Abstract
Introduction
Organs procured following brain stem death (BSD) are the main source of organ grafts for transplantation. However, BSD is associated with inflammatory responses that may damage the organ and affect both the quantity and quality of organs available for transplant. Therefore, we aimed to investigate plasma and bronchoalveolar lavage (BAL) pro-inflammatory cytokine profiles and cardiovascular physiology in a clinically relevant 6-h ovine model of BSD.
Methods
Twelve healthy female sheep (37–42 Kg) were anaesthetized and mechanically ventilated prior to undergoing BSD induction and then monitored for 6 h. Plasma and BAL endothelin-1 and cytokines (IL-1β, 6, 8 and tumour necrosis factor alpha (TNF-α)) were assessed by ELISA. Differential white blood cell counts were performed. Cardiac function during BSD was also examined using echocardiography, and cardiac biomarkers (A-type natriuretic peptide and troponin I were measured in plasma.
Results
Plasma concentrations big ET-1, IL-6, IL-8, TNF-α and BAL IL-8 were significantly (p < 0.01) increased over baseline at 6 h post-BSD. Increased numbers of neutrophils were observed in the whole blood (3.1 × 109 cells/L [95% confidence interval (CI) 2.06–4.14] vs. 6 × 109 cells/L [95%CI 3.92–7.97]; p < 0.01) and BAL (4.5 × 109 cells/L [95%CI 0.41–9.41] vs. 26 [95%CI 12.29–39.80]; p = 0.03) after 6 h of BSD induction vs baseline. A significant increase in ANP production (20.28 pM [95%CI 16.18–24.37] vs. 78.68 pM [95%CI 53.16–104.21]; p < 0.0001) and cTnI release (0.039 ng/mL vs. 4.26 [95%CI 2.69–5.83] ng/mL; p < 0.0001), associated with a significant reduction in heart contractile function, were observed between baseline and 6 h.
Conclusions
BSD induced systemic pro-inflammatory responses, characterized by increased neutrophil infiltration and cytokine production in the circulation and BAL fluid, and associated with reduced heart contractile function in ovine model of BSD.
Keywords: Brain stem death, Transplantation, Endothelin-1, Inflammation, Cytokines, Troponin
At a glance of commentary
Scientific background on the subject
The majority of organs are donated by patients exposed to brain stem death (BSD) who have suffered irreversible brain injury that subsequently leads to cessation of brain stem function. BSD causes devastating haemodynamic, neurohumoral and immunoloical perturbations, which can result in graft dysfunction of the donor organ.
What this study adds to the field
This study showed that BSD is associated with early systemic and lung inflammatory response, characterized by increased neutrophil infiltration and cytokine production in the circulation and BAL fluid, and associated with reduced heart contractile function in ovine model of BSD.
Organ transplantation remains the mainstay treatment for end-stage organ failures. However, the availability of suitable organs for transplantation is insufficient for this demand [[1], [2], [3], [4], [5], [6]]. Approximately 50% of donated organs are non-transplantable [1,2,4,5]. The majority of organs are donated by patients exposed to brain stem death (BSD) who have suffered irreversible brain injury that subsequently leads to cessation of brain stem function [[1], [2], [3], [4], [5]]. BSD is also considered the main cause of pre-transplantation injury. BSD causes devastating haemodynamic, neurohumoral and immunological perturbations, which can result in graft dysfunction of the donor organ, thus increasing chances of rejection and poor patient outcomes post-transplantation in the recipient [2,6,7]. BSD is often accompanied by a systemic inflammatory response, which triggers inflammatory signalling cascades, increases in expression of transcriptional regulators and infiltration and activation of immune cells [[7], [8], [9], [10], [11]]. During the initial phase of BSD, the ischemic brain damage releases inflammatory mediators that could induce a systemic inflammatory response [12]. The catecholamine storm elicited from BSD [13] also induces a systemic inflammatory response by either changing the metabolism to anaerobic [14] (metabolic changes after brain death modulate the inflammatory response) [15] or inducing ischemia in the gut, thereby releasing cytokines [16,17]. Moreover, neuropeptides released from central nervous system may also form the link between BSD and a systemic inflammatory response [18].
Previous human and porcine model studies, have identified up-regulation of inflammatory mediators such as TNFα, IL-6 and macrophage inflammatory protein 1-alpha in blood circulation following the onset of BSD [16,[19], [20], [21]]. These mediators further exacerbate the inflammatory response within tissues, potentially leading to impaired graft function [22,23]. Several studies have shown that elevated IL-8 in bronchoalveolar lavage (BAL) fluid [24] and lung tissue [25] of brain dead patients was associated with early graft failure in the transplant recipient. Neutrophils also appear to be a major contributor to the pro-inflammatory reaction, tissue injury and graft dysfunction in the BSD donor [26,27]. BSD also contributes to increased oxidative activity, up-regulation of oxidative enzymes [28] and generation of chloramine, a biomarker for protein oxidative damage which has been positively correlated with IL-6 in patients suffering from BSD [29]. Previous studies using animal models of BSD and brain-dead donors have also suggested the importance of ET-1 in lung allograft survival and rejection [30,31]. Release of ET-1 may contribute to pulmonary inflammation and lung injury pathogenesis [32,33].
BSD induces changes that compromise cardiac function and affect the graft response after transplantation [34,35]. The hyperdynamic cardiac response to BSD causes increased pulmonary and systemic after-load, leading to biventricular distension, myocardial cell death and eventual heart failure [36]. Increased ventricular stretching, by either increased pre-load or after-load, has been shown to be associated with increased plasma levels of A-type natriuretic peptide (ANP) in humans that can cause endothelial activation and subsequent shedding of individual components of the glycocalyx, and histologically detectable degradation [37]. Elevated cardiac troponin I (cTnI) in the serum of heart donors has been linked to graft failure after transplant [38,39]. Furthermore, inflammatory process induced after BSD may also contribute to myocardial dysfunction [35].
We recently published the first 24-h ovine model capable of elucidating the role of the endothelin axis in BSD related pulmonary inflammation [10]. We have shown an early elevation and then restitution of both ET-1 and big ET-1 concentrations in plasma after brain death. Here we further characterise our ovine model of BSD by examining the plasma and bronchoalveolar lavage (BAL) pro-inflammatory cytokines profile and cardiovascular changes, at different time courses that occur during BSD.
Methodology
Animal BSD model
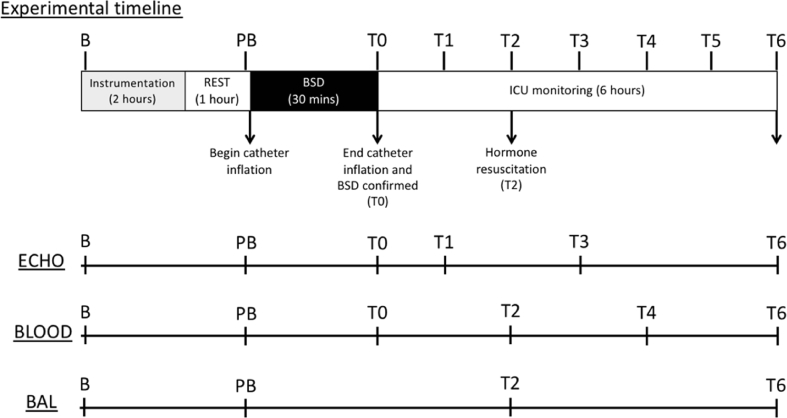
Twelve female merino sheep (37–42 Kg, 2 years old) underwent BSD procedures for 6 h as previously developed and described by our group [10]. The animals only had access to drinking water during the night prior to the experiment. Following anaesthetic induction, all animals were mechanically ventilated and standard instrumentation procedures were performed [40]. Briefly, a cranial burr hole was created midway between the midline and lateral edge of the cranium followed by the extradural placement of 5.3 mm Foley catheter (Brad BIOCATH, United Kingdom). One hour after completion of all invasive procedures, BSD was induced by slowly inflating the intracranial catheter with 30 mL saline over 30 min to increase intracranial pressure (ICP) above the mean arterial pressure (MAP). Confirmation of BSD was achieved by continuously negative cerebral perfusion pressure (defined as MAP-ICP) for greater than 30 min, loss of pupillary and corneal reflexes and lack of respiratory efforts. All sheep received hormone resuscitation 2 h following confirmation of BSD; triiodothyronine (4 μg bolus and 3 μg/h infusion), vasopressin (1 unit bolus followed by 0.5–4.0 U/hr infusion, adjusted to SVR 800–1200 dyn s.cm-5) and methylprednisolone (15 mg/kg) [10]. Sheep were monitored and hemodynamically managed for 6 h after BSD confirmation, then humanely sacrificed with sodium pentobarbitone (162.5 mg/kg). Fig. 1 below provides a schematic representation of the experimental timeline. All animal experiments were performed at the Medical Engineering Research Facility (Queensland University of Technology; QUT) and approved by the QUT Animal Ethics Research Committee (animal ethics number 1400000922).
Fig. 1.
Experimental timeline. Once the sheep were brought into theatre, a baseline blood sample (B) was taken following placement of the central venous line and prior to induction of anaesthesia. Following induction of anaesthesia, the sheep were mechanically ventilated and remaining instrumentation procedures were completed [40]. The sheep were rested for 1 h following completion of instrumentation, after which pre-BSD (PB) echocardiography (ECHO), blood, and bronchoalveolar lavage (BAL) samples were collected. BSD was then induced by inflation of the catheter slowly over 30 min, at which point BSD was confirmed by standard clinical observations [41]. Once BSD was confirmed (T0), blood samples and ECHO measures were taken immediately, and the sheep were monitored in ICU settings for 6 additional hours (T0-T6). The sheep received standard BSD hormone resuscitation 2 h following confirmation of BSD (T2). Blood, BAL and ECHO measures were collected progressively as per the sampling schedule listed above. The sheep were humanely euthanised at T6 following collection of all relevant samples.
Blood collection and analysis
Whole blood was collected from the facial artery during procedures (Baseline, pre-BSD, 0, 2, 4 and 6 h post BSD). Blood gas measurements were detected in fresh whole blood using ABL800 FLEX analyser (Radiometer, Australia). Full blood count analysis was performed on EDTA whole blood using Coulter Act (Australia), and a thin film of blood smear was prepared for differential white blood cell (WBC) count. The cells were observed using a light microscope (ZEISS, Australia) under total × 400-magnification power. Whole blood was centrifuged twice at 3000×g for 15 min (4 °C), platelet poor plasma samples were aliquoted immediately and stored at −80 °C until analysis.
BAL fluid collection and analysis
BAL samples were collected using bronchoscopy at baseline, 2- and 6-h post-BSD. Samples were centrifuged at 1500×g for 10 min at room temperature, and the supernatant was collected and stored at −80 °C until analysis. The total cell number was estimated using Coulter Counter (Invitrogen, Australia). Cell suspensions (1 × 106 cells/mL; 100 μL) were prepared and deposited on cytocentrifuge chambers (Shandon, Australia) and centrifuged at 500×g for 5 min. Differential WBC was determined by counting in a circular pattern from right to left until 200 cells were obtained under total × 400 and × 1000 magnification power (results expressed as a percentage of total cell number).
Cytokines
Our group has recently validated the antibodies and developed ELISA assays for sheep cytokines IL-1β, IL-6, IL-8 [41] and TNF-α [42]. EDTA plasma and BAL samples were analysed in duplicate and absorbance was read at 450 nm, with background correction read at 670 nm. A standard curve was created and a four-parameter logistic (4- PL) curve fit used to determine sample and standard concentrations (GraphPad Prism 8, CA, USA).
Endothelin
Big ET-1 and ET-1 concentrations were measured in EDTA plasma and BAL samples using commercially available ELISA kits (Cat # BI-20052 and BI-20082H; United Bioscience, Austria). Samples were analysed in duplicate and absorbance read at 450 nm with background correction read at 630 nm on a microplate reader (BMG Labtech, VIC, Australia).
Cardiac biomarkers
ANP was measured in EDTA plasma using radioimmunoassay with high performance liquid chromatography (PerkinElmer, New Zealand) according to previously published methods [43]. cTnI was quantified in heparined plasma using a commercial ELISA kit (Cat# CTNI-9-HSP; Fisher Biotech, Australia). Samples were analysed in duplicate and read at absorbance 450 nm using a microplate reader (BMG Labtech, VIC, Australia).
Cardiovascular function
Cardiac function was assessed by direct epicardial two-dimensional (2D) echocardiography scanning in the right-lateral decubitus position with a 3 S transducer and a spacer (Vivid.i, GE Healthcare©, U.S.A). Three-beat ECG-gated loops in the parasternal short axis (PSAX) were recorded at a frame rate of 50–80 frames per second (FPS) at the left-ventricular base, mid-papillary and apical level. Data were stored and transferred (Transcend® hard disc drive, Taiwan) for offline analysis on a vendor independent platform using 2D Cardiac Performance Analysis software (TomTec Imaging Systems, Munich, Germany). Measurements at baseline and at 6-h were compared for the end-diastolic area (EDA), end-systolic area (ESA), fractional area change (FAC), global circumferential strain (GCS), global radial strain (GRS) and torsion.
Reduced thiols
Serum and BAL reduced thiols concentrations were quantified with 5,5-dithiobis (2-nitrobenzoic acid) (DTNB) reagent in triplicate at absorbance 412 nm using a 96-well plate reader according to the method of Hawkins et al. [44]. Reduced glutathione (0–0.5 mM) served as an external standard and data were expressed relative to the total protein concentration (nmol/mg protein).
Chloramine
Chloramine is a stable product of protein oxidation and was quantified according to previously published methods [45]. 50 μl of developing reagent (2 mM 3, 3′, 5, 5′-Tetramethylbenzidine (TMB) in 400 mM acetate buffer, pH 5.4, containing 10% dimethylformamide and 100 μM sodium iodide; 150 μl) was added to 96-well plate wells containing 200 μl of the serum. Samples were incubated on a plate shaker at 50 rpm for 25 min. The plate was read at absorbance 650 nm using a 96-well plate reader and results were expressed in nmols/mg of protein.
Statistics
Normality of distribution was assessed by inspecting the corresponding histogram, pp-plot and using a Kolmogorov–Smirnov test. Each data set, except the echocardiography, were not normally distributed and therefore non-parametric Friedman tests followed by post-hoc Dunn's multiple comparison were used to assess differences over time. As the echocardiography data was normally distributed, a paired t-test was used to compare between baseline and 6 h. All statistical analysis was performed using GraphPad Prism 8 (GraphPad Software, Santiago, USA). A p-value < 0.05 was considered statistically significant. All data are expressed as mean ± 95% confidence interval (CI).
Results
WBC counts during BSD in sheep blood and BAL samples
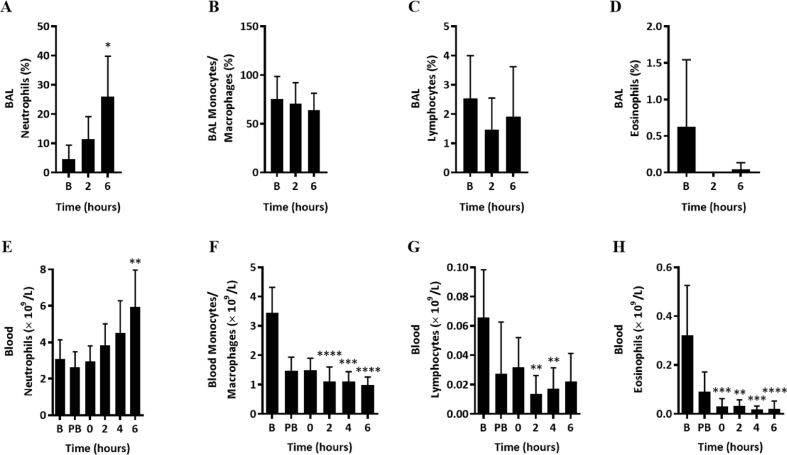
Differential WBC counts were performed on BAL fluid and blood samples collected prior to BSD and post-BSD. Upon BSD induction, blood neutrophil counts were consistently increased during the BSD period and significantly elevated at 6 h post-BSD in BAL (Fig. 2A; p = 0.0239) and blood (Fig. 2E; p = 0.0048, Fig. S1). In contrast, other WBC including monocyte/macrophages, lymphocytes, and eosinophils counts were unchanged in BAL (Fig. 2B, C & D) and significantly reduced in whole blood (Fig. 2F, G & H) during BSD.
Fig. 2.
Differential WBC count in BAL ((A) neutrophils, (B) monocytes/macrophages, (C) lymphocytes and (D) eosinophils) and blood ((E) neutrophils, (F) monocytes/macrophages, (G) lymphocytes and (H) eosinophils) during BSD in sheep (n = 12) at time baseline (B), pre-BSD (PB), 0, 2, 4 and 6 h. Data are expressed as mean ± 95%CI. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 and ∗∗∗∗p < 0.0001 compared to baseline.
Differential time-courses of pro-inflammatory cytokine release during BSD
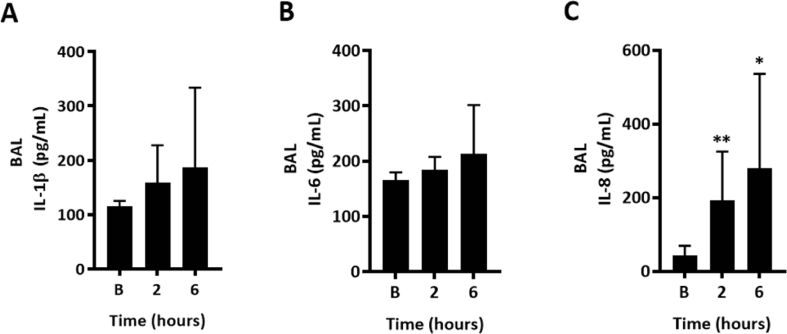
Plasma IL-6 concentrations were significantly increased in blood plasma at 6 h following BSD induction compared to baseline (Fig. 3A; p < 0.0006). Interestingly, plasma concentrations of IL-8 were reduced post-BSD when compared to baseline (Fig. 3B; p < 0.0001). No significant difference in plasma IL-1β and TNF-α concentrations were found at any time point (Fig. 3C and D). In BAL, there were no differences in IL-1β and Il-6 concentrations between time points (Fig. 4A and B). However, IL-8 concentrations were significantly increased throughout the observation period of BSD, in contradistinction to the plasma (Fig. 4C). TNF-α concentration was below the detection limit and data were not reported.
Fig. 3.
Plasma IL-6 (A), IL-8 (B), IL-1β (C) and TNF-α (D) in BSD-induced sheep (n = 12) at baseline (B), pre-BSD (PB), 0, 2, 4 and 6 h. Data are expressed as mean ± 95%CI. ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001 compared to baseline.
Fig. 4.
BAL IL-1β (A), IL-6 (B) and IL-8 (C) in BSD-induced sheep (n = 12) at time baseline (B), pre-BSD (PB), 0, 2, 4 and 6 h. BAL TNF-α concentration was below the detection limit (data not shownData are expressed as mean ± 95%CI. ∗p < 0.05, ∗∗p < 0.01 compared to baseline.
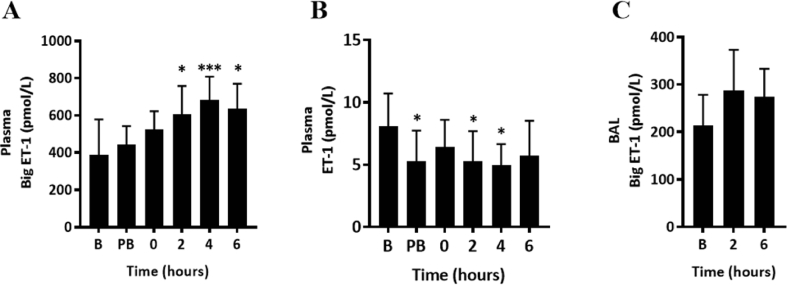
BSD increased big ET-1 release in plasma
We investigated biomarkers of endothelial dysfunction in blood and BAL fluid during BSD (Fig. 5). Circulating plasma big ET-1 concentrations were progressively increased by ∼40% at 4 and 6 h post-BSD (Fig. 5A; p < 0.05). However, plasma ET-1 concentrations were predominantly unchanged, with an exception at pre-BSD, 2 and 4 h post-BSD (Fig. 5B) that was not sustained. No significant change was observed in BAL concentrations of big ET-1 (Fig. 5C). ET-1 concentration in BAL was below the detection limit and data was not reported.
Fig. 5.
Big ET-1 and ET-1 concentrations in plasma (A and B) and BAL (C), respectively, in BSD-induced sheep (n = 12) at time baseline (B), pre-BSD (PB), 0, 2, 4 and 6 h. Data are expressed as mean ± 95%CI. ∗p < 0.05, ∗∗∗p < 0.001 compared to baseline.
Elevated serum levels of cardiac injury biomarkers with significant change to contractility during BSD
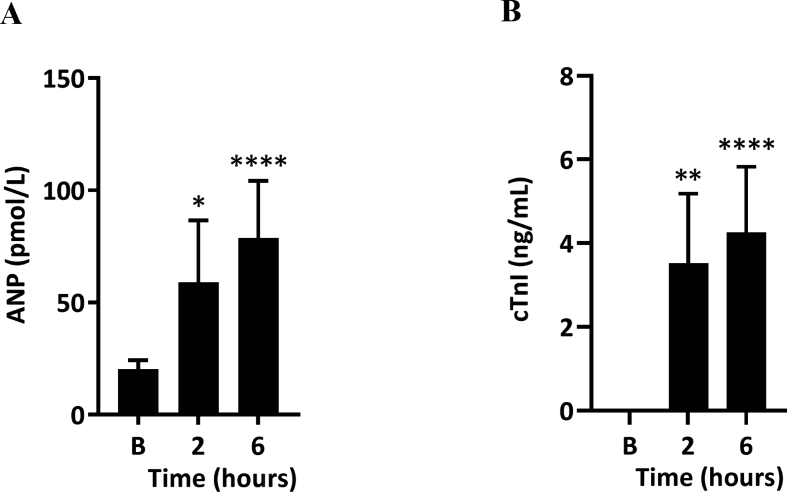
There was a significant rise in the plasma level of ANP at 2 (p = 0.0128) and 6 h (p < 0.0001) after BSD induction (Fig. 6A). Similarly, the level of cTnI in circulation rose from undetectable levels at baseline to significant levels at 2 (p = 0.0066) and 6 h (p < 0.0001) post-BSD (Fig. 6B). Echocardiographs showed a significant reduction in the mean Global Circumferential Strain (GCS, Fig. 7A; p = 0.0383), Torsion (Fig. 7C; P = 0.004) and fractional area change (FAC, Fig. 7F; p = 0.0314) relative to baseline at 6 h. We also noted a significant increase in end-systolic area (ESA, Fig. 7E; p = 0.0278). No alteration was detected in Global Radial Strain (GRS, Fig. 7B; p = 0.5625), Torsion (Fig. 7C; p = 0.2965)and end-diastolic area (EDA, Fig. 7D; p = 0.2992) at 6 h post-BSD.
Fig. 6.
A-type natriuretic peptide, ANP (A) and Cardiac troponin I, cTnI (B) concentrations in BSD-induced sheep (n = 12) at time baseline (B), 2 and 6 h. Baseline levels of cTnI were under the detection limit of 0.039 ng/mL. Data are expressed as mean ± 95%CI. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗∗p < 0.0001 compared to baseline.
Fig. 7.
Echocardiography data comparing (A) Global Circumferential Strain (GCS) (n = 8), (B) Global Radial Strain (GRS) (n = 6), (C) Torsion (n = 6), (D) End-Diastolic Area (EDA) (n = 8), (E) End-Systolic Area (ESA) (n = 7) and (F) Fractional Area Change (FAC) (n = 7) at baseline and 6 h in BSD-induced sheep. Data are expressed as mean ± 95%CI ∗p < 0.05.
Oxidative stress markers were unchanged upon BSD
We used Chloramine and reduced thiols as biomarkers for oxidative damage. We found no change to levels of chloramine and reduced thiol during BSD in the serum and BAL (Table S1).
Effect of instrumentation on inflammation
In order to assess the effects of instrumentation, plasma samples from one of our previous ovine models of transfusion-related acute lung injury (TRALI), were analysed for cytokine production. The in vivo transfusion protocol has been previously described in detail [46] and used similar instrumentation protocol. Sheep that received saline infusion showed a significant increase in IL-6 at 2- and 4-h compared to baseline (8–15 times higher in BSD, Fig. S2B); however, no significant differences were noted during the instrumentation between BSD-induced and saline-infused sheep. This might indicate that the marked cytokine profiles after BSD (released early before hormone resuscitation started) were associated with persistent inflammation throughout the study.
Discussion
Here we extend our investigations of our recently published 24-h-ovine model that elucidated the role of the endothelin axis in BSD related pulmonary inflammation [10] where we suggested that poor organ function in BSD is related to a pro-inflammatory process. We have extended this work to examine the profile of plasma and BAL pro-inflammatory cytokines, endothelins and cardiovascular changes, at different time courses that occur during BSD. We demonstrated that BSD induced a significant increase in neutrophil content, Big ET-1, pro-inflammatory cytokines IL-6 (in plasma) and IL-8 (in BAL) concentrations in blood and BAL. Increased levels of cardiac biomarkers cTnI and ANP indicated cardiac dysfunction that was associated with significant changes in contractility. Our study show that BSD is associated with early systemic and lung inflammatory response and impaired cardiac function in 6-h-ovine model of BSD.
Pro-inflammatory cytokines
Explosive BSD induction presents tremendous increases in hemodynamics or catecholamine levels [47], which has been suggested to be responsible for the inflammatory phenomena occurring after BSD [32]. The association we see between rapid (<2 h) neutrophil recruitment to the lungs (Fig. 2A) and elevation of IL-8 in BAL (Fig. 4C) in our ovine model of BSD is consistent with observations in humans [24]. The increased circulating blood neutrophils in the ovine model at 6-h post-BSD (Fig. 2E), the unchanged monocyte/macrophage, lymphocyte, and eosinophil counts in BAL and their reduction in whole blood (Fig. 2) are also consistent with previous findings in our rat model of BSD [30,32]. Our ovine model also produced a significant and progressive increase in serum IL-6 concentrations for up to 6-h post-BSD (Fig. 3A), consistent with previous findings, from human and rat brain-dead donors, which showed that serum IL-6 was significantly elevated [48]. Although IL-1β and TNF-α act early in the inflammatory cascade [49], we failed to detect any increase in serum IL-1β and TNF-α after BSD in this study (Fig. 3C and D). This discrepancy could have been due to changes in serum levels of TNF-α and IL-1β post-BSD depend on the rate of BSD induction [50,51]. Damman and his colleagues, while also utilizing gradual BSD induction in a rat, showed that the levels of TNF-α and IL-1β in serum did not change significantly over the 4-h in animal models and human brain-dead donors [48]. Conversely, rapid BSD induction in rodent [51] and pig [52] studies identified progressive and significant elevations in serum IL-1β and TNF-α (during the rat study, the initial spike of serum TNF-α decreased after 1-h post-BSD). The levels of TNF-α and IL-1β in the lung did not change over the 6-h of our BSD study (Fig. 4A), consistent with recent studies from larger, higher level mammals such as brain-dead pigs, which showed that the levels of TNF-α and IL-1β expression in the lung did not significantly change [53,54]. However, previous studies detect significant increases in TNF-α and IL-1β mRNA in lungs rejected for transplant [55] as well as in rodent [56] and in porcine [19] BSD models. The divergence in results may be due to organ-to-organ variations in the expression of these cytokines after BSD or variations between individuals, which may account for the organ-specific variation in outcomes after transplantations as shown previously [19,54]. Interestingly, our model diverges from the rat model of BSD in which the rat model shows up-regulation of macrophage in both the injured brain and the peripheral organs up to 4 [30] and 6-h after induction of brain death [57], whereas no difference was seen in our ovine model. The divergence may be due to different BSD models (rat vs. sheep, rodent lungs different to sheep [58]), ventilation, haemodynamic management/optimisation and hormone resuscitation which can ultimately affect the temporal inflammatory profile [32,57].
Endothelins
A significant elevation of plasma big ET-1 concentrations has been detected throughout the BSD induction (Fig. 5A), whereas plasma ET-1 levels were unchanged during BSD (Fig. 5B). The peak concentrations were seen at 1- and 6-h for ET-1 and big ET-1, respectively. We have previously shown an initial increase in plasma big ET-1 and ET-1 up to 6 h post-BSD in ovine BSD model, followed by normalisation over 24 h [10]. Others have shown that ET-1 peaks as early as 30 min in canine model of BSD [59]. These findings reflect the possibility of an early release with rapid clearance of ET-1 [60], implying that plasma ET-1 could have been cleared before sampling at 2-h post BSD. While the concentrations of ET-1 and big ET-1 were low in BAL fluid, ET-1 levels trended to increase with the progression of BSD (Fig. 5D). Our data is consistent with previous findings that showed unchanged expression levels of ET-1 in lung samples obtained from pig BSD model [53]. However, Salama et al. have shown that the ET-1 mRNA overexpression in donor's lung tissue (whose primary insult was trauma) contributes to primary graft dysfunction development [61]. The differences observed across studies may be due to the shorter half-life of ET-1 (rapid clearing by the ET receptor in the lungs), and the ability to potentiate catecholamine-mediated vasoconstriction may complicate delineation of this pathway profile in brain death [62]. This clearing effect could also explain the low concentrations of ET-1 in BAL fluid observed in our study. It has been shown that ET-1 contributes to oxidative damage, through activated neutrophils, in severe brain injury and BSD [[63], [64], [65]]. However, unchanged levels of chloramine and reduced thiol concentrations were observed in our early-phase of BSD model, which could possibly be due to a slower acute oxidative damage process in this BSD model. A clear picture for the role of the endothelin axis in BSD remains to be elucidated and requires further attention, since conflicting data shows opposing changes in both serum and lung tissue.
Cardiac damage as a consequence of BSD
Echocardiographs revealed that BSD caused a significant reduction in the myocardial strain in the circumferential axis (Fig. 7A) as well as chamber dilation with increased end-systolic area measurements (Fig. 7E). Contractile function significantly declined post-BSD as seen by a reduction in the fractional area change (Fig. 7F). Our data is consistent with recent findings, using a rat model of BSD, that show similar elevation in end-diastolic pressure over the 6-h of their study [66]. Our data also demonstrates a significant elevation in both plasma ANP and cTnI concentrations after BSD induction (Fig. 6), in agreement with previous studies in brain-dead patients [38,67] and animal models of BSD [39,68]. Interestingly, a previous BSD study in pigs identified a reduction in ANP over time. The authors raised the possible hypothesis that intact brain function is required for ANP release [39]. The differences in the results we observed may be due to different BSD models, since acute brain death induction was used in their study. Further studies are required to delineate changes in ANP after BSD prior to its use as a prognostic tool for donor heart selection.
Pro-inflammatory cytokines, TNF α, IL-6 and IL-1β, have been implicated in developing heart failure [69]. Interestingly, Plenz et al. have shown that donor heart dysfunction early after transplantation has been associated with mRNA expression of IL-6 [70]. Moreover, Skrabal et al. have detected an increase in IL-6 mRNA in the hearts of BD animals, but not in other donor organs such as lung and kidney [19]. Although we did not measure IL-6 expression in the heart, it is quite possible that the elevated IL-6 in the circulation plays a role in cardiac dysfunction in our study.
Study limitations
Several important limitations have been observed in the current study, with lack of control being the major limitation. However, our TRALI ovine model indicates that the marked cytokine profiles after BSD were associated with persistent inflammation throughout the study. In addition, a previous study by our group has shown that instrumentation does not cause significant effect on endothelial axis and cardiac injury biomarkers (myoglobin and creatine kinase MB isoenzyme) [10]. Another limitation was no direct evidence indicate that any of the biochemical inflammatory changes are responsible for changes seen in cardiac and pulmonary function. However, pharmacological interference with the pro-inflammatory response may represent a useful option for the treatment of damaged lung grafts [71]. Furthermore, sampling times may have missed very early release of ET-1, therefore, early sampling in future studies is recommended. Moreover, further investigation of cytokine expression (mRNA) in donor organs tissues will better characterise pro-inflammatory response after BSD. Finally, ANP and cTnI were chosen for cardiac injury assessment in this study but the correlation between cTnI levels and recipient outcome remains controversial [72]. A recent study recommended inclusion of creatine kinase (CK), a heart injury biomarker, in the assessment of donor hearts for transplantation [73].
Conclusion
In our ovine model of BSD, we conclude that BSD induced systemic pro-inflammatory responses, characterized by increased neutrophil infiltration and cytokine production in the circulation and BAL fluid. Deleterious effects occurred on both pulmonary endothelial and cardiac functions. These pathophysiological changes could lead to graft dysfunction in the donor organ. These findings have potential clinical implications in developing specific strategies to intervene neutrophil/ET-1 mediated inflammation in BSD donor prior to organ transplantation.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Conflicts of interest
The authors have declared that there are no conflicts of interest.
Funding
This study is supported by the University of Queensland, Metro North Hospital and Health Service. It was funded by the National Health and Medical Research Council (1079421) CRE ACTIONS, and the Health Innovation, Investment and Research Office of Queensland Health.
Acknowledgement
We acknowledge the generous contribution of Professor Christopher Pemberton, Sara Raudsepp and Anthony Mitchell (Christchurch Heart Institute) for ANP analysis.
Footnotes
Peer review under responsibility of Chang Gung University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bj.2021.10.007.
Contributor Information
K. Walweel, Email: k.walweel@uq.edu.au.
J.Y. Suen, Email: j.suen1@uq.edu.au.
J.F. Fraser, Email: fraserjohn001@gmail.com.
Appendix ASupplementary data
The following is the Supplementary data to this article:
References
- 1.Linden P.K. History of solid organ transplantation and organ donation. Crit Care Clin. 2009;25:165–184. doi: 10.1016/j.ccc.2008.12.001. ix. [DOI] [PubMed] [Google Scholar]
- 2.Pratschke J., Wilhelm M.J., Kusaka M., Basker M., Cooper D.K., Hancock W.W., et al. Brain death and its influence on donor organ quality and outcome after transplantation. Transplantation. 1999;67:343–348. doi: 10.1097/00007890-199902150-00001. [DOI] [PubMed] [Google Scholar]
- 3.Busson M., N'Doye P., Benoit G., Hannoun L., Adam R., Pavie A., et al. Donor factors influencing organ transplant prognosis. Transplant Proc. 1995;27:1662–1664. [PubMed] [Google Scholar]
- 4.Li S., Wang S., Murugan, Al-Khafaji A., Lebovitz D.J., Souter M., et al. Donor biomarkers as predictors of organ use and recipient survival after neurologically deceased donor organ transplantation. J Crit Care. 2018;48:42–47. doi: 10.1016/j.jcrc.2018.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Israni A.K., Zaun D., Bolch C., Rosendale J.D., Snyder J.J., Kasiske B.L. Deceased organ donation. Am J Transplant. 2016;16 Suppl 2:195–215. doi: 10.1111/ajt.13673. [DOI] [PubMed] [Google Scholar]
- 6.Machado C. Diagnosis of brain death. Neurol Int. 2010;2:e2. doi: 10.4081/ni.2010.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barklin A. Systemic inflammation in the brain-dead organ donor. Acta Anaesthesiol Scand. 2009;53:425–435. doi: 10.1111/j.1399-6576.2008.01879.x. [DOI] [PubMed] [Google Scholar]
- 8.Murray K.N., Parry-Jones A.R., Allan S.M. Interleukin-1 and acute brain injury. Front Cell Neurosci. 2015;9:18. doi: 10.3389/fncel.2015.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stiegler P., Sereinigg M., Puntschart A., Bradatsch A., Seifert-Held T., Wiederstein-Grasser I., et al. Oxidative stress and apoptosis in a pig model of brain death (BD) and living donation (LD) J Transl Med. 2013;11:244. doi: 10.1186/1479-5876-11-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Watts R.P., Bilska I., Diab S., Dunster K.R., Bulmer A.C., Barnett A.G., et al. Novel 24-h ovine model of brain death to study the profile of the endothelin axis during cardiopulmonary injury. Intensive Care Med Exp. 2015;3:31. doi: 10.1186/s40635-015-0067-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Watts R.P., Thom O., Fraser J.F. Inflammatory signalling associated with brain dead organ donation: from brain injury to brain stem death and posttransplant ischaemia reperfusion injury. J Transplant. 2013;2013:521369. doi: 10.1155/2013/521369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McKeating E.G., Andrews P.J., Signorini D.F., Mascia L. Transcranial cytokine gradients in patients requiring intensive care after acute brain injury. Br J Anaesth. 1997;78:520–523. doi: 10.1093/bja/78.5.520. [DOI] [PubMed] [Google Scholar]
- 13.Barklin A., Larsson A., Vestergaard C., Koefoed-Nielsen J., Bach A., Nyboe R., et al. Does brain death induce a pro-inflammatory response at the organ level in a porcine model? Acta Anaesthesiol Scand. 2008;52:621–627. doi: 10.1111/j.1399-6576.2008.01607.x. [DOI] [PubMed] [Google Scholar]
- 14.Mertes P.M., el Abassi K., Jaboin Y., Burtin P., Pinelli G., Carteaux J.P., et al. Changes in hemodynamic and metabolic parameters following induced brain death in the pig. Transplantation. 1994;58:414–418. doi: 10.1097/00007890-199408270-00004. [DOI] [PubMed] [Google Scholar]
- 15.Hotamisligil G.S. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 16.Rech T.H., Crispim D., Rheinheimer J., Barkan S.S., Osvaldt A.B., Grezzana Filho T.J., et al. Brain death-induced inflammatory activity in human pancreatic tissue: a case-control study. Transplantation. 2014;97:212–219. doi: 10.1097/TP.0b013e3182a949fa. [DOI] [PubMed] [Google Scholar]
- 17.Ott L., McClain C.J., Gillespie M., Young B. Cytokines and metabolic dysfunction after severe head injury. J Neurotrauma. 1994;11:447–472. doi: 10.1089/neu.1994.11.447. [DOI] [PubMed] [Google Scholar]
- 18.Barklin A., Larsson A., Vestergaard C., Kjaergaard A., Wogensen L., Schmitz O., et al. Insulin alters cytokine content in two pivotal organs after brain death: a porcine model. Acta Anaesthesiol Scand. 2008;52:628–634. doi: 10.1111/j.1399-6576.2008.01606.x. [DOI] [PubMed] [Google Scholar]
- 19.Skrabal C.A., Thompson L.O., Potapov E.V., Southard R.E., Joyce D.L., Youker K.A., et al. Organ-specific regulation of pro-inflammatory molecules in heart, lung, and kidney following brain death. J Surg Res. 2005;123:118–125. doi: 10.1016/j.jss.2004.07.245. [DOI] [PubMed] [Google Scholar]
- 20.Weiss S., Kotsch K., Francuski M., Reutzel-Selke A., Mantouvalou L., Klemz R., et al. Brain death activates donor organs and is associated with a worse I/R injury after liver transplantation. Am J Transplant. 2007;7:1584–1593. doi: 10.1111/j.1600-6143.2007.01799.x. [DOI] [PubMed] [Google Scholar]
- 21.Sillesen M., Rasmussen L.S., Jin G., Jepsen C.H., Imam A., Hwabejire J.O., et al. Assessment of coagulopathy, endothelial injury, and inflammation after traumatic brain injury and hemorrhage in a porcine model. J Trauma Acute Care Surg. 2014;76:12–19. doi: 10.1097/TA.0b013e3182aaa675. discussion 19-20. [DOI] [PubMed] [Google Scholar]
- 22.Avlonitis V.S., Fisher A.J., Kirby J.A., Dark J.H., et al. Pulmonary transplantation: the role of brain death in donor lung injury. Transplantation. 2003;75:1928–1933. doi: 10.1097/01.TP.0000066351.87480.9E. [DOI] [PubMed] [Google Scholar]
- 23.Strieter R.M., Kunkel S.L. Acute lung injury: the role of cytokines in the elicitation of neutrophils. J Invest Med. 1994;42:640–651. Erratum in: J Investig Med 1995;43:204. [PubMed] [Google Scholar]
- 24.Fisher A.J., Donnelly S.C., Hirani N., Haslett C., Strieter R.M., Dark J.H., et al. Elevated levels of interleukin-8 in donor lungs is associated with early graft failure after lung transplantation. Am J Respir Crit Care Med. 2001;163:259–265. doi: 10.1164/ajrccm.163.1.2005093. [DOI] [PubMed] [Google Scholar]
- 25.Fisher A.J., Donnelly S.C., Hirani N., Burdick M.D., Strieter R.M., Dark J.H., et al. Enhanced pulmonary inflammation in organ donors following fatal non-traumatic brain injury. Lancet. 1999;353:1412–1413. doi: 10.1016/S0140-6736(99)00494-8. [DOI] [PubMed] [Google Scholar]
- 26.Scozzi D., Ibrahim M., Menna C., Krupnick A.S., Kreisel D., Gelman A.E. The role of neutrophils in transplanted organs. Am J Transplant. 2017;17:328–335. doi: 10.1111/ajt.13940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meyer K.C. Neutrophils, myeloperoxidase, and bronchiectasis in cystic fibrosis: green is not good. J Lab Clin Med. 2004;144:124–126. doi: 10.1016/j.lab.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 28.Liao Y., Liu P., Guo F., Zhang Z.Y., Zhang Z. Oxidative burst of circulating neutrophils following traumatic brain injury in human. PLoS One. 2013;8 doi: 10.1371/journal.pone.0068963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leber B., Stadlbauer V., Stiegler P., Stanzer S., Mayrhauser U., Koestenbauer S., et al. Effect of oxidative stress and endotoxin on human serum albumin in brain-dead organ donors. Transl Res. 2012;159:487–496. doi: 10.1016/j.trsl.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 30.Sutherland A.J., Ware R.S., Winterford C., Fraser J.F. The endothelin axis and gelatinase activity in alveolar macrophages after brain-stem death injury: a pilot study. J Heart Lung Transplant. 2007;26:1040–1047. doi: 10.1016/j.healun.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 31.Fagan K.A., McMurtry I.F., Rodman D.M. Role of endothelin-1 in lung disease. Respir Res. 2001;2:90–101. doi: 10.1186/rr44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Avlonitis V.S., Wigfield C.H., Kirby J.A., Dark J.H. The hemodynamic mechanisms of lung injury and systemic inflammatory response following brain death in the transplant donor. Am J Transplant. 2005;5:684–693. doi: 10.1111/j.1600-6143.2005.00755.x. [DOI] [PubMed] [Google Scholar]
- 33.Kowalczyk A., Kleniewska P., Kolodziejczyk M., Skibska B., Goraca A. The role of endothelin-1 and endothelin receptor antagonists in inflammatory response and sepsis. Arch Immunol Ther Exp (Warsz) 2015;63:41–52. doi: 10.1007/s00005-014-0310-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Owen V.J., Burton P.B., Michel M.C., Zolk O., Böhm M., Pepper J.R., et al. Myocardial dysfunction in donor hearts. A possible etiology. Circulation. 1999;99:2565–2570. doi: 10.1161/01.cir.99.19.2565. [DOI] [PubMed] [Google Scholar]
- 35.Birks E.J., Yacoub M.H., Burton P.S., Owen V., Pomerance A., O'Halloran A., et al. Activation of apoptotic and inflammatory pathways in dysfunctional donor hearts. Transplantation. 2000;70:1498–1506. doi: 10.1097/00007890-200011270-00018. [DOI] [PubMed] [Google Scholar]
- 36.Tahsili-Fahadan P., Geocadin R.G. Heart-brain Axis: effects of neurologic injury on cardiovascular function. Circ Res. 2017;120:559–572. doi: 10.1161/CIRCRESAHA.116.308446. [DOI] [PubMed] [Google Scholar]
- 37.Bruegger D., Jacob M., Rehm M., Loetsch M., Welsch U., Conzen P., et al. Atrial natriuretic peptide induces shedding of endothelial glycocalyx in coronary vascular bed of Guinea pig hearts. Am J Physiol Heart Circ Physiol. 2005;289:H1993–H1999. doi: 10.1152/ajpheart.00218.2005. [DOI] [PubMed] [Google Scholar]
- 38.Potapov E.V., Wagner F.D., Loebe M., Ivanitskaia E.A., Müller C., Sodian R., et al. Elevated donor cardiac troponin T and procalcitonin indicate two independent mechanisms of early graft failure after heart transplantation. Int J Cardiol. 2003;92:163–167. doi: 10.1016/s0167-5273(03)00083-4. [DOI] [PubMed] [Google Scholar]
- 39.Potapov E.V., Blömer T., Michael R., Hennig F., Müller C., Loebe M., et al. Effect of acute brain death on release of atrium and B-type natriuretic peptides in an animal model. Transplantation. 2004;77:985–990. doi: 10.1097/01.tp.0000119165.32200.1a. [DOI] [PubMed] [Google Scholar]
- 40.Chemonges S., Shekar K., Tung J.P., Dunster K.R., Diab S., Platts D., et al. Optimal management of the critically ill: anaesthesia, monitoring, data capture, and point-of-care technological practices in ovine models of critical care. BioMed Res Int. 2014;2014:468309. doi: 10.1155/2014/468309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bouquet M., Passmore M.R., See Hoe L.E., Tung J.P., Simonova G., Boon A.C., et al. Development and validation of ELISAs for the quantitation of interleukin (IL)-1β, IL-6, IL-8 and IL-10 in ovine plasma. J Immunol Methods. 2020;486:112835. doi: 10.1016/j.jim.2020.112835. [DOI] [PubMed] [Google Scholar]
- 42.Passmore M.R., Fung Y.L., Simonova G., Foley S.R., Dunster K.R., Diab S.D., et al. Inflammation and lung injury in an ovine model of extracorporeal membrane oxygenation support. Am J Physiol Lung Cell Mol Physiol. 2016;311:L1202–L1212. doi: 10.1152/ajplung.00296.2016. [DOI] [PubMed] [Google Scholar]
- 43.Yandle T.G., Espiner E.A., Nicholls M.G., Duff H. Radioimmunoassay and characterization of atrial natriuretic peptide in human plasma. J Clin Endocrinol Metab. 1986;63:72–79. doi: 10.1210/jcem-63-1-72. [DOI] [PubMed] [Google Scholar]
- 44.Hawkins C.L., Morgan P.E., Davies M.J. Quantification of protein modification by oxidants. Free Radic Biol Med. 2009;46:965–988. doi: 10.1016/j.freeradbiomed.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 45.Dypbukt J.M., Bishop C., Brooks W.M., Thong B., Eriksson H., Kettle A.J. A sensitive and selective assay for chloramine production by myeloperoxidase. Free Radic Biol Med. 2005;39:1468–1477. doi: 10.1016/j.freeradbiomed.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 46.Tung J.P., Fung Y.L., Nataatmadja M., Colebourne K.I., Esmaeel H.M., Wilson K., et al. A novel in vivo ovine model of transfusion-related acute lung injury (TRALI) Vox Sang. 2011;100:219–230. doi: 10.1111/j.1423-0410.2010.01381.x. [DOI] [PubMed] [Google Scholar]
- 47.Shivalkar B., Van Loon J., Wieland W., Tjandra-Maga T.B., Borgers M., Plets C., et al. Variable effects of explosive or gradual increase of intracranial pressure on myocardial structure and function. Circulation. 1993;87:230–239. doi: 10.1161/01.cir.87.1.230. [DOI] [PubMed] [Google Scholar]
- 48.Damman J., Nijboer W.N., Schuurs T.A., Leuvenink H.G., Morariu A.M., Tullius S.G., et al. Local renal complement C3 induction by donor brain death is associated with reduced renal allograft function after transplantation. Nephrol Dial Transplant. 2011;26:2345–2354. doi: 10.1093/ndt/gfq717. [DOI] [PubMed] [Google Scholar]
- 49.Lattmann T., Hein M., Horber S., Ortmann J., Teixerira M.M., Souza D.G., et al. Activation of pro-inflammatory and anti-inflammatory cytokines in host organs during chronic allograft rejection: role of endothelin receptor signaling. Am J Transplant. 2005;5:1042–1049. doi: 10.1111/j.1600-6143.2005.00807.x. [DOI] [PubMed] [Google Scholar]
- 50.Avlonitis V.S., Wigfield C.H., Kirby J.A., Dark J.H. Treatment of the brain-dead lung donor with aprotinin and nitric oxide. J Heart Lung Transplant. 2010;29:1177–1184. doi: 10.1016/j.healun.2010.05.024. [DOI] [PubMed] [Google Scholar]
- 51.Atkinson C., Varela J.C., Tomlinson S. Complement-dependent inflammation and injury in a murine model of brain dead donor hearts. Circ Res. 2009;105:1094–1101. doi: 10.1161/CIRCRESAHA.109.194977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhu C., Li J., Zhang G., Zhang Y., Zhai W., Shi J., et al. Brain death disrupts structure and function of pig liver. Transplant Proc. 2010;42:733–736. doi: 10.1016/j.transproceed.2010.02.028. [DOI] [PubMed] [Google Scholar]
- 53.Valenza F., Coppola S., Froio S., Ruggeri G.M., Fumagalli J., Villa A.M., Rosso L., et al. A standardized model of brain death, donor treatment, and lung transplantation for studies on organ preservation and reconditioning. Intensive Care Med Exp. 2014;2:12. doi: 10.1186/2197-425X-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Belhaj A., Dewachter L., Rorive S., Remmelink M., Weynand B., Melot C., et al. Mechanical versus humoral determinants of brain death-induced lung injury. PLoS One. 2017;12:e0181899. doi: 10.1371/journal.pone.0181899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cypel M., Kaneda H., Yeung J.C., Anraku M., Yasufuku K., de Perrot M., et al. Increased levels of interleukin-1beta and tumor necrosis factor-alpha in donor lungs rejected for transplantation. J Heart Lung Transplant. 2011;30:452–459. doi: 10.1016/j.healun.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 56.Pilla E.S., Pereira R.B., Forgiarini Junior L.A., Forgiarini L.F., Paludo Ade O., Kulczynski J.M., et al. Effects of methylprednisolone on inflammatory activity and oxidative stress in the lungs of brain-dead rats. J Bras Pneumol. 2013;39:173–180. doi: 10.1590/S1806-37132013000200008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Takada M., Nadeau K.C., Hancock W.W., Mackenzie H.S., Shaw G.D., Waaga A.M., et al. Effects of explosive brain death on cytokine activation of peripheral organs in the rat. Transplantation. 1998;65:1533–1542. doi: 10.1097/00007890-199806270-00001. [DOI] [PubMed] [Google Scholar]
- 58.Jobe A.H. Animal models, learning lessons to prevent and treat neonatal chronic lung disease. Front Med (Lausanne) 2015;2:49. doi: 10.3389/fmed.2015.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Oishi Y., Nishimura Y., Tanoue Y., Kajihara N., Imasaka K., Morita S., et al. Endothelin-1 receptor antagonist prevents deterioration of left ventricular function and coronary flow reserve in brain-dead canine heart. J Heart Lung Transplant. 2005;24:1354–1361. doi: 10.1016/j.healun.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 60.Parker J.D., Thiessen J.J., Reilly R., Tong J.H., Stewart D.J., Pandey A.S. Human endothelin-1 clearance kinetics revealed by a radiotracer technique. J Pharmacol Exp Therapeut. 1999;289:261–265. [PubMed] [Google Scholar]
- 61.Salama M., Andrukhova O., Hoda M.A., Taghavi S., Jaksch P., Heinze G., et al. Concomitant endothelin-1 overexpression in lung transplant donors and recipients predicts primary graft dysfunction. Am J Transplant. 2010;10:628–636. doi: 10.1111/j.1600-6143.2009.02957.x. [DOI] [PubMed] [Google Scholar]
- 62.Fukuroda T., Kobayashi M., Ozaki S., Yano M., Miyauchi T., Onizuka M., et al. Endothelin receptor subtypes in human versus rabbit pulmonary arteries. J Appl Physiol. 1994;76:1976–1982. doi: 10.1152/jappl.1994.76.5.1976. [DOI] [PubMed] [Google Scholar]
- 63.Schuurs T.A., Morariu A.M., Ottens P.J., 't Hart N.A., Popma S.H., Leuvenink H.G., et al. Time-dependent changes in donor brain death related processes. Am J Transplant. 2006;6:2903–2911. doi: 10.1111/j.1600-6143.2006.01547.x. [DOI] [PubMed] [Google Scholar]
- 64.Hohl A., da Silva Gullo J., Silva C.C.P., Bertotti M.M., Felisberto F., Nunes J.C., et al. Plasma levels of oxidative stress biomarkers and hospital mortality in severe head injury: a multivariate analysis. J Crit Care. 2012;27 doi: 10.1016/j.jcrc.2011.06.007. 523.e11-9. [DOI] [PubMed] [Google Scholar]
- 65.Nayak C., Nayak D., Bhat S., Raja A., Rao A. Relationship between neurological outcome and early oxidative changes in erythrocytes in head injury patients. Clin Chem Lab Med. 2007;45:629–633. doi: 10.1515/CCLM.2007.123. [DOI] [PubMed] [Google Scholar]
- 66.Sato M., Yamanaka H., Iwasaki M., Miyata Y., Kamibayashi T., Fujino Y., et al. Altered phosphatidylinositol 3-kinase and calcium signaling in cardiac dysfunction after brain death in rats. Ann Thorac Surg. 2016;102:556–563. doi: 10.1016/j.athoracsur.2016.02.014. [DOI] [PubMed] [Google Scholar]
- 67.Riou B., Dreux S., Roche S., Arthaud M., Goarin J.P., Léger P., et al. Circulating cardiac troponin T in potential heart transplant donors. Circulation. 1995;92:409–414. doi: 10.1161/01.cir.92.3.409. [DOI] [PubMed] [Google Scholar]
- 68.Ryan J.B., Wilson M.K., Hicks M., Nicholson A., Kesteven S.H., Junius F., et al. A brain dead donor model of porcine orthotopic cardiac transplantation for assessment of cardiac allograft preservation. Heart Lung Circ. 2000;9:78–81. doi: 10.1046/j.1444-2892.2000.00028.x. [DOI] [PubMed] [Google Scholar]
- 69.Mann D.L. Innate immunity and the failing heart: the cytokine hypothesis revisited. Circ Res. 2015;116:1254–1268. doi: 10.1161/CIRCRESAHA.116.302317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Plenz G., Eschert H., Erren M., Wichter T., Böhm M., Flesch M., et al. The interleukin-6/interleukin-6-receptor system is activated in donor hearts. J Am Coll Cardiol. 2002;39:1508–1512. doi: 10.1016/s0735-1097(02)01791-6. [DOI] [PubMed] [Google Scholar]
- 71.Walweel K., Skeggs K., Boon A.C., Hoe L.E., Bouquet M., Obonyo N.G., et al. Endothelin receptor antagonist improves donor lung function in an ex vivo perfusion system. J Biomed Sci. 2020;27:96. doi: 10.1186/s12929-020-00690-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dronavalli V.B., Banner N.R., Bonser R.S. Assessment of the potential heart donor: a role for biomarkers? J Am Coll Cardiol. 2010;56:352–361. doi: 10.1016/j.jacc.2010.02.055. [DOI] [PubMed] [Google Scholar]
- 73.Kilic A., Emani S., Sai-Sudhakar C.B., Higgins R.S., Whitson B.A. Donor selection in heart transplantation. J Thorac Dis. 2014;6:1097–1104. doi: 10.3978/j.issn.2072-1439.2014.03.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.