Abstract
We sought to assess the degree of cross-protective immunity in a mouse model of chlamydial genital tract infection. Following resolution of genital infection with the mouse pneumonitis (MoPn) biovar of Chlamydia trachomatis, mice were challenged intravaginally with either MoPn or human serovar E or L2. The majority of animals previously infected with MoPn were solidly immune to challenge with either of the two human biovars. Surprisingly, approximately 50% of animals became reinfected when homologously challenged with MoPn, although the secondary infection yielded significantly lower numbers of the organism isolated over a shorter duration than in the primary infection. Primary infection with serovar E also protected against challenge with MoPn or serovar L2, although the degree of immune protection was lower than that resulting from primary infection with MoPn. Blast transformation and assessment of delayed-type hypersensitivity indicated that mice previously infected with either human or murine biovars produced broadly cross-reactive T cells that recognized epitopes of either murine or human biovars of C. trachomatis. Immunoblotting demonstrated that primary MoPn infection produced immunoglobulin G (IgG) antibody to antigens of MoPn as well as at least three distinct antigenic components of human serovar E, one of which was identical in molecular weight to the major outer membrane protein (MOMP). Primary infection with serovar E produced IgG antibody reactive against serovar E but not MoPn MOMP and against at least one ca. 60-kDa protein of both chlamydial strains. Our results indicate that primary genital infection of mice with murine C. trachomatis induces immunity against challenge with either of two human biovars.
It has been concluded that human infection with a given serovar of Chlamydia trachomatis confers serovar-specific immunity, but broadly cross-reactive genus-specific epitopes are likely to elicit immunopathological injury (23, 36). This conclusion is based on several observations. Jawetz et al. found in a small trial that humans recovering from inclusion conjunctivitis were immune to rechallenge with the same serovar but were susceptible to rechallenge with a heterologous serovar (18). Additionally, early vaccine trials established that whole inactivated elementary bodies (EBs) administered parenterally yielded brief (lasting 1.5 to 3 years) protective immunity to ocular infection that was limited to the immunizing serovar (15–17, 37). In nonhuman primate models of trachoma and pelvic inflammatory disease, immunity is short lived and serovar specific (16, 27). Lastly, antibodies that neutralize chlamydial infectivity in vitro are usually directed against serovar-specific epitopes (reviewed in references 3, 6, 23, and 36). Collectively, one could assume from these findings that immunity resulting from prior chlamydial infection, if it exists at all, leads to serovar-specific immunity. However, it has not been definitively determined that prior genital tract infection in humans leads to protective immunity or, if protective immunity exists at this anatomical site, whether it is serovar specific (4, 22, 32).
That immunopathological injury results from previous or persistent exposure to broadly cross-reacting chlamydial antigens is also a well-accepted theory. Although serovar-specific immunity was elicited by a killed EB vaccine, it appeared to potentiate the disease state upon exposure to a heterologous serovar (15, 17). Further in vivo evidence is the observation that chlamydial Hsp60, which has 90% homology across the genus Chlamydia, elicited damaging inflammation in the eyes of guinea pigs (24) and the fallopian tubes of monkeys (27) that had been previously sensitized. Regarding murine chlamydial disease, mouse strains susceptible to infertility following chlamydial genital tract infection are also characterized as immunological responders to Hsp60, whereas strains resistant to the development of infertility are nonresponders (6, 12, 13, 33, 34, 38). Lastly, and perhaps more relevant, is the observation that a high incidence of antibody to Hsp60 epitopes is observed in women with ectopic pregnancy and tubal infertility (6, 14, 36). To summarize, the consensus has been that effective immunoprophylaxis would need to include and elicit immune responses against epitopes that are specific to each of the serovars that circulate in the vaccinated population, presumably those of the major outer membrane protein (MOMP). The same vaccine would need to omit epitopes that could possibly provide cross-serovar protection, because they may lead to untoward hypersensitizing responses against chlamydial antigens (e.g., hsp60).
Two murine models for the study of disease and immunity resulting from chlamydial genital tract infection have been established. One version of this model uses human serovars of C. trachomatis either inoculated directly into the uterus or ovarian bursa of mice or instilled intravaginally (36). Alternatively, a more commonly used model is intravaginal inoculation of mice with the mouse pneumonitis (MoPn) biovar of C. trachomatis (2). In the first case, successful infection of mice with human serovars is highly dependent upon prior treatment of mice with progesterone (P4); the MoPn model is less dependent on P4, especially if multiple infecting inoculations are conducted on two or three consecutive days or higher doses are used. However, mice sustain a more consistent infection with one MoPn inoculation if the estrus cycle is arrested with prior treatment with P4. Additionally, approximately 2 log scales fewer chlamydiae are recovered from mice inoculated with human serovars at the peak of infection, and less inflammation is observed than in those infected with MoPn (12). One may reasonably conclude from these findings that human C. trachomatis is less virulent in mice than is MoPn.
It has been found that following resolution of primary infection, mice rechallenged intravaginally with MoPn are immune for a limited period. Of those animals that shed viable organisms after challenge infection, each shed smaller quantities for an abbreviated period of time (20, 28). Of equal importance is the finding that viable MoPn organisms were required to establish protection against intravaginal challenge regardless of the route of administration (intranasal, oral, vaginal, or subcutaneous), whereas UV-inactivated organisms by any route were ineffective in this regard (20). Hence, at present, any study of protective immunity in mice requires viable chlamydiae.
In the present study, we combined the mouse-MoPn and mouse-human serovar intravaginal infection models to determine if protective immunity is biovar or serovar specific and to determine the extent of cross-protective immunity resulting from infection of mice with the mouse and human biovars.
MATERIALS AND METHODS
C. trachomatis.
All chlamydial strains were grown in HeLa 229 cells and partially purified by differential centrifugation. Stocks were assessed for viable chlamydiae by culture of sequential 10-fold dilutions in HeLa 229 monolayers and subsequent enumeration of inclusions by indirect immunofluorescence (10). The remainder were frozen at −70°C in SPG buffer (0.25 M sucrose, 10 mM phosphate, and 5 mM l-glutamic acid [pH 7.3]) until needed. MoPn (Weiss strain) was originally obtained from Todd Cotter, who acquired it from stocks maintained in the laboratory of Harlan Caldwell (Rocky Mountain Laboratory, Hamilton, Mont.).
Mice.
Five- to 6-week-old C3H/HeN mice were obtained from Harlan Sprague-Dawley, Indianapolis, Ind. Mice were given food and water ad libitum and were housed under a 14:10 dark-light cycle and allowed to acclimate for 10 days prior to inclusion in experiments. C3H/HeN mice were used as the model strain due to their increased susceptibility to chlamydial infection and disease compared to other strains of mice (7, 12, 13, 33, 34, 38).
Intravaginal infection and challenge of mice.
Although not essential for establishing MoPn genital tract infection in mice, progesterone pretreatment was used to ensure a 100% infection rate in these studies. Mice were treated subcutaneously with 2.5 mg of medroxyprogesterone acetate (P4 [Depo-Provera; UpJohn, Kalamazoo, Mich.]) after 10 days of acclimation, i.e., at 6 to 7 weeks old, and again 1 week later, which correlated with 10 and 3 days prior to infection, respectively (10). Three days following the second P4 treatment, mice were infected intravaginally with 200 50% infective doses (ID50) (correlating to 104 inclusion-forming units [IFU] for MoPn and 106 IFU for serovar E) of either MoPn or human serovar E C. trachomatis in 10 μl of SPG. Following culture-confirmed resolution of infection, at 46 and 53 days postinfection, mice were again pretreated with P4 as described above. At day 56 postinfection, mice were challenged intravaginally with 2,000 ID50 of either MoPn or human C. trachomatis serovar E or L2.
Assessment of infection.
Infection was assessed by sequential collection of cervical-vaginal swabs (Calgiswab, type 1; Spectrum Diagnostics, Houston, Tex.) at 4, 7, 10, and 14 days postinfection and every 7 days thereafter until cessation of chlamydial shedding. Swab specimens were cultured immediately for detection of viable chlamydia or were frozen at −70°C immediately. Once thawed, specimens were vortexed vigorously and swab fluid was diluted 1:4 in minimal essential medium and inoculated onto HeLa 229 cell monolayers in 24-well plates. After centrifugation at 1,500 × g for 1 h at 37°C, followed by an additional 2 h at 36.5°C, the swab fluid was replaced with medium and the monolayers were incubated at 36.5°C in a humidified atmosphere of 5% CO2. Following an incubation time appropriate for each serovar or strain tested, monolayers were fixed in methanol and stained by indirect immunofluorescence antibody and examined with an inverted fluorescence microscope. A detailed description of the isolation procedure and the protocol for staining and enumeration of chlamydial inclusions has been provided elsewhere (11).
Preparation of chlamydial EBs for use as antigen in immunological assays.
Chlamydial EBs were purified from HeLa 229 cells by ultracentrifugation over a Renograffin density gradient, as previously described (8). Purified EBs were then inactivated with UV light (28), standardized according to protein content (BCA protein assay kit; Pierce Chemical Co., Rockford, Ill.), and frozen in sterile phosphate-buffered saline (PBS) at −80°C.
T-lymphocyte blast transformation.
At 56 days post-primary infection, mice were sacrificed and spleens and iliac lymph nodes (ILN) were excised. Single-cell suspensions were prepared by mincing tissues over stainless steel 40 to 60 mesh screens in cold Hanks balanced salt solutions, washed by centrifugation, and passed over nylon wool-packed columns in order to enrich for T cells. Nylon wool-enriched T lymphocytes were washed three times by centrifugation and resuspended in culture medium (RPMI 1640 with 10% fetal bovine serum, penicillin, streptomycin, l-glutamine, nonessential amino acids, and sodium pyruvate) to 2 × 106/ml. One hundred microliters of this suspension per well was placed in 96-well culture plates with an equal concentration and volume of mitomycin C-treated syngeneic splenic feeder cells that had been previously pulsed with 5 μg of UV-inactivated EBs per ml. T-lymphocyte proliferation was determined by incorporation of [3H]thymidine for the last 18 to 24 h of a 5-day culture period and expressed as mean counts per minute of quadruplicate cultures. A detailed description of chlamydial antigen-driven T-lymphocyte blast transformation assays is provided elsewhere (29).
Assessment of delayed-type hypersensitivity (DTH) to chlamydial antigen.
At 56 days post-primary infection, 5 μg of UV-inactivated chlamydial antigen in 50 μl of sterile pyrogen-free PBS was injected into the hind footpad of mice. The contralateral hind footpad received 5 μg of HeLa 229 cell antigen in 50 μl of PBS. Footpad thickness measurements were made with a dial micrometer (MTI, Aurora, Ill.) just prior to injection and at 4 to 6, 12, 24, 48, and 72 h postinjection (1, 30). Increases in footpad thickness were assessed by subtracting footpad thickness just prior to injection of antigen from subsequent measurements and are expressed as mean increases in footpad thickness of five animals.
Immunoblotting.
Purified chlamydial antigens were solubilized and resolved by electrophoresis according to the manufacturer’s instructions (Nupage electrophoresis system; Novex, San Diego, Calif.). Samples were loaded onto 4 to 12% gradient bis-Tris gels at 1.5 μg/well. Gel conditions were 200 V (constant) for 55 min. Resolved proteins were electrophoretically transferred to a 0.2-μm-pore-size nitrocellulose membrane (Novex) at 25 V (constant) for 120 min. Membranes were blocked in casein blocking buffer overnight at 4°C with gentle rocking. Immune sera were diluted 1:50 and 1:400 in casein blocking buffer for serovar E- and MoPn-immune animals, respectively. Samples were incubated on membranes with gentle rocking for 60 min at room temperature, followed by three wash steps with 0.5% Tween in PBS. Secondary antibody (peroxidase-conjugated goat anti-mouse immunoglobulin G [IgG]; Kirkegaard and Perry Laboratories, Gaithersburg, Md.) was diluted to 1:10,000 in casein blocking buffer and incubated on the membranes for an additional 60 min at room temperature. The membranes were then washed five times with PBS-Tween for 10 minutes each, followed by a wash with PBS only. Membranes were developed with SuperSignal chemiluminescent substrate solution, according to the manufacturer’s instructions (Pierce Chemical Co.), for 5 min, rinsed briefly in distilled water, and placed on glass plates. Bound antibody was detected by exposure of blots to Biomax MS film (Kodak, Rochester, N.Y.) for 30 s.
Statistics.
The two-tailed t test was used to determine significant differences in IFU counts, [3H]thymidine incorporation, and footpad swelling. Differences were considered significant at P of <0.05.
RESULTS
Prior infection with the MoPn biovar of C. trachomatis protects against subsequent intravaginal challenge with human serovars.
So that primary and challenge inocula could be standardized in our model, we first determined the ID50 for each of the strains tested in C3H/HeN mice (data not shown). In our first experiments, all mice were first inoculated intravaginally with MoPn according to the protocol detailed in Materials and Methods. The primary infection followed a normal course of vaginal shedding of the organism, as assessed by collection of cervical-vaginal swabs and subsequent isolation in HeLa cell culture as has been described elsewhere (10). All animals were culture negative on days 42, 49, and 56 post-primary infection (data not shown). The animals were again treated with P4 at 10 and 3 days prior to challenge. Nonimmune challenge controls were similarly treated with P4 but received no primary infection. On day 56, all mice were challenged intravaginally with 10 times the ID50 used in the primary inoculum.
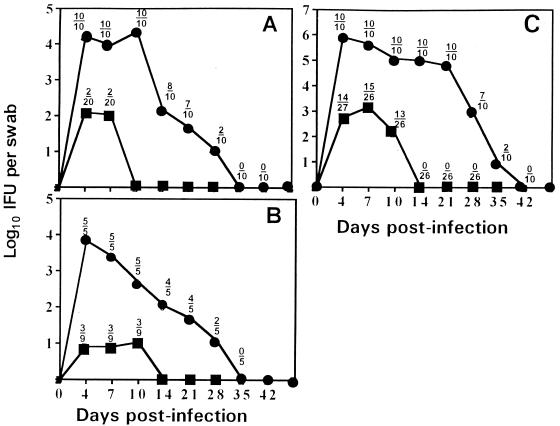
As seen in Fig. 1A, mice previously infected with MoPn were immune to subsequent intravaginal challenge with human serovar E. Only 10% of animals that recovered from MoPn infection became infected upon challenge with serovar E. The two animals that became infected shed at least 2 logs fewer viable chlamydia than the nonimmune controls and were isolation positive only on days 4 and 7, whereas the nonimmune controls sustained a normal infection course and shed viable organisms for up to 35 days. Similarly, Fig. 1B shows that prior infection with MoPn also protects against subsequent intravaginal challenge with human serovar L2. Interestingly, prior infection with MoPn provided less protection against homotypic challenge than against heterotypic challenge with other chlamydial strains (Fig. 1C). Approximately 50% of animals became infected following homotypic challenge, albeit shedding significantly fewer viable organisms (P of <0.0001 for day 4 through day 10) over an abbreviated infection course (10 to 14 days) compared to nonimmune animals. This finding was consistent over the course of three experiments.
FIG. 1.
Primary murine genital tract infection with MoPn C. trachomatis protects against homotypic and heterotypic challenge. The course of infection is shown by solid lines, with each point representing mean IFU from cervical-vaginal swabs of culture-positive mice collected at the time points indicated. Above each point is the ratio of the number of culture-positive animals to the total number of animals in each group. Symbols: ●, previously uninfected controls; ■, mice previously infected with MoPn. (A) Mice challenged with human serovar E (total of two experiments). (B) Mice challenged with human serovar L2 (total of one experiment). (C) Mice challenged with MoPn (total of three experiments).
When the order of challenge was reversed, we found that mice first infected with human serovar E and then challenged intravaginally at day 56 with MoPn, serovar L2, or again with serovar E displayed at least partial immunity. As shown in Fig. 2A, although nine of nine mice previously infected with serovar E became infected when challenged with MoPn, the numbers of IFU isolated from these animals were significantly lower at 7, 10, 14, and 21 days postinfection than at the identical time points for the nonimmune challenge controls (P of <0.01 at each time point). Additionally, the time course of MoPn infection was abbreviated in each animal previously infected with serovar E compared to primary infection in the nonimmune challenge control group, with higher percentages of mice resolving the infection by days 21, 28, and 35. Heterotypic intravaginal challenge with serovar L2 (Fig. 2B) or homotypic challenge with serovar E (Fig. 2C) resulted in reductions in the numbers of culture-positive animals, the numbers of IFU shed, and the duration of shedding of viable organisms in culture-positive animals compared to primary infection in the nonimmune challenge control groups.
FIG. 2.
Primary murine genital infection with C. trachomatis serovar E protects against homotypic and heterotypic challenge. The course of infection is shown by solid lines, with each point representing mean IFU from cervical-vaginal swabs of culture-positive mice collected at the time points indicated. Above each point is the ratio of the number of culture-positive animals to the total number of animals in each group. Symbols: ●, previously uninfected controls; ■, mice previously infected with serovar E. (A) Mice challenged with MoPn (total of two experiments). (B) Mice challenged with human serovar L2 (total of one experiment). (C) Mice challenged with serovar E (total of one experiment).
We also attempted to establish intravaginal infection with human serovar A C. trachomatis. However, due to the fastidious nature of this organism, we experienced difficulty in establishing concentrated stocks to determine the ID50 in mice for this organism and therefore excluded it from challenge studies. Infection was established in <50% of animals with a dose of approximately 2 × 104 IFU in a total volume of 10 μl—the highest concentration of stock attained for serovar A. Infection in these mice followed a course similar to that described above for other human serovars (data not shown). Serovar A-infected mice were included in the DTH determinations described below.
Species-conserved epitopes are recognized by murine T lymphocytes following infection with either the mouse biovar or a human serovar of C. trachomatis.
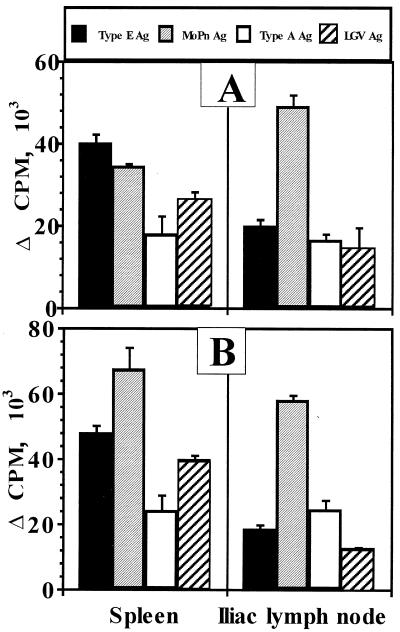
At day 56 postinfection with MoPn or human serovar E, T-lymphocyte reactivity in the spleen or ILN in response to purified UV-inactivated MoPn or serovar A, E, or L2 EBs was assessed in order to determine the existence of T-cell epitope conservation across human serogroups and the mouse biovar. We chose day 56 in order to assess the response at the time of challenge. Spleen and ILN T cells were selected as a measure of systemic and local draining lymph node responses, respectively (20, 21). Figure 3A shows the results of blast transformation of T lymphocytes from mice previously infected with human serovar E. T lymphocytes proliferated in response to EBs of serovar E, MoPn, serovar A, and L2. Proliferative responses to serovar E, as well as heterotypic EBs, were significant compared to the proliferative response to HeLa 229 antigen or background proliferation in medium only (P of <0.0001 in each case). Likewise, Fig. 3B indicates that spleen- and ILN-derived T lymphocytes from mice previously infected with MoPn yielded significant proliferative responses to EBs from each of the human serovars tested (P of <0.0001 in each case).
FIG. 3.
Proliferative responses of T lymphocytes to homotypic and heterotypic chlamydial antigens (Ag). Proliferation was assessed by [3H]thymidine incorporation in quadruplicate cultures of nylon wool-enriched T lymphocytes derived from either spleen or ILN in response to UV-inactivated, gradient-purified EBs of C. trachomatis serovar E (closed bars), MoPn (shaded bars), serovar A (open bars), or serovar L2 (hatched bars). (A) Response at day 56 postinfection with serovar E. (B) Response at day 56 postinfection with MoPn. Data are expressed as mean counts per minute above HeLa 229 antigen stimulation for each experimental group (serovar E infected and MoPn infected) and T-cell origin (spleen and ILN). The responses to Hela 229 antigen (and background unstimulated controls) were as follows: ILN (A), 297 ± 91 (176 ± 63); spleen (A), 1,151 ± 217 (927 ± 242); ILN (B), 303 ± 143 (148 ± 54); spleen (B), 2,524 ± 760 (532 ± 158).
As a measure of T-lymphocyte responses to chlamydial antigen in vivo, DTH reactions were assessed following infection with MoPn. This was accomplished by injection of UV-inactivated EBs of various chlamydial strains into the footpad of mice in parallel with those involved in the challenge experiments. Again, we selected day 56 postinfection to coincide with the time of challenge infection. The pattern of reactions observed was typical compared to those in previous studies (1, 30). Chlamydial antigen induced a biphasic response with an early (4- to 6-h) footpad swelling that subsided by 12 h and then peaked at 24 to 48 h. This was followed by a slow and steady decline thereafter. The early (4- to 6-h) response has been interpreted previously to be an Arthus-like reaction because of the time frame involved and because it is absent in B-cell-depleted mice (30). Uninfected mice had a very mild erythematous reaction to chlamydial antigen which was not significant compared to the contralateral footpad controls (data not shown).
Table 1 shows the 24-h DTH responses of mice previously infected with MoPn to EBs of MoPn and human serovars A, E, and L2. In each case, responses were significantly greater than that of HeLa 229 cell antigen only. Nonetheless, MoPn antigen yielded significantly greater responses than antigen of human serovar A, E, or L2 (P of <0.002, <0.02, and <0.004, respectively). Among the human serovars, antigens of serovar E elicited a response significantly greater than that of serovar A (P < 0.04) but not greater than that of serovar L2 (P > 0.5). From these observations and the results of T-lymphocyte blast transformation studies described above, we conclude that T cells responding during murine infection with MoPn recognize epitopes that are conserved across serovar and biovar boundaries. These data may also suggest that T-cell epitopes of MoPn are more conserved within serovar E than within serovars A and L2. However, more-detailed studies would be required to confirm this possibility.
TABLE 1.
DTH responses at day 56
| Primary infection | Mean (SD) increase in footpad thicknessa in response to:
|
Pb | |
|---|---|---|---|
| MoPn antigen | HeLa 229 antigen | ||
| MoPn | 7.1 (0.5) | 1.3 (0.5) | <0.0001 |
| Serovar A | 2.9 (1.8) | 0.3 (0.4) | <0.02 |
| Serovar E | 5.3 (1.1) | 0.2 (0.2) | <0.0001 |
| Serovar L2 | 3.6 (1.8) | 0.2 (0.3) | <0.003 |
Increase in footpad thickness (in millimeters) was measured at 24 h after injection of 5 μg of MoPn antigen and is the mean of 5 mice. Responses in all mice had significantly diminished by 72 h.
By two-tailed t test.
Species-conserved epitopes are recognized by antibodies formed in response to murine genital tract infection with either the mouse biovar or a human serovar of C. trachomatis.
On the day of infection and at the time of challenge, plasma samples were obtained to assess antibody reactivity by immunoblotting. Figure 4 shows the IgG antibody reactivity pattern at day 56 postinfection with either MoPn or human serovar E. Lanes C and D are EB antigens of MoPn and serovar E, respectively, probed with plasma from an MoPn-immune mouse. Three distinct antigens common to both organisms were detected. One antigen migrated to between 40 and 45 kDa and is most likely the MOMP, which is known to contain species-conserved epitopes (6, 19, 36). Another antigen migrated to ca. 60 kDa, and we speculate that this antigen may be chlamydial hsp60 because it displays >90% homology across the genus Chlamydia (6, 36). However, chlamydial Omp2 also comigrates with Hsp60; therefore, this band could represent antibody binding either or both of these proteins. The third antigen is of higher molecular mass (we estimate approximately 98 kDa), and its identity is unknown at this time. Lanes A and B are also resolved EB antigens of serovars E and MoPn, respectively, but were probed with immune plasma from a serovar E-immune mouse. IgG antibodies recognized serovar E MOMP, but not that of MoPn, and the ca. 60-kDa protein(s) of both strains. The high-molecular-weight antigen identified in this MoPn-infected mouse was not recognized. Similar results were obtained for four mice each of the serovar E- and MoPn-immune groups, for a total of 8 animals individually assessed. These observations clearly indicate that at least two, and possibly three, distinct antigenic components of MoPn and human serovar E share epitopes recognized by IgG antibodies produced during the course of murine genital tract infection with either strain.
FIG. 4.
IgG antibody reactivity at day 56 postinfection with a mouse or human biovar of C. trachomatis. Solubilized gradient-purified EBs of serovar E (lanes A and D) or MoPn (lanes B and C) resolved on 4 to 12% gradient gels and transferred to nitrocellulose were probed with immune plasma collected at day 56 postinfection from a mouse infected with MoPn (lanes C and D) or a mouse infected with serovar E (lanes A and B). Similar results were obtained from a total of eight mice tested (four each MoPn- and serovar E-infected animals). The exposed film was developed and scanned on a Microtek Scanmaker E3 with Photoshop 3.0.5 software. The scanned image was prepared and labeled with QuarkXpress 3.32.
DISCUSSION
The data in this study conclusively demonstrate that immunity resulting from chlamydial genital tract infection in the mouse is not solely restricted to the infecting serovar or biovar. The fact that we used organisms from two different human C. trachomatis biovars (lymphogranuloma venereum and trachoma) and the mouse biovar indicates that the immune mechanisms elicited during primary infection that protect against challenge infection are broadly cross-protective. It follows, therefore, that some protective antigenic epitopes recognized following primary infection are common to each of the three biovars (mouse, human oculogenital, and lymphogranuloma venereum) tested in our experiments. In support of this finding is the observation that specific T lymphocytes produced in response to primary infection with either human serovar E or the murine biovar responded to EB antigens of human serovars A, E, and L2 and the mouse biovar. This was evident by both in vivo (DTH) and in vitro (proliferative response) assessments. Additionally, the IgG antibodies produced in response to infection recognized the MOMP, a ca. 60-kDa antigen(s), and a higher-molecular-mass antigen (ca. 98 kDa) common to human and mouse C. trachomatis biovars.
Our results support previous studies that determined that primary infection with human serovars in the murine genital tract tends to be more brief in duration than primary infection with MoPn by about 7 days (12). Additionally, primary infection with human serovars yields approximately 2 logs fewer IFU at its peak than primary infection with MoPn. Lastly, we found that the ID50 of human serovar E in mice is on the order of 2 logs higher than that of MoPn. One could reasonably conclude from these comparisons that the human chlamydial biovars are relatively weak pathogens in mice compared to the mouse biovar. Hence, it would be easier to induce heterotypic protection against challenge infection with human serovars when mice are infected first with a strain more virulent in the mouse. A higher antigenic burden and possibly a further degree of dissemination in this scenario would yield a broader and more potent immune response that would provide more protection against challenge with the weaker human pathogen. Further supporting this explanation is the fact that primary infection with a human serovar apparently provides a lesser degree of protection against the more virulent mouse biovar (Fig. 2A). Indeed, we have found that antibody levels in response to human serovar infection in mice are much lower than those following MoPn infection (unpublished data). However, it should be emphasized that in each case, the challenge inoculum was increased by 1 log scale over the primary infecting inoculum on an ID50 basis, thus skewing the challenge in favor of reinfection. Therefore, the exceptionally high challenge inocula may have overcome an otherwise protective immune response, and a greater degree of protection might be observed at lower challenge doses.
In humans, repeated genital infections with C. trachomatis are common. However, persons repeatedly exposed to infection display reduced shedding of viable organisms (4). This reduction in the isolation rate is not prominent if the interval between positive cultures is greater than 6 months. In human ocular infections, protection from infection is observed only if the infecting or immunizing serovar is the same as the challenge serovar (18). Thus, it has been assumed that immunity to natural infection in humans is brief, and many believe it is mediated by serovar-specific antibody (3). However, it is commonly accepted that immunopathological injury as a result of chlamydial infection arises from antigens conserved among serovars and even among the chlamydial genus (6). An unexpected observation in our study was that homotypic challenge with MoPn provided a lesser degree of protection than what was observed following heterotypic challenge. While we are not able to provide an explanation of these results, they were consistent for a total of three experiments.
Whether the nature of the cross-biovar protection observed in the present study was antibody mediated, T cell mediated, or both cannot reasonably be correlated with the type of data provided. Due to the observed cross-reactive antibody, one could speculate that antibody played a contributing role in immune protection in this study. However, other studies have demonstrated that, although capable of neutralizing chlamydia in vitro (4, 5, 9, 25), the role played by antibody in mice is not critical to protection because mice deficient in B cells resolve infection in a manner similar to B-cell-intact mice (30). Nonetheless, Cotter et al. found a contribution of passively acquired antibody in protection from ascending infection, prevention of infection following very low doses (5 or fewer ID50), and reduction in inflammation (10).
In the present study, where high challenge doses were used, it is more likely that the observed cross-protective immunity was a T-cell-mediated effect, because previous studies of immune mechanisms of chlamydial infection of mice have proven an essential role of T cells in resolution of infection (29–31). Further support of this hypothesis is that most antibody epitopes defined to date have been localized to variable regions of the MOMP that are unique among serovars or serogroups, as reviewed in reference 36. However, T-cell epitopes are often localized to regions of high amino acid sequence conservation within species (19, 26). It is likely that several broadly cross-reacting T-cell epitopes exist within the MOMP. In support of this hypothesis, Kaltenboek et al. identified candidate T-cell epitopes within the MOMP that are common between the human and mouse biovars (19). Similarly, Ortiz and coworkers found a single immunodominant MOMP sequence containing up to six possible epitopes that elicit HLA-restricted responses in the majority of infected female patients (26). Comparison of these two studies indicates that the sequence identified by Ortiz et al. is conserved in the MoPn MOMP. Interestingly, parsimony analysis of the MOMP indicates that MoPn may be an ancestral strain of human serovars of C. trachomatis (19).
In summary, we conclude that broadly cross-protective immunity against C. trachomatis in mice arises following genital tract infection. This immunity is not limited to the biovar of the primary infection but protects against challenge with other biovars as well. Future explorations with this model of heterotypic immunity in mice should include determination of whether the immunity elicited protects against the disease process (e.g., inflammation and infertility) and definition of the T-cell epitopes elicited as a result of primary infection that are conserved among the human and mouse biovars.
ACKNOWLEDGMENTS
We thank Bob Kelley and Dana Dawson for assistance with the graphics in Fig. 4.
This work was supported by Public Health Service grants AI37807 (to K.H.R.) and AI19782 (to G.I.B.).
REFERENCES
- 1.Barron A L, Rank R G, Moses E B. Immune response in mice infected in the genital tract with mouse pneumonitis agent (Chlamydia trachomatis biovar) Infect Immun. 1984;44:82–85. doi: 10.1128/iai.44.1.82-85.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barron A L, White H J, Rank R G, Soloff B L, Moses E B. A new animal model for the study of Chlamydia trachomatis genital infections: infection of mice with the agent of mouse pneumonitis. J Infect Dis. 1981;143:63–66. doi: 10.1093/infdis/143.1.63. [DOI] [PubMed] [Google Scholar]
- 3.Brunham R C. Vaccine design for the prevention of Chlamydia trachomatis infection. In: Orfila J, Byrne G I, Chernesky M A, Grayston J T, Jones R B, Ridgway G L, Saikku P, Schachter J, Stamm W E, Stephens R S, editors. Chlamydial infections: proceedings of the Eighth International Symposium on Human Chlamydial Infections. Bologna, Italy: Societa Editrice Esculapio; 1994. pp. 73–82. [Google Scholar]
- 4.Brunham R C, Kuo C-C, Cles L, Holmes K K. Correlation of host immune response with quantitative recovery of Chlamydia trachomatis from the human endocervix. Infect Immun. 1983;39:1491–1494. doi: 10.1128/iai.39.3.1491-1494.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brunham R C, Peeling R, Maclean I, McDowell J, Persson K, Osser S. Postabortal Chlamydia trachomatis salpingitis: correlating risk with antigen-specific serological responses and with neutralization. J Infect Dis. 1987;55:749–755. doi: 10.1093/infdis/155.4.749. [DOI] [PubMed] [Google Scholar]
- 6.Brunham R C, Peeling R W. Chlamydia trachomatis antigens: role in immunity and pathogenesis. Infect Agents Dis. 1997;3:218–233. [PubMed] [Google Scholar]
- 7.Byrne G I, Padilla M, Lacey D, Paulnock D, Xiu L G. Mouse model for protective immunity to chlamydia. In: Bowie W R, Caldwell H D, Jones R P, Mardh P A, Ridgway G L, Schachter J, Stamm W E, Ward M E, editors. Chlamydial infections: proceedings of the Seventh International Symposium on Human Chlamydial Infections. Cambridge, United Kingdom: Cambridge University Press; 1990. pp. 236–240. [Google Scholar]
- 8.Caldwell H D, Kromhout J, Schachter J. Purification and partial characterization of the major outer membrane protein of Chlamydia trachomatis. Infect Immun. 1981;31:1161–1176. doi: 10.1128/iai.31.3.1161-1176.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caldwell H D, Perry L J. Neutralization of Chlamydia trachomatis infectivity with antibodies to the major outer membrane protein. Infect Immun. 1982;38:745–754. doi: 10.1128/iai.38.2.745-754.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cotter T W, Meng Q, Shen Z, Zhang Y, Su H, Caldwell H D. Protective efficacy of major outer membrane protein specific immunoglobulin A (IgA) and IgG monoclonal antibodies in a murine model of Chlamydia trachomatis genital tract infection. Infect Immun. 1996;63:4704–4714. doi: 10.1128/iai.63.12.4704-4714.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cotter T W, Ramsey K H, Miranpuri G S, Poulsen C E, Byrne G I. Dissemination of Chlamydia trachomatis chronic genital tract infection in gamma interferon gene knockout mice. Infect Immun. 1997;65:2145–2152. doi: 10.1128/iai.65.6.2145-2152.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Darville T, Andrews C W, Laffoon K K, Shymasani W, Kishen L R, Rank R G. Mouse strain-dependent variation in the course and outcome of chlamydial genital tract infection is associated with differences in host response. Infect Immun. 1997;65:3065–3078. doi: 10.1128/iai.65.8.3065-3073.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De La Maza L M, Pal S, Khamesipour A, Peterson E M. Intravaginal inoculation of mice with the Chlamydia trachomatis mouse pneumonitis biovar results in infertility. Infect Immun. 1994;62:2094–2097. doi: 10.1128/iai.62.5.2094-2097.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eckert L O, Hawes S E, Wolner-Hanssen P, Money D M, Peeling R W, Brunham R C, Stevens C E, Eschenbach D A, Stamm W E. Prevalence and correlates of antibody to chlamydial heat shock protein in women attending sexually transmitted disease clinics and women with confirmed pelvic inflammatory disease. J Infect Dis. 1997;175:1453–1458. doi: 10.1086/516479. [DOI] [PubMed] [Google Scholar]
- 15.Grayston J T, Wang S-P. New knowledge of chlamydiae and the diseases they cause. J Infect Dis. 1975;132:87–105. doi: 10.1093/infdis/132.1.87. [DOI] [PubMed] [Google Scholar]
- 16.Grayston J T, Wang S-P. The potential for vaccine against infection of the genital tract with Chlamydia trachomatis. Sex Transm Dis. 1978;5:73–77. doi: 10.1097/00007435-197804000-00011. [DOI] [PubMed] [Google Scholar]
- 17.Grayston J T, Wang S-P, Yang Y F, Woolridge R L. The effects of trachoma vaccine on the course of experimental infection in blind volunteers. J Exp Med. 1962;115:1009–1022. doi: 10.1084/jem.115.5.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jawetz E, Rose L, Hanna L, Thygeson P. Experimental inclusion conjunctivitis in man. JAMA. 1965;194:150–162. [PubMed] [Google Scholar]
- 19.Kaltenboeck B, Kousoulas K G, Storz J. Structures of and allelic diversity and relationships among the major outer membrane protein (ompA) genes of the four chlamydial species. J Bacteriol. 1993;175:487–502. doi: 10.1128/jb.175.2.487-502.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kelly K A, Robinson E A, Rank R G. Initial route of antigen administration alters the T-cell cytokine profile produced in response to the mouse pneumonitis biovar of Chlamydia trachomatis following genital infection. Infect Immun. 1996;64:4976–4983. doi: 10.1128/iai.64.12.4976-4983.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kincy Cain T, Rank R G. Local Th1-like responses are induced by intravaginal infection of mice with the mouse pneumonitis biovar of Chlamydia trachomatis. Infect Immun. 1995;63:1784–1789. doi: 10.1128/iai.63.5.1784-1789.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kunimoto D, Brunham R C. Human immune response and Chlamydia trachomatis infection. Rev Infect Dis. 1985;7:665–673. doi: 10.1093/clinids/7.5.665. [DOI] [PubMed] [Google Scholar]
- 23.Morrison R P. Immune responses to Chlamydia are protective and pathogenic. In: Bowie W R, Caldwell H D, Jones R P, Mardh P A, Ridgway G L, Schachter J, Stamm W E, Ward M E, editors. Chlamydial infections: proceedings of the Seventh International Symposium on Human Chlamydial Infections. Cambridge, United Kingdom: Cambridge University Press; 1990. pp. 163–172. [Google Scholar]
- 24.Morrison R P, Belland R J, Lyng K, Caldwell H D. Chlamydial disease pathogenesis: the 57 kD chlamydial hypersensitivity antigen is a stress response protein. J Exp Med. 1989;170:1271–1283. doi: 10.1084/jem.170.4.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murdin A D, Su H, Klein M H, Caldwell H D. Poliovirus hybrids expressing neutralizing epitopes from variable domains I and IV of the major outer membrane protein of Chlamydia trachomatis elicit broadly cross-reactive C. trachomatis-neutralizing antibodies. Infect Immun. 1995;63:1116–1121. doi: 10.1128/iai.63.3.1116-1121.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ortiz L, Demick K P, Petersen J W, Polka M, Rudersdorf R A, Van der Pol B, Jones R, Angevine M, DeMars R. Chlamydia trachomatis major outer membrane protein (MOMP) epitopes that activate HLA class II-restricted T cells from humans. J Immunol. 1996;157:4554–4567. [PubMed] [Google Scholar]
- 27.Patton D L, Sweeney Y T C, Kuo C C. Demonstration of delayed hypersensitivity in Chlamydia trachomatis salpingitis in monkeys: a pathogenic mechanism of tubal damage. J Infect Dis. 1994;169:680–683. doi: 10.1093/infdis/169.3.680. [DOI] [PubMed] [Google Scholar]
- 28.Ramsey K H, Newhall W J, Rank R G. Humoral immune response to chlamydial genital infection of mice with the agent of mouse pneumonitis. Infect Immun. 1989;57:2441–2446. doi: 10.1128/iai.57.8.2441-2446.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramsey K H, Rank R G. Resolution of chlamydial genital infection of mice with antigen-specific T-lymphocyte lines. Infect Immun. 1991;59:925–931. doi: 10.1128/iai.59.3.925-931.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramsey K H, Soderberg L S F, Rank R G. Resolution of chlamydial genital infection in B-cell-deficient mice and immunity to reinfection. Infect Immun. 1988;56:1320–1325. doi: 10.1128/iai.56.5.1320-1325.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rank R G, Soderberg L S F, Barron A L. Chronic chlamydial genital infection in congenitally athymic nude mice. Infect Immun. 1985;48:847–849. doi: 10.1128/iai.48.3.847-849.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schachter J. Overview of human diseases. In: Barron A L, editor. Microbiology of chlamydia. Boca Raton, Fla: CRC Press; 1988. pp. 18–35. [Google Scholar]
- 33.Tuffrey M, Alexander F, Woods C, Taylor-Robinson D. Genetic susceptibility to chlamydial salpingitis and subsequent infertility in mice. J Reprod Fertil. 1992;95:31–38. doi: 10.1530/jrf.0.0950031. [DOI] [PubMed] [Google Scholar]
- 34.Tuffrey M, Falder T, Gale J, Taylor-Robinson D. Salpingitis in mice induced by human strains of Chlamydia trachomatis. Br J Exp Pathol. 1986;67:605–616. [PMC free article] [PubMed] [Google Scholar]
- 35.Tuffrey M, Taylor-Robinson D. Progesterone as a key factor in the development of a mouse model for genital tract infection with Chlamydia trachomatis. FEMS Microbiol Lett. 1981;12:111–114. [Google Scholar]
- 36.Ward M E. The immunobiology and immunopathology of chlamydial infections. APMIS. 1995;103:769–796. doi: 10.1111/j.1699-0463.1995.tb01436.x. [DOI] [PubMed] [Google Scholar]
- 37.Woolridge R L, Grayston J T, Chang I H Y C Y, Cheng K H. Long-term follow-up of the initial (1959–1960) trachoma vaccine field trial on Taiwan. Am J Ophthalmol. 1967;63:1650–1655. doi: 10.1016/0002-9394(67)94159-1. [DOI] [PubMed] [Google Scholar]
- 38.Yang X, Hayglass K T, Brunham R C. Genetically determined differences in IL-10 and IFN-γ responses correlate with clearance of Chlamydia trachomatis mouse pneumonitis infection. J Immunol. 1996;156:4334–4338. [PubMed] [Google Scholar]