Abstract

Catechols have been largely investigated as antiaggregating agents toward β-amyloid peptide. Herein, as a follow up of a previous series of hydroxycinnamic derivatives, we synthesized a small set of dihydroxy isomers for exploring the role of the reciprocal position of the two hydroxyl functions at a molecular level. Para- and ortho-derivatives effectively reduced amyloid fibrillization, while the meta-analogue was devoid of any activity in this respect. Electrochemical analyses showed that the antiaggregating potency correlates with the oxidation potential, hence indicating the proelectrophilic character as a prerequisite for activity. Interestingly, mass spectrometry studies and quantum mechanical calculations revealed different modes of action for active para- and ortho-derivatives, involving covalent or noncovalent interactions with β-amyloid. The distinctive mode of action is also translated into a different cytotoxicity profile. This work clearly shows how apparently minimal structural modifications can completely change the compound behavior and generate alternative mechanisms of action of proelectrophilic chemical probes.
Keywords: β-Amyloid, Alzheimer’s disease, Natural compounds, Covalent inhibition, Polyphenols
Alzheimer’s disease (AD) is the most common protein misfolding disease, for which the amyloidogenic pathway represents a relevant feature. Amyloid oligomerization is a critical step of β-amyloid (Aβ) toxicity, as it shifts soluble nontoxic monomers into insoluble deposits through midterm stages of oligomerization and amyloid fibril formation. Soluble oligomers, rather than the insoluble end products of the amyloid cascade, contribute to Aβ synaptotoxicity.1 A variety of natural compounds have attracted the attention of the scientific community for their ability to interfere with protein misfolding.2 Several efforts have been made to understand their mode of action at a molecular level. Particularly, structure-dependent categorization of phenolic compounds has been performed suggesting both covalent and noncovalent mechanisms of amyloid binding.3
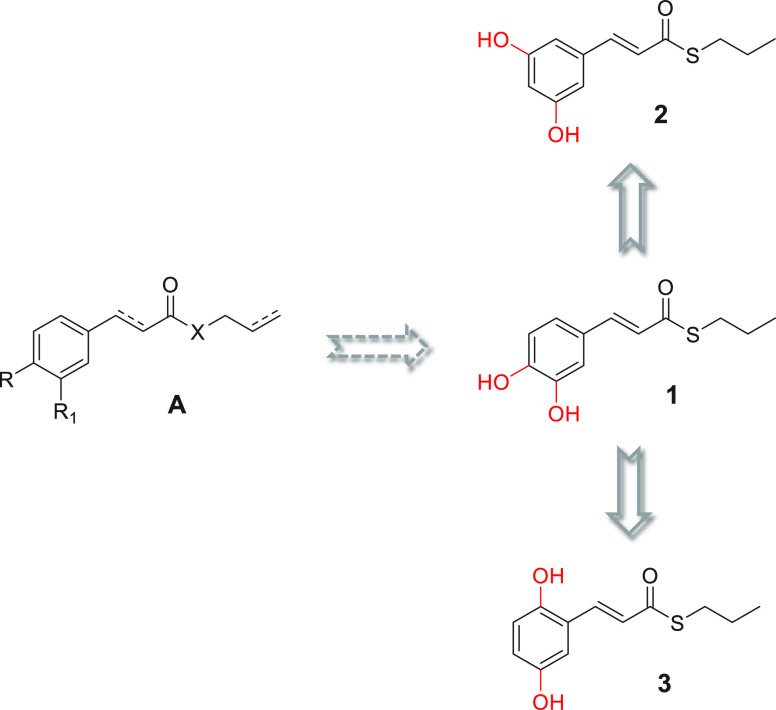
We previously reported a series of molecules based on the general formula A (Figure 1) as versatile chemical tools for investigating the molecular mechanisms potentially involved in Aβ damage.4,5
Figure 1.
Drug design strategy.
Compound 1 emerged as the most potent antiaggregating agent (IC50 = 3.99 μM). SAR studies on those compounds revealed the importance of the thioester moiety and of the catechol function, which provided a peculiar “on–off” pattern of control of the antiaggregating effect.4,5
The presence of vicinal hydroxyl groups in the polyphenols baicalein, epigallocatechin, and (+)-taxifolin have been highlighted as a key structural feature for the inhibition of amyloidosis processes involving amylin, α-synuclein, and Aβ42.3,6 Thus, we sought to more deeply examine the role of the two vicinal hydroxyl functions of compound 1 and how they influence the mode of action of this class of compounds. To this aim, we synthesized a new set of derivatives where the hydroxyl function in position 4 of 1 was moved to position 5 or 6, achieving the corresponding meta- and para-derivatives 2 and 3 (Figure 1).
Synthesis of compounds 2 and 3 was carried out through a straightforward synthetic route with minor modifications to the procedure previously exploited to obtain compound 1.5 As outlined in Scheme 1, selected dimethoxy benzaldehydes underwent a Knoevenagel condensation with malonic acid to give the corresponding α,β-unsaturated carboxylic acids 4 and 5. Treatment of 4 and 5 with HOBt and EDC as activating agents and further coupling reaction with 1-propanethiol allowed obtaining intermediates 6 and 7. Final treatment with BBr3 exerted demethylation, giving the final compounds 2 and 3. 1H NMR spectra showed that compounds 1–3, featuring a carbon–carbon double bond between the benzene ring and the carbonyl function, have an E configuration as indicated by the large spin coupling constants (around 16 Hz) of α-H and β-H on double bonds.
Scheme 1. Synthesis of Thioester Isomers 2 and 3.

Reagents and conditions: (i) malonic acid, pyridine, aniline, toluene, reflux, 4h; (ii) HOBt, EDC, 1-propanethiol, DCM, rt, o/n; (iii) BBr3 1 M in DCM, DCM, 0 °C to rt, 2h.
The antiaggregating properties of the newly synthesized compounds were tested by a thioflavin-T (ThT) assay, a commonly used fluorimetric assay for in vitro screening of antiaggregating agents.7 Results from this evaluation confirmed that the reciprocal position of the two hydroxyl functions is a critical feature, since it strongly modulates the on–off switch of the antiaggregating efficacy. In particular, while para-hydroquinone 3, as the corresponding ortho-quinone 1, was able to effectively decrease amyloid fibrilization, the meta-derivative 2 was completely devoid of any inhibitory activity (Table 1). Compound 3 emerged as a strong antiaggregating agent, exerting an almost complete inhibition of amyloid aggregation when screened at equimolar concentration with Aβ42. 3 was only 2.5-fold less potent than the lead compound 1. To further confirm these data and get further insight into the inhibition mechanism, the antiaggregating potencies of compounds 1–3 were also explored by mass spectrometry (MS) analysis. While ThT assay allows detecting/quantifying amyloid fibrils, MS allows detecting and quantifying the monomeric forms of Aβ42 and monitoring its disappearance as it is incorporated in growing Aβ oligomers and fibrils.8 Hence, in an MS assay a higher residual amount of monomeric Aβ42 is expected to be detected when an active inhibitor of the amyloid aggregation is co-incubated with Aβ42. Compounds 1 and 3 proved to exert such an action, confirming the ability to inhibit Aβ42 self-aggregation with the same trend outlined from the ThT assay, whereas compound 2 was inactive (Table 1).
Table 1. Antiaggregating Activities of Compounds 1–3 Compared with Corresponding Anodic Peaks (Ep) and Standard Redox Potentials (E1/2).
| inhibition of Aβ42 self-aggregation |
||||
|---|---|---|---|---|
| cpd | % inhibition by ThT [I] = 50 μMa (IC50 ± SEM/μM) | % inhibition by MS ± SD [I] = 50 μMb | Ep(ox) (V) | E1/2(ox)c (V) |
| 1 | >90 (3.99 ± 0.39)d | 56.9 ± 3.5 | 0.19 | 0.10 |
| 2 | <10 | <10 | 0.76 | 0.66 |
| 3 | >90 (10.2 ± 1.3) | 43.6 ± 0.7 | 0.32 | 0.10 |
Inhibition of Aβ42 50 μM self-aggregation by [I] = 50 μM (tested compound/Aβ42 = 1/1). For compounds showing a % inhibition greater than 50%, the IC50 value was determined. Values are the mean of two independent experiments each performed in duplicate. SEM = standard error of the mean.
Studies were performed by incubating Aβ42 samples under the assay conditions used for the ThT assay, with and without tested compounds at 50 μM (tested compound/Aβ42 = 1/1). Experiments were performed in duplicate. SD = standard deviation.
Halfwave (E1/2) redox potential values estimated by digital simulation of the experimental voltammetric curves.
IC50 already reported in ref (5).
The finding that ortho- and para-hydroquinones, but not the meta-hydroquinone, exert notable antiaggregating effects suggests that the oxidative properties of these compounds might be crucial for their inhibitory ability.
It is known that ortho- and para-hydroquinones can undergo full oxidation to semiquinone radicals and quinones while oxygen is reduced to hydrogen peroxide. Instead, resorcinol derivatives can convert to radical semiquinone but not to quinones.9 Cyclic voltammetry has been extensively exploited to depict the redox properties of catechols.10 Thus, to dissect the role of oxidative properties in a compound’s antiaggregating ability, the oxidation potential of isomers 1–3 was analyzed by performing cyclic voltammetry experiments, and the results were compared with functional biological activity. With potential scanned positively, catechols are converted into the corresponding ortho-benzoquinones, generating a characteristic anodic peak. On the reverse scan, the counterpart cathodic peak represents the reconversion of ortho-benzoquinones back to catechols. This is verified for the ortho- and para-derivatives, although affected by some degree of side reactivity following up the electron transfer (whose investigation is outside the scope of this work). An irreversible oxidation was observed for the meta-analogue 2 (Figure 2), in agreement with previous reports on resorcinol derivatives.9
Figure 2.

Cyclic voltammetric curves of 1 (black line), 2 (blue line), and 3 (red line) recorded in phosphate buffer 0.2 M electrolyte solution at a glassy carbon (GC) electrode and scan rate of 100 mV/s. The voltammetric curves represent the first potential sweep cycle as the subsequent cycles involve a passivation of the electrode surface by a film deposition, presumably formed by the oxidation byproducts.
Furthermore, a correlation was found by comparing the antiaggregating activities of the compounds with their anodic peak values (Epan), which match for smaller values to a higher inclination to oxidation, suggesting that a redox-controlled conversion to the active quinone form may plausibly be involved in the inhibition of amyloid aggregation. The same trend can be observed for the standard redox potentials (the experimental E1/2 values), instead of the peak potentials (Table 1).
The inhibitory activity of quinones toward Aβ assembly is well-documented,11 and both covalent and noncovalent mechanisms of inhibition have been proposed.3,12 Quinones are electrophilic species that can undergo nucleophilic addition from nucleophilic residues in proteins. However, the charge distribution of the quinone ring has been shown to exert a fundamental role in establishing a favorable dipole interaction between the central electron-poor quinone ring and the electron-rich peptide carbonyls as well as aromatic recognition sites within the amyloidogenic proteins.13
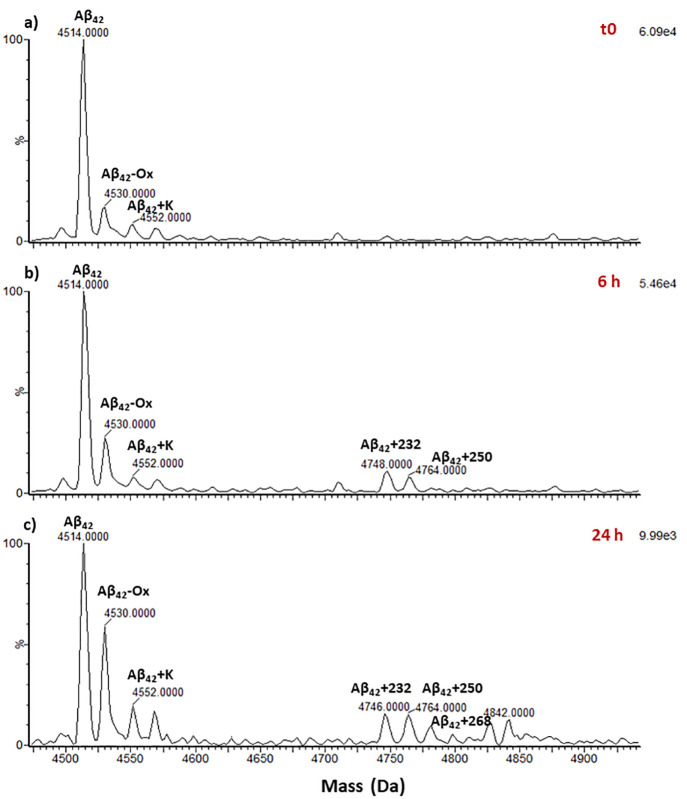
To achieve a conclusive answer on the possibility that quinone bioconversion is or is not followed by covalent interaction with β-amyloid peptide, the interaction of active compounds 1 and 3 with Aβ42 was studied by mass spectrometry. To this aim, Aβ42 samples were incubated (same assay conditions used for the ThT assay) with and without 1 or 3 at 50 μM (compound/Aβ42 = 1/1). No covalent adduct formation was detected when Aβ was co-incubated with 1, strengthening the relevance of noncovalent interactions in triggering the biological response exerted by 1. Conversely, the formation of a covalent adduct between Aβ42 and 3 was detected. In particular, the deconvoluted MS spectrum of Aβ42 at t0, that is immediately after the addition of compound 3 (Figure 3a), showed an intense signal at 4514 Da, which stands for the molecular weight of the monomeric native form of the peptide. Minor signals corresponding to the oxidized form of Aβ42 and its adduct with potassium ions were also detected.
Figure 3.
Deconvoluted mass spectra of Aβ42 after incubation for 0 h, corresponding to t0 (a), 6 h (b) and 24 h (c) with compound 3. Aβ42+K stands for the adduct between Aβ42 and K+ while Aβ42-Ox refers to the oxidized form of the protein.
Co-incubation of Aβ42 with compound 3 led to the appearance of new species at higher molecular weights (Figure 3b,c). In particular, species characterized by a mass increment of 232 and 250 Da (signals at 4748 and 4764) were detected upon a 6 h incubation, while a further signal at 4782 (mass increment of 268 Da) was observed upon a 24 h incubation.
Further LC-MS/MS analyses revealed that Lys16 residue of Aβ42 is involved in the formation of both covalent adducts (Figure S1a,b). These results may lead to the hypothesis that the two adducts are related to each other and that A250 may result from a rearrangement of A232. In detail, the A232 adduct may form upon the double attack of the nucleophilic Lys16 on the oxidized quinone form of 3 (MW = 236 Da), affording a 4,7-dioxo-4,7-dihydro-1H-indole bicyclic structure. The formation of this adduct could first involve the attack of Lys16 to the para-quinone ring of 3, followed by subsequent cyclization with the vinyl position (route a, Scheme 2) or, conversely, an initial addition to the vinyl position and a subsequent cyclization with the quinone moiety (route b, Scheme 2).
Scheme 2. Hypothesized Double Attack of Lys16 to Oxidized 3 Possibly Justifying the Formation of A232 Adduct.
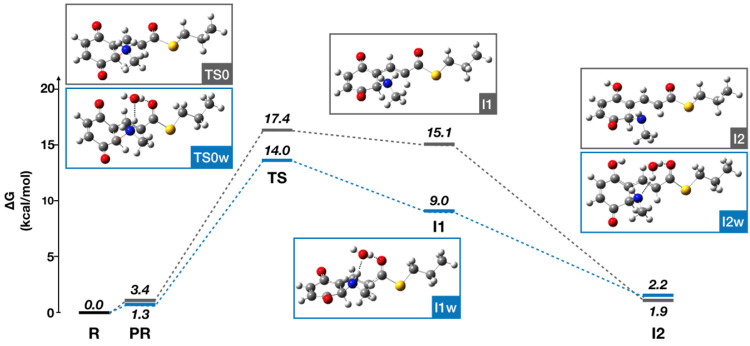
To deepen this hypothesis and examine the molecular mechanism implicated in covalent adduct formation between compound 3 and Aβ42, quantum mechanical (QM) computations were performed. Given the lack of precise structural information on the inhibitor–Aβ complex, this study was performed using a reduced model, where the side chain of the Lys16 residue was simulated with methylamine. The free energy profile determined by density functional theory calculations (M062X/6-31G(d,p)) for the attack to the para-quinone ring of 3 reveals a barrier of 17.4 kcal/mol (relative to the separated reactants).
Additional calculations were also performed with the inclusion of a water molecule in order to check the potential effect in assisting the attack of methylamine to 3, for which a decrease to 14.0 kcal/mol was observed (Figure 4). This can be ascribed to the formation of hydrogen bonds between the water molecule with the amine N (3.02 Å) and with the carbonyl oxygens of the para-quinone ring (3.22 Å) and the thioester moiety (3.17 Å). It is worth noting that the transition state (TS) is formed earlier than in the attack without the water molecule, as reflected in the distances from the N atom to the para-quinone carbon in the presence (2.02 Å) and absence (1.88 Å) of the water molecule.
Figure 4.
Free energy profiles in the gas phase determined from density functional theory calculations for the attack to 3 and performed using a reduced model where the side chain of Lys16 was simulated with methylamine and assisted (or not) by a water molecule.
The second hypothesis, i.e., an initial attack to the vinyl position (route b, Scheme 2), was found to be less favored, as the free energy barrier was estimated to be 25.3 and 19.0 kcal/mol in the absence and presence of a water molecule, respectively (Figure S2).
These results suggest that the adduct between Aβ42 and compound 3 could likely result from the attack of the nucleophilic Lys16 to the para-quinone ring of 3. Keeping in mind the hydrophobic character of 3 (estimated logP close to 3.9 according to IEFPCM/MST continuum solvation calculations), this process may be promoted by the initial formation of a transient complex with Aβ monomers or dimers, as suggested for other catechol derivatives.12,14 The covalent adduct may subsequently evolve through an intramolecular cyclization, in conjunction with an oxidative process, to yield A232.
Finally, the interaction of Aβ42 and the ortho-quinone derivative 1 was also examined. In this case, the TS structures for the water-assisted nucleophilic attack of methylamine to the quinone and vinyl positions were destabilized by 20.5 and 24.4 kcal/mol (see Figure S3), which may contribute to explaining the lack of covalent modification upon incubation with this compound (see above).
It has been previously proven that Aβ can be easily modified by ROS at specific amino acidic residues, and the change of redox state can impair the biological behavior and the oligomerization process. Indeed, the oxidized form of Aβ42 (Aβ42 Ox) was shown to be less prone to aggregate than the native one (Aβ42 Native), accounting for its slower aggregation rate.15,16 In this respect, the peculiar profile of polyphenols, which can act as either antioxidant or pro-oxidant agents, offers an additional method of intervention in amyloid aggregation.17 A prototypical example is the pro-oxidant effect exerted by the natural polyphenol myricetin toward Aβ42.16
On this basis, we sought to verify whether the pro-electrophiles 1 and 3 could partially carry out their inhibitory activity through an oxidation-based mechanism. To estimate whether 1 or 3 may favor amyloid oxidation, variations in the content of Aβ42 Ox was monitored by MS analysis over time in the presence and absence of each inhibitor. As shown in Figure 5, Aβ42 oxidation increased over time when it was co-incubated with 3, while no significant increase in Aβ42 Ox was detected when Aβ42 was co-incubated with 1. This highlights a further peculiar different feature in the mode of action of these two compounds.
Figure 5.

Evaluation of the oxidized form of Aβ42 in the absence and in the presence of 1 or 3 at equimolar concentration with Aβ42. The ratio between the signal corresponding to the oxidized form of Aβ42 and the signal of the internal standard (reserpine) is reported as a function of the incubation time.
Electrophilic features raise potential toxicity concerns, as covalent binding with nucleophiles in proteins is generally viewed as a basis for off-target interactions.18 Thus, cellular toxicity elicited by compounds 1–3 in SH-SY5Y human neuroblastoma cells was explored. Cells were treated with 1–3 at 5 and 10 μM. Cell viability was evaluated after a 24 h incubation by MTT assay, and results are reported in Figure 6. Electrophile 1 was well tolerated at tested concentrations, as was also the newly synthesized nonelectrophile 2, which lacked any toxicity. Conversely, electrophile 3, for which a covalent mode of action was shown, reduced cell viability in a dose-dependent manner, up to more than 50% when tested at 10 μM. Notably, a correlation could be found between cytotoxicity and the ability to covalently trap nucleophiles in proteins, whereas the (pro)electrophilic feature alone was shown to be less relevant in this respect.
Figure 6.
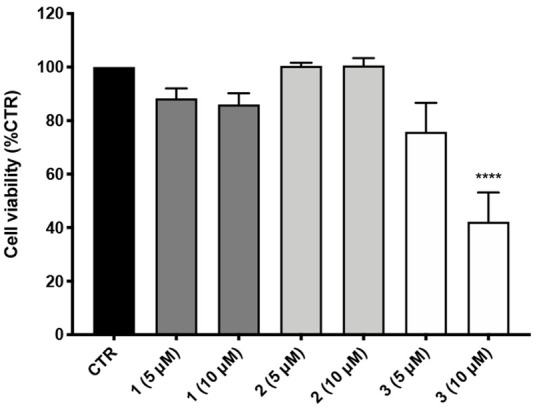
Cellular toxicity of compounds 1–3 in human neuroblastoma SH-SY5Y cells. Cells were treated with compounds 1–3 for 24 h at the concentrations of 5 and 10 μM. Cell viability was assessed by MTT assay. Data are expressed as percentage of cell viability ± SEM versus CTR; ****p < 0.0001 versus CTR; Dunnett’s multiple comparison test, n = 4.
In conclusion, we investigated the molecular mechanisms underpinning the antiaggregating activity of previously synthesized catechol-based compounds through the development of the corresponding meta- and para-dihydroxy-analogues of 1 and the study of their antiaggregating properties. Our results allowed disclosing the pro-electrophilic character as a prerequisite for compound activity. Indeed, the ortho- and para-derivatives, which can be easily oxidized to the corresponding quinones, strongly inhibited β-amyloid aggregation, whereas the meta-analogue was devoid of any relevant activity. Interestingly, MS analyses highlighted a different mode of action for compounds 1 and 3, suggesting that the strong inhibitory activity of 3 arises from a variety of processes, including the formation of a covalent adduct with Lys16 residue of Aβ42 and an oxidation-based mechanism. Conversely, noncovalent interactions seem to represent the driving mechanism in the antiaggregating action of 1. Notably, the different cytotoxic profile of compounds 1–3 is in line with the covalent or noncovalent mode of action, highlighting the concept that (pro)electrophilic features are not per se sufficient to covalently engage a target protein. Furthermore, the results highlight that apparently minimal structural modifications can generate alternative mechanisms of action and trigger distinctive toxicity profiles. These results support the view that electrophiles should not be a priori considered as structural alerts or toxicophores. A deep understanding and fine-tuning of chemical reactivity could be useful to explore the investigational and therapeutic potential of electrophile-modulator signals.
Glossary
Abbreviations
- AD
Alzheimer’s disease
- Aβ
amyloid-β peptide
- BBr3
boron tribromide
- DMF
N,N-dimethylformamide
- DMSO
dimethyl sulfoxide
- EDC
1-ethyl-3-(3-(dimethylamino)propyl)carbodiimide hydrochloride
- ESI-MS
electrospray ionization mass spectrometry
- GST
glutathione S-transferase
- HPLC
high-performance liquid chromatography
- HOBt
1-hydroxy benzotriazole hydrate
- MW
molecular weight
- NMR
nuclear magnetic resonance
- ppm
parts per million
- ROS
reactive oxygen species
- SAR
structure–activity relationships
- TLC
thin-layer chromatography
- TMS
tetramethylsilane
- ThT
thioflavin T; UV, ultraviolet.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsmedchemlett.2c00410.
Materials and methods for synthetic procedures with characterization of final compounds, ThT- and MS-based studies, cyclic voltammetry analysis, and computational (with optimized geometries) and cellular experiments (PDF)
This research was funded by the Italian Ministry of University and Research (MIUR), PRIN 2017 (2017MT3993_007), INSTM and Spanish Ministerio de Ciencia e Innovación (PID2020-117646RB-I00 MCIN/AEI/10.13039/501100011033 and MDM-2017-0767 and AEI/FEDER UE), Barcelona Supercomputing Center (BCV-2022-2-0008), and Consorci de Serveis Universitaris de Catalunya (CSUC; Molecular Recognition project).
The authors declare no competing financial interest.
Supplementary Material
References
- Jan A.; Adolfsson O.; Allaman I.; Buccarello A. L.; Magistretti P. J.; Pfeifer A.; Muhs A.; Lashuel H. A. Abeta42 neurotoxicity is mediated by ongoing nucleated polymerization process rather than by discrete Abeta42 species. J. Biol. Chem. 2011, 286 (10), 8585–8596. 10.1074/jbc.M110.172411. [DOI] [PMC free article] [PubMed] [Google Scholar]; Bernstein S. L.; Dupuis N. F.; Lazo N. D.; Wyttenbach T.; Condron M. M.; Bitan G.; Teplow D. B.; Shea J. E.; Ruotolo B. T.; Robinson C. V.; Bowers M. T. Amyloid-β protein oligomerization and the importance of tetramers and dodecamers in the aetiology of Alzheimer’s disease. Nat. Chem. 2009, 1 (4), 326–331. 10.1038/nchem.247. [DOI] [PMC free article] [PubMed] [Google Scholar]; Laurén J.; Gimbel D. A.; Nygaard H. B.; Gilbert J. W.; Strittmatter S. M. Cellular prion protein mediates impairment of synaptic plasticity by amyloid-beta oligomers. Nature 2009, 457 (7233), 1128–1132. 10.1038/nature07761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2 Pagano K.; Tomaselli S.; Molinari H.; Ragona L. Natural Compounds as Inhibitors of Aβ Peptide Aggregation: Chemical Requirements and Molecular Mechanisms. Front Neurosci 2020, 14, 619667. 10.3389/fnins.2020.619667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M.; Murakami K.; Uno M.; Nakagawa Y.; Katayama S.; Akagi K.; Masuda Y.; Takegoshi K.; Irie K. Site-specific inhibitory mechanism for amyloid β42 aggregation by catechol-type flavonoids targeting the Lys residues. J. Biol. Chem. 2013, 288 (32), 23212–23224. 10.1074/jbc.M113.464222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoni E.; Serafini M. M.; Bartolini M.; Caporaso R.; Pinto A.; Necchi D.; Fiori J.; Andrisano V.; Minarini A.; Lanni C.; Rosini M. Nature-Inspired Multifunctional Ligands: Focusing on Amyloid-Based Molecular Mechanisms of Alzheimer’s Disease. ChemMedChem. 2016, 11 (12), 1309–1317. 10.1002/cmdc.201500422. [DOI] [PubMed] [Google Scholar]
- Simoni E.; Serafini M. M.; Caporaso R.; Marchetti C.; Racchi M.; Minarini A.; Bartolini M.; Lanni C.; Rosini M. Targeting the Nrf2/Amyloid-Beta Liaison in Alzheimer’s Disease: A Rational Approach. ACS Chem. Neurosci. 2017, 8 (7), 1618–1627. 10.1021/acschemneuro.7b00100. [DOI] [PubMed] [Google Scholar]
- Velander P.; Wu L.; Ray W. K.; Helm R. F.; Xu B. Amylin Amyloid Inhibition by Flavonoid Baicalein: Key Roles of Its Vicinal Dihydroxyl Groups of the Catechol Moiety. Biochemistry 2016, 55 (31), 4255–4258. 10.1021/acs.biochem.6b00578. [DOI] [PubMed] [Google Scholar]; Bu X. L.; Rao P. P. N.; Wang Y. J. Anti-amyloid Aggregation Activity of Natural Compounds: Implications for Alzheimer’s Drug Discovery. Mol. Neurobiol 2016, 53 (6), 3565–3575. 10.1007/s12035-015-9301-4. [DOI] [PubMed] [Google Scholar]; Tu L. H.; Young L. M.; Wong A. G.; Ashcroft A. E.; Radford S. E.; Raleigh D. P. Mutational analysis of the ability of resveratrol to inhibit amyloid formation by islet amyloid polypeptide: critical evaluation of the importance of aromatic-inhibitor and histidine-inhibitor interactions. Biochemistry 2015, 54 (3), 666–676. 10.1021/bi501016r. [DOI] [PMC free article] [PubMed] [Google Scholar]; Palhano F. L.; Lee J.; Grimster N. P.; Kelly J. W. Toward the molecular mechanism(s) by which EGCG treatment remodels mature amyloid fibrils. J. Am. Chem. Soc. 2013, 135 (20), 7503–7510. 10.1021/ja3115696. [DOI] [PMC free article] [PubMed] [Google Scholar]; Zhu M.; Rajamani S.; Kaylor J.; Han S.; Zhou F.; Fink A. L. The flavonoid baicalein inhibits fibrillation of alpha-synuclein and disaggregates existing fibrils. J. Biol. Chem. 2004, 279 (26), 26846–26857. 10.1074/jbc.M403129200. [DOI] [PubMed] [Google Scholar]
- Bartolini M.; Bertucci C.; Bolognesi M. L.; Cavalli A.; Melchiorre C.; Andrisano V. Insight into the kinetic of amyloid beta (1–42) peptide self-aggregation: elucidation of inhibitors’ mechanism of action. Chembiochem 2007, 8 (17), 2152–2161. 10.1002/cbic.200700427. [DOI] [PubMed] [Google Scholar]; Naiki H.; Higuchi K.; Hosokawa M.; Takeda T. Fluorometric determination of amyloid fibrils in vitro using the fluorescent dye, thioflavin T1. Anal. Biochem. 1989, 177 (2), 244–249. 10.1016/0003-2697(89)90046-8. [DOI] [PubMed] [Google Scholar]
- Bartolini M.; Naldi M.; Fiori J.; Valle F.; Biscarini F.; Nicolau D. V.; Andrisano V. Kinetic characterization of amyloid-beta 1–42 aggregation with a multimethodological approach. Anal. Biochem. 2011, 414 (2), 215–225. 10.1016/j.ab.2011.03.020. [DOI] [PubMed] [Google Scholar]
- Satoh T.; McKercher S. R.; Lipton S. A. Nrf2/ARE-mediated antioxidant actions of pro-electrophilic drugs. Free Radic Biol. Med. 2013, 65, 645–657. 10.1016/j.freeradbiomed.2013.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y.; Huang Q.; Yu X.; Liu Y.; Li L.; Li B.; Zhang X.; Chen S.; Liu Z.; Zhao X.; Ma J. Study of reactions of Nε-(carboxymethyl) lysine with o-benzoquinones by cyclic voltammetry. Food Chem. 2020, 307, 125554. 10.1016/j.foodchem.2019.125554. [DOI] [PubMed] [Google Scholar]
- Ono K.; Hasegawa K.; Naiki H.; Yamada M. Preformed beta-amyloid fibrils are destabilized by coenzyme Q10 in vitro. Biochem. Biophys. Res. Commun. 2005, 330 (1), 111–116. 10.1016/j.bbrc.2005.02.132. [DOI] [PubMed] [Google Scholar]
- Hofmann J.; Ginex T.; Espargaró A.; Scheiner M.; Gunesch S.; Aragó M.; Stigloher C.; Sabaté R.; Luque F. J.; Decker M. Azobioisosteres of Curcumin with Pronounced Activity against Amyloid Aggregation, Intracellular Oxidative Stress, and Neuroinflammation. Chemistry 2021, 27 (19), 6015–6027. 10.1002/chem.202005263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherzer-Attali R.; Pellarin R.; Convertino M.; Frydman-Marom A.; Egoz-Matia N.; Peled S.; Levy-Sakin M.; Shalev D. E.; Caflisch A.; Gazit E.; Segal D. Complete phenotypic recovery of an Alzheimer’s disease model by a quinone-tryptophan hybrid aggregation inhibitor. PLoS One 2010, 5 (6), e11101 10.1371/journal.pone.0011101. [DOI] [PMC free article] [PubMed] [Google Scholar]; Convertino M.; Pellarin R.; Catto M.; Carotti A.; Caflisch A. 9,10-Anthraquinone hinders beta-aggregation: how does a small molecule interfere with Abeta-peptide amyloid fibrillation?. Protein Sci. 2009, 18 (4), 792–800. 10.1002/pro.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T.; Zhang J.; Derreumaux P.; Mu Y. Molecular mechanism of the inhibition of EGCG on the Alzheimer Aβ(1–42) dimer. J. Phys. Chem. B 2013, 117 (15), 3993–4002. 10.1021/jp312573y. [DOI] [PubMed] [Google Scholar]
- Hou L.; Kang I.; Marchant R. E.; Zagorski M. G. Methionine 35 oxidation reduces fibril assembly of the amyloid abeta-(1–42) peptide of Alzheimer’s disease. J. Biol. Chem. 2002, 277 (43), 40173–40176. 10.1074/jbc.C200338200. [DOI] [PubMed] [Google Scholar]
- Fiori J.; Naldi M.; Bartolini M.; Andrisano V. Disclosure of a fundamental clue for the elucidation of the myricetin mechanism of action as amyloid aggregation inhibitor by mass spectrometry. Electrophoresis 2012, 33 (22), 3380–3386. 10.1002/elps.201200186. [DOI] [PubMed] [Google Scholar]
- Forester S. C.; Lambert J. D. The role of antioxidant versus pro-oxidant effects of green tea polyphenols in cancer prevention. Mol. Nutr Food Res. 2011, 55 (6), 844–854. 10.1002/mnfr.201000641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basagni F.; Lanni C.; Minarini A.; Rosini M. Lights and shadows of electrophile signaling: focus on the Nrf2-Keap1 pathway. Future Med. Chem. 2019, 11 (7), 707–721. 10.4155/fmc-2018-0423. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.