Abstract
Aging is a multifactorial process involving many steps including senescence. The immune system plays a critical role in aging where chronic inflammation and senescence has been shown to be detrimental. Natural killer (NK) cells are the predominant innate lymphocyte subset that mediate various responses to include surveillance and elimination of senescent cells. Here, we use autologous propagated and activated NK (aNK) cells from 5 patients to demonstrate that aNK cells decrease senescent cells in vitro and immunosenescence in humans based on markers p16 and β-galactosidase. In addition, inflammatory cytokine panel data suggest a role for removal of immunosenescence to reduce the aging-related inflammatory response.
Keywords: NK cells, Senescence, Inflammation, Aging
Abbreviations: aNK, Autologous propagated and activated natural killer cells
Highlights
-
•
aNK cells exhibit an enhanced ability to eliminate tumorigenic cells and senescent cells in vitro in comparison to PBMCs.
-
•
Cytotoxic effects of aNK cells on senescent fibroblasts shown in time-lapsed video illustrate cooperation among attackers.
-
•
Infusion of aNK cells resulted in lower expression of senescence markers (p16 and β-galactosidase) in PBMCs.
-
•
After aNK cell infusion there is a decrease of proinflammatory markers, such as IL-6 and IFN-γ.
-
•
aNK cells are safe, help reduce immunosenescence, and may reduce the detrimental features of aging such as inflammation.
1. Introduction
Aging is a multifactorial process involving oxidative stress, inflammation, autophagy, mitochondrial injury, telomerase damage, and senescence [1,2]. Steady accumulation of senescent cells with age has adverse consequences, i.e., these non-proliferating cells occupy key cellular niches and secrete pro-inflammatory cytokines, contributing to aging-related diseases, frailty, and morbidity [3,4]. Hence, the cellular senescence theory of aging posits that human aging is a consequence of the accumulation of senescent cells. In agreement with this, the elimination of senescent cells from transgenic progeroid mice [5] and non-progeroid, naturally aged mice [6,7] led to greater resistance against aging-associated diseases and prolonged lifespan.
The immune system plays a critical role in the aging process with several studies suggesting a direct correlation with elevated levels of interleukin 6 (IL-6), tumor necrosis factor alpha (TNF-α), and diseases of aging such as heart disease and dementia [[8], [9], [10]]. Thus, the theory of inflammaging where immunosenescence plays a critical role in the aging process [11].
Senescent immune system cells are potentially among the most harmful of all senescent cells because they spread tissue damage and rapid aging across other body organs and systems. Therefore, an aged senescent immune system represents a key therapeutic target to maintain and extend healthy aging [12]. Indeed, the immune system has mechanisms for keeping senescence under control. Accumulating senescent cells are normally cleared by the immune system [[13], [14], [15]]. However, the ability of the immune system to fight its own senescence is limited [16].
As a predominant innate lymphocyte subset that mediates anti-tumor and anti-viral responses, NK cells have been also implicated in the surveillance of senescent cells, depending on the pathophysiological context [2,17]. There are several examples of NK cells eliminating senescent cells in vitro and in mice models, wherein NK cells participate in fibrosis reversion and senescent cell clearance in mice models in vivo [18,19]. Similar results were obtained with senescent hepatocyte AML12 cells [20], in agreement with previous reports [19,21], where NK cells preferentially targeted senescent cells, as shown by significantly higher levels of active caspase 3 (measuring cell apoptosis) in senescent vs non-senescent fibroblasts when cultured with NK cells. These results were confirmed using a CD107a-based degranulation assay as a surrogate marker of cytotoxicity, indicating that NK cells can target senescent fibroblasts in vitro [22].
Presently, there is a lack of data on NK cell effect on immunosenescence in humans. Here we investigated the role of autologous propagated and activated (aNK) cells on immunosenescence with 5 case studies. Specifically, we assessed whether aNK cell therapy is safe, reduces senescent immune cells, and suppresses the inflammatory response in humans. Our data suggest aNK cell therapy is safe and reduces immunosenescence in humans. Moreover, the presented data demonstrate a role for reduction of immunosenescence in decreasing the age-related inflammatory response.
2. Materials and methods
2.1. Patient guidelines and study setup
All studies were approved in accordance with the ethical committee of Amazonia, S.A. de C.V., Galenia Hospital, Cancun, Mexico. The study was explained to all 5 patients and an informed consent was obtained from each patient. All patients were healthy and evaluated prior to enrollment in this study to assure safety. Cohort 1 consisted of 3 individuals, all males of ages 70 (A), 50 (B), and 41 (C), whose bloods were collected for tests and assays outlined in Table 1. Following expansion of NK cells, each individual from cohort 1 received 1 × 109 NK cells based on safety profile of previous studies [23]. Cohort 2 consisted of 2 individuals, 1 female of age 50 (D) and 1 male of age 52 (E). Cohort 2 received 2 infusions of 2 × 109 NK cells each time and immunosenescence was assessed.
Table 1.
Blood analysis schedule of individuals infused with aNK cells.
| Procedure | Days Relative to NK cell infusion | ||||||
|---|---|---|---|---|---|---|---|
| −7 | −1 | 0 | 3 | 14 | 30 | 90 | |
| aNK cell infusion | + | ||||||
| CBC plus Homocysteine, Sedimentation rate, HS-CRP, Fibrinogen, Liver and Kidney markers | + | + | + | + | + | + | |
| Plasma cytokine profiles | + | + | + | + | + | + | |
| PBMCs senescence markers | + | + | + | ||||
2.2. PBMCs and NK cell isolation and propagation
Whole blood for NK cell preparation was collected in sodium heparin tubes (BD Medical). NK cell expansion and stimulation was achieved as previously described [24]. Briefly, PBMCs were isolated by density gradient centrifugation using Ficoll-Paque (Cytiva) and cultured for 3 days, at 106 cells/ml, in serum-free medium OpTmizer CTS T-cell expansion SFM (Invitrogen) containing 700 IU/ml recombinant human IL-2 (rhIL-2; Chiron), 0.01 kE/ml OK432 (Chugai Pharmaceutical), and 10% heat-inactivated autologous human plasma in a flask containing immobilized anti-CD16 monoclonal antibody (Beckman Coulter). The cells were transferred to an untreated flask containing OpTmizer medium supplemented with IL-2 and 10% heat-inactivated autologous human plasma, or heat-inactivated human serum type AB, and cultured further. Fresh medium was added to the flask every 2–3 days to bring the cell density down to 106 cells/ml. After 10–14 days in culture, cells were harvested by centrifugation and cryopreserved in CryoStor CS10 Freeze Media (BioLife Solutions). For the determination of cell concentration and viability, cells were stained with Cellometer ViaStain (Nexcelom Bioscience) and analyzed using Cellometer K2 cell counter (Nexcelom Bioscience).
2.3. NK cell cytotoxicity in vitro
Cytotoxicity assay (lactate dehydrogenase [LDH] release) was performed using CytoTox 96 Non-Radioactive Cytotoxicity Assay (Promega) according to the manufacturer's instructions. Essentially, K562 cells (ATCC) were incubated with NK cells or PBMCs from the same donor at an effector to target ratios 2:1, 6:1, and 10:1 for 4 h; the number of PBMC cells were adjusted to yield the equivalent CD56-positive cells in the cultured NK cell population.
For neutral red uptake assay, senescent skin fibroblasts were obtained from 70-year old male donor by serial passaging. At passage 10, cells were considered senescent due to growth arrest. Cells were stained with 33 μg/ml neutral red (Sigma) in medium (DMEM-LG, no phenol red, supplemented with 10% FBS and GlutaMAX) for 4 h (37 °C, 5% CO2); rinsed with PBS, exposed to effector cells at effector to target ratios 1:1, 3:1, and 10:1 in medium for 4 h (37 °C, 5% CO2); rinsed with PBS; and air-dried. Lysis solution was added and incubated at ambient temperature with gentle agitation for 15 min. Absorbance at 450 nm (A450) was determined with a microtiter plate reader (Molecular Devices SpectraMax Plus 384). Skin fibroblast cells were dissociated enzymatically and counted to determine the number of effector cells to fit the set effector to target ratios. The number of PBMC cells were adjusted to yield the equivalent CD56-positive cells in the cultured NK cell population.
2.4. Video of aNK cells cytotoxicity on senescent human fibroblasts in vitro
To record aNK cell cytotoxicity in real-time, fibroblasts were cultured to near confluence, exposed to cultured aNK cells at effector:target ratio of 10:1, incubated for 1 h in a CO2 incubator, and transferred to room temperature to be observed with a 10 × phase contrast objective. Time-lapsed photomicrography was performed at 1 exposure per min for 4 h 9 min with an Olympus IX2-UCB-2 unit and Camedia 2.5.1 software. The resulting video clip (72 exposures), edited for clarity, is shown at 1 exposure per second (Supplementary Material 1).
Supplementary video related to this article can be found at https://doi.org/10.1016/j.bbrep.2022.101380
The following is/are the supplementary data related to this article:
2.5. Inflammatory cytokine profiles in plasma samples
For cytokine profiles, blood was collected in EDTA tubes (BD Medical). Cytokine profiles in donors’ plasma included the following markers: Alpha-1-Antitrypsin, Alpha-2-Macroglobulin, β-2-Microglobulin, BDNF, CRP, Complement C3, Eotaxin-1, Factor VII, Ferritin, Fibrinogen, GMCSF, Haptoglobin, Intercellular Adhesion Molecule 1, IFN-γ, IL-1α, IL-1β, IL-1RA, IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12 Subunit p40, IL-12 Subunit p70, IL-15, IL-17, IL-18, IL-23, IL-27, MIP-1α, MIP-1β, MMP-3, MMP-9, MCP 1, Stem Cell Factor, RANTES, Tissue Inhibitor of Metalloproteinases 1, TNFα, TNFβ, TNFr2, VCAM-1, VEGF, Vitamin D-Binding Protein, von Willebrand Factor. The tests were performed by Myriad RBM (Supplementary Material 2).
2.6. Flow cytometry
Cells used in this study had a greater than 90% viability. One million cells were stained according to standard protocols with CD56 and CD3 antibodies from Miltenyi Biotec: PE-conjugated mouse IgG1 clone IS5–21F5, PE-conjugated anti-human CD56 clone AF12-7H3, APC-conjugated mouse IgG2a clone S43.10, and APC-conjugated anti-human CD3 clone BW264/56. The cell pellet was dispersed in 200 μl of MACS buffer and analyzed using the BD Accuri C6 Plus Flow Cytometer with BD Accuri C6 software v 1.0.264.21.
2.7. Protein assay and ELISA for senescence markers
Protein concentration was determined using the Pierce Detergent Compatible Bradford Assay Kit according to the manufacturer's instructions. Human p16 levels were determined using a kit from MyBiosource according to manufacturer's instructions. Similarly, β-galactosidase activity was analyzed with a cellular senescence assay kit from Cell Biolabs according to manufacturer's instructions.
2.8. Statistical analysis
Statistical summary (Mean, standard deviation) was obtained for all data and charted accordingly. To detect any significant differences among the experimental groups, all data were subjected to non parametric Kruskal Wallis test, followed by post-hoc Dunn's test. Statistical significance was denoted on charts with “*“, “**“, or “***“, indicating p-value as 0.01 < p ≤ 0.05, 0.001 < p ≤ 0.01, or p ≤ 0.001, respectively.
3. Results
3.1. Phenotypic characterization of NK cells
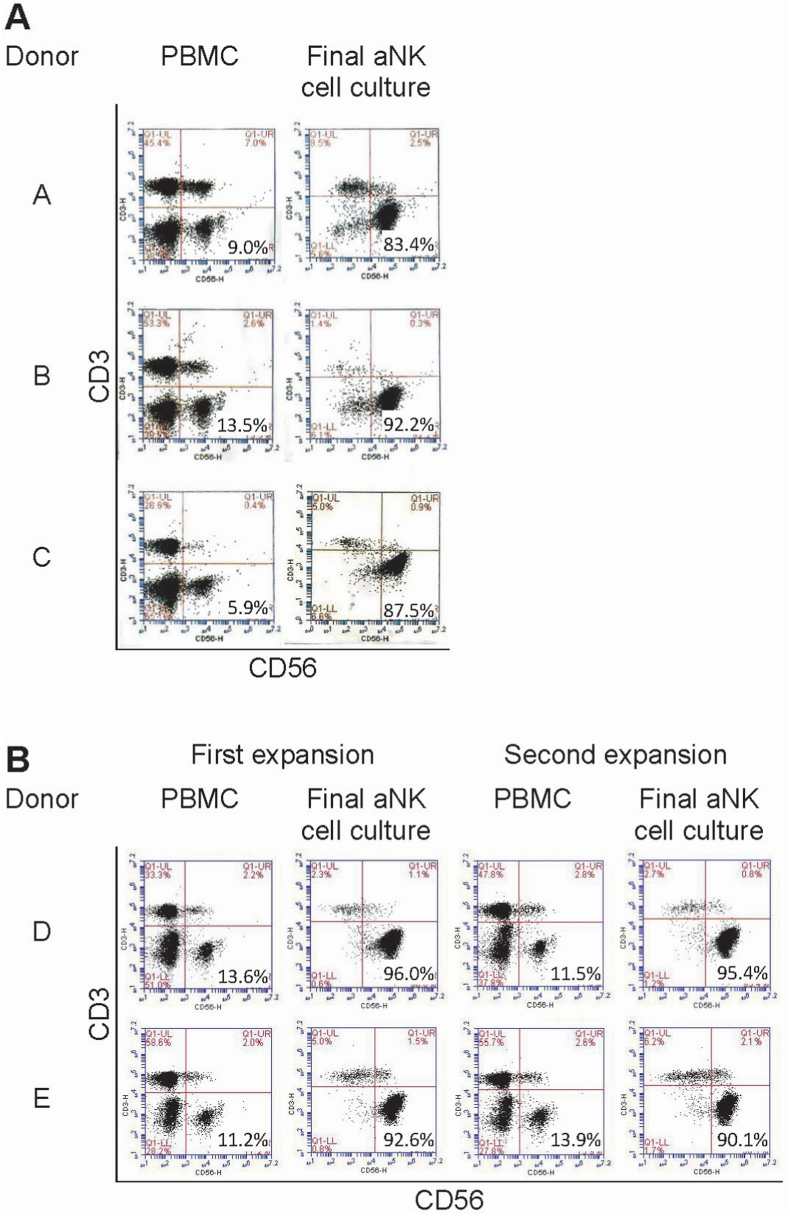
Fig. 1 illustrates results from flow cytometry of cells from individual donors. PBMCs had a median of 11.2% CD3(-)CD56(+) NK cells (range 5.9–13.6). After a mean of 15 days in culture (range 14–19) aNK cells were harvested at the peak of the exponential phase of expansion, with a median of 91.0% CD3(-)CD56(+) aNK cells (range 83.4–96.0) and a mean expansion factor of 198 (range 128–373) (Fig. 1A and B).
Fig. 1.
Flow cytometry CD3 vs. CD56 dot plots of PBMCs and aNK cells. A: Results from cohort 1 with donors A, B, and C. B: Results from cohort 2 with donors D and E. The 5 patients had a median frequency of 11.2% CD3(−)CD56(+) NK cells (range 5.9–13.6) in their PBMCs, and following culture expansion, there was an increase of the median frequency to 91.0% CD3(−)CD56(+) aNK cells (range 83.4–96.0).
3.2. Cytotoxicity of NK cells in vitro
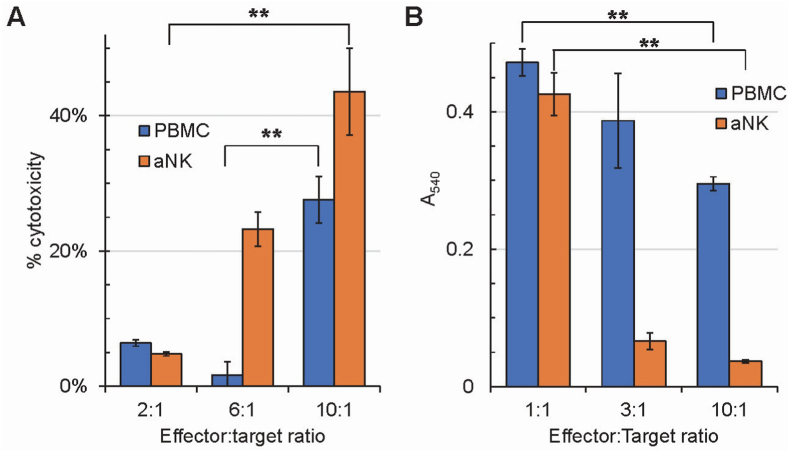
To demonstrate that this method of expansion and stimulation of NK cells is effective, we tested the cytotoxicity of PBMCs and aNK cells on K562 cells in vitro with an LDH release assay (Fig. 2A). aNK cells acquired higher levels of cytotoxic activity against K526 cells, in a dose-dependent manner in vitro, than NK cells that were part of PBMCs from which they were derived.
Fig. 2.
Cytotoxic effects of PBMCs and aNK cells on K562 cells (A) and senescent human skin fibroblasts (B). Based on flow cytometry results, PBMC numbers were adjusted to contain equivalent NK cell numbers in reactions with aNK cells. A: Kruskal-Wallis test indicates that there is significant difference between at least two experimental PBMC groups (p = 0.02651) and aNK groups (p = 0.02732); post-hoc Dunn's test identifies PBMC groups 6:1 vs 10:1 and aNK groups 2:1 vs 10:1 to have significant differences (p = 0.007049 and 0.00729, respectively). B: Kruskal-Wallis test indicates that there is significant difference between at least two experimental PBMC groups (p = 0.02732) and aNK groups (p = 0.02732); post-hoc Dunn's test identifies PBMC groups 1:1 vs 10:1 and aNK groups 1:1 vs 10:1 to have significant differences (p = 0.00729 and 0.00729, respectively). The cytotoxicity effects on K562 cells and senescent human skin fibroblasts are enhanced in aNK cells in comparison to PBMCs, and dose-dependent. Data were obtained in triplicates for each effector to target ratio, for PBMC and aNK cells; error bar = standard deviation. **: 0.001 < p ≤ 0.01 (Kruskal-Wallis test followed by post-hoc Dunn's test). If not indicated, results are not statistically significant.
The NK cell cytotoxic effects were also evaluated by a neutral red uptake and release assay, using senescent skin fibroblasts (Fig. 2B). As observed with K562 cells (Fig. 2A), aNK cells showed higher levels of cytotoxicity against senescent skin fibroblasts than naïve NK cells.
In addition, we documented the in vitro cytotoxicity of aNK cells activity by video (Supplementary material 1). Here, we illustrated a time course video of the elimination of senescent fibroblasts by aNK cells. Notably, the video shows a high level of cooperation among attackers, with simultaneous initiation of cytotoxicity by aNK cells and elimination of senescent fibroblast on separate targets.
3.3. Reduction of senescence marker expression in human PBMCs following aNK cell infusion
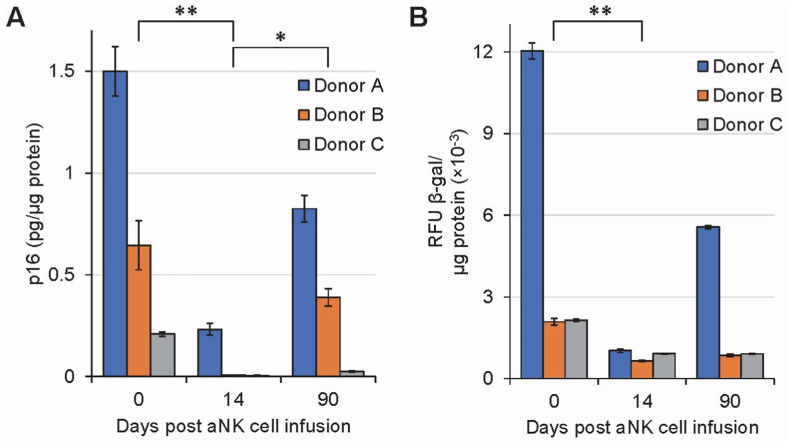
Three individuals (Cohort 1) were infused with 1.0 × 109 autologous aNK cells. No adverse effects were noted, and all blood test results were within normal physiological range (Data not shown). At various time points, PBMCs were isolated from donors A, B, and C and assessed for senescence markers p16 and β-galactosidase prior to aNK cell infusion, and on days 14 and 90 post infusion (Fig. 3). Following aNK cell infusion, there was a reduction in both p16 and β-galactosidase levels for all three individuals. The highest levels of both markers were observed for all three donors prior to aNK cell infusion (day 0). Ninety days after aNK cell infusion, there was an increase of senescence markers towards baseline, albeit still lower than baseline in all three donors. Of note, the older the individual, the higher the levels of both p16 and β-galactosidase prior to aNK cell infusion (Fig. 3). Similarly, following aNK cell infusion, there was a larger reduction for the older individual of both p16 and β-galactosidase on day 14. The data suggests that aNK cells may reduce senescence marker expression in PBMCs.
Fig. 3.
Effects of aNK cell infusion on senescence markers p16 (A) and β-galactosidase (B) in human PBMCs isolated from donors A, B, and C, of ages, 70, 51, and 41 years old, respectively. Donors were infused with 109 aNK cells on day 0. Data was obtained in duplicates for each donor at each time point. Data within each time point were pooled from the three donors and analyzed with Kruskal-Wallis test followed by post-hoc Dunn's test. Error bar = Standard deviation. Expression of senescent markers p16 and β-galactosidase decreases following infusion of aNK cells and trends toward pre-infusion levels by 90 days post-infusion. Significant differences were denoted by *: 0.01 < p ≤ 0.05; **: 0.001 < p ≤ 0.01. If not indicated, results are not statistically significant.
3.4. Cytokine profiles in plasma of donors A, B, and C
To understand other effects of aNK cells, we ran a 50-cytokine inflammatory profile on donors A, B, and C. There were varying results for all 3 donors. Of the 50 cytokines performed, there were 27 that were detectable in all 3 donors. Since many showed no relevant changes or patterns, here, we present 7 different cytokines/proteins that had a trend in all three donors following aNK cell infusion (Supplementary Material 2). T-cell specific RANTES trended higher following aNK cell infusion while ferritin, monocyte chemotactic protein-1, IL-6 and IFN-γ decreased. Of note, donor C, who has inflammatory bowel disease (IBD), had inflammatory markers, IL-6, IL-17A, IFN-γ and IL-27 upregulated prior to aNK cell infusion and a significant decrease to almost undetectable in many instances post infusion (Supplementary Material 2). IL-17A was not detected in donors A and B.
3.5. Reduction of senescence marker expression in human PBMCs following two aNK cell infusions
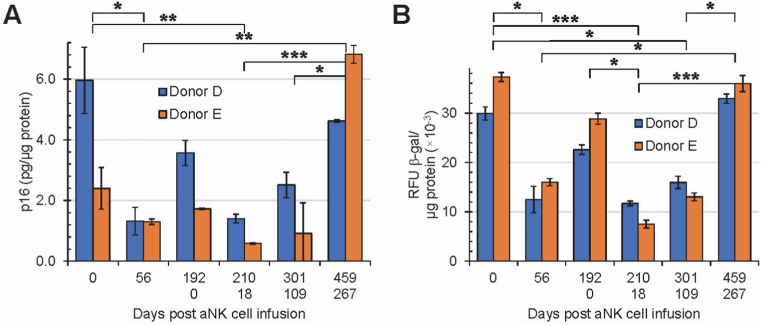
In support of the effects of a single dose infusion of aNK cells on senescence markers (Cohort 1), two individuals (Cohort 2, donors D and E) were infused with 2.0 × 109 aNK cells per dose separated 192 days apart. Blood was collected prior to infusion (baseline) on the days of infusion (0 and 192). We assessed p16 and β-galactosidase on days 0, 56, 192/0, 210/18, 301/109 and 459/267 for a total study period of 459 days (Fig. 4). For cohort 2, the effects of the first aNK cell infusion on senescence markers were similar to those for cohort 1. The initial levels of p16 and β-galactosidase decreased on day 56 followed by an increase on day 192. The second infusion (Day 192/0) resulted in another decrease in p16 for both donors (Fig. 4A), and the effect lasted longer for both markers, approximately eight vs six months compared with the first infusion. The decrease in β-galactosidase levels for both donors was not as large as it was for p16 compared with the first infusion (Fig. 4B). The data support the administration of one aNK cell infusion and the possibility that an additional infusion may have a longer lasting decrease of immunosenescence.
Fig. 4.
Effects of aNK cell infusion on senescence markers p16 (A) and β-galactosidase (B) for human PBMCs in vivo. Donors D and E were infused with 2 × 109 of aNK cells twice, as indicated by the upper row (Days 0 through 192, first infusion) and lower row (Days 0 through 267, second infusion) of the x-axis label. Total study period 459 days. Error bar = Standard deviation. Data was obtained in duplicates for each donor at each time point. Data within each time point were pooled from the two donors and analyzed with Kruskal-Wallis test followed by post-hoc Dunn's test. Expression of senescent markers p16 and β-galactosidase decreases following infusion of aNK cells and trends toward pre-infusion levels by 6 months post-infusion; the pattern is repeated with a second infusion. Significant differences were denoted by *: 0.01 < p ≤ 0.05; **: 0.001 < p ≤ 0.01; ***: p ≤ 0.001. If not indicated, results are not statistically significant.
3.6. Blood test results
Standard blood tests scheduled as shown in Table 1 did not reveal any significant changes following aNK cell infusion. All infusions were well tolerated with no adverse effects according to safety and toxicity blood testing (data not shown).
4. Discussion
Aging is a multifactorial process involving many critical steps including senescence, with senescence playing a pivotal role in the molecular mechanisms leading to increased inflammation, disease, and frailty as the manifestation of aging [[1], [2], [3]]. It is well understood that the immune system is key to aging and longevity where immunosenescence plays a major role. When immune cells undergo senescence, they lose their capacity to ward off negative effects in aging physiology [[12], [13], [14], [15]]. PBMCs represent the major subsets of immune cells, such as lymphocytes (B-cells, T-cells, and NK-cells), monocytes, and dendritic cells, all constantly interacting with each other to maintain homeostasis [25]. Hence, analyzing PBMCs allows us to gather a deeper understanding of the immune system in general.
NK cells directly communicate with various PBMCs and are known to target senescent cells both in vitro and in vivo, [[18], [19], [20], [21], [22]]. Recently, Tang, et al., described studying specific T cell subsets that were reduced following NK cell infusion, yet universally accepted markers of senescence such as β-galactosidase and p16 were not determined. The study followed 37 patients for 1 month following NK cell infusion. Moreover, the study illustrated a decrease in various well-defined inflammatory cytokines indicative of reducing inflammatory burden in age-related diseases [26].
Here, we used aNK cells to assess their ability to remove immunosenescence following specific dosage. Our in vitro experiments demonstrate that aNK cells exhibit an ability to eliminate senescent cells superior to NK cells found in PBMCs (Fig. 2). The effect of aNK cells on senescent fibroblasts was also documented in a cell culture video capture (Supplementary Material 1). The video shows aggressive targeting and physical elimination of senescent cells by aNK cells after they interrogate fibroblasts to select targets for elimination. We hypothesize there was a concerted response to senescent fibroblast by several aNK cells. Note the high level of cooperation among aNK cells attackers. The data suggests signal exchange among aNK cells prompting them to coordinate cytotoxic activity. Whether the same occurs in vivo, remains to be determined.
In support for the removal of immunosenescent cells in humans, we performed studies on 5 individuals infused with aNK cells. We assessed levels of senescent markers, p16 and β-galactosidase, in PBMCs isolated from 5 healthy individuals before and after infusion of aNK cells. In addition, in cohort 1 (n = 3), we assessed safety, toxicity and an inflammatory profile using a 50-protein marker array at 2 baselines and followed up to 90 days post aNK (Table 1).
Cohort 1 had elevated levels of both senescence markers p16 and β-galactosidase in PBMCs prior to aNK cell infusion. Donor A, the oldest, had the highest levels of both markers while donor C, the youngest, had the lowest levels of markers (Fig. 3), indicating a direct correlation of high p16 and β-galactosidase levels with increased age. On day 14 after aNK cell infusion, there was a drop in both markers for all three donors regardless of age indicating reduction of immunosenescence. On day 90 post aNK cell infusion, there was an increase of both senescence markers towards baseline levels. This observed trend in all 3 donors may be due to a transient effect of treatment because of a limited time of activity for infused aNK cells [27].
To assess whether the immunosenescence effect would last longer, donors D and E, cohort 2, were infused with 2 separate and larger doses of aNK cells. Results obtained for cohort 2 support and extend our cohort 1 data to a longer period post aNK cell infusion (Fig. 4). When cohort 2 was given a second aNK cell infusion, the effect lasted longer, up to a measured total of 267 days (Fig. 4). At this point from the start of the first aNK cell infusion, levels of immunosenescence for donor E were higher than recorded at the first infusion (459 days prior). This is not surprising granted the number of uncontrollable variables that can increase or decrease immunosenescence such as diet and nutrition [28]. Based on our p16 and β-galactosidase data in cohort 2, it can be inferred that the reversal of markers to higher levels occurred somewhere between 4 and 6 months, implying therapeutic intervention to reduce immunosenescence in humans will likely need to be repeated for optimal removal of immunosenescence. This is the first time following two aNK cell infusions immunosenescence has been documented for greater than 1 year.
To further extend our understanding of other systemic changes occurring following aNK cell infusion, we performed inflammatory protein profiling in cohort 1 (Supplementary material 2). T-cell specific RANTES trended higher while the remainder 6 inflammatory cytokines all trended lower following aNK cell infusion (Supplementary material 2). NK cells are affected by several interleukins and chemokines such as T-cell specific RANTES, which are found early on when NK cells are activated [29] and previously shown to be secreted by senescent fibroblast among other inflammatory stimuli [30]. The trend towards increasing T-cell specific RANTES may be due to immunosenescence being eliminated and activation of nearby neutrophils or macrophages to clear those cells [31].
There was a decreasing trend of inflammatory proteins ferritin, monocyte chemotactic protein-1 (MCP-1), IL-6 and IFN-γ following aNK cell infusion. Ferritin has been previously reported to be stored in senescent cells [32] which suggests that the decrease in ferritin levels in our data is a result of clearing of immunosenescence. Inflammatory proteins such as MCP-1, IL-6 and IFN-γ play a key role in inflammation and have been shown to be upregulated in chronic inflammation and diseases of aging [[33], [34], [35]]. Tang et al., illustrated a decrease in IL-6, IL-8, IL-1- α, IL-17, MIP-1 α, MIP-1β, and MMP1. This decrease was shown only to 30 days out. Nevertheless, those data support our findings that removal of senescent cells may lower these age-associated inflammatory proteins. Importantly, IFN-γ is known to play a key role in NK cell activation and recruitment.
Interestingly, IL-17A which has been implicated in several autoimmune diseases [36,37] was highly upregulated in Donor C prior to aNK cell infusion. This donor suffers from IBD. IL-17A also plays a role in age associated disease [34]. Following aNK cell infusion, IL-17A was reduced in donor C. In support of this data, studies illustrate that IL-27 plays an important role in IBD [38,39]. While there are conflicting reports of the role of IL-27 in IBD, our data shows a clear reduction in IL-27 in donor C following aNK cell infusion (Supplementary Material 2). This data suggests a role for aNK cell therapy in controlling inflammatory disease.
In summary, our data suggest that one or two infusions of aNK are safe and may reduce immunosenescence for up to 6 months. This represents a new therapeutic approach to reduce the detrimental features of aging such as inflammation and immunosenescence. This study is limited due to the observation of only 5 cases. Further studies are needed with larger cohorts and controlled placebos.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Nickolas Chelyapov, Toai Nguyen, Rafael Gonzalez report a relationship with Thebiobox, LLC that includes: employment. Nickolas Chelyapov, Rafael Gonzalez have patent #16/178,369 METHODS AND COMPOSITIONS OF NATURAL KILLER CELL ADOPTIVE TRANSFER THERAPY issued to RESTEM.
Acknowledgement
We thank Medicina Biocelular Avanzada for study support. This study was funded by Thebiobox, LLC.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbrep.2022.101380.
Contributor Information
Nickolas Chelyapov, Email: nchelyapov@thebiobox.com.
Toai T Nguyen, Email: ttnguyen@thebiobox.com.
Rafael Gonzalez, Email: rgonzalez@restem.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- 1.Liochev S.I. Which is the most significant cause of aging? Review, Antioxidants. 2015;4:793–810. doi: 10.3390/antiox4040793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antonangeli F., Zingoni A., Soriani A., Santoni A. Senescent Cells: living or dying is a matter of NK cells. J. Leukoc. Biol. 2019;105(6):1275–1283. doi: 10.1002/JLB.MR0718-299R. [DOI] [PubMed] [Google Scholar]
- 3.He S., Sharpless N.E. Senescence in health and disease. Cell. 2017;169(6):1000–1011. doi: 10.1016/j.cell.2017.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boccardi V., Mecocci P. The importance of cellular senescence in frailty and cardiovascular diseases. Adv. Exp. Med. Biol. 2020;1216:79–86. doi: 10.1007/978-3-030-33330-0_9. [DOI] [PubMed] [Google Scholar]
- 5.Baker D., Wijshake T., Tchkonia T., LeBrasseur N., et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479(7372):232–236. doi: 10.1038/nature10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.M. Xu, A.K. Palmer, H. Ding, et al., Targeting senescent cells enhances adipogenesis and metabolic function in old age. eLife 4: e12997. 10.7554/eLife.12997. [DOI] [PMC free article] [PubMed]
- 7.Baker D.J., Childs B.G., Durik M., et al. Naturally occurring p16Ink4a-positive cells shorten healthy lifespan. Nature. 2016;530(7589):184–189. doi: 10.1038/nature16932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bruunsgaard H., Andersen-Ranberg K., Jeune B., et al. A high plasma concentration of TNF-α is associated with dementia in centenarians. J. Gerontol.: MEDICAL SCIENCES. 1999;54A(7):M357–M364. doi: 10.1093/gerona/54.7.m357. [DOI] [PubMed] [Google Scholar]
- 9.Bruunsgaard H., Skinhej P., Pedersen A.N., et al. Ageing, tumour necrosis factor-alpha (TNF-a) and atherosclerosis. Clin. Exp. Immunol. 2000;121:255–260. doi: 10.1046/j.1365-2249.2000.01281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kalogeropoulos A., Georgiopoulou V., Psaty B.M., et al. Inflammatory markers and incident heart failure risk in older adults: the health, aging, and body composition study. J. Am. Coll. Cardiol. 2010;55(19):2129–2137. doi: 10.1016/j.jacc.2009.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frasca D., Blomberg B.B. Inflammaging decreases adaptive and innate immune responses in mice and humans. Biogerontology. 2016;17:7–19. doi: 10.1007/s10522-015-9578-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yousefzadeh M.J., Flores R.R., Zhu Y., et al. An aged immune system drives senescence and ageing of solid organs. Nature. 2021;594:100–105. doi: 10.1038/s41586-021-03547-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schroth S.J., Thiemermann C., Henson S.M. Senescence and the aging immune system as major drivers of chronic kidney disease. Review, Front. Cell Developmental Biol. 2020;8 doi: 10.3389/fcell.2020.564461. Article 564461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kale A., Sharma A., Stolzing A., et al. Role of immune cells in the removal of deleterious senescent cells. Immun. Ageing. 2020;17:16. doi: 10.1186/s12979-020-00187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Langhi Prata L.G.P., Ovsyannikova I.G., Tchkonia T., Kirkland J.L. Senescent cell clearance by the immune system: emerging therapeutic opportunities. Semin. Immunol. 2018;40 doi: 10.1016/j.smim.2019.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirokawa K., Utsuyama M., Kikuchi Y., Kitagawa M. In: Handbook on Immunosenescence. Fulop T., Franceschi C., Hirokawa K., Pawelec G., editors. Springer; 2009. Assessment of age-related decline of immunological function and possible methods for immunological restoration in elderly; pp. 1547–1570. [DOI] [Google Scholar]
- 17.Caligiuri M.A. 2008. Human Natural Killer Cells 112 Blood; pp. 461–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sagiv A A., Burton D.G., Moshayev Z., et al. NKG2D ligands mediate immunosurveillance of senescent cells. Aging. 2016;8:328–344. doi: 10.18632/aging.100897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krizhanovsky V., Yon M., Dickins R.A., et al. Senescence of activated stellate cells limits liver fibrosis. Cell. 2008;134:657–667. doi: 10.1016/j.cell.2008.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zang J., Ye J., Zhang C., Sha M., Gao J. Senescent hepatocytes enhance natural killer cell activity via the CXCL-10/CXCR3 axis. Exp. Ther. Med. 2019;18:3845–3852. doi: 10.3892/etm.2019.8037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xue W., Zender L., Miething C., et al. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature. 2007;445:656–660. doi: 10.1038/nature05529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pereira B.I., Devine O.P., Vukmanovic-Stejic M., et al. Senescent cells evade immune clearance via HLA-E-mediated NK and CD8+ T cell inhibition. Nat. Commun. 2019;10(1) doi: 10.1038/s41467-019-10335-5. Article number: 2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kundu S., Gurney M., O'Dwyer M. Generating natural killer cells for adoptive transfer: expanding horizons. Cytotherapy. 2021;23:559–566. doi: 10.1016/j.jcyt.202012.002. [DOI] [PubMed] [Google Scholar]
- 24.Deng X., Terunuma H., Nieda M., Xiao W., Nicol A. Synergistic cytotoxicity of ex vivo expanded natural killer cells in combination with monoclonal antibody drugs against cancer cells. Int. Immunopharm. 2012;14:593–605. doi: 10.1016/j.intimp.2012.09.014. [DOI] [PubMed] [Google Scholar]
- 25.Grievink H.W., Luisman T., Kluft C., Moerland M., Malone K.E. Comparison of three isolation techniques for human peripheral blood mononuclear cells: cell recovery and viability, population composition, and cell functionality. Biopreserv. Biobanking. 2016;14(5):410–415. doi: 10.1089/bio.2015.0104. [DOI] [PubMed] [Google Scholar]
- 26.Tang X., Dang B., Zang A., et al. Characterization of age-related immune features after autologous NK cell infusion: protocol for an open-label and randomized control trial. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.940577. 1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vivier E., Tomasello E., Baratin M., Walzer T., Ugolini S. Functions of natural killer cells. Nat. Immunol. 2008;9(5):503–510. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 28.Aiello A., Farzaneh F., Candore G., et al. Immunosenescence and its hallmarks: how to oppose aging strategically? A review of potential options for therapeutic intervention. Front. Immunol. 2019;10 doi: 10.3389/fimmu.2019.02247. Article 2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marischen L., Englert A., Schmitt A.-L., Einsele H., Loeffler J. Human NK cells adapt their immune response towards increasing multiplicities of infection of Aspergillus fumigatus. BMC Immunol. 2018;19 doi: 10.1186/s12865-018-0276-6. Article number:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Budamagunta V., Manohar-Sindhu S., Yang Y. Senescence-associated hyper-activation to inflammatory stimuli in vitro. Aging. 2021;13(15):19088–19107. doi: 10.18632/aging.203396. https://www.aging-us.com/article/203396/text [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shanmugham L.N., Petrarca C., Castellani M.L., et al. Rantes potentiates human macrophage aggregation and activation responses to calcium ionophore (A23187) and activates arachidonic acid pathways. J. Biol. Regul. Homeost. Agents. 2006;20(1–2):15–23. doi: 10.1111/acel.12706. [DOI] [PubMed] [Google Scholar]
- 32.Masaldan S., Clatworthy S.A.S., Gamel C., et al. Iron accumulation in senescent cells is coupled with impaired ferrinopathy and inhibition of ferroptosis. Redox Biol. 2018;14:100–115. doi: 10.1016/j.redox.2017.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yousefzadeh M.J., Schafer M.J., Hooten N.N., et al. Circulating levels of monocyte chemoattractant protein-1 as a potential measure of biological age in mice and frailty in humans. Aging Cell. 2017 doi: 10.1111/acel.12706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Volpe E.A., Henriksson J.T., Wang C., et al. Interferon-gamma deficiency protects against aging-related goblet cell loss. Oncotarget. 2016;7(40):64605–64614. doi: 10.18632/oncotarget.11872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gonzalez R., et al. Stem cells targeting inflammation as potential anti-aging strategies and therapies. Cell Tissue Transplant. Ther. 2015;7:1–8. doi: 10.4137/CTTT.S19477. [DOI] [Google Scholar]
- 36.Lowes M.A., Kikuchi T., Fuentes-Duculan J., et al. Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. J. Invest. Dermatol. 2008;128:1207–1211. doi: 10.1038/sj.jid.5701213. [DOI] [PubMed] [Google Scholar]
- 37.Yang W., Yao Y., Yang Y.Q., Lu F.T., et al. Differential modulation by IL-17A of Cholangitis versus Colitis in IL-2Ralpha deleted mice. PLoS One. 2014;9(8) doi: 10.1371/journal.pone.0105351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cox J.H., Kljavin N.M., Ramamoorthi N., et al. IL-27 promotes T cell-dependent colitis through multiple mechanisms. J. Exp. Med. 2010;208(1):115–123. doi: 10.1084/jem.20100410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Andrews C., McLean M.H., Durum S.K. IL-27 as a novel therapy for inflammatory bowel disease: a critical review of the literature. Inflamm. Bowel Dis. 2016;22(9):2255–2264. doi: 10.1097/MIB.0000000000000818. https://doi:10.1097/MIB.0000000000000818 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.