Abstract
Spinal cord injury (SCI) results in massive neuronal death, axonal disruption, and cascading inflammatory response, which causes further damage to impaired neurons. The survived neurons with damaged function fail to form effective neuronal circuits. It is mainly caused by the neuroinflammatory microenvironment at injury sites and regenerated axons without guidance. To address this challenge, a ferrofluid hydrogel (FFH) was prepared with Ferric tetrasulfide (Fe3S4), carboxymethyl chitosan, and gold. Its internal structural particles can be oriented in a magnetic field to acquire anisotropy. Moreover, Fe3S4 can release hydrogen sulfide (H2S) with anti-inflammatory effects under acidic conditions. Regarding in vitro experiments, 0.01g/ml Fe3S4 FFH significantly reduced the inflammatory factors produced by LPS-induced BV2 cells. Oriented and longer axons of the induced neural stem cells loaded on anisotropic FFH were observed. In vivo experiments showed that FFH reduced the activated microglia/macrophage and the expression of pro-inflammatory factors in SCI rats through the NF-κB pathway. Moreover, it significantly promoted directional axonal regrowth and functional recovery after SCI. Given the critical role of inhibition of neuroinflammation and directional axonal growth, anisotropic Fe3S4 FFH is a promising alternative for the treatment of SCI.
Keywords: Spinal cord injury, Anisotropic, Ferrofluid, Hydrogen sulfide
Graphical abstract
Figure 1 Schematic representation of the Fe3S4 FFH synthesis. During the process of synthesis, the particles inside the FFH can be oriented and stabilized in a magnetic field. Axons can be directed to grow under this physical signal. The release of H2S is slow and sustained, providing a beneficial effect to improve the inflammatory microenvironment. Then, its effect on the differentiation of induced neural stem cells (iNSCs) and the treatment of SCI was explored. CMC = carboxymethyl chitosan, Fe3S4 = Ferric tetrasulfide, Au = gold.
Highlights
-
•
An anisotropic Fe3S4 ferrofluid hydrogel with allied particles was prepared, in which the axonal growth can be guided.
-
•
This hydrogel can continuously release H2S to improve the inflammatory microenvironment and promote axonal regrowth.
-
•
The anisotropy and anti-inflammatory effects of this hydrogel provide a promising candidate for the treatment of SCI.
1. Introduction
Spinal cord injury (SCI) is an extremely devastating traumatic injury with massive neuronal death and axonal disruption [1,2]. A key factor limiting the effectiveness of SCI treatment is regenerated axons without guidance [3,4]. Recently, several anisotropic hydrogels with allied particles formed a directional microtube, in which the axonal growth can be guided. This contributes to the proliferation and differentiation of neural stem cells(NSCs) and also increases functional connections [5]. There are many approaches to achieving directional alignment of particles, such as electrospinning, 3D printing, and electricity [[6], [7], [8], [9]]. However, their application is limited due to disadvantages, such as poor mechanical properties, low biocompatibility, complex fabrication process, and additional ionization. Recent advances in magnetism made magnetic anisotropy with benefits including a homogeneous, contact-free approach and ease of operation [[10], [11], [12]].
After the primary injury, subsequent inflammation further creates a harsh environment for survived neurons and impedes axonal regeneration in the injured site [13,14]. Current strategies, such as drugs, surgery, and hyperbaric oxygen, were used to provide a favorable condition for axonal regeneration in SCI repair [15,16]. But the effort is not satisfying. Therefore, better-designed protective strategies are highly urgent matters. Readily deliverable biological gases can preserve nerve cells by regulating oxidative, inflammatory, and apoptosis processes. Multimodal homeostatic and highly accessible biological gases may represent a promising pharmaceutical approach to treating SCI [17]. But the continual stalling of strategies due to limitations of administration methods, pungent smell, and instability keep therapeutics from clinical application. A slow-release and direct-acting gas at the injury site may be a natural candidate.
Ferric tetrasulfide (Fe3S4), commonly used in ferrofluids, can release hydrogen sulfide (H2S) in weak acidic conditions. As paramagnetic, ferrofluid is formed by the homogeneous dispersion of magnetic substances in a liquid. Its particles can be arranged in a magnetic field and maintained stably over time [18]. Anisotropic hydrogel with linear channels can promote linear axon extension [5]. As an endogenous gaseous signal, H2S can freely cross the cell membrane without transport carriers [19]. H2S can directly scavenge reactive oxygen species (ROS) and upregulate c-glutamylcysteine synthetase, increasing the transport of cysteine, which is the major source of glutathione. H2S also reduces the inflammatory response and promotes the proliferation and differentiation of NSCs [20]. Despite its high solubility and rapid clearance, H2S has acted as a neuroprotective agent in multiple neurological disorders.
Although many biomaterials that guide axonal regeneration or reduce inflammation have been studied for SCI, few reports have combined anisotropy and anti-inflammatory effects. Based on the previous findings, our study aimed to address SCI repair limitations by developing a dual-function hydrogel to orderly guide axonal regeneration and inhibit harsh inflammation. Fe3S4 ferrofluid hydrogel (FFH) was prepared with Fe3S4, carboxymethyl chitosan, and gold. During synthesis, the particles inside the FFH can be oriented and stabilized in a magnetic field. Axons can be guided under this physical signal. The release of H2S was slow and sustained, providing a beneficial effect to the inflammatory microenvironment. Then, its effect on the differentiation of induced neural stem cells (iNSC) and the treatment of SCI was explored.
2. Methods
2.1. Synthesis of Fe3S4 ferrofluid hydrogel
0.2 g Fe3S4 particles were dispersed uniformly in 2 ml deionized water. 10 ml of deionized water and 0.15 g of highly-purified carboxymethyl chitosan (Damao, China) were added to the magnetic stirrer, followed by high-speed stirring with the chloroauric acid solution (Aladdin, China). Then the mixture was transferred to a water bath for 3h and then cooled to room temperature. The Fe3S4 dispersion was added to the above mixture and maintained with high-speed mechanical stirring for 2h. Subsequently, it was transferred to an ultrasonic dispersion apparatus with high frequency shaking for 30min to obtain Fe3S4 ferrofluid. 0.06 g of aldehyde-based chitosan, 0.45 g of carboxymethyl chitosan, and 2 mg of carbon-dotted copper were mixed in 10ml of deionized water and mechanically stirred until a viscous gel was obtained. Subsequently, 1g of Fe3S4 ferrofluid was added and stirred continuously until a hydrogel was obtained. Meanwhile, an appropriate amount of Lactide (Meilunbio, China) was added and stirred until homogeneous. The hydrogel was placed in a magnetic field, in which the particles were rearranged during the synthesis to form aligned structures.
2.2. Characterization of hydrogel swelling properties
The equilibrium swelling rate (Ws) of the hydrogels in 37 °C PBS solution for different times was determined. The weight of Fe3S4 ferrofluid hydrogel before swelling was recorded as W0. The weights of the hydrogel at each specific time after soaking in PBS solution were recorded as Wt. The swelling ratio was recorded as Ws=(Wt-W0)/W0*100%
The remaining percentage of the hydrogel in PBS at different times was used to evaluate the degradability of hydrogel. The weight of the dried Fe3S4 FFH was recorded as W0. The Fe3S4 FFH was immersed in 10ml of PBS solution, which was changed daily. After drying at different times, the weight was recorded as Wx. The remaining rate was recorded as Wx/W0*100%
2.3. Characterization of hydrogel anisotropy
The hydrogel was laid flat on a slide with a thickness of 1 mm and the fine structure was observed under a light microscope. Then the optical scattering pattern was observed by a red laser (wavelength 650 nm) that directed towards hydrogel at a vertical angel.
2.4. Concentration of H2S released by Fe3S4 FFH
The concentrations of H2S released in vitro were determined by the methylene blue standard curve. Firstly, Na2S standard solutions (concentrations of 5, 10, 20, 40, 60, 80, and 100 μM) were prepared with sodium sulfide (Na2S) and distilled water. 1 ml of each concentration of Na2S standard solution was taken, and repeated three times. The reaction solution was fully reacted with methylene blue reagent for 30min at room temperature. The absorption spectrum was detected by a UV spectrophotometer. Then a standard curve was plotted as a control. Then 1g of Fe3S4 FFH and 10 ml of deionized water were mixed, 1 ml of each sample was taken at different times, and the reaction with methylene blue reagent was also carried out at room temperature for 30min. The maximum absorbance at 670 nm was detected. The concentration of H2S was calculated according to the previous standard curve.
2.5. Culture and induction of iNSC
In this experiment, human iPSC-derived iNSC were used to explore the effect of Fe3S4 FFH. iPSC was obtained from the Key Laboratory of Stem Cell and Tissue Engineering, Ministry of Education. A reported induction protocol for the spinal cord derived iNSC was used [[21], [22], [23]]. The iPSCs were cultured with mTeSR medium (Stemcell, Canada) to proliferate in the form of colonies. ReLeSR (Stemcell, Canada) was used every 5 days at a 1:3 ratio for passaging. When 70% of cells were contacted, a neural induction medium (NIM) was added. Accutase (Gibco, USA) was used to passage every 3 days at a ratio of 1:3. After 10 days, when the cells were induced into iNSC, NIM was replaced by a neural maintenance medium (NMM). iNSC differentiation was induced in vitro with a neural differentiation medium (NDM). Neurospheres were identified by immunofluorescence. iPSCs were labeled with Nanog and Oct4, iNSC with Nestin and Pax6, neurons with TUJ1, astrocytes with GFAP, and oligodendrocytes with MBP.
2.6. Live/dead staining and CCK-8
iNSC in neurospheres form was cultured on the Fe3S4 FFH. On the 4th day, Calcein-AM/PI (Dojindo, Japan) was added to iNSC for 15min. A confocal reflection microscope (Leica, Germany) was used to capture images. The CCK-8(Dojindo, Japan) solution was added to culture plates at a ratio of 1:10 to detect cell proliferation on day 1,4,7. After incubation for 2 h, 100μl of the supernatant mixed solution was transferred into 96-well plates and measured with an enzyme-labelling instrument (SpectraMax M5, USA) at the 450 nm wavelength.
2.7. BV2 culture and stimulation
BV2 cells were cultured with DMEM high glucose(Gibco, USA) supplemented with 10% fetal bovine serum (FBS)(Gibco, USA) and 1% penicillin-streptomycin(PS)(Gibco, USA)at 37 °C in 5% CO2. For inflammatory stimulation, BV2 cells were reseeded on hydrogels with different Fe3S4 ferrofluid concentrations in the serum-free medium for 12h to avoid excessive activation. 100ng/ml LPS (Gibco, USA) was added to the medium for 24 h and BV2 cells were collected for RT-PCR analysis.
2.8. Directional axon extension analysis
PC12 cells were cultured with DMEM low glucose(Gibco, USA) supplemented with 10% FBS and 1% PS at 37 °C in 5% CO2. PC12 cells were reseeded on CCH, FFH(−), and FFH(+) hydrogels for 12h and switched to the differentiation medium (DMEM low glucose supplemented with 1% FBS and 1% PS) for 7 days for axonal outgrowth. iNSC were also reseeded on CCH, FFH(−), and FFH(+) hydrogels for 12h and switched to NDM for 7 days. Immunofluorescence staining was performed at the end of the experiment. The length and angle of axons were calculated using Image J.
3. Ethics statement
All animal procedures were approved by the Animal Ethics Committee of the South China Agricultural University(2021d066) and performed according to the protocols approved by the Centre of Laboratory Animals.
3.1. Complete transection of spinal cord and grouping
Adult female SD rats (6–8 weeks old, 200–220g) were supplied by the Guangdong Medical Laboratory Animal Center. All rats were divided into four groups: SCI group without treatment after injury, CCH group implanted carboxymethyl chitosan after injury, FFH(−) group implanted Fe3S4 FFH without anisotropy after injury, FFH(+) group implanted Fe3S4 FFH with anisotropy after injury. After anesthesia, a laminectomy at the T10 level was carried out to expose the spinal cord. The dura was incised longitudinally, then 3 mm of the spinal cord was removed, and different hydrogels were placed into the defective area. The dura was kept and sutured, and the dorsal incision was closed (Fig. S1). Manual emiction was given twice daily after the operation until their automatic micturition function recovered. Gentamicin(4000U/Kg/day) was given for 3 days after surgery to prevent additional infections.
3.2. Behavioral tests
Basso–Beattie–Bresnahan (BBB) test was performed to assess the hindlimb function of rats weekly after SCI. Rats moved freely in an open field for 3min to assess the joint move range of the hindlimbs, the capacity of weight-bearing, and the resting state of the feet. Inclined grid climbing test was also performed to assess the accuracy of foot placement and coordination.
3.3. Electrophysiological analysis
The evoked potentials of rats under anesthesia were recorded by the BL-420s biological signal acquisition system (TECHMAN, China). The lamina rostral to the injury was removed as previously described. The stimulating electrode was placed on the spinal cord rostral to the injury. The recording electrode was connected to the left sciatic nerve. The ground electrode was clamped on the tail. Both the stimulating and receiving electrode was connected to the BL-420S. The waveform was recorded to analyze the amplitude and latency. Parameters setting: single square wave stimulation, 10mv, 50Hz.
3.4. Immunofluorescence
Rats were transcardially perfused sequentially with 0.9% saline and 4% paraformaldehyde after being deeply anesthetized. Spinal cord sections were washed with 1 PBS solution, then incubated with 10% normal goat serum (Beyotime, China) and 0.3% Triton X-100 (Bio-Froxx, Germany) in PBS for 1 h at room temperature. The primary antibodies (details in Supplementary Table 1) were diluted in antibody-dilution buffer (New Cell &Molecular, China), then added and incubated overnight at 4 °C. The next day, Alexa Fluor-coupled secondary antibodies were diluted and incubated for 1 h. DAPI (Servicebio, China) was then incubated for 15 min.
3.5. RT-PCR
mRNA from cells and spinal cord tissue was extracted using an RNA extraction kit (Sigma, Germany) and then converted into cDNA by a reverse transcription kit (Takara, Japan). Quantitative PCR was performed using the SYBR Green PCR Master Mix and QuantStudio 5 detection system (Thermofisher, USA). Actin-β was used as the housekeeping gene for normalization. Each sample was measured three times for each gene. The primers used were shown in Supplementary Table 2.
3.6. Western-Blot
Spinal cords of the injury site including one segment caudal and rostral were collected in RIPA lysis buffer and phosphatase inhibitor buffer (Beyotime, China). After centrifuging at 12,000g for 35min, the supernatant was collected. The protein concentration was quantified using a BCA protein analysis kit (Thermo Fisher, USA). SDS-PAGE protein loading buffer (5X) was added to the samples and heated for 5min at 100 °C. Equal amounts of protein(20 μg) were separated by 10% sodium dodecyl sulfate-polyacrylamide gel in electrophoresis and then transferred to polyvinylidene fluoride (PVDF) membranes. After being blocked in 5% BSA at room temperature for 1 h, the PVDF membranes were incubated with the primary antibodies overnight at 4 °C. After washing in 1X Tris-buffered saline (TBS) with 1% Tween 20 the next day, the PVDF membranes were incubated with the secondary antibodies for 1 h at room temperature. An enhanced chemiluminescence kit (Thermo Fisher, USA) was used to detect the bands.
3.7. Hemolysis analysis
Blood samples were collected and co-incubated with FFH for 4 h, followed by centrifugation at 12000g for 5 min at 4 °C. PBS and Triton-100X were used as the blank and positive group, respectively. The absorbance was measured, and the hemolysis percentage was calculated using the following Equation:
3.8. Statistics
To statistically analyze the collected data, SPSS 22.0 statistical analysis software and GraphPad Prism software were applied. And multiple groups were compared using analysis of one-way ANOVA and Student's t-test. P < 0.05 was considered a statistically significant difference.
4. Results
4.1. Fe3S4 ferrofluid hydrogel with paramagnetic properties and proper degradability
Fe3S4 ferrofluid hydrogel (FFH) was synthesized according to Fig. 1. When a magnet was held close to the hydrogel, the surface of which was attracted to bulge upward (Fig. 2A, B), indicating that the hydrogel was paramagnetic. The orientation of Fe3S4 FFH particles was unified in the magnetic field to achieve anisotropy. The structures of the intermediate and final products were observed under a scanning electron microscope (SEM). Fe3S4 particles were agglomerated and of different sizes, with uneven distribution and irregular shapes (Fig. 2C). As the intermediate product, particles in Fe3S4 ferrofluid (FF) were connected to rod-like polymer crystals, leading to a tightly connected rivet-like structure on the surface (yellow box) (Fig. 2D). Fe3S4 FFH had a porous structure (red box) (Fig. 2E), through which water and nutrients were transported to cells. After swelling in phosphate-buffered saline (PBS) solution and achieving equilibrium after 100 h, both Fe3S4 FFH and Carboxymethyl chitosan-based hydrogel (CCH) increased in volume (Fig. S2A). Fe3S4 FFH and CCH could absorb water more than 10 and 18 times their weight, respectively (Fig. 2F). The hydrogels should be biodegradable after serving as an extracellular matrix without hindering tissue regeneration. The weight remaining of Fe3S4 FFH in PBS solution after 7 days was approximately 56.35% ± 2.23%, which was higher than that in CCH (48.68% ± 7.66%). In addition, the degradation rate of FFH gradually decreased as time progressed (Fig. 2G).
Fig. 1.
Schematic representation of the Fe3S4 FFH synthesis and animal experiment. After spinal cord transection, the hydrogel was transplanted into the injury site of rats. CMC = carboxymethyl chitosan, Fe3S4 = Ferric tetrasulfide, Au = gold.
Fig. 2.
(A, B) Fe3S4 ferrofluid hydrogel could be attracted by a magnet, forming an upward bulge (white triangle), (C–E) The SEM images of Fe3S4 particles, Fe3S4 FF and Fe3S4 FFH were shown respectively. The white triangle indicates the particles of different sizes, irregular arrangement, and uneven distribution, and the yellow box indicates the “rivet” structure on the surface of the ferrofluid, with an enlarged view shown in the lower right corner. The red box indicates the porous structure of the Fe3S4 FFH, with an enlarged view in the lower right corner. (F) Swelling curves of Fe3S4 FFH and CCH in PBS solutions, (G) Degradation curves of Fe3S4 FFH and CCH in PBS solutions. (H) The concentration of H2S released from the FF and FFH at PH 6.4. (I) Effect of anisotropy on mechanical properties. (J, K) Microscopic images of FFH (−) and FFH (+). (L, M) Laser scattering test. Inside the white box are the patterns formed by the laser irradiating the hydrogels vertically. The white dotted lines indicate the patterns formed on the background after the laser scattering.
4.2. Slow release of H2S and anisotropy in FFH
According to Methods 4, the concentrations of H2S released from Fe3S4 FF and Fe3S4 FFH at different times were calculated. In Fe3S4 FF, H2S release increased rapidly and maintained a concentration of more than 30 μM after 50 h (unstable). However, H2S released from Fe3S4 FFH was stable at 10 μM after equilibrium (Fig. 2H). Using different concentrations of the direct donor Na2S, CCK-8 experiments showed that 10uM-1mM H2S promoted the cell proliferation of iNSC (Fig. S3). Fe3S4 FFHs with or without anisotropy were classified into the FFH (+) and FFH (−) groups, respectively. When stretching on the universal testing machine (Fig. S2B), anisotropy made the maximum strain of Fe3S4 FFH decrease slightly, but the maximum stress increased to more than 30 KPa (Fig. 2I). This means that the mechanical property of the hydrogel was enhanced in the anisotropic direction. Subsequently, a microscope was used to examine the microstructures of hydrogels. The particles in the FFH (−) were arranged irregularly, whereas those in the FFH (+) were almost in the same direction (Fig. 2J and K). In addition, the anisotropy had unique scattering patterns in light experiments. When the hydrogel of FFH (−) was irradiated with a laser, the pattern formed through the hydrogel was circular. It was due to the randomly scattered light in all directions. In contrast, when FFH (+) was irradiated, the scattering was enhanced along the orientation of the particles, resulting in a clear shuttle-shaped pattern (white dotted lines in Fig. 2L, M) The patterns in the white box were formed on the glass slide by laser irradiation. The patterns in the white dotted line were formed on a white background after laser scattering. This further supported the anisotropy of Fe3S4 FFH (+).
4.3. The biocompatibility and anti-inflammatory effect of Fe3S4 FFH
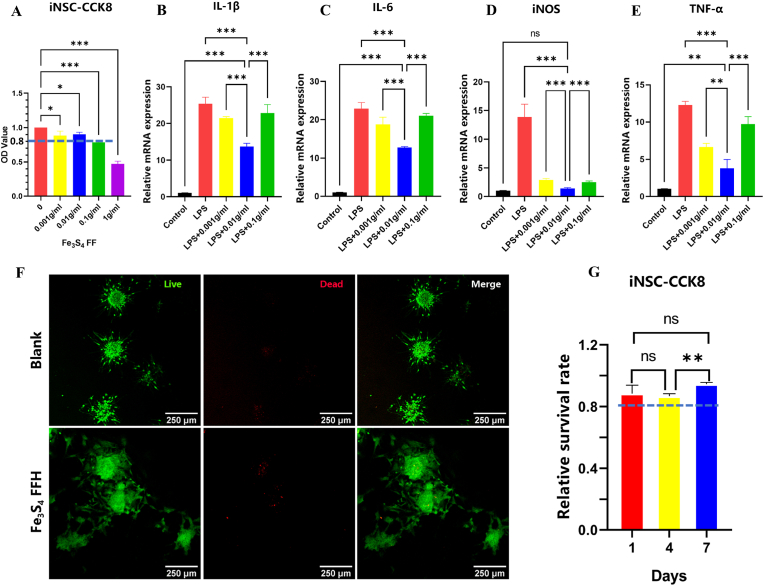
The viability of iNSC on 1 g/ml FFHs was less than 80% of the control (Fig. 3A). Therefore, it was excluded from further study. To select the optimal concentration of Fe3S4 FF, we further explored the function of anti-inflammation. The results showed that the mRNA of IL-1β, IL-6, iNOS, and TNF-α were significantly elevated after LPS stimulation while decreased in hydrogels with different Fe3S4 FF concentrations. The best outcome was observed in the 0.01 g/ml group, indicating the best anti-inflammatory effect (Fig. 3B–E).
Fig. 3.
(A) The cell activity of iNSC on hydrogels with different Fe3S4 FF concentrations, *P < 0.05, ***P < 0.001, ns means no significance, n = 3; (B–E) RT-PCR to explore mRNA of the proinflammatory factors in LPS pre-treated BV2 cells(100 ng/ml,24 h) on hydrogels with different Fe3S4 FF concentrations, (**P < 0.01, ***P < 0.001, ns means no significance, n = 3). (F) Live/Dead staining of iNSC 4 days after plantation, (G) CCK-8 to detect the cell viability of iNSC cultured on 0.01 g/ml Fe3S4 FFH for 1, 4 and 7 days (**P < 0.01, ns means no significance, n = 3).
Hydrogel with 0.01 g/ml Fe3S4 FF was served as the FFH group, and a blank plate as control. After iNSC plantation, live/dead staining was performed on day 4. Live cells were stained green, whereas dead cells were stained red. The results showed that few cells were stained red in both groups (Fig. 3F). The results of CCK-8 suggested that the survival rate of iNSC in the FFH group was more than 80% of the control group on days 1, 4 and 7 (Fig. 3G), indicating that hydrogel with the concentration of 0.01 g/ml FFH had good biocompatibility. Hemocompatibility was an important indicator for evaluating the hemolytic activity of implanted materials in vivo. As shown in Fig. 4, serum extracted from 0.01 g/ml FFH co-incubated whole blood showed slight yellow, close to that of the PBS control group. But the Triton-X100 group was bright red in color. The hemolysis ratio of FFH was 0.3143%. Therefore, the hydrogel with 0.01 g/ml Fe3S4 FF was selected for the subsequent experiments.
4.4. iNSC differentiation and directional axonal extension on FFH
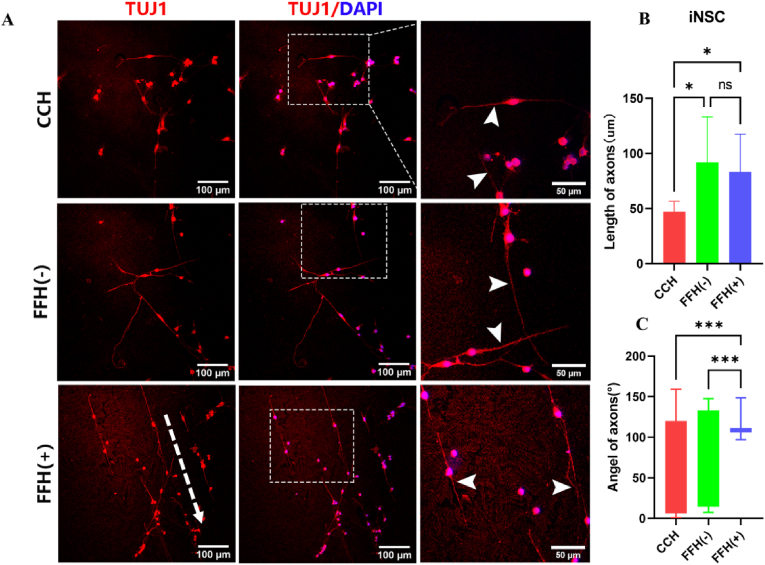
The immunofluorescence staining showed that most iNSC was stained by TUJ1, indicating that the iNSC successfully differentiated into neurons with axons (Fig. 4A). The length of axons in the CCH group was 26.8 ± 5.34 μm (Fig. 4B), shorter than that of the FFH (−) group (52.14 ± 23.51 μm) and FFH (+) group (47.34 ± 19.35 μm) (P < 0.05). The growth angle of axons in the FFH (−) group ranged in (±57.27°), which was close to that of the CCH group (±55.40°) (Fig. 4C). Most neuronal axons in the FFH (+) group exhibited almost the same orientation and more concentrated growth angle (±13.99°). Almost the same results were found in PC12 cells (Fig. S6).
Fig. 4.
(A)The axonal growth of iNSC on CCH, FFH (−), and FFH (+),(B–C)Quantitative statistics of axon length and angle on the three hydrogels(*P < 0.05, ***P < 0.001, ns means no significance).
4.5. Mobility assessment of SCI rats
The hindlimbs of the rats in 4 groups were completely paralyzed after injury. At week 3, rats in the SCI group failed to move the joints of their hindlimbs, with their legs straight and claws dragging on the ground. In other groups, the claws were able to turn over occasionally by large joints. From week 4, the hindlimb locomotor capacity of rats in the FFH (+) group experienced rapid recovery. At week 5, the BBB score of the FFH (+) group was significantly higher than that of the FFH (−) group (P < 0.01) (Fig. 5A). To evaluate the nerve conduction of injured spinal cord, the amplitude, and latency of evoked potentials were recorded 8 weeks post-injury. The waveforms after single electrical stimulation were shown in Fig. 5B. The amplitude of evoked potentials in the FFH (+) group was (1.01 ± 0.01 mV), which was significantly higher than that of the SCI group (0.23 ± 0.03 mV), CCH group(0.51 ± 0.02 mV), and FFH (−) group (0.64 ± 0.01 mV). On the contrary, the FFH (+) and FFH (−)groups had the shortest latency (Fig. 5C).
Fig. 5.
(A) BBB score of the hindlimb in rats within 8 weeks after injury, *#ɸ P < 0.05, **##ɸɸ P < 0.01, *** ###ɸɸɸ P < 0.001, * means the comparison of the FFH (+) and SCI group, # means the comparison of the FFH (+) and CCH group, ɸ means the comparison of the FFH (+) and FFH (−) group, n = 4.(B) Evoked potentials of the left hindlimb in rats at 8 weeks post-injury, the green dashed line indicates the amplitude, the blue dashed line indicates the latency. (C) Statistics of the amplitude and latency, ***P < 0.001, ns means no significance; n = 3. (D) Pictures of the left claw during inclined grid climbing test at week 8. (E) Observation of spinal cord tissue after 8 weeks. The blue dotted line refers to the approximate reaction range of the damaged area. The minimum scale of the ruler is 1 mm. (F) HE staining of the spinal cord 8 weeks post-injury. The blue dotted line refers to the approximate reaction range of the damaged area, n = 1.
At the 8th week, the images of left claw during inclined grid climbing test were shown in Fig. 5D. The SCI group was still in a contracture state. In the FFH (+) group, the toes could be straightened, and some rats were able to land on their feet without full weight bearing. Videos of rats in SCI and FFH (+) groups crawling were available in Supplementary Video1,2.
The appearance and HE staining of spinal cord showed the morphology of the spinal cord. The damaged area was filled with tissues instead of leaving a cavity and significantly thinner than uninjured (Fig. 5E and F). Moreover, the HE staining of the heart, liver, spleen, lung, and kidney revealed that Fe3S4 FFH did not result in pathological changes to viscera (Fig. S7).
4.6. Anti-inflammatory effect of Fe3S4 FFH after SCI
Inflammation after SCI was mainly mediated by macrophage/microglia. As a marker of activated macrophage/microglia, CD68 immunofluorescence was performed on normal and 7-days injured rats. Only little CD68 staining was found in normal rats, indicating the resting state of macrophage/microglia in the uninjured spinal cord (Fig. 8A). The fluorescence intensity in the SCI group was the strongest among the four groups, indicating extensive macrophage/microglia activation after SCI (Fig. 6A and B). It was lower in the FFH (−) and FFH (+) groups than in the CCH group. However, no significant difference was observed between the FFH (−) and FFH (+) groups. Western blotting (WB) also showed the highest CD68 expression in the SCI group, followed by CCH. The FFH (−) and FFH (+) groups had the lowest CD68 expression (Fig. 6C). Fe3S4 FFH also significantly reduced the expression of proinflammatory factors (iNOS, IL-1β, TNF-α) at 7-day post injury (Fig. 6D–F). We further analyzed these factors 1-day post-injury, and similar results were obtained (Fig. S9). These results revealed that H2S can alleviate the inflammation in the early stage of SCI.
Fig. 6.
(A) CD68 immunofluorescence staining in the spinal cord of four groups at 7 days after injury, (B) Quantitative of CD68 immunofluorescence intensity in four groups, **P < 0.01, ***P < 0.001, ns means no significance, n = 3. (C) CD68 expression in the spinal cord by WB, n = 3. (D–F) The mRNA of IL-1β, iNOS, and TNF-α detected by RT-PCR 7 days post injury, ***P < 0.001, ns means no significance, n = 3. (G) The relationship between the anti-inflammatory effect and the NF-κB pathway was verified by WB, n = 3.
The expression of P65 and IκB in the injured tissues was examined by WB to investigate the mechanism underlying the anti-inflammatory effects of Fe3S4 FFH. There was no significant difference in the expression of P65 and IκB among the four groups. However, the levels of phosphorylated IκB and P65 were lowest in the FFH (−) and FFH (+) groups (Fig. 6G). This trend was consistent with the pro-inflammatory factors described above. Therefore, the anti-inflammatory effect of Fe3S4 FFH was related to the inhibition of the NF-κB pathway.
4.7. Axonal regeneration in SCI rats by anisotropic Fe3S4 FFH
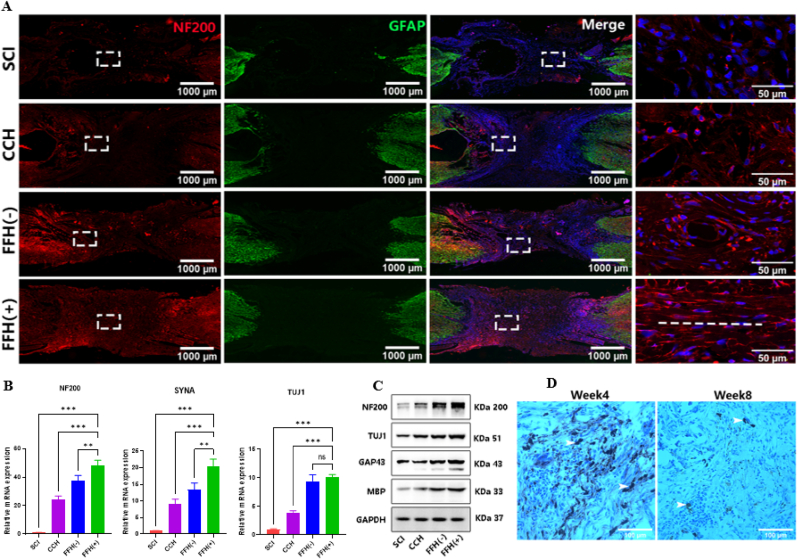
Immunofluorescence staining was performed to assess the neural regeneration (Fig. 7A, S8B). GFAP was used to outline the edge of the injury area and NF200 to demonstrate the regenerated neurofilaments (Fig. 7A). The absence of GFAP at the injury sites showed that the spinal cords were completely transected. For NF200 staining, large defects and cavities were observed in the SCI group, with weak neurofilament fluorescence and fewer connections at both ends.
Fig. 7.
(A) The immunofluorescence staining of spinal cords 8 weeks after injury. Nerve filaments and astrocytes were labeled with NF200 and GFAP respectively. The enlarged picture in the white box was displayed in the right column, and the white dotted line indicates the anisotropic direction, n = 1. (B) RT-PCR and (C) WB to detect the expression levels of several axon-related proteins in the four groups 8 weeks after SCI. **P < 0.01, ***P < 0.001, ns means no significance, n = 3. (D) Prussian iron staining in the injured spinal cord at 4 and 8 weeks, white arrows indicate iron, which disappeared by 8 weeks, n = 1.
In the CCH group, slightly higher expression of neurofilaments was observed than that in the SCI group. In FFH (−) and FFH (+) groups, the neurofilaments increased significantly with massive neural connections at both ends. However, the NF200 staining of the injured area was stronger in the FFH (+) group, indicating a higher density of neurofilaments. In addition, the magnification image in the FFH (+) group showed a more consistent orientation of neurofilament compared with other groups.
To explore the phenotypes of newly formed neurons, we further performed the 5-HT(descending neurons of cortico-spinal tract) and CGRP staining(ascending sensory neurons) (Fig. S10). Little 5-HT(+) and CGRP(+) staining was observed in the SCI group and CCH group, which may due to the limited neural regeneration. Although lots of CGRP (+) axons were visible in both FFH (−) and FFH (+) group, indicating large numbers of nascent sensory nerves, the 5-HT (+) axons in FFH (−) group were fewer than that in FFH (+) group. It can be inferred that the anisotropy promoted the regeneration of the cortico-spinal tract. However, the detailed mechanisms involved need further study. Protein expressions of TUJ1(neurons), NF200 (neurofilaments), GAP43(growth cone), and MBP (myelin sheath) were also used to reflect the neural regeneration at the injury site by Western Blot. It was consistent with the immunofluorescence analysis (Fig. 7C). We also quantified the mRNA of the TUJ1, NF200, and SYNA (synapses) to assess the neural regeneration, in which FFH (+) group was the highest (Fig. 7B). We further analyzed the expression of axon guidance molecules, Semaphorin(Sema3a), Netrin(Ntn1), Slit(Slit1), Ephrin B(Efnb1), to determine whether they participate in the orientated growth of axons. There was no difference in Semaphorin, a potent inhibitory cue, between FFH(+) and FFH (−) groups. But both FFH groups were slightly downregulated compared to the CCH group. This indicated that the released H2S might be able to inhibit Semaphorin to promote axon regrowth. Netrin, Slit, and Ephrin B showed no difference among each group (Fig. S11). This indicated that the anisotropy of FFH had no significant effect on the expression of axon guidance molecules. The orientated growth of axons was mostly due to the topography of the hydrogel.
To explore the fate of Fe3S4 FFH, Prussian iron staining was carried out. The results showed that a large amount of iron was stained in the damaged area at 4 weeks but mostly disappeared at 8 weeks (Fig. 7D). It indicated the degradation of the Fe3S4 ferrofluid hydrogel. The images of spinal cords at different times after transplantation also showed that the FFH hydrogel was gradually degraded in the first 6 weeks and disappeared at 8 weeks after transplantation (Fig. S13).
5. Discussion
5.1. The anisotropy of hydrogel
The anisotropic hydrogels can be synthesized by surface patterning, 3D printing, light, current, and magnetism [[6], [7], [8], [9]]. Surface patterning only creates oriented grooves on the surface of the material. 3D printing can arrange all particles in the same orientation, but the fabrication process is complicated. Light-induced anisotropy is mostly applied to photosensitive materials. Electricity allows the internal particles within hydrogels to be oriented in the direction of the current. But additional electrolysis or dissociation may be caused. The magnetic field-induced anisotropic hydrogel is synthesized in a uniform, broad, and contact-free manner. With the recent development in superconducting magnetism, the orientation of internal particles can be done by magnet field conveniently. Once the orientation arrangement is completed during the gelatinization, the particle arrangement becomes stable [24]. Gently handling and placing the hydrogel will not disturb the orientation arrangement.
5.2. The effect of anisotropy on neuronal regeneration
Without guidance signals, axons regenerated at the lesion site are disordered after SCI, with limited capacity to form a relay [25]. This might be a reason for the limited effectiveness of current therapies. The reinnervation and functional recovery after SCI depend on the longitudinally directed regrowth of injured axons [25,26]. Various anisotropic hydrogels can elicit directed axonal regeneration through the directional arrangement of internal particles, which can increase the effective connections [27,28]. Studies have demonstrated that axonal guidance by physical signals, such as highly anisotropic polymer hydrogels, can induce highly oriented axonal growth and form neuronal networks and functional connectivity [5,29,30]. An anisotropic hydrogel containing rod-shaped microgels also achieved in-situ orientation under a magnetic field. This narrow and long microgel enabled strong axonal guidance to promote axonal growth. Another nanohydrogel with hierarchically anisotropic microstructures provided multiple physical cues to repair SCI. Its aligned microstructure promoted cell migration and orientation, which further stimulated angiogenesis and neuronal extension [31]. It also found that the direction of neuronal growth on the hybrid hydrogel was determined within the first 3 days, followed by enhanced alignment [31]. Long directional axons and interrupted conduction bundles formed a relay with the help of implants. This played an important role in restoring spinal cord conductivity. There might be an angular range that allowed for effective connections between the protrusions of the neurons, and the same orientation of the axons increased this possibility.
5-HT represents descending neurons of the cortico-spinal tract and CGRP represents ascending sensory neurons [32,33]. These two indexes can show anterograde or retrograde axonal regeneration. Better 5-HT fluorescence were observed in the FFH(+) group, indicating that the anisotropy might be able to facilitate cortico-spinal tract regeneration.
We also explored the relationship between topology and axon guidance molecules, the endogenous factors that guide axonal growth by attracting or repelling axons, but discovered no positive results. Probably because axon guidance molecules mainly attract or repel axon migration during the development of the nervous system and will not be affected by topography [34,35].
5.3. Advantages of slowly released H2S
Despite being a protective agent, H2S also has some concerns that hinder its application. A high concentration of H2S would cause brain damage and decrease learning and memory function [36]. Besides, H2S can aggravate antiproliferative and proapoptotic effects during atherosclerosis and exhibit pro-inflammatory effects in pancreatitis, sepsis, and hemorrhagic shock [36]. Strong H2S odor or acute exposure also leads to eye irritations, neurological disorders, skin symptoms cardiovascular abnormalities, and respiratory symptoms [37]. It is generally agreed that the concerning outcomes of hydrogen sulfide are related to the concentration and administration routes [36]. High concentrations of H2S aggravated inflammation while low concentrations alleviated inflammation. Based on the above concerns, the sustained release system was critical for its application.
Commonly used donors of H2S include inorganic salts such as sodium hydrosulfide (NaHS), and sodium sulfide (Na2S). They failed to simulate the biological process of H2S because of their extremely rapid release, which often results in excessive concentration. Although there were H2S compounds with a slow-release property such as GYY4137 and AP39, the short half-life period limits their application [[38], [39], [40]]. Therefore, maintaining an effective concentration of H2S in vivo require additional dosage continuously. We demonstrated the feasibility of Fe3S4 FFH to release H2S within an acute therapeutic window (24–72 h). The releasing of H2S rose rapidly to 10 μM in the first 50 h, followed by an equilibrium. It is consistent with the pathophysiology process of the inflammation after SCI and avoid frequent supplementation [41,42].
With a high clearance rate and consumption in tissues, the concentration of H2S in most tissues was estimated in the low nanomolar range [43]. However, free H2S, acid-labile sulfide, and sulfane-sulfur were interchangeable, and measurement in biological samples can be confounded by side reactivity, leading to highly variable estimates of H2S concentration [44]. Although we failed to provide the concentration of H2S released from FFH in vivo, we demonstrated that the toxic threshold of H2S was 1 mM (Fig. S3), which was 100-fold higher than released from FFH in vitro. We also proved that FFH showed no toxicity to the viscera (Fig. S7), indicating no long-term toxicity. Based on the above reasons, we believed the acute toxidrome of H2S from FFH was minimal. And more accurate measurements are required in future to assess the H2S in vivo.
5.4. The effect of H2S in the treatment of SCI
H2S was a novel neuro-modulator and neuroprotective agent, with anti-inflammatory, antioxidant, and anti-apoptotic effects in many neurological diseases [44,45]. In the nervous system, H2S reduces inflammation through direct anti-inflammatory effects and indirect effects such as inhibition of the NF-κB pathway, making it a potent immunomodulatory agent [46]. Following SCI, the early phase of inflammation was comprised principally of neutrophils (peaking 1-day post-injury), and macrophages/microglia (peaking 7 days post-injury) [47], and the latter was maintained for several months [48]. In this study, CD68 was used to label activated microglia/macrophages at the injury site on day 7 after SCI. It was worth noting that, there was no significant differences in CD68 expression between FFH(−) and FFH(+) groups. This indicated that the anisotropy had no effect on inflammation. The anti-inflammatory effect of H2S in the nervous system was shown in reducing the inflammatory mediators, such as NO, TNF-α, and IL-1β or inhibiting oxidative stress [49,50]. In RT-PCR, Fe3S4 FFH reduced the mRNA levels of pro-inflammatory factors such as IL-1β, TNF-α, and iNOS on day 1 and day 7 after SCI. This will help to reduce the damage of inflammation on neurons.
Besides anti-inflammation, H2S was proved to promote axonal regrowth [51]and upregulate the expression of axonal guidance molecules such as NGF and ARTN [52]. NaSH was proved to enhance the proliferation of NSCs through the extracellular signal-regulated kinase (ERK) 1/2 pathway, and regulate the differentiation of NSCs by some factors [53]. In this study, iNSC and PC12 loaded on FFH sprouted longer axons than those loaded on CCH. This indicated that H2S can promote axonal extension in vitro. It also increased the CGRP(+) axons and promoted the expression of TUJ1, NF200, and SYNA in vivo. Moreover, Semaphorin, a potent inhibitory axon guidance molecule, was slightly downregulated after ferrofluid hydrogel transplantation. This indicated that the released H2S might be able to inhibit Semaphorin to promote axon regrowth. However, the detailed mechanisms involved need further study.
6. Conclusion
We designed an anisotropic Fe3S4 ferrofluid hydrogel with H2S sustained releasing property in this study. The Fe3S4 FFH possesses great biocompatibility and releases H2S at a concentration of 10 μM, displaying excellent anti-inflammatory and neurotrophic effects. Moreover, the preorientation of Fe3S4 particles by magnetic field promotes axonal directional extension in vitro. The in vivo experiments proved that Fe3S4 FFH could attenuate microglia/macrophage activation through the NF-κB pathway. Further, the anisotropy of FFH significantly stimulated directional axonal regeneration and motor function recovery in SCI rats. The immunomodulatory and anisotropic dual effects make Fe3S4 FFH a promising candidate for the treatment of SCI.
CRediT authorship contribution statement
Ruofei Wang: Conceptualization, Methodology, Formal analysis, Writing – original draft. Xiaxiao Wu: Conceptualization, Methodology, Investigation, Data curation, Data curSation. Zhenming Tian: Methodology, Validation, Writing. Tian Hu: Methodology, Investigation. Chaoyang Cai: Methodology, Investigation, Resources. Guanping Wu: Methodology, Data curation. Gangbiao Jiang: Conceptualization, Validation, Supervision. Bin Liu: Conceptualization, Formal analysis, Supervision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could appear to influence the work reported in this paper.
Acknowledgments
This work was supported by the following grants: the National Key Research and Development Program of China (2017YFA0105400); the National Natural Science Foundation of China (82072455, 81772349); the Guangdong Basic and Applied Basic Research Foundation (2019A1515012181). The graphical abstract was created with BioRender.com.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bioactmat.2022.10.020.
Contributor Information
Gang-biao Jiang, Email: jgb3h@163.com.
Bin Liu, Email: liubin6@mail.sysu.edu.cn.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Golpich M., Amini E., Mohamed Z., Azman Ali R., Mohamed Ibrahim N., Ahmadiani A. Mitochondrial dysfunction and biogenesis in neurodegenerative diseases: pathogenesis and treatment. CNS Neurosci. Ther. 2017;23(1) doi: 10.1111/cns.12655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cao Y., Lv G., Wang Y.-S., Fan Z.-K., Bi Y.-L., Zhao L., Guo Z.-P. Mitochondrial fusion and fission after spinal sacord injury in rats. Brain Res. 2013;1522:59–66. doi: 10.1016/j.brainres.2013.05.033. [DOI] [PubMed] [Google Scholar]
- 3.Günther M.I., Weidner N., Müller R., Blesch A. Cell-seeded alginate hydrogel scaffolds promote directed linear axonal regeneration in the injured rat spinal cord. Acta Biomater. 2015;27:140–150. doi: 10.1016/j.actbio.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 4.Yao S., Yu S., Cao Z., Yang Y., Yu X., Mao H.-Q., Wang L.-N., Sun X., Zhao L., Wang X. Hierarchically aligned fibrin nanofiber hydrogel accelerated axonal regrowth and locomotor function recovery in rat spinal cord injury. Int. J. Nanomed. 2018;13:2883–2895. doi: 10.2147/IJN.S159356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rose J.C., Cámara-Torres M., Rahimi K., Köhler J., Möller M., De Laporte L. Nerve cells decide to orient inside an injectable hydrogel with minimal structural guidance. Nano Lett. 2017;17(6):3782–3791. doi: 10.1021/acs.nanolett.7b01123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li G., Zhao X., Zhao W., Zhang L., Wang C., Jiang M., Gu X., Yang Y. Porous chitosan scaffolds with surface micropatterning and inner porosity and their effects on Schwann cells. Biomaterials. 2014;35(30):8503–8513. doi: 10.1016/j.biomaterials.2014.05.093. [DOI] [PubMed] [Google Scholar]
- 7.Wu S., Ni S., Jiang X., Kuss M.A., Wang H.-J., Duan B. Guiding mesenchymal stem cells into myelinating schwann cell-like phenotypes by using electrospun core-sheath nanoyarns. ACS Biomater. Sci. Eng. 2019;5(10):5284–5294. doi: 10.1021/acsbiomaterials.9b00748. [DOI] [PubMed] [Google Scholar]
- 8.Wang L., Wu Y., Hu T., Ma P.X., Guo B. Aligned conductive core-shell biomimetic scaffolds based on nanofiber yarns/hydrogel for enhanced 3D neurite outgrowth alignment and elongation. Acta Biomater. 2019;96:175–187. doi: 10.1016/j.actbio.2019.06.035. [DOI] [PubMed] [Google Scholar]
- 9.Yu T., Wen L., He J., Xu Y., Li T., Wang W., Ma Y., Ahmad M.A., Tian X., Fan J., Wang X., Hagiwara H., Ao Q. Fabrication and evaluation of an optimized acellular nerve allograft with multiple axial channels. Acta Biomater. 2020;115:235–249. doi: 10.1016/j.actbio.2020.07.059. [DOI] [PubMed] [Google Scholar]
- 10.Cheng F.-M., Chen H.-X., Li H.-D. Recent progress on hydrogel actuators. J. Mater. Chem. B. 2021;9(7):1762–1780. doi: 10.1039/d0tb02524k. [DOI] [PubMed] [Google Scholar]
- 11.Sano K., Ishida Y., Aida T. Synthesis of anisotropic hydrogels and their applications. Angew Chem. Int. Ed. Engl. 2018;57(10):2532–2543. doi: 10.1002/anie.201708196. [DOI] [PubMed] [Google Scholar]
- 12.Babu S., Albertino F., Omidinia Anarkoli A., De Laporte L. Controlling structure with injectable biomaterials to better mimic tissue heterogeneity and anisotropy. Adv. Healthc Mater. 2021;10(11) doi: 10.1002/adhm.202002221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hutson T.H., Di Giovanni S. The translational landscape in spinal cord injury: focus on neuroplasticity and regeneration. Nat. Rev. Neurol. 2019;15(12):732–745. doi: 10.1038/s41582-019-0280-3. [DOI] [PubMed] [Google Scholar]
- 14.Fischer I., Dulin J.N., Lane M.A. Transplanting neural progenitor cells to restore connectivity after spinal cord injury. Nat. Rev. Neurosci. 2020;21(7):366–383. doi: 10.1038/s41583-020-0314-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Badhiwala J.H., Wilson J.R., Witiw C.D., Harrop J.S., Vaccaro A.R., Aarabi B., Grossman R.G., Geisler F.H., Fehlings M.G. The influence of timing of surgical decompression for acute spinal cord injury: a pooled analysis of individual patient data. Lancet Neurol. 2021;20(2):117–126. doi: 10.1016/S1474-4422(20)30406-3. [DOI] [PubMed] [Google Scholar]
- 16.Ahuja C.S., Wilson J.R., Nori S., Kotter M.R.N., Druschel C., Curt A., Fehlings M.G. Traumatic spinal cord injury. Nat. Rev. Dis. Prim. 2017;3 doi: 10.1038/nrdp.2017.18. [DOI] [PubMed] [Google Scholar]
- 17.Zafonte R.D., Wang L., Arbelaez C.A., Dennison R., Teng Y.D. Medical gas therapy for tissue, organ, and CNS protection: a systematic review of effects, mechanisms, and challenges. Adv. Sci. 2022;9(13) doi: 10.1002/advs.202104136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cervantes O., Lopez Z.D.R., Casillas N., Knauth P., Checa N., Cholico F.A., Hernandez-Gutiérrez R., Quintero L.H., Paz J.A., Cano M.E. A ferrofluid with surface modified nanoparticles for magnetic hyperthermia and high ROS production. Molecules. 2022;27(2) doi: 10.3390/molecules27020544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mathai J.C., Missner A., Kügler P., Saparov S.M., Zeidel M.L., Lee J.K., Pohl P. No facilitator required for membrane transport of hydrogen sulfide. Proc. Natl. Acad. Sci. U. S. A. 2009;106(39):16633–16638. doi: 10.1073/pnas.0902952106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu L.-F., Lu M., Hon Wong P.T., Bian J.-S. Hydrogen sulfide: neurophysiology and neuropathology. Antioxidants Redox Signal. 2011;15(2):405–419. doi: 10.1089/ars.2010.3517. [DOI] [PubMed] [Google Scholar]
- 21.Du C., Feng Y., Qiu D., Xu Y., Pang M., Cai N., Xiang A.P., Zhang Q. Highly efficient and expedited hepatic differentiation from human pluripotent stem cells by pure small-molecule cocktails. Stem Cell Res. Ther. 2018;9(1):58. doi: 10.1186/s13287-018-0794-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ke Q., Li L., Yao X., Lai X., Cai B., Chen H., Chen R., Zhai Z., Huang L., Li K., Hu A., Mao F.F., Xiang A.P., Tao L., Li W. Enhanced generation of human induced pluripotent stem cells by ectopic expression of Connexin 45. Sci. Rep. 2017;7(1):458. doi: 10.1038/s41598-017-00523-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumamaru H., Kadoya K., Adler A.F., Takashima Y., Graham L., Coppola G., Tuszynski M.H. Generation and post-injury integration of human spinal cord neural stem cells. Nat. Methods. 2018;15(9):723–731. doi: 10.1038/s41592-018-0074-3. [DOI] [PubMed] [Google Scholar]
- 24.Liu K., Han L., Tang P., Yang K., Gan D., Wang X., Wang K., Ren F., Fang L., Xu Y., Lu Z., Lu X. An anisotropic hydrogel based on mussel-inspired conductive ferrofluid composed of electromagnetic nanohybrids. Nano Lett. 2019;19(12):8343–8356. doi: 10.1021/acs.nanolett.9b00363. [DOI] [PubMed] [Google Scholar]
- 25.Pawar K., Prang P., Müller R., Caioni M., Bogdahn U., Kunz W., Weidner N. Intrinsic and extrinsic determinants of central nervous system axon outgrowth into alginate-based anisotropic hydrogels. Acta Biomater. 2015;27:131–139. doi: 10.1016/j.actbio.2015.08.032. [DOI] [PubMed] [Google Scholar]
- 26.Ghasemi-Mobarakeh L., Prabhakaran M.P., Morshed M., Nasr-Esfahani M.-H., Ramakrishna S. Electrospun poly(epsilon-caprolactone)/gelatin nanofibrous scaffolds for nerve tissue engineering. Biomaterials. 2008;29(34):4532–4539. doi: 10.1016/j.biomaterials.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 27.Gao M., Lu P., Bednark B., Lynam D., Conner J.M., Sakamoto J., Tuszynski M.H. Templated agarose scaffolds for the support of motor axon regeneration into sites of complete spinal cord transection. Biomaterials. 2013;34(5):1529–1536. doi: 10.1016/j.biomaterials.2012.10.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saglam A., Perets A., Canver A.C., Li H.-L., Kollins K., Cohen G., Fischer I., Lazarovici P., Lelkes P.I. Angioneural crosstalk in scaffolds with oriented microchannels for regenerative spinal cord injury repair. J. Mol. Neurosci. 2013;49(2):334–346. doi: 10.1007/s12031-012-9863-9. [DOI] [PubMed] [Google Scholar]
- 29.Prang P., Müller R., Eljaouhari A., Heckmann K., Kunz W., Weber T., Faber C., Vroemen M., Bogdahn U., Weidner N. The promotion of oriented axonal regrowth in the injured spinal cord by alginate-based anisotropic capillary hydrogels. Biomaterials. 2006;27(19):3560–3569. doi: 10.1016/j.biomaterials.2006.01.053. [DOI] [PubMed] [Google Scholar]
- 30.Lu Q., Zhang F., Cheng W., Gao X., Ding Z., Zhang X., Lu Q., Kaplan D.L. Nerve guidance conduits with hierarchical anisotropic architecture for peripheral nerve regeneration. Adv. Healthc Mater. 2021;10(14) doi: 10.1002/adhm.202100427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rose J.C., Gehlen D.B., Omidinia-Anarkoli A., Fölster M., Haraszti T., Jaekel E.E., De Laporte L. How much physical guidance is needed to orient growing axons in 3D hydrogels? Adv. Healthc Mater. 2020;9(21) doi: 10.1002/adhm.202000886. [DOI] [PubMed] [Google Scholar]
- 32.Bardoni R., Tawfik V.L., Wang D., François A., Solorzano C., Shuster S.A., Choudhury P., Betelli C., Cassidy C., Smith K., de Nooij J.C., Mennicken F., O'Donnell D., Kieffer B.L., Woodbury C.J., Basbaum A.I., MacDermott A.B., Scherrer G. Delta opioid receptors presynaptically regulate cutaneous mechanosensory neuron input to the spinal cord dorsal horn. Neuron. 2014;81(6):1312–1327. doi: 10.1016/j.neuron.2014.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jacobson P.B., Goody R., Lawrence M., Mueller B.K., Zhang X., Hooker B.A., Pfleeger K., Ziemann A., Locke C., Barraud Q., Droescher M., Bernhard J., Popp A., Boeser P., Huang L., Mollon J., Mordashova Y., Cui Y.-F., Savaryn J.P., Grinnell C., Dreher I., Gold M., Courtine G., Mothe A., Tator C.H., Guest J.D., Elezanumab A human anti-RGMa monoclonal antibody, promotes neuroprotection, neuroplasticity, and neurorecovery following a thoracic hemicompression spinal cord injury in non-human primates. Neurobiol. Dis. 2021;155 doi: 10.1016/j.nbd.2021.105385. [DOI] [PubMed] [Google Scholar]
- 34.Van Battum E.Y., Brignani S., Pasterkamp R.J. Axon guidance proteins in neurological disorders. Lancet Neurol. 2015;14(5):532–546. doi: 10.1016/S1474-4422(14)70257-1. [DOI] [PubMed] [Google Scholar]
- 35.Chédotal A. Roles of axon guidance molecules in neuronal wiring in the developing spinal cord. Nat. Rev. Neurosci. 2019;20(7):380–396. doi: 10.1038/s41583-019-0168-7. [DOI] [PubMed] [Google Scholar]
- 36.Wang R. Physiological implications of hydrogen sulfide: a whiff exploration that blossomed. Physiol. Rev. 2012;92(2):791–896. doi: 10.1152/physrev.00017.2011. [DOI] [PubMed] [Google Scholar]
- 37.Kolluru G.K., Shackelford R.E., Shen X., Dominic P., Kevil C.G. Sulfide regulation of cardiovascular function in health and disease. Nat. Rev. Cardiol. 2022 doi: 10.1038/s41569-022-00741-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Szczesny B., Módis K., Yanagi K., Coletta C., Le Trionnaire S., Perry A., Wood M.E., Whiteman M., Szabo C. AP39, a novel mitochondria-targeted hydrogen sulfide donor, stimulates cellular bioenergetics, exerts cytoprotective effects and protects against the loss of mitochondrial DNA integrity in oxidatively stressed endothelial cells in vitro. Nitric Oxide. 2014;41:120–130. doi: 10.1016/j.niox.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Robinson H., Wray S. A new slow releasing, H2S generating compound, GYY4137 relaxes spontaneous and oxytocin-stimulated contractions of human and rat pregnant myometrium. PLoS One. 2012;7(9) doi: 10.1371/journal.pone.0046278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sparatore A., Santus G., Giustarini D., Rossi R., Del Soldato P. Therapeutic potential of new hydrogen sulfide-releasing hybrids. Expet Rev. Clin. Pharmacol. 2011;4(1):109–121. doi: 10.1586/ecp.10.122. [DOI] [PubMed] [Google Scholar]
- 41.Pineau I., Lacroix S. Proinflammatory cytokine synthesis in the injured mouse spinal cord: multiphasic expression pattern and identification of the cell types involved. J. Comp. Neurol. 2007;500(2):267–285. doi: 10.1002/cne.21149. [DOI] [PubMed] [Google Scholar]
- 42.Yang L., Conley B.M., Cerqueira S.R., Pongkulapa T., Wang S., Lee J.K., Lee K.-B. Effective modulation of CNS inhibitory microenvironment using bioinspired hybrid-nanoscaffold-based therapeutic interventions. Adv. Mater. 2020;32(43) doi: 10.1002/adma.202002578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Furne J., Saeed A., Levitt M.D. Whole tissue hydrogen sulfide concentrations are orders of magnitude lower than presently accepted values. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008;295(5):R1479–R1485. doi: 10.1152/ajpregu.90566.2008. [DOI] [PubMed] [Google Scholar]
- 44.Panthi S., Chung H.-J., Jung J., Jeong N.Y. 2016. Physiological Importance of Hydrogen Sulfide: Emerging Potent Neuroprotector and Neuromodulator, Oxidative Medicine and Cellular Longevity 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Greco V., Spalloni A., Corasolla Carregari V., Pieroni L., Persichilli S., Mercuri N.B., Urbani A., Longone P. Proteomics and toxicity analysis of spinal-cord primary cultures upon hydrogen sulfide treatment. Antioxidants. 2018;7(7) doi: 10.3390/antiox7070087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tamizhselvi R., Sun J., Koh Y.-H., Bhatia M. Effect of hydrogen sulfide on the phosphatidylinositol 3-kinase-protein kinase B pathway and on caerulein-induced cytokine production in isolated mouse pancreatic acinar cells. J. Pharmacol. Exp. Therapeut. 2009;329(3):1166–1177. doi: 10.1124/jpet.109.150532. [DOI] [PubMed] [Google Scholar]
- 47.Beck K.D., Nguyen H.X., Galvan M.D., Salazar D.L., Woodruff T.M., Anderson A.J. Quantitative analysis of cellular inflammation after traumatic spinal cord injury: evidence for a multiphasic inflammatory response in the acute to chronic environment. Brain. 2010;133(Pt 2):433–447. doi: 10.1093/brain/awp322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lei F., He W., Tian X., Zhou Q., Zheng L., Kang J., Song Y., Feng D. GSK-3 inhibitor promotes neuronal cell regeneration and functional recovery in a rat model of spinal cord injury. BioMed Res. Int. 2019;2019 doi: 10.1155/2019/9628065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scheid S., Goeller M., Baar W., Wollborn J., Buerkle H., Schlunck G., Lagrèze W., Goebel U., Ulbrich F. Hydrogen sulfide reduces ischemia and reperfusion injury in neuronal cells in a dose- and time-dependent manner. Int. J. Mol. Sci. 2021;22(18) doi: 10.3390/ijms221810099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang J.-F., Li Y., Song J.-N., Pang H.-G. Role of hydrogen sulfide in secondary neuronal injury. Neurochem. Int. 2014;64:37–47. doi: 10.1016/j.neuint.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 51.Nagasawa K., Tarui T., Yoshida S., Sekiguchi F., Matsunami M., Ohi A., Fukami K., Ichida S., Nishikawa H., Kawabata A. Hydrogen sulfide evokes neurite outgrowth and expression of high-voltage-activated Ca2+ currents in NG108-15 cells: involvement of T-type Ca2+ channels. J. Neurochem. 2009;108(3):676–684. doi: 10.1111/j.1471-4159.2008.05808.x. [DOI] [PubMed] [Google Scholar]
- 52.Moniaga C.S., Kamata Y., Ogawa H., Suga Y., Tominaga M., Takamori K. Hydrogen sulfide modulates the expression of axon-guidance molecules in human keratinocytes. J. Dermatol. Sci. 2020;97(3):232–235. doi: 10.1016/j.jdermsci.2020.01.007. [DOI] [PubMed] [Google Scholar]
- 53.Liu D., Wang Z., Zhan J., Zhang Q., Wang J., Zhang Q., Xian X., Luan Q., Hao A. Hydrogen sulfide promotes proliferation and neuronal differentiation of neural stem cells and protects hypoxia-induced decrease in hippocampal neurogenesis. Pharmacol. Biochem. Behav. 2014;116:55–63. doi: 10.1016/j.pbb.2013.11.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.