Abstract

To optimize the pharmacological properties of an anticancer pyrrole–imidazole (Py-Im) polyamide (PIP-1), we characterized the acid dissociation constants of PIP-1, three other structurally related hairpin-shaped polyamides, and a cyclic polyamide bearing the same core sequence as PIP-1 via potentiometric titration. The acidities of the carboxylic acid at the C-terminus and the tertiary amine in the triamine linker remained very similar among the polyamides tested, whereas the pKa of the N-methylimidazole (Im) moieties varied with the peptide sequence and molecular architecture. A nearly 0.2 pH unit pKa shift of terminal Im toward the neutral state compared to internal Im was observed. Furthermore, according to the dissociation constants, a speciation diagram of PIP-1 as a function of pH is presented, which allows an assessment of the net charge and distribution of protonated species in the range of physiological pH.
Keywords: Py-Im polyamide, pKa, N-methylimidazole, potentiometric titration, protonation profiling
Inspired by naturally occurring netropsin and distamycin A,1,2 Dervan and co-workers pioneered the design and development of pyrrole–imidazole (Py-Im) polyamides as a class of cell-permeable small molecules that bind to the minor groove of double-stranded DNA (dsDNA) in a sequence-specific fashion.3−5 A set of pairing rules describes the recognition of Watson–Crick base pairs by pairs of these heterocyclic amino acids within the minor groove via the formation of distinct hydrogen bonds: Im/Py pairs distinguish G/C from C/G base pairs, whereas Py/Py pairs are degenerate for both A/T and T/A base pairs (also called W base pairs).6,7 Py-Im polyamides can induce an expansion of the minor groove and a corresponding compression in the opposing major groove,8,9 thereby interfering the DNA–protein interaction10,11 and the transcriptional machinery.12,13 The application of these synthetic molecules in biological systems leads to altered expression of cancer-related genes, including PSA,10 TGFβ1,14 NF-κB,15 MMP-9,16 ABCA1,17 EVI1,18 LOX-1,19 and Plk1.20 In particular, Dervan’s group reported the eight-ring hairpin-shaped Py-Im polyamide PIP-1 targeting the androgen response element (ARE) half-site 5′-WGWWCW-3′, which can disrupt the interaction of ARE with androgen receptor (AR) or glucocorticoid receptor (GR) and shows efficacy against enzalutamide-resistant VCaP and LREX′ prostate cancer models in cell culture and xenografts.11,21 Furthermore, Lown and co-workers achieved great progress in the design and synthesis of unit modules mimicking pyrrole and imidazole moieties in biological applications.22−24
The acid dissociation constant (pKa) is considered to be one of the most important physical-chemical characteristics of acid–base drugs. The pKa of drugs is correlated to lipophilicity, solubility, target binding ability, and membrane permeability.25 Furthermore, it also profoundly affects some pharmacokinetic and pharmacodynamic processes, such as absorption, distribution, metabolism, and excretion (ADME)26 and even is closely related to side effects of drugs.27 Despite their widespread applications in medicinal chemistry and chemical biology, the pKa values of Py-Im polyamides have not been systematically explored to date. In general, there are three distinct ionizable functional groups in Py-Im polyamides: carboxylic acid, tertiary amine, and N-methylimidazole (Im) residues. The carboxylic acid belongs to the C-terminal isophthalic acid (IPA) (Figure 1), which plays a key role in nuclear localization of polyamides.28 The tertiary amine is located in the triamine linker, which is designed to recognize W base pairs of DNA in the minor groove (Figure 1).29 Since the IPA and the triamine linker have been fully optimized for biological applications, their chemical environments are relatively similar across an array of polyamides. In sharp contrast, the chemical environment of the Im motifs varies considerably with the peptide sequence and molecular architecture, thereby defining the protonation profiles of the macrospecies under different pH conditions. Therefore, the determination of pKa values of Im residues will be the focus to unveil polyamides’ protonation states under physiological conditions. In the present work, the pKa values of PIP-1, an ARE-targeting polyamide currently under preclinical investigation, were systematically studied via the potentiometric titration technique. The influence of the peptide sequence and molecular architecture on Im residue’s pKa was also probed. Leveraging the protonation profile of PIP-1, its net charge and distribution of different protonated species under various physiological conditions were also discussed.
Figure 1.

Structure of PIP-1, an ARE-targeting polyamide. Pale-green blocks present the ionic groups, and the bottom right corner shows the consensus ARE. Main symbols: blue ○, Py; red ●, Im; green ⬡, isophthalic acid; black curve with appended “NHAc”, γ-aminobutyric acid (γ-turn); notched bond with + sign, N,N-dimethyl-1,3-propanediamine.
The anticancer drug PIP-1 is an enneapeptide prepared by the solid-phase peptide synthesis (SPPS) technique in approximately 20 steps, including iterative peptide condensation and deprotection.30,31 Considering the low overall yield (typically less than 10%), it is ideal to perform the potentiometric titration of PIP-1 at low concentration (10–5 M). Under this circumstance, N-methylimidazole was chosen as a model substrate to validate the titration method. After excluding the interference of CO2 dissolved in solution by purging with argon, the pKa of N-methylimidazole was determined to be 7.18 ± 0.02 (n = 3) in water at 1 × 10–5 M via potentiometric titration based on Benet analysis.32 The pKa of N-methylimidazole was previously reported to be 6.95 (in H2O, by potentiometric titration),33 7.20 (in H2O, by potentiometric titration),34 or 7.25 (in H2O, by spectrophotometric titration).35 Our experimental result is consistent with the literature reports, which indicates that potentiometric titration is a well-suited method to measure pKa values at 10–5 M.
Next, the validated potentiometric titration technique was utilized to determine pKa values of tripeptide PIP-2 (Figure 2), which possesses the same ionizable groups as PIP-1 but could be produced in higher yield due to its shorter sequence. Due to its multi-ionizable feature, the second-derivative method36 in combination with meaningful inflection points from titration curves and guided by chemical knowledge was used to deduce pKa values from the titration curves of PIP-2. Three distinct pKa values were obtained (3.55 ± 0.09, 6.51 ± 0.05, and 9.70 ± 0.07; Figure 2C, E). Compared with Bordwell’s pKa table37 as well as the results predicted by the machine-learning software iBond 2.0,38 3.55 ± 0.09, 6.51 ± 0.05, and 9.70 ± 0.07 were assigned as the experimental pKa values of the carboxylic acid, N-methylimidazole, and tertiary amine groups, respectively. Those pH values where the second derivative was equal to zero that could not be correlated with meaningful inflection points from the standpoint of chemical knowledge, such as 5.6, 7.7, and 8.5 in Figure 2C, were discarded.
Figure 2.

Titration of PIP-2. (A) Chemical structure of PIP-2. (B, D) Titration curves. (C, E) Second-derivative plots.
There are two Im residues in PIP-1 located at different positions in the peptide sequence (Figure 1). To assign the pKa values of these two residues, two hairpin-shaped polyamides PIP-3 and PIP-4 bearing only one Im at the same position as one of the Im residues of PIP-1 were designed and prepared (Table 1). Three pKa values were obtained for PIP-3 and also for PIP-4 via potentiometric titration, as illustrated in Table 1. Like the titration results for PIP-2, the pKa of the carboxylic acid falls into the region around 3.60 (3.60 ± 0.01 for PIP-3 and 3.68 ± 0.04 for PIP-4) and the pKa of the tertiary amine was found to be around 9.65 (9.63 ± 0.02 for both PIP-3 and PIP-4). Therefore, 6.45 ± 0.07 and 6.26 ± 0.09 could be assigned to the corresponding terminal Im residue in PIP-3 and internal Im residue in PIP-4. Interestingly, the pKa of the internal Im residue inside the hairpin-shaped polyamide pocket in PIP-4 is approximately 0.2 pH units smaller than that of terminal Im residue in PIP-3.
Table 1. Summary of the pKa Values of Three Hairpin-Shaped Polyamides (PIP-3, PIP-4, and PIP-1) and Cyclic Polyamide PIP-5.

Concentrations of polyamides were calculated using a molar absorptivity ε = 69500 M–1 cm–1 at 310 nm by UV–vis spectroscopy. All of the compounds were prepared in neat DMSO and then diluted with water to a final concentration of 0.1% DMSO.
Errors represent standard deviations of three independent measurements.
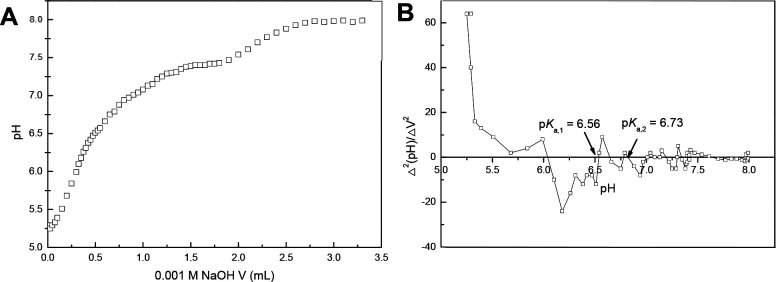
Four pKa values (3.57 ± 0.05, 6.54 ± 0.01, 6.74 ± 0.03, and 9.65 ± 0.08) were derived from the titration curve of PIP-1 via second-derivative analysis and were assigned to the carboxylic acid, internal Im, terminal Im, and tertiary amine, respectively (Figure 3). As expected, there was no apparent difference observed regarding the pKa values of the carboxylic acids and tertiary amines of PIP-1, PIP-3, and PIP-4, while a slight shift of the pKa of the Im residues occurred due to the altered peptide sequences. To probe the influence of the molecular architecture, PIP-5, a cyclic PIP molecule bearing the same peptide sequence as PIP-1 (Table 1), was synthesized,31,39 and its pKa was investigated via potentiometric titration. Since the two Im residues in PIP-5 are symmetric, only one pKa value (6.73 ± 0.04) was obtained (Figure 4). Of note, the pKa of the Im in PIP-5 is closer to the value of the terminal Im residue than that of the internal Im in PIP-1, indicating that its chemical environment and the role as a hydrogen-bond acceptor are more similar to the terminal Im than the internal Im.
Figure 3.
Titration of PIP-1. (A) Titration curve. (B) Second-derivative plot.
Figure 4.
Titration of PIP-5. (A) Titration curve. (B) Second-derivative plot.
The potentiometric titrations revealed that the pKa values of Im residues in polyamides were decreased compared with that of N-methylimidazole, probably due to the introduction of electron-withdrawing amide groups on the Im residues. Interestingly, the pKa of the internal Im (6.26 in PIP-4) is approximately 0.2 pH units smaller than the pKa of the terminal Im (6.45 in PIP-3). A similar trend was also observed from PIP-1, a hairpin-shaped polyamide possessing two Im residues (6.54 for the internal Im and 6.74 for the terminal Im). The increase in the pKa values of both the internal and terminal N-methylimidazoles when they are both present in PIP-1 with respect to the PIP-3 and PIP-4 variants clearly indicates that in PIP-1 the closed hairpinlike conformation is more abundant. Based on the potentiometric titration results, the ratios of the different species of PIP-1 with different extents of ionization are plotted in Figure 5. In an acidic environment (pH < 3), PIP-1 is present in the state of A0B+C+D+. Upon treatment with base, the −COOH group is deprotonated first, and thus, the macrospecies of PIP-1 transforms to A–B+C+D+. Further increasing the pH of the environment results in deprotonation of the two Im residues. Complete deprotonation of PIP-1 occurs at pH > 10, and PIP-1 is present in the state of A–B0C0D0 eventually.
Figure 5.

Ionization profiling of PIP-1. Colored regions represent different ionizable moieties of PIP-1: blue blocks represent ionic groups with protons, while the pale-green blocks represent removal of a proton from an ionic group.
Moreover, the variation of PIP-1’s net charge was investigated in the physiological pH range (Figure 6). At pH 2–8, PIP-1 possesses overall positive charge; on the contrary, it bears net negative charge in the pH range from 8 to 12. At pH 8, the positive charge is equal to the negative charge, and thus, the isoelectric point of PIP-1 is determined to be pH 8.
Figure 6.

Variation of the net charge of PIP-1 in the physiological pH range and determination of PIP-1’s isoelectric point.
To shed light on the absorption and distribution properties of PIP-1, its speciation in aqueous solution was readily revealed by the distribution curve as a function of pH (Figure 7A). In particular, we were interested in the relative abundance of species at pH 4.7, 7.2, and 8.0,40 the pH values inside lysosomes, the cytosol, and mitochondria40 (Figure 7B). Although the cellular uptake mechanism of polyamides is not yet fully understood, endocytosis is generally believed to be the major entry pathway due to polyamides’ large molecular weights (>1000 Da in general). In this regard, the distribution of different charged species may play a detrimental role in determining the efficiency of lysosome escape and also provide a basis for the development of a polyamide-specific drug delivery system. At pH 4.7, there are three kinds of species of PIP-1 (Figure 7B), and the major one is A–B+C+D+, which accounts for 92.8%. The other two minor species, A0B+C+D+ and A–B0C+D+, account for only 5.9% and 1.3%, respectively. At pH 7.2, two major species of PIP-1 are present in the cytosol: A–B0C0D+ and A–B0C+D+. Of note, approximately 70% of PIP-1 (A–B0C0D+) exists in the molecular form bearing no net charge, and the remaining 30% (24.3% A–B0C+D+ and 5.3% A–B+C+D+) is in cationic form. Sugiyama and co-workers demonstrated that with the guidance of mitochondria-penetrating peptides (MMPs), polyamides could specifically target mitochondria instead of the nucleus.41 The pH of the mitochondrial matrix is about 8.0, wherein neutral, cationic, and anionic forms of PIP-1 are all present. The neutral A–B0C0D+ occupies the predominant proportion of PIP-1 (92.5%) under this alkaline circumstance, while the other two ionic microspecies, A–B0C+D+ and A–B0C0D0, account for only 5.1% and 2.3%, respectively. An increase in the ratio of the neutral microspecies A–B0C0D+ among PIP-1 would probably lead to an increase in the octanol–water partition coefficient (LogP), a critical property in the drug discovery industry.
Figure 7.

(A) Distribution of microspecies as a function of pH for PIP-1. (B) Proportion of microspecies of PIP-1 at pH 4.7, 7.2, and 8.0.
In summary, we have systematically explored the pKa values of the anticancer Py-Im polyamide drug PIP-1, which exhibits great efficacy against enzalutamide-resistant prostate cancer cells in vivo and in vitro by targeting the ARE domain. The potentiometric titrations revealed that the pKa values of Im residues in polyamides varied with the peptide sequence and molecular architecture, while the pKa values of carboxylic acids and tertiary amines stayed similar across a range of polyamides. Moreover, the pKa of the internal Im residue is depressed compared to the terminal counterpart. Interestingly, under the circumstance of the same core sequence, the acidity of the Im residues in cyclic PIP-5 was found to be closer to that of the terminal Im in hairpin-shaped PIP-1 rather than the internal Im. Furthermore, the net charge and distribution of protonation species of PIP-1 as a function of pH under physiological conditions was characterized. Ultimately, the isoelectric point of PIP-1 was determined to be 8.0. The data presented in this work may provide a basis to understand the pharmacokinetic behaviors of polyamide drugs and to facilitate the informed design of polyamide delivery.
Acknowledgments
We are very grateful to Dr. Yang Yu (SUSTech) for HRMS. We acknowledge the assistance of SUSTech Core Research Facilities. This paper is dedicated to Prof. Peter B. Dervan on the occasion of his winning the 2022 Priestley Medal.
Glossary
Abbreviations
- Py
N-methylpyrrole
- Im
N-methylimidazole
- pKa
acid dissociation constant
- AR
androgen receptor
- ARE
androgen receptor element
- GR
glucocorticoid receptor
- PSA
prostate-specific antigen
- MMP
mitochondrial-penetrating peptide
Any further relevant data are available from the authors upon reasonable request.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsmedchemlett.2c00348.
Experimental procedures and characterization data of compounds (PDF)
Author Contributions
T.J. conceived and supervised the project. X.L. and Z.S. performed the experiments. T.J. and X.L. analyzed the experimental results and wrote the manuscript.
This work was supported by the Natural Science Foundation of Guangdong Province (2022A1515011770), the Guangdong-Joint Foundation of Shenzhen (2021B1515120046), and the Guangdong Provincial Key Laboratory of Catalysis (2020B121201002).
The authors declare no competing financial interest.
Supplementary Material
References
- Coll M.; Frederick C. A.; Wang A. H.; Rich A. A bifurcated hydrogen-bonded conformation in the d(A.T) base pairs of the DNA dodecamer d(CGCAAATTTGCG) and its complex with distamycin. Proc. Natl. Acad. Sci. U. S. A. 1987, 84, 8385–8389. 10.1073/pnas.84.23.8385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelton J. G.; Wemmer D. E. Structural characterization of a 2:1 distamycin A.d(CGCAAATTGGC) complex by two-dimensional NMR. Proc. Natl. Acad. Sci. U. S. A. 1989, 86, 5723–5727. 10.1073/pnas.86.15.5723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dervan P. B.; Edelson B. S. Recognition of the DNA minor groove by pyrrole-imidazole polyamides. Curr. Opin. Struct. Biol. 2003, 13, 284–299. 10.1016/S0959-440X(03)00081-2. [DOI] [PubMed] [Google Scholar]
- Hsu C. F.; Phillips J. W.; Trauger J. W.; Farkas M. E.; Belitsky J. M.; Heckel A.; Olenyuk B. Z.; Puckett J. W.; Wang C. C.; Dervan P. B. Completion of a Programmable DNA-Binding Small Molecule Library. Tetrahedron 2007, 63, 6146–6151. 10.1016/j.tet.2007.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishijima S.; Shinohara K.; Bando T.; Minoshima M.; Kashiwazaki G.; Sugiyama H. Cell permeability of Py-Im-polyamide-fluorescein conjugates: Influence of molecular size and Py/Im content. Bioorg. Med. Chem. 2010, 18, 978–983. 10.1016/j.bmc.2009.07.018. [DOI] [PubMed] [Google Scholar]
- Kielkopf C. L.; White S.; Szewczyk J. W.; Turner J. M.; Baird E. E.; Dervan P. B.; Rees D. C. A structural basis for recognition of A.T and T.A base pairs in the minor groove of B-DNA. Science 1998, 282, 111–115. 10.1126/science.282.5386.111. [DOI] [PubMed] [Google Scholar]
- Dervan P. B. Molecular recognition of DNA by small molecules. Bioorg. Med. Chem. 2001, 9, 2215–2235. 10.1016/S0968-0896(01)00262-0. [DOI] [PubMed] [Google Scholar]
- Chenoweth D. M.; Dervan P. B. Allosteric modulation of DNA by small molecules. Proc. Natl. Acad. Sci. U. S. A. 2009, 106, 13175–13179. 10.1073/pnas.0906532106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenoweth D. M.; Dervan P. B. Structural basis for cyclic Py-Im polyamide allosteric inhibition of nuclear receptor binding. J. Am. Chem. Soc. 2010, 132, 14521–14529. 10.1021/ja105068b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickols N. G.; Dervan P. B. Suppression of androgen receptor-mediated gene expression by a sequence-specific DNA-binding polyamide. Proc. Natl. Acad. Sci. U. S. A. 2007, 104, 10418–10423. 10.1073/pnas.0704217104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F.; Nickols N. G.; Li B. C.; Marinov G. K.; Said J. W.; Dervan P. B. Antitumor activity of a pyrrole-imidazole polyamide. Proc. Natl. Acad. Sci. U. S. A. 2013, 110, 1863–1868. 10.1073/pnas.1222035110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J.; Lahiri I.; Wang W.; Wier A.; Cianfrocco M. A.; Chong J.; Hare A. A.; Dervan P. B.; DiMaio F.; Leschziner A. E.; Wang D. Structural basis for the initiation of eukaryotic transcription-coupled DNA repair. Nature 2017, 551, 653–657. 10.1038/nature24658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh J.; Jia T.; Xu J.; Chong J.; Dervan P. B.; Wang D. RNA polymerase II trapped on a molecular treadmill: Structural basis of persistent transcriptional arrest by a minor groove DNA binder. Proc. Natl. Acad. Sci. U. S. A. 2022, 119, e2114065119. 10.1073/pnas.2114065119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda H.; Fukuda N.; Ueno T.; Tahira Y.; Ayame H.; Zhang W.; Bando T.; Sugiyama H.; Saito S.; Matsumoto K.; Mugishima H.; Serie K. Development of gene silencing pyrrole-imidazole polyamide targeting the TGF-β1 promoter for treatment of progressive renal diseases. J. Am. Soc. Nephrol. 2006, 17, 422–432. 10.1681/ASN.2005060650. [DOI] [PubMed] [Google Scholar]
- Raskatov J. A.; Meier J. L.; Puckett J. W.; Yang F.; Ramakrishnan P.; Dervan P. B. Modulation of NF-κB-dependent gene transcription using programmable DNA minor groove binders. Proc. Natl. Acad. Sci. U. S. A. 2012, 109, 1023–1028. 10.1073/pnas.1118506109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.; Nagase H.; Watanabe T.; Nobusue H.; Suzuki T.; Asami Y.; Shinojima Y.; Kawashima H.; Takagi K.; Mishra R.; Igarashi J.; Kimura M.; Takayama T.; Fukuda N.; Sugiyama H. Inhibition of MMP-9 transcription and suppression of tumor metastasis by pyrrole-imidazole polyamide. Cancer Sci. 2010, 101, 759–766. 10.1111/j.1349-7006.2009.01435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunemi A.; Ueno T.; Fukuda N.; Watanabe T.; Tahira K.; Haketa A.; Hatanaka Y.; Tanaka S.; Matsumoto T.; Matsumoto Y.; Nagase H.; Soma M. A novel gene regulator, pyrrole-imidazole polyamide targeting ABCA1 gene increases cholesterol efflux from macrophages and plasma HDL concentration. J. Mol. Med. 2014, 92, 509–521. 10.1007/s00109-013-1118-x. [DOI] [PubMed] [Google Scholar]
- Syed J.; Pandian G. N.; Sato S.; Taniguchi J.; Chandran A.; Hashiya K.; Bando T.; Sugiyama H. Targeted Suppression of EVI1 Oncogene Expression by Sequence-Specific Pyrrole-Imidazole Polyamide. Chem. Biol. 2014, 21, 1370–1380. 10.1016/j.chembiol.2014.07.019. [DOI] [PubMed] [Google Scholar]
- Yao E.-H.; Fukuda N.; Ueno T.; Matsuda H.; Matsumoto K.; Nagase H.; Matsumoto Y.; Takasaka A.; Serie K.; Sugiyama H.; Sawamura T. Novel Gene Silencer Pyrrole-Imidazole Polyamide Targeting Lectin-Like Oxidized Low-Density Lipoprotein Receptor-1 Attenuates Restenosis of the Artery After Injury. Hypertension 2008, 52, 86–92. 10.1161/HYPERTENSIONAHA.108.112797. [DOI] [PubMed] [Google Scholar]
- Liu K.; Fang L.; Sun H.; Pan Z.; Zhang J.; Chen J.; Shao X.; Wang W.; Tan Y.; Ding Z.; Ao L.; Wu C.; Liu X.; Li H.; Wang R.; Su W.; Li H. Targeting Polo-like Kinase 1 by a Novel Pyrrole-Imidazole Polyamide-Hoechst Conjugate Suppresses Tumor Growth In Vivo. Mol. Cancer Ther. 2018, 17, 988–1002. 10.1158/1535-7163.MCT-17-0747. [DOI] [PubMed] [Google Scholar]
- Kurmis A. A.; Yang F.; Welch T. R.; Nickols N. G.; Dervan P. B. A Pyrrole-Imidazole Polyamide Is Active against Enzalutamide-Resistant Prostate Cancer. Cancer Res. 2017, 77, 2207–2212. 10.1158/0008-5472.CAN-16-2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissinger K.; Krowicki K.; Dabrowiak J. C.; Lown J. W. Molecular recognition between oligopeptides and nucleic acids. Monocationic imidazole lexitropsins that display enhanced GC sequence dependent DNA binding. Biochemistry 1987, 26, 5590–5595. 10.1021/bi00392a002. [DOI] [PubMed] [Google Scholar]
- Lown J. W.; Krowicki K.; Bhat U. G.; Skorobogaty A.; Ward B.; Dabrowiak J. C. Molecular recognition between oligopeptides and nucleic acids: novel imidazole-containing oligopeptides related to netropsin that exhibit altered DNA sequence specificity. Biochemistry 1986, 25, 7408–7416. 10.1021/bi00371a024. [DOI] [PubMed] [Google Scholar]
- Reddy B. S. P.; Sondhi S. M.; Lown J. W. Synthetic DNA minor groove-binding drugs. Pharmacol. Ther. 1999, 84, 1–111. 10.1016/S0163-7258(99)00021-2. [DOI] [PubMed] [Google Scholar]
- Navo C. D.; Jiménez-Osés G. Computer Prediction of pKa Values in Small Molecules and Proteins. ACS Med. Chem. Lett. 2021, 12, 1624–1628. 10.1021/acsmedchemlett.1c00435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manallack D. T. The pKa Distribution of Drugs: Application to Drug Discovery. Perspect. Med. Chem. 2007, 1, 25–38. 10.1177/1177391X0700100003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore N. D.; Tammela T. L.; Massard C.; Bono P.; Aspegren J.; Mustonen M.; Fizazi K. Safety and Antitumour Activity of ODM-201 (BAY-1841788) in Chemotherapy-naive and CYP17 Inhibitor-naive Patients: Follow-up from the ARADES and ARAFOR Trials. Eur. Urol. Focus 2018, 4, 547–553. 10.1016/j.euf.2017.01.015. [DOI] [PubMed] [Google Scholar]
- Jacobs C. S.; Dervan P. B. Modifications at the C-terminus to improve pyrrole-imidazole polyamide activity in cell culture. J. Med. Chem. 2009, 52, 7380–7388. 10.1021/jm900256f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrksich M.; Parks M. E.; Dervan P. B. Hairpin Peptide Motif. A New Class of Oligopeptides for Sequence-Specific Recognition in the Minor Groove of Double-Helical DNA. J. Am. Chem. Soc. 1994, 116, 7983–7988. 10.1021/ja00097a004. [DOI] [Google Scholar]
- Fang L.; Yao G.; Pan Z.; Wu C.; Wang H. S.; Burley G. A.; Su W. Fully automated synthesis of DNA-binding Py-Im polyamides using a triphosgene coupling strategy. Org. Lett. 2015, 17, 158–161. 10.1021/ol503388a. [DOI] [PubMed] [Google Scholar]
- Chenoweth D. M.; Harki D. A.; Dervan P. B. Oligomerization route to Py-Im polyamide macrocycles. Org. Lett. 2009, 11, 3590–3593. 10.1021/ol901311m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benet L. Z.; Goyan J. E. Nonlogarithmic titration curves for the determination of dissociation constants and purity. J. Pharm. Sci. 1965, 54, 1179–1182. 10.1002/jps.2600540818. [DOI] [PubMed] [Google Scholar]
- Guthrie J. P.; Pike D. C. Hydration of acylimidazoles: tetrahedral intermediates in acylimidazole hydrolysis and nucleophilic attack by imidazole on esters. The question of concerted mechanisms for acyl transfers. Can. J. Chem. 1987, 65, 1951–1969. 10.1139/v87-326. [DOI] [Google Scholar]
- Paiva A. C.; Juliano L.; Boschcov P. Ionization of methyl derivatives of imidazole, histidine, thyreotropin releasing factor, and related compounds. J. Am. Chem. Soc. 1976, 98, 7645–7648. 10.1021/ja00440a033. [DOI] [PubMed] [Google Scholar]
- Catalan J.; Claramunt R. M.; Elguero J.; Laynez J.; Menendez M.; Anvia F.; Quian J. H.; Taagepera M.; Taft R. W. Basicity and acidity of azoles: the annelation effect in azoles. J. Am. Chem. Soc. 1988, 110, 4105–4111. 10.1021/ja00221a001. [DOI] [Google Scholar]
- Vogel A. I.A Text-Book of Quantitative Inorganic Analysis Including Elementary Instrumental Analysis; Lowe & Brydone: London, 1961; pp 911–931. [Google Scholar]
- Hans Reich’s Collection: Bordwell pKa Table. https://organicchemistrydata.org/hansreich/resources/pka/ (accessed 2022-09-10).
- Yang Q.; Li Y.; Yang J. D.; Liu Y.; Zhang L.; Luo S.; Cheng J. P. Holistic Prediction of the pKa in Diverse Solvents Based on a Machine-Learning Approach. Angew. Chem., Int. Ed. 2020, 59, 19282–19291. 10.1002/anie.202008528. [DOI] [PubMed] [Google Scholar]
- Chenoweth D. M.; Harki D. A.; Dervan P. B. Solution-phase synthesis of pyrrole-imidazole polyamides. J. Am. Chem. Soc. 2009, 131, 7175–7181. 10.1021/ja901307m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey J. R.; Grinstein S.; Orlowski J. Sensors and regulators of intracellular pH. Nat. Rev. Mol. Cell Biol. 2010, 11, 50–61. 10.1038/nrm2820. [DOI] [PubMed] [Google Scholar]
- Hidaka T.; Pandian G. N.; Taniguchi J.; Nobeyama T.; Hashiya K.; Bando T.; Sugiyama H. Creation of a Synthetic Ligand for Mitochondrial DNA Sequence Recognition and Promoter-Specific Transcription Suppression. J. Am. Chem. Soc. 2017, 139, 8444–8447. 10.1021/jacs.7b05230. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.