Abstract
Citrobacter rodentium is a classically noninvasive pathogen of mice that is similar to enteropathogenic Escherichia coli (EPEC) in man. Following oral infection of young mice, the organism colonizes the distal colon, and within 1 week the colonic mucosa doubles in thickness and there is massive epithelial cell hyperplasia. Since T-cell responses in mouse models of inflammatory bowel disease (IBD) also cause epithelial hyperplasia, we have investigated the possibility that C. rodentium promotes similar T-cell responses in the mucosa, thereby increasing epithelial shedding, transmission, and replication of the organism. Beginning 6 days after infection, bacteria were observed to be in close association with the epithelial surface and were also visible scattered throughout the lamina propria and in the submucosa. There was a CD3+-cell infiltrate into the colonic lamina propria and epithelium as well as mucosal thickening and crypt hyperplasia. The majority of CD3+ cells were CD4+ and were not γδ+. Reverse transcription-PCR analysis of cytokines also revealed a highly polarized Th1 response (interleukin-12, gamma interferon, and tumor necrosis factor alpha) in the mucosa and a large increase in the epithelial cell mitogen keratinocyte growth factor. None of the changes were seen in mice inoculated with bacteria lacking intimin (which is necessary for colonization), but they were seen in mice inoculated with C. rodentium complemented with intimin from EPEC. This is the first example of a classically noninvasive bacterial pathogen which elicits a strong mucosal Th1 response and which produces pathology similar to that seen in mouse models of IBD, which is also characterized by a strong Th1 response. These results also suggest that the colonic mucosa responds in a stereotypic way to Th1 responses.
Citrobacter rodentium, formerly Citrobacter freundii biotype 4280, causes transmissible murine colonic hyperplasia (6, 45, 46). The lesions produced at the epithelial surface are indistinguishable from those of enteropathogenic Escherichia coli (EPEC) infection, a major cause of infantile diarrhea in humans in developing countries (37). Murine colonic hyperplasia is a naturally occurring disease of laboratory mice, and infection is characterized by crypt hyperplasia and dilation, epithelial cell proliferation, mucosal thickening, and an uneven apical enterocyte surface. In experimentally infected mice, large numbers of bacteria colonize the distal colon and are observed adhering to the epithelial surface. The earliest signs of hyperplasia are seen 4 days after oral infection, with mucosal thickening reaching a maximum between 10 and 12 days postinfection (23). An inflammatory response also takes place, but it has not been characterized.
Binding of EPEC or C. rodentium to the enterocyte induces a specific attachment and effacement (A/E) lesion. There is dissolution of the brush border and pedestal formation (34). The proteins necessary for the formation of the A/E lesion are delivered to the host cell by a type III secretion system encoded by the chromosomal locus of enterocyte effacement (17, 45). The locus of chromosomal effacement contains 41 open reading frames which include the intimin-encoding eae gene (30) and the espA, espB, and espD genes (14, 32, 36), which encode the secreted proteins that result in the A/E lesion and in host cell cytoskeletal rearrangements and signal transduction. Intimate attachment of the bacteria to the host enterocyte is mediated through the bacterial outer membrane protein intimin, which binds to a bacterially derived receptor, translocated intimin receptor (Tir), that is embedded in the host cell surface (33). The eae genes of several different bacterial strains encode intimin proteins that have highly conserved N-terminal regions but show significant heterogeneity at the C termini (24). At least four different intimin proteins have been characterized to date, namely intimins α, β, γ, and δ. All display diversity but contain two stretches of identical amino acid sequence that may be critical to binding. Intimins α and β were found to be expressed largely by strains belonging to EPEC clones 1 and 2, respectively, while intimin γ was determined to be expressed by enterohemorrhagic E. coli serotype O157:H7 and intimin δ was found to be expressed by EPEC O86:H34 (1). An intimin mutant of C. rodentium, which normally expresses intimin β, does not cause A/E lesions in mice, but when compensated with intimin α derived from EPEC, pathogenicity is restored (23). In addition to participating in the formation of the A/E lesion, intimin has been shown to bind in vitro to β1 integrins on T cells (25). The function of such an interaction is not understood. The intimin homologue invasion of Yersinia pseudotuberculosis costimulates T-cell proliferation in vitro through interaction with the β1 integrin VLA-4 (18).
In this study, we inoculated mice with wild-type C. rodentium or an intimin-β-negative mutant of C. rodentium, and the latter was compensated with intimin α from an EPEC isolate. We have characterized for the first time the host immune response that follows infection with this organism.
MATERIALS AND METHODS
Animals.
Female Swiss NIH and C3H mice (21 days old) were obtained from Harlan/Olac, Scunthorpe, United Kingdom. All mice were housed under specific-pathogen-free conditions with unlimited access to food and water.
Bacterial strains and challenge of mice with bacteria.
The bacterial strains used in this study were described previously (23) and are listed in Table 1. Mice (Swiss NIH or C3H) were orally inoculated by gavage with either wild-type C. rodentium, C. rodentium DBS255, or C. rodentium DBS255(pCVD438). For inoculations, bacteria were grown overnight in L broth containing 100 μg of nalidixic acid per ml or 30 μg of chloramphenicol per ml. Bacteria were diluted with phosphate-buffered saline, pH 7.2, to an optical density at 600 nm of 1.7 and delivered to mice in a 100-μl volume containing ca. 1.5 × 107 CFU. Mice were killed at various time points postchallenge, and tissues were snap frozen in liquid nitrogen and stored at −70°C for further analysis.
TABLE 1.
Bacterial strains used in this study
| Strain | Description | Intimin derivative expressed |
|---|---|---|
| C. rodentium biotype 4280 | Wild-type C. rodentium | β |
| DBS255 | C. rodentium eae mutant | None |
| E2348/69 | Wild-type EPEC (O127ab:H6) | α |
| DBS255(pCVD438) | DBS255 complemented with eae from E2348/69 | α |
Immunohistochemistry.
Three-step avidin-peroxidase staining was performed on 5-μm-thick frozen sections of distal colonic tissue as described previously (51), using monoclonal antibodies 145-2C11 (anti-CD3), YTS 191 (anti-CD4), YTS 169 (anti-CD8), and M5-114 (anti-major histocompatability complex [MHC] class II). Biotin-conjugated rabbit anti-rat immunoglobulin G (IgG; DAKO, High Wycombe, United Kingdom) and goat anti-hamster IgG (Vector Laboratories, Peterborough, United Kingdom) were used at a 1:50 dilution in Tris-buffered saline, pH 7.6, containing 4% (vol/vol) normal mouse serum (Harlan Seralab, Oxon, United Kingdom). Avidin peroxidase (Sigma) was used at a dilution of 1:200 in Tris-buffered saline. A two-step protocol was performed with polyclonal rabbit anti-E. coli antibody (Sigma) and rabbit anti-intimin antibody (1) together with horseradish peroxidase-conjugated swine anti-rabbit IgG secondary antibody. Peroxidase activity was detected with 3,3′-diaminobenzidine tetrahydrochloride (DAB; Sigma) in 0.5-mg/ml Tris-HCl, pH 7.6, containing 0.01% H2O2. Endogenous-peroxidase-containing cells were visualized by incubation of sections with DAB substrate and H2O2 alone. The density of positive cells in the lamina propria was determined by image analysis as described previously (38). Crypt length was measured by micrometry, with 10 measurements being taken in the distal colons of individual mice. Only well-oriented crypts were counted. All measurements were carried out by L.M.H. The measurements were not blinded, but all were independently confirmed by T.T.M.
RNA extraction and quantitative RT-PCR.
Total cellular RNA was isolated from frozen distal colonic tissue by homogenization of the tissue in TRIzol (Gibco Life Technologies, Paisley, United Kingdom) and incubation at room temperature for 5 min. RNA was extracted with chloroform (Sigma) and then centrifuged for 15 min at 12,000 × g and 4°C. The aqueous phase was precipitated with an equal volume of isopropanol (Sigma) and subsequently centrifuged for 15 min at 12,000 × g and 4°C. The pellet was washed with 70% ethanol and resuspended in 50 μl of water. Total RNA was determined by spectrometric analysis. Constructs encoding standard RNAs (pMCQ1, pMCQ2, pMCQ3, and pMCQ4), kindly provided by M. F. Kagnoff, Department of Medicine, University of California, San Diego (16), and a construct encoding keratinocyte growth factor (KGF) RNA, generated by M. Bajaj-Elliott, Department of Paediatric Gastroenterology, St. Bartholomew’s Hospital, London, United Kingdom, (3), were used for quantitative competitive reverse transcription (RT)-PCR. To generate standard RNA, plasmids were linearized with SalI (pMCQ1), NotI (pMCQ2, -3, and -4), or HindIII (pKGF) and transcribed in vitro with T7 RNA polymerase under conditions recommended by the supplier (Promega, Southampton, United Kingdom). Serial 10-fold dilutions of standard RNA (1 pg to 1 fg) were co-reverse transcribed with total cellular RNA (2 μg) at 42°C for 50 min in a 20-μl reaction volume containing 50 mM Tris (pH 8.3), 75 mM KCl, 3 mM MgCl2, 3 mM dithiothreitol, 10 mM deoxynucleoside triphosphate mix, and 0.5 μg oligo(dT) (Pharmacia Biotech, St. Albans, Hertfordshire, United Kingdom), using 100 U of reverse transcriptase (Superscript II; RNase H negative; Gibco). The reaction was terminated by heat inactivation at 70°C for 10 min. PCR amplification was routinely carried out in 50-μl reaction volumes (10 mM Tris [pH 9], 50 mM KCl, 1.5 mM MgCl2, 200 μM each deoxynucleoside triphosphate, and 10 pmol each of 5′ and 3′ primers) as described elsewhere (19, 20), using 1 U of Taq polymerase (Pharmacia Biotech). Forty amplification cycles consisting of a 45-s denaturation at 94°C, a 45-s annealing at 58°C, and a 75-s extension at 72°C were used. After amplification, PCR products were analyzed on 1% agarose gels and bands were visualized by ethidium bromide staining. Band intensities were quantified by densitometry (Seescan, Cambridge, United Kingdom). The sensitivity of this technique enables the detection of >103 mRNA transcripts per μg of total RNA.
Statistics.
The significance of differences between means was determined by using the Mann-Whitney U test.
RESULTS
Colonic hyperplasia and bacterial colonization caused by infection with C. rodentium.
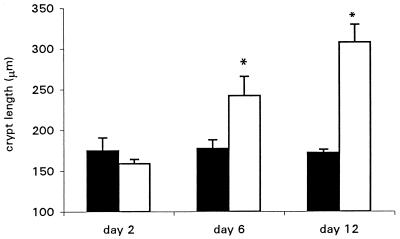
Swiss NIH mice were killed on days 2, 6, and 12 postinfection, and their colons were examined for signs of bacterial colonization and mucosal hyperplasia by immunohistochemistry. The colons from wild-type-bacterium- and mutant-bacterium-infected mice were indistinguishable on day 2. Macroscopic thickening of the distal colon was observed in approximately 60% of the mice infected with wild-type bacteria by day 6 and in all such mice by day 12. Microscopic examination showed massive epithelial cell hyperplasia with a two- to fourfold increase in mucosal thickness and crypt length; the latter is quantified in Fig. 1. In preliminary experiments it was observed that male and female mice were equally susceptible to infection and displayed similar pathologies. All results presented here represent infection of female mice.
FIG. 1.
Swiss NIH mice infected with wild-type C. rodentium showed severe colonic hyperplasia compared to mice infected with an intimin mutant strain. Crypt lengths were measured on days 2, 6, and 12 postinfection. Mean crypt lengths in the colons of mice infected with wild-type C. rodentium (open bars) were significantly greater than those of mice infected with the control mutant strain (closed bars). Error bars represent standard errors. Each group contained five mice. ∗, P < 0.05).
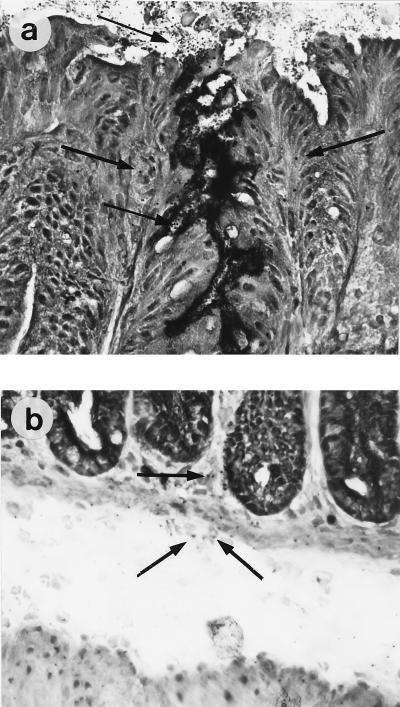
The presence of bacteria adhering to the epithelial surface was visualized by immunohistochemistry with an anti-E. coli antibody that cross-reacts with C. rodentium or with anti-intimin antibody. The two types of immunostaining revealed identical patterns of expression. In mice infected with intimin mutants, no bacteria were present at any time point, while in wild-type-C. rodentium-infected mice, bacteria were observed in close association with enterocytes both at the tips of the villi and deep down in crypts (Fig. 2a). Colonization of the epithelium was directly associated with mucosal thickening and crypt hyperplasia on days 6 and 12, and no bacteria were observed on day 2 postinfection. In addition to the bacteria at the enterocyte surface, organisms were seen scattered throughout the lamina propria and in the edematous submucosa (Fig. 2b). Viable bacteria were occasionally isolated from the mesenteric lymph nodes (results not shown). Other features of pathogenesis included loss of goblet cells, increased shedding of epithelial cells into the lumen, and an uneven apical surface.
FIG. 2.
Bacteria were visualized on the epithelial surface (a) and in the mucosa and submucosa (b) of C. rodentium-infected Swiss NIH mice. The clear area between the muscularis mucosa and the external muscle layer is the submucosa, which becomes edematous during Citrobacter infection. The arrows indicate bacteria. Immunoperoxidase immunohistochemistry was performed with anti-intimin antibody. Magnification, ×400.
T-cell infiltrate resembling inflammatory bowel disease (IBD).
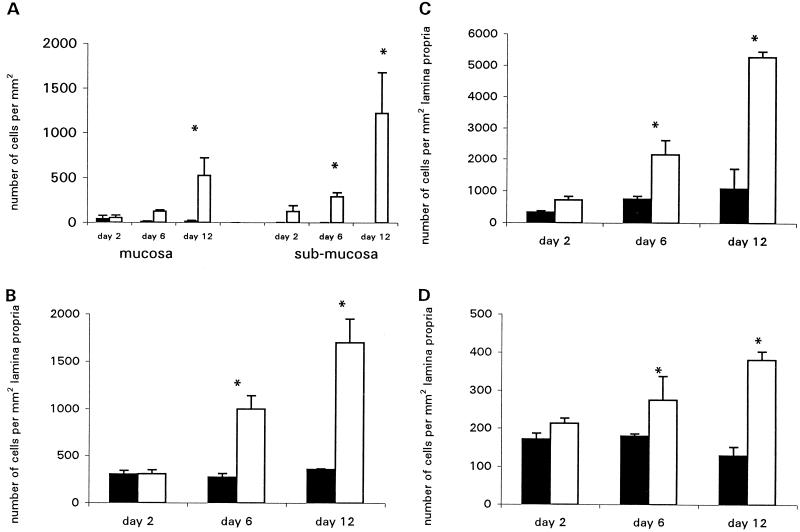
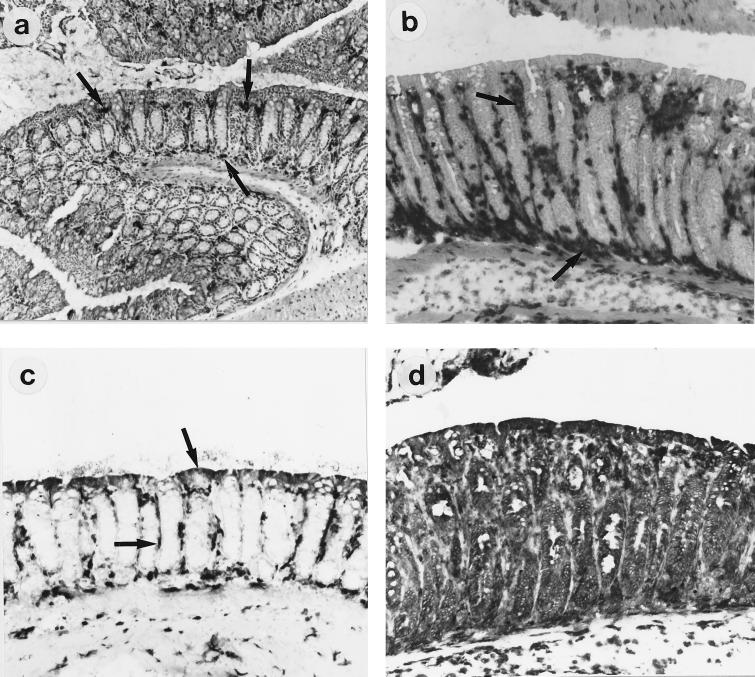
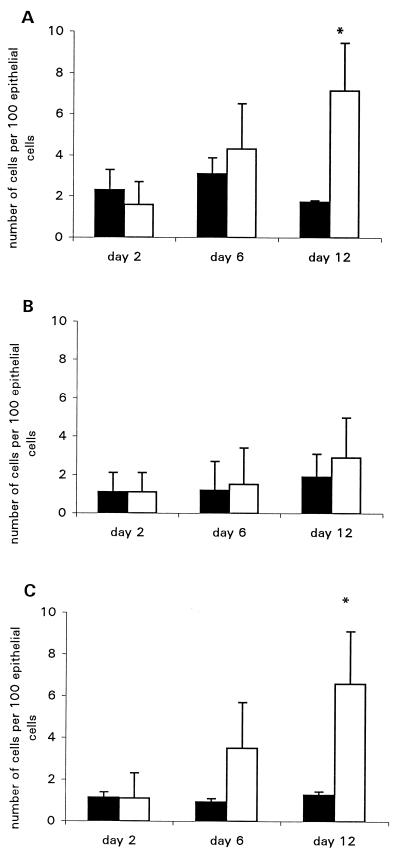
Infiltrating T cells and macrophages were visualized by immunohistochemistry analysis for CD3+, CD4+, and CD8+ cells on frozen sections. Once colonization had taken place, a striking inflammatory infiltrate was observed. Numbers of endogenous-peroxidase-containing cells were increased, particularly in the submucosa (Fig. 3A). The predominant cell types infiltrating the mucosa were CD4+ and CD3+ T cells and CD4+ macrophages. Since the density of CD4+ cells exceeded that of CD3+ cells, we assume, but have not formally shown, that the excess CD4+ cells are macrophages. There was also a slight increase in numbers of CD3+ cells and CD4+ cells in mice given intimin-negative C. rodentium over the course of the experiment (Fig. 3B and C). Rather than this being a weak inflammatory response to the bacteria, we feel that this reflects the normal age-associated increase in numbers of mucosal T cells and macrophages which occurs around this time in all mice (21). CD8+ cells also increased in number, as quantified in Fig. 3D (CD4+ cells are visualized in Fig. 4a and b). The infiltrate was located predominantly in the lamina propria at the base of the crypts. There was only a marginal increase in the number of intraepithelial lymphocytes, and these were almost exclusively of the CD8+ phenotype (Fig. 5). An increase in γδ intraepithelial lymphocytes was not seen (data not shown). No significant difference between the wild type and controls was observed on day 2. In addition, in mice infected with intimin-negative C. rodentium, only a few MHC class II-positive cells were observed scattered throughout the lamina propria, and surface epithelial cells were also evident. However, in mice infected with intimin-expressing C. rodentium there was strong MHC class II expression on crypt and surface epithelial cells as well as on most of the cells in the lamina propria, suggesting that gamma interferon (IFN-γ) is being produced by mucosal T cells (Fig. 4c and d).
FIG. 3.
Mean cell counts for peroxidase-containing cells in the mucosa and submucosa (A) and for CD3+ (B), CD4+ (C), and CD8+ (D) cells infiltrating the lamina propria of Swiss NIH mice were determined on days 2, 6, and 12 postinfection. In addition to the massive increase in numbers of CD3+ and CD4+ cells in the mucosa of C. rodentium-infected mice, there was also a slight increase in numbers of these cells in control mice. This probably reflected the increase which is seen in normal mice around this time (21). Closed bars represent mice infected with an intimin mutant strain; open bars represent mice infected with wild-type C. rodentium. Error bars indicate standard errors. Each group contained five mice. ∗, P < 0.05).
FIG. 4.
Immunoperoxidase immunohistochemistry of colon tissue of infected Swiss NIH mice. (a and b) CD4+ cells in mice infected with an intimin mutant strain (a) or wild-type C. rodentium (b). In colon tissues of control mice, only a few CD4+ cells are visible in the lamina propria (arrows). In Citrobacter-infected mice, there is a massive accumulation of CD4+ cells at the crypt bases and in the lamina propria (arrows). (c and d) MHC class II-positive cells in the lamina propria of intimin-mutant-infected mice (c) and wild-type-C. rodentium-infected mice (d). In control mice, MHC class II-positive cells can be seen in the lamina propria, and the surface epithelium is also positive (arrows). In Citrobacter-infected mice, every surface and crypt epithelial cell is strongly class II positive, as are all cells in the lamina propria. Serial sections stained with control antibodies showed only a few cells expressing endogenous peroxidase in the mucosa, although these cells were more abundant in the submucosa (see Fig. 3A). Original magnification, ×100.
FIG. 5.
Quantitation of intraepithelial cells. Counts are expressed as the number of positive intraepithelial lymphocytes per 100 epithelial cells. Each group contained five Swiss NIH mice. Closed bars represent mice infected with the intimin mutant strain; open bars represent mice infected with wild-type C. rodentium. Values are means ± standard errors. (A) CD3+ cells; (B) CD4+ cells; (C) CD8+ cells. ∗, P < 0.05.
Infiltrating T cells are of a Th1 phenotype.
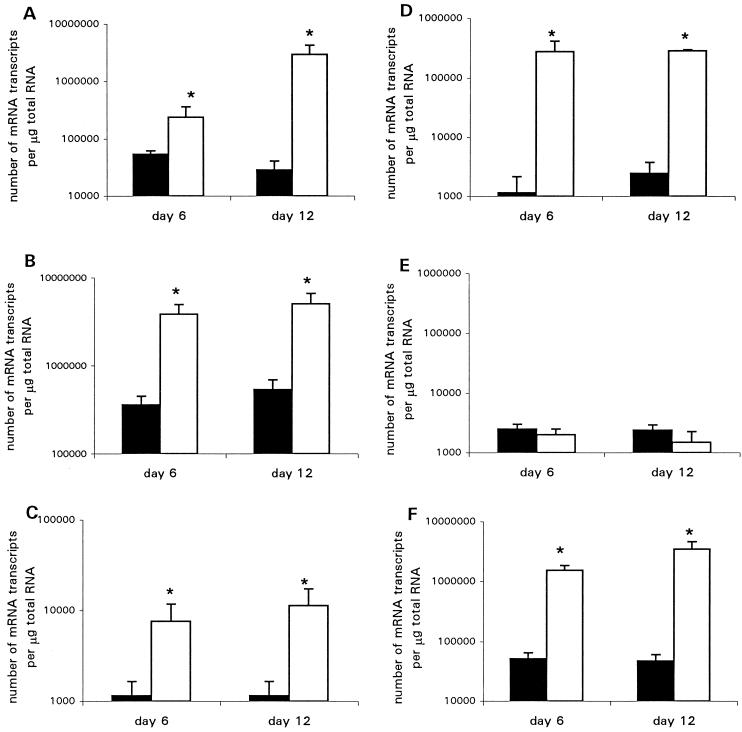
The cytokine profile in the distal colons of infected mice was determined by competitive quantitative RT-PCR. Mice infected with wild-type C. rodentium showed a striking increase in mRNA transcripts for interleukin-1 (IL-1), tumor necrosis factor alpha (TNF-α), IL-12, and IFN-γ. Results for mice killed on days 6 and 12 are shown in Fig. 6. Transcript numbers for the Th2 type cytokine IL-4 in the colons of wild-type-infected mice were not significantly different from those of control mice (Fig. 6A to E).
FIG. 6.
Cytokine and KGF mRNA transcripts in distal colonic tissue as measured by RT-PCR on days 6 and 12 postinfection. Closed bars represent mice infected with the intimin mutant strain; open bars represent mice infected with wild-type C. rodentium. Each group contained five Swiss NIH mice. Values are means ± standard errors. (A) IL-1; (B) TNF-α; (C) IL-12; (D) IFN-γ; (E) IL-4; (F) KGF. ∗, P < 0.05.
Elevated production of the epithelial cell growth factor KGF.
In response to proinflammatory cytokines, stromal cells produce KGF, which interacts with the KGF receptor on epithelial cells and increases epithelial cell proliferation (4). Because of the overwhelming increase in epithelial cell proliferation in wild-type-infected mice, levels of mRNA transcripts for KGF in colonic tissue were measured by quantitative RT-PCR. A dramatic increase in transcript levels was observed in mice displaying hyperplasia. Results for mice killed on days 6 and 12 are shown in Fig. 6F.
Infection of different strains of mice and infection with intimin-α-expressing C. rodentium.
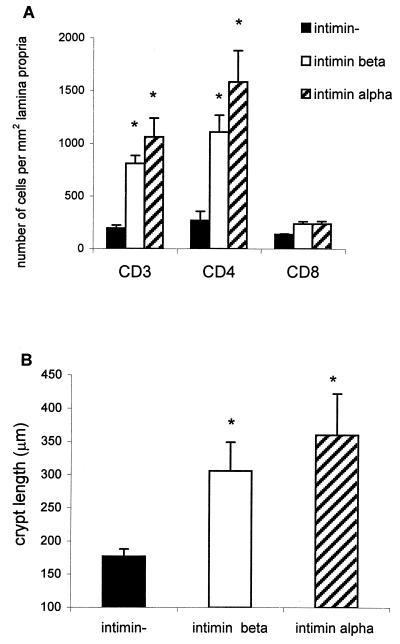
All of the results presented in this article so far represent infection of Swiss NIH mice with wild-type C. rodentium expressing intimin β. C3H mice were also infected with this organism as well as with a strain of C. rodentium expressing intimin α. Colonic hyperplasia was seen when either strain of bacterium was used, with bacteria being evident on the epithelial surface upon immunostaining (data not shown). In addition, immunohistochemistry was performed on the colonic tissues from these mice, and CD3+, CD4+, and CD8+ cells were counted. When C3H mice were infected with either intimin-α- or intimin-β-expressing C. rodentium, a significant increase in CD3+ and CD4+ cells in the lamina propria was seen, although this increase was smaller than that observed in Swiss NIH mice (Fig. 7A). There was also a significant increase in crypt length in C3H mice infected with wild-type C. rodentium expressing intimin β and in mice infected with C. rodentium expressing human intimin α (Fig. 7B).
FIG. 7.
(A) Cell counts on day 12 postinfection of C3H mice with wild-type intimin-β-expressing C. rodentium (open bars), intimin-α-expressing DBS255(pCVD438) (hatched bars), and intimin mutant (closed bars) strains. (B) Crypt lengths (day 12) in the colons of wild-type-C. rodentium-infected mice (open bars) or mice infected with C. rodentium expressing human intimin α were significantly greater than those of mice infected with the control mutant strain (closed bars). Each group contained five mice. Values are means ± standard errors. ∗, P < 0.05.
DISCUSSION
In the present study, we have characterized the inflammatory infiltrate in the colon following oral infection of young mice with C. rodentium. First, we demonstrated that infection with C. rodentium elicited a mucosal T-cell infiltrate associated with colonic hyperplasia. Second, we demonstrated that the infiltrate consisted predominantly of CD4+ T cells with a Th1 phenotype. Third, the epithelial cell hyperplasia was found to be associated with KGF production. Finally, we showed that C. rodentium expressing either wild-type intimin β or EPEC intimin α produced a similar response following infection.
Murine colonic hyperplasia caused by C. rodentium was first identified more than 2 decades ago (5), but the role of inflammatory mediators in this response has not been reported. Studies of EPEC in humans tend to concentrate on the resulting diarrhea rather than the extent of mucosal damage owing to the ethical problem of taking biopsy specimens during infections. However, there have been occasional reports of small-intestinal mucosal injury in children with chronic diarrhea caused by EPEC. In one such study, biopsy samples from five of six children showed villus atrophy and crypt hyperplasia (28). Others have also reported a high incidence of villus atrophy in jejunal specimens, as well as colitis in EPEC-infected children (20). Extensive mucosal damage, including villus atrophy and crypt hyperplasia, was also seen in piglets infected with EPEC, even after the diarrhea had subsided (50). In addition, although mucosal damage was present when the large intestine was infected, diarrhea occurred only when the small bowel was affected. These studies highlight the fact that different strains of EPEC are capable of infecting both the small and large intestine, although infection of the jejunum and duodenum has been more closely associated with human disease.
The A/E epithelial lesion formed by C. rodentium and EPEC involves an intimate, noninvasive association of the bacteria with the host cell (14, 30, 32, 33, 36). Despite this highly characterized A/E lesion, EPEC can also enter the mucosa, a feature we visualized with C. rodentium. Donnenberg et al. (13) examined the invasiveness of EPEC compared to those of enteroinvasive E. coli, enterotoxigenic E. coli, and enterohemorrhagic E. coli and found, for the particular strains studied, that EPEC was actually more efficient at invading Hep-2 cell lines than was enteroinvasive E. coli. In infected piglets, EPEC was shown to translocate into the lamina propria, causing mucosal injury but not diarrhea (50), and it has been found within the epithelium in a small-bowel biopsy specimen from a child with acute diarrhea (19). Translocation of bacteria into the lamina propria may occur through leaky tight junctions or a damaged epithelium. Increased epithelial permeability following coincubation of EPEC with T84 monolayers has been reported, and it has also been shown that EPEC adherence disrupts T84 barrier function, resulting in increased paracellular conductance (47). The reduction in the junctional integrity may be triggered by the activation of sodium ion transporters (12). Colonization and invasion of intestinal epithelial cell lines at different stages of differentiation have also been examined. EPEC efficiently colonizes only fully differentiated cells, while invasion occurs only in recently differentiated, but not fully differentiated, cell lines (26).
In C. rodentium-infected mice, we also observed via immunohistochemistry that bacteria do transverse the epithelial barrier and enter the colonic mucosa, where interaction with host immune cells is highly likely. It is possible that these bacteria are nonviable and are passively leaking into the lamina propria. Although we have detected viable C. rodentium in the mesenteric lymph nodes of orally infected mice, we have no way of telling how many of the bacteria that we visualized in the mucosa and draining lymphatics are alive. A similar process is believed to occur in IBD; enteric bacteria have been isolated from the mesenteric lymph nodes of patients with Crohn’s disease (2).
Peyer’s patches and lymphoid follicles of the large intestine are the primary sites of antigen uptake from the lumen. Antigen is taken up by M cells in the follicle-associated epithelium overlying the organized lymphoid follicles (40). Many invasive pathogens use M cells as a way of transiting across the mucosal barrier (27, 31). Yersinia enterocolitica infects M cells by using invasin, the intimin homologue, to bind to β1 integrins on the apical surface of these cells (11). It may also cause systemic infection by a Peyer’s patch-independent mechanism (41). It is well established that rabbit EPEC adheres to M cells via either plasmid-encoded pili, AF/R1, or, in afrA-negative strains, some other unknown ligand (52). The M-cell receptors, however, are unknown. It is highly likely that C. rodentium and EPEC also interact with β1 integrins on M cells through intimin, although this has not been formally shown.
The striking T-cell response which accompanies A/E lesion formation and crypt hyperplasia indicates that C. rodentium is capable of activating mucosal T cells, which under normal conditions are hyporesponsive and require strong costimulatory signals before cytokine production occurs (49). This lack of responsiveness is essential for the prevention of inappropriate immune activation to innocuous gut antigens, including the normal flora. However, it is now well established that a breakdown in tolerance is a feature of IBD, as demonstrated by Duchmann and colleagues, who found that both peripheral blood and lamina propria T cells isolated from patients with Crohn’s disease were activated by autologous gut microflora antigens (15).
The T-cell infiltration, cytokine production, and epithelial cell proliferation seen in C. rodentium-infected mice resemble those seen in murine models of IBD. These include mice with a variety of T-cell defects, including mice homozygous for null mutations in the genes encoding IL-2 (44), IL-10 (35), T-cell receptor alpha (TcRα) or TcRβ (39), and Gαi2 (43). In addition, T-cell-reconstituted tgɛ26 mice transgenic for the human CD3ɛ gene (29) and mice transgenic for IL-7 (53) develop chronic IBD. In these models, disease is a result of immune dysregulation and is mediated principally by CD4+ T cells of a Th1 phenotype and in certain cases, including TcRα knockout mice, of a Th2 phenotype (48). As in models of IBD, infection of mice with C. rodentium is dependent on host genetic background and gut microflora (7).
We have observed an increase in mRNA for KGF in C. rodentium-infected mice. Increasing epithelial cell proliferation is advantageous for the colonizing organism since it results in increased substrate surface area for formation of A/E lesions. Following T-cell activation, cytokine production triggers a cascade of events, including production of KGF (4). KGF is produced by mesenchymal cells and interacts with the KGF receptor on epithelial cells, resulting in proliferation (42). Elevated KGF mRNA levels have been detected in gastrointestinal tissue isolated from patients with Crohn’s disease, ulcerative colitis, and celiac disease (9, 22, 54). In addition, the role of KGF in repair of mucosal injury has been examined in several animal models of IBD (54). In agreement with the concomitant KGF and cytokine production detected in this study, others have shown that proinflammatory cytokines, including IL-1, IL-6, and TNF-α, upregulate KGF production by fibroblasts (8, 10).
The interaction of VLA (very late activation antigen) integrins on T cells with intimin has been demonstrated previously (25), and although it is widely believed that intimin does not interact with β1 integrins on epithelial cells (33), the function of intimin-integrin interaction in infection remains unknown. It is conceivable that costimulation of T cells by intimin expressed on the outer surface of C. rodentium results in mucosal T-cell proliferation.
In conclusion, we have demonstrated for the first time that C. rodentium, the mouse equivalent of human EPEC, induces a T-cell response that is similar to that seen in IBD. We do not, however, suggest that IBD is a result of a specific pathogenic agent but rather imply that the gut behaves in a stereotypic fashion to Th1 responses.
ACKNOWLEDGMENTS
This work was supported by the Wellcome Trust. L. M. Higgins was supported by the Crohn’s in Childhood Research Association (CICRA).
REFERENCES
- 1.Adu-Bobie J, Frankel G, Bain C, Guedes Goncalves A, Trabulsi L R, Douce G, Knutton S, Dougan G. Detection of intimins α, β, γ, and δ, four intimin derivatives expressed by attaching and effacing microbial pathogens. J Clin Microbiol. 1998;36:662–668. doi: 10.1128/jcm.36.3.662-668.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ambrose N S, Johnson M, Burdon D W, Keighley M R B. Incidence of pathogenic bacteria from mesenteric lymph nodes and ileal serosa during Crohn’s disease surgery. Br J Surg. 1984;71:623–625. doi: 10.1002/bjs.1800710821. [DOI] [PubMed] [Google Scholar]
- 3.Bajaj-Elliott M, Breese E, Poulsom R, Fairclough P D, MacDonald T T. Keratinocyte growth factor in inflammatory bowel disease: increased mRNA transcripts in ulcerative colitis compared with Crohn’s disease in biopsies and isolated mucosal myofibroblasts. Am J Pathol. 1997;151:1469–1476. [PMC free article] [PubMed] [Google Scholar]
- 4.Bajaj-Elliott M, Poulsom R, Pender S L F, Wathen N C, MacDonald T T. Interactions between stromal cell-derived keratinocyte growth factor and epithelial transforming growth factor in immune-mediated crypt cell hyperplasia. J Clin Investig. 1998;102:1473–1480. doi: 10.1172/JCI2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barthold S W, Coleman G L, Bhatt P N, Osbaldiston G W, Jonas A M. The etiology of transmissable murine colonic hyperplasia. Lab Anim Sci. 1976;26:889–894. [PubMed] [Google Scholar]
- 6.Barthold S W, Coleman G L, Jacoby R O, Livstone E M, Jonas A M. Transmissable murine colonic hyperplasia. Vet Pathol. 1978;15:223–236. doi: 10.1177/030098587801500209. [DOI] [PubMed] [Google Scholar]
- 7.Barthold S W, Osbaldiston G W, Jonas A M. Dietary, bacterial, and host genetic interactions in the pathogenesis of transmissable murine colonic hyperplasia. Lab Anim Sci. 1977;27:938–945. [PubMed] [Google Scholar]
- 8.Brauchle M, Angermeyer K, Hüber G, Werner S. Large induction of keratinocyte growth factor expression by serum growth factors and proinflammatory cytokines in cultured fibroblasts. Oncogene. 1994;9:3199–3204. [PubMed] [Google Scholar]
- 9.Brauchle M, Madlener M, Wagner A D, Angermeyer K, Lauer U, Hofschneider P H, Gregor M, Werner S. Keratinocyte growth factor is highly overexpressed in inflammatory bowel disease. Am J Pathol. 1996;149:521–529. [PMC free article] [PubMed] [Google Scholar]
- 10.Chedid M, Rubin J S, Csaky K G, Aaronson S A. Regulation of keratinocyte growth factor gene expression by interleukin 1. J Biol Chem. 1994;269:10753–10757. [PubMed] [Google Scholar]
- 11.Clark M A, Hirst B H, Jepson M A. M-cell surface β1 integrin expression and invasin-mediated targeting of Yersinia pseudotuberculosis to mouse Peyer’s patch M cells. Infect Immun. 1998;66:1237–1243. doi: 10.1128/iai.66.3.1237-1243.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collington G K, Booth I W, Knutton S. Rapid modulation of electrolyte transport in Caco-2 cell monolayers by enteropathogenic Escherichia coli (EPEC) infection. Gut. 1998;42:200–207. doi: 10.1136/gut.42.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donnenberg M S, Donohue-Rolfe A, Keusch G T. Epithelial cell invasion: an overlooked property of enteropathogenic Escherichia coli (EPEC) associated with the EPEC adherence factor. J Infect Dis. 1989;160:452–459. doi: 10.1093/infdis/160.3.452. [DOI] [PubMed] [Google Scholar]
- 14.Donnenberg M S, Yu J, Kaper J B. A second chromosomal gene necessary for intimate attachment of enteropathogenic Escherichia coli to epithelial cells. J Bacteriol. 1993;175:4670–4680. doi: 10.1128/jb.175.15.4670-4680.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duchmann R, Kaiser I, Hermann E, Mayet W, Ewe K, Meyer Zum Büschenfelde K H. Tolerance exists towards resident intestinal flora but is broken in active inflammatory bowel disease (IBD) Clin Exp Immunol. 1995;102:448–455. doi: 10.1111/j.1365-2249.1995.tb03836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eckmann L, Fierer J, Kagnoff M F. Genetically resistant (Ityr) and susceptible (Itys) congenic mouse strains show similar cytokine responses following infection with Salmonella dublin. J Immunol. 1996;156:2894–2900. [PubMed] [Google Scholar]
- 17.Elliott S J, Wainwright L A, McDaniel T K, Jarvis K G, Deng Y K, Lai L C, McNamara B P, Donnenberg M S, Kaper J B. The complete sequence of the locus of enterocyte effacement (LEE) from enteropathogenic Escherichia coli E2348/69. Mol Microbiol. 1998;28:1–4. doi: 10.1046/j.1365-2958.1998.00783.x. [DOI] [PubMed] [Google Scholar]
- 18.Ennis E, Isberg R R, Shimizu Y. Very late antigen 4-dependent adhesion and costimulation of resting human T cells by the bacterial β1 integrin ligand invasin. J Exp Med. 1993;177:207–212. doi: 10.1084/jem.177.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fagundes Neto U, Freymuller E, Gatti M S, Schmitz L G, Scaletsky I. Enteropathogenic Escherichia coli O111ab:H2 penetrates the small bowel epithelium in an infant with acute diarrhoea. Acta Paediatr. 1995;84:453–455. doi: 10.1111/j.1651-2227.1995.tb13670.x. [DOI] [PubMed] [Google Scholar]
- 20.Fagundes Neto U, de Castro Ferreira V, Patricio F R S, Mostaço V L, Trabulsi L R. Protracted diarrhea: the importance of the enteropathogenic E. coli (EPEC) strains and Salmonella in its genesis. J Pediatr Gastroenterol Nutr. 1989;8:207–211. [PubMed] [Google Scholar]
- 21.Ferguson A, Parrott D M V. The effect of antigen deprivation on thymus-dependent and thymus-independent lymphocytes in the small intestine of the mouse. Clin Exp Immunol. 1973;12:477–488. [PMC free article] [PubMed] [Google Scholar]
- 22.Finch P W, Pricolo V, Wu A, Finkelstein S D. Increased expression of keratinocyte growth factor messenger RNA associated with inflammatory bowel disease. Gastroenterology. 1996;110:441–451. doi: 10.1053/gast.1996.v110.pm8566591. [DOI] [PubMed] [Google Scholar]
- 23.Frankel G, Phillips A D, Novakova M, Field H, Candy D C A, Schauer D B, Douce G, Dougan G. Intimin from enteropathogenic Escherichia coli restores murine virulence to a Citrobacter rodentium eaeA mutant: induction of an immunoglobulin A response to intimin and EspB. Infect Immun. 1996;64:5315–5325. doi: 10.1128/iai.64.12.5315-5325.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frankel G, Candy D C A, Everest P, Dougan G. Characterization of the C-terminal domains of intimin-like proteins of enteropathogenic and enterohemorrhagic Escherichia coli, Citrobacter freundii, and Hafnia alvei. Infect Immun. 1994;62:1835–1842. doi: 10.1128/iai.62.5.1835-1842.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frankel G, Lider O, Hershkoviz R, Mould A P, Kachalsky S G, Candy D, Cahalon L, Humphries M J, Dougan G. The cell-binding domain of intimin from enteropathogenic Escherichia coli binds to β1 integrins. J Biol Chem. 1996;271:20359–20364. doi: 10.1074/jbc.271.34.20359. [DOI] [PubMed] [Google Scholar]
- 26.Gabastou J M, Kernéis S, Bernet-Camard M F, Barbat A, Coconnier M H, Kaper J B, Servin A L. Two stages of enteropathogenic Escherichia coli intestinal pathogenicity are up- and down-regulated by epithelial cell differentiation. Differentiation. 1995;59:127–134. doi: 10.1046/j.1432-0436.1995.5920127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grutzkau A, Hanski C, Hahn H, Riecken E O. Involvement of M cells in the bacterial invasion of Peyer’s patches: a common mechanism shared by Yersinia enterocolitica and other enteroinvasive bacteria. Gut. 1990;31:1011–1015. doi: 10.1136/gut.31.9.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hill S M, Phillips A D, Walker-Smith J A. Enteropathogenic Escherichia coli and life threatening chronic diarrhoea. Gut. 1991;32:154–158. doi: 10.1136/gut.32.2.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Höllander G A, Simpson S J, Mizoguchi E, Nichogiannopoulou A, She J, Gutierrez-Ramos J C, Bhan A K, Burakoff S J, Wang B, Terhorst C. Severe colitis in mice with aberrant thymic selection. Immunity. 1995;3:27–38. doi: 10.1016/1074-7613(95)90156-6. [DOI] [PubMed] [Google Scholar]
- 30.Jerse A E, Kaper J B. The eae gene of enteropathogenic Escherichia coli encodes a 94-kilodalton membrane protein, the expression of which is influenced by the EAF plasmid. Infect Immun. 1991;59:4302–4309. doi: 10.1128/iai.59.12.4302-4309.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jones B D, Ghori N, Falkow S. Salmonella typhimurium initiates murine infection by penetrating and destroying the specialized epithelial M cells of the Peyer’s patches. J Exp Med. 1994;180:15–23. doi: 10.1084/jem.180.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kenny B, Lai L C, Finlay B B, Donnenberg M S. EspA, a protein secreted by enteropathogenic Escherichia coli, is required to induce signals in epithelial cells. Mol Microbiol. 1996;20:313–323. doi: 10.1111/j.1365-2958.1996.tb02619.x. [DOI] [PubMed] [Google Scholar]
- 33.Kenny B, BeVinney R, Stein M, Reinscheid D J, Frey E A, Finlay B B. Enteropathogenic E. coli (EPEC) transfers its receptor for intimate adherence into mammalian cells. Cell. 1997;91:511–520. doi: 10.1016/s0092-8674(00)80437-7. [DOI] [PubMed] [Google Scholar]
- 34.Knutton S, Baldwin T, Williams P H, McNeish A S. Actin accumulation at sites of bacterial adhesion to tissue culture cells: basis of a new diagnostic test for enteropathogenic and enterohemorrhagic Escherichia coli. Infect Immun. 1989;57:1290–1298. doi: 10.1128/iai.57.4.1290-1298.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kühn R, Löhler J, Rennick D, Rajewsky K, Müller W. Interleukin-10 deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 36.Lai L-C, Wainwright L A, Stone K D, Donnenberg M S. A third secreted protein that is encoded by the enteropathogenic Escherichia coli pathogenicity island is required for transduction of signals and for attaching and effacing activities in host cells. Infect Immun. 1997;65:2211–2217. doi: 10.1128/iai.65.6.2211-2217.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Levine M M. Escherichia coli that cause diarrhea: enterotoxigenic, enteropathogenic, enteroinvasive, enterohemorrhagic, and enteroadherent. J Infect Dis. 1987;155:377–389. doi: 10.1093/infdis/155.3.377. [DOI] [PubMed] [Google Scholar]
- 38.McDonald S A C, Palmen M J H J, Van Rees E P, MacDonald T T. Characterisation of the mucosal cell-mediated immune response in IL-2 knockout mice before and after the onset of colitis. Immunology. 1997;91:73–80. doi: 10.1046/j.1365-2567.1997.00217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mombaerts P, Mizoguchi E, Grusby M J, Glimcher L H, Bahn A K, Tonegawa S. Spontaneous development of inflammatory bowel disease in T cell receptor mutant mice. Cell. 1993;75:275–282. doi: 10.1016/0092-8674(93)80069-q. [DOI] [PubMed] [Google Scholar]
- 40.Owen R L, Jones A L. Epithelial cell specialisation within human Peyer’s patches: an ultrastructural study of intestinal lymphoid follicles. Gastroenterology. 1974;66:189–203. [PubMed] [Google Scholar]
- 41.Pepe J C, Wachtel M R, Wagar E, Miller V L. Pathogenesis of defined invasion mutants of Yersinia enterocolitica in a BALB/c mouse model of infection. Infect Immun. 1995;63:4837–4848. doi: 10.1128/iai.63.12.4837-4848.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rubin J S, Osada H, Finch P W, Taylor W G, Rudikoff S, Aaronson S A. Purification and characterization of a newly identified growth factor specific for epithelial cells. Proc Natl Acad Sci USA. 1989;86:802–806. doi: 10.1073/pnas.86.3.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rudolph U, Finegold M J, Rich S S, Harriman G R, Srinivasan Y, Brabert P, Boulay G, Bradley A, Birnbaumer L. Ulcerative colitis and adenocarcinoma of the colon in Gαi2 deficient mice. Nat Genet. 1995;10:143–150. doi: 10.1038/ng0695-143. [DOI] [PubMed] [Google Scholar]
- 44.Sadlack B, Merz H, Schorle H, Schimpl A, Feller A C, Horak I. Ulcerative colitis in mice with a disrupted interleukin-2 gene. Cell. 1993;75:253–261. doi: 10.1016/0092-8674(93)80067-o. [DOI] [PubMed] [Google Scholar]
- 45.Schauer D B, Falkow S. Attaching and effacing locus of a Citrobacter freundii biotype that causes transmissable murine colonic hyperplasia. Infect Immun. 1993;61:2486–2492. doi: 10.1128/iai.61.6.2486-2492.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schauer D B, Zabel B A, Pedraza I F, O’Hara C M, Steigerwalt A G, Brenner D J. Genetic and biochemical characterization of Citrobacter rodentium sp. nov. J Clin Microbiol. 1995;33:2064–2068. doi: 10.1128/jcm.33.8.2064-2068.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Spitz J, Yuhan R, Koutsouris A, Blatt C, Alverdy J, Hecht G. Enteropathogenic Escherichia coli adherence to intestinal epithelial monolayers diminishes barrier function. Am J Physiol. 1995;268:G374–G379. doi: 10.1152/ajpgi.1995.268.2.G374. [DOI] [PubMed] [Google Scholar]
- 48.Takahashi I, Kiyono H, Hamada S. CD4+ T-cell population mediates development of inflammatory bowel disease in T cell receptor alpha-chain deficient mice. Gastroenterology. 1997;112:1876–1886. doi: 10.1053/gast.1997.v112.pm9178680. [DOI] [PubMed] [Google Scholar]
- 49.Targan S R, Deem R L, Liu M, Wang S, Nel A. Definition of lamina propria T cell responsive state: enhanced cytokine responsiveness of T cells stimulated through the CD2 pathway. J Immunol. 1995;154:664–675. [PubMed] [Google Scholar]
- 50.Tzipori S, Gibson R, Montanaro J. Nature and distribution of mucosal lesions associated with enteropathogenic and enterohemorrhagic Escherichia coli in piglets and the role of plasmid-mediated factors. Infect Immun. 1989;57:1142–1150. doi: 10.1128/iai.57.4.1142-1150.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Viney J, MacDonald T T, Kilshaw P J. T cell receptor expression in intestinal intraepithelial lymphocyte subpopulations of normal and athymic mice. Immunology. 1989;66:583–587. [PMC free article] [PubMed] [Google Scholar]
- 52.Von Moll L K, Cantey J R. Peyer’s patch adherence of enteropathogenic Escherichia coli strains in rabbits. Infect Immun. 1997;65:3788–3793. doi: 10.1128/iai.65.9.3788-3793.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Watanabe M, Ueno Y, Yajima T, Okamoto S, Hayashi T, Yamazaki M, Iwao Y, Ishii H, Habu S, Uehira M, Nishimoto H, Iahikawa H, Hata J I, Hibi T. Interleukin 7 transgenic mice develop chronic colitis with decreased interleukin 7 protein accumulation in the colonic mucosa. J Exp Med. 1998;187:389–402. doi: 10.1084/jem.187.3.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zeeh J M, Procaccino F, Hoffmann P, Aukerman S L, McRoberts J A, Soltani S, Pierce G F, Lakshmanan J, Lacey D, Eyselein V E. Keratinocyte growth factor ameliorates mucosal injury in an experimental model of colitis in rats. Gastroenterology. 1996;110:1077–1083. doi: 10.1053/gast.1996.v110.pm8612996. [DOI] [PubMed] [Google Scholar]