Abstract
Objectives
The Mitochondrial Unfolded Protein Response (UPRmt) is a compartment-specific mitochondrial quality control (MQC) mechanism that uses the transcription factor ATF5 to induce the expression of protective enzymes to restore mitochondrial function. Acute exercise is a stressor that has the potential to temporarily disrupt organellar protein homeostasis, however, the roles of ATF5 and the UPRmt in maintaining basal mitochondrial content, function and exercise-induced MQC mechanisms in skeletal muscle are not known.
Methods
ATF5 KO and WT mice were examined at rest or after a bout of acute endurance exercise. We measured protein content in whole muscle, nuclear, cytosolic and mitochondrial fractions, in addition to mRNA transcript levels in whole muscle. Using isolated mitochondria, we quantified rates of oxygen consumption and ROS emission to observe the effects of the absence of ATF5 on organelle function.
Results
ATF5 KO mice exhibited a larger and less functional muscle mitochondrial pool, most likely a culmination of enhanced biogenesis via increased PGC-1 expression, and attenuated mitophagy. The absence of ATF5 resulted in a reduction in antioxidant proteins and increases in mitochondrial ROS emission, cytosolic cytochrome c, and the expression of mitochondrial chaperones. KO muscle also displayed enhanced exercise-induced stress kinase signaling, but a blunted mitophagic and UPRmt gene expression response, complemented by significant increases in the basal mRNA abundance and nuclear localization of ATF4. Instead of promoting its nuclear translocation, acute exercise caused the enrichment of ATF5 in mitochondrial fractions. We also identified PGC-1 as an additional regulator of the basal expression of UPRmt genes.
Conclusion
The transcription factor ATF5 retains a critical role in the maintenance of mitochondrial homeostasis and the appropriate response of muscle to acute exercise for the optimization of mitochondrial quality control.
Keywords: Skeletal muscle, Mitochondria, Mitochondrial unfolded protein response (UPRmt), Protein homeostasis, Exercise, Mitochondrial quality control
Graphical abstract
Highlights
-
•
A lack of ATF5 increases mitochondrial content but reduces function.
-
•
Absence of ATF5 triggers a compensatory increase in PGC-1α and ATF4 expression.
-
•
ATF5 is required for the normal mitophagic and UPRmt mRNA response to exercise.
-
•
ATF5 optimizes the quality of the mitochondrial pool in skeletal muscle.
Abbreviations
- ANOVA
Analysis of variance
- ARE
ATF5-specific response element
- ATF
Activating transcription factor
- ATFS-1
Activating transcription factor associated with stress-1
- ATG7
Autophagy-related protein 7
- bZIP
Basic leucine zipper
- C/EBP
CCAAT-enhancer binding protein
- CCA
Chronic contractile activity
- cDNA
Complimentary DNA
- CHOP
C/EBP homologous protein
- ClpP
Caseinolytic mitochondrial matrix peptidase proteolytic subunit
- COX
Cytochrome c oxidase
- Cpn10
Chaperonin 10 (10 kDa heat shock protein)
- CPT1b
Carnitine palmitoyl transferase 1 b
- eIF2α
Eukaryotic translation-initiation factor-2 alpha
- ER
Endoplasmic reticulum
- ETC
Electron transport chain
- FA
Fatty acids
- FUNDC1
FUN14 domain containing 1
- GAPDH
Glyceraldehyde-3-phosphate dehydrogenase
- GPx1
Glutathione peroxidase 1
- HAF-1
ABC (ATP Binding Cassette) transporter
- H2DCF-DA
2′,7′ dichlorodihydrofluorescein diacetate
- HO-1
Heme oxygenase-1
- HRP
Horseradish peroxidase
- HSP
Heat shock protein
- HSP60
60 kDa heat shock protein
- H2B
Histone 2 B
- IMF
Intermyofibrillar
- IMM
Inner mitochondrial membrane
- IMS
Intermembrane space
- ISR
Integrated Stress Response
- JNK
c-jun N-terminal kinase
- KO
Knockout
- kDa
Kilodaltons
- LAMP1
Lysosomal-associated membrane protein 1
- LC3
Microtubule-associated proteins 1 A/1 B light chain 3 A
- LDHA
Muscle-specific lactate dehydrogenase
- LONP1
Lon protease 1
- MnSOD
Manganese-dependent superoxide dismutase
- mRNA
Messenger RNA
- mtDNA
Mitochondrial DNA
- mtHSP70
75 kDA mitochondrial heat shock protein
- mPTP
Mitochondrial permeability transition pore
- NQO1
NAD(P)H quinone dehydrogenase 1
- NR
Nicotinamide riboside
- NuGEMPs
Nuclear genes encoding mitochondrial proteins
- OMM
Outer mitochondrial membrane
- OSNs
Olfactory sensory neurons
- OXPHOS
Oxidative phosphorylation
- PCR
Polymerase chain reaction
- PFK-1
Phosphofructokinase-1
- PGC-1α
Peroxisome proliferator activator receptor (PPAR) γ coactivator-1 alpha
- PIM
Protein import machinery
- PKR
Protein kinase RNA-activated
- PQC
Protein quality control
- RNA
Ribonucleic acid
- ROS
Reactive oxygen species
- SDS-PAGE
Sodium dodecyl sulfate polyacrylamide gel electrophoresis
- SS
Subsarcolemmal
- TFAM
Mitochondrial transcription factor A
- uORF
Upstream open reading frame
- UPR
Unfolded protein response
- UPRER
Endoplasmic reticulum UPR
- UPRmt
Mitochondrial UPR
- V-ATPase
Vacuolar-type ATPase
- VDAC1
Voltage-dependent anion channel 1
- WT
Wild-type
1. Introduction
Skeletal muscle is an extremely malleable tissue and is able to alter its structural, physiological and metabolic phenotype in response to altered external demands. This “plasticity” of muscle [1] is due in part to the adaptability of the mitochondrial reticulum, being a large contributor to improvements in muscle endurance resulting from regular exercise [2,3]. Mitochondria not only produce cellular energy, but they also lie at the crossroads of a multitude of bidirectional signaling cascades with the nucleus. This “retrograde” (mitochondria-to-nuclear) communication induces specific transcriptional programs to monitor and preserve the organelle network [4,5], and serves as an important component of the mitochondrial quality control (MQC) systems. These include biogenesis (synthesis), mitophagy (degradation), antioxidant capacity and the maintenance of intra-organellar protein homeostasis [6,7].
Muscle contractions during voluntary exercise promote mitochondrial biogenesis and mitophagy, enhancing the quality of the mitochondrial pool [2,[8], [9], [10], [11]]. At the onset of exercise, early signaling events are initiated, converging in part on the activation of the transcriptional co-activator PGC-1 [[12], [13], [14], [15]]. This “master regulator” of organelle biogenesis coordinates the transcription of mitochondrial genes encoded by the nuclear genome (NuGEMPs), which require import into the mitochondria via specialized protein import machinery (PIM) components [[16], [17], [18]]. The mitochondrial proteome consists of ∼1200 proteins, and over 99% of these are transcribed in the nucleus, while less than 1% are encoded by mtDNA [19]. The regulation of these proteins, including their proper import, translation, folding, and degradation is referred to as protein homeostasis or “proteostasis” [20,21]. As an integral component of MQC, adequate proteostasis is critical for sustaining mitochondrial function, as dysregulation is linked to a variety of pathological conditions [22].
The status of the mitochondrial protein folding environment is reliant on the equilibrium between the abundance of internal misfolded proteins and the organelle's intrinsic folding capacity. This is determined by the amount of chaperone and protease enzymes, which possess the dedicated functions of refolding and degrading misfolded proteins, respectively. Unaccustomed exercise may elicit a proteostatic imbalance, generated by an increase in misfolded proteins leading to acute organellar proteotoxic stress [23,24], ratifying a need for protein quality control strategies. As an adaptive mechanism, the cell activates a compartment-specific transcriptional program called the mitochondrial unfolded protein response (UPRmt) [[25], [26], [27]], named in relation to the UPRER [28]. Utilizing novel mitochondria-to-nuclear communication, the transcription of UPRmt genes, including mitochondrial-specific chaperones and proteases, are increased to restore proteostasis within the organelle [[29], [30], [31]]. UPRmt activation and the adequate expression of MQC enzymes are a critical adaptive mechanism for the maintenance of organelle quality and homeostasis [[32], [33], [34]].
Activating transcription factor 5 (ATF5) is a major regulator of the UPRmt in mammalian cells, promoting the transcriptional induction of downstream UPRmt targets during mitochondrial stress [35]. Basally, ATF5 is degraded in the mitochondrion, similar to ATFS-1 in C. elegans [28,29], while under stressful conditions, ATF5 exhibits retrograde translocation to the nucleus [35]. As part of the ATF/CREB family, ATF5 retains a DNA-binding domain allowing it to interact with the promoters of UPRmt target genes, in addition to a bZIP domain facilitating its heterodimerization with other bZIP transcription factors [36,37]. Primarily abundant in the liver, ATF5 has been investigated in a plethora of cellular conditions. It is responsive during amino acid starvation [[38], [39], [40]], in mediating cell survival and proliferation in cancer and neurodegeneration [[41], [42], [43], [44]], and in retaining an influential role in the maturation of olfactory sensory neurons (OSNs) [45]. However, evidence is beginning to emerge, supporting its role in mediating stress responses in muscle. ATF5 is upregulated in skeletal muscle during -adrenergic stimulation [46], in mitochondrial myopathy [47], in myoblasts upon impaired protein translation [47], and during differentiation [48]. Furthermore, ATF5 has been found to be integral in UPRmt activation and rescuing of mitochondrial function upon cardiac insult [[49], [50], [51]], providing opportunistic evidence for pursuing ATF5 function and UPRmt activation in skeletal muscle.
The molecular mechanisms governing mitochondrial biogenesis in response to exercise are complex, and seminal work is beginning to reveal potential roles that acute mitochondrial proteotoxicity and the UPRmt may have in mediating changes in cell signaling upon contractile activity [[52], [53], [54]]. Despite these findings, no work has focused on the role of ATF5 in the maintenance of basal mitochondrial homeostasis in muscle, or its function in mediating the UPRmt in response to acute exercise. Therefore, we utilized WT and ATF5 KO mice to examine 1) the influence of ATF5 on mitochondrial content and function, 2) whether ATF5 is required for various MQC processes including biogenesis, mitophagy, antioxidant capacity, apoptosis, and 3) the role of ATF5 in exercise-induced UPRmt activation and mitochondrial biogenesis signaling.
2. Materials and methods
2.1. Animals
ATF5 whole-body KO mice were generated by crossing ATF5tm1(KOMP) (Velocigene Project 11,612) heterozygotes in a C57BL6/N background, generously provided by Dr. Stavros Lomvardas from Columbia University, with FVB WT females. Animals were housed in a 12:12-h light–dark cycle and given food and water ad libitum. To genotype progeny, ear clippings were obtained from each animal to make crude DNA extracts. They were subsequently mixed with JumpStart REDTaq polymerase (P0982, Sigma), forward and reverse primers (50 M) (Table S1) for the WT and altered ATF5 gene, and subjected to amplification by PCR. Reaction products were run on a 1% agarose gel and visualized with the use of ethidium bromide. Experimental animals were used at 5.5–7 months of age and separated into either Control (CON) or Exercise (EX) groups (n = 9–13/group). For the PGC-1 KO experiments, TA muscles were extracted from WT and whole-body PGC-1 KO mice at 4–5 months of age [55], snap-frozen in liquid nitrogen and stored at −80C for later analysis by qPCR (n = 3–5/group).
2.2. Exercise protocols
Two different acute exercise protocols were used in this study, where mice performed one-time exercise of either a continuous, submaximal bout or an exhaustive exercise test. All exercised animals ran at a fixed 10% slope and were acclimatized to the treadmill for three days prior to exercising. The first was a bout of acute continuous exercise, where mice ran at a pace of 15 m/min for 60 min, followed by 18 m/min for 30 min (90 min total). The second was acute exhaustive exercise, which was completed as follows: 0 m/min for 5 min, 5 m/min for 5 min 10 m/min for 5 min, 15 m/min for 5 min, 20 m/min for 5 min, 25 m/min for 5 min, increasing the speed by 1 m/min every 3 min until exhaustion. Exhaustion was defined as the animal remaining on the shock pad for 10 s, despite encouragement to run. Using a small tail bleed, blood lactate was measured with a Lactate Scout+ analyzer (EKF Diagnostics). The tissues were collected immediately after exercise and stored at −80C for later protein, mRNA, enzyme analyses, or used fresh for cellular fractionations.
2.3. Acute in situ muscle stimulation
The in situ muscle stimulation protocol was performed as described previously [56]. The right gastrocnemius was attached to a force transducer by the Achilles tendon and stimulated indirectly via innervation of the sciatic nerve while being maintained at a temperature of 37C. Both twitch and tetanic contractions (6 V, 100 Hz, 100 msec duration) were assessed, and the data were expressed per milligram of gastrocnemius muscle mass. Twitch kinetics measured included maximum twitch force, time to peak tension, and half relaxation time.
2.4. Cytochrome c oxidase activity
Activity of the cytochrome c oxidase (COX) enzyme was used as a marker of mitochondrial content in muscle. Using a portion of the TA, tissues were placed in COX enzyme extraction buffer (100 mM Na–K-Phosphate, 2 mM EDTA, pH 7.2) on ice and diluted 40-fold. They were subsequently organized in metal brackets that were stored at −20C and homogenized with stainless steel beads at 30 Hz using a TissueLyser II (Qiagen). Samples were lysed twice for 1 min, followed by three to five 30-second rounds until fully homogenized. A test solution containing 20 mg of horse heart cytochrome c (C2506, Sigma) was prepared and incubated at 30°C. Using a multipipette, 240 l of the test solution was added to 50 l of whole muscle homogenate in a 96-well plate. The maximal oxidation rate of cytochrome c was then measured spectrophotometrically in a Synergy HT (Bio-Tek Instruments) plate reader, analyzing the change in absorbance at 550 nm and temperature of 30°C. For each sample, the COX activity measurement was determined as an average of three trials.
2.5. Nuclear and cytosolic fractionation
Nuclear and cytosolic fractions were prepared from one freshly extracted whole TA, using NE-PER Extraction Reagents (38,835, Thermo Fisher Scientific) supplemented with phosphatase and protease inhibitors. Approximately ∼30–50 mg of tissue was minced on ice and homogenized in CER-I. Homogenates were then left to stand on ice for 10 min. Following the addition of CER-II, samples were briefly vortexed and centrifuged at 16,000 g for 10 min at 4C. The supernatant (cytosolic fraction) was collected. The remaining pellets, containing nuclei and cellular debris, were washed 3 times in cold 1 × PBS and resuspended in NER. Nuclear fractions were then sonicated 3 times for 3 s each and incubated on ice for 40 min. Samples were vortexed every 10 min during the incubation, then underwent centrifugation at 16, 000 g for 10 min. The resulting supernates (nuclear fractions) were collected. Both cytosolic and nuclear subfractions were stored in −80C until further analysis.
2.6. RNA isolation and reverse transcription
Approximately 50–70 mg of lysed gastrocnemius muscle tissue was combined with TRIzol® reagent (15,596,018, Life Technologies) and mixed with chloroform. Samples were centrifuged at 16,000 g for 15 min at 4°C, and the upper aqueous phase was transferred into a new tube with isopropanol and left overnight at −20°C to precipitate. Samples were once again centrifuged at 16,000 g for 10 min. The resulting supernatant fraction was discarded, and the pellet was suspended in 30 l of molecular grade sterile H2O. RNA concentrations and purities were measured using the NanoDrop 2000 (Thermo Fisher Scientific). The Superscript III Reverse Transcriptase enzyme (Invitrogen) was used to reverse-transcribe 1.5 g of RNA into cDNA.
2.7. mRNA expression using real-time PCR
The mRNA expression of ATF5, ATF4, CHOP, PGC-1, COX-IV, HSP60, LONP1, mtHSP70 and ClpP was measured using the 7500 Real-Time PCR system (Applied Biosystems Inc.) and SYBR Green qPCR Master Mix (B21203, BiMake) in a 96-well plate. For normalization of transcript levels, GAPDH and -2 Microglobulin were used as housekeeping genes for the ATF5 KO experiments, while -2 Microglobulin and ATF4 were used in the PGC-1 study. Each well contained SYBR green, forward and reverse primers for the gene of interest (GOI) (20 M) (Table S1), 10 ng of cDNA and sterile H2O to yield a final reaction volume of each well of 25 l. Primer optimizations were run beforehand to control for primer dimers and nonspecific amplification by analyzing melt curves generated by the instrument. All samples were run in duplicates in tandem with negative control wells that contained sterile H2O in the place of cDNA.
2.8. Real-time PCR quantification
For the quantification of mRNA levels, the threshold cycle (CT) value of the GOI was first subtracted from the average CT value of both endogenous reference genes to obtain the CT value for the GOI: CT = CT (GOI) - CT (reference). Next, the CT value of the exercised tissue (EX) was subtracted from the CT value of the control tissue (CON) for each genotype to obtain the CT values for the WT EX, KO CON, and KO EX samples: CT = CT (EX) - CT (CON). Results are expressed as fold changes above mRNA levels of WT CON animals using the CT method, calculated as 2-CT.
2.9. Immunoblotting
Protein extracts prepared from a portion of the gastrocnemius muscle or other tissues, as well as isolated mitochondrial subfractions were separated on 10–15% SDS-PAGE gels via electrophoresis at 120 V for approximately ∼90 min, and subsequently transferred onto nitrocellulose membranes, stained with Ponceau Red and cut at the appropriate molecular weights. Blots were blocked in 5% skim milk or 5% BSA for phosphorylated proteins in TBS-T solution (25 mM Tris–HCl, 1 mM NaCl, 0.1% Tween-20, pH 7.5) for 1 h at room temperature with gentle agitation. They were then coated with the appropriate primary antibodies (Table S2) and incubated overnight at 4°C. Membranes were then washed 3 × 5 min in TBS-T and incubated with HRP-linked secondary antibodies for 1 h at room temperature. After another series of wash steps, blots were visualized with enhanced chemiluminescence using an iBright CL1500 Imaging System (Thermo Fisher Scientific). Quantifications were carried out using ImageJ (NIH) software and normalized to the corresponding loading controls or Ponceau. Corrected values were additionally normalized over a ‘Standard’ whole muscle sample that was run on every gel. For the cytochrome c blots, cytochrome c was probed for in cytosolic fractions isolated from the muscle of WT and ATF5 KO animals. Quantified values were corrected for VDAC and normalized for PFK-1. Approximate molecular weights in kDa (kilodaltons) of proteins are indicated on each blot.
2.10. Mitochondrial isolations
Approximately 700–1000 mg of fresh skeletal muscle tissue (one gastrocnemius, two quadriceps, and two triceps) was extracted from anesthetized animals, placed in ice-cold buffer, and subsequently minced and homogenized. Mitochondrial isolations were performed as previously described [57]. To separate SS and IMF mitochondria, homogenates were subjected to differential centrifugation at 800 g for 10 min. The IMF fraction was briefly treated with the Nagarse protease from Bacillus lichenformis (P5380, Sigma) to liberate the mitochondrial pool. After additional centrifugation steps, both SS and IMF pellets were suspended in resuspension buffer (100 mM KCl, 10 mM MOPS, 0.2% BSA pH of 7.4). Protein concentrations were determined using the Bradford method. Fresh mitochondrial fractions were utilized immediately to measure respiration and ROS emission.
2.11. Mitochondrial respiration
Oxygen consumption (O2/mg/min) over time in SS and IMF mitochondria was measured using a Clark Electrode (Strathkelvin Instruments). A small volume of either the SS or IMF fraction (50 l) was incubated with 250 l of VO2 buffer (250 mM sucrose, 50 mM KCl, 25 mM Tris base, 10 mM K2HPO4, pH 7.4) while being continuously stirred at 30°C. Respiration rates were determined (nanoatoms O2/min/mg) in the presence of 10 mM glutamate for State IV (passive) respiration, and 0.44 mM ADP for State III (active) respiration. Finally, NADH was added to test mitochondrial membrane integrity.
2.12. Mitochondrial ROS emission
SS and IMF mitochondria (75 g) were incubated in a black polystyrene 96-well plate with VO2 buffer and 50 mM H2DCF-DA (D399, Thermo Fisher Scientific) at 37°C for 30 min. The fluorescence emission (480–520 nm) was measured in a Synergy HT (Biotek) plate reader using Gen5 software. ROS emission was assessed with the addition of 10 mM glutamate in the absence (State IV) or presence (State III) of 0.44 mM ADP. Data were normalized to the corresponding respiration rates.
2.13. Statistical analysis
Data were analyzed using GraphPad Prism 8.0 software and values are reported as means SEM. Basal comparisons of WT and KO animals were carried out with unpaired Student's t-tests. A 2-way ANOVA was used in the comparisons of exercise conditions and genotypes, followed by a Bonferroni post-hoc test where necessary. Statistical significance was set at P < 0.05.
3. Results
3.1. ATF5 KO mice do not exhibit differences in muscle contractile properties or exercise performance
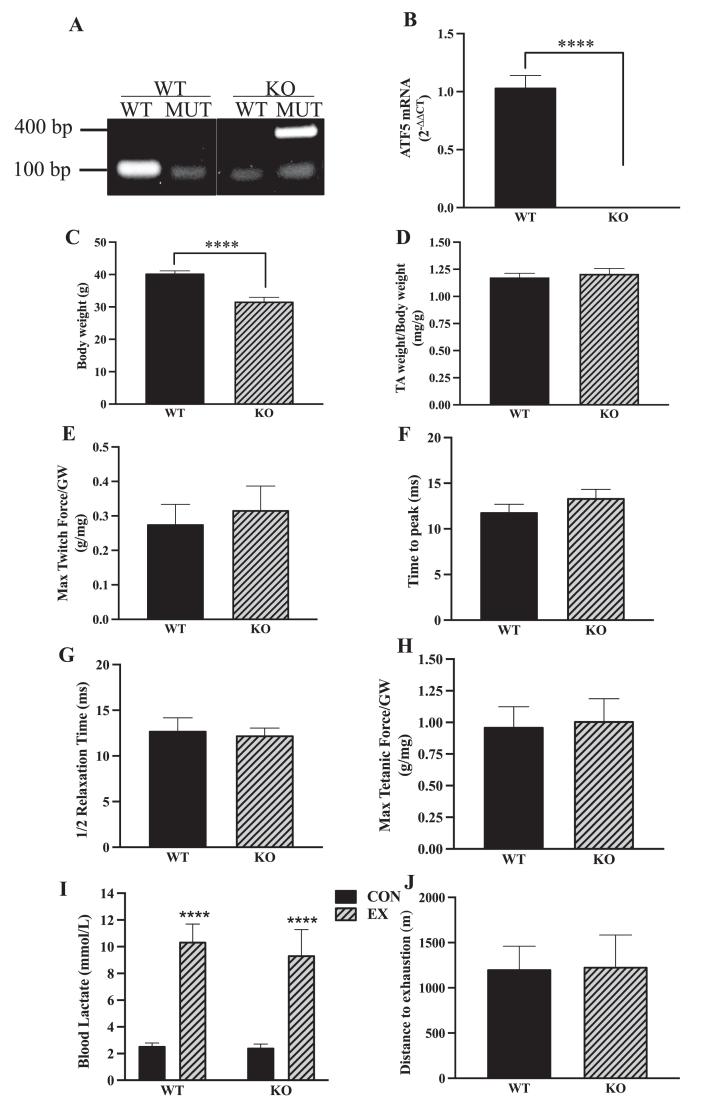
The ATF5 KO mouse model was confirmed with DNA genotyping, through the presence of the mutant ATF5 gene and the absence of the WT gene (Figure 1A) [50]. It was additionally confirmed with the abolishing of the ATF5 transcript in KO muscle (P < 0.0001) (Fig. 1B). The phenotypic characteristics of ATF5 KO mice have been described, in part, previously [50]. In our study, deletion of the ATF5 gene resulted in a reduction in body weight of approximately 21% (P < 0.0001) (Fig. 1C) without any changes occurring in relative muscle mass, observed by expressing TA weight per unit of body weight (Fig. 1D). Further characterization of the ATF5 KO phenotype indicated no difference in the muscle weights of gastrocnemius, soleus or heart between genotypes, but a 40% reduction in the weights of the epididymal fat pad (P = 0.08) was evident in comparison to age-matched WT mice (Fig. S1). In WT animals, ATF5 protein expression is abundant in striated muscles, including the gastrocnemius, soleus and heart. ATF5 content was higher in the gastrocnemius muscle relative to the more oxidative soleus muscle and heart tissues (P < 0.05) (Fig. S2). Acute stimulation of the gastrocnemius was performed to assess the contractile properties of muscle from WT and ATF5 KO mice. Twitch kinetics including maximum force (Fig. 1E), time to peak tension (Fig. 1F) and half relaxation time (Fig. 1G), in addition to maximum tetanic force (Fig. 1H) were not significantly different between genotypes. To examine the involvement of ATF5 in determining exercise performance, WT and ATF5 KO mice were subjected to an incremental, exhaustive exercise bout. Animals were run to exhaustion, indicated by 3.8-4-fold increases in blood lactate post-exercise (P < 0.0001) (Fig. 1I). No differences in exercise tolerance were observed between genotypes, measured through distance to exhaustion in the exhaustive exercise bout (Fig. 1J).
Figure 1.
ATF5 KO animals exhibit reduced body weights with no changes in muscle contractile properties, exercise tolerance or performance. The whole-body ATF5 KO mouse model was confirmed through A) DNA genotyping targeting the ATF5 WT (100 bp) and MUT (400 bp) alleles with designated primer sets and B) measuring ATF5 mRNA levels with qPCR. (WT: n = 16, KO: n = 23). Phenotypic characteristics of the genotypes were assessed, including C) Body weight and D) TA weight normalized for body weight, (WT: n = 18, KO: n = 26–27). Twitch kinetics were assessed with E) Maximum twitch force expressed as grams of force per milligram of muscle weight (g/mg), F) Time to peak and G) Half relaxation time (1/2 R T) in milliseconds (ms). Muscle force was further examined with H) Maximum tetanic force (g/mg) (n = 5). I) Blood lactate levels in CON and EX animals subjected to acute exhaustive exercise (n = 4–13). J) Exercise performance was observed from distance to exhaustion recordings from an incremental exhaustive exercise test on the treadmill (n = 4–7). ∗∗∗∗P < 0.0001, unpaired t-test. bp, base pairs; CON, control; EX, exercised; GW, gastrocnemius weight; KO, knockout; MUT, mutant; WT, wild-type.
3.2. Basal protein expression of select UPRmt chaperones is augmented with no changes in autophagy and lysosomal proteins in the absence of ATF5
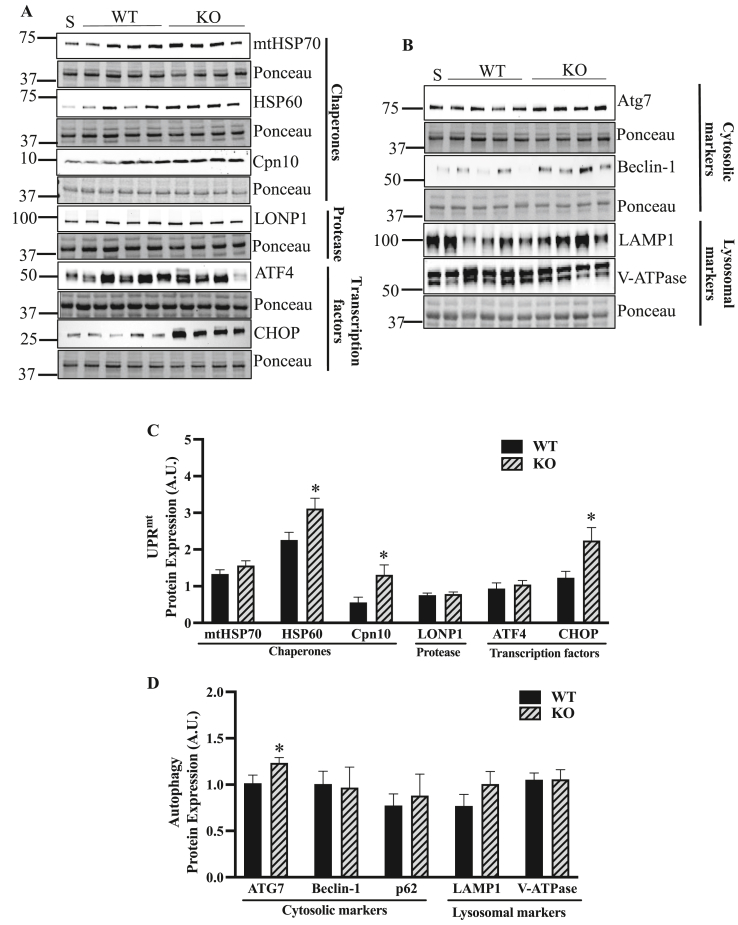
Since it has been suggested that ATF5 is required for the expression of its downstream UPRmt targets during mitochondrial stress [35], it was of interest to investigate the requirement of ATF5 in the basal expression of these proteins. Interestingly, expression of the UPRmt chaperones HSP60 and Cpn10 was upregulated in the muscle of ATF5 KO animals by 1.4-fold (P < 0.05) and 2.4-fold (P < 0.05), respectively, along with a 1.8-fold increase in the UPRmt transcription factor CHOP (P < 0.05) (Figure 2A,C). However, no changes were observed in protein expression of the chaperone mtHSP70, the protease LONP1 or the transcription factor ATF4. Probing for autophagy and lysosomal markers to evaluate any basal differences in protein content between genotypes, we observed a 22% increase in ATG7 protein (P < 0.05) in KO animals, with no changes in Beclin-1 or the lysosomal markers LAMP1 and V-ATPase (Figure 2B,D).
Figure 2.
Increased protein expression of select UPRmt markers in the absence of ATF5 with no changes in autophagy or lysosomal proteins in whole muscle. A) Representative blots of UPRmt proteins in whole muscle with corresponding Ponceau stains. Proteins include chaperones mtHSP70, HSP60, Cpn10, the protease LONP1, and transcription factors ATF4 and CHOP. B) Representative blots of basal autophagy proteins in whole muscle, including cytosolic markers Atg7, Beclin-1 and lysosomal markers LAMP1 and V-ATPase with Ponceau stains. Corresponding quantifications of C) UPRmt and D) autophagy and lysosomal proteins corrected for Ponceau (n = 8–10). ∗P < 0.05, unpaired t-test. A. U., arbitrary units; KO, knockout; S, standard; WT, wild-type.
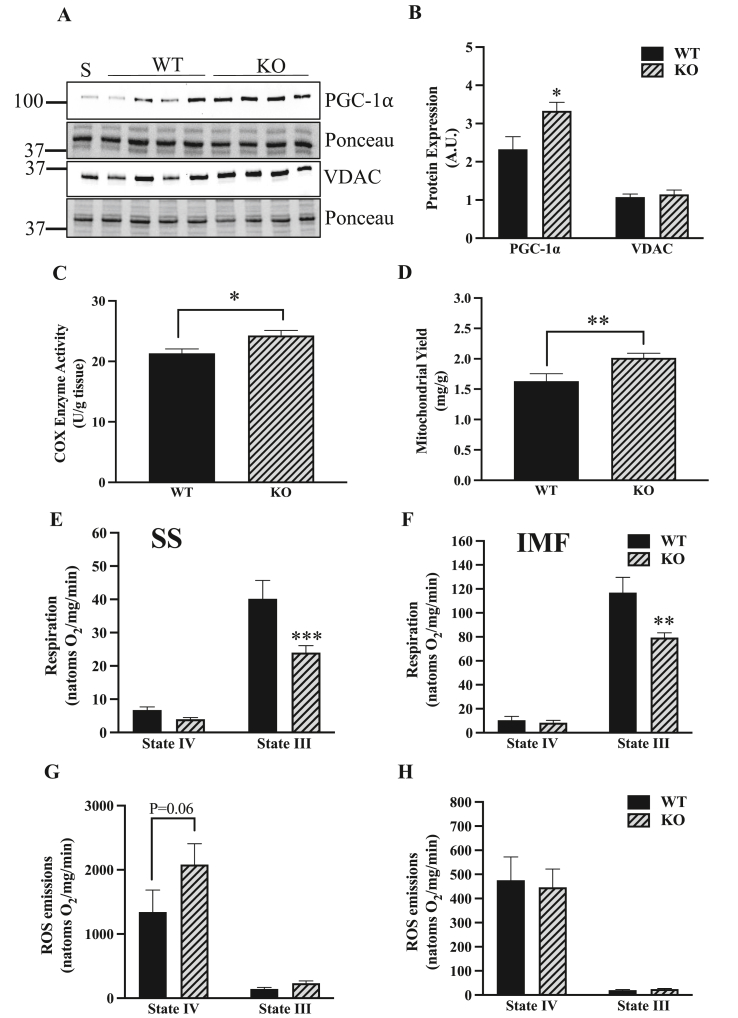
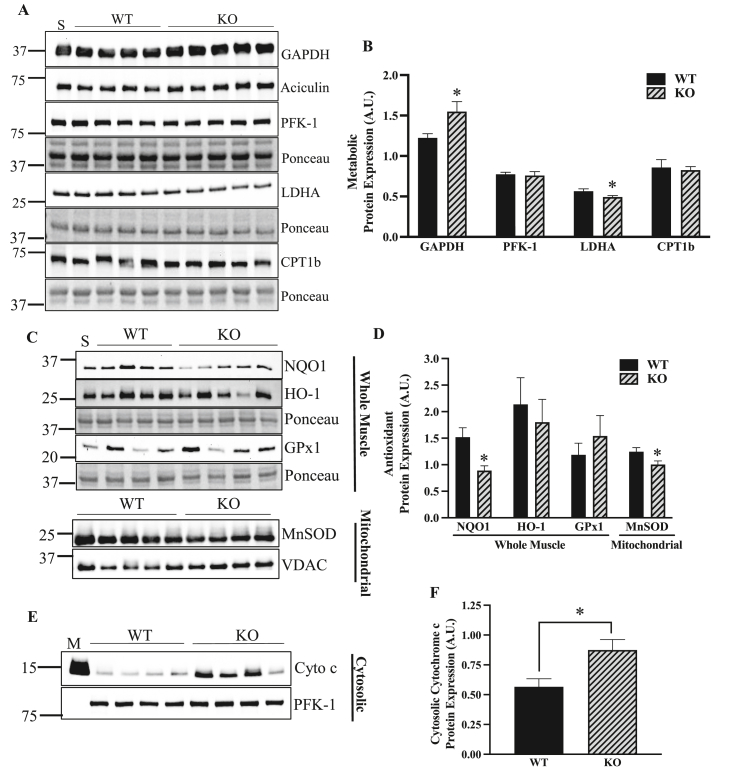
3.3. The muscle of ATF5 KO animals displays enhanced mitochondrial content with reduced basal function
To analyze the influence of ATF5 on the maintenance of the mitochondrial pool, mitochondrial content was measured via protein content and enzyme activity analyses. In the muscle of ATF5 KO mice, there was a 43% increase in whole muscle PGC-1 protein (P < 0.05) with no change in VDAC (Figure 3A,B), indicating an increased drive for mitochondrial biogenesis. We also measured COX enzyme activity and performed mitochondrial yield calculations from mitochondrial isolations. KO muscle exhibited 14% greater COX activity relative to WT muscle (P < 0.05) (Fig. 3C) and an increased mitochondrial yield of 24% (P < 0.01) (Fig. 3D). We then investigated how the absence of ATF5 influences organelle function, as ATF5 is known to be required for the maintenance of basal mitochondrial function in HEK 293T cells in culture [35]. To investigate whether this holds true in muscle, oxygen consumption and ROS emission were measured in SS and IMF mitochondria isolated from the skeletal muscle of WT and ATF5 KO animals. In the absence of ATF5, significant reductions in mitochondrial respiration in State III conditions, but not State IV, were observed in both mitochondrial fractions derived from KO muscle. State III respiration was reduced by 40% in SS mitochondria (P < 0.001) (Fig. 3E), and by 32% in the IMF pool (P < 0.01) (Fig. 3F) in comparison to WT animals. Furthermore, SS mitochondria from KO muscle exhibited a trending increase in State IV ROS emission of 55% (P = 0.06) (Fig. 3G) without any changes observed in IMF mitochondria (Fig. 3H).
Figure 3.
The muscle of ATF5 KO mice presents a more abundant mitochondrial pool with reduced organellar function. A) Representative blots in whole muscle of the transcription factor involved in mitochondrial biogenesis PGC-1⍺, the mitochondrial marker VDAC and corresponding Ponceau stains. B) Quantifications of PGC-1⍺ and VDAC corrected for Ponceau (n = 8–10). Mitochondrial content was assessed from C) COX Enzyme Activity values (n = 9–10) and D) Mitochondrial Yields (SS and IMF combined) derived from mitochondrial isolation experiments (WT: n = 18, KO: n = 27). Mitochondrial respiration in E) SS and F) IMF mitochondria expressed in natoms O2/mg/min in both passive (State IV) and active (State III) respiratory conditions. Organelle function was also assessed by measuring ROS emission in G) SS and H) IMF subfractions (n = 18–27). ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001 unpaired t-test, WT vs KO basally or in given respiratory state. A. U., arbitrary units; KO, knockout; S, standard; WT, wild-type.
3.4. The absence of ATF5 yields altered glycolytic enzyme levels, reduced antioxidant protein expression and increased cytosolic cytochrome c
ATF5 is known to have an impact on metabolism, as it regulates adipocyte differentiation and its knockdown in the white adipose tissue of mice improves whole-body insulin sensitivity [58]. To investigate how the absence of ATF5 regulates metabolic enzymes, the protein content of glycolytic enzymes GAPDH, PFK-1, LDHA and the -oxidation enzyme CPT1b was measured in whole muscle protein extracts. The muscle of ATF5 KO mice exhibited a 27% increase in GAPDH (P < 0.05) with a 14% reduction in the LDHA enzyme (P < 0.05) (Figure 4A,B), the LDH isoform responsible for the conversion of pyruvate to lactate. No changes in protein content were observed for PFK-1 or CPT1b. We also measured the levels of the cytosolic antioxidants NQO1, HO-1, GPx1 and the mitochondrial antioxidant MnSOD in SS mitochondrial fractions. In whole muscle, NQO1 protein was reduced by 42% (P < 0.05) in ATF5 KO samples with no changes in HO-1 and GPx1 (Figure 4C,D). Furthermore, a reduction of 19% was observed in MnSOD in the SS pool derived from KO muscle (P < 0.05) (Figure 4C,D). The increases in mitochondrial ROS emission, coupled with these reductions in antioxidant enzymes in KO mice prompted us to question whether there was an increase in mitochondrially-mediated apoptotic signaling. Thus, we probed for cytochrome c in cytosolic samples fractionated from muscles of WT and ATF5 KO animals (Figure 4E,F). Interestingly, there was a 55% increase in cytosolic cytochrome c (P < 0.05), potentially indicating increased mPTP opening and apoptotic signaling in the absence of ATF5.
Figure 4.
ATF5 regulates the expression of glycolytic and antioxidant enzymes, in addition to apoptotic cytochrome c release. A) Representative blots in whole muscle of the glycolytic enzymes GAPDH, PFK-1, LDHA and the β-oxidation protein CPT1b with Ponceau stains. B) Quantifications of the enzymes corrected for loading control or Ponceau (n = 7–10). C) Representative blots of NQO1 and HO-1 in whole muscle samples with respective Ponceau stains, and MnSOD with VDAC in SS mitochondria. D) Quantifications of antioxidant proteins corrected for Ponceau stains or the respective loading control (n = 6–9). E) Representative blot of cytochrome c and PFK-1 in cytosolic samples. F) Quantification of cytosolic cytochrome c protein corrected for PFK-1 (n = 8). An amount of 5 g was loaded to probe for GAPDH and was normalized to Aciculin, while 25 g was used to target the other proteins and were normalized to Ponceau stains. ∗P < 0.05 unpaired t-test. A.U., arbitrary units; M, IMF mitochondrial sample; KO, knockout; S, standard; WT, wild-type.
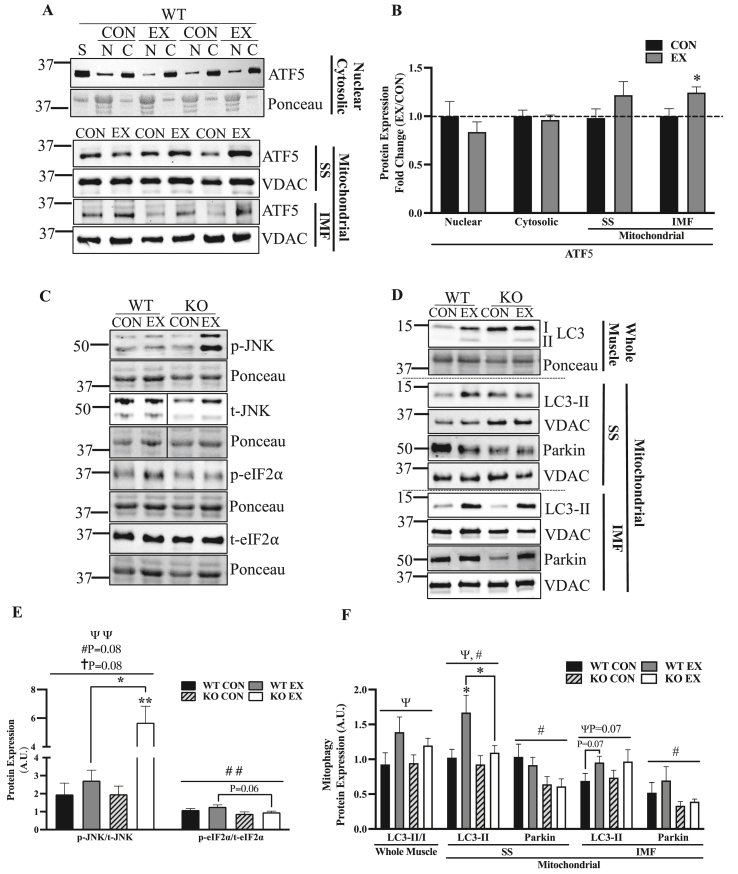
3.5. Acute exercise shifts ATF5 to the mitochondrial compartment, while the muscle of ATF5 KO mice exhibits blunted basal and exercise-induced mitophagy, and enhanced stress signaling with exercise
It has been previously documented in mammalian cells that various forms of mitochondrial stress induce the nuclear translocation of ATF5 [35]. Thus, we sought to investigate whether acute exercise was a sufficient stimulus to alter the cellular localization of ATF5 using western blotting in nuclear, cytosolic and mitochondrial fractions isolated from the muscle of control and exercised WT animals (Figure 5A,B). Probing for ATF5 in nuclear and cytosolic fractions, there was no indication of its nuclear translocation. This prompted us to measure ATF5 levels in mitochondrial fractions, in which ATF5 protein content was enriched in the IMF fraction by 24% (P < 0.05) (Figure 5A,B).
Figure 5.
Acute exercise enhances mitochondrial ATF5 in WT mice, while there is reduced basal mitochondrial Parkin, attenuated exercise-induced mitochondrial LC3-II and enhanced stress signaling with exercise in ATF5 KO animals. A) Representative blots of ATF5 in nuclear, cytosolic and SS and IMF mitochondrial fractions from CON or EX animals subjected to acute exercise (continuous and exhaustive). The blot from nuclear and cytosolic samples is shown with the corresponding Ponceau stain, while that from mitochondrial samples is shown with VDAC. B) Graphical representation of ATF5 protein in different cellular fractions and conditions. Blots corrected for Ponceau or VDAC and displayed as a fold change (EX/CON) (n = 6–9). Protein loaded for each fraction is as follows: Nuclear, 60 g; Cytosolic, 10 g; Mitochondrial, 25 g. Representative blots of C) whole muscle p-JNK, p-eIF2⍺, t-JNK and t-eIF2⍺ and D) autophagy proteins LC3-I, LC3-II and Parkin in whole muscle and SS and IMF mitochondrial samples in CON and EX animals subjected to acute exercise (continuous and exhaustive). Blots are shown with corresponding Ponceau stains or VDAC blots. E) Quantifications of phosphorylated/total for JNK and eIF2⍺ corrected for Ponceau (n = 5–12). F) Quantification of LC3 in whole muscle represented as the LC3-II/I ratio, and LC3-II and Parkin in SS and IMF mitochondria (n = 6–11). P < 0.05, P < 0.01 main effect of exercise; #P < 0.05, ##P < 0.01 main effect of genotype; P < 0.05 interaction effect of exercise and genotype; ∗P < 0.05, ∗∗P < 0.01Bonferroni post-hoc analysis, CON vs EX of same genotype unless otherwise indicated. A. U., arbitrary units; C, cytosolic; CON, control; EX, exercised; KO, knockout; N, nuclear; p, phosphorylated; S, standard; t, total; WT, wild-type. Lines in blot indicate where two different areas were spliced together from the same blot.
The phosphorylation of the subunit of ribosomal eIF2 at Serine 51 is a pivotal event during ISR activation, inhibiting global protein translation and selectively translating mRNAs with uORFs. These include the transcription factors ATF5, ATF4 and CHOP, which induce the transcription of mitochondrial-protective genes in the nucleus. On the other hand, JNK is a kinase that is highly responsive to acute exercise [59] and has been identified to be implicated in UPRmt signaling during mitochondrial stress [60]. Thus, we sought to investigate whether there are discrepancies in acute exercise-induced UPRmt upstream signaling, represented by eIF2 and JNK phosphorylation. While there was no significant effect of acute exercise on eIF2 phosphorylation, KO muscle had 29% less phosphorylated eIF2 relative to WT, with a significant effect of genotype across groups (P < 0.01) (Figure 5C,E). Exercise significantly increased JNK phosphorylation, and the magnitude of the effect appeared to depend on genotype (P = 0.08), displaying a greater increase in KO animals. Post-hoc analyses showed that JNK phosphorylation increased by 2.9-fold (P < 0.01) in KO mice, but only by 1.4-fold in WT muscle (Figure 5C,E).
The response to acute exercise was further examined by investigating exercise-induced mitophagy. The more abundant mitochondrial pool in skeletal muscle of ATF5 KO mice could be a result of the increase in mitochondrial biogenesis indicated by more PGC-1 protein. However, we sought to investigate whether the increase in mitochondrial content could be a product of a reduction in mitochondrial degradation via mitophagy. Thus, we probed for LC3 in whole muscle samples and measured LC3-II and Parkin recruitment to mitochondria basally, and following acute exercise (Figure 5D,F). There was a main effect of acute exercise in the LC3-II/LC3-I ratio in whole muscle (P < 0.05), with increases of 50% and 27% in WT and ATF5 KO mice, respectively. To evaluate exercise-induced mitophagy, we probed for LC3-II in SS and IMF mitochondrial fractions. In SS mitochondria, there were main effects of exercise (P < 0.05) and genotype (P < 0.05) with a 64% increase in mitochondrial LC3-II in WT mice (P < 0.05), but an increase of only 18% in KO animals. In the IMF fraction, there was a trending main effect of exercise (P = 0.07) with a 39% increase in mitochondrial LC3-II in WT samples (P = 0.07) and a similar 32% increase in KO muscle (Figure 5D,F). Although acute exercise did not exert any changes in mitochondrial Parkin, there were significant effects of genotype in both SS and IMF mitochondria (P < 0.05) (Figure 5D,F). In KO muscle, basal mitochondrial Parkin was reduced by 33–38% and by 36–44% in SS and IMF fractions, respectively, when compared to WT muscle.
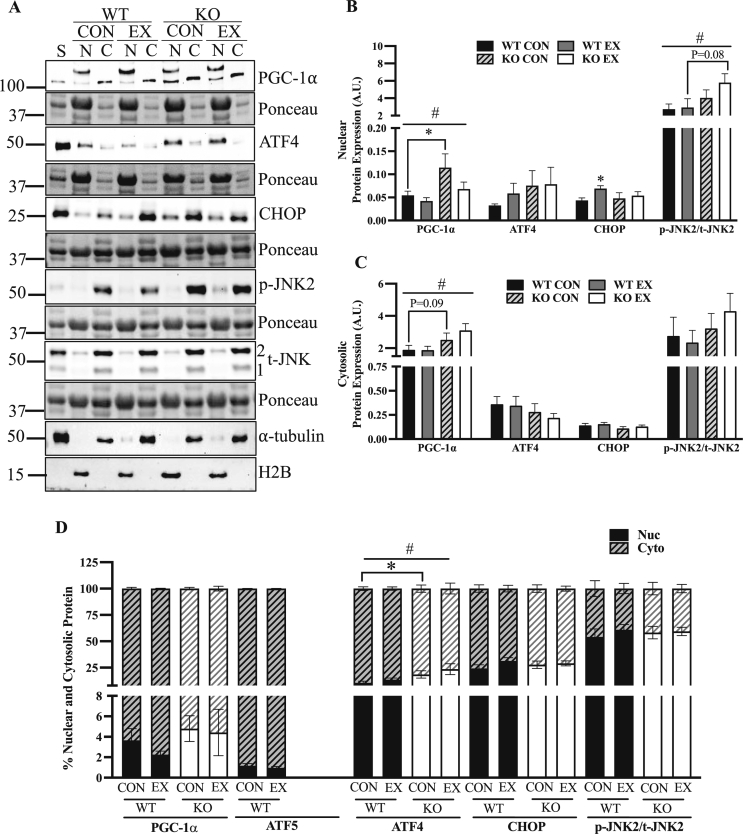
3.6. ATF5 KO muscle exhibits changes in the subcellular localization of PGC-1, ATF4 and phosphorylated JNK2
In addition to whole muscle protein content analysis that was described above, the subcellular localization of PGC-1 ATF4, CHOP and p-JNK2 was measured in nuclear and cytosolic fractions isolated from WT and KO muscle following the exhaustive exercise test. Verification of the validity of the PGC-1α protein band is provided using siRNA knockdown in C2C12 myotubes (Fig. S3). The data show depletion of PGC-1α protein with siRNA treatment at a level of approximately 100 kDa, in line with the band in scrambled-treated myotubes, as well as whole muscle samples and nuclear extracts (not shown). A main effect of genotype was observed for both cellular compartments for PGC-1 protein. Nuclear PGC-1 was enhanced by 2.1-fold basally in the KO animals in comparison to WT counterparts, (P < 0.05) (Figure 6A,B), while collectively, cytosolic PGC-1 was increased by 49% when combining values from both control and exercised animals (P < 0.05) (Figure 6A,C). However, proportions of nuclear PGC-1 relative to total PGC-1 did not differ significantly between genotypes (Fig. 6D). With regards to the cellular localization of ATF4, 10–14% of total ATF4 was present in the nucleus in WT muscle, while KO muscle exhibited its enhanced nuclear translocation, with its nuclear proportions being 19–24% (P < 0.05) (Fig. 6D). We did not observe any influence of acute exercise on the cellular localization of ATF4. However, in WT animals, nuclear CHOP increased by 59% following acute exercise (P < 0.05) but only by 13% in KO mice. We also investigated the cellular localization of phosphorylated JNK2, as total JNK1 did not appear in nuclear fractions (Fig. 6A). Approximately 55–60% of p-JNK2 in the cell was found in the nucleus (Fig. 6D). Although there were no effects of exercise, collectively, the KO animals exhibited 74% more nuclear p-JNK2 than the WT mice (P < 0.05) (Fig. 6B).
Figure 6.
A lack of ATF5 in muscle elicits alterations in the cellular localization of proteins. A) Representative blots of PGC-1⍺, ATF4, CHOP, p-JNK2 (∼54 kDa) and t-JNK2 (∼54 kDa) in nuclear and cytosolic fractions of CON and EX animals subjected to acute exhaustive exercise. Blots are shown with corresponding Ponceau stains and blots of ⍺-tubulin and H2B are also shown as indicators of sample purity. The band just at/below the 100 kDa mark was used to quantify PGC-1α, as in Figure 3. Quantifications shown for B) Nuclear and C) Cytosolic protein corrected for Ponceau stains. D) Cellular translocation data expressed as a percentage of protein in either cellular compartment (n = 4–9). #P < 0.05 main effect of genotype; ∗P < 0.05 Bonferroni post-hoc analysis, CON vs EX of same genotype unless otherwise indicated. A.U., arbitrary units; C, cytosolic; CON, control; EX, exercised; KO, knockout; N, nuclear; p, phosphorylated; S, standard; t, total; WT, wild-type.
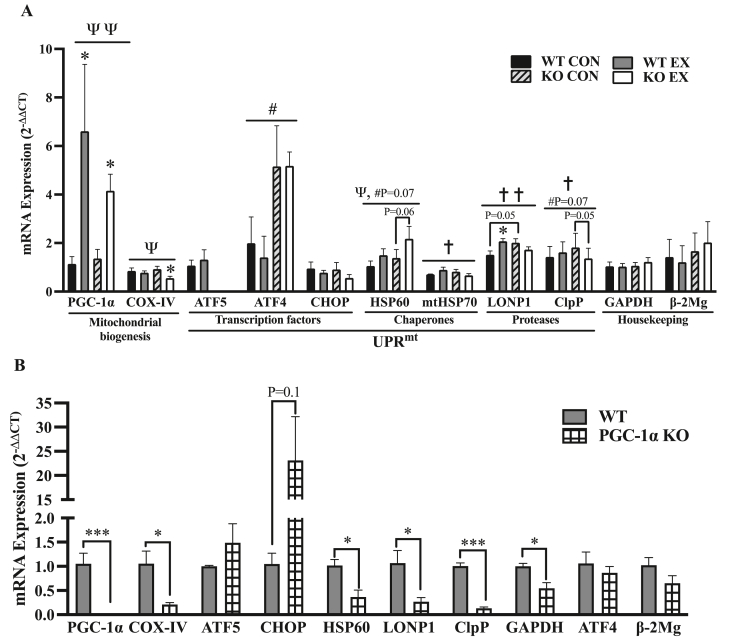
3.7. ATF5 KO animals exhibit attenuated exercise-induced UPRmt signaling, while PGC-1 KO mice display reduced basal expression of UPRmt genes
In mammalian cells in culture and in cardiac injury in rodents, ATF5 is known to be required for the stress-induced transcriptional activation of its downstream targets, including HSP60, LONP1 and mtHSP70 [35,50]. Thus, we investigated whether ATF5 KO animals exhibit impaired transcriptional signaling through the measurement of mRNA levels in control animals, as well as those subjected to a bout of continuous endurance exercise, with tissues collected immediately post-exercise (Figure 7A). As an indicator of mitochondrial biogenesis signaling, there was a main effect of exercise (P < 0.01) as PGC-1 mRNA was enhanced in both animal models after acute exercise, increasing by 5.8-fold (P < 0.05) and 3.1-fold (P < 0.05) in WT and KO animals, respectively (Fig. 7A). A main effect of exercise was also observed for COX-IV mRNA (P < 0.05), as a 40% reduction was observed in KO muscle post-exercise (P < 0.05) with no changes occurring in WT animals (Fig. 7A).
Figure 7.
ATF5 is required for the increase in UPRmt mRNAs with acute exercise, while PGC-1⍺ coordinates their basal expression. A) Quantification of mRNA levels of genes involved in the mitochondrial gene expression response to acute continuous exercise. Targeted genes include mitochondrial biogenesis markers PGC-1⍺, COX-IV; UPRmt transcription factors ATF5, ATF4, CHOP; UPRmt chaperones HSP60, mtHSP70; UPRmt proteases LONP1 and ClpP (n = 4–5). GAPDH and β2-Microglobulin served as housekeeping genes. P < 0.05, P < 0.01 main effect of exercise; #P < 0.05 main effect of genotype; P < 0.05, P < 0.01 interaction effect of exercise and genotype; ∗P < 0.05 Bonferroni post-hoc analysis, CON vs EX of same genotype unless otherwise indicated. B) Quantification of basal mRNA levels of mitochondrial and UPRmt genes in WT and PGC-1⍺ KO muscle. PGC-1⍺, COX-IV, ATF5, ATF4, CHOP, HSP60, LONP1 and ClpP (n = 3–5). β2-Microglobulin and ATF4 served as housekeeping genes. ∗P < 0.05, unpaired t-test. Values are expressed using the 2−ΔΔCT method. CON, control; EX, exercised; KO, knockout; WT, wild-type.
No significant changes were observed in ATF5 or ATF4 mRNA with exercise. However, ATF4 mRNA was 2.6-fold and 3.7-fold higher in KO control and exercised samples, respectively, relative to WT mice (P < 0.05). No changes in CHOP mRNA were observed between WT and KO mice basally, or with exercise. There was a main effect of exercise for HSP60 mRNA (P < 0.05) as it was increased by 43% and 57% in WT and KO mice, respectively, and was more apparent in the KO animals (P = 0.06). In addition, there was a trending main effect of genotype (P = 0.07), with 32% and 45% more HSP60 mRNA in control and exercised KO animals, respectively.
The gene expression response of UPRmt markers downstream of ATF5 other than HSP60, such as mtHSP70, LONP1 and ClpP, was also evaluated in WT and KO animals. Basal mRNA levels of LONP1 and ClpP were elevated by 33% (P = 0.05) and 27% (P = 0.07) in KO muscle, respectively. In contrast to what was observed with HSP60, the absence of ATF5 in muscle had a divergent response of these UPRmt mRNAs to exercise, culminating in significant interaction effects of exercise and genotype for mtHSP70 (P < 0.05), LONP1 (P < 0.01) and ClpP (P < 0.05). These targets increased by 13–37% in WT animals but were reduced by 14–25% in KO mice.
As the “master regulator” of mitochondrial biogenesis, PGC-1 coordinates the expression of many mitochondrial genes, and has also been shown to mediate increases in genes of the UPRER with acute exercise in mouse muscle [53]. Since PGC-1 protein was upregulated in both nuclear and cytosolic compartments in ATF5 KO animals, we investigated whether PGC-1 coordinates the expression of UPRmt genes by measuring mRNA levels of target genes in WT and PGC-1 KO muscle tissues (Fig. 7B). PGC-1 mRNA was absent in PGC-1 KO mice as expected (P < 0.001), while that of its bona fide downstream target, COX-IV of the electron transport chain, was reduced by 50% (P < 0.05). The glycolytic enzyme GAPDH was also reduced by 46% (P < 0.05) in the absence of PGC-1. Although no significant changes were observed in the expression of the transcription factors ATF5 or ATF4, CHOP appeared to be enhanced by 23-fold in PGC-1 KO muscle (P = 0.1). The downstream UPRmt targets HSP60, LONP1 and ClpP were significantly downregulated in the absence of PGC-1, by 64% (P < 0.05), 43% (P < 0.05) and 87% (P < 0.001), respectively.
4. Discussion
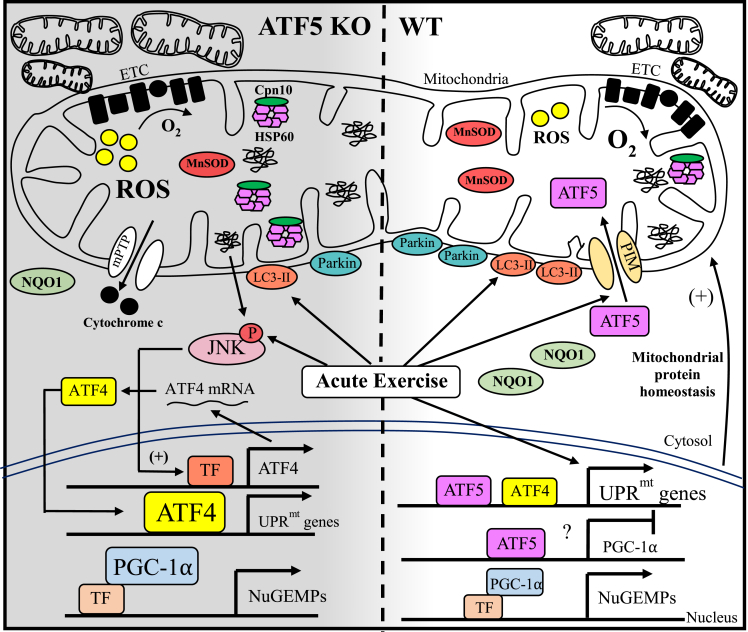
The maintenance of mitochondrial quality via the balance of biogenesis and mitophagy pathways in muscle has received considerable attention in recent years. In contrast, investigations into the contribution of protein quality control mechanisms to the maintenance of organelle function in mammalian organ systems have only recently begun to emerge, specifically with regard to the UPRmt. ATF5 is a transcription factor that has been found to be a mediator of the UPRmt by translocating to the nucleus during various forms of mitochondrial stress to stimulate the transcription of chaperones and proteases [35]. Recent findings have emphasized the importance of an ATF5-dependent UPRmt in rescuing cardiac function during injury [50,51]. In light of this, we questioned whether the requirement of this mechanism is conserved in skeletal muscle during exercise stress. Other groups have shown that there is UPRmt induction with aerobic exercise training [54,61], while our research in this area has shown that an induction of UPRmt occurs in response to muscle contractile activity, and that this actually precedes signaling towards mitochondrial biogenesis in a rodent model [52]. This suggests the importance of upregulating the expression of proteostatic machinery, possibly via the action of ATF5, for mitochondrial adaptations to exercise to occur. Thus, to initiate our foray into the study of the function of ATF5 in skeletal muscle, we used whole-body ATF5 KO mice and we evaluated basal and exercise-induced mitochondrial quality control processes. We appreciate that future studies using conditional muscle-specific knockout or overexpressing transgenic animals will be required to verify the specificity of our findings. Our results indicate that ATF5 is required for the maintenance of basal mitochondrial function, as well as exercise-induced UPRmt mRNA and mitophagic response. Furthermore, ATF5 may have a regulatory role in substrate metabolism, antioxidant capacity and apoptosis, revealing ATF5 to have an influential role in muscle physiology (Figure 8).
Figure 8.
Working model illustrating the altered muscle physiology of ATF5 KO mice. The absence of ATF5 in skeletal muscle yields a more abundant mitochondrial pool composed of organelles that are less functional. This is characterized by reductions in oxygen consumption and enhanced ROS emission in comparison to WT mitochondria. Increases in ROS may be exacerbated by decreases in the expression of the antioxidant enzymes MnSOD and NQO1, inducing an increase in apoptotic cytochrome c release into the cytosol via the mPTP. The increased abundance of nuclear PGC-1 in the absence of ATF5 may be contributing to the transcription of nuclear genes encoding mitochondrial proteins (NuGEMPs) as well as increases in mitochondrial content observed, suggesting that ATF5 may be a negative regulator of PGC-1 in WT conditions. An enlarged mitochondrial pool in KO muscle may also be attributed to decrements in basal mitophagy indicated by reduced mitochondrial Parkin. ATF5 KO animals also exhibit a blunted mitochondrial quality control response to acute exercise stress, with an attenuated induction of mitochondrial LC3-II and the transcription of UPRmt mRNAs. However, the increased mRNA levels and nuclear localization of ATF4 may explain the enhanced expression of chaperones HSP60 and Cpn10 basally in the muscle of ATF5 KO mice. Despite the attenuated mitochondrial response (above), an increased stress kinase signaling was evident post-exercise in these animals, represented by enhanced JNK phosphorylation. The activation of JNK could also result from mitochondrial proteotoxicity to induce the transcription of ATF4 and the increase in ATF4 expression. Finally, acute exercise appears to induce the import of ATF5 into mitochondria, rather than to the nucleus. Solid arrows indicate evidence that signaling is occurring.
Given the function of ATF5 in regulating the UPRmt, we originally hypothesized that the absence of this transcription factor would result in a decrease in mitochondrial content. However, our findings indicate that the muscle of ATF5 KO animals displayed increased mitochondrial content in comparison to WT animals. We then assessed whether these mitochondria were equally functional, by measuring respiration and ROS emission in isolated mitochondria. Our data show that mitochondria from ATF5 KO muscle displayed reduced rates of oxygen consumption, and increases in ROS emission, most likely a product of reduced antioxidant capacity, in comparison to mitochondria from WT animals. These findings support other work that measured mitochondrial respiration in the absence of ATF5 in a mammalian cell model [35], and they indicate that in the absence of ATF5, skeletal muscle possesses a larger mitochondrial pool comprised of more dysfunctional mitochondria. The result of this compensatory adaptation is little to no change in exercise capacity between the two genotypes. Whether this altered mitochondrial content and function is confounded by inherent differences in the habitual activity or inactivity level of the KO animals remains to be determined. However, no differences in muscle mass were found between genotypes, suggesting that marked differences in muscle activity or inactivity were unlikely. This lack of difference was observed despite ATF5 being reported to promote muscle atrophy when overexpressed [62]. However, it is unlikely that ATF5 contributes to muscle fibre type determination, since in situ assessments of contractile properties of the gastrocnemius, including force production and contraction/relaxation time were equal in WT and ATF5 KO mice, indicating no differences in calcium kinetics or slow- and fast-twitch fiber type proportions. The reduction in body weight of the global ATF5 KO mice can be explained, in part, by the reduced size of epididymal fat, a phenomenon that has also been characterized by others [58].
To further investigate the underlying reasons for this increase in organelle content, we examined the expression and localization of PGC-1. We found increased expression in whole muscle samples, enhanced in both the nuclear and cytosolic fractions isolated from ATF5 KO muscle, relative to WT samples. Thus, an increased transcriptional drive for mitochondrial biogenesis in the absence of ATF5 is contributing to a larger mitochondrial pool, albeit composed of more dysfunctional organelles. This indicates that ATF5 may be a negative regulator of PGC-1 and oxidative genes, similar to what has been observed with respect to its fellow bZIP transcription factor ATF4, which has recently been shown to be a negative regulator of Tfam and consequently, the expression of mtDNA-derived proteins [32]. Supporting this, ATFS-1, the nematode homologue of ATF5, is known to negatively regulate the expression of OXPHOS genes encoded by both nuclear and mitochondrial genomes [63]. Interestingly, ATF5 protein expression appears to be inversely related to the oxidative capacity of striated muscles in WT mice, supporting the possibility that ATF5 is a negative regulator of the expression of mitochondrial genes.
An accumulation of poor quality mitochondria in the absence of ATF5 may also be a consequence of impaired mitophagy, the process whereby defective mitochondria are degraded and processed by the lysosomes [64]. The involvement of ATF5 in mitophagy is suggested by the fact that ATF5 levels are increased when mitophagy is inhibited in the myocardium, along with other UPRmt proteins, possibly playing a compensatory role in the maintenance of mitochondrial homeostasis during cardiac stress [65]. Both SS and IMF mitochondrial subfractions isolated from ATF5 KO muscle display reduced levels of Parkin, an E3 ubiquitin ligase involved in mitophagy [9]. Based on this, organelles in ATF5 KO muscle may be less equipped to undergo basal mitophagic degradation, contributing to the enlarged pool of less functional mitochondria. Acute exercise also stimulates the recruitment of LC3-II to induce mitophagy to clear out dysfunctional organellar components of the muscle pool [15,66]. The SS fraction isolated from ATF5 KO muscle displayed attenuated recruitment of LC3-II following acute exercise, a similar phenomenon that we have observed in other animal models [9,15] corresponding with a reduction in mitophagy flux. Thus, in the absence of ATF5, mitochondria are less primed to be cleared via mitophagy to maintain the normal and exercise-induced regulation of mitochondrial quality control. The lack of changes in lysosomal proteins in ATF5 KO muscle suggests that ATF5 influences upstream autophagic signaling, perhaps with respect to autophagosomal formation, instead of the degradative end-stages. In future studies, direct mitophagy flux measurements are warranted to improve our interpretation and to see if the endurance training-induced increase in mitochondrially-localized Parkin that we have previously observed [9] can rescue the apparent stress-induced mitophagy defect observed here.
Among the many cellular events that encompass the physiological response to the stress of acute exercise, the activation (phosphorylation) of stress-inducible kinases is at the forefront of this molecular cascade. Our data show that acute exercise increases JNK activation, a member of the MAPK family of kinases, that has been shown to contribute to increases in PGC-1 gene expression following exercise [12,67,68]. We detected two isoforms of JNK in muscle, JNK1 (∼46 kDa) and JNK2 (∼54 kDa) with similar but yet distinct functions in some signaling pathways [69]. The JNK activation response to acute exercise is exaggerated in ATF5 KO muscle, in addition to its enhanced nuclear abundance. Interestingly, we did not detect JNK1 in the nucleus, and thus, only quantified nuclear JNK2. Regardless, JNK2 is the isoform that is implicated in UPRmt signaling and it is responsive to mitochondrial proteotoxicity, lying downstream of the ISR kinase, PKR [60]. JNK activation is also highly responsive to ROS production [70,71], and could be a consequence of the elevated ROS levels that we observed in SS mitochondria from ATF5 KO muscle. We have shown that enhancing ROS levels induces increases in mitochondrial cytochrome c release and JNK phosphorylation in muscle to induce apoptosis [71]. Thus, the elevations in ROS emission and increased JNK signaling could explain a greater release of cytochrome c in ATF5 KO mice, culminating in its higher abundance in the cytosol. This could indicate an increase in apoptotic activation in ATF5 KO muscle, coinciding with the anti-apoptotic function of ATF5 that has been observed in other studies [43,72]. As an alternative explanation, it is also possible that the greater cytochrome c content in the cytosol is due to a deficit in the mitochondrial protein import pathway in KO mice. Although this remains to be shown directly, this possibility seems unlikely since the expression of protein import machinery components is not reduced in muscle of KO mice (unpublished observations).
In contrast to our initial hypothesis, protein markers previously established to be downstream targets of ATF5, such as mitochondrial chaperones and proteases [35], were increased basally in ATF5 KO muscle. To investigate the reason for this, we studied the expression and localization of ATF4, a well-established transcription factor that regulates the UPRER [73]. Sharing close homology with ATF5, ATF4 controls the transcription of ATF5, is activated by endoplasmic reticulum (ER) and mitochondrial stress [74,75], and its protein levels are upregulated in mammalian cells in the absence of ATF5 [76]. Whether ATF4 truly regulates the transcription of chaperones and proteases of the UPRmt remains controversial [74,75], however our results indicate that muscle from ATF5 KO animals exhibited enhanced ATF4 mRNA levels, in addition to its increased nuclear localization. These changes suggest that either ATF5 negatively regulates the transcription of ATF4, or that signals consequent to the lack of ATF5 promote ATF4 expression and localization.
The ATF5 protein harbours both a mitochondrial targeting and nuclear localization sequence that regulates its organelle-partitioning depending on the cellular environment, as does its worm homologue ATFS-1 [77]. A previous study showed that cellular proteotoxicity induces the nuclear translocation of ATF5 due to its failed mitochondrial import [35]. However, acute exercise failed to induce this, and instead increased the abundance of ATF5 in IMF mitochondria. Thus, it is possible that a greater accumulation of misfolded proteins is required to inhibit the mitochondrial import of ATF5 and prompt its nuclear translocation immediately following exercise stress. This is a similar phenomenon to what has been observed in a yeast model, identifying an “early” and “late” UPRmt with diverging efficiencies of mitochondrial protein import depending on the amount of time elapsed post-stress, as well as and the intensity of the stimulus imposed [78]. It is also known that chronically-imposed exercise induces an increase in mitochondrial protein import [79,80]. Whether the import flux is regulated by acute exercise to facilitate ATF5 accumulation inside the organelle remains a compelling question.
Previous research has identified that the expression of UPRmt markers, including chaperones and proteases, is extremely responsive to inducible mitochondrial stressors, in an ATF5-dependent manner [35]. Thus, we sought to investigate whether acute exercise influences UPRmt signaling skeletal muscle, and whether ATF5 is required for this response. The exercise protocol chosen was a sufficient exercise stimulus, as it augmented the transcript levels of PGC-1α in both genotypes, a shown previously [15,55]. Our data also indicate that ATF5 is not required for the induction of PGC-1α signaling following acute exercise, confirming what has also been shown in cardiomyocytes during cardiac stress [49].
However, WT and ATF5 KO mice displayed markedly divergent exercise-induced changes in mRNA transcripts of the UPRmt downstream markers mtHSP70, LONP1 and ClpP. The upregulation in UPRmt transcripts in WT muscle suggests that acute exercise is a sufficient stimulus to improve the protein folding capacity of mitochondria, similar to what occurs with respect to mRNA expression of UPRER markers [53]. Despite the relatively low abundance of ATF5 detected in the nuclear fractions, these levels are clearly sufficient to induce this exercise stress response in WT animals, which is absent in KO muscle. These results coincide with the concept that ATF5 may be part of a team of transcription factors that regulates the basal expression of UPRmt markers [35], but that it is required for normal stress-induced changes in these downstream proteins. Other studies have confirmed that ATF5 is dispensable for the expression of downstream targets under basal conditions, but that it is required during a plethora of mitochondrial stressors, including proteotoxicity and ROS, to activate the UPRmt and rescue mitochondrial function in mammalian cells and the mouse heart [35,50]. Interestingly, digressing from this collective response is the change in HSP60 gene expression, an established UPRmt chaperone which is extremely responsive to acute exercise. However, these changes occurred in WT animals and were even more pronounced in the absence of ATF5, suggesting redundancies in the transcriptional regulation of HSP60 during exercise stress that do not obligate ATF5. This alternative regulation of HSP60 in comparison to the other proteins is also supported by the enhanced basal expression of HSP60 protein and that of its co-chaperone, Cpn10. Interestingly, these proteins were upregulated even when normalized for COX activity, indicating a compositional change of mitochondria favouring the maintenance of organellar proteostasis. Our data also reveal that HSP60 mRNA and that of other downstream UPRmt markers were severely downregulated in PGC-1α KO muscle. Since PGC-1α is upregulated in ATF5 KO tissue, PGC-1α could be driving the increase in HSP60 mRNA and protein, with a heavier reliance of other UPRmt genes on the presence of ATF5 for their transcription during exercise stress. PGC-1α has been shown to be required for the induction of UPRER genes post-exercise, but is not required for their basal expression. Thus, the novelty of our data supports PGC-1α as having a compartment-specific role within the cell to regulate the expression of protein folding under basal physiological conditions.
5. Conclusion
Taken together, our results indicate that the skeletal muscle of ATF5 KO animals exhibits 1) enhanced acute exercise-induced stress signaling, 2) increased basal expression of UPRmt proteins, PGC-1 and ATF4, 3) a more abundant mitochondrial pool, with reduced organelle function, and 4) an altered UPRmt gene expression and mitophagy response to acute exercise. This illustrates that ATF5 is a critical regulator of mitochondrial quality control in skeletal muscle. In future investigations, it is worth pursuing the question of whether ATF5 is required to mediate beneficial mitochondrial adaptations to endurance training. Finally, it will also be compelling to examine the importance of ATF5 expression in coordinating the action of the UPRmt upon impaired mitochondrial protein import and induced proteotoxicity, to preserve organelle function in skeletal muscle.
Declarations competing of interest
None.
Acknowledgements
We would like to thank Dr. Stavros Lomvardas at Columbia University for providing us with ATF5 (+/−) heterozygous KO mice, in addition to Hani J. Shayya for assistance with breeding and genotyping. We are also grateful to Neushaw Moradi, Victoria Sanfrancesco and Jenna Wong for their expert technical assistance. This work was supported by funding from the Canadian Institutes of Health Research (CIHR) and the Natural Sciences and Engineering Research Council of Canada (NSERC) to D.A. Hood. D.A. Hood is also the holder of a Canada Research Chair in Cell Physiology.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.molmet.2022.101623.
Contributor Information
Mikhaela B. Slavin, Email: mslavin@yorku.ca.
Rita Kumari, Email: ritayu83@yorku.ca.
David A. Hood, Email: dhood@yorku.ca.
Conflicts of interest
There are no conflicts of interest to be declared
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- 1.Plasticity of muscle: Proceedings of a symposium held at the university of konstanz. De Gruyter; Germany: 2019. September 23–28, 1979. [Google Scholar]
- 2.Jacobs R.A., Flück D., Bonne T.C., Bürgi S., Christensen P.M., Toigo M., et al. Improvements in exercise performance with high-intensity interval training coincide with an increase in skeletal muscle mitochondrial content and function. J Appl Physiol. 2013;115(6):785–793. doi: 10.1152/japplphysiol.00445.2013. Bethesda, Md.: 1985. [DOI] [PubMed] [Google Scholar]
- 3.Calvo J.A., Daniels T.G., Wang X., Paul A., Lin J., Spiegelman B.M., et al. Muscle-specific expression of PPARgamma coactivator-1alpha improves exercise performance and increases peak oxygen uptake. J Appl Physiol. 2008;104(5):1304–1312. doi: 10.1152/japplphysiol.01231.2007. Bethesda, Md.: 1985. [DOI] [PubMed] [Google Scholar]
- 4.Callegari S., Dennerlein S. Sensing the stress: a role for the UPRmt and UPRam in the quality control of mitochondria. Front Cell Dev Biol. 2018;6:31. doi: 10.3389/fcell.2018.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ryan M.T., Hoogenraad N.J. Mitochondrial-nuclear communications. Annu Rev Biochem. 2007;76(1):701–722. doi: 10.1146/annurev.biochem.76.052305.091720. [DOI] [PubMed] [Google Scholar]
- 6.Quiles J.M., Gustafsson A.B. Mitochondrial quality control and cellular proteostasis: two sides of the same coin. Front Physiol. 2020;11 doi: 10.3389/fphys.2020.00515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Slavin M.B., Memme J.M., Oliveira A.N., Moradi N., Hood D.A. Regulatory networks coordinating mitochondrial quality control in skeletal muscle. Am J Physiol Cell Physiol. 2022;322(5):C913–C926. doi: 10.1152/ajpcell.00065.2022. [DOI] [PubMed] [Google Scholar]
- 8.Holloszy J.O. Biochemical adaptations in muscle. J Biol Chem. 1967;242(9):2278–2282. doi: 10.1016/S0021-9258(18)96046-1. [DOI] [PubMed] [Google Scholar]
- 9.Chen C.C.W., Erlich A.T., Hood D.A. Role of Parkin and endurance training on mitochondrial turnover in skeletal muscle. Skeletal Muscle. 2018;8(1):10. doi: 10.1186/s13395-018-0157-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dun Y., Liu S., Zhang W., Xie M., Qiu L. Exercise combined with rhodiola sacra supplementation improves exercise capacity and ameliorates exhaustive exercise-induced muscle damage through enhancement of mitochondrial quality control. Oxid Med Cell Longev. 2017;2017 doi: 10.1155/2017/8024857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beyfuss K., Erlich A.T., Triolo M., Hood D.A. The role of p53 in determining mitochondrial adaptations to endurance training in skeletal muscle. Sci Rep. 2018;8(1) doi: 10.1038/s41598-018-32887-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akimoto T., Pohnert S.C., Li P., Zhang M., Gumbs C., Rosenberg P.B., et al. Exercise stimulates Pgc-1alpha transcription in skeletal muscle through activation of the p38 MAPK pathway. J Biol Chem. 2005;280(20):19587–19593. doi: 10.1074/jbc.M408862200. [DOI] [PubMed] [Google Scholar]
- 13.Brandt N., Gunnarsson T.P., Hostrup M., Tybirk J., Nybo L., Pilegaard H., et al. Impact of adrenaline and metabolic stress on exercise-induced intracellular signaling and PGC-1α mRNA response in human skeletal muscle. Physiological Reports. 2016;4(14) doi: 10.14814/phy2.12844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pilegaard H., Saltin B., Neufer P.D. Exercise induces transient transcriptional activation of the PGC-1alpha gene in human skeletal muscle. J Physiol. 2003;546(Pt 3):851–858. doi: 10.1113/jphysiol.2002.034850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vainshtein A., Tryon L.D., Pauly M., Hood D.A. Role of PGC-1α during acute exercise-induced autophagy and mitophagy in skeletal muscle. Am J Physiol Cell Physiol. 2015;308(9):C710–C719. doi: 10.1152/ajpcell.00380.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hood D.A., Adhihetty P.J., Colavecchia M., Gordon J.W., Irrcher I., Joseph A.-M., et al. Mitochondrial biogenesis and the role of the protein import pathway. Med Sci Sports Exerc. 2003;35(1):86–94. doi: 10.1097/00005768-200301000-00015. [DOI] [PubMed] [Google Scholar]
- 17.Hood D.A., Joseph A.-M. Mitochondrial assembly: protein import. Proc Nutr Soc. 2004;63(2):293–300. doi: 10.1079/PNS2004342. [DOI] [PubMed] [Google Scholar]
- 18.Hoogenraad N.J., Ryan M.T. Translocation of proteins into mitochondria. IUBMB Life. 2001;51(6):345–350. doi: 10.1080/152165401753366096. [DOI] [PubMed] [Google Scholar]
- 19.Garcia I., Jones E., Ramos M., Innis-Whitehouse W., Gilkerson R. The little big genome: the organization of mitochondrial DNA. Front Biosci. 2017;22:710–721. doi: 10.2741/4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Romanello V., Sandri M. The connection between the dynamic remodeling of the mitochondrial network and the regulation of muscle mass. Cell Mol Life Sci: CM. 2021;78(4):1305–1328. doi: 10.1007/s00018-020-03662-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oliveira A.N., Hood D.A. Effect of Tim23 knockdown in vivo on mitochondrial protein import and retrograde signaling to the UPRmt in muscle. Am J Physiol Cell Physiol. 2018;315(4):C516–C526. doi: 10.1152/ajpcell.00275.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jishi A., Qi X. Altered mitochondrial protein homeostasis and proteinopathies. Front Mol Neurosci. 2022;15 doi: 10.3389/fnmol.2022.867935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haynes C.M., Ron D. The mitochondrial UPR – protecting organelle protein homeostasis. J Cell Sci. 2010;123(22):3849–3855. doi: 10.1242/jcs.075119. [DOI] [PubMed] [Google Scholar]
- 24.Jovaisaite V., Auwerx J. The mitochondrial unfolded protein response - synchronizing genomes. Curr Opin Cell Biol. 2015;33:74–81. doi: 10.1016/j.ceb.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fiorese C.J., Haynes C.M. Integrating the UPRmt into the mitochondrial maintenance network. Crit Rev Biochem Mol Biol. 2017;52(3):304–313. doi: 10.1080/10409238.2017.1291577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anderson N., Haynes C.M. Folding the mitochondrial UPR into the integrated stress response. Trends Cell Biol. 2020;30(6):428–439. doi: 10.1016/j.tcb.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Melber A., Haynes C.M. UPRmt regulation and output: a stress response mediated by mitochondrial-nuclear communication. Cell Res. 2018;28(3):281–295. doi: 10.1038/cr.2018.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ron D., Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8(7):519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 29.Zhao Q., Wang J., Levichkin I.V., Stasinopoulos S., Ryan M.T., Hoogenraad N.J. A mitochondrial specific stress response in mammalian cells. EMBO J. 2002;21(17):4411–4419. doi: 10.1093/emboj/cdf445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aldridge J.E., Horibe T., Hoogenraad N.J. Discovery of genes activated by the mitochondrial unfolded protein response (mtUPR) and cognate promoter elements. PLoS One. 2007;2(9):e874. doi: 10.1371/journal.pone.0000874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martinus R.D., Garth G.P., Webster T.L., Cartwright P., Naylor D.J., Høj P.B., et al. Selective induction of mitochondrial chaperones in response to loss of the mitochondrial genome. Eur J Biochem. 1996;240(1):98–103. doi: 10.1111/j.1432-1033.1996.0098h.x. [DOI] [PubMed] [Google Scholar]
- 32.Zhao K., Huang X., Zhao W., Lu B., Yang Z. LONP1-mediated mitochondrial quality control safeguards metabolic shifts in heart development. Development. 2022;149(6):dev200458. doi: 10.1242/dev.200458. [DOI] [PubMed] [Google Scholar]
- 33.Richards B.J., Slavin M., Oliveira A.N., Sanfrancesco V.C., Hood D.A. Mitochondrial protein import and UPRmt in skeletal muscle remodeling and adaptation. Semin Cell Dev Biol: S1084952122000040. 2022 doi: 10.1016/j.semcdb.2022.01.002. [DOI] [PubMed] [Google Scholar]
- 34.Fan F., Duan Y., Yang F., Trexler C., Wang H., Huang L., et al. Deletion of heat shock protein 60 in adult mouse cardiomyocytes perturbs mitochondrial protein homeostasis and causes heart failure. Cell Death Differ. 2020;27(2):587–600. doi: 10.1038/s41418-019-0374-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fiorese C.J., Schulz A.M., Lin Y.-F., Rosin N., Pellegrino M.W., Haynes C.M. The transcription factor ATF5 mediates a mammalian mitochondrial UPR. Curr Biol: CB (Curr Biol) 2016;26(15):2037–2043. doi: 10.1016/j.cub.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hansen M.B., Mitchelmore C., Kjaerulff K.M., Rasmussen T.E., Pedersen K.M., Jensen N.A. Mouse Atf5: molecular cloning of two novel mRNAs, genomic organization, and odorant sensory neuron localization. Genomics. 2002;80(3):344–350. doi: 10.1006/geno.2002.6838. [DOI] [PubMed] [Google Scholar]
- 37.Greene L.A., Lee H.Y., Angelastro J.M. The transcription factor ATF5: role in neurodevelopment and neural tumors. J Neurochem. 2009;108(1):11–22. doi: 10.1111/j.1471-4159.2008.05749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Al Sarraj J., Vinson C., Thiel G. Regulation of asparagine synthetase gene transcription by the basic region leucine zipper transcription factors ATF5 and CHOP. Biol Chem. 2005;386(9):873–879. doi: 10.1515/BC.2005.102. [DOI] [PubMed] [Google Scholar]
- 39.Yamazaki T., Ohmi A., Kurumaya H., Kato K., Abe T., Yamamoto H., et al. Regulation of the human CHOP gene promoter by the stress response transcription factor ATF5 via the AARE1 site in human hepatoma HepG2 cells. Life Sci. 2010;87(9–10):294–301. doi: 10.1016/j.lfs.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 40.Shimizu Y.I., Morita M., Ohmi A., Aoyagi S., Ebihara H., Tonaki D., et al. Fasting induced up-regulation of activating transcription factor 5 in mouse liver. Life Sci. 2009;84(25–26):894–902. doi: 10.1016/j.lfs.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 41.Lai C., Zhang J., Tan Z., Shen L.F., Zhou R.R., Zhang Y.Y. Maf1 suppression of ATF5-dependent mitochondrial unfolded protein response contributes to rapamycin-induced radio-sensitivity in lung cancer cell line A549. Aging. 2021;13(5):7300–7313. doi: 10.18632/aging.202584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun X., Angelastro J.M., Merino D., Zhou Q., Siegelin M.D., Greene L.A. Dominant-negative ATF5 rapidly depletes survivin in tumor cells. Cell Death Dis. 2019;10(10):709. doi: 10.1038/s41419-019-1872-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hu D., Liu Z., Qi X. UPRmt activation protects against MPP+-induced toxicity in a cell culture model of Parkinson's disease. Biochem Biophys Res Commun. 2021;569:17–22. doi: 10.1016/j.bbrc.2021.06.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hernández I.H., Torres-Peraza J., Santos-Galindo M., Ramos-Morón E., Fernández-Fernández M.R., Pérez-Alvarez M.J., et al. The neuroprotective transcription factor ATF5 is decreased and sequestered into polyglutamine inclusions in Huntington's disease. Acta Neuropathol. 2017;134(6):839–850. doi: 10.1007/s00401-017-1770-2. [DOI] [PubMed] [Google Scholar]
- 45.Dalton R.P., Lyons D.B., Lomvardas S. Co-opting the unfolded protein response to elicit olfactory receptor feedback. Cell. 2013;155(2):321–332. doi: 10.1016/j.cell.2013.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brown D., Ryan K., Daniel Z., Mareko M., Talbot R., Moreton J., et al. The Beta-adrenergic agonist, Ractopamine, increases skeletal muscle expression of Asparagine Synthetase as part of an integrated stress response gene program. Sci Rep. 2018;8 doi: 10.1038/s41598-018-34315-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Forsström S., Jackson C.B., Carroll C.J., Kuronen M., Pirinen E., Pradhan S., et al. Fibroblast growth factor 21 drives dynamics of local and systemic stress responses in mitochondrial myopathy with mtDNA deletions. Cell Metabol. 2019;30(6):1040–1054. doi: 10.1016/j.cmet.2019.08.019. e7. [DOI] [PubMed] [Google Scholar]
- 48.Brearley M.C., Li C., Daniel Z.C.T.R., Loughna P.T., Parr T., Brameld J.M. Changes in expression of serine biosynthesis and integrated stress response genes during myogenic differentiation of C2C12 cells. Biochemistry and Biophysics Reports. 2019;20 doi: 10.1016/j.bbrep.2019.100694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang B., Tan Y., Zhang Z., Feng P., Ding W., Wang Q., et al. Novel PGC-1α/ATF5 Axis partly activates UPRmt and mediates cardioprotective role of tetrahydrocurcumin in pathological cardiac hypertrophy. Oxid Med Cell Longev. 2020;2020 doi: 10.1155/2020/9187065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang Y.T., Lim Y., McCall M.N., Huang K.-T., Haynes C.M., Nehrke K., et al. Cardioprotection by the mitochondrial unfolded protein response requires ATF5. Am J Physiol Heart Circ Physiol. 2019;317(2):H472–H478. doi: 10.1152/ajpheart.00244.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smyrnias I., Gray S.P., Okonko D.O., Sawyer G., Zoccarato A., Catibog N., et al. Cardioprotective effect of the mitochondrial unfolded protein response during chronic pressure overload. J Am Coll Cardiol. 2019;73(14):1795–1806. doi: 10.1016/j.jacc.2018.12.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Memme J.M., Oliveira A.N., Hood D.A. Chronology of UPR activation in skeletal muscle adaptations to chronic contractile activity. Am J Physiol Cell Physiol. 2016;310(11):C1024–C1036. doi: 10.1152/ajpcell.00009.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu J., Ruas J.L., Estall J.L., Rasbach K.A., Choi J.H., Ye L., et al. The unfolded protein response mediates adaptation to exercise in skeletal muscle through a PGC-1α/ATF6α complex. Cell Metabol. 2011;13(2):160–169. doi: 10.1016/j.cmet.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cordeiro A.V., Brícola R.S., Braga R.R., Lenhare L., Silva V.R.R., Anaruma C.P., et al. Aerobic exercise training induces the mitonuclear imbalance and UPRmt in the skeletal muscle of aged mice. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 2020;75(12):2258–2261. doi: 10.1093/gerona/glaa059. [DOI] [PubMed] [Google Scholar]
- 55.Erlich A.T., Brownlee D.M., Beyfuss K., Hood D.A. Exercise induces TFEB expression and activity in skeletal muscle in a PGC-1α-dependent manner. Am J Physiol Cell Physiol. 2018;314(1):C62–C72. doi: 10.1152/ajpcell.00162.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Crilly M.J., Tryon L.D., Erlich A.T., Hood D.A. The role of Nrf2 in skeletal muscle contractile and mitochondrial function. J Appl Physiol. 2016;121(3):730–740. doi: 10.1152/japplphysiol.00042.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Takahashi M., Hood D.A. Protein import into subsarcolemmal and intermyofibrillar skeletal muscle mitochondria. J Biol Chem. 1996;271(44):27285–27291. doi: 10.1074/jbc.271.44.27285. [DOI] [PubMed] [Google Scholar]
- 58.Jiang J.-H., Zhao Y., Xiao L., Zhu C.-S., Li X., Li X. Activating transcription factor 5 regulates lipid metabolism in adipocytes. Sci Bull. 2016;61:1802. doi: 10.1007/s11434-016-1200-1. –9. [DOI] [Google Scholar]
- 59.Boppart M.D., Aronson D., Gibson L., Roubenoff R., Abad L.W., Bean J., et al. Eccentric exercise markedly increases c-Jun NH2-terminal kinase activity in human skeletal muscle. J Appl Physiol. 1999;87(5):1668–1673. doi: 10.1152/jappl.1999.87.5.1668. [DOI] [PubMed] [Google Scholar]
- 60.Rath E., Berger E., Messlik A., Nunes T., Liu B., Kim S.C., et al. Induction of dsRNA-activated protein kinase links mitochondrial unfolded protein response to the pathogenesis of intestinal inflammation. Gut. 2012;61(9):1269–1278. doi: 10.1136/gutjnl-2011-300767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cordeiro A.V., Peruca G.F., Braga R.R., Brícola R.S., Lenhare L., Silva V.R.R., et al. High-intensity exercise training induces mitonuclear imbalance and activates the mitochondrial unfolded protein response in the skeletal muscle of aged mice. GeroScience. 2020;43(3):1513–1518. doi: 10.1007/s11357-020-00246-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brearley-Sholto M.C., Loczenski-Brown D.M., Jones S., Daniel Z.C.T.R., Ebling F.J.P., Parr T., et al. Effect of AAV-mediated overexpression of ATF5 and downstream targets of an integrated stress response in murine skeletal muscle. Sci Rep. 2021;11(1) doi: 10.1038/s41598-021-99432-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nargund A.M., Fiorese C.J., Pellegrino M.W., Deng P., Haynes C.M. Mitochondrial and nuclear accumulation of the transcription factor ATFS-1 promotes OXPHOS recovery during the UPR(mt) Mol Cell. 2015;58(1):123–133. doi: 10.1016/j.molcel.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hood D.A., Tryon L.D., Vainshtein A., Memme J., Chen C., Pauly M., et al. Exercise and the regulation of mitochondrial turnover. Progress in Molecular Biology and Translational Science. 2015;135:99–127. doi: 10.1016/bs.pmbts.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 65.Wang Y., Jasper H., Toan S., Muid D., Chang X., Zhou H. Mitophagy coordinates the mitochondrial unfolded protein response to attenuate inflammation-mediated myocardial injury. Redox Biol. 2021;45 doi: 10.1016/j.redox.2021.102049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Laker R.C., Drake J.C., Wilson R.J., Lira V.A., Lewellen B.M., Ryall K.A., et al. Ampk phosphorylation of Ulk1 is required for targeting of mitochondria to lysosomes in exercise-induced mitophagy. Nat Commun. 2017;8(1):548. doi: 10.1038/s41467-017-00520-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Goodyear L.J., Chang P.Y., Sherwood D.J., Dufresne S.D., Moller D.E. Effects of exercise and insulin on mitogen-activated protein kinase signaling pathways in rat skeletal muscle. Am J Physiol. 1996;271(2 Pt 1):E403–E408. doi: 10.1152/ajpendo.1996.271.2.E403. [DOI] [PubMed] [Google Scholar]
- 68.Whitham M., Chan M.H.S., Pal M., Matthews V.B., Prelovsek O., Lunke S., et al. Contraction-induced interleukin-6 gene transcription in skeletal muscle is regulated by c-Jun terminal kinase/activator protein-1. J Biol Chem. 2012;287(14):10771–10779. doi: 10.1074/jbc.M111.310581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Matsuguchi T., Chiba N., Bandow K., Kakimoto K., Masuda A., Ohnishi T. JNK activity is essential for Atf4 expression and late-stage osteoblast differentiation. J Bone Miner Res: The Official Journal of the American Society for Bone and Mineral Research. 2009;24(3):398–410. doi: 10.1359/jbmr.081107. [DOI] [PubMed] [Google Scholar]
- 70.Enslen H., Tokumitsu H., Stork P.J., Davis R.J., Soderling T.R. Regulation of mitogen-activated protein kinases by a calcium/calmodulin-dependent protein kinase cascade. Proc Natl Acad Sci USA. 1996;93(20):10803–10808. doi: 10.1073/pnas.93.20.10803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vainshtein A., Kazak L., Hood D. Effects of endurance training on apoptotic susceptibility in striated muscle. J Appl Physiol. 2011;110:1638–1645. doi: 10.1152/japplphysiol.00020.2011. Bethesda, Md. : 1985. [DOI] [PubMed] [Google Scholar]
- 72.Dluzen D., Li G., Tacelosky D., Moreau M., Liu D.X. BCL-2 is a downstream target of ATF5 that mediates the prosurvival function of ATF5 in a cell type-dependent manner. J Biol Chem. 2011;286(9):7705–7713. doi: 10.1074/jbc.M110.207639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Han J., Back S.H., Hur J., Lin Y.-H., Gildersleeve R., Shan J., et al. ER-stress-induced transcriptional regulation increases protein synthesis leading to cell death. Nat Cell Biol. 2013;15(5):481–490. doi: 10.1038/ncb2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jiang D., Cui H., Xie N., Banerjee S., Liu R.-M., Dai H., et al. ATF4 mediates mitochondrial unfolded protein response in alveolar epithelial cells. Am J Respir Cell Mol Biol. 2020;63(4):478–489. doi: 10.1165/rcmb.2020-0107OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Quirós P.M., Prado M.A., Zamboni N., D'Amico D., Williams R.W., Finley D., et al. Multi-omics analysis identifies ATF4 as a key regulator of the mitochondrial stress response in mammals. J Cell Biol. 2017;216(7):2027–2045. doi: 10.1083/jcb.201702058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Suárez-Rivero J.M., Pastor-Maldonado C.J., Povea-Cabello S., Álvarez-Córdoba M., Villalón-García I., Talaverón-Rey M., et al. UPRmt activation improves pathological alterations in cellular models of mitochondrial diseases. Orphanet J Rare Dis. 2022;17(1):204. doi: 10.1186/s13023-022-02331-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nargund A.M., Pellegrino M.W., Fiorese C.J., Baker B.M., Haynes C.M. Mitochondrial import efficiency of ATFS-1 regulates mitochondrial UPR activation. Science (New York, N.Y.) 2012;337(6094):587–590. doi: 10.1126/science.1223560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.N d Increased mitochondrial protein import and cardiolipin remodelling upon early mtUPR. https://journals.plos.org/plosgenetics/article?id=10.1371/journal.pgen 1009664. accessed. [DOI] [PMC free article] [PubMed]
- 79.Takahashi M., Chesley A., Freyssenet D., Hood D.A. Contractile activity-induced adaptations in the mitochondrial protein import system. Am J Physiol. 1998;274(5):C1380–C1387. doi: 10.1152/ajpcell.1998.274.5.C1380. [DOI] [PubMed] [Google Scholar]
- 80.Gordon J.W., Rungi A.A., Inagaki H., Hood D.A. Effects of contractile activity on mitochondrial transcription factor A expression in skeletal muscle. J Appl Physiol. 2001;90(1):389–396. doi: 10.1152/jappl.2001.90.1.389. Bethesda, Md.: 1985. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.