Summary
Background
Anti-Müllerian hormone (AMH) is produced by granulosa cells in small growing ovarian follicles. In adult women, serum concentrations of AMH reflect the ovarian reserve of resting primordial follicles, and low AMH is associated with risk of early menopause. In contrast, patients with polycystic ovary syndrome (PCOS) have elevated AMH. The primary aim of this study was to evaluate the individual tracking of serum AMH concentrations, as well as whether AMH in early childhood reflects ovarian activity in adolescence.
Methods
In this large longitudinal study of healthy girls were examined from infancy to adolescence (1997–2019) including physical examination, assessment of serum concentrations of reproductive hormones (in infancy, median age 0.3 yrs; mid-childhood, 7.2 yrs; puberty, 11.3 yrs; and adolescence, 15.9 yrs), transabdominal ultrasound (TAUS, puberty and adolescence) and magnetic resonance imaging (MRI, puberty) of the ovaries.
Findings
Each girl maintained her relative AMH concentration (expressed as standard deviation (SD) scores) over time; mean variation of individual age adjusted AMH concentrations was 0.56 ± 0.31 SD.
Serum concentrations of AMH in adolescence correlated with AMH in infancy and childhood; infancy: r = 0.347; mid-childhood: r = 0.637; puberty: r = 0.675, all p < 0.001.
AMH correlated negatively with FSH concentrations in all age groups (infancy: r = −0.645, p < 0.001; mid-childhood: r = −0.222, p < 0.001; puberty: r = −0.354, p < 0.001; adolescence: n = 275, r = −0.175, p = 0.004).
Serum AMH concentrations in mid-childhood correlated with the number of follicles in puberty (TAUS and MRI) as well as in adolescence (TAUS); e.g. total number of follicles: TAUS puberty (r = 0.607), MRI puberty (r = 0.379), TAUS adolescence (r = 0.414), all p < 0.001.
AMH concentration in infancy as well as in mid-childhood predicted low AMH (<10 pmol/L) in adolescence; AMH infancy <7.5 pmol/L as predictor of low AMH in adolescence: sensitivity 0.71, specificity 0.70, AUC 0.759; AMH mid-childhood < 8.4 pmol/L as predictor of low AMH in adolescence: sensitivity 0.88, specificity 0.87, AUC 0.949.
Girls with high serum AMH concentration in mid-childhood (AMH >30.0 pmol/L vs. other girls) had higher adolescent LH (median 4.53 vs. 3.29 U/L p = 0.041), LH/FSH ratio (1.00 vs 0.67, p = 0.019), testosterone (1.05 vs 0.81 nmol/L, p = 0.005), total number of follicles (23 vs. 19, p = 0.004), and higher prevalence of irregular cycles (10/15 = 67% vs. 28/113 = 25%, p = 0.002).
Interpretation
The present findings suggest remarkably stable ovarian activity from small growing follicles in healthy girls, supporting AMH in early life as a useful clinical tool to predict future ovarian activity.
Funding
The work was supported by The Center on Endocrine Disruptors (CeHoS) under The Danish Environmental Protection Agency and The Ministry of Environment and Food (grant number: MST-621-00 065), the EU (QLK4-CT1999-01422; QLK4-2001-00269), the Novo Nordisk Foundation and The Danish Ministry of Science Technology and Innovation (2107-05-0006). A.S.B. is funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) – 464240267. KM receives honoraria from Novo Nordisk A/S for teaching at the Danish annual postgraduate course of pituitary diseases.
Keywords: Anti-Müllerian hormone, AMH, Ovarian function, Girls, Female reproductive endocrinology
Research in context.
Evidence before this study
Serum concentration of Anti-Müllerian Hormone (AMH) reflects the number of small antral ovarian follicles. Concentrations decline as menopause approaches, and very low AMH is associated with increased risk of early menopause. Little is known about the clinical implications of individual serum AMH concentrations during childhood and adolescence.
We searched PubMed in January 2022 for English language publications with the terms “Anti-Müllerian Hormone”, and “AMH”, and “girls”, and “longitudinal”. We identified two longitudinal studies reporting serum AMH concentrations in healthy girls. In one study from our own group, individual AMH was evaluated during a limited time span covering the pubertal transition. In the other study, longitudinal data were used to assess trends over time, however individual trajectories were not evaluated.
Added value of this study
In this large longitudinal study over two decades, individual serum concentrations of AMH were stable from infancy to adolescence. This novel finding of an individual set point of ovarian activity from small ovarian follicles evident from early childhood raises several physiological, clinical and scientific points of interest.
Implication of all the available evidence
The present study supports AMH as a useful clinical tool to predict future ovarian activity. Thus, evaluation of AMH concentration in infancy candidates as a useful parameter when evaluating ovarian function including assessment of the ovarian reserve of primordial follicles later in life.
Introduction
Anti-Müllerian hormone (AMH) is produced by granulosa cells in small follicles prior to FSH dependent growth.1,2 Serum AMH concentrations reflect the number of small antral follicles throughout life; i.e. in infancy,3 peripubertal girls,4 adolescents5 and adult women.6 In adult women, the number of small growing follicles are in equilibrium with the number of resting primordial follicles constituting the total number of germ cells established in fetal life.7 Thus, risk of early menopause is increased in women with low age-specific AMH.8
When AMH was first characterized, it was a marker of testicular tissue in young boys due to high levels produced by immature Sertoli cells,9 however, after introduction of highly sensitive AMH assays, serum concentrations of AMH are now measurable in all healthy girls.10,11 This is due to recruitment of primordial follicles to small growing follicles through childhood even prior to pubertal onset. Minor fluctuations of AMH around pubertal onset have been observed, but overall, AMH concentrations are stable through pubertal development12,13 which indicates an individual set-point of ovarian activity. To our knowledge, there are currently no longitudinal data revealing whether this set-point of activity from small antral follicles tracks all the way from infancy and mid-childhood over puberty to adulthood.
AMH reduces follicle growth as well as intrafollicular aromatase activity and hereby inhibits estradiol production from small growing ovarian follicles.14, 15, 16 High AMH is a frequent finding in women with polycystic ovarian syndrome (PCOS)17: These patients have an increased number of small AMH producing follicles due to adversely altered gonadotropin secretion18; AMH production in granulosa cells from follicles in PCOS patients is intensified19; and AMH may even adversely affect hypothalamic GnRH secretion by increasing LH secretion from the pituitary.20 Daughters of mothers with PCOS have elevated AMH concentrations,21 and it has been speculated if high AMH in childhood predicts PCOS in adult life.
Minipuberty is a transient activation of the hypothalamic-pituitary-gonadal hormone axis (HPG) during infancy where gonads are stimulated to hormone production reaching concentrations comparable with adults.22,23 Gonadotropins stimulate growth of ovarian follicles, and AMH is detectable in sera from all infant girls.11 Peak concentrations around 3.5 months of age vary considerably between girls reflecting the number of antral follicles.3 The transient activation of the HPG axis is used as an early window of opportunity to assess gonadal and pituitary function in children with suspected congenital hypogonadotropic hypogonadism as well as in children born with differences of sexual development (DSD conditions).24,25 Little is known about the biological significance and possible long-term consequences of minipuberty. In mid-childhood, the HPG axis is centrally inhibited until reactivation at time of pubertal onset. In this quiescent period of ovarian activity, follicles are continuously recruited from the primordial follicle pool growing to early stages independent of FSH stimulation. Reactivation of the HPG axis with increasing concentrations of FSH and LH marks pubertal onset, inducing further follicle growth to large antral stages producing estradiol. Thelarche precedes menarche by 2–3 years which can be followed by years of irregular menstrual bleedings due to juvenile anovulatoric cycles.26
In this longitudinal study of 695 healthy girls, we aimed to evaluate whether: 1) Serum concentrations of AMH in infancy and mid-childhood are associated with AMH concentrations and ovarian morphology in puberty and adolescence; 2) High AMH concentrations in infancy and childhood are associated with altered profiles of reproductive hormones, ovarian morphology as well as a risk of irregular menstrual cycles in adolescence.
Methods
Participants
Participating girls are part of the Copenhagen Mother-Child Cohort (http://www.edmarc.net/mother-child-cohort.html), a population-based longitudinal birth cohort of healthy Danish children born between 1997 and 2002. Healthy pregnant women were recruited consecutively during the first trimester of pregnancy. A total of 1210 live born girls were included at birth and invited to participate in examinations during infancy, mid-childhood, puberty (three peripubertal examinations) and adolescence.4,5,27,28
Height was measured using a portable calibrated stadiometer (Holtain Ltd, Crymych, UK) and weight was measured to the nearest 0.1 kg using the same electronic scale (Brabantia scale no. 483127, Brabantia, Hadsten, Denmark) for all participants. For measurements of height and weight, the mean of three measurements was calculated and used for all statistical analyses. Body mass index (BMI) was calculated (kg/m2). Pubertal stage was assessed according to Tanner and Marshall.29 In adolescence, 131/317 were examined at cycle day 2 through 5; 175/317 girls were examined at cycle day 1 through 7.5
AMH concentrations from this population have previously been reported for nested cross sectional cohorts of girls in infancy, mid-childhood, puberty, and adolescence. The selection of girls for AMH analyses in previous studies from this cohort are briefly described. 2010: To establish a reliable reference range of AMH concentrations, approximately 50 girls from each age-group were randomly selected for AMH analyses due to funding restrictions.11 2014: A nested cohort of 121 girls examined at infancy and mid-childhood were randomly invited to participate in a substudy of the concurrent pubertal examinations including transabdominal ultrasound (TAUS), magnetic resonance imaging (MRI).4 2019: All adolescents previously participating in the study were invited for examination including TAUS.5 All girls with at least two available AMH measurements were included in the present study. No girls were excluded due to age. Data from visits at which adolescents were on hormonal contraception were excluded from all analyses (including hormones as well as ovarian morphology) (n = 61). AMH from all time points (including three examinations during puberty) were included to evaluate the intraindividual variation of AMH. To evaluate correlations between AMH at different time points, we included data from infancy (median age 0.3 yrs), mid-childhood (7.2 yrs), first examination in puberty (11.3 yrs) and at adolescence (15.9 yrs). The total number of AMH samples was 1183; total number of girls, n = 437; paired AMH samples: infancy – mid-childhood, n = 183; infancy – puberty, n = 212; infancy – adolescence, n = 115; mid-childhood – puberty, n = 251; mid-childhood – adolescence, n = 145; puberty – adolescence, n = 204. None of the included girls suffered from conditions affecting ovarian function or hormone production of the HPG axis.
We included all available data on transabdominal ultrasound (TAUS) and magnetic resonance imaging (MRI) of the ovaries. These scans were performed at puberty (TAUS and MRI) and adolescence (TAUS). After exclusion of girls on hormonal contraception (n = 65) and with ovarian cysts (n = 10), a total of MRI puberty, n = 78; TAUS puberty, n = 83 and TAUS adolescence, n = 137 were included.
Hormone analyses
All blood samples were drawn between 8:00 AM and 2:00 PM from an antecubital vein, clotted, and centrifuged; serum was stored at −20 °C until hormone analyses.
During the study period, two AMH assays (Beckman Coulter, Inc. Brea, CA) have been used. The Access immunoassay substituted the Immunometric assay generation I when this was no longer commercially available. The Beckman Coulter enzyme immunometric assay generation I (Research Resource Identifier, RRID: AB_2923005) had a detection limit of 2.0 pmol/L; interassay coefficients of variation (CV) 11.6%. The Access immunoassay (RRID: AB_2892998) has a detection limit of 0.14 pmol/L and interassay CV < 5%. Comparative studies between the two assays revealed negligible differences (1.3%) and no adjustments of results have therefore been performed.
Serum FSH (infancy and mid-childhood) were measured by Delfia (Wallac, Inc, Turku, Finland) with detection limit of 0.06 IU/L, interassay CV < 5%.
Serum FSH (puberty and adolescence) and LH were measured by Delfia (PerkinElmer, Boston, MA. RRID: AB_2783738 and AB_2783737) with detection limits of 0.05 IU/L and interassay CV < 4%.
Serum inhibin B was determined using the Beckman Coulter GenII assay (Beckman Coulter, Inc. Brea, CA. RRID: AB_2827405) with a detection limit of 3 pg/mL and interassay CV 10.3%.
Serum estradiol was measured by RIA (Pantex Corp, Immunodiagnostic Systems Ltd, Santa Monica, CA. RRID: AB_2905658) with a detection limit of 18 pmol/L and interassay CV 14.9%.
SHBG was measured by Access2 (Beckman Coulter, Brea, CA. RRID: AB_2893035) with a limit of detection at 0.33 nmol/L and interassay CV 5.2%.
Androgens were quantified by Turbo Flow-liquid chromatography-tandem mass spectrometry. Detection limits were 0.10 nmol/L (testosterone) and 0.18 nmol/L (androstenedione).30 The relative standard deviations for low- and high-quality control samples were 16.5% and 7.2% (testosterone); 22.9% and 18.3% (androstenedione), respectively.
Transabdominal ultrasonography and MRI of internal genitalia
2D and 3D ovarian and uterine TAUS were conducted through a full urinary bladder. 3D images were obtained from stored 2D image sequences in 3 planes: sagittal, transverse, and coronal. All scans in girls were performed by two trained examiners with the Voluson E8 Ultrasound System (GE Healthcare Medical Systems, Zipf, Austria) including a multifrequency transabdominal probe (RM6C, 3–8 MHz). Manual counting of follicles as well as measurement of ovarian length, width and depth were performed by four experienced operators.
All images were stored, and image analyses were done with 4-dimensional View software (GE Medical System, v 9.1. Little Chalfont, England). The number of follicles was counted in each ovary. Follicle numbers were evaluated by Tomographic Ultrasound Imaging (TUI) where a 3D model of the ovary was sliced (4 mm thickness) and follicles were manually counted in subgroups. TAUS Puberty: small follicles (1.0–4.4 mm), medium (4.5–9.4 mm), large (≥9.5 mm). TAUS Adolescence: small (2.0–4.9 mm), medium (5.0–7.9 mm), large (≥8 mm). We report the sum of follicles from both ovaries. Analyses of images from TAUS Adolescence of 12 girls (4%) were technically challenging because of poor image quality; these ultrasound data were excluded from all analyses. Interobserver CV was 16.5% for follicle count of follicles 2–7.9 mm, 15.3% for all follicles.
Menstrual cycles
At examination in adolescence, the girls completed an electronic questionnaire including age at menarche, menstrual cycle length within the past 6 months (grouped as: < 21 days, 21–35 days, > 35 days, “too irregular to tell”, “on hormonal contraception” or “unknown”), number of menstrual bleedings in the past 6 months. Participants completed a menstrual calendar either from time of invitation until examination or retrospectively, if this information was kept. Information was available for a minimum of three cycles.
Menstrual cycles in the 145 girls with AMH data at mid-childhood and adolescence were categorized as either: regular menstrual cycles, irregular menstrual cycles, or other cycle patterns.
Regular menstrual cycles (n = 90): A reported cycle length of 21–35 days and 5 to 8 menstrual bleedings within the past 6 months was defined as regular.
Irregular menstrual cycles (n = 38): was defined as oligomenorrhea or secondary amenorrhea.
Oligomenorrhea: Reported cycle length of “>35 days” or “too irregular to tell” in combination with menstrual calendar data of 1–3 bleedings in the past 6 months. Girls reporting either “don't know” or “4 bleedings in the past 6 months” were categorized as irregular only if their menstrual calendar showed oligomenorrhea.
Secondary amenorrhea: Girls who had experienced menarche but had no menstrual bleeding within the past 6 months.
Other cycle patterns (n = 17): Girls who had not experienced menarche or who had menarche during the past 6 months were included in this group. If answers on questionnaire and menstrual calendar were not in accordance, the girls were also included in this group.
Statistics
Data are presented as median and interquartile range (IQR).
To assess if absolute AMH concentrations changed according to age, Wilcoxon signed rank test was used.
To adjust AMH concentrations for age, reference curves were created using the generalized additive model for location, scale, and shape (GAMLSS) including the GAMLSS R package.31 The age-specific distribution of AMH for age was summarized by 3 curves: L (age-dependent skewness), M (age-dependent median), and S (age-dependent CV). Age-specific standard deviation (SD) scores were calculated using the following equation: SD score = ((X/M)L-1)/(L × S), where X is the measurement and L ≠ 0.
We calculated the within-girl (intra-individual) variation of AMH SDS, reported as mean ± 1 SD.
The girls of most clinical interest were girls with the highest AMH concentrations (potential risk of future PCOS) and the girls with the lowest AMH (potential risk of imminent POI). A priori power calculation was not possible due to lack of similar studies. Based on the individual mean SD scores, we divided the girls in AMH quintiles (5 groups). Quintiles were grouped in low AMH SDS (Q1), medium AMH SDS (Q2-4) and high AMH SDS (Q5).
The majority of variables evaluated were not normally distributed. In addition, a number of variables included cases with undetectable levels of hormone concentrations, and in some of the analyses, the number of girls were limited. We therefore decided to apply non-parametric statistical analyses as a conservative approach. Univariate correlations, e. g between AMH concentrations in infancy or mid-childhood and AMH concentrations or number of follicles in puberty or adolescence were evaluated with Spearman's Rho.
To evaluate if high concentrations of AMH in mid-childhood (AMH ≥30 pmol/L matching the Q5 AMH SDS cut off) were associated with reproductive hormone concentrations and ovarian morphology in adolescence, girls with the highest mid-childhood AMH were compared with other girls by the Mann Whitney U (MWU) test (continuous variables) and Pearson chi square test (categorical variables). The same method was used to assess if low AMH (AMH ≤10 pmol/L matching the Q1 AMH SDS cut off) in mid-childhood was associated with ovarian activity in adolescence.
We conducted a Receiver Operating Characteristic (ROC) curve to test the predictive value of low AMH in infancy and mid-childhood for low AMH (AMH ≤10 pmol/L) in adolescence. The analyses provide AUC, sensitivity, specificity for the AMH cut-off (in infancy and mid-childhood, respectively) which most accurately predicts AMH <10 pmol/L in adolescence.
All statistical analyses were performed using the R software/environment and IBM SPSS Statistics 25.0 (SPSS, Chicago, IL). P-Values < 0.05 were considered statistically significant.
Ethics
The longitudinal Copenhagen Mother-Child cohort was approved by the local ethics committee (KF 01–030/97, KF 01 276 357, KF 02-125/95, H-1–2009–074) and the Danish Data Protection Agency (1997–1200–074/2005–41–5545, 2010–41–4757). All parents and teenagers received written and oral information. Informed consent was obtained before inclusion.
Role of the funding source
Study sponsors have not been involved in any aspect of decision making concerning the present study.
Results
Age and anthropometrics of the 437 girls at examinations from infancy to adolescence are listed in Table 1.
Table 1.
Population characteristics of 437 girls included in the study.
| Infancy (n = 276) | Mid-childhood (n = 307) | Puberty (n = 366) | Adolescence (n = 237) | |
|---|---|---|---|---|
| Age, years | 0.26 (0.24–0.28) | 7.2 (6.5–7.8) | 11.3 (10.5–11.8) | 15.9 (15.5–16.4) |
| Weight, kg | 5.9 (5.5–6.3) | 24.2 (21.5–27.9) | 38.4 (32.6–44.9) | 58.7 (53.1–65.5) |
| Height, cm | 60.7 (59.1–62.0) | 125.0 (118.9–131.4) | 149.0 (143.0–156.1) | 167.8 (162.8–172.9) |
| BMI, kg/m2 | 15.9 (15.1–17.1) | 15.6 (14.7–16.8) | 17.2 (15.9–18.9) | 20.7 (19.4–22.9) |
| Tanner stage 1 | 77 (21.0%) | |||
| Tanner stage 2 | 146 (39.0%) | |||
| Tanner stage 3 | 100 (27.3%) | |||
| Tanner stage 4 | 37 (10.1%) | |||
| Tanner stage 5 | 6 (1.6%) | |||
| Gestational age | 40 (39.0–40.9) | 40.1 (39.0–41.0) | 40.1 (39.0–40.8) | 40.0 (39.1–40.8) |
| SGA | 18 (6.5%) | |||
| LGA | 14 (5.1%) |
Median (IQR).
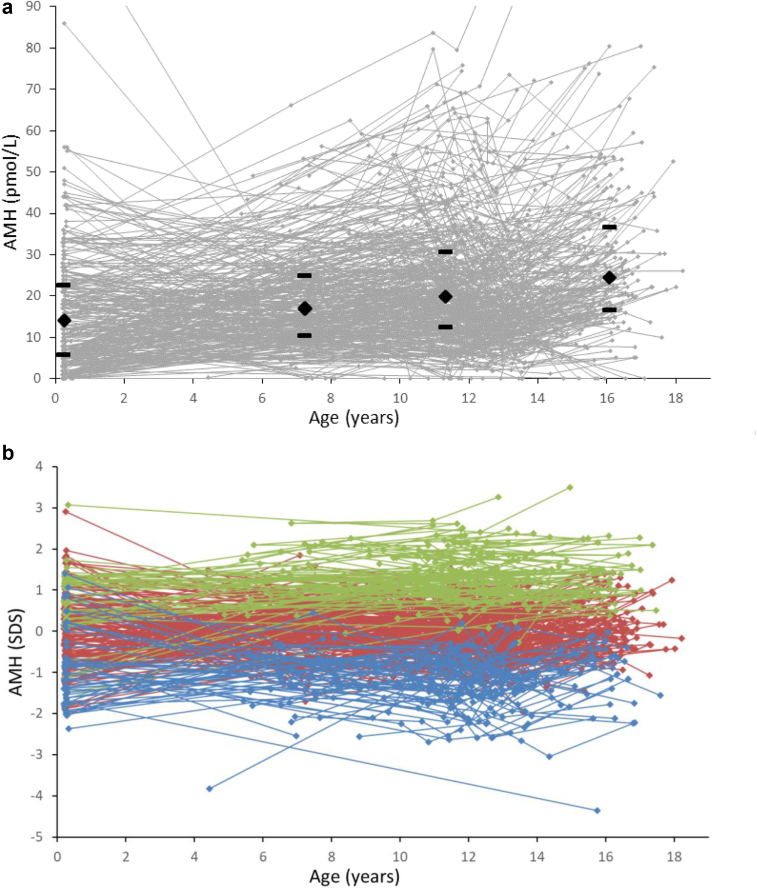
Individual longitudinal measurement of AMH (pmol/L) during infancy, childhood, puberty and adolescence are visualized in Fig. 1a. Absolute concentrations of AMH increased consecutively with age; AMH infancy to mid-childhood (n = 115): median (IQR) 14.0 (6.0–22.8) to 17.1 (10.5–25.1) pmol/L, p = 0.015; AMH mid-childhood to puberty (n = 251): 17.1 (10.5–25.1) to 19.8 (12.6–30.8) pmol/L, p < 0.001; AMH puberty to adolescence (n = 204): 19.8 (12.6–30.8) to 24.5 (16.8–36.8) pmol/L, p < 0.001.
Fig. 1.
Serum AMH concentrations in absolute values (pmol/L) (a) and standard deviation scores (SDS) (b) according to age. Dots indicate individual values and longitudinal courses are connected by lines. At each age group, i. e infancy, mid-childhood, puberty and adolescence, median and IQR is marked with black diamants and brackets. All girls were divided into AMH quintiles (5 groups), based on the individual mean SD scores. Blue: 1st quintile, red: 2nd, 3rd, 4th quintile, green: 5th quintile.
Age-adjusted AMH concentrations (expressed as SDS) are shown in Fig. 1b. The girls with the highest and lowest AMH SD scores maintained their relative concentrations from infancy to adolescence; girls with high AMH remained high, and girls with low AMH remained low. The mean individual variation of AMH SDS was 0.56 ± 0.31 SD.
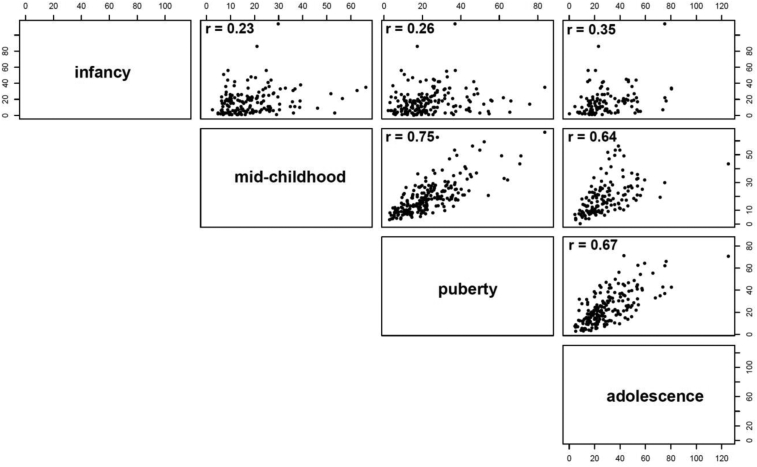
Serum concentrations of AMH (pmol/L) in infancy, mid-childhood, and puberty correlated strongly with AMH in adolescence; infancy: n = 115, r = 0.347, p < 0.001; mid-childhood: n = 145, r = 0.637, p < 0.001; puberty: n = 204, r = 0.675, p < 0.001, respectively (Fig. 2).
Fig. 2.
Correlation plots between serum AMH concentrations (pmol/L) in infancy, mid-childhood, puberty and adolescence, all p < 0.001. Numbers refer to Spearman's Rho, r values.
AMH concentrations (pmol/L) in mid-childhood correlated with the number of ovarian follicles in puberty (TAUS and MRI) as well as in adolescence (TAUS), with respect to total number of follicles as well as follicle subgroups, e.g. total number of follicles: TAUS puberty (n = 78, r = 0.607), MRI puberty (n = 83, p = 0.379), TAUS adolescence (n = 142, p = 0.414), all p < 0.001 (Table 2).
Table 2.
Correlations of AMH levels (pmol/L) in infancy and mid-childhood with total follicle count as well as follicle subgroups in puberty and adolescence.
| AMH infancy |
AMH mid-childhood |
||||||
|---|---|---|---|---|---|---|---|
| n | Correlation coefficient | P-value | n | Correlation coefficient | P-value | ||
| Puberty | Number of follicles 2–3 mm by MRI | 74 | 0.229 | 0.050 | 78 | 0.461 | <0.001 |
| Number of follicles 4–6 mm by MRI | 74 | 0.126 | 0.286 | 78 | 0.551 | <0.001 | |
| Number of follicles ≥ 7 mm by MRI | 74 | 0.010 | 0.934 | 78 | 0.236 | 0.038 | |
| Total number of follicles by MRI | 74 | 0.138 | 0.240 | 78 | 0.607 | <0.001 | |
| Number of follicles < 4 mm by TAUS | 77 | 0.253 | 0.026 | 83 | 0.313 | 0.004 | |
| Number of follicles 5–9 mm by TAUS | 77 | 0.152 | 0.188 | 83 | 0.315 | 0.004 | |
| Total number of follicles by TAUS | 77 | 0.283 | 0.013 | 83 | 0.379 | <0.001 | |
| Adolescence | Number of follicles 2–4 mm by TAUS | 108 | 0.174 | 0.072 | 144 | 0.255 | <0.002 |
| Number of follicles 5–7 mm by TAUS | 106 | 0.013 | 0.892 | 142 | 0.334 | <0.001 | |
| Number of follicles ≥ 8 mm by TAUS | 108 | −0.079 | 0.413 | 144 | 0.108 | 0.197 | |
| Total number of follicles by TAUS | 106 | 0.168 | 0.084 | 142 | 0.414 | <0.001 | |
TAUS = transabdominal ultrasound.
MRI = magnetic resonance imaging.
AMH (pmol/L) in infancy correlated with the total number of follicles in puberty (TAUS: n = 77, r = 0.283, p = 0.013). This was driven primarily by small follicles (TAUS (<4 mm): n = 77, r = 0.253, p = 0.026 and MRI (2–3 mm): n = 74, r = 0.229, p = 0.050). AMH in infancy did not correlate with the number of follicles in adolescence (Table 2).
The total number of follicles in puberty (MRI) correlated with the total number of follicles in adolescence (n = 42, r = 0.445, p = 0.003).
AMH correlated negatively with FSH concentrations in all age groups (infancy: n = 262, r = −0.645, p < 0.001; mid-childhood: n = 307, r = −0.222, p < 0.001; puberty: n = 365, r = −0.354, p < 0.001; adolescence: n = 275, r = −0.175, p = 0.004).
AMH concentrations in adolescence were not associated with age of menarche (n = 208, r = −0.016, p = 0.819).
Girls with low AMH in mid-childhood (AMH ≤10.0 pmol/L, n = 40 vs. other girls, n = 145) had lower concentrations of inhibin B (61.0 vs. 79.0 pg/mL, p = 0.007), testosterone (0.72 vs. 0.89 nmol/L, p = 0.002), androstenedione (2.45 vs. 3.11 nmol/L, p = 0.001), AMH (15.8 vs. 30.4 pmol/L, p < 0.001) number of follicles, e.g. total follicles (17 vs. 20, p < 0.001) in adolescence (Table 3).
Table 3.
Reproductive hormone concentrations and follicle counts in adolescence depending on AMH in mid-childhood (AMH ≤10.0 pmol/L vs. AMH >10.0 pmol/L).
| Adolescence | Mid-childhood AMH ≤10 pmol/L |
Mid-childhood AMH >10 pmol/L |
p-value | ||||
|---|---|---|---|---|---|---|---|
| n | median | IQR | n | median | IQR | ||
| AMH, pmol/L | 40 | 15.8 | 10.3–21.0 | 105 | 30.4 | 21.7–42.3 | <0.001 |
| FSH, U/L | 39 | 4.86 | 3.89–5.77 | 101 | 5.79 | 4.49–6.99 | 0.009 |
| LH, U/L | 39 | 3.00 | 1.97–4.43 | 101 | 3.47 | 2.26–5.22 | 0.305 |
| Inhibin B, pg/mL | 39 | 61.0 | 39.0–81.0 | 101 | 79.0 | 58.0–97.5 | 0.007 |
| Testosterone, nmol/L | 39 | 0.72 | 0.57–0.90 | 100 | 0.89 | 0.71–1.14 | 0.002 |
| Androstenedione, nmol/L | 39 | 2.45 | 1.89–3.49 | 100 | 3.11 | 2.50–4.11 | 0.001 |
| Number of follicles 2–4 mm | 40 | 12 | 10–15 | 104 | 14 | 11–16 | 0.037 |
| Number of follicles 5–7 mm | 39 | 4 | 2–5 | 103 | 6 | 3–8 | <0.001 |
| Number of follicles ≥8 mm | 40 | 0 | 0–1 | 104 | 1 | 1–2 | 0.157 |
| Total number of follicles | 39 | 17 | 15–20 | 103 | 20 | 18–23 | <0.001 |
| BMI | 40 | 21.28 | 19.48–23.85 | 111 | 20.56 | 19.29–22.49 | 0.316 |
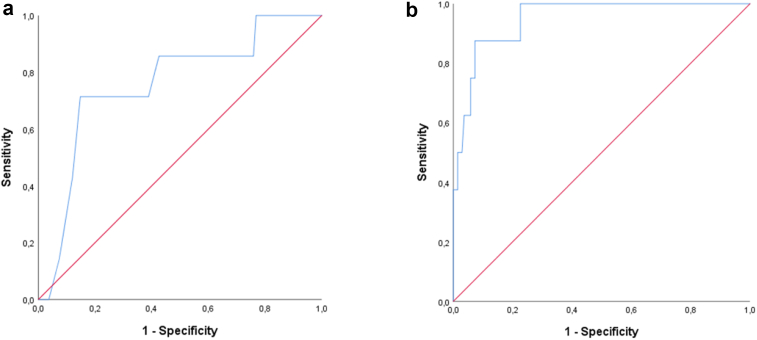
Low AMH in infancy and mid-childhood predicted low AMH in adolescence (AMH ≤10.0 pmol/L); AMH infancy <7.5 pmol/L (n = 7 vs. 108): AUC 0.759, sensitivity 0.71, specificity 0.70; AMH mid-childhood < 8.4 pmol/L (n = 8 vs. 137): AUC 0.949, sensitivity 0.88, specificity 0.87 (Fig. 3). Thus, there is a 94.9% chance that AMH in mid-childhood will predict if AMH is below or above 10 pmol/L in adolescence; 88% of girls with AMH <10 pmol/L in adolescence had AMH <8.4 pmol/L in mid-childhood, and 87% of girls with AMH >10 pmol/L in adolescence had AMH >8.4 pmol/L in mid-childhood.
Fig. 3.
Receiver operating characteristic (ROC) curves of low AMH in a) infancy (AMH <7.5 pmol/L, sensitivity 0.71, specificity 0.70, AUC 0.759) b) mid-childhood (AMH <8.5 pmol/L, sensitivity 0.88, specificity 0.87, AUC 0.949) as predictors of low AMH in adolescence (AMH ≤10 pmol/L).
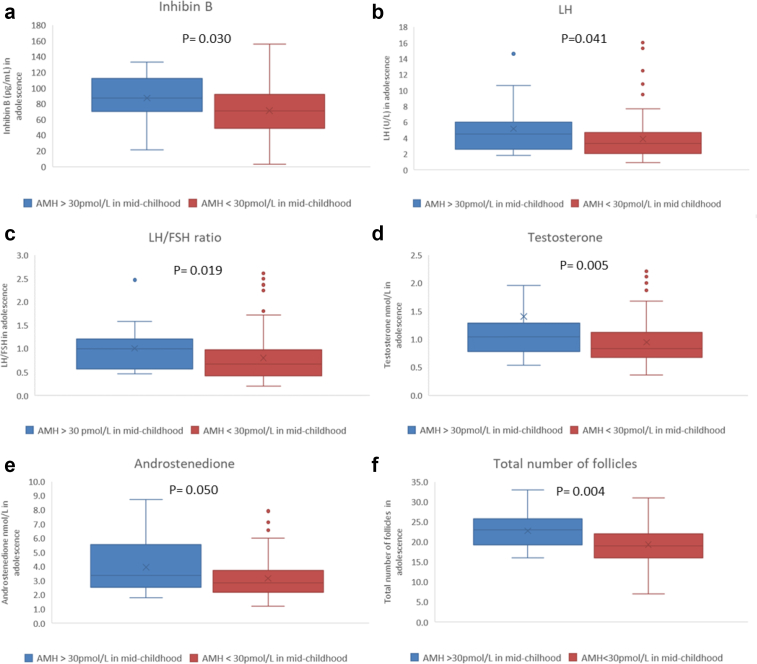
Girls with high AMH in mid-childhood (AMH ≥30.0 pmol/L, n = 21 vs. other girls, n = 124) had higher adolescent concentrations of LH (median 4.53 vs. 3.29 U/L p = 0.041), LH/FSH ratio (1.00 vs 0.67, p = 0.019), inhibin B (87 vs. 71 pg/mL, p = 0.030), testosterone (1.05 vs 0.81 nmol/L, p = 0.005), androstenedione (3.37 vs. 2.84, p = 0.050), AMH (38.7 vs 22.4 pmol/L, p < 0.001), number of total follicles (23 vs. 19, p = 0.004) as well as a higher prevalence of irregular menstrual cycles vs. regular menstrual cycles: (10/15 = 67% vs. 28/113 = 25%, p = 0.002) (Fig. 4). BMI as well as concentrations of SHBG and free testosterone did not differ between the two groups: BMI 21.2 vs 20.7 kg/m2, p = 0.755; SHBG 69.25 vs 63.20 nmol/L, p = 0.416; free testosterone 1.46 vs 1.32 pmol/L, p = 0.168, respectively
Fig. 4.
Reproductive hormone concentrations: a) inhibin B, b) LH, c) LH/FSH ratio, d) testosterone, e) androstenedione, as well as (f) follicle counts in adolescence depending on AMH in mid-childhood (AMH ≥30 pmol/L vs. AMH <30 pmol/L).
Discussion
In this long-term longitudinal study of serum AMH concentrations in healthy girls through infancy (median age 0.3 yrs), mid-childhood (7.2 yrs), puberty (11.3 yrs) and adolescence (15.9 yrs), each girl maintained her relative AMH level. AMH in adolescence correlated strongly with AMH concentrations in puberty and mid-childhood as well as infancy. In mid-childhood, serum concentrations of AMH predicted the number of ovarian follicles as the girls reached puberty and adolescence. From infancy to adolescence, AMH was negatively associated with FSH. This individual setpoint of ovarian activity deriving from small growing follicles which exert negative feedback on the pituitary already from infancy highlights several physiological, clinical and scientific points of interest.
The primordial follicle pool is established in fetal life. Hereafter the number of primordial follicles declines continuously until time of menopause.32 It has therefore been argued, that relatively stable concentrations of AMH during childhood excludes AMH as a clinical marker of the follicle reserve in girls.33 However, based on the present data, we speculate that this individual setpoint of ovarian activity tracking throughout the entire childhood to some degree may reflect the size of the primordial follicle pool. From birth to menopause, primordial follicles are constantly recruited into small antral stages producing AMH. In adult women, the number of small antral follicles are in equilibrium with the number of metabolically inactive primordial follicles.34 If the same dynamics exist in childhood, girls with continuously low AMH may have less primordial follicles than girls with high AMH concentrations.
Growth of small AMH producing follicles is not dependent on FSH stimulation, and therefore minipuberty as well as pubertal reactivation of the HPG axis affects AMH concentrations much less than other ovarian hormones e.g. inhibin B and estradiol produced from larger follicles.23,35 In contemporary longitudinal cohorts of healthy girls, we and other groups have previously observed minor fluctuations of AMH during pubertal development with increase of concentrations prior to pubertal onset (defined by initial breast development) followed by a limited decrease in the first years following pubertal onset.35,36 Compiled data from several cohorts suggest a further increase of AMH in adolescence.37 The present study supports previous findings of relatively limited dynamics of absolute AMH concentration through infancy, childhood and adolescence. The consistent negative correlation between AMH and FSH through childhood was also observed in another cohort of healthy Danish peripubertal girls,35 suggesting that ovarian activity from smoldering growth of small antral follicles exerts negative feedback on the pituitary even in quiescent periods through childhood.
The pubertal reactivation of the HPG axis is coordinated by networks of inducers and inhibitors of hypothalamic GnRH secretion.38 Although AMH may exert regulation of GnRH secretion,20 we were not able to find associations with pubertal onset (initial breast development) in a previous study of healthy girls followed frequently through puberty.12 In line with these findings, AMH was not associated with age at menarche in the present study.
It is uncertain, whether AMH concentrations in an individual healthy girl predicts the length of her reproductive lifespan. Even in adulthood, the predictive value of AMH for the number of primordial follicles7 as well as the specific age at menopause8 is limited. Furthermore, time to pregnancy is not affected in young women with low AMH.39 Low AMH in otherwise healthy girls therefore probably does not indicate a risk of future premature ovarian insufficiency (POI) nor reduced fecundability. Longitudinal studies from early childhood to adulthood are needed to clarify this further.
The clinical utility of the present findings remains to be explored in patients with risk of premature loss of ovarian follicles; e.g. Turner Syndrome, other DSD conditions, galactosemia, autoimmune disorders, patients with familiar dispositions, and cancer patients after gonadotoxic therapy. In cross sectional studies, low AMH is a marker of POI in adolescent Turner Syndrome patients.11,40 For patients at risk of POI, limited longitudinal data support that very low or undetectable AMH in early childhood predicts hypergonadotropic hypogonadism at time of expected puberty.41 The relatively high accuracy of low AMH in mid-childhood to predict low AMH in adolescence in the present study supports an assumption that low AMH in young patients at risk of POI is of concern. However, large longitudinal studies of patients in risk of POI are needed to assess the predictive value AMH concerning age at menopause. This could have considerable impact on decisions of whether a patient would benefit from early fertility preservation, i.e. patients with Turner syndrome.42
Although we expect a limited predictive value of individual AMH concentrations concerning the number of primordial follicles and age at menopause in an individual healthy girl, the remarkable correlations of AMH in infancy and mid-childhood with ovarian activity later in adolescence suggests that AMH is a valuable tool in scientific population-based studies. AMH in infancy and mid-childhood may serve as a key outcome when evaluating risk factors for fetal ovarian development during which the ovarian reserve of primordial follicles is established.
PCOS is a major endocrinopathy among women at reproductive age. The exact prevalence is not clarified, but it is estimated that 5–20% of apparently healthy girls will develop PCOS in adulthood.43 The definition of PCOS is continuously discussed, and the criteria of PCOS have been revised several times.44 Diagnosing PCOS in adolescence is particularly challenging as multifollicular ovarian morphology as well as irregular juvenil anovulatoric cycles are normal physiological findings the first years after menarche.26 It has therefore been suggested to postpone diagnosing PCOS to at least eight years after menarche.45 Despite these challenges, we could demonstrate that AMH in early childhood (highest quintile) was associated with adolescent hormone concentrations, ovarian morphology and menstrual cycle patterns resembling PCOS in adulthood. Our study cannot predict whether these girls will fulfil the criteria for PCOS as young women or, in fact, have a normal ovarian function. However, the link between early childhood AMH and a PCOS-resembling phenotype in adolescents is intriguing. Although clinical findings of PCOS are not present before pubertal reactivation of the HPG axis, this indicates that the ovarian physiology of future PCOS patients may already be altered in early childhood. This supports previous evidence that the etiology of PCOS is multifactorial, including genetic predisposition, intergenerational links as well as environmental factors.46 Thus, long term longitudinal studies are needed to clarify if AMH in childhood is predictive of PCOS in adulthood.
This is a unique large and long-term study of healthy Danish girls. Due to inclusion criteria (e.g. Caucasian, healthy pregnant women), the limited participation-rate (approximately 25%), the limited geographical area included (The Copenhagen area) as well as over-representation of mothers with an academic degree, the girls are probably not representative for the entire Danish background population.47,48 At examinations of adolescents, participants did not differ from non-participants concerning gestational age, birth weight, weight for gestational age, maternal pre-pregnancy BMI or social class.5 Thus, selection-bias concerning the girls who kept participating in the study compared to the girls withdrawing from the longitudinal part of the study seems limited. A previous study has shown that AMH levels are comparable between different ethnical groups implying that findings in the present study may be applied to girls of other ethnicity.49
AMH measurements cannot be directly compared between commercially available assays, and reports of different converting factors suggest that the assays have been modified continuously.50 Therefore, the absolute AMH concentrations reported in this study should be used with caution for other AMH assays. Nevertheless, the relative concentrations (SDS) based on a well-defined reference range are clinically useful. It was not possible to examine all adolescents at cycle day 2 through 5. We do not expect this to affect the primary outcome of this study, as serum concentrations of AMH do not vary considerably during the menstrual cycle.51 However, serum concentrations of other reproductive hormones may be affected by this variation in timing of sampling. Furthermore, we may have missed adolescents with a phenotype resembling PCOS where symptoms were disguised by hormonal contraceptive therapy as these girls were excluded from analyses. Traditionally, regular cycles are defined as a cycle length of 21–35 days. However, The American College of Obstetricians and Gynecologists (ACOG) suggests that regular cycles in adolescents to be defined by cycle length of 21–45 days.52 This definition could affect analyses of high AMH in mid-childhood associated with irregular cycles in adolescence, however, our data do not allow any reclassification.5
We cannot exclude recall – or self-reporting bias concerning menstrual cycle pattern as well as age at menarche. However, we do not expect AMH concentrations to affect the risk of misclassification. Thus, misclassification is likely equally distributed between AMH subgroups and therefore not affecting the results.
In this unique long-term longitudinal study of healthy girls with repeated blood sampling, serum AMH concentrations in infancy, mid-childhood and puberty correlate strongly with AMH concentrations measured in the same girls in adolescence. In mid-childhood, AMH concentrations even reflect the number of ovarian follicles in puberty and adolescence. This suggests a remarkably stable ovarian follicular activity, supporting AMH in early life as a useful clinical tool to predict future ovarian activity.
Contributors
KM, CH and MBF had full access to the study data and take responsibility for the integrity of the data and the accuracy of the data analysis. KM designed the study and applied for grants. CH, CWV and MA coordinated the study and examined the girls. CH, MBF and ASB did the statistical analyses. CH, MBF, AJ, ATP and KM interpreted the data. CH and MBF drafted the original manuscript, which was reviewed and edited by all other authors. All authors approved the final submitted version.
Data sharing statement
All data collected for the study will be available with publication. Requests should be addressed to casper.hagen@regionh.dk.
Declaration of interests
The work was supported by The Center on Endocrine Disruptors (CeHoS) under The Danish Environmental Protection Agency and The Ministry of Environment and Food (grant number: MST-621-00 065), the EU (QLK4-CT1999-01422; QLK4-2001-00269), the Novo Nordisk Foundation and The Danish Ministry of Science Technology and Innovation (2107-05-0006). A.S.B. is funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) – 464240267. KM receives honoraria from Novo Nordisk A/S for teaching at the Danish annual postgraduate course of pituitary diseases.
References
- 1.Jeppesen J.V., Anderson R a, Kelsey T.W., et al. Which follicles make the most anti-Mullerian hormone in humans? Evidence for an abrupt decline in AMH production at the time of follicle selection. Mol Hum Reprod. 2013;19:519–527. doi: 10.1093/molehr/gat024. [DOI] [PubMed] [Google Scholar]
- 2.Stubbs S a, Hardy K., Da Silva-Buttkus P., et al. Anti-müllerian hormone protein expression is reduced during the initial stages of follicle development in human polycystic ovaries. J Clin Endocrinol Metab. 2005;90:5536–5543. doi: 10.1210/jc.2005-0907. [DOI] [PubMed] [Google Scholar]
- 3.Kuiri-Hänninen T., Kallio S., Seuri R., et al. Postnatal developmental changes in the pituitary-ovarian axis in preterm and term infant girls. J Clin Endocrinol Metab. 2011;96:3432–3439. doi: 10.1210/jc.2011-1502. [DOI] [PubMed] [Google Scholar]
- 4.Hagen C.P., Mouritsen A., Mieritz M.G., et al. Circulating AMH reflects ovarian morphology by Magnetic Resonance Imaging and 3D-ultrasound in 121 healthy girls. J Clin Endocrinol Metab. 2014 doi: 10.1210/jc.2014-3336. [DOI] [PubMed] [Google Scholar]
- 5.Assens M., Dyre L., Henriksen L.S., et al. Menstrual pattern, reproductive hormones, and transabdominal 3D ultrasound in 317 adolescent girls. J Clin Endocrinol Metab. 2020;105:E3257–E3266. doi: 10.1210/clinem/dgaa355. [DOI] [PubMed] [Google Scholar]
- 6.de Vet A., Laven J.S., de Jong F.H., Themmen A.P., Fauser B.C. Antimullerian hormone serum levels: a putative marker for ovarian aging. Fertil Steril. 2002;77:357–362. doi: 10.1016/s0015-0282(01)02993-4. [DOI] [PubMed] [Google Scholar]
- 7.Hansen K.R., Hodnett G.M., Knowlton N., Craig L.B. Correlation of ovarian reserve tests with histologically determined primordial follicle number. Fertil Steril. 2011;95:170–175. doi: 10.1016/j.fertnstert.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 8.Depmann M., Eijkemans M.J.C., Broer S.L., et al. Does AMH relate to timing of menopause? results of an individual patient data meta-analysis. J Clin Endocrinol Metab. 2018;103:3593–3600. doi: 10.1210/jc.2018-00724. [DOI] [PubMed] [Google Scholar]
- 9.Lee M.M., Donahoe P.K., Silverman B.L., et al. Measurements of serum mullerian inhibiting substance in the evaluation of children with nonpalpable gonads. N Engl J Med. 1997;336:1480–1486. doi: 10.1056/NEJM199705223362102. [DOI] [PubMed] [Google Scholar]
- 10.Lee M.M., Donahoe P.K., Hasegawa T., et al. Mullerian inhibiting substance in humans: normal levels from infancy to adulthood. J Clin Endocrinol Metab. 1996;81:571–576. doi: 10.1210/jcem.81.2.8636269. [DOI] [PubMed] [Google Scholar]
- 11.Hagen C.P., Aksglaede L., Sørensen K., et al. Serum levels of anti-Müllerian hormone as a marker of ovarian function in 926 healthy females from birth to adulthood and in 172 Turner syndrome patients. J Clin Endocrinol Metab. 2010;95:5003–5010. doi: 10.1210/jc.2010-0930. [DOI] [PubMed] [Google Scholar]
- 12.Hagen C.P., Aksglaede L., Sorensen K., et al. Individual serum levels of anti-Mullerian hormone in healthy girls persist through childhood and adolescence: a longitudinal cohort study. Hum Reprod. 2012;27:861–866. doi: 10.1093/humrep/der435. [DOI] [PubMed] [Google Scholar]
- 13.Jeffery A., Streeter A.J., Hosking J., Wilkin T.J., Nelson S.M. Anti-Müllerian hormone in children: a ten-year prospective longitudinal study (EarlyBird 39) J Pediatr Endocrinol Metab. 2015;28:1153–1162. doi: 10.1515/jpem-2014-0517. [DOI] [PubMed] [Google Scholar]
- 14.Durlinger A.L., Gruijters M.J., Kramer P., et al. Anti-Mullerian hormone inhibits initiation of primordial follicle growth in the mouse ovary. Endocrinology. 2002;143:1076–1084. doi: 10.1210/endo.143.3.8691. [DOI] [PubMed] [Google Scholar]
- 15.Durlinger A.L., Gruijters M.J., Kramer P., et al. Anti-Mullerian hormone attenuates the effects of FSH on follicle development in the mouse ovary. Endocrinology. 2001;142:4891–4899. doi: 10.1210/endo.142.11.8486. [DOI] [PubMed] [Google Scholar]
- 16.Eilso N.M., Rasmussen I.A., Fukuda M., Westergaard L.G., Yding A.C. Concentrations of anti-Mullerian hormone in fluid from small human antral follicles show a negative correlation with CYP19 mRNA expression in the corresponding granulosa cells. Mol Hum Reprod. 2010;16:637–643. doi: 10.1093/molehr/gaq001. [DOI] [PubMed] [Google Scholar]
- 17.Lauritsen M.P., Bentzen J.G., Pinborg a, et al. The prevalence of polycystic ovary syndrome in a normal population according to the Rotterdam criteria versus revised criteria including anti-Mullerian hormone. Hum Reprod. 2014;0:1–11. doi: 10.1093/humrep/det469. [DOI] [PubMed] [Google Scholar]
- 18.Coulam C.B., Adamson S.C., Annegers J.F. Incidence of premature ovarian failure. Obstet Gynecol. 1986;67:604–606. [PubMed] [Google Scholar]
- 19.Pellatt L., Hanna L., Brincat M., et al. Granulosa cell production of anti-Müllerian hormone is increased in polycystic ovaries. J Clin Endocrinol Metab. 2007;92:240–245. doi: 10.1210/jc.2006-1582. [DOI] [PubMed] [Google Scholar]
- 20.Cimino I., Casoni F., Liu X., et al. Novel role for anti-Müllerian hormone in the regulation of GnRH neuron excitability and hormone secretion. Nat Commun. 2016;7:1–12. doi: 10.1038/ncomms10055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sir-Petermann T., Maliqueo M., Codner E., et al. Early metabolic derangements in daughters of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2007;92:4637–4642. doi: 10.1210/jc.2007-1036. [DOI] [PubMed] [Google Scholar]
- 22.Busch A.S., Ljubicic M.L., Upners E.N., et al. Dynamic changes of reproductive hormones in male minipuberty: temporal dissociation of leydig and Sertoli cell activity. J Clin Endocrinol Metab. 2022;107:1560–1568. doi: 10.1210/clinem/dgac115. [DOI] [PubMed] [Google Scholar]
- 23.Ljubicic M.L., Busch A.S., Upners E.N., et al. A biphasic pattern of reproductive hormones in healthy female infants –the COPENHAGEN minipuberty study. J Clin Endocrinol Metab. 2022:1–8. doi: 10.1210/clinem/dgac363. [DOI] [PubMed] [Google Scholar]
- 24.Johannsen T.H., Main K.M., Ljubicic M.L., et al. Sex differences in reproductive hormones during mini-puberty in infants with normal and disordered sex development. J Clin Endocrinol Metab. 2018;103:3028–3037. doi: 10.1210/jc.2018-00482. [DOI] [PubMed] [Google Scholar]
- 25.Aksglaede L., Davis S.M., Ross J.L., Juul A. Minipuberty in Klinefelter syndrome: current status and future directions. Am J Med Genet Part C Semin Med Genet. 2020;184:320–326. doi: 10.1002/ajmg.c.31794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Metcalf M.G., Skidmore D.S., Lowry G.S., Mackenzie J.A. Incidence of ovulation in the years after the menarche. J Endocrinol. 1983;97:213–219. doi: 10.1677/joe.0.0970213. [DOI] [PubMed] [Google Scholar]
- 27.Chellakooty M., Schmidt I.M., Haavisto A.M., et al. Inhibin A, Inhibin B, follicle-stimulating hormone, luteinizing hormone, estradiol, and sex hormone-binding globulin levels in 473 healthy infant girls. J Clin Endocrinol Metab. 2003;88:3515–3520. doi: 10.1210/jc.2002-021468. [DOI] [PubMed] [Google Scholar]
- 28.Boas M., Hegedus L., Feldt-Rasmussen U., Skakkebaek N.E., Hilsted L., Main K.M. Association of thyroid gland volume, serum insulin-like growth factor-I, and anthropometric variables in euthyroid prepubertal children. J Clin Endocrinol Metab. 2009;94:4031–4035. doi: 10.1210/jc.2009-0939. [DOI] [PubMed] [Google Scholar]
- 29.Marshall W.A., Tanner J.M. Variations in pattern of pubertal changes in girls. Obstet Gynecol Surv. 1970;25:694–696. [Google Scholar]
- 30.Søeborg T., Frederiksen H., Mouritsen A., et al. Sex, age, pubertal development and use of oral contraceptives in relation to serum concentrations of DHEA, DHEAS, 17α-hydroxyprogesterone, δ4-androstenedione, testosterone and their ratios in children, adolescents and young adults. Clin Chim Acta. 2014;437:6–13. doi: 10.1016/j.cca.2014.06.018. [DOI] [PubMed] [Google Scholar]
- 31.Rigby R.A., Stasinopoulos D.M. Automatic smoothing parameter selection in GAMLSS with an application to centile estimation. Stat Methods Med Res. 2014;23:318–332. doi: 10.1177/0962280212473302. [DOI] [PubMed] [Google Scholar]
- 32.Baker T.G.A. Quantitative and cytological study of germ cells in human ovaries. Proc RSoc L B Biol Sci. 1963;158:417–433. doi: 10.1098/rspb.1963.0055. [DOI] [PubMed] [Google Scholar]
- 33.von Wolff M., Roumet M., Stute P., Liebenthron J. Serum anti-Mullerian hormone (AMH) concentration has limited prognostic value for density of primordial and primary follicles, questioning it as an accurate parameter for the ovarian reserve. Maturitas. 2020;134:34–40. doi: 10.1016/j.maturitas.2020.02.001. [DOI] [PubMed] [Google Scholar]
- 34.Gougeon a, Ecochard R., Thalabard J.C. Age-related changes of the population of human ovarian follicles: increase in the disappearance rate of non-growing and early-growing follicles in aging women. Biol Reprod. 1994;50:653–663. doi: 10.1095/biolreprod50.3.653. [DOI] [PubMed] [Google Scholar]
- 35.Hagen C.P., Aksglaede L., Sørensen K., et al. Individual serum levels of anti-Müllerian hormone in healthy girls persist through childhood and adolescence: a longitudinal cohort study. Hum Reprod. 2012;27:861–866. doi: 10.1093/humrep/der435. [DOI] [PubMed] [Google Scholar]
- 36.Lashen H., Dunger D.B., Ness A., Ong K.K. Peripubertal changes in circulating antimüllerian hormone levels in girls. Fertil Steril. 2013:1–5. doi: 10.1016/j.fertnstert.2013.01.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kelsey T.W., Wright P., Nelson S.M., Anderson R.A., Wallace W.H. A validated model of serum anti-mullerian hormone from conception to menopause. PLoS One. 2011;6 doi: 10.1371/journal.pone.0022024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Day F.R., Thompson D.J., Helgason H., et al. Genomic analyses identify hundreds of variants associated with age at menarche and support a role for puberty timing in cancer risk. Nat Genet. 2017;49:834–841. doi: 10.1038/ng.3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hagen C.P., Vestergaard S., Juul A., et al. Low concentration of circulating antimüllerian hormone is not predictive of reduced fecundability in young healthy women: a prospective cohort study. Fertil Steril. 2012;98:1602–1608.e2. doi: 10.1016/j.fertnstert.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 40.Visser J a, Hokken-Koelega A.C.S., Zandwijken G.R.J., Limacher A., Ranke M.B., Flück C.E. Anti-Müllerian hormone levels in girls and adolescents with Turner syndrome are related to karyotype, pubertal development and growth hormone treatment. Hum Reprod. 2013;28:1899–1907. doi: 10.1093/humrep/det089. [DOI] [PubMed] [Google Scholar]
- 41.Lunding S.A., Aksglaede L., Anderson R a, et al. AMH as predictor of premature ovarian insufficiency: a longitudinal study of 120 turner syndrome patients. J Clin Endocrinol Metab. 2015;100:E1030–E1038. doi: 10.1210/jc.2015-1621. [DOI] [PubMed] [Google Scholar]
- 42.Oktay K., Bedoschi G., Berkowitz K., et al. Fertility preservation in women with turner syndrome: a comprehensive review and practical guidelines. J Pediatr Adolesc Gynecol. 2016;29:409–416. doi: 10.1016/j.jpag.2015.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Deswal R., Narwal V., Dang A., Pundir C. The prevalence of polycystic ovary syndrome: a brief systematic review. J Hum Reprod Sci. 2020;13:261–271. doi: 10.4103/jhrs.JHRS_95_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS) Hum Reprod. 2004;19:41–47. doi: 10.1093/humrep/deh098. [DOI] [PubMed] [Google Scholar]
- 45.Teede H.J., Misso M.L., Costello M.F., et al. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Fertil Steril. 2018;110:364–379. doi: 10.1016/j.fertnstert.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Franks S., Berga S.L. Does PCOS have developmental origins? Fertil Steril. 2012;97:2–6. doi: 10.1016/j.fertnstert.2011.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boisen K.A., Kaleva M., Main K.M., et al. Difference in prevalence of congenital cryptorchidism in infants between two Nordic countries. Lancet (London, England) 2004;363:1264–1269. doi: 10.1016/S0140-6736(04)15998-9. [DOI] [PubMed] [Google Scholar]
- 48.Damgaard I.N., Jensen T.K., Petersen J.H., Skakkebaek N.E., Toppari J., Main K.M. Risk factors for congenital cryptorchidism in a prospective birth cohort study. PLoS One. 2008;3 doi: 10.1371/journal.pone.0003051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Elchuri S.V., Patterson B.C., Brown M.R., Buchanan I., Mertens A.C., Meacham L.R. Anti-Mullerian hormone levels in American girls by age and race/ethnicity. J Pediatr Endocrinol Metab. 2015;28:189–193. doi: 10.1515/jpem-2014-0242. [DOI] [PubMed] [Google Scholar]
- 50.Kumar A., Kalra B., Patel A., McDavid L., Roudebush W.E. Development of a second generation anti-Müllerian hormone (AMH) ELISA. J Immunol Methods. 2010;362:51–59. doi: 10.1016/j.jim.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 51.La Marca A., Malmusi S., Giulini S., et al. Anti-Müllerian hormone plasma levels in spontaneous menstrual cycle and during treatment with FSH to induce ovulation. Hum Reprod. 2004;19:2738–2741. doi: 10.1093/humrep/deh508. [DOI] [PubMed] [Google Scholar]
- 52.ACOG Committee Opinion No 651: menstruation in girls and adolescents: using the menstrual cycle as a vital sign. Obstet Gynecol. 2015;126:e143–e146. doi: 10.1097/AOG.0000000000001215. [DOI] [PubMed] [Google Scholar]