Abstract
Background
To evaluate the protective efficacy of a hepatitis B (HB) vaccination program in Taiwan among high-risk children.
Methods
Children born to HBeAg-positive mothers from 2001 to 2010 were invited back. Blood samples for hepatitis B virus (HBV) seromarkers were taken and the children underwent hepatobiliary ultrasonography. Perinatal factors including delivery mode and vaccination history were collected from their medical records. According to the results of HBV serological markers, the children were initially classified into five groups: HBV naïve, HB vaccine responder, HBsAg carrier, recovered from HBV infection, and anti-HBc-positive alone. Children in the HBV naïve and anti-HBc-positive alone groups who presented with an anamnestic response after a booster HB vaccine were re-assigned to the vaccine responder and recovered from infection groups, respectively.
Results
All of the 196 enrolled children received postnatal hepatitis B immunoglobulin (HBIG) and HB vaccinations, of whom one was HBV naïve (0.5%), 109 were vaccine responders (55.6%), 21 were carriers (10.7%), and 65 recovered from infection (33.2%). Among the 21 carriers, 14 (66.7%) presented in the immunotolerant phase. Cesarean section was the only significant perinatal factor between the carriers (5.3%) and those who recovered from infection (37.7%) (p = 0.007).
Conclusion
In this study, there was a 43.9% HBV infection rate and 10.7% HBsAg carrier rate in high-risk Taiwanese children even after receiving HBIG and HB vaccinations. C-section may protect newborns from becoming HBsAg carriers, while HBV genotype and time of HBIG injection did not contribute to the HBV carrier rate.
Keywords: Mass vaccination, Hepatitis B e Antigens, Hepatitis B Antibodies
At a glance commentary
Scientific background on the subject
In Taiwan, HB vaccinations combined with postnatal HBIG injections have significantly decreased the prevalence of HBV infection with a success rate of 55.6% in high-risk infants of HBeAg-positive mothers. However, even though the prevalence of HB among parturients in Taiwan continues to decrease, investigations on the long-term efficacy of HB vaccination among children born to HBeAg-positive mothers are still needed.
What this study adds to the field
Our study showed there was a 43.9% HBV infection rate and 10.7% HBsAg carrier rate in high-risk Taiwanese children born to HBeAg-positive mothers even after receiving HBIG and HB vaccinations. Cesarean section may protect newborns from becoming HBsAg carriers, while HBV genotype and time of HBIG injection did not contribute to the HBV carrier rate.
In Taiwan, a universal hepatitis B (HB) vaccination program was launched in July 1984, and this pioneer immunoprophylaxis program has been shown to be a good example of the control of hepatitis B virus (HBV) infection, especially in an endemic area [1,2]. In addition to introducing a four-dose serum-derived or three-dose recombinant vaccine for all babies, hepatitis B immunoglobulin (HBIG) is also administered within 24 h after birth to newborns born to HBeAg-positive mothers. Although the prevalence of HBV infection in children and the incidence of hepatocellular carcinoma (HCC) and fulminant hepatitis in vaccinated children have successfully been reduced in the last 20 years [3], HBV-carrier infants and the breakthrough of HBV infection in vaccinated children have still been reported [[4], [5], [6]].
Vertical transmission of HBV from infected mothers to their fetuses or newborns, either in utero or peripartum, remains a major source of perpetuating the reservoir of chronically infected individuals globally [7]. In addition, many studies have investigated the long-term impact of HB vaccine failure on infants born to HBeAg-positive mothers, because the reduction in incidence rate ratio since the launch of the universal HB vaccination program for HCC (68%–75%) is not as high as that for chronic HBV infection (90%) [8,9]. Even though the prevalence of HB among parturients in Taiwan continues to decrease and has already dramatically decreased to 2.6% in those vaccinated against HB [10], investigations on the long-term efficacy of HB vaccination among children born to HBeAg-positive mothers are still needed.
The rate of HBV breakthrough infection in Asia among infants born to chronic HBV-infected mothers has been reported to be 1%–11.8%, and HBeAg-seropositive vaccine failure HBV-carrier children have been associated with delayed HBeAg seroconversion [9]. In addition, approximately 97% of HCC cases among children born after the launch of the vaccination program have been reported to be HBV-related due to vaccine failure and/or not receiving HBIG at birth in long-term follow-up studies [2,11]. Therefore, many studies have investigated the factors that may be associated with HB vaccine failure, especially in countries with a history of nationwide vaccination programs for more than 30 years as in Taiwan, in order to decrease maternal–fetal transmission and increase HB vaccination efficacy.
The aims of this study were to investigate the success rate of the HB vaccination program in Taiwan, elucidate the HBV infection and HBsAg carrier rates, and identify the associated perinatal factors among children born to HBeAg-positive mothers.
Materials and methods
All experiments were performed in accordance with relevant guidelines and regulations. Informed consent was obtained from all participants for chart review and blood sampling to check their HB status. This study was approved by the Institutional Review Board of Chang Gung Memorial Hospital in Taiwan (number 99–3556B) and was funded by a grant from Chang Gung Memorial Hospital (CMRPG8C0492).
Subjects
From 2001 to 2010, 8696 deliveries were recorded by the Department of Obstetrics in Kaohsiung Chang Gung Memorial Hospital in Southern Taiwan. Among these deliveries, 1595 infants born to 1256 mothers were positive for HBsAg and all of them didn't have any antiviral therapy before delivery. Two hundred and sixty-three mothers who were confirmed to be HBeAg-positive during pregnancy and all of their children were invited back to the hospital to be checked in April 2011. In this study, we only recruited the children born to mothers who were confirmed to be HBsAg and HBeAg positive during pregnancy.
Cross-sectional study
All participants were asked about their HB vaccination history and underwent upper abdominal ultrasonography and blood sampling for both HBV seromarkers and liver function tests. Ultrasonography was performed by five hepatologists using Philips (ATL) HDI 5000 SonoCT system (Philips Medical system, DA Best, The Netherlands) and Toshiba Aplio 50 SSA-700A (Toshiba, Tokyo, Japan) system. The HB serological markers included HBsAg, anti-HBs and anti-HBc. HBV DNA was also checked in the participants who were positive for anti-HBc, and quantitative tests of HBsAg and HBeAg were also performed for the participants who were positive for HBsAg. We also analyzed the genotype of HBV in the mothers and children with positive HBV DNA. Levels of HBsAg, anti-HBs, anti-HBc and HBeAg were determined using a microparticle enzyme immunoassay (MEIA) (AxSYM, Abbott GmbH & Co. KG, Wiesbaden, Germany). Liver function tests, including alanine transaminase (ALT) were performed using an automated biochemical analyzer (model TBA-200FR; Toshiba Co., Tokyo, Japan) with GPT-JS kits (Denka Seiken Co. Ltd., Niigata, Japan). In addition, we recorded and summarized perinatal factors from medical records, including maternal gestational diabetes, gestational age at birth, mode of delivery, duration of the second stage of labor, the time interval from birth to HBIG injection, birth body weight, birth body height, and Apgar score 10 min after birth.
Quantitative HBsAg was studied using a chemiluminescent microparticle immunoassay (CMIA) (Architect HBsAg; Abbott Ireland Diagnostics Division, Sligo, Ireland) according to the manufacturer's instructions. The kits have a lower limit of detection of 0.05 IU/ml. HBV DNA was quantified using the COBAS TaqMan HBV test (CAP-CTM; Roche Molecular Systems, Inc., Branchburg, NJ, USA), which has a lower limit of detection of 20 IU/ml.
HBV genotyping
The HBV genotypes were determined using the restriction fragment length polymorphism (RFLP) on the surface gene (between nucleotide positions 256 and 796), as described previously [12]. Surface genes were directly sequenced if the results of HBV genotype by RFLP were ambiguous or indicated a mixed infection.
Initial grouping of the study subjects
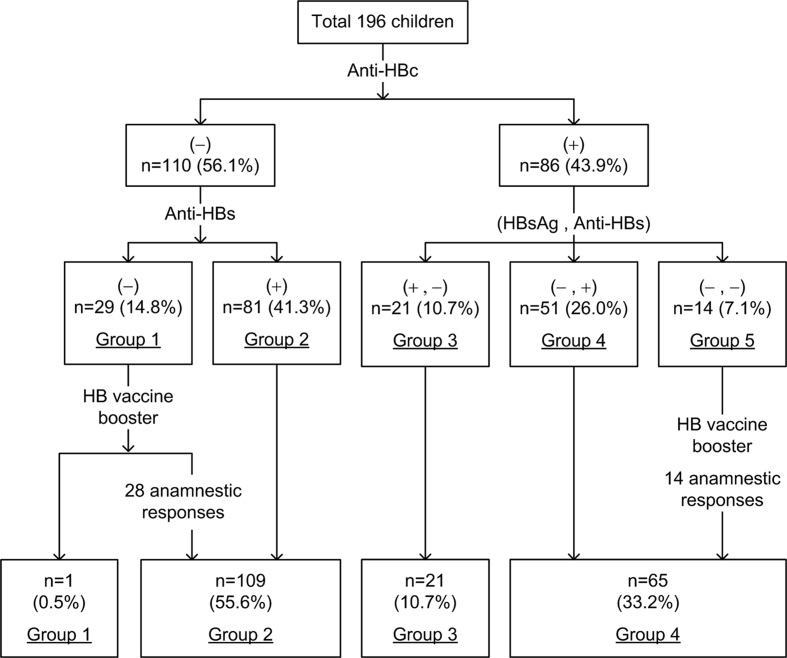
According to the results of HBsAg, anti-HBs and anti-HBc, all subjects were classified into five groups [Fig. 1]. Subjects who were negative for all three markers were classified into the HB vaccine naïve group (group 1). Subjects who were only positive for anti-HBs were classified into the HB vaccine responder group (group 2). Subjects who were positive for HBsAg and anti-HBc were classified into the HBsAg carrier group (group 3). Subjects who recovered from HBV infection, including those who were positive for anti-HBs and anti-HBc were classified into group 4, and those only positive for anti-HBc were categorized into group 5.
Fig. 1.
Recruited high-risk children grouped by serum anti-HBc status.
Re-grouping after a booster dose of HB vaccination
For the subjects in group 1 and 5, an additional booster dose of HB vaccination was given followed by detection of anti-HBs 3 weeks later. The subjects with an elevation in the titer of anti-HBs to more than 50 U/L were defined as having a positive anamnestic response. The subjects in group 1 with a positive anamnestic response were then re-assigned to group 2, while the subjects in group 5 were re-assigned to group 4 if they had a positive anamnestic response. Moreover, we administered a complete three-dose HB vaccine to the subjects without an anamnestic response.
Clinical phases of HBsAg carriers
Based on the natural history of chronic HBV infection, HBsAg carriers present in three phases, including immune tolerance, immune clearance and inactive carrier state. In this study, subjects were defined as being carriers in the immunotolerant phase if they had an HBV DNA level >10 7 IU/ml, normal ALT level and normal liver parenchyma on ultrasonography, and were positive for HBeAg. Subjects who were negative for HBeAg and had an HBV DNA level <200 IU/ml were defined as being carriers in the inactive carrier state. The remaining subjects were grouped as carriers in the immune clearance phase.
Statistics
Continuous and categorical variables were expressed as mean, standard deviation, and percentage. Chi-square and Fisher's exact tests were used to analyze comparisons between groups. A p value of <0.05 was considered to be statistically significant.
Results
A total of 121 (45.2%) mothers responded to our invitation, and 196 children born to these mothers met our inclusion criteria, including 89 (45.4%) boys and 107 (54.6%) girls with a mean age of 6.5 ± 3.7 years. All of them received HBIG injections within 24 h after delivery as well as three doses of recombinant HB vaccination at month 0, 1 and 6 after birth.
As shown in Fig. 1, 29 (14.8%) of the children were vaccine-naïve (group 1) and 81 (41.3%) had a vaccine response (group 2). Furthermore, 21 (10.7%) of the children were classified as being HBsAg carriers (group 3), and 51 (26.0%) had recovered from HBV infection (group 4). The remaining 14 (7.1%) children were only positive for anti-HBc (group 5).
After receiving a booster dose of HB vaccine, 28 (96.6%) of the 29 subjects in group 1 showed an anamnestic response and were consequently re-assigned to group 2. All 14 subjects in group 5 had an anamnestic response, and thus they were re-classified into group 4. The only subjects without an anamnestic response acquired anti-HBs after the catch-up vaccination.
After re-allocating these high-risk children of HBeAg-positive mothers, only 109 (55.6%) were classified as having a successful HB vaccination (group 2). Accordingly, 174 (88.8%) subjects were classified as having protection against HBV (those in group 2 and group 4). There were 86 (43.9%) subjects, including groups 3, 4, and 5 who had been or were infected with HBV, and the HBsAg carrier rate was 10.7% (n = 21, group 3).
There were no significant differences in sex (p = 0.717), time interval from birth to HBIG injection (p = 0.514), gestational age at birth (p = 0.975), mode of delivery (p = 0.162), maternal gestational diabetes (p = 0.329), birth body weight (p = 0.643), birth body height (p = 0.233), duration of the second stage of labor (p = 0.685), and Apgar score 10 min after birth (p = 1.000) between the vaccine responders (group 2) and the subjects infected with HBV (group 3 & 4). Moreover, when limited to the HBsAg carrier group (group 3), only mode of delivery was a significant factor to protect high-risk newborns from becoming HBsAg carriers with 37.7% of the recovered from HBV infection group delivered by Cesarean section (C-section) and only 5.3% of the HBsAg carrier group delivered by C-section (p = 0.007). In addition, there were no significant differences in aforementioned factors between the subjects who recovered from HBV infection and the HBsAg carriers [Table 1].
Table 1.
Analysis of risk factors of HB vaccine efficacy and HBV infection rate among high-risk children born to HBeAg-positive mothers.
| Factors | Comparison | HB vaccine responders n = 109 (55.6%) | HBsAg carrier n = 21 (10.7%) | Subjects recovered from HBV infection n = 65 (33.2%) | Subjects infected with HBV n = 86 (43.9) | ap | bp | cp |
|---|---|---|---|---|---|---|---|---|
| Sex | Male | 51 (46.8) | 10 (47.6) | 28 (43.1) | 38 (44.2) | 0.944 | 0.716 | 0.717 |
| Female | 58 (53.2) | 11 (52.4) | 37 (56.9) | 48 (55.8) | ||||
| Time interval from birth to HBIG | ≤3 h | 70 (76.1) | 14 (73.7) | 39 (70.9) | 53 (71.6) | 0.777 | 0.817 | 0.514 |
| >3 h | 22 (23.9) | 5 (26.3) | 16 (29.1) | 21 (28.4) | ||||
| Gestational age at birth | ≥37 weeks | 84 (87.5) | 19 (100) | 50 (83.3) | 69 (87.3) | 0.212 | 0.107 | 0.975 |
| <37 weeks | 12 (12.5) | 0 (0) | 10 (16.7) | 10 (12.7) | ||||
| Mode of delivery | CS | 20 (20.8) | 1 (5.3) | 23 (37.7) | 24 (30.0) | 0.190 | 0.007∗ | 0.162 |
| NSD | 76 (79.2) | 18 (94.7) | 38 (62.3) | 56 (70.0) | ||||
| Maternal gestational diabetes | No | 95 (99.0) | 19 (100) | 57 (95.0) | 76 (96.2) | 1.000 | 1.000 | 0.329 |
| Yes | 1 (1.0) | 0 (0) | 3 (5.0) | 3 (3.8) | ||||
| Baby's birth body weight | ≥2500 g | 86 (89.6) | 16 (84.2) | 53 (88.3) | 69 (87.3) | 0.448 | 0.696 | 0.643 |
| <2500 g | 10 (10.4) | 3 (15.8) | 7 (11.7) | 10 (12.7) | ||||
| Baby's birth body height | ≥45 cm | 89 (92.7) | 17 (89.5) | 52 (86.7) | 69 (87.3) | 0.642 | 1.000 | 0.233 |
| <45 cm | 7 (7.3) | 2 (10.5) | 8 (13.3) | 10 (12.7) | ||||
| Second stage of labor | ≥1 h | 27 (35.5) | 7 (38.9) | 11 (28.9) | 18 (32.1) | 0.789 | 0.457 | 0.685 |
| <1 h | 49 (64.5) | 11 (61.1) | 27 (71.1) | 38 (67.9) | ||||
| Apgar score at 10 min | ≥9 | 92 (95.8) | 19 (100) | 56 (93.3) | 75 (94.9) | 1.000 | 0.567 | 1.000 |
| <9 | 4 (4.2) | 0 () | (6.7) | 4 (5.1) |
∗p < 0.01.
HB vaccination responders versus HBsAg carriers.
HBsAg carriers versus subjects who had recovered from HBV infection.
HB vaccination responders versus subjects infected with HBV.
HBV genotypes were obtained in 115 mother and 19 children, and they were all either genotype B or C. There was no significant difference in the proportion of genotype B between the mothers (75.7%) and children (63.2%) (p = 0.267).
Among the 21 HBsAg carriers, 14 (66.7%) presented in the immunotolerant phase, four (19%) in the immune clearance phase, and three (14.3%) in the inactive carrier state. The ratios of boys to girls were 7:7, 3:1 and 0:3, respectively (p = 0.231), and their mean ages were 5.1 ± 2.5 years, 7.0 ± 2.9 years and 9.3 ± 3.8 years, respectively (p = 0.068).
Discussion
Our study showed there was a 43.9% HBV infection rate and 10.7% HBsAg carrier rate in high-risk Taiwanese children born to HBeAg-positive mothers even after receiving HBIG and HB vaccinations. C-section may protect newborns from becoming HBsAg carriers, while HBV genotype and time of HBIG injection did not contribute to the HBV carrier rate.
Taiwan launched the world's first successful universal HBV vaccination program to cover all newborn since 1986 and encouragingly, the prevention rate has been increased to 95% with the concurrent use of HB vaccine and HBIG [4,13,14]. In the current study, 21.9% (group 1 and group 5) of the subjects born to HBeAg-positive mothers had no response to the HB vaccination and undetectable anti-HBs antibodies (non-responders) even if they had received timely postnatal HBIG injections and three-dose HB vaccination. However, more than 97% of these non-responders achieved seroprotective anti-HBs after receiving one booster dose, indicating the occurrence of an anamnestic anti-HB response in most of them, which is relatively higher than in previous studies conducted in Taiwan [15], although some studies have reported the absence of an anamnestic anti-HBs response in approximately 25% of adolescents after one booster dose [16,17].
In Taiwan, the overall breakthrough HBV infection rate in children born to HBsAg-positive mothers has been reported to be 2.46%, compared to 9.26% for children born to HBeAg-positive and HBsAg-positive mothers, and 0.23% for those born to HBeAg-negative and HBsAg-positive mothers [1]. In our study, although all of the children born to mothers who were verified to be positive for HBsAg and HBeAg during pregnancy received postnatal HBIG within 24 h plus complete three-dose HB vaccinations including a timely birth dose, we still found that up to 43.8% of the children had detectable serum anti-HBc, indicating the occurrence of a present or previous history of HBV infection related to immunoprophylaxis failure. Our study confirmed similar chronic HBV infection rate as previous study published by Huey-Ling Chen et al., in 2012 which disclosed 9.26% chronic HBV infection rate, however, the infection rate in our study was relatively higher than in previous reports [5,18], and we proposed the higher positive anti-HBc rate from Southern Taiwan was owing to the higher breakthrough infection rate during their childhood even these high-risk children have received complete HB vaccination. As we know, this finding hasn't been reported before. Furthermore, 10.7% of the subjects were chronic HBV carriers with one-fifth already having HBeAg seroconversion. Even so, only 66.7% of the HBV carriers presented in the immunotolerant phase. We found a similar HBV carrier rate to that reported by Wen et al. in Northern Taiwan however all of the carrier children in our study had an older mean age than those in Wen et al.’s study [19]. Considering that rate of HBV seroconversion increases with age, we demonstrated a higher HB vaccination failure rate in infants born to HBeAg-positive mothers in Southern Taiwan. This discrepancy in infection rates among vaccinated cohorts between Northern and Southern Taiwan may be related to the difference in intensity of medical institutions [20]. In addition, among the 21 carriers, we found a trend of increasing age among the subjects in the three immune phases including immune tolerance, immune clearance and inactive phase (p = 0.068), although it did not reach a statistically significant difference, possibly due to the small sample size.
The factors possibly associated with mother-to-child transmission of HBV despite immunoprophylaxis have been frequently discussed in the past 10 years, of which maternal viral load is the most important factor contributing to vaccine failure [6,18,[21], [22], [23]]. A large-scale study by Zou et al. showed a linear correlation between maternal HBV DNA levels and immunoprophylaxis failure rates in 1,043 mother–infant pairs when maternal predelivery HBV DNA levels were higher than 6 log10 copies/mL, the failure rate was 3.2%, and the rate further increased to 6.7% and 7.6% for DNA levels of 7–7.99 log10 copies/mL and ≥8 log10 copies/mL, respectively [5]. Therefore, there is growing evidence suggesting that treatment with antiviral drugs during the third trimester should be given to pregnant women with HBV DNA levels >6 log10 copies/mL (>200,000 IU/mL) to reduce viral load prior to parturition and to provide appropriate safety for both the mothers and infants [[24], [25], [26], [27]]. One meta-analysis published in 2016 also showed that, compared to the use of HBIG and vaccination alone, antiviral therapy could improve HBV suppression in women with chronic HBV infection and high viral load to reduce the rate of mother-to-child transmission without increasing adverse maternal or fetal outcomes [28].
Overall, 33.1% of the children with positive anti-HBc in our study recovered from infection and 26% also had detectable anti-HBs, meaning that the antibodies came from past HBV infection. However, 7.1% of the subjects had anti-HBc only with no HBsAg or anti-HBs being detected, indicating a past history of acquired infection transiently during the disappearance of anti-HBs instead of during the phase of occult infection. Even so, all of these children had an anamnestic response after receiving one booster dose of HB vaccination. We also disclosed different intervals between delivery and postnatal HBIG injection within 24 h didn't influence the perinatal infection rate.
We found that of the high-risk newborns delivered by C-section, more had recovered from HBV infection (37.7%) than were HBV carriers (5.3%), and C-section was the only significant perinatal factor affecting the protective efficacy of HB vaccine. It is unclear whether the method of parturition affects vertical transmission of HBV, although HBsAg and HBV cannot traverse the placenta. However, HBeAg is small enough to cross the placenta and the occurrence of HBV transmission during the intrapartum period can occur in three ways, including instrumental trauma during delivery, micro-transfusion of maternal and fetal blood, or by neonatal contact with vaginal fluid or epithelium [29]. Some retrospective studies have supported the use of C-section for infants born to HBsAg-positive mothers to reduce perinatal infection [[30], [31], [32]]. For example, a meta-analysis conducted in China reported a statistically significant decrease in vertical transmission rate of HBV to 4.37% for mothers who underwent a C-section compared to 9.31% for those who underwent vaginal delivery (relative risk: 0.51, 95% CI: 0.44–0.60, p < 0.001) [33]. Transplacental leakage allows for the direct exchange of maternal and fetal blood and is the most common mode of intrauterine infection by HBV, and it can occur during early pregnancy due to an immature placenta or late due to uterine contractions during vaginal delivery [34]. Therefore, C-section may decrease the risk of maternal fetal transfusion by essentially eliminating uterine contraction and also limiting direct contact with the maternal genital tract and its secretions which occur during the second stage of labor. However, in this study, the duration of the second stage of labor among the children born by vaginal delivery was not associated with vaccine efficacy. Owning to a lack of randomized studies to specifically control for other risk factors including maternal HBeAg status and concerning the negative impact and risks of C-section compared to vaginal delivery, C-section is not currently recommended by the Society for Maternal-Fetal Medicine or WHO for HBsAg-positive mothers solely to prevent vertical transmission [3]. In recent years, the use of antiviral medication during the 3rd trimester has been suggested for high-risk mothers with a high prenatal viral load [35]. Further studies are warranted to investigate the protective effect of C-section on vertical transmission in mothers receiving antiviral treatment.
In this study, we also analyzed several perinatal factors including neonatal sex, birth body weight, height, and Apgar score 10 min after delivery to identify possible risk factors associated with immunoprophylaxis failure among children born to HBeAg-positive mothers. Chronic carriers of HBV have been reported to have a minimally increased risk of preterm birth and low birth weight, with a more pronounced increase in HBeAg-positive women. However, we did not find an association between vaccine efficacy and fetal sex or birth body weight. This may be because HBsAg-positive mothers have been reported to have a higher chance of perinatal HBV transmission with a lower immune response to HB vaccine [[36], [37], [38]].
We also investigated whether different HBV genotypes affected the transmission rate from HBeAg-positive mothers, and found no significant difference in the distribution of HBV genotype B between 115 mothers (75%) and 19 children (63.2%) (p = 0.267). A previous study reported that the distribution of ten known HBV genotypes varies markedly both within and between continents, and that genotypes B and C are common in Asia and North America [39]. Current evidence suggests that current recombinant vaccines are effective in preventing infections caused by all known HBV genotypes, although some evidence suggests that the severity of clinical disease may be influenced by HBV genotype [40,41].
There are several limitations in this study. As we know, there is a strong correlation between risk of vertical transmission and mother's HBV DNA level. However, in our study, all the 196 children enrolled were born from 133 HBeAg-positive mothers delivered during 2001–2010 and their hepatitis B status were routinely tested by serum HBsAg and HBeAg during the second or third trimester when they visited the prenatal clinic. Since HBV DNA level was not routinely checked in HBeAg-positive parturients during prenatal care in Taiwan until antiviral therapy covered by national health insurance since Feb. 2018 for these high-risk mothers, we could not provide the information of HBV DNA level of these mothers. In addition, the prevalence of HBsAg carrier among parturient mothers in Taiwan has been gradually decreasing to 12% and the prevalence rate of HBeAg among HBsAg-positive parturients to 19% in 2010 under a nationwide HB vaccination program in Taiwan launched since July 1984. Thus, the number of high-risk children enrolled in this study is not big enough as some of previous studies, however, the data indeed comes from the biggest tertiary medical center at Southern Taiwan during a large-scaled 10-year retrospective analysis. These might be other limitation of this study.
In conclusion, HB vaccination combined with postnatal HBIG injections in Taiwan have significantly decreased the prevalence of HBV infection, with a success rate of 55.6% in high-risk infants of HBe-positive mothers in this study. However, our cohort still had an HBV infection rate of 43.9% and an HBsAg carrier rate of 10.7%, although only 66.7% of the HBsAg-carrier children presented in the immunotolerant phase. Concerning perinatal factors affecting the protective efficacy of HB vaccine, of the high-risk newborns delivered by C-section, more had recovered from HBV infection (37.7%) than were HBV carriers (5.3%). On the other hand, HBV genotypes and the time interval from birth to HBIG injection did not contribute to the vaccination failure rate.
Funding sources
The study was funded by Chang Gung Memorial Hospital in Taiwan (grant number CMRPG8C0492).
Conflicts of interest
All authors have no conflicts of interest that could be perceived to bias their work, making known all financial support and any other personal connections.
Acknowledgements
We especially thank the Biostatistics Center, Kaohsiung Chang Gung Memorial Hospital for assistance with the statistical analysis in this study. The study was funded by Chang Gung Memorial Hospital in Taiwan (grant number CMRPG8C0492). The corresponding author confirmed that all authors have agreed with the submission in its present form and have no conflicts of interest that could be perceived to bias their work, making known all financial support and any other personal connections.
Footnotes
Peer review under responsibility of Chang Gung University.
References
- 1.Chen H.L., Lin L.H., Hu F.C., Lee J.T., Lin W.T., Yang Y.J., et al. Effects of maternal screening and universal immunization to prevent mother-to-infant transmission of HBV. Gastroenterology. 2012;142:773–781.e2. doi: 10.1053/j.gastro.2011.12.035. [DOI] [PubMed] [Google Scholar]
- 2.Ni Y.H., Chang M.H., Wu J.F., Hsu H.Y., Chen H.L., Chen D.S. Minimization of hepatitis B infection by a 25-year universal vaccination program. J Hepatol. 2012;57:730–735. doi: 10.1016/j.jhep.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 3.Society for Maternal-Fetal Medicine (SMFM) Dionne-Odom J., Tita A.T., Silverman N.S. #38: hepatitis B in pregnancy screening, treatment, and prevention of vertical transmission. Am J Obstet Gynecol. 2016;214:6–14. doi: 10.1016/j.ajog.2015.09.100. [DOI] [PubMed] [Google Scholar]
- 4.Hsu H.M., Chen D.S., Chuang C.H., Lu J.C., Jwo D.M., Lee C.C., et al. Efficacy of a mass hepatitis B vaccination program in Taiwan. Studies on 3464 infants of hepatitis B surface antigen-carrier mothers. JAMA. 1988;260:2231–2235. [PubMed] [Google Scholar]
- 5.Zou H., Chen Y., Duan Z., Zhang H., Pan C. Virologic factors associated with failure to passive-active immunoprophylaxis in infants born to HBsAg-positive mothers. J Viral Hepat. 2012;19:e18–e25. doi: 10.1111/j.1365-2893.2011.01492.x. [DOI] [PubMed] [Google Scholar]
- 6.Lin X., Guo Y., Zhou A., Zhang Y., Cao J., Yang M., et al. Immunoprophylaxis failure against vertical transmission of hepatitis B virus in the Chinese population: a hospital-based study and a meta-analysis. Pediatr Infect Dis J. 2014;33:897–903. doi: 10.1097/INF.0000000000000315. [DOI] [PubMed] [Google Scholar]
- 7.Chen H.L., Wen W.H., Chang M.H. Management of pregnant women and children: focusing on preventing mother-to-infant transmission. J Infect Dis. 2017;216:S785–S791. doi: 10.1093/infdis/jix429. [DOI] [PubMed] [Google Scholar]
- 8.Chang M.H., Chen T.H., Hsu H.M., Wu T.C., Kong M.S., Liang D.C., et al. Prevention of hepatocellular carcinoma by universal vaccination against hepatitis B virus: the effect and problems. Clin Cancer Res. 2005;11:7953–7957. doi: 10.1158/1078-0432.CCR-05-1095. [DOI] [PubMed] [Google Scholar]
- 9.Tai C.S., Wu J.F., Chen H.L., Ni Y.H., Hsu H.Y., Chang M.H. The Impact of Hepatitis B Vaccine Failure on Long-term Natural Course of Chronic Hepatitis B Virus Infection in Hepatitis B e Antigen-Seropositive Children. J Infect Dis. 2017;216:662–669. doi: 10.1093/infdis/jix339. [DOI] [PubMed] [Google Scholar]
- 10.Wu C.H., Hsu T.Y., Kung F.T., ChangChien C.C., Tsai C.C., Lu S.N. Changes in the prevalence of HBsAg and HBeAg: a study of 8696 parturients in a well vaccinated area. Sci Rep. 2017;7:1212. doi: 10.1038/s41598-017-01234-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ni Y.H., Huang L.M., Chang M.H., Yen C.J., Lu C.Y., You S.L., et al. Two decades of universal hepatitis B vaccination in taiwan: impact and implication for future strategies. Gastroenterology. 2007;132:1287–1293. doi: 10.1053/j.gastro.2007.02.055. [DOI] [PubMed] [Google Scholar]
- 12.Lee C.M., Chen C.H., Lu S.N., Tung H.D., Chou W.J., Wang J.H., et al. Prevalence and clinical implications of hepatitis B virus genotypes in southern Taiwan. Scand J Gastroenterol. 2003;38:95–101. doi: 10.1080/00365520310000500. [DOI] [PubMed] [Google Scholar]
- 13.Chen D.S., Hsu N.H., Sung J.L., Hsu T.C., Hsu S.T., Kuo Y.T., et al. A mass vaccination program in Taiwan against hepatitis B virus infection in infants of hepatitis B surface antigen-carrier mothers. JAMA. 1987;257:2597–2603. [PubMed] [Google Scholar]
- 14.Beasley R.P., Hwang L.Y., Lee G.C., Lan C.C., Roan C.H., Huang F.Y., et al. Prevention of perinatally transmitted hepatitis B virus infections with hepatitis B immune globulin and hepatitis B vaccine. Lancet. 1983;2:1099–1102. doi: 10.1016/s0140-6736(83)90624-4. [DOI] [PubMed] [Google Scholar]
- 15.Lu C.Y., Ni Y.H., Chiang B.L., Chen P.J., Chang M.H., Chang L.Y., et al. Humoral and cellular immune responses to a hepatitis B vaccine booster 15-18 years after neonatal immunization. J Infect Dis. 2008;197:1419–1426. doi: 10.1086/587695. [DOI] [PubMed] [Google Scholar]
- 16.Kao J.T., Wang J.H., Hung C.H., Yen Y.H., Hung S.F., Hu T.H., et al. Long-term efficacy of plasma-derived and recombinant hepatitis B vaccines in a rural township of Central Taiwan. Vaccine. 2009;27:1858–1862. doi: 10.1016/j.vaccine.2009.01.027. [DOI] [PubMed] [Google Scholar]
- 17.Ko S.C., Schillie S.F., Walker T., Veselsky S.L., Nelson N.P., Lazaroff J., et al. Hepatitis B vaccine response among infants born to hepatitis B surface antigen-positive women. Vaccine. 2014;32:2127–2133. doi: 10.1016/j.vaccine.2014.01.099. [DOI] [PubMed] [Google Scholar]
- 18.Yin Y.Z., Zhou J., Zhang P.Z., Hou H.Y. [Identification of risk factors related to the failure of immunization to interrupt hepatitis B virus perinatal transmission] Zhonghua Gan Zang Bing Za Zhi. 2013;21:105–110. doi: 10.3760/cma.j.issn.1007-3418.2013.02.008. Chinese. [DOI] [PubMed] [Google Scholar]
- 19.Wen W.H., Chang M.H., Zhao L.L., Ni Y.H., Hsu H.Y., Wu J.F., et al. Mother-to-infant transmission of hepatitis B virus infection: significance of maternal viral load and strategies for intervention. J Hepatol. 2013;59:24–30. doi: 10.1016/j.jhep.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 20.Chen C.J., Wang L.Y., Yu M.W. Epidemiology of hepatitis B virus infection in the Asia-Pacific region. J Gastroenterol Hepatol. 2000;15 Suppl:E3–E6. doi: 10.1046/j.1440-1746.2000.02124.x. [DOI] [PubMed] [Google Scholar]
- 21.Liu C.P., Zeng Y.L., Zhou M., Chen L.L., Hu R., Wang L., et al. Factors associated with mother-to-child transmission of hepatitis B virus despite immunoprophylaxis. Intern Med. 2015;54:711–716. doi: 10.2169/internalmedicine.54.3514. [DOI] [PubMed] [Google Scholar]
- 22.Tseng Y.R., Wu J.F., Ni Y.H., Chen H.L., Chen C.C., Wen W.H., et al. Long-term effect of maternal HBeAg on delayed HBeAg seroconversion in offspring with chronic hepatitis B infection. Liver Int. 2011;31:1373–1380. doi: 10.1111/j.1478-3231.2011.02574.x. [DOI] [PubMed] [Google Scholar]
- 23.Zhang L., Gui X., Wang B., Ji H., Yisilafu R., Li F., et al. A study of immunoprophylaxis failure and risk factors of hepatitis B virus mother-to-infant transmission. Eur J Pediatr. 2014;173:1161–1168. doi: 10.1007/s00431-014-2305-7. [DOI] [PubMed] [Google Scholar]
- 24.Xu W.M., Cui Y.T., Wang L., Yang H., Liang Z.Q., Li X.M., et al. Lamivudine in late pregnancy to prevent perinatal transmission of hepatitis B virus infection: a multicentre, randomized, double-blind, placebo-controlled study. J Viral Hepat. 2009;16:94–103. doi: 10.1111/j.1365-2893.2008.01056.x. [DOI] [PubMed] [Google Scholar]
- 25.Han G.R., Cao M.K., Zhao W., Jiang H.X., Wang C.M., Bai S.F., et al. A prospective and open-label study for the efficacy and safety of telbivudine in pregnancy for the prevention of perinatal transmission of hepatitis B virus infection. J Hepatol. 2011;55:1215–1221. doi: 10.1016/j.jhep.2011.02.032. [DOI] [PubMed] [Google Scholar]
- 26.Zhang H., Pan C.Q., Pang Q., Tian R., Yan M., Liu X. Telbivudine or lamivudine use in late pregnancy safely reduces perinatal transmission of hepatitis B virus in real-life practice. Hepatology. 2014;60:468–476. doi: 10.1002/hep.27034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Samadi Kochaksaraei G., Castillo E., Osman M., Simmonds K., Scott A.N., Oshiomogho J.I., et al. Clinical course of 161 untreated and tenofovir-treated chronic hepatitis B pregnant patients in a low hepatitis B virus endemic region. J Viral Hepat. 2016;23:15–22. doi: 10.1111/jvh.12436. [DOI] [PubMed] [Google Scholar]
- 28.Brown R.S., Jr., McMahon B.J., Lok A.S., Wong J.B., Ahmed A.T., Mouchli M.A., et al. Antiviral therapy in chronic hepatitis B viral infection during pregnancy: a systematic review and meta-analysis. Hepatology. 2016;63:319–333. doi: 10.1002/hep.28302. [DOI] [PubMed] [Google Scholar]
- 29.Pan C.Q., Duan Z.P., Bhamidimarri K.R., Zou H.B., Liang X.F., Li J., et al. An algorithm for risk assessment and intervention of mother to child transmission of hepatitis B virus. Clin Gastroenterol Hepatol. 2012;10:452–459. doi: 10.1016/j.cgh.2011.10.041. [DOI] [PubMed] [Google Scholar]
- 30.Pan C.Q., Zou H.B., Chen Y., Zhang X., Zhang H., Li J., et al. Cesarean section reduces perinatal transmission of hepatitis B virus infection from hepatitis B surface antigen-positive women to their infants. Clin Gastroenterol Hepatol. 2013;11:1349–1355. doi: 10.1016/j.cgh.2013.04.026. [DOI] [PubMed] [Google Scholar]
- 31.Hu Y., Chen J., Wen J., Xu C., Zhang S., Xu B., et al. Effect of elective cesarean section on the risk of mother-to-child transmission of hepatitis B virus. BMC Pregnancy Childbirth. 2013;13:119. doi: 10.1186/1471-2393-13-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peng S., Wan Z., Liu T., Li X., Du Y. Cesarean section reduces the risk of early mother-to-child transmission of hepatitis B virus. Dig Liver Dis. 2018;50:1076–1080. doi: 10.1016/j.dld.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 33.Yang M., Qin Q., Fang Q., Jiang L., Nie S. Cesarean section to prevent mother-to-child transmission of hepatitis B virus in China: a meta-analysis. BMC Pregnancy Childbirth. 2017;17:303. doi: 10.1186/s12884-017-1487-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen Y., Wang L., Xu Y., Liu X., Li S., Qian Q., et al. Role of maternal viremia and placental infection in hepatitis B virus intrauterine transmission. Microb Infect. 2013;15:409–415. doi: 10.1016/j.micinf.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 35.Chen H.L., Lee C.N., Chang C.H., Ni Y.H., Shyu M.K., Chen S.M., et al. Efficacy of maternal tenofovir disoproxil fumarate in interrupting mother-to-infant transmission of hepatitis B virus. Hepatology. 2015;62:375–386. doi: 10.1002/hep.27837. [DOI] [PubMed] [Google Scholar]
- 36.Li Z., Xie Z., Ni H., Zhang Q., Lu W., Yin J., et al. Mother-to-child transmission of hepatitis B virus: evolution of hepatocellular carcinoma-related viral mutations in the post-immunization era. J Clin Virol. 2014;61:47–54. doi: 10.1016/j.jcv.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 37.Linder N., Handsher R., German B., Sirota L., Bachman M., Zinger S., et al. Controlled trial of immune response of preterm infants to recombinant hepatitis B and inactivated poliovirus vaccines administered simultaneously shortly after birth. Arch Dis Child Fetal Neonatal Ed. 2000;83:F24–F27. doi: 10.1136/fn.83.1.F24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sood A., Singh D., Mehta S., Midha V., Kumar R. Response to hepatitis B vaccine in preterm babies. Indian J Gastroenterol. 2002;21:52–54. [PubMed] [Google Scholar]
- 39.Kurbanov F., Tanaka Y., Mizokami M. Geographical and genetic diversity of the human hepatitis B virus. Hepatol Res. 2010;40:14–30. doi: 10.1111/j.1872-034X.2009.00601.x. [DOI] [PubMed] [Google Scholar]
- 40.Cassidy A., Mossman S., Olivieri A., De Ridder M., Leroux-Roels G. Hepatitis B vaccine effectiveness in the face of global HBV genotype diversity. Expert Rev Vaccines. 2011;10:1709–1715. doi: 10.1586/erv.11.151. [DOI] [PubMed] [Google Scholar]
- 41.Velkov S., Ott J.J., Protzer U., Michler T. T1he global hepatitis B virus genotype distribution approximated from available genotyping data. Genes (Basel) 2018;9:495. doi: 10.3390/genes9100495. [DOI] [PMC free article] [PubMed] [Google Scholar]