nature18966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiser A.; Šali A. Modeller: generation and refinement of homology-based protein structure models. Methods Enzymol. 2003, 374, 461–491. 10.1016/S0076-6879(03)74020-8. [DOI] [PubMed] [Google Scholar]
- Jumper J.; Evans R.; Pritzel A.; Green T.; Figurnov M.; Ronneberger O.; Tunyasuvunakool K.; Bates R.; Žídek A.; Potapenko A.; Bridgland A.; Meyer C.; Kohl S. A. A.; Ballard A. J.; Cowie A.; Romera-Paredes B.; Nikolov S.; Jain R.; Adler J.; Back T.; Petersen S.; Reiman D.; Clancy E.; Zielinski M.; Steinegger M.; Pacholska M.; Berghammer T.; Bodenstein S.; Silver D.; Vinyals O.; Senior A. W.; Kavukcuoglu K.; Kohli P.; Hassabis D. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. 10.1038/s41586-021-03819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J.; MacKerell A. D. Jr. CHARMM36 all-atom additive protein force field: validation based on comparison to NMR data. J. Comput. Chem. 2013, 34, 2135–2145. 10.1002/jcc.23354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J.; Rauscher S.; Nawrocki G.; Ran T.; Feig M.; de Groot B. L.; Grubmüller H.; MacKerell A. D. Jr. CHARMM36m: an improved force field for folded and intrinsically disordered proteins. Nat. Methods 2017, 14, 71–73. 10.1038/nmeth.4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanommeslaeghe K.; MacKerell A. D. Jr. Automation of the CHARMM General Force Field (CGenFF) I: bond perception and atom typing. J. Chem. Inf. Model. 2012, 52, 3144–3154. 10.1021/ci300363c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanommeslaeghe K.; Raman E. P.; MacKerell A. D. Jr. Automation of the CHARMM General Force Field (CGenFF) II: assignment of bonded parameters and partial atomic charges. J. Chem. Inf. Model. 2012, 52, 3155–3168. 10.1021/ci3003649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W.; He X.; Vanommeslaeghe K.; MacKerell A. D. Jr. Extension of the CHARMM General Force Field to sulfonyl-containing compounds and its utility in biomolecular simulations. J. Comput. Chem. 2012, 33, 2451–2468. 10.1002/jcc.23067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolinsky T. J.; Nielsen J. E.; McCammon J. A.; Baker N. A. PDB2PQR: an automated pipeline for the setup of Poisson-Boltzmann electrostatics calculations. Nucleic Acids Res. 2004, 32, W665–W667. 10.1093/nar/gkh381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson M. H. M.; Søndergaard C. R.; Rostkowski M.; Jensen J. H. PROPKA3: consistent treatment of internal and surface residues in empirical pKa predictions. J. Chem. Theory Comput. 2011, 7, 525–537. 10.1021/ct100578z. [DOI] [PubMed] [Google Scholar]
- Sommer B. Membrane packing problems: a short review on computational membrane modeling methods and tools. Comput. Struct. Biotechnol. J. 2013, 5, e201302014 10.5936/csbj.201302014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomize M. A.; Lomize A. L.; Pogozheva I. D.; Mosberg H. I. OPM: orientations of proteins in membranes database. Bioinformatics 2006, 22, 623–625. 10.1093/bioinformatics/btk023. [DOI] [PubMed] [Google Scholar]
- Jorgensen W. L.; Chandrasekhar J.; Madura J. D.; Impey R. W.; Klein M. L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 1983, 79, 926–935. 10.1063/1.445869. [DOI] [Google Scholar]
- Harvey M. J.; Giupponi G.; Fabritiis G. D. ACEMD: accelerating biomolecular dynamics in the microsecond time scale. J. Chem. Theory Comput. 2009, 5, 1632–1639. 10.1021/ct9000685. [DOI] [PubMed] [Google Scholar]
- Berendsen H. J. C.; Postma J. P. M.; Gunsteren W. F. v.; DiNola A.; Haak J. R. Molecular dynamics with coupling to an external bath. J. Chem. Phys. 1984, 81, 3684–3690. 10.1063/1.448118. [DOI] [Google Scholar]
- Loncharich R. J.; Brooks B. R.; Pastor R. W. Langevin dynamics of peptides: the frictional dependence of isomerization rates of N-acetylalanyl-N’-methylamide. Biopolymers 1992, 32, 523–535. 10.1002/bip.360320508. [DOI] [PubMed] [Google Scholar]
- Forester T. R.; Smith W. SHAKE, rattle, and roll: efficient constraint algorithms for linked rigid bodies. J. Comput. Chem. 1998, 19, 102–111. . [DOI] [Google Scholar]
- Kräutler V.; van Gunsteren W. F.; Hünenberger P. H. A fast SHAKE algorithm to solve distance constraint equations for small molecules in molecular dynamics simulations. J. Comput. Chem. 2001, 22, 501–508. . [DOI] [Google Scholar]
- Essmann U.; Perera L.; Berkowitz M. L.; Darden T.; Lee H.; Pedersen L. G. A smooth particle mesh Ewald method. J. Chem. Phys. 1995, 103, 8577–8593. 10.1063/1.470117. [DOI] [Google Scholar]
- Trott O.; Olson A. J. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchi M.; Ballone P. Adiabatic bias molecular dynamics: a method to navigate the conformational space of complex molecular systems. J. Chem. Phys. 1999, 110, 3697–3702. 10.1063/1.478259. [DOI] [Google Scholar]
- Humphrey W.; Dalke A.; Schulten K. VMD: visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
- Cuzzolin A.; Deganutti G.; Salmaso V.; Sturlese M.; Moro S. AquaMMapS: an alternative tool to monitor the role of water molecules during protein-ligand association. ChemMedChem 2018, 13, 522–531. 10.1002/cmdc.201700564. [DOI] [PubMed] [Google Scholar]
- Ballesteros J. A.; Weinstein H.. [19] Integrated methods for the construction of three-dimensional models and computational probing of structure-function relations in G protein-coupled receptors. In Methods in Neurosciences; Stuart C. S., Ed.; Academic Press: San Diego, 1995; Vol. 25, pp. 366–428. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.