Abstract
Background:
Although the clinical application of osimertinib, a third-generation epidermal growth factor receptor-tyrosine kinase inhibitor (EGFR-TKI), has been a new step forward in the first-line treatment of non-small cell lung cancer (NSCLC), an increasing number of patients with progression on osimertinib represents a great challenge clinically. The patterns of resistance mechanisms and subsequent treatment strategies after first-line osimertinib resistance are not well established.
Methods:
Between January 1, 2016 and October 31, 2020, a consecutive of 56 EGFR-mutant lung cancer patients treated with osimertinib as first-line therapy at Daping Hospital (Chongqing, China) were retrospective screened. The samples of pre-osimertinib and osimertinib-resistance were all detected by next-generation sequencing (NGS) panels. Statistical analyses were carried out using SPSS 23.0 software. Survival analyses were performed using the Kaplan–Meier method and compared using a log-rank test between groups.
Results:
Among 47 patients with osimertinib effectiveness analysis, the median progression free survival (mPFS) was 15.4 months (95% confidence interval [CI]: 12.2-24.9 months), and median overall survival (mOS) was 35.5 months (95% CI: 23.9 months -NA). A total of 21 patients underwent repeated NGS tests upon osimertinib resistance. MET amplification was the most common resistance mechanism (6/21, 28.6%), followed by C797S mutation (5/21, 23.8%). A total of 15 patients received subsequent treatments, with mPFS of 7.3 months (95% CI 5.0 months -NA). Among them, 7 patients with EGFR C797 S or/and MET amplification received subsequent second-line targeted therapy, achieving mPFS of 7.3 months (95% CI 4.5 months -NA). Of note, 3 patients received immunotherapy as second- or third-line treatment after osimertinib resistance, achieving median clinical benefit of 37.3 months.
Conclusions:
MET amplification and C797S mutation are main resistance mechanisms, which could be targeted by crizotinib and gefitinib, respectively. More than 50% patients could receive subsequent anticancer targetable therapies after first-line osimertinib resistance. Immunotherapy may also be an acceptable choice after osimertinib resistance.
Keywords: NSCLC, osimertinib, first-line treatment, resistance, subsequent treatments
Introduction
Epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) have significantly prolonged survival in non-small cell lung cancer (NSCLC) patients with EGFR sensitizing mutations.1 Osimertinib is an oral, irreversible, third-generation EGFR-TKI that can target both sensitizing EGFR mutations and the T790M mutations.2,3 Moreover, osimertinib has shown significant central nervous system (CNS) activity and a favorable safety profile.4,5 Initially approved for the treatment of patients with EGFR T790M mutations upon disease progression on first- or second- generation EGFR-TKIs, osimertinib is now emerging as the standard first-line treatment of advanced EGFR-mutant NSCLC patients on the basis of results from FLAURA trial, documenting a progression free survival (PFS) of 18.9 months and overall survival (OS) of 38.6 months.5 Nevertheless, the Chinese population subset analysis of FLAURA trial demonstrated that 71 Chinese patients had PFS of 17.8 months and OS of 33.1 months, which were inferior to that for the overall FLAURA study population.6 Although osimertinib is recommended as first-line treatment in EGFR-mutant NSCLC patients according to National comprehensive cancer network guidelines (NCCN), there is ongoing controversy regarding its application in Chinese population subset. Besides, significant survival benefit of first-line osimertinib compared with first generation TKIs in first-line setting is in dispute. In addition, the subsequent second-line therapeutic strategies after osimertinib resistance remain largely unknown. Currently, as limited treatments are available in patients progressing on osimertinib, platinum-based combination chemotherapy remains the standard of care. Although several early phase trials are showing promising results for strategies to target-specific resistance mechanisms like MET-inhibitors, little is known on the effectiveness of subsequent therapeutic strategies for overcoming the resistance of first-line osimertinib.
Besides, patterns of resistance mechanisms to osimertinib are highly heterogeneous. Most data about osimertinib resistance mechanisms are based on analyses in the second-line setting, therefore mechanisms of resistance to osimertinib as first-line therapy are not yet fully understood.7,8 Also, different mechanisms of osimertinib resistance may exist among patients of different races.9
The current study aimed to explore the mechanisms of acquired resistance to first-line osimertinib in Chinese NSCLC patients with EGFR mutation and investigate the effectiveness of various subsequent treatments after osimertinib resistance, such as combinatorial therapy of other targeted drugs, chemotherapy, and immunotherapy.
Materials and Methods
Study design and patients
This was a retrospective cohort study. Between January 1, 2016 and October 31, 2020, a total of 56 NSCLC patients receiving osimertinib as first-line therapy were screened, who were treated at the Daping Hospital of Army Medical University (Chongqing, China). Eligible patients had (locally) advanced stage NSCLC with a sensitive EGFR mutation, and received osimertinib as first-line therapy. Patients were excluded if they received previous treatments, or lost-to-follow-up before first effectiveness evaluation. The demographic and clinical characteristics of patients were collected, including age, sex, smoking history, histopathology, metastatic sites prior to osimertinib, and genetic information. The primary aims of the study were to assess osimertinib effectiveness as first-line treatment and characterize the mechanisms of resistance to first-line osimertinib. We also explored the clinical outcomes of patients with various subsequent treatments after osimertinib resistance.
Response to treatment was determined by Response Evaluation Criteria in Solid Tumor (RECIST), version 1.1. The data cutoff date for analysis was Jun 1, 2022. Disease control rate (DCR) was defined as the percentage of patients who had complete response (CR), partial response (PR) or stable disease (SD). Objective response rate (ORR) was defined as the proportion of patients achieving a CR or PR. PFS was calculated from the date of osimertinib initiation treatment until either the date of progressive disease (PD) according to the RECIST1.1 or death from any cause. PFS2 and PFS3 were defined as the duration from the start of second- or third-line treatment until PD or lost to follow-up or death, respectively. Duration of therapy (DOT) was defined as time from drug treatment initiation until treatment termination, including treatment beyond progression. OS was calculated from the date of osimertinib initiation treatment until the date of death or data cutoff.
Capture-based targeted next-generation sequencing (NGS)
The samples of pre-osimertinib and after radiological identified progression were all detected by next-generation sequencing (NGS) panels, and molecular results were analyzed. Various sample types, including tissue biopsy, plasma, malignant pleural effusion supernatant, and cerebrospinal fluid were collected for NGS tests using different commercially available gene panels.
Comprehensive genomic profiling was mainly performed by NGS with panel A (Burning Rock Biotech, Guangzhou, China). Sequencing was performed using Illumina NextSeq 500 using paired-end reads with target sequencing depth of 10,000X. The sequencing data were analyzed using bioinformatics pipeline optimized for somatic variant calling. Target capture was performed using commercially available panel consisting of 168 lung cancer–related genes. A total of 16 samples were tested by panel B (3D Medicines Inc, Shanghai, China), with a 68 cancer-related gene panel at a mean coverage depth of >30,000X.
Statistical analysis
Statistical analyses were carried out using SPSS 23.0 statistical software (SPSS, Inc., Chicago, IL, US). Survival analyses were performed using the Kaplan–Meier method and compared using a log-rank test between groups. All P values were two sided and a P value <.05 was considered to be statistically significant.
Ethical Statement
This study was approved by the ethics committee of Daping Hospital affiliated to Army Medical University, Chongqing, China (NO. 2021144). The need for consent was waived by the ethics committee after evaluation of the study design.
Results
Patient characteristics
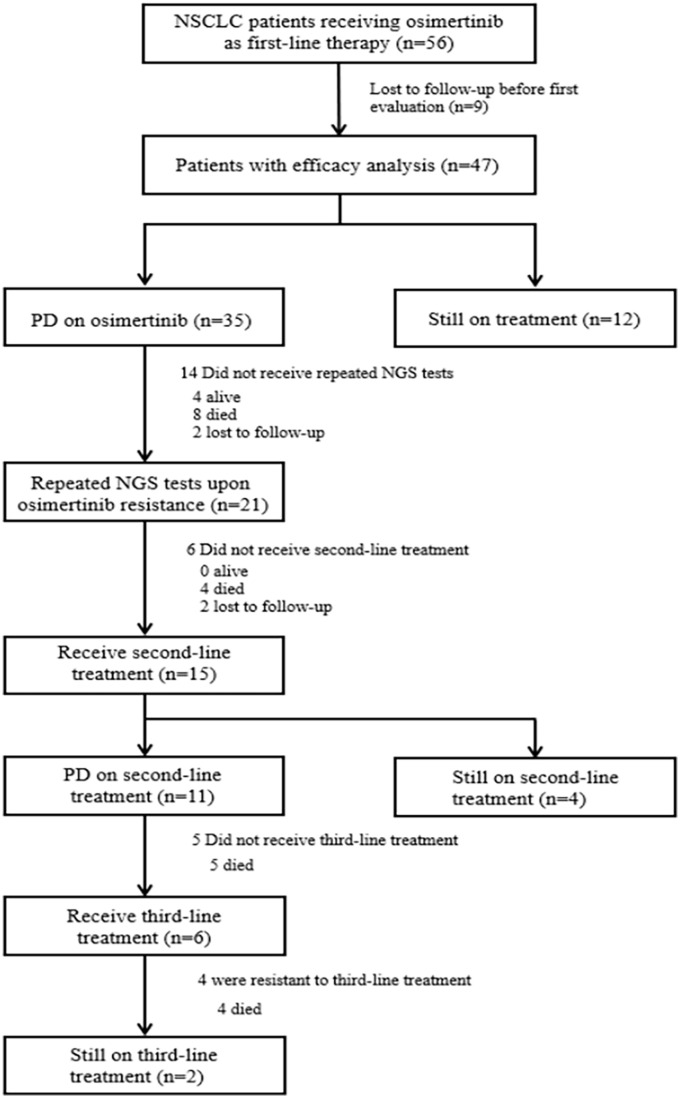
Of 56 patients screened, a total of 9 patients were lost to follow-up before the first effectiveness evaluation. Among the remaining 47 patients, 30 patients developed disease progression while the other 12 patients were still on first-line treatment at data cutoff date of Jun 1, 2022. On osimertinib resistance, 21 patients received repeated NGS tests to identify drug resistance mechanisms, among whom 15 received subsequent second-line treatments. A study flowchart was presented in Figure 1. The clinical characteristics of 47 patients were summarized in Table 1. The median age was 64 (range: 38–86) years, 59.6% (28/47) of the patients were women, 63.8%(30/47) were non-smokers, and 95.7% (45/47) had adenocarcinoma. In total, 91.5% (43/47) patients were in advanced stage, with bone being the most common metastasis site (26/47, 55.3%). Brain metastases were identified in 19 patients (40.4%), and 9 of them were symptomatic. However, none of them underwent radiation therapy or surgery during osimertinib treatment. EGFR mutations were identified in all patients, including 22 EGFR 19del (46.8%), 20 L858R (42.6%), 3 L858R/T790M (6.4%), 2 G719A (4.3%), 1 L861R (2.1%), and 2 20ins (4.3%).
Figure 1.
Study flowchart. Flow chart describing the enrolment and therapy of patients in the study.
Table 1.
Characteristics of enrolled patients.
| Total patients (N = 47) | Patients with paired NGS tests (N = 21) | |
|---|---|---|
| Age-median (range), years | 64 (38–86) | 44 (38–81) |
| Sex-no. (%) | ||
| Male | 19 (40.4) | 6 (28.6) |
| Female | 28(59.6) | 15 (71.4) |
| Smoking- no. (%) | ||
| Never | 30(63.8) | 14(66.7) |
| Former | 17(36.2) | 7(33.3) |
| Histology-no. (%) | ||
| Adenocarcinoma | 45(95.7) | 20(95.2) |
| NSCLC NOS | 2(4.3) | 1(4.8) |
| Primary EGFR mutation-No. (%) | ||
| Exon 19 deletion | 22(46.8) | 10(47.6) |
| L858R | 20(42.6) | 10(47.6) |
| L861R | 1(2.1) | 0 |
| G719A | 2(4.3) | 1(4.8) |
| 20 INS | 2(4.3) | 0 |
| L858R/T790M | 3(6.4) | 1(4.8) |
| PD-L1 expression- No. (%) | ||
| Negative | 8(17.0) | 3(14.3) |
| 1%–49% | 4(8.5) | 3(14.3) |
| ⩾50% | 4(8.5) | 3(14.3) |
| Unknown | 31(66.0) | 12(57.1) |
| Disease stage- No. (%) | ||
| IIIb/ IIIc | 4(8.5) | 2(9.5) |
| IV | 43(91.5) | 19(90.5) |
| Metastasis sites- No. (%) | ||
| Lung | 22(46.8) | 9(42.9) |
| Bone | 26(55.3) | 10(47.6) |
| Pleural | 17(36.2) | 10(47.6) |
| Brain | 19(40.4) | 9(42.9) |
| Liver | 8(17.0) | 6(28.6) |
The demographics, histology, metastasis sites, PD-L1 expression and sensitizing EGFR mutation for the two cohorts are shown.
Abbreviations: EGFR, epidermal growth factor receptor; NGS, next-generation sequencing; PD-L1, programmed death- ligand 1.
Effectiveness and Survival Analysis of Enrolled Patients
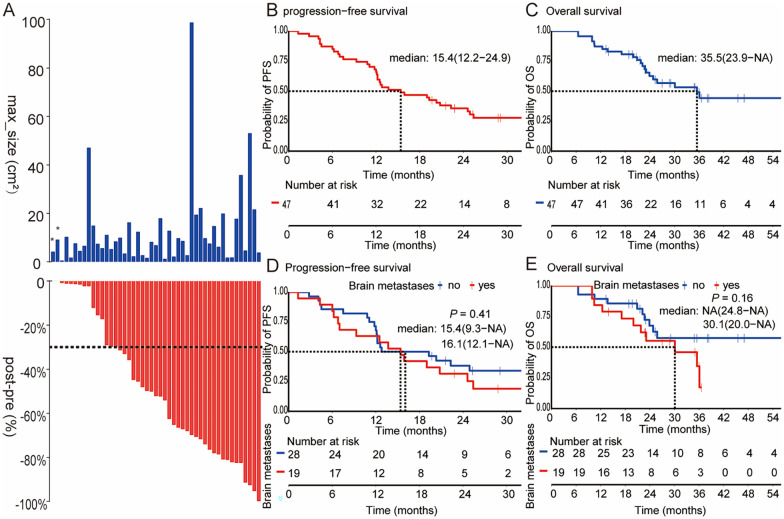
In total, 47 EGFR-mutant NSCLC patients were available for osimertinib effectiveness and survival analysis. Irrespective of baseline tumor size, osimertinib resulted in tumor shrinkage in most patients (Figure 2A), with an ORR of 51.1% (24/47), and a DCR of 95.7% (45/47). Of note, the two patients with EGFR 20ins showed primary resistance to osimertinib, and new metastases were identified at the time of first imaging evaluation. One patient with EGFR L858R achieved a CR response. With a median follow-up of 23.1 months (range: 6.5 to 73.9 months), the median PFS was 15.4 months (95% confidence interval [CI]: 12.2–24.9 months) and the median OS was 35.5 months (95% CI: 23.9 months—not arrived [NA]), as shown in Figure 2B and C. Meanwhile, PFS and OS of patients with brain metastases were not shorter than that without brain metastases (PFS 15.4 vs 16.1 months, P = 0.41; OS 30.1 months vs NA, P = 0.16, Figure 2D and E). These results suggest that osimertinib has strong infiltration ability across blood-brain barrier and against brain metastases.
Figure 2.
The effectiveness of first-line osimertinib and Kaplan-Meier estimates of survivals for overall patients and subgroups. (A) The max lesion size at baseline and (B) best percentage change in target lesion size from baseline. (B) PFS and (C) OS among 47 NSCLC patients with EGFR sensitive mutations treated with first-line osimertinib. (D) PFS and (E) OS in patients with brain metastases and without brain metastases.
*These two patient harboring EGFR 20ins receiving first-line osimertinib while developed disease progression at the first evaluable-for response.
PFS indicates progression-free survival; OS, overall survival; NSCLC, non-small cell lung cancer; EGFR, epidermal growth factor receptor.
Resistance Mechanisms to First-Line Osimertinib Treatment
A total of 21 patients underwent paired NGS tests before osimertinib treatment and after drug resistance. Their clinical characteristics were summarized in Table 1. A total of 10 patients carried EGFR 19 del, 10 with EGFR L858R (one with EGFR L858R/T790M), 1 with G719A. Baseline samples for genetic testing included tissue (12/21, 57.1%), plasma (5/21, 23.8%), pleural effusion (3/21, 14.3%) and cerebrospinal fluid (1/21, 4.8%). At the time of disease progression on osimertinib, tissue samples from repeated biopsy were available in 9 patients (9/21, 42.9%), and plasma samples were obtained from 11 patients (11/21, 52.4%), for whom re-biopsy of primary tumor was difficult to perform. Cerebrospinal fluid was obtained from one patient with leptomeningeal metastasis as the progression site (1/21, 4.8%).
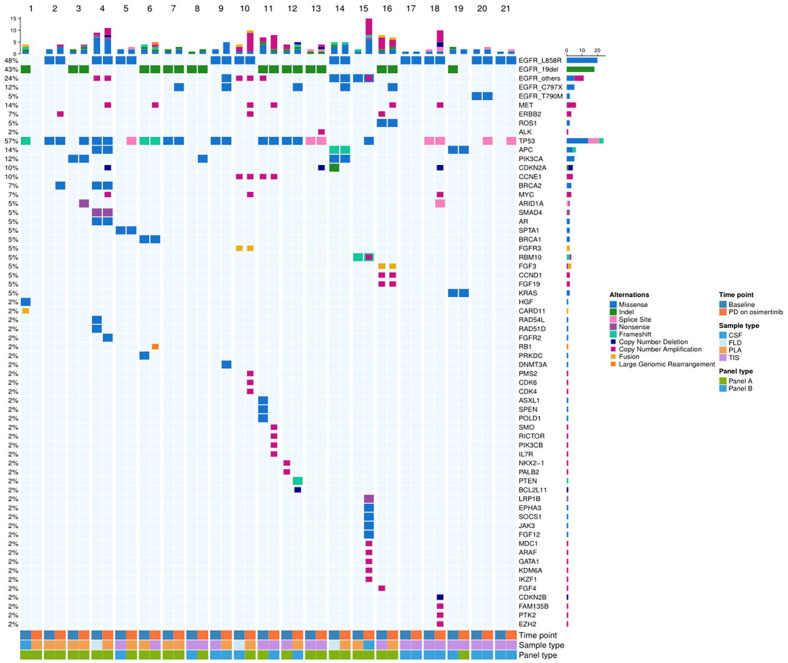
The paired genetic profiles at baseline and upon osimertinib resistance for each patient were presented in Figure 3. On osimertinib resistance, EGFR activating mutations were detected in 19 patients (90.5 %) and lost in 2 patients (9.5%). The most frequently detected concurrent gene was TP53 (14/21, 66.7%). EGFR-dependent resistance mechanisms included C797S mutation (5/21, 23.8%), L718Q (1/21, 4.8%), and EGFR amplification (1/21, 4.8%). As expected, EGFR T790M mutation was not detected in this cohort. EGFR-independent resistant mechanisms included MET amplification (6/21, 28.6%), ERBB2 amplification (2/21, 9.5%), PTEN (1/21, 4.8%), and PIK3CA (1/21, 4.8%).
Figure 3.
Genomic alterations of the 21 patients with paired NGS tests. Pre- and post-osimertinib somatic mutation profiles of 21 patients were shown according to next generation sequencing test results. Patients were arranged on the x-axis while genetic profiles detected were spread along the y-axis. Numbers on the left represent the percentage of patients with a specific gene. Top plot represents the overall mutations detected in one patient. Different colors indicate different genomic alterations. Panel A, Burning Rock Biotech; Panel B, 3D Medicines Inc.
CSF indicates cerebrospinal fluid; FLD, pleural effusions; PLA, plasma; TIS, tissue.
The cut-off value of MET copy number (CN) in panel A was 2.25, while in panel B was 5. MET CN of 4 patients were more than 4 MET copies but less than 7 MET copies, and 2 patients were more than 7 MET copies (Figure S1).
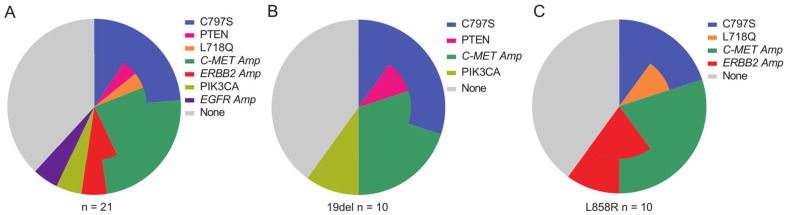
Meanwhile, 4 patients had more than one resistance mechanisms. The genetic profiles of each patient were summarized in Table S1. Besides, a total of 8 patients (38.1%) failed to find any resistance mechanisms. Patients with EGFR 19del and L858R showed similar resistance mechanisms. A schematic representation of the main resistance mechanisms to osimertinib was shown in Figure 4.
Figure 4.
Main resistance mechanisms to first-line osimetinib. The three pie charts depict main resistance mechanisms. Resistance mechanisms of osimetinib were reported according to the EGFR mutation type. The different color represents different genomic alterations. In some cases, different molecular aberrations might co-exist in one patient.
EGFR indicates epidermal growth factor receptor.
Subsequent Treatments and Prognosis on Osimertinib Resistance
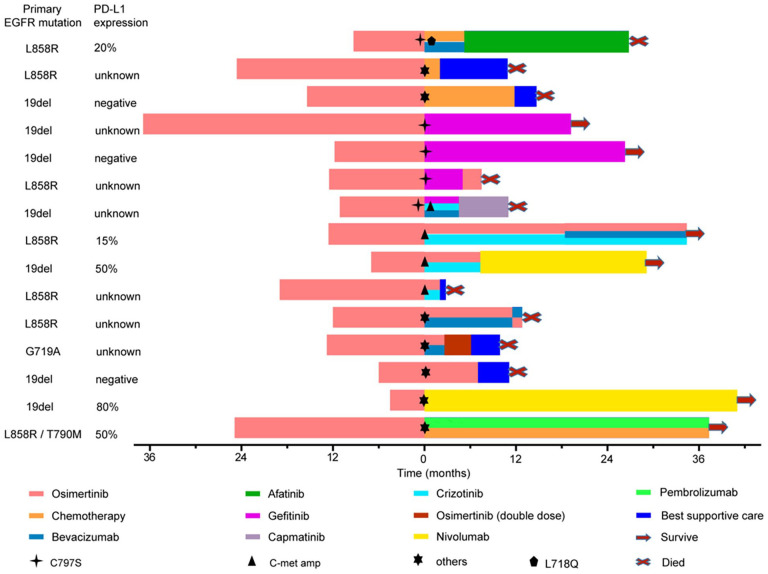
Of 21 patients who experienced repeated NGS tests after osimertinib progression, 4 patients without any subsequent treatment died within 6 months, 2 patients were lost to follow-up, and 15 patients received subsequent second-line treatment, achieving mPFS2 of 7.3 months (95% CI: 5.0 months -NA) [Figure 5 and Figure S2).
Figure 5.
The courses of treatment given to 15 patients. Starting point (0 on X axis) indicate the time of disease progression on first-line osimertinib. Different treatments are shown in different colors.
Of 5 patients with EGFR C797 S mutation, 3 patients received gefitinib monotherapy, and 2 of them were still on gefitinib treatment at data cutoff, whose DOT were 18.3 and 25.9 months, respectively. Another one patient developed gefitinib resistance after 5 months. One patient with C797S and L718Q received platinum-based pemetrexed plus bevacizumab, while disease progressed again after 5.2 months. Then the patient began to receive oral afatinib as subsequent third-line treatment, and developed drug resistance after 20.6 months. Then the patient did not receive any further treatment, achieving an OS of 36.1 months. One patient harboring MET amplification and C797S concurrently was treated with gefitinib plus crizotinib, achieving PFS2 of 4.5 months. When disease progressed again, capmatinib plus gefitinib and bevacizumab were given to the patient, achieving PFS3 of 4.4 months. However, the patient died shortly after drug resistance, with an OS of 22.2 months.
Of other 5 patients with MET amplification, 2 patients did not receive subsequent treatment, while 3 patients were treated with osimertinib plus crizotinib, achieving mPFS2 of 7.3 months. All of them developed disease progression again and two of them received third-line treatment. One patient underwent osimertinib and crizotinib plus bevacizumab. Another one with nivolumab treatment has lasted for 20.0 months and is still ongoing at data cutoff date.
In summary, a total of 7 patients with EGFR C797 S or/and MET amplification received subsequent second-line targeted therapy, achieving mPFS of 7.3 months (95% CI: 4.5 months -NA) (Figure S3).
For the remaining 7 patients, 3 patients continued with osimertinib or combination therapy with bevacizumab, whose mPFS2 was 7.0 months. Two patients received platinum-based plus pemetrexed chemotherapy and achieved PFS2 of 11.8 months and 2.0 months, respectively. Another 2 patients received immunotherapy (one in combination with chemotherapy) as second-line treatment, and they were still on treatment at data cutoff, with DOT of 39.4 months and 36.3 months, respectively.
Discussion
This is a single-center retrospective cohort study of Chinese patients. The outcomes demonstrated that Chinese patients with EGFR-mutant NSCLC treated with first-line osimertinib achieved a PFS of 15.4 months. It seems that, compared with first-line dacomitinib and gefitinib, the advantage of osimertinib as first-line setting in Chinses population was not obvious10 However, osimertinib exhibited potent intracranial effectiveness against brain metastases.3,11 In our study, patients with brain metastases receiving first-line osimertinib achieved similar prognosis compared with those without brain metastases.
Although PFS in our article was inferior to that in the FLAURA China study (15.4 vs 17.8 months, the median OS was longer (35.5 vs 33.1 months).6 The possible reasons for this difference were the higher proportion of patients with the combinatorial therapy of TKIs in subsequent second-line anticancer treatment and the lower proportion of cytotoxic chemotherapy in our study than that in the FLAURA China study (46.7% vs 33%, 20% vs 57%, respectively). Besides, there are 3 patients with strong programmed death-ligand 1(PD-L1) expression in our study who received subsequent immune checkpoint inhibitors (ICIs) and achieved a long duration of survival. A better characterization and understanding of resistance mechanisms to first-line osimertinib is helpful to guide subsequent precision treatment instead of traditional chemotherapy, and improve the prognosis.
Multiple biological mechanisms of acquired resistance to osimertinib, both EGFR-dependent and -independent, have been previously identified.12 The patterns of molecular resistance were not exactly the same in patients treated with osimertinib as first or later lines.7,13 The acquired EGFR C797S mutation in first-line osimertinib set-ting occurred less frequently than that in second-line regime.14,15 MET amplification was the most commonly reported acquired off-target resistance mechanism in first-line osimertinib setting.16,17 Although spatially heterogeneous, MET amplification often co-occurs with additional acquired focal copy-number amplifications and is associated with early progression.18 Previous studies reported that the incidence of EGFR C797S mutation and MET amplification were 7% and 15%.19,20 It means that a low proportion of patients could receive second-line targeted therapy and most cases underwent chemotherapy after osimertinib resistance. This is one of the main reasons why osimertinib applied in first-line is controversial. As described in our cohort, the incidence of EGFR C797S mutation and MET amplification were 23.8% and 28.6%, which were both higher than previous reports. The gefitinib and crizotinib might be more effective therapeutic strategy to overcome EGFR C797S mutation and MET amplification following osimertinib resistance than chemotherapy.13,21,22 For those patients received first-generation EGFR-TKIs as first-line therapy, about 49% to 63% patients were found to have secondary EGFR T790M mutation after disease progression and then received subsequent osimertinib treatment, achieving mOS of 25.7 months.23-25 Similarly, according to our study, for patients with first-line osimertinib regime, there are still over 50% patients having an opportunity to receive subsequent combinatorial therapy of TKIs after osimertinib resistance, achieving mOS of 35.5 months. Currently, some new highly selective, potent small-molecule MET inhibitors like capmatinib, savolitinib, tepotinib have shown promising antitumor activity to against MET-mediated acquired resistance to osimertinib.22,26-28 Therefore, our study indicated that various subsequent effective therapeutic strategies for overcoming first-line osimertinib resistance could be available in future.
Current data suggest that ICIs is limited effectiveness in EGFR-mutant cancers.29,30 However, unexpected favorable outcome to ICIs as later lines treatment after progressed on at least one EGFR TKI have been reported.31 The study from Ken Masuda et al32 showed that the level of PD-L1 expression also could be helpful to predict the effectiveness of programmed death-1 (PD-1) inhibitors even in EGFR mutant patients. However, it is hard to apply a single biomarker to screen the potential population in EGFR-mutated NSCLC who could benefit from immunotherapy. It is important to establish an evaluation system on the basis of multiplexed and multiomics for those patients.33 In our study, a total of 3 patients with strong PD-L1 expression (tumor proportion score ⩾ 50%) received ICIs and achieved such a long survival, being significantly superior than other subsequent treatment regimens. Therefore, PD-1 inhibitors could be an effective treatment option for EGFR mutant patients with strong PD-L1 expression after TKIs resistance. More clinical details of these 3 patients are shown in TableS2.
Other rare mechanisms including PIK3CA/PTEN mutation, ERBB2 amplifications and EGFR amplifications were detected. For those patients, chemotherapy was still the standard treatment. Previous studies have shown that the combinatorial treatment consisting of EGFR-TKI and bevacizumab could improve PFS compared with TKI alone.34 Due to lack of effective subsequent therapies, more studies are needed in the future for those patients who are failure to be identified drug resistance mechanisms.
There were some limitations to this study. This was a retrospective study with small sample size. As limited application of repeated biopsy, the genetic heterogeneity between the tissue and plasma might lead to deviation for identification of resistance mechanism. Another potential resistance mechanism, such as small cell or squamous cell transformation, was not discussed in this study because it can’t be confirmed by plasma genetic detection. Besides, MET FISH were not conducted for patients who were presumed to have defined MET amplification by NGS.
Conclusions
Osimertinib is recommended as the preferred choice for Chinese NSCLC patients with EGFR sensitive mutation, although acquired resistance is still inevitable. In the current study, more than 50% patients were found to have MET amplification or/and C797S mutation, and then received subsequent second-line TKI-containing regimen after osimertinib resistance. The effectiveness of crizotinib and gefitinib against MET amplification and C797S mutation-mediated first line osimertinib resistance in patients with EGFR-mutant NSCLC were identified in our study. Besides, PD-1 inhibitors might be an effective treatment for patients with strong PD-L1 expression after failure of TKIs. Understanding the resistance mechanism of osimertinib could be feasible to guide subsequent treatment, and help formulating precision and individual treatment.
Supplemental Material
Supplemental material, sj-docx-1-onc-10.1177_11795549221134735 for First-Line Osimertinib in Patients With EGFR-Mutated Non-Small Cell Lung Cancer: Effectiveness, Resistance Mechanisms, and Prognosis of Different Subsequent Treatments by Naifu Nie, Jianghua Li, Jian Zhang, Jie Dai, Zhulin Liu, Zhenyu Ding, Yubo Wang, Mengxiao Zhu, Chen Hu, Rui Han, Huan Tang, Li Li and Yong He in Clinical Medicine Insights: Oncology
Supplemental material, sj-docx-2-onc-10.1177_11795549221134735 for First-Line Osimertinib in Patients With EGFR-Mutated Non-Small Cell Lung Cancer: Effectiveness, Resistance Mechanisms, and Prognosis of Different Subsequent Treatments by Naifu Nie, Jianghua Li, Jian Zhang, Jie Dai, Zhulin Liu, Zhenyu Ding, Yubo Wang, Mengxiao Zhu, Chen Hu, Rui Han, Huan Tang, Li Li and Yong He in Clinical Medicine Insights: Oncology
Supplemental material, sj-tif-3-onc-10.1177_11795549221134735 for First-Line Osimertinib in Patients With EGFR-Mutated Non-Small Cell Lung Cancer: Effectiveness, Resistance Mechanisms, and Prognosis of Different Subsequent Treatments by Naifu Nie, Jianghua Li, Jian Zhang, Jie Dai, Zhulin Liu, Zhenyu Ding, Yubo Wang, Mengxiao Zhu, Chen Hu, Rui Han, Huan Tang, Li Li and Yong He in Clinical Medicine Insights: Oncology
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research is funded by National Natural Science Foundation of China [81972189, 82272908], and funding from Daping Hospital [2019CXLCA003, 2019CXLCB011] and a Science Foundation for Outstanding Young People of the Army Medical University.
Author Contributions: H.Y. and L.L. conceived of the study, and participated in its design and coordination. N.N.F., L.J.H. and Z.J. carried out immunoassays and performed the statistical analysis. D.J., L.Z.L., D.Z.Y., W.Y.B., Z.M.X., H.C., H.R., and TH participated in provision of study materials or patients. N.N.F., L.J.H., Z.J., D.J., L.Z.L., D.Z.Y., W.Y.B., Z.M.X., H.C., H.R., and T.H. participated in collection and assembly of data. All authors participated in writing and read and approved the final and the final version.
ORCID iDs: Li Li  https://orcid.org/0000-0003-3499-8465
https://orcid.org/0000-0003-3499-8465
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Bollinger MK, Agnew AS, Mascara GP. Osimertinib: a third-generation tyrosine kinase inhibitor for treatment of epidermal growth factor receptor-mutated non-small cell lung cancer with the acquired Thr790Met mutation. J Oncol Pharm Pract. 2018;24:379-388. [DOI] [PubMed] [Google Scholar]
- 2. Cross DA, Ashton SE, Ghiorghiu S, et al. AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov. 2014;4:1046-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wu YL, Ahn MJ, Garassino MC, et al. CNS efficacy of osimertinib in patients with T790M-positive advanced non-small-cell lung cancer: data from a randomized phase III trial (AURA3). J Clin Oncol. 2018;36:2702-2709. [DOI] [PubMed] [Google Scholar]
- 4. Ramalingam SS, Vansteenkiste J, Planchard D, et al. Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N Engl J Med. 2020;382:41-50. [DOI] [PubMed] [Google Scholar]
- 5. Soria JC, Ohe Y, Vansteenkiste J, Reungwetwattana T, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. 2018;378:113-125. [DOI] [PubMed] [Google Scholar]
- 6. Cheng Y, He Y, Li W, et al. Osimertinib versus comparator EGFR TKI as first-line treatment for EGFR-mutated advanced NSCLC: FLAURA China, a randomized study. Target Oncol. 2021;16:165-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mehlman C, Cadranel J, Rousseau-Bussac G, et al. Resistance mechanisms to osimertinib in EGFR-mutated advanced non-small-cell lung cancer: a multicentric retrospective French study. Lung Cancer. 2019;137:149-156. [DOI] [PubMed] [Google Scholar]
- 8. Schoenfeld AJ, Yu HA. The evolving landscape of resistance to osimertinib. J Thorac Oncol. 2020;15:18-21. [DOI] [PubMed] [Google Scholar]
- 9. Fuchs V, Roisman L, Kian W, et al. The impact of osimertinib’ line on clonal evolution in EGFRm NSCLC through NGS-based liquid biopsy and overcoming strategies for resistance. Lung Cancer. 2021;153:126-133. [DOI] [PubMed] [Google Scholar]
- 10. Wu YL, Cheng Y, Zhou X, et al. Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): a randomised, open-label, phase 3 trial. Lancet Oncol. 2017;18:1454-1466. [DOI] [PubMed] [Google Scholar]
- 11. Ballard P, Yates JW, Yang Z, et al. Preclinical comparison of osimertinib with other EGFR-TKIs in EGFR-mutant NSCLC brain metastases models, and early evidence of clinical brain metastases activity. Clin Cancer Res. 2016;22:5130-5140. [DOI] [PubMed] [Google Scholar]
- 12. Lazzari C, Gregorc V, Karachaliou N, et al. Mechanisms of resistance to osimertinib. J Thorac Dis. 2020;12:2851-2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rangachari D, To C, Shpilsky JE, et al. EGFR-mutated lung cancers resistant to osimertinib through EGFR C797S respond to first-generation reversible EGFR inhibitors but eventually acquire EGFR T790M/C797S in preclinical models and clinical samples. J Thorac Oncol. 2019;14:1995-2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Oxnard GR, Hu Y, Mileham KF, et al. Assessment of resistance mechanisms and clinical implications in patients with EGFR T790M-positive lung cancer and acquired resistance to osimertinib. JAMA Oncol. 2018;4:1527-1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schmid S, Li JJN, Leighl NB. Mechanisms of osimertinib resistance emerging treatment options. Lung Cancer. 2020;147:123-129. [DOI] [PubMed] [Google Scholar]
- 16. Piotrowska Z, Isozaki H, Lennerz JK, et al. Landscape of acquired resistance to osimertinib in EGFR-mutant NSCLC and clinical validation of combined EGFR and RET inhibition with osimertinib and BLU-667 for acquired RET fusion. Cancer Discov. 2018;8:1529-1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yang Z, Yang N, Ou Q, et al. Investigating novel resistance mechanisms to third-generation EGFR tyrosine kinase inhib-itor osimertinib in non-small cell lung cancer Patients. Clin Cancer Res. 2018;24:3097-3107. [DOI] [PubMed] [Google Scholar]
- 18. Roper N, Brown AL, Wei JS, et al. Clonal evolution and heterogeneity of osimertinib acquired resistance mechanisms in EGFR mutant lung cancer. Cell Rep Med. 2020;1:100007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cho BC, Cheng Y, Zhou C, et al. Mechanisms of acquired resistance to first-line osimertinib: preliminary data from the phase III FLAURA study. Ann Oncol. 2018;29:483. [Google Scholar]
- 20. Ramalingam SSCY, Zhou C, Ohe Y, et al. LBA50 Mechanisms of acquired resistance to first-line osimertinib: preliminary data from the phase III FLAURA study. Ann Oncol. 2018;29:740. [Google Scholar]
- 21. Niederst MJ, Hu H, Mulvey HE, et al. The allelic context of the C797S mutation acquired upon treatment with third-generation EGFR inhibitors impacts sensitivity to subsequent treatment strategies. Clin Cancer Res. 2015;21:3924-3933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang Y, Tian P, Xia L, et al. The clinical efficacy of combinatorial therapy of EGFR-TKI and crizotinib in overcoming MET amplification-mediated resistance from prior EGFR-TKI therapy. Lung Cancer. 2020;146:165-173. [DOI] [PubMed] [Google Scholar]
- 23. Yu HA, Arcila ME, Rekhtman N, et al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res. 2013;19:2240-2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sequist LV, Waltman BA, Dias-Santagata D, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med. 2011;3:75ra26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Oxnard GR, Arcila ME, Sima CS, et al. Acquired resistance to EGFR tyrosine kinase inhibitors in EGFR-mutant lung cancer: distinct natural history of patients with tumors harboring the T790M mutation. Clin Cancer Res. 2011;17:1616-1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sequist LV, Han JY, Ahn MJ, et al. Osimertinib plus savolitinib in patients with EGFR mutation-positive, MET-amplified, non-small-cell lung cancer after progression on EGFR tyrosine kinase inhibitors: interim results from a multicentre, open-label, phase 1b study. Lancet Oncol. 2020;21:373-386. [DOI] [PubMed] [Google Scholar]
- 27. Wu YL, Cheng Y, Zhou J, et al. Tepotinib plus gefitinib in patients with EGFR-mutant non-small-cell lung cancer with MET overexpression or MET amplification and acquired resistance to previous EGFR inhibitor (INSIGHT study): an open-label, phase 1b/2, multicentre, randomised trial. Lancet Respir Med. 2020;8:1132-1143. [DOI] [PubMed] [Google Scholar]
- 28. Haura EB, Cho BC, Lee JS, et al. JNJ-61186372 (JNJ-372), an EGFR-cMet bispecific antibody, in EGFR-driven advanced non-small cell lung cancer (NSCLC). J Clin Oncol. 2019;37:9009-9009. [Google Scholar]
- 29. Lee CK, Man J, Lord S, et al. Clinical and molecular characteristics associated with survival among patients treated with checkpoint inhibitors for advanced non-small cell lung carcinoma: a systematic review and meta-analysis. JAMA Oncol. 2018;4:210-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lisberg A, Cummings A, Goldman JW, et al. A phase II study of pembrolizumab in EGFR-mutant, PD-L1+, tyrosine kinase inhibitor naive patients with advanced NSCLC. J Thorac Oncol. 2018;13:1138-1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bylicki O, Guisier F, Monnet I, et al. Efficacy and safety of programmed cell-death-protein-1 and its ligand inhibitors in pre-treated patients with epidermal growth-factor receptor-mutated or anaplastic lymphoma kinase-translocated lung adeno-carcinoma. Medicine (Baltimore). 2020;99:e18726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Masuda K, Horinouchi H, Tanaka M, et al. Efficacy of anti-PD-1 antibodies in NSCLC patients with an EGFR mutation and high PD-L1 expression. J Cancer Res Clin Oncol. 2021;147:245-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Qiao M, Jiang T, Liu X, et al. Immune checkpoint inhibitors in EGFR-mutated NSCLC: dusk or dawn? J Thorac Oncol. 2021;16:1267-1288. [DOI] [PubMed] [Google Scholar]
- 34. Saito H, Fukuhara T, Furuya N, et al. Erlotinib plus bevacizumab versus erlotinib alone in patients with EGFR-positive ad-vanced non-squamous non-small-cell lung cancer (NEJ026): interim analysis of an open-label, randomised, multicentre, phase 3 trial. Lancet Oncol. 2019;20:625-635. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-onc-10.1177_11795549221134735 for First-Line Osimertinib in Patients With EGFR-Mutated Non-Small Cell Lung Cancer: Effectiveness, Resistance Mechanisms, and Prognosis of Different Subsequent Treatments by Naifu Nie, Jianghua Li, Jian Zhang, Jie Dai, Zhulin Liu, Zhenyu Ding, Yubo Wang, Mengxiao Zhu, Chen Hu, Rui Han, Huan Tang, Li Li and Yong He in Clinical Medicine Insights: Oncology
Supplemental material, sj-docx-2-onc-10.1177_11795549221134735 for First-Line Osimertinib in Patients With EGFR-Mutated Non-Small Cell Lung Cancer: Effectiveness, Resistance Mechanisms, and Prognosis of Different Subsequent Treatments by Naifu Nie, Jianghua Li, Jian Zhang, Jie Dai, Zhulin Liu, Zhenyu Ding, Yubo Wang, Mengxiao Zhu, Chen Hu, Rui Han, Huan Tang, Li Li and Yong He in Clinical Medicine Insights: Oncology
Supplemental material, sj-tif-3-onc-10.1177_11795549221134735 for First-Line Osimertinib in Patients With EGFR-Mutated Non-Small Cell Lung Cancer: Effectiveness, Resistance Mechanisms, and Prognosis of Different Subsequent Treatments by Naifu Nie, Jianghua Li, Jian Zhang, Jie Dai, Zhulin Liu, Zhenyu Ding, Yubo Wang, Mengxiao Zhu, Chen Hu, Rui Han, Huan Tang, Li Li and Yong He in Clinical Medicine Insights: Oncology