Abstract
Background
Thrombocytopenia is a common extrahepatic manifestation in chronic liver disease. However, there have been rare studies of impacts of risk for hepatitis C virus-associated thrombocytopenia (HCV-TP) and hepatitis B virus-associated thrombocytopenia (HBV-TP). The aim of this study is to evaluate different impacts of risk factors for HCV-TP and HBV-TP.
Methods
We retrospectively collected 1803 HCV patients and 1652 HBV patients to examine the risk factors for time to moderate and severe thrombocytopenia (platelet counts <100 × 109/L and <50 × 109/L, respectively) by Cox proportional hazards models. Moreover, we prospectively enrolled 63 HCV-TP patients, 11 HBV-TP patients, and 27 HCV controls to detect specific antiplatelet antibodies by enzyme-linked immunosorbent assay and analyze their effects.
Results
Prevalence of platelet <100 × 109/L was 11.86% and 6.35% in HCV and HBV patients without cancer history, respectively. HCV-to-HBV incidence rate ratio for thrombocytopenia was 6.95. Initial thrombocytopenia was the most significant risk factor for HCV-TP and HBV-TP regardless of thrombocytopenia severity. Splenomegaly and cirrhosis were significant risk factors for moderate, but not severe HCV-TP. Hyperbilirubinemia was an important moderate and severe HBV-TP risk factor. Antiplatelet antibodies were correlated with HCV-TP severity, of which anti-glycoprotein IIb/IIIa antibody being associated with smaller spleen size. The antiplatelet autoantibody might contribute to thrombocytopenia either independently or with splenomegaly as the important risk in HCV-TP patients without advanced cirrhosis.
Conclusion
HCV was associated with higher thrombocytopenia incidence than HBV. Thrombocytopenia risk factors varied with virus type and severity. Different management for HCV-TP and HBV-TP was suggested.
Keywords: Antiplatelet antibody, Cirrhosis, Hepatitis B virus, Hepatitis C virus, Spleen, Thrombocytopenia
At a glance commentary.
Scientific background on the subject
Thrombocytopenia is a common clinical presentation in patients with chronic liver disease. The major causes include cirrhosis-related thrombopoietin hypoproduction, splenomegaly-associated platelet sequestration, and immune modulation-related platelet destruction. The impacts of these factors have not been clarified in patients with hepatitis B virus and hepatitis C virus infection.
What this study adds to the field
This study demonstrated thrombocytopenia risk factors varied according to virus type and severity. Initial thrombocytopenia was the most significant risk in HCV and HBV patients. Antiplatelet antibody was important risk for HCV-associated thrombocytopenia, especially those with unadvanced cirrhosis and severe thrombocytopenia. Different treatment for HCV and HBV associated thrombocytopenia was suggested.
Hepatitis B virus (HBV) and hepatitis C virus (HCV) infections are both global health threats. Viral hepatitis caused more than 1 million deaths globally in 2015. The worldwide prevalence of chronic HBV and HCV infection was estimated approximately 3.5% and 1.0%, respectively [1,2]. In addition to the mortality associated complications of chronic hepatitis and cirrhosis, many extrahepatic manifestations may occur, such as thrombocytopenia [3,4]. There have been many reports and reviews on HCV-associated thrombocytopenia (HCV-TP) [[5], [6], [7]]. However, only few cohort studies have focused on HBV-associated thrombocytopenia (HBV-TP) [[8], [9], [10]].
The pathophysiology of thrombocytopenia in chronic liver disease is complex and multifactorial [4,6,11]. The mechanisms include antiplatelet antibody-mediated Fc-dependent phagocytosis, T-cell–mediated immune response, splenic sequestration, viral suppression of megakaryocyte production, insufficient thrombopoietin production, and interaction of cytokines [[11], [12], [13], [14], [15], [16]]. In addition to certain direct mechanisms, some indirect risk factors are also related to thrombocytopenia. For instance, liver fibrosis is an important predictive factor for thrombocytopenia [17]. To predict the level of liver fibrosis, researchers have constructed indexes, such as fibrosis-4 index and the aspartate aminotransferase (AST)-to-platelet ratio index, by using noninvasive biomarkers, including peripheral platelet count and liver enzymes. However, HBV and HCV infections are associated with different cutoff levels of fibrosis [18]. In contrast, these data suggest there may be different predictive value of clinical parameters related to platelet count in patients with different viral hepatitis.
HCV patients can have more severe thrombocytopenia than HBV patients [3]. HCV has been suggested as a cause of secondary immune thrombocytopenia (ITP), and HCV-ITP has been well documented to be associated with immune destruction [[19], [20], [21]]. For HBV-TP, only a few studies have focused on the immunological interactions between HBV and platelet count [14,22]. In addition, there has been no study about the interaction of the indirect clinical parameters and the direct immune destruction. The area of this study population is a hyperendemic region of HBV and HCV infection, with crude prevalence of 17.9% and 10.3%, respectively [23]. We collected patients with HBV or HCV infection to explore the impact of hepatitis virus and associated risk factors on thrombocytopenia.
Material and Methods
Our study included two parts: retrospective and prospective analyses.
Retrospective analysis
We first selected patients with seropositive for anti-HCV antibody (anti-HCV Ab) or the HBV surface antigen (HBsAg) between Jan, 2004 and Jun, 2017 at Chang Gung Memorial Hospital, Chiayi, Taiwan. We excluded patients with either missing one test or seropositive for both anti-HCV Ab and HBsAg. Patients having cancer history were excluded. Patients were included only when they had at least one documented white blood cell (WBC) count of 3.5 × 106/L to 11 × 106/L and hemoglobin (Hb) level of >12.0 g/dL in men and >11 g/dL in women within one year before and 2 years after documentation of seropositivity for anti-HCV Ab or HBsAg. We intended to avoid confounding factors including hematological disorder, end-stage cirrhosis, bleeding, or infection related pancytopenia. Finally, 3455 eligible subjects were enrolled for analysis. This study, performed according to the Helsinki Declaration, was approved by the Institutional Review Board of Chang Gung Memorial Hospital (approval number, 104–7526B).
Demographic data, medical records, and drug history of all eligible patients were collected. Regarding drug history, we defined positive antiviral treatment as HCV and HBV patients having received interferon therapy, and HBV patients having received a nucleoside analog (NA), including lamivudine, entecavir, tenofovir, adefovir, and telbivudine, at a >90-day interval before thrombocytopenic events or last follow-up date.
Laboratory and image examination
We reviewed hemogram data with complete blood cell count, including WBC count, Hb levels, and platelet count. The initial platelet counts were categorized into five levels: levels 1 (≥150 × 109/L), 2 (100–149 × 109/L), 3 (50–99 × 109/L), 4 (30–49 × 109/L), and 5 (<30 × 109/L). Blood biochemistry data included levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), albumin, total bilirubin, and ammonia and prothrombin time (PT). We longitudinally followed these two cohorts to explore the changes in the platelet count. First thrombocytopenic events were recorded when the patient had at least two episodes of platelet count of <100 × 109/L or <50 × 109/L at an >1-month interval. Then we analyzed the incidence and risk factors of these two thrombocytopenic events in HCV and HBV patients, respectively. Interferon treatment–associated thrombocytopenia events, occurring from the time of first dose to 3 months after last dose in each treatment course, were excluded. Patients with initial platelet counts less than the target thrombocytopenic levels at the time of enrollment and missing follow-up records were also excluded.
We analyzed abdominal ultrasonography reports and recorded the status of cirrhosis, ascites, and the spleen index. The status of cirrhosis was defined according to a comprehensive evaluation of liver parenchymal texture, surface nodularity, and edge [24]. The spleen index was calculated by multiplying the length of the long axis with that of the short axis crossing each other at a right angle over the splenic hilum. The splenomegaly was defined as spleen index ≥20 [25]. Child–Pugh classification, which is defined as the total scores of PT prolongation, total bilirubin level, albumin level, ascites volume, and presence of encephalopathy, was categorized as A, B, and C by scores of 5–6, 7–9, and 10–15, respectively. The first laboratory value and ultrasonographic report within 180 days after enrollment were collected as the initial baseline data for analysis.
Prospective analysis
In the second part of our study, we prospectively enrolled HCV-TP and HBV-TP patients with platelet count <100 × 109/L as well as HCV control patients without thrombocytopenia. Only patients with chronic hepatitis or early cirrhosis without active bleeding were enrolled because there was only a small proportion of advanced cirrhotic patients in our retrospectively analyzed population. Patients with advanced liver cirrhosis (i.e., Child–Pugh class B or C) were excluded. After patients provided written informed consent, blood sample collection and laboratory examination were conducted as mentioned in the retrospective analysis, and abdominal ultrasonography was performed. This study was approved by the Institutional Review Board of Chang Gung Memorial Hospital (approval number, 201901413B0) and performed in accordance with the Helsinki Declaration.
Antiplatelet antibody examination
We used a commercial qualitative solid-phase sandwich enzyme-linked immunosorbent assay (ELISA) kit (PakPlus assay, Immucor, USA) to detect antiplatelet antibodies. This kit can identify autoantibodies to platelet glycoprotein (GP) IIb/IIIa, Ia/IIa, Ib/IX, and IV, and human leukocyte antigen (HLA) class I. Patient serum was added to a 96-microwell plate coated with the aforementioned GPs so that the autoantibodies, if presented, would bind to the GPs. Then, alkaline phosphatase (ALP)–labeled antihuman immunoglobulins G/A/M were added to activate the substrate p-nitrophenyl phosphate. We also constructed standards for standard curve as reference to predict positive result. Sample preparation and ELISA were performed according to the manufacturers’ instructions.
Statistical analysis
The differences between HCV and HBV groups at baseline were compared by using the two-sample t-test or the chi-square test. For comparisons with insufficient sample sizes, the Wilcoxon rank sum and Fisher's exact test were employed instead. To analyze the risk factors associated with time to thrombocytopenic events, we first used simple Cox proportional hazards model for each covariate (Supplementary Tables S1 and S2). Secondly, all baseline variables were included in a multiple Cox model, and the significant risk factors in Table 2 and Table 3 were selected by a stepwise procedure. Data were managed and analyzed using SAS version 9.4 (SAS Institute, Inc., Cary, NC, USA). All statistical tests were two-sided, and p < 0.05 was statistically significant.
Table 2.
Risk factors for thrombocytopenic event <100 × 109/L using multiple Cox proportional hazards models.a
| HCV (n = 1436) | HBV (n = 1432) | |||||
|---|---|---|---|---|---|---|
| Platelet events | 59 | 26 | ||||
| Follow-up (year) | 2670.72 | 8173.59 | ||||
| Incidence (per 100,000 person-years) |
2209.14 |
318.10 |
||||
| Characteristic | AHR | 95% CI | p | AHR | 95% CI | p |
| Initial platelet level | ||||||
| 1 (≥150 × 109/L) | 1.00 | 1.00 | ||||
| 2 (≥100–149 × 109/L) | 7.90 | 3.80–16.43 | <0.001 | 8.75 | 3.50–21.86 | <0.001 |
| Total bilirubin —mg/dL | ||||||
| ≥2.0 | 5.83 | 2.35–14.46 | <0.001 | |||
| <2.0 | 1.00 | |||||
| Spleen index | ||||||
| ≥20 | 2.92 | 1.53–5.56 | <0.001 | |||
| <20 | 1.00 | |||||
| Cirrhosis | ||||||
| Positive | 3.27 | 1.60–6.67 | <0.001 | |||
| Negative | 1.00 | |||||
Abbreviations: HCV: hepatitis C virus; HBV: hepatitis B virus; AHR: adjusted hazard ratio; CI: confidence interval.
Only the covariates with statistical significance in the multiple Cox model were presented.
Table 3.
Risk factors for thrombocytopenic events <50 × 109/L using multiple Cox proportional hazards models.a
| HCV (n = 1603) | HBV (n = 1504) | |||||
|---|---|---|---|---|---|---|
| Platelet events | 14 | 7 | ||||
| Follow-up (year) | 3054.06 | 8587.05 | ||||
| Incidence (per 100,000 person-years) |
458.41 |
81.52 |
||||
| Characteristic | AHR | 95% CI | p | AHR | 95% CI | p |
| Initial platelet level | ||||||
| 1 or 2 (≥100 × 109/L) | 1.00 | 1.000 | ||||
| 3 (50–99 × 109/L) | 13.24 | 2.99–58.59 | <0.001 | 50.42 | 7.69–330.30 | <0.001 |
| ALP —U/L | ||||||
| ≥200 | 7.92 | 1.57–40.01 | <0.001 | 5.27 | 1.34–20.76 | 0.015 |
| <200 | 1.00 | 1.00 | ||||
| Total bilirubin —mg/dL | ||||||
| ≥2.0 | 5.47 | 1.12–26.68 | 0.032 | |||
| <2.0 | 1.00 | |||||
| PT prolongation —second | ||||||
| ≥4.0 | 5.85 | 1.36–25.13 | 0.015 | |||
| <4.0 | 1.00 | |||||
Abbreviations: HCV: hepatitis C virus; HBV: hepatitis B virus; AHR: adjusted hazard ratio; ALP: alkaline phosphatase; PT: prothrombin time; CI: confidence interval.
Only the covariates with statistical significance in the multiple Cox model were presented.
Results
Retrospective analysis
HCV patients had significantly lower platelet counts and higher prevalence of thrombocytopenia than did HBV patients
A total of 1803 HCV patients and 1652 HBV patients were enrolled [Table 1]. The demographic variables were different between HCV and HBV groups at proportion of male (47.92% vs. 61.32%, p < 0.001) and mean age (61.61 vs. 50.22 years, p < 0.001). When comparing laboratory results, the mean AST, ALT, albumin, total bilirubin, and PT-prolong time in seconds were similar between these two groups. Although HCV patients had higher ALP levels, the mean levels were within normal limit. HCV patients had significantly lower mean platelet count than did HBV patients (176.83 × 109/L vs. 194.79 × 109/L, p < 0.001). This trend was also observed when the platelet counts were categorized into five levels (p < 0.001). The proportion of HCV patients with normal platelet count (level 1) was lower, whereas that of HCV patients with mild (level 2) and moderate (level 3) thrombocytopenia was higher than HBV patients. More specifically, the prevalence of thrombocytopenia, defined as either plate count <100 × 109/L or <50 × 109/L, was higher in HCV than HBV group (11.86% vs. 6.35% or 1.77% vs. 1.57%, respectively).
Table 1.
Baseline characteristics of HCV and HBV cohorts.
| Characteristic | HCV (n = 1803) | HBV (n = 1652) | p value |
|---|---|---|---|
| Sex — Male | 864 (47.92) | 1013 (61.32) | <0.001 |
| Age — years | 61.61 ± 14.15 | 50.22 ± 14.92 | <0.001 |
| Platelet (x109/L) | 176.83 ± 72.97 | 194.79 ± 70.39 | <0.001 |
| Platelet level | |||
| 1 (≥150 × 109/L) | 1145 (63.51) | 1270 (76.88) | <0.001 |
| 2 (≥100–149 × 109/L) | 444 (24.63) | 277 (16.77) | |
| 3 (≥50–99 × 109/L) | 182 (10.09) | 79 (4.78) | |
| 4 (≥30–49 × 109/L) | 22 (1.22) | 19 (1.15) | |
| 5 (<30 × 109/L) | 10 (0.55) | 7 (0.42) | |
| WBC (x106/L) | 7.04 ± 3.22 | 7.28 ± 2.98 | 0.021 |
| Hemoglobin - g/dL | 13.21 ± 1.95 | 13.76 ± 2.01 | <0.001 |
| AST — U/L | 92.00 ± 209.98 | 95.89 ± 299.87 | 0.668 |
| ALT — U/L | 122.17 ± 247.51 | 110.69 ± 307.71 | 0.232 |
| ALP —U/L | 88.75 ± 49.14 | 79.92 ± 39.43 | <0.001 |
| Albumin — g/dL | 3.86 ± 0.71 | 3.86 ± 0.79 | 0.920 |
| Total bilirubin —mg/dL | 1.36 ± 2.42 | 1.37 ± 2.14 | 0.899 |
| PT prolong—second | 0.73 ± 3.11 | 0.93 ± 4.08 | 0.177 |
| Spleen index | 15.79 ± 7.73 | 13.91 ± 6.73 | <0.001 |
| Splenomegaly-Positive | 357 (23.47) | 182 (12.88) | <0.001 |
| Cirrhosis -Positive | 173 (10.80) | 125 (8.49) | 0.031 |
| Child-Pugh classification (cirrhosis positive only)— n (%) | |||
| A | 72 (59.02) | 29 (34.94) | <0.001 |
| B | 42 (34.43) | 38 (45.78) | |
| C | 8 (6.56) | 16 (19.28) | |
Abbreviations: HCV: hepatitis C virus; HBV: hepatitis B virus; WBC: white blood cell; AST: aspartate aminotransferase; ALT: alanine aminotransferase; ALP: alkaline phosphatase; PT: prothrombin time; SD: standard deviation.
The descriptive statistics were presented as mean ± sd or frequency (%).
HCV patients had a larger mean spleen index and a higher proportion of splenomegaly than did HBV patients (15.79 vs. 13.91, p < 0.001; 23.47% vs. 12.88%, p < 0.001). In addition, the HCV cohort demonstrated a higher percentage of liver cirrhosis, but most of them had early-stage cirrhosis (i.e., Child-Pugh class A). By contrast, in the HBV cohort, a higher percentage of patients had advanced cirrhosis.
The incidence of thrombocytopenic event was higher in HCV group than HBV group
We analyzed the time from diagnosis of HCV or HBV to first thrombocytopenic events with two endpoints, platelet counts of <100 × 109/L and <50 × 109/L, respectively. Total 1436 HCV patients and 1432 HBV patients had platelet count ≥100 × 109/L initially. In these patients, 59 moderate thrombocytopenic events (platelet counts of <100 × 109/L) occurred during the follow-up of 2670.72 person-years in the HCV cohort and 26 events in the follow-up of 8173.59 person-years in the HBV cohort (Table 2, upper panel). The crude incidence rates were 2209.14 and 318.10 per 100,000 person-years in the HCV and HBV cohorts, respectively, resulting in an HCV-to-HBV incidence rate ratio (IRR) at 6.95 [95% confidence interval (CI) = 4.38–11.02]. The mean time to event was 1.52 and 4.55 years in HCV-TP and HBV-TP patients, respectively. Similar trend was observed in the analysis of severe thrombocytopenic events, defined as platelet count <50 × 109/L (Table 3, upper panel). The IRR was 5.62 (95% CI = 2.27–13.93) with crude incidence of 458.41 and 81.52 per 100,000 person-years for HCV and HBV patients, respectively. These consistent results implied that HCV patients were more likely to develop thrombocytopenia than HBV patients. However, the comparison between two crude incidence rates was not adjusted by any covariates. As a result, the multiple Cox models were employed to examine all possible risk factors associated with thrombocytopenia for HCV and HBV cohorts, respectively, in the following section.
Risk factors for thrombocytopenia were different between HCV and HBV patients and related to thrombocytopenic levels
In the univariate analysis of time to moderate thrombocytopenia of platelet count <100 × 109/L, the common risk factors for both HCV and HBV groups were initial platelet level 2, AST, total bilirubin, PT prolongation, spleen index, and cirrhosis (Supplementary Table S1). Male gender was a risk factor for HBV-TP. In contrast, post-antiviral treatment was a protective factor in HCV patients. In the multiple Cox proportional hazards model of the HCV group, lower initial platelet level, splenomegaly, and cirrhosis were risk factors [adjusted hazard ratio (AHR) = 7.90, 2.92, and 3.27, respectively, Table 2]. In the HBV group, the results revealed initial thrombocytopenia and hyperbilirubinemia to be risk factors (AHRs = 8.75 and 5.83, respectively). In short, initial mild thrombocytopenia was the most important risk factor for both moderate HCV-TP and HBV-TP. The ultrasonographic splenomegaly and cirrhosis were more consistent risk factors in HCV patients, whereas laboratory hyperbilirubinemia was a reliable predictor in HBV patients.
Regarding analysis of severe thrombocytopenic events, the univariant analysis was presented in Supplementary Table S2. Because there were too few or no events in patients with initial thrombocytopenia level 1/2, age of more than 65 years, normal AST and ALT in HBV group, the validity of the Cox model fit was questionable. As a result, we could not present a robust estimated HR for these variables. High ALP level, hyperbilirubinemia, and cirrhosis were all risk factors in both HCV and HBV groups. In addition, PT prolongation was significant risk for severe HCV-TP but not HBV-TP patients, whereas splenomegaly was important in severe HBV-TP patients but negligible in severe HCV-TP patients. The multiple Cox models demonstrated that initial thrombocytopenia level 3 and high ALP level were associated with the time to severe thrombocytopenia, of which initial thrombocytopenia level 3 was the most important risk factor with highest AHR at 13.24 and 50.42 in HCV and HBV group, respectively [Table 3]. Similar in moderate HBV-TP analysis, hyperbilirubinemia was a consistent risk factor for severe HBV-TP patients. However, the impact of ultrasonographic splenomegaly and cirrhosis was negligible.
To explore the late effect of these common risk factors, we compared the characteristics of HCV and HBV patients who had first thrombocytopenic events between status at enrollment and at time of events. Among patients with moderate thrombocytopenia, HCV patients initially had significantly larger spleen index and higher percentage of splenomegaly (Supplement Table S3 and Fig. 1A). However, the percentage of splenomegaly slightly increased from baseline 52.84% to 54.17% at event in moderate HCV-TP patients, whereas it increased more greater from 25.00% to 47.37% in moderate HBV-TP patients. The moderate HBV-TP patients also had much increase of cirrhosis from 25.00% to 45.00% higher than 31.48% to 42.00% in moderate HCV-TP patients [Fig. 1B]. The spleen index and splenomegaly also progressed with higher difference from 24.70 to 30.92 and from 57.14% to 100.00% in severe HBV-TP patients (vs. 23.86 to 25.84 and 42.86% to 57.14% in severe HCV-TP patients, respectively) (Supplementary Table S3 and Fig. 1A). HBV-TP patients even had a higher percentage of cirrhosis regardless of thrombocytopenic levels and splenomegaly at severe thrombocytopenia than did HCV-TP patients. In short, although splenomegaly and cirrhosis were not shown to be significant initial risk factors in HBV patients, the much greater increase of the percentage from baseline values to the time of event suggested their greater late effects in HBV-TP patients than in HCV-TP patients.
Fig. 1.
Comparison of splenomegaly and cirrhosis between baseline and thrombocytopenic events in HCV and HBV patients. A. Splenomegaly, B. Cirrhosis. At baseline, HCV patients with thrombocytopenic events had a higher proportion of splenomegaly and cirrhosis compared with HBV patients. However, HBV patients had relatively larger increases in the proportion of splenomegaly and cirrhosis from baseline value to value at the thrombocytopenic event. Finally, compared with HCV-TP patients, HBV-TP patients had a higher proportion of splenomegaly at thrombocytopenia (platelet count <50 × 109/L) and a higher proportion of cirrhosis irrespective of the thrombocytopenia level.
Prospective analysis
We enrolled 63 HCV-TP patients, 11 HBV-TP patients, and 27 control HCV patients and their baseline characteristics were presented in Supplementary Table S4. Compared with the HCV controls, HCV-TP patients were older and had lower WBC counts, Hb and albumin levels, and higher ALP and total bilirubin levels. However, no significant differences were found between HCV-TP and HBV-TP patients.
Anti-GPIIb/IIIa-positive HCV-TP patients had small spleen size
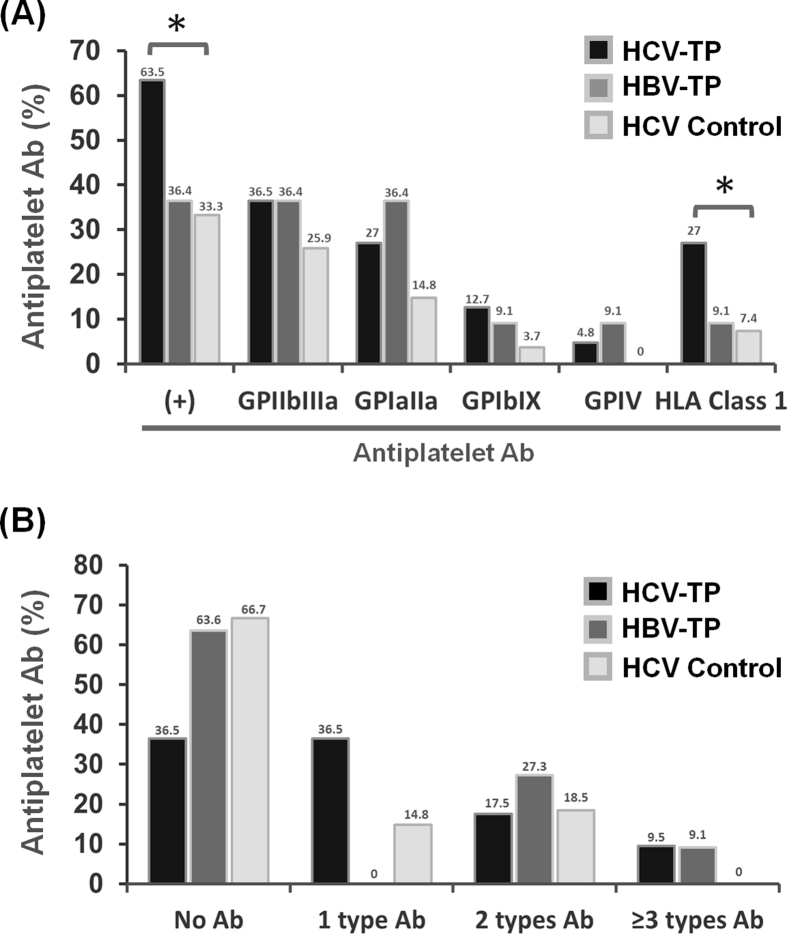
The antiplatelet antibody analyses in all three patient groups were illustrated in Fig. 2. Compared with the controls, HCV-TP patients had higher rates of positive antiplatelet antibodies and anti-HLA class I antibodies [Fig. 2A]. In the analysis of antibody complexity, HCV-TP patients had more complex profiles than HCV controls, not having more than two types of antibodies [Fig. 2B]. In the comparison of platelet levels with antiplatelet antibodies in total HCV patients, we found that positivity for anti-GP antibodies tended to increase with the platelet count reduction, with the result for anti-GPIb/XI antibodies reaching statistical significance [Fig. 3A]. In the comparison of complexity according to platelet levels, severe HCV-TP patients with platelet level 4/5 had a significantly lower rate of negative antiplatelet antibody and a higher rate of at least three types of antibodies than those with platelet level 1 [Fig. 3B].
Fig. 2.
Antiplatelet antibody results in HCV-TP patients, HBV-TP patients, and HCV controls. A. Antiplatelet antibody profiles. Compared with HCV controls, HCV-TP patients had significantly higher positive rates for antiplatelet antibody and HLA class I antibody. ∗ denotes statistical significance compared with HCV controls. B. Antiplatelet antibody complexity. The results revealed the number of types of antiplatelet antibodies. Significant differences were observed between HCV-TP patients and HCV controls (p = 0.024) but not between HCV-TP and HBV-TP groups using the Pearson chi-square method.
Fig. 3.
Antiplatelet antibody results according to platelet levels in HCV patients. A. Antiplatelet antibody profile. Compared with the HCV patients with platelet count level 1 (normal), patients with platelet level 3 (moderate thrombocytopenia) had significantly higher positive rates of HLA class I antibody, while patients with platelet level 4/5 (severe thrombocytopenia) had higher positive anti-GPIb/IX antibody rates. In addition, there was a trend that positive rates of all anti-GP antibody increased as severity of thrombocytopenia progressed. B. Antiplatelet antibody complexity. Compared with HCV patients with platelet level 1, the patients with platelet count level 4/5 had different profiles in the number of types of antiplatelet antibodies by using the Pearson chi-square method (p = 0.015). In addition, these patients had a significantly lower percentage of negative antiplatelet antibodies and a higher percentage of ≥3 types of antibodies. ∗ denotes statistical significance when comparing the HCV patients with platelet count level 1.
Because we found splenomegaly to not be a significant factor for severe HCV-TP and the percentage of splenomegaly at time of event was much lower in severe HCV-TP than in HBV-TP in the retrospective analysis, we analyzed the association between splenomegaly, antiplatelet antibodies, and platelet levels in this prospective analysis and found that HCV-TP patients with positive anti-GPIIb/IIIa antibodies had a significantly lower percentage of splenomegaly than patients without anti-GPIIb/IIIa antibody (30.4% vs. 60.0%, p = 0.024). Eleven of 24 (45.8%) severe HCV-TP patients had positive anti-GPIIb/IIIa compared with 12 of 39 (30.4%) moderate HCV-TP patients did [Fig. 3A]. In contrast, the percentage of splenomegaly was lower in severe HCV-TP patients (41.67%) than in moderate HCV-TP (53.85%). Meanwhile, that of cirrhosis was equivalent in severe and moderate HCV-TP patients (Supplement Table S2). We then analyzed the correlation between different combinations of antiplatelet antibodies and platelet count in HCV patients (Supplement Fig. S1). The platelet counts were lower in patients testing positive for the combination of any antiplatelet antibody with anti-GPIb/IX antibody than other combinations. These data correlated the result that severe HCV-TP patients had significantly higher positive rate of anti-GPIb/IX antibody than did HCV control. However, the number of patients with these combinations was too small to yield any conclusive results. Accordingly, these results suggested antiplatelet antibody might be a very important role in HCV-TP patients without advanced cirrhosis.
Discussion
This study explored the risk factors of thrombocytopenia and effect of antiplatelet antibodies in HCV and HBV patients. The prevalence of thrombocytopenia of platelet count <100 × 109/L was 11.86% and 6.35% in HCV and HBV patients, respectively and higher than reported in a community-based study in A-Lein, Taiwan (10.2% and 1.9%, respectively) [8]. This difference might be due to this study being institutional, with a larger cohort size and an endemic area. Taiwan has obvious endemic variants of HCV and HBV infection. The area of this study population, Chiayi county, is especially hyperendemic HCV and HBV infection [23]. In addition to prevalence, the incidence rates of thrombocytopenia were higher in HCV than in HBV patients regardless of thrombocytopenia severity. These results explained the reason that HCV patients had lower incidence globally but raised concern about the thrombocytopenia.
The univariant analysis revealed cirrhosis, ALP, hyperbilirubinemia, PT prolongation, splenomegaly, and initial thrombocytopenia to be significant common risk factors for moderate and/or severe thrombocytopenia in HCV and HBV patients. This result agrees with those of the studies on fibrosis predictive models, which revealed platelet count to be associated with fibrosis levels [26,27]. In contrast, liver fibrosis was strongly associated with thrombocytopenia. Progressive cirrhosis has been associated with decreased production of thrombopoietin, a regulator of megakaryocyte maturation and platelet production [28,29]; this mechanism may explain the correlation between cirrhosis and platelet count. On the other hand, hyperbilirubinemia and PT prolongation contribute Child-Pugh scoring and correlate to status of cirrhosis, becoming important risk factors. ALP has been reported to be correlated with cirrhosis [30]. High ALP level may also indicate biliary cirrhosis in addition to viral hepatitis, leading to more severe cirrhotic status [31]. Another mechanism could be splenomegaly, which leads to platelet sequestration [16,32]. In our Cox models, initial thrombocytopenia level 2 was associated with the highest AHR in both moderate HCV-TP and HBV-TP patients. The analysis of time to severe thrombocytopenia also demonstrated that initial thrombocytopenia level 3 persisted as a most significant factor in both groups. These data imply thrombocytopenia in patients with viral hepatitis progresses with thrombocytopenia severity and the clinical progression might be predictable.
However, significantly high HCV-to-HBV IRR of thrombocytopenia regardless of severity suggests there should be more mechanism of thrombocytopenia in HCV patients in addition to cirrhosis and splenomegaly. Compared with HBV patients, HCV patients had obvious discrepancy of proportion between splenomegaly and cirrhosis initially, with much higher proportion of splenomegaly than cirrhosis. According to Cox proportion models, splenomegaly was a significant risk for moderate thrombocytopenia. However, in the comparison of moderate HCV-TP patients, they had only slight increase of spleen index and percentage of splenomegaly from initial enrollment to time at thrombocytopenia event. These data imply splenomegaly is a significant risk factor but not the leading cause of thrombocytopenia. In a previous study, splenomegaly might be not only associated with cirrhosis but also anti-HLA class I antibody in HCV-TP patients [33]. In this retrospective analysis, the results confirmed the findings indirectly. Thus, we suggest splenomegaly is not only caused by cirrhotic portal hypertension, leading to sequestration of platelet, but also the result of production of antiplatelet antibody in HCV patients.
Of the possible pathomechanisms of HCV-ITP [6,16], immune-mediated platelet destruction is one of the most documented etiology. In our prospective study, we found the positive rates of all specific anti-GP antibodies increased as the platelet count decreased in chronic hepatitis and early cirrhotic HCV-TP patients. In addition to possible progressive antibody-mediated platelet destruction, decreasing rates of negative antibody may also suggest unbalanced T cell-mediated immune response [16]. Interestingly, HCV-TP patients with the anti-GPIIb/IIIa antibody exhibited a significantly lower percentage of splenomegaly than those without anti-GPIIb/IIIa antibody. Then we found severe HCV-TP patients had higher percentage of positive anti-GPIIb/IIIa antibody, but lower percentage of splenomegaly and equivalent cirrhotic status compared with moderate HCV-TP patients. These data suggested that the most common antiplatelet antibody, anti-GPIIb/IIIa antibody was a more important risk factor than splenomegaly in severe HCV-TP patients without advanced cirrhosis (Child-Pugh B or C). This data correlated the result that splenomegaly was negligible in the Cox proportion models of severe HCV-TP patients. These data implied that the antiplatelet antibody-mediated platelet destruction might contribute to an important risk factor in the HCV-TP patients without advanced cirrhosis, resulting in HCV-associated immune thrombocytopenia. In addition, severe HCV-TP patients had a higher positive rates and complexity of antiplatelet antibodies than HCV controls. Therefore, we speculate that a certain percentage of HCV patients have antiplatelet autoantibodies before presentation of thrombocytopenia. When the antibodies specificity to a major platelet antigen and/or the complexity of antibodies increase, clinical thrombocytopenia develops and progresses, either independently or with splenomegaly and cirrhosis. HCV patients having severe thrombocytopenia without advanced cirrhosis may be especially affected by these immunomodulatory effects.
Interferon eradicates HCV viremia but induces reversible thrombocytopenia, which affects tolerability and clinical outcomes [34]. Nevertheless, interferon therapy can alleviate thrombocytopenia after treatment completion and sustained virological response. The response was independent of not only cirrhotic status but also initial platelet levels [[35], [36], [37]]. This impact correlated to the only protective effect of interferon therapy in our univariant analysis of moderate HCV-TP patients.
HBV-TP patients had a much longer mean time to thrombocytopenia event than did HCV-TP patients. The liver enzyme, hyperbilirubinemia, PT prolongation, and splenomegaly were considered to be related to cirrhotic status in HBV patients [38], becoming risk factors for moderate HBV-TP. Among these factors, only hyperbilirubinemia revealed to be risk in the multivariant analysis. For severe thrombocytopenia, the impact of cirrhosis associated hyperbilirubinemia persisted. In the comparison of retrospective HCV-TP and HBV-TP patients, the percentages of splenomegaly and cirrhosis, which were lower in HBV-TP patients initially, continue to increase until becoming even higher than those in HCV-TP patients at the time of the thrombocytopenic event. The prospective study also found a much higher percentage of splenomegaly in severe HBV-TP patients than in severe HCV-TP patients. Accordingly, we suppose that laboratory initial thrombocytopenia and hyperbilirubinemia are early predictors for thrombocytopenia, whereas ultrasonographic cirrhosis and splenomegaly contribute to thrombocytopenia later in the relatively longer course of HBV-TP patients. Because of different risk factors and clinical course in HBV-TP and HCV-TP patients, different strategy of management for these two groups was suggested. We also detected antiplatelet autoantibodies in HBV-TP patients, similar to previous studies [14]. However, because of the relatively low incidence rate of isolated thrombocytopenia in HBV patients, the number of HBV-TP patients enrolled was too small to definitively determine the influence of antiplatelet antibodies.
Our study had some limitations. Because we used the objective laboratory data with strict criteria, this approach may underestimate the incidence rates of thrombocytopenic events. Second, although the number of enrolled patients was large, our sample was heterogenous with variant liver disease status. Third, because we could not definitely clarify the association between cancer related factors and thrombocytopenic events in our retrospective study, patients with cancer history were all excluded. This led much smaller number of thrombocytopenic events in our analysis. Finally, because the incidence of HBV-TP and referral were low, the number of patients in the prospective HBV-TP cohort was relatively small. Future studies with a larger cohort size are thus warranted.
In conclusion, our study reported HCV patients had higher cross-sectional prevalence and longitudinal follow-up incidence rates of thrombocytopenia than HBV patients. The predicting risk factors for thrombocytopenia varied with viral type and thrombocytopenia severity. Direct antiplatelet antibody-mediated platelet destruction was an important factor in HCV-TP patients without advanced cirrhosis.
Conflicts of interest
All authors declared no conflicts of interest.
Acknowledgement
This work was supported by Chang Gung Medical Foundation, Taiwan, grants to C.E. Huang (CMRPG6F0211 and CMRPG6J0391) and to M.C. Chen (CMRPD1J0101-0102). All authors approved the final version of the article, including the authorship list.
Footnotes
Peer review under responsibility of Chang Gung University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bj.2021.09.001.
Contributor Information
Chih-Cheng Chen, Email: ccchen@cgmh.org.tw.
Min-Chi Chen, Email: mcc@mail.cgu.edu.tw.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Seto W.K., Lo Y.R., Pawlotsky J.M., Yuen M.F. Chronic hepatitis B virus infection. Lancet. 2018;392:2313–2324. doi: 10.1016/S0140-6736(18)31865-8. [DOI] [PubMed] [Google Scholar]
- 2.Polaris Observatory HCV Collaborators Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol. 2017;2:161–176. doi: 10.1016/S2468-1253(16)30181-9. [DOI] [PubMed] [Google Scholar]
- 3.Tejima K., Masuzaki R., Ikeda H., Yoshida H., Tateishi R., Sugioka Y., et al. Thrombocytopenia is more severe in patients with advanced chronic hepatitis C than B with the same grade of liver stiffness and splenomegaly. J Gastroenterol. 2010;45:876–884. doi: 10.1007/s00535-010-0233-5. [DOI] [PubMed] [Google Scholar]
- 4.Stasi R., Chia L.W., Kalkur P., Lowe R., Shannon M.S. Pathobiology and treatment of hepatitis virus-related thrombocytopenia. Mediterr J Hematol Infect Dis. 2009;1 doi: 10.4084/MJHID.2009.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Louie K.S., Micallef J.M., Pimenta J.M., Forssen U.M. Prevalence of thrombocytopenia among patients with chronic hepatitis C: a systematic review. J Viral Hepat. 2011;18:1–7. doi: 10.1111/j.1365-2893.2010.01366.x. [DOI] [PubMed] [Google Scholar]
- 6.Weksler B.B. Review article: the pathophysiology of thrombocytopenia in hepatitis C virus infection and chronic liver disease. Aliment Pharmacol Ther. 2007;26 doi: 10.1111/j.1365-2036.2007.03512.x. Suppl 1:13–9. [DOI] [PubMed] [Google Scholar]
- 7.Rawi S., Wu G.Y. Pathogenesis of thrombocytopenia in chronic HCV infection: a review. J Clin Transl Hepatol. 2020;8:184–191. doi: 10.14218/JCTH.2020.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang C.S., Yao W.J., Wang S.T., Chang T.T., Chou P. Strong association of hepatitis C virus (HCV) infection and thrombocytopenia: implications from a survey of a community with hyperendemic HCV infection. Clin Infect Dis. 2004;39:790–796. doi: 10.1086/423384. [DOI] [PubMed] [Google Scholar]
- 9.Behnava B., Alavian S.M., Asl M.A. The prevalence of thrombocytopenia in patients with chronic hepatitis B and C. Hepat Mon. 2006;6:67–69. [Google Scholar]
- 10.Joo E.J., Chang Y., Yeom J.S., Lee Y.G., Ryu S. Hepatitis B infection is associated with an increased incidence of thrombocytopenia in healthy adults without cirrhosis. J Viral Hepat. 2017;24:253–258. doi: 10.1111/jvh.12642. [DOI] [PubMed] [Google Scholar]
- 11.Moore A.H. Thrombocytopenia in cirrhosis: a review of pathophysiology and management options. Clin Liver Dis (Hoboken) 2019;14:183–186. doi: 10.1002/cld.860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang C.E., Chen Y.Y., Chang J.J., Kuan F.C., Lee K.D., Lu C.H., et al. Thrombopoietic cytokines in patients with hepatitis C virus-associated immune thrombocytopenia. Hematology. 2017;22:54–60. doi: 10.1080/10245332.2016.1204493. [DOI] [PubMed] [Google Scholar]
- 13.Olariu M., Olariu C., Olteanu D. Thrombocytopenia in chronic hepatitis C. J Gastrointestin Liver Dis. 2010;19:381–385. [PubMed] [Google Scholar]
- 14.Christodoulou D., Katsanos K., Zervou E., Theopistos V., Papathanasopoulos A., Christou L., et al. Platelet IgG antibodies are significantly increased in chronic liver disease. Ann Gastroenterol. 2011;24:47–52. [PMC free article] [PubMed] [Google Scholar]
- 15.Pradella P., Bonetto S., Turchetto S., Uxa L., Comar C., Zorat F., et al. Platelet production and destruction in liver cirrhosis. J Hepatol. 2011;54:894–900. doi: 10.1016/j.jhep.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 16.Zufferey A., Kapur R., Semple J.W. Pathogenesis and therapeutic mechanisms in immune thrombocytopenia (ITP) J Clin Med. 2017;6:16. [Google Scholar]
- 17.Karasu Z., Tekin F., Ersoz G., Gunsar F., Batur Y., Ilter T., et al. Liver fibrosis is associated with decreased peripheral platelet count in patients with chronic hepatitis B and C. Dig Dis Sci. 2007;52:1535–1539. doi: 10.1007/s10620-006-9144-y. [DOI] [PubMed] [Google Scholar]
- 18.Houot M., Ngo Y., Munteanu M., Marque S., Poynard T. Systematic review with meta-analysis: direct comparisons of biomarkers for the diagnosis of fibrosis in chronic hepatitis C and B. Aliment Pharmacol Ther. 2016;43:16–29. doi: 10.1111/apt.13446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neunert C., Lim W., Crowther M., Cohen A., Solberg L Jr, Crowther M.A., et al. The American Society of Hematology 2011 evidence-based practice guideline for immune thrombocytopenia. Blood. 2011;117:4190–4207. doi: 10.1182/blood-2010-08-302984. [DOI] [PubMed] [Google Scholar]
- 20.Zhang W., Nardi M.A., Borkowsky W., Li Z., Karpatkin S. Role of molecular mimicry of hepatitis C virus protein with platelet GPIIIa in hepatitis C-related immunologic thrombocytopenia. Blood. 2009;113:4086–4093. doi: 10.1182/blood-2008-09-181073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Panzer S., Seel E., Brunner M., Kormoczi G.F., Schmid M., Ferenci P., et al. Platelet autoantibodies are common in hepatitis C infection, irrespective of the presence of thrombocytopenia. Eur J Haematol. 2006;77:513–517. doi: 10.1111/j.0902-4441.2006.t01-1-ejh2888.x. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y., Wang L., Gao W., Chen X., Su Y. Detection of Treg/Th17 cells and related cytokines in peripheral blood of chronic hepatitis B patients combined with thrombocytopenia and the clinical significance. Exp Ther Med. 2018;16:1328–1332. doi: 10.3892/etm.2018.6311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen C.H., Yang P.M., Huang G.T., Lee H.S., Sung J.L., Sheu J.C. Estimation of seroprevalence of hepatitis B virus and hepatitis C virus in Taiwan from a large-scale survey of free hepatitis screening participants. J Formos Med Assoc. 2007;106:148–155. doi: 10.1016/S0929-6646(09)60231-X. [DOI] [PubMed] [Google Scholar]
- 24.Gerstenmaier J.F., Gibson R.N. Ultrasound in chronic liver disease. Insights Imaging. 2014;5:441–455. doi: 10.1007/s13244-014-0336-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chien C.H., Lin Y.L., Chien R.N., Hu C.C., Yen C.L., Lee T.S., et al. Transient elastography for spleen stiffness measurement in patients with cirrhosis: role in degree of thrombocytopenia. J Ultrasound Med. 2016;35:1849–1857. doi: 10.7863/ultra.15.09064. [DOI] [PubMed] [Google Scholar]
- 26.Vallet-Pichard A., Mallet V., Nalpas B., Verkarre V., Nalpas A., Dhalluin-Venier V., et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology. 2007;46:32–36. doi: 10.1002/hep.21669. [DOI] [PubMed] [Google Scholar]
- 27.Yen Y.H., Kuo F.Y., Kee K.M., Chang K.C., Tsai M.C., Hu T.H., et al. APRI and FIB-4 in the evaluation of liver fibrosis in chronic hepatitis C patients stratified by AST level. PLoS One. 2018;13 doi: 10.1371/journal.pone.0199760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peck-Radosavljevic M., Wichlas M., Zacherl J., Stiegler G., Stohlawetz P., Fuchsjager M., et al. Thrombopoietin induces rapid resolution of thrombocytopenia after orthotopic liver transplantation through increased platelet production. Blood. 2000;95:795–801. [PubMed] [Google Scholar]
- 29.Eissa L.A., Gad L.S., Rabie A.M., El-Gayar A.M. Thrombopoietin level in patients with chronic liver diseases. Ann Hepatol. 2008;7:235–244. [PubMed] [Google Scholar]
- 30.Wai C.T., Greenson J.K., Fontana R.J., Kalbfleisch J.D., Marrero J.A., Conjeevaram H.S., et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518–526. doi: 10.1053/jhep.2003.50346. [DOI] [PubMed] [Google Scholar]
- 31.Lammers W.J., van Buuren H.R., Hirschfield G.M., Janssen H.L., Invernizzi P., Mason A.L., et al. Levels of alkaline phosphatase and bilirubin are surrogate end points of outcomes of patients with primary biliary cirrhosis: an international follow-up study. Gastroenterology. 2014;147:1338–1349. doi: 10.1053/j.gastro.2014.08.029. e5; quiz e15. [DOI] [PubMed] [Google Scholar]
- 32.Aster R.H. Pooling of platelets in the spleen: role in the pathogenesis of "hypersplenic" thrombocytopenia. J Clin Invest. 1966;45:645–657. doi: 10.1172/JCI105380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang C.E., Chen W.M., Wu Y.Y., Shen C.H., Hsu C.C., Li C.P., et al. Comparison of antiplatelet antibody profiles between hepatitis C virus-associated immune thrombocytopenia and primary immune thrombocytopenia. Platelets. 2021;32:1043–1050. doi: 10.1080/09537104.2020.1820975. [DOI] [PubMed] [Google Scholar]
- 34.Maan R., van der Meer A.J., Hansen B.E., Feld J.J., Wedemeyer H., Dufour J.F., et al. Effect of thrombocytopenia on treatment tolerability and outcome in patients with chronic HCV infection and advanced hepatic fibrosis. J Hepatol. 2014;61:482–491. doi: 10.1016/j.jhep.2014.04.021. [DOI] [PubMed] [Google Scholar]
- 35.García-Suárez J., Burgaleta C., Hernanz N., Albarran F., Tobaruela P., Alvarez-Mon M. HCV-associated thrombocytopenia: clinical characteristics and platelet response after recombinant alpha2b-interferon therapy. Br J Haematol. 2000;110:98–103. doi: 10.1046/j.1365-2141.2000.02132.x. [DOI] [PubMed] [Google Scholar]
- 36.Rajan S., Liebman H.A. Treatment of hepatitis C related thrombocytopenia with interferon alpha. Am J Hematol. 2001;68:202–209. doi: 10.1002/ajh.1180. [DOI] [PubMed] [Google Scholar]
- 37.Kee K.M., Wang J.H., Hung C.H., Chen C.H., Lee C.M., Lu S.N. Improvement of thrombocytopenia in hepatitis C-related advanced fibrosis patients after sustained virological response. Dig Dis Sci. 2013;58:556–561. doi: 10.1007/s10620-012-2380-4. [DOI] [PubMed] [Google Scholar]
- 38.Shetty A., Jun Yum J., Saab S. The gastroenterologist's guide to preventive management of compensated cirrhosis. Gastroenterol Hepatol. 2019;15:423–430. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.