Abstract
Distinct Roles of Rodent Thalamus and Corpus Callosum in Seizure Generalization
Brodovskaya A, Batabyal T, Shiono S, Sun H, Kapur J. Ann Neurol. 2022;91(5):682-696. doi:10.1002/ana.26338
Objective:
Bilateral synchronous cortical activity occurs during sleep, attention, and seizures. Canonical models place the thalamus at the center of bilateral cortical synchronization because it generates bilateral sleep spindle oscillations and primarily generalized absence seizures. However, classical studies suggest that the corpus callosum mediates bilateral cortical synchronization.
Methods:
We mapped the spread of right frontal lobe-onset, focal to bilateral seizures in mice and modified it using chemo and optogenetic suppression of motor thalamic nucleus and corpus callosotomy.
Results:
Seizures from the right cortex spread faster to the left cortex than to the left thalamus. The 2 thalami have minimal monosynaptic commissural connections compared to the massive commissure corpus callosum. Chemogenetic and closed-loop optogenetic inhibition of the right ventrolateral thalamic nucleus did not alter inter-hemispheric seizure spread. However, anterior callosotomy delayed bilateral seizure oscillations.
Interpretation:
Thalamocortical oscillations amplify focal onset motor seizures, and corpus callosum spreads them bilaterally.
Commentary
How fast, and by what pathways, does focal seizure activity spread? Are there preferential pathways for ictal activity in a focus to spread to the other hemisphere? Is thalamus a necessary way station in bilateral spread of seizure activity? Do focal to generalized tonic–clonic (GTC) convulsive seizures utilize the same pathways as primary generalized seizures? These are long-standing questions whose resolution has been limited by appropriate technology. Historically, the centrencephalic model of Penfield proposed that the diencephalon, particularly the thalamus, controls bilateral spread of seizure activity by activating both hemispheres. 1 This model provides a reasonable explanation for certain neuronal oscillations such as sleep spindles and the 3-Hz spike-waves of absence seizures. But the situation may be different for a focal motor seizure that generalizes and leads to a GTC. The availability of modern techniques such as chemogenetics and optogenetics now allows testing of hypotheses related to focal to bilateral seizure spread.
In this paper, Brodovskaya and colleagues explore pathways by which focal seizure activity spreads to the opposite hemisphere, causing a GTC. 2 The investigators injected cobalt (Co2+), a proconvulsant divalent cation, into the right frontal cortex of C57Bl/6 mice to create a seizure focus. Seizure activity in the form of epileptic local field potentials (LFPs) was then monitored as it progressed to nearby and distant sites. Electrodes to record LFPs were placed simultaneously in adjacent premotor cortex as well as the analogous left premotor cortex, and left and right ventral lateral (VL) thalamic nuclei. Ventral lateral was chosen because of its extensive reciprocal connections with motor cortex. 3
Local field potential recordings showed that ictal activity spread quickly (∼25 ms) to both premotor cortices and to the ipsilateral thalamus, but ictal LFPs often did not appear in the contralateral thalamus for 200 ms or more. This observation suggested that the interhemispheric ictal activity spread along nonthalamic routes, possibly via the corpus callosum (CC), to the opposite motor cortex over hundreds of milliseconds before reaching the contralateral thalamus. Furthermore, using an assay to assess c-Fos expression, ipsilateral thalamic neurons were much more highly activated than contralateral thalamic neurons, suggesting that the 2 sides of the thalamus were not strongly interconnected, an observation consistent with the lack of direct interthalamic spread. Therefore, the role of the thalamus mediating bilateral ictal spread was rightfully questioned. The remainder of the article describes elegant methods by which the authors support this hypothesis.
To further evaluate the role of the thalamus in bilateral seizure spread, the authors used several methods to inactivate the VL nucleus of the thalamus. When VL was inhibited, rapid spread of the ictal activity to the contralateral motor cortex was still observed. Specifically, they used a chemogenetic approach to express kappa-opioid receptors (KORD; a form of DREADD—designer receptor exclusively activated by designer drugs). In mice, neuronal activity of the right VL could be suppressed by the KORD-specific agonist salvinorin B, which activates G-protein-coupled inwardly rectifying potassium channels, resulting in neuronal hyperpolarization. Under these conditions, Co2+-induced seizures spread rapidly from the right premotor cortex to the left premotor cortex, but the seizures were shorter and less intense. The authors concluded that the thalamus is not necessary for the interhemispheric transfer of ictal activity. However, the thalamus does alter cortical seizure expression by acting as an “amplifier” in a site-specific manner—when the anterior part of VL is suppressed, seizure amplitude decreased, and when the posterior part of VL is inhibited, shorter seizures were observed.
Second, because of the limited temporal resolution of chemogenetics, the authors suppressed VL using closed-loop optogenetics. They employed a neural net model to detect seizures automatically and interrupt them by a light pulse. With this optogenetic technique, even when the right VL was suppressed, ictal activity still proceeded to the left hemisphere, again, supporting the interhemispheric transmission of ictal activity via nonthalamic routes.
More direct evidence of the primacy of the CC as the pathway for focal to bilateral seizure activity was obtained by interrupting callosal fibers. By severing the CC with knife cuts at its genu (anterior), but not its body (posterior), seizure activity spread to the contralateral hemisphere was prevented. This result clearly demonstrates that the spread of seizure activity from a frontal lobe focus occurs via the CC. Does this mean that the centrencephalic theory is dead? Not entirely. As discussed above, the centrencephalic theory posits that the thalamus generates bilateral seizures—that conclusion is challenged by the present data, but the thalamus does maintain an active role in the modulation of focal to bilateral motor seizures by amplifying seizure amplitude and duration.
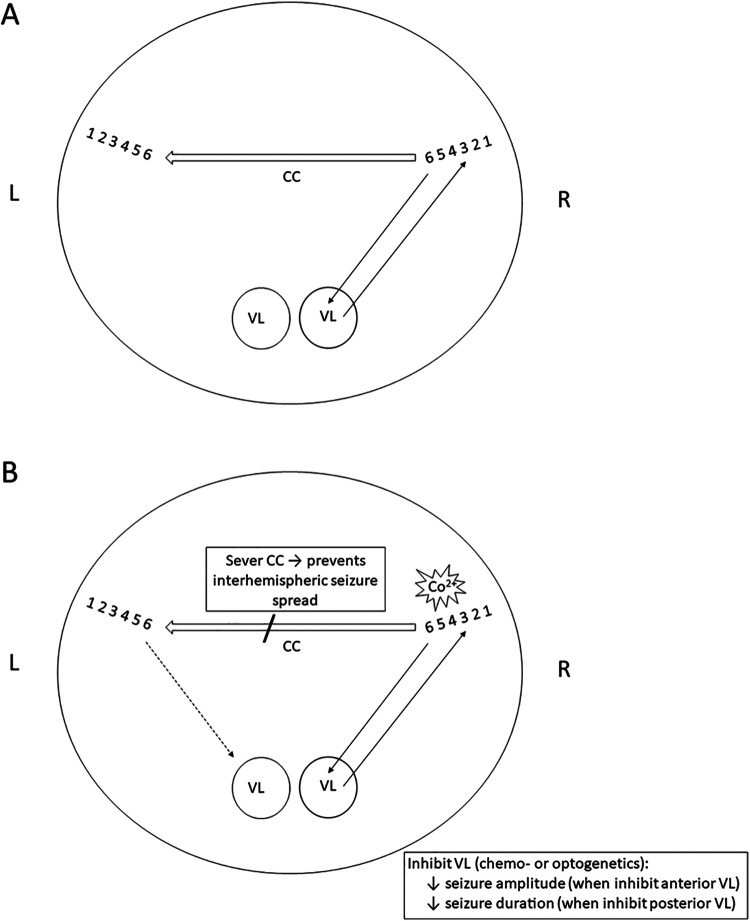
The circuit proposed here by Brodovskaya et al implies that ictal activity generated in a cortical focus utilizes extant neural pathways to spread to the other hemisphere via the CC and subcortically to the thalamus. Their model is summarized schematically in Figure 1. Neuronal lamination of right and left premotor cortex is illustrated by numerals 1 to 6, interconnected by the CC. Left and right VL thalamic nuclei receive ipsilateral cortical input and VL sends thalamocortical projections back to motor cortex. Co2+ exposure creates a seizure focus, with ictal LFPs reaching the left motor cortex and right VL quickly and simultaneously but ictal LFPs appear in the left VL only after a delay. Focal activity spreads to the contralateral hemisphere even when VL is inactivated by chemogenetic or optogenetic techniques, but the thalamus nevertheless modulates seizure expression.
Figure 1.
Schematic of coronal brain section to illustrate potential pathways of focal to bilateral seizure spread. A, Neuronal lamination of right (R) and left (L) premotor cortex is illustrated by numerals 1 to 6, interconnected by the corpus callosum (CC, large arrow). Left and right ventral lateral (VL) nuclei of the thalamus are shown. B, Cobalt (Co2+) produces a seizure focus (star). Seizure activity reaches the left motor cortex and right VL quickly and simultaneously but arrives at the left VL but only after a long delay (dashed line). Cutting the CC prevents the bilateral spread of ictal activity. Suppression of VL via chemogenetic or optogenetic techniques decreases seizure amplitude and duration, confirming that the thalamus modulates (“amplifies”) focal seizure expression.
Several differences exist between this proposed mechanism for focal to GTC seizures and for primary generalized seizures such as absence seizures, which involve circuit interactions between somatosensory cortex, reticular thalamic neurons, and thalamocortical neurons. 4 A major difference is the cortical region involved in expression of the 2 seizure types. Absence seizures involve somatosensory cortex whereas focal seizures arise from motor areas that mediate GTCs. Specific cortical layers that predominate in the 2 seizure types, as well as different thalamic nuclei, produce diversity in seizure semiology and mechanisms of propagation. Corticothalamic input reaches VL via layers 5 to 6; thalamocortical input innervates layers 2 to 3. Activity progressing contralaterally via the CC arises from layers 2 to 3. 3 Future work will elucidate the roles of specific cortical layers in the spread of seizure activity and establish their clinical significance.
Other pivotal studies have revealed variation in focal pathways of seizure spread and modulation as well. In a model of focal seizures caused by stroke in somatosensory cortex, relevant (intralaminar) thalamic nuclei were shown using optogenetic techniques to be critical for the bilateral spread of a seizure. 5 Furthermore, it is important to note that the Co2+ model represents a form of acute reactive seizure, not chronic epilepsy. In epilepsy, circuits are modified chronically by a number of structural and physiological processes such as neuronal loss, synaptic plasticity, and axonal sprouting, and the routes by which focal seizures spread in epilepsy require further study. 6
When considering the clinical implications of this work, the role of corpus callosotomy for seizure control naturally arises. This surgical technique, once used more avidly, has recently been limited to certain patients with atonic (drop) seizures in which the ictal activity spreads quickly between hemispheres, with the idea that interrupting this rapid bilateral seizure spread might avert drop seizures and prevent physical injury. The limited scope and effectiveness of corpus callosotomy could potentially be improved with novel techniques and approaches. 7 Certainly, basic investigations into the distinct pathways underlying various epilepsies could open such opportunities as the relative importance of the various neural pathways involved in bilateral seizure spread emerge.
Footnotes
ORCID iD: Carl E. Stafstrom, MD, PhD  https://orcid.org/0000-0002-4432-2453
https://orcid.org/0000-0002-4432-2453
References
- 1. Penfield W, Jasper HH. Epilepsy and the Functional Anatomy of the Human Brain. Little Brown; 1954. [Google Scholar]
- 2. Brodovskaya A, Batabyal T, Shiono S, Sun H, Kapur J. Distinct roles of rodent thalamus and corpus callosum in seizure generalization. Ann Neurol. 2022;91(5):682–696. doi:10.1002/ana.26338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brodovskaya A, Kapur J. Circuits generating secondarily generalized seizures. Epilepsy Behav. 2019;101(Pt B):106474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Huguenard J. Current controversy: Spikes, bursts, and synchrony in generalized absence epilepsy: unresolved questions regarding thalamocortical synchrony in absence epilepsy. Epilepsy Curr. 2019;19(2):105–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Paz JT, Davidson TJ, Frechette ES, et al. Closed-loop optogenetic control of thalamus as a tool for interrupting seizures after cortical injury. Nat Neurosci. 2013;16(1):64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pitkänen A, Lukasiuk K, Dudek FE, Staley KJ. Epileptogenesis. Cold Spring Harb Perspect Med. 2015;5(10):a022822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Markosian C, Patel S, Kosach S, Goodman RR, Tomycz JD. Corpus callosotomy in the modern era: origins, efficacy, technical variations, complications, and indications. World Neurosurg. 2022;159:146–155. [DOI] [PubMed] [Google Scholar]