Abstract
A series of exceptionally selective CDK2 inhibitors are described. Starting from an HTS hit, we successfully scaffold hopped to a 5,7-dihydro-6H-pyrrolo[2,3-d]pyrimidin-6-one core structure, which imparted a promising initial selectivity within the CDK family. Extensive further SAR identified additional factors that drove selectivity to above 200× for CDKs 1/4/6/7/9. General kinome selectivity was also greatly improved. Finally, use of in vivo metabolite identification allowed us to pinpoint sulfonamide dealkylation as the primary metabolite, which was ameliorated through the deuterium effect.
Keywords: CDK2, metabolism, kinase, biotransformation
The cyclin-dependent kinases (CDKs) comprise a large family of serine/threonine kinases involved in the regulation of cell cycle progression via the binding of specific cyclins. The promise of inhibiting cell division has made the CDKs a long-standing target in oncology.1,2 Early work led to the identification of numerous pan-selective inhibitors, with several compounds progressing into clinical trials (Figure 1).3−6 However, toxicities associated with certain CDK family members, in particular CDK17 and CDK9, led to the search for more selective inhibitors. Blockade of CDK4/6 has proven to be particularly successful; e.g., palbociclib (Ibrance), ribociclib (Kisqali), and abemaciclib (Verzenio) are all FDA approved drugs to treat HR+/HER2- metastatic breast cancer.8 Recent research indicates that resistance to CDK4/6-based therapies frequently proceeds through a compensatory pathway involving CDK2.9,10 Therefore, selective inhibition of CDK2 represents a promising potential therapeutic option for patients progressing after CDK4/6 therapy. In addition, a number of cancers are driven by CDK2 hyperactivity11 or amplification of CCNE1,12−14 the gene responsible for producing the cyclin partner to CDK2, cyclin E.
Figure 1.
Pan-selective CDK inhibitors in clinical trials.
Although a number of preclinical compounds with varying degrees of CDK2 selectivity have been reported,15,16 at the time we started this work, there were no reports of a CDK2 inhibitor with excellent potency and selectivity across the CDK family.17−19 We thus set out to identify a single-digit nanomolar CDK2 inhibitor possessing excellent (>100×) selectivity against CDK1, CDK4, CDK6, CDK7, and CDK9 in the primary enzyme assay, to test in vivo effects of CDK2 inhibition independently from CDK4/6.
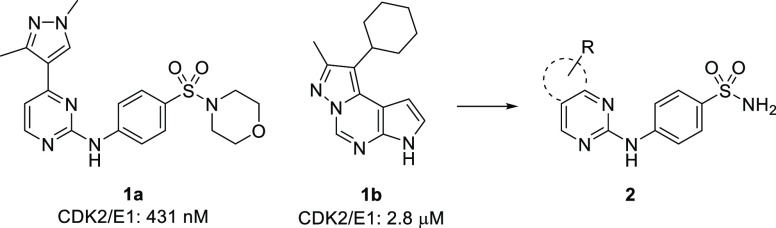
The program started with a high throughput screen of our in house compound collection. Compounds were screened in a CDK2/cyclin E1 homogeneous time-resolved fluorescence (HTRF) binding assay using eIF4E-binding protein-1 peptide as substrate, at 1 mM ATP concentration. A number of structurally distinct hits were identified. Two in particular are shown in Figure 2. Pyrimidine pyrazole 1a was the parent compound of a series of related congeners with varying substitution at the 1-position of the pyrazole. Given the tolerance for diverse pyrazole substitution, we reasoned that the combination of the aryl sulfonamide and an appropriately placed lipophilic substituent off of the 4-position of the pyrimidine were responsible for the potency of 1a. Compound 1b gave credence to this hypothesis. Albeit less potent, the cyclohexyl substituent was located in a similar area of space as the pyrazole, likely enforced by the rigid framework of the tricycle. In an effort to access distinct chemical space, we merged these two scaffolds as shown in Figure 2, in essence opening the pyrrole ring of 1b and replacing it with the 4-aminobenzenesulfonamide of 1a.20,21 We then set about identifying a bicyclic scaffold that would allow us to project an appropriate substituent to the pocket shared by 1a and 1b.
Figure 2.
Initial hit and scaffold merging design.
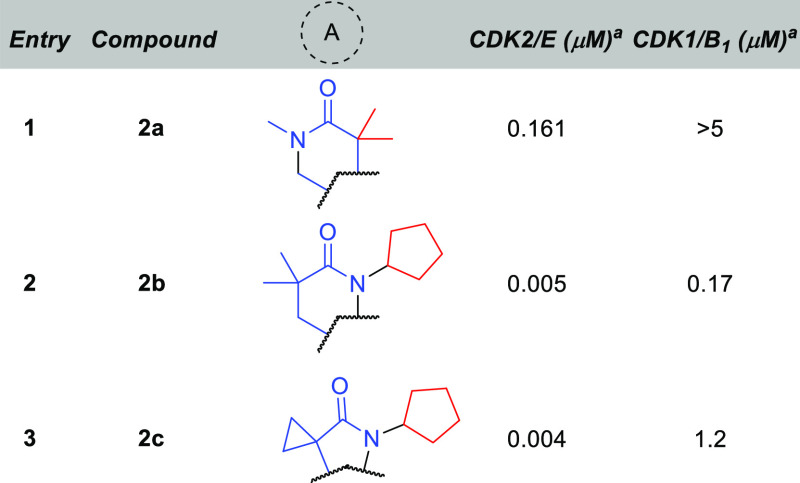
Select examples of our heterocycle screen can be seen in Table 1, with CDK1 serving as an initial surrogate of CDK family selectivity. For synthetic ease, we chose to use the unsubstituted sulfonamide as a starting point. Cyclic lactam 2a was an initial indication that our hypothesis had potential, with reasonable potency against CDK2 and promising levels of selectivity against CDK1. However, it was reversing the lactam that provided us with our first significant breakthrough. Potency was greatly enhanced for both the γ- and δ-lactam. But it was the selectivity profile that proved truly surprising: the γ-lactam was 10× less active against CDK1! The source of this selectivity, especially versus the δ-lactam, remains unclear, but it supports our design hypothesis that conformational rigidity would be beneficial to distinguishing minor differences in CDK family binding pockets.
Table 1. Bicyclic Core Screen.

Measured at 1 mM [ATP].
Compound 2c proved to be potent and selective and was therefore further profiled in cellular assays. We used a p-Rb HTRF assay in COV318 cells, with and without human whole blood added. While compound 2c showed promising potency in the cellular assay (102 nM), potency was completely abrogated in the presence of human whole blood. Literature reports that had utilized the primary aminobenzenesulfonamide had noted its propensity to bind red blood cells.22 We thus set out to find alternatives to the benzenesulfonamide. To our dismay, a wide variety of aryl, heteroaryl, and heterocycloalkyl replacements proved to be significantly less active and/or completely devoid of selectivity. In fact, of the groups surveyed, only 1-methanesulfonyl-4-aminopiperdine23 (3a, Figure 3) showed a promising level of selectivity, albeit the cutoff for our CDK1 assay precluded the exact number from being determined.
Figure 3.
1-Methanesulfonyl-4-aminopiperdine 3a as a route to improved hWB potency.
In spite of the diminished potency (∼20× relative to the aminobenzenesulfonamide), 3a showed considerable promise. We reasoned that the lack of a primary sulfonamide would provide improved translation from the cellular to the whole blood setting if sufficient potency could be achieved. We were also drawn to the aminopiperidine as a potential source of general kinome selectivity, as sp3 substitution is rarely tolerated in the hinge region of kinases.
We first elected to keep the 1-methylsulfonylpiperdine constant and explored the SAR on the lactam (R3, R3') (Table 2, entries 2–4). With even small modifications (e.g., methyl/ethyl, cyclobutyl, entries 2–3) leading to reduction in enzyme potency, we quickly discovered that limited space was available in this area. Only dimethyl substitution was tolerated (entry 4), but generally showed lower cellular potency (data not shown). As a result, the spirocyclopropyl moiety was retained in the scaffold. We next examined the lactam nitrogen substitution (R2). Expansion of the cyclopentyl ring provided similar levels of potency (Table 2, entry 5); however, replacement with an aryl moiety was less tolerated (entry 6). Introduction of polarity was similarly detrimental, e.g., tetrahydropyran 3g (2.2 μM, entry 7) vs cyclohexyl 3e (82 nM, entry 5). We eventually discovered that steric bulk around the nitrogen substituent improved potency. Racemic 2-methylcyclopentyl was the most potent of the cyclic congeners. Upon separation of the mixture, the most potent isomer (3h, entry 8, absolute configuration not assigned) exhibited single digit nanomolar potency and 77× selectivity vs CDK1, as well as submicromolar cellular potency (528 nM). We also found that open chain variants, while generally less potent than cyclic analogues, similarly benefited from α-substitution (entry 9 vs entry 10).
Table 2. Initial SAR on the Sulfonylpiperidine-Substituted Pyrimidine Lactam Series.


Measured at 1 mM [ATP].
Extensive additional screening of the R2 substituent proved to be fruitless and we realized that additional improvements in potency necessitated further modifications to the molecule. Hence, we switched our attention to the aminopiperidine. As seen earlier, most changes proved to have a drastic negative effect on activity, including changing ring size, adding substitution, or changing the capping group on the nitrogen. The only region that tolerated changes was sulfonamide substitution (R1); in particular, heteroaryl-substituted sulfonamides, e.g., N-methylpyrazole 3k (entry 11). Nevertheless, overall potency in this series remained low. In addition, as potency for the series increased, it became clear that achieving the target selectivity we had set out at the beginning of the program would be a challenge.
We thus decided to revisit benzenesulfonamide 2c. Our first priority was to resolve hWB potency loss. We did not find literature reports of nonprimary benzenesulfonamides binding to red blood cells, leading us to believe this problem was unique to the primary sulfonamide. To test this hypothesis, we utilized the most potent lactam substituent and added a methyl group on the sulfonamide (Table 3, entry 2). Gratifyingly, this change was sufficient to provide hWB potency in the aryl sulfonamide series.24 Surprisingly, methylsulfonamide substitution boosted selectivity within the CDK family as well. Having resolved the hWB issue and firmly in the desired potency and selectivity range, we next sought to improve the ADME profile, as the turnover in human liver microsomes also increased significantly with methyl substitution. We first examined the possibility of reducing clearance via modulation of the sulfonamide (entries 3–4). Unfortunately, addition of polarity off the sulfonamide methyl did not substantially affect microsomal stability. On the other hand, the effects on potency and selectivity were more pronounced. Compounds with basic amines had excellent potency, e.g. 4c exhibited hWB potency of 44 nM (entry 4). Similarly, CDK family selectivity was sensitive to sulfonamide substitution, with smaller (e.g., primary sulfonamide in compound 2c) or larger substitution (entries 3–4) proving to be less selective than methyl (entry 2).
Table 3. Optimization of the Pyrimidine Lactam (R2) and Benzenesulfonamide (R1) Enhances hWB Potency and Selectivity.

Measured at 1 mM [ATP].
The methyl sulfonamide was therefore retained and focus shifted to modifying the R2 substituent. In addition to addressing microsomal stability, an additional issue arose—kinome selectivity. In spite of the excellent selectivity within the CDK family, the benzenesulfonamides had reduced selectivity against the general kinome, and compound 4a in particular showed <1 μM potency against 12/54 kinases in our in-house panel and <100 nM potency against 2/54 (see Supporting Information).
Fortunately, the correlation between steric bulk adjacent to the lactam nitrogen and CDK family selectivity identified in the piperdine sulfonamide series transferred to the aryl sulfonamide series. Moreover, the potency boost provided by the aryl sulfonamide allowed for a greatly expanded scope of viable substitution. Small alkyl substituents with branching (Table 3, entry 5), 2-substituted phenyls (e.g., entry 6), and select polar substituents (entries 7–8), all insufficiently potent in the piperidine sulfonamide series, now achieved submicromolar activity in hWB. 3-Hydroxycyclohexane 4g was particularly potent and selective. The (1S,3R) isomer showed 0.25 nM enzyme potency and >1000× selectivity against CDK1 (Table 3, entry 8). Additionally, the kinome selectivity for 4g was excellent. In the 54-membered kinase panel, TrkA (114 nM, > 400× vs CDK2) was the most potent off-target kinase, and only four other kinases showed <1000 nM potency (see Supporting Information).
Having achieved several compounds with balanced in vitro ADME profiles, potency, and selectivity, we next examined the pharmacokinetic (PK) profile of analogues 4d–f in rats (see Supporting Information). Disappointingly, all three compounds had low exposure (<100 nM·h) and high clearance (>100% hepatic blood flow). Compounds 4d and 4e were presumed to suffer from solubility-limited absorption, as both precipitated from the dosing solution. The PK profile of lactam 4f was more perplexing, with a large disconnect between the measured stability in human liver microsomes (0.8 L/h/kg) and the observed in vivo clearance in excess of hepatic blood flow. Therefore, we performed MetID profiling for compound 4f in rat and human microsomes to identify the major metabolic pathways responsible for clearance. The results revealed that the major metabolite in rats was oxidation of the cyclopentyl ring, while in higher species, demethylation of the sulfonamide predominated. Sulfonamides stabilize α carbon radicals, increasing the rate of oxidation at the α carbon. The resulting hemiaminal can readily expel the sulfonamide, which is an excellent leaving group, thus further facilitating oxidative dealkylation.25 Accordingly, sulfonamide metabolism frequently causes challenges in medicinal chemistry applications.26−29 We sought to address the main source of metabolism through two approaches. First, since oxidation α to the sulfonamide is mechanistically the first step to dealkylation, we reasoned that removal of the liable protons would alleviate the problem. However, substitution of the sulfonamide methyl, with either polar functionality or steric blocking groups, led to decreased cellular potency and CDK family selectivity (see, for instance, 4b in Table 3). Methyl substitution on the sulfonamide was unequivocally optimal. Hence, we opted for a more direct method: to replace the protons with deuterium, which can hinder oxidation-driven metabolism.30 Indeed, the resulting deuterated compounds 5f and 5g showed improved turnover in human liver microsomes, with minimal turnover in 5f (<0.5 L/h/kg) (Figure 4).
Figure 4.
Strategies to improve metabolic stability.
A second approach involved the removal of the aryl sulfonamide altogether and return to the piperidine sulfonamide series. Though this series was put on hold due to insufficient potency and selectivity, the discovery of (1S,3R)-hydroxycyclohexyl at R2 allowed us to revive the scaffold. Though both scaffolds contain an alkyl sulfonamide, there was ample precedent for cyclic sulfonamides showing reduced metabolic turnover relative to their acyclic congeners.26,28,31 Using the lessons learned in previous SAR, we prepared a small library of heteroaryl sulfonamides. Of these, N-methylpyrazole piperidine sulfonamide 6 (Figure 4) displayed the best balance of potency (320 nM hWB), selectivity (660× for CDK1), and ADME profile (improved turnover: 1.0 L/h/kg). Notably, the (1S, 3R)-hydroxycyclohexyl piece resolved the CDK family selectivity issue in the piperidine sulfonamide series.
With two methods to mitigate the metabolic instability of the methylsulfonamide, we next examined the PK of compounds 5f, 5g, and 6 (Table 4).
Table 4. PK Parameters for Compounds 5f, 5g, and 6.
| compound | routea | dose (mg/kg) | Cmax (nM) | AUC (nM·h) | %F | %HBF | Vss (L/kg) |
|---|---|---|---|---|---|---|---|
| 5f | iv (r) | 0.5 | >100 | 3.2 | |||
| 5f | po (r) | 3.0 | 752 | 666 | 92 | ||
| 5g | iv (r) | 0.5 | >100 | 1.1 | |||
| 5g | po (r) | 3.0 | 992 | 1150 | 100 | ||
| 5g | iv (c) | 0.5 | 70 | 3.5 | |||
| 5g | po (c) | 2.0 | 138 | 739 | 100 | ||
| 6 | iv (r) | 0.5 | 96 | 2.0 | |||
| 6 | po (r) | 3.0 | 562 | 1134 | 59 | ||
| 6 | iv (c) | 0.3 | 48 | 1.2 | |||
| 6b | po (c) | 2.0 | 56 | 388 | 12 |
r = rat, c = cyno.
Adjusted from 1 mg/kg dose for comparison.
Gratifyingly, rat plasma exposure improved for all three compounds, with 5g and 6 showing >1 μM·h exposure. Therefore, we progressed 5g and 6 into cyno PK. After a 2.0 mg/kg dose, compound 6 showed poor exposure and low oral bioavailability. Meanwhile, benzenesulfonamide 5g showed a reasonable PK profile, with an AUC of 739 nM·h.
To further understand the effect of deuteration on sulfonamide metabolism, we performed a detailed MetID analysis of 5f and compared it to the protonated congener 4f across species (Table 5). In human microsomes, compound 5f showed a significant reduction in the demethylation metabolite relative to the nondeuterated 4f, thus confirming our hypothesis. In human microsomes, demethylation was reduced from 26% to 10%, leading to overall good stability, with 85% parent compound remaining after 30 min. In cyno microsomes, the rate of demethylation was also reduced, from 98% to 71%, albeit only 20% parent remained after 30 min due to the particularly rapid rate of cyno metabolism of 4f.
Table 5. Metabolic Profiling of Compounds 4f and 5f.

| entry | compound | R1 | metabolite | rat (%)a | cyno (%)a | human (%)a |
|---|---|---|---|---|---|---|
| 1 | 4f | CH3 | oxidation | 74 | 8 | 6 |
| 2 | 4f | CH3 | demethylation | 35 | 98 | 26 |
| % remaining | 0.1 | 0.1 | 68 | |||
| 3 | 5f | CD3 | oxidation | 54 | 12 | 5 |
| 4 | 5f | CD3 | demethylation | 23 | 71 | 10 |
| % remaining | 4.4 | 20 | 85 |
In some cases, total is >100% due to double metabolic activation. See SI
Overall, the profiles of compounds 5f, 5g, and 6 are promising (Table 6). All are active against CDK2 across assays, including submicromolar hWB potency (300–800 nM) with excellent selectivity over our target CDK family members (CDK1, 4, 6, 7, and 9). Furthermore, the selectivity extends into cell, with ∼30-fold selectivity against CDK1 in a CDK1 driven cell line for analogue 5g. All three compounds possess promising initial general kinome selectivity. The CYP inhibition and hERG inhibition are minimal and solubility is generally excellent. Combined with a reasonable PK profile in rodent, these compounds represent valuable tools to study the effects of CDK2-selective inhibition in in vivo contexts where CDK2 is believed to drive cancer cell growth.
Table 6. Summary Profiles of 5f, 5g, and 6.
| parameter | 5f | 5g | 6 |
|---|---|---|---|
| CDK2/E (nM)a | 1.2 | 0.3 | 2.0 |
| selectivity(1/7/9)b | 490/NR/2800 | 1200/17000/8200 | 660/1100/2500 |
| selectivity(4/6)b | 240/95 | 770/230 | 110/35 |
| off-targetsc | 5/54 | 5/54 | 0/54 |
| hWB (nM) | 746 | 642 | 316 |
| hERG (% Inh)d | 16 | 9.7 | 7.2 |
| CYPe | >25 μM | >25 μM | >25 μM |
| CYP TDIf | none | none | none |
| solubility (μg/mL) | >400g | N/A | >400 |
Measured at 1 mM [ATP].
Fold selectivity over the corresponding CDK isoform as measured by enzyme IC50.
# kinases <1 μM in a 54 membered kinase panel
At 10 μM concentration
CYP isomforms tested: CYP3A4, CYP1A2, CYP2C8, CYP2C19, CYP2B6, CYP2D6, CYP2C9.
CYP isoforms tested: CYP3A4, CYP2D6, CYP2C9, CYP2C19
Solubility determined for 4f; NR = not recorded.
In conclusion, we report the design and development of a series of highly active and selective 5,7-dihydro-6H-pyrrolo[2,3-d]pyrimidin-6-ones starting from a library hit. We discovered an unexpected selectivity trend where the 5,7-dihydro-6H-pyrrolo[2,3-d]pyrimidin-6-ones showed significantly improved selectivity relative to the respective 5,8-dihydropyrido[2,3-d]pyrimidin-7(6H)-one congeners. Extensive SAR then allowed us to identify further avenues to augment selectivity of the core: methyl substitution on the benzenesulfonamide and a variety of lactam substituents, in particular (1S, 3R)-hydroxycyclohexane.
Combined, these factors allowed us to identify compounds with unprecedented selectivity against the CDK family. Furthermore, we used MetID studies to identify sulfonamide dealkylation as a major metabolic hotspot. Deuteration proved particularly effective at reducing dealkylation, with the resulting pyrimidine lactam 5g demonstrating improved rat and cyno PK profiles. We envision that our deuteration strategy to reduce sulfonamide dealkylation will prove to be useful in other medicinal chemistry applications where substitution-based approaches are not tolerated.
Acknowledgments
Susan Petusky, Michelle Conlin, Amy Hehman, Rina Pan, Robert Landman, and Gengjie Yang are gratefully acknowledged for biological and ADME assay support. We would like to thank Karl Blom, Yingrui Dai, Robby Kirk, Ronald Magboo, and Min Li for analytical assistance. We thank Laurine Galya, James Hall, and Scott Leonard for assistance with obtaining and analyzing NMR data. We thank Onur Atasoylu for help with molecular modelling.
Glossary
ABBREVIATIONS
- ADME
absorption, distribution, metabolism, and excretion
- AUC
area under the drug concentration–time curve
- CDK
cyclic dependent kinase
- CYP
cytochrome p450
- CYP TDI
time dependent inhibition of cytochrome p450
- F%
fraction of an administered dose of unchanged drug that reaches systemic circulation
- FDA
Federal Drug Administration
- hWB
human whole blood
- SAR
structure–activity relationship
- MetID
metabolite identification
- hERG
human ether-a-go-go-related gene
- IV
intravenous
- Vss
volume of distribution at steady state
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsmedchemlett.2c00408.
Synthetic procedures, characterization of final compounds, protocols for in vitro and in vivo assays, full kinase profiles of selected compounds and full metabolic profiles of 4f, 5f, and 6 (PDF)
Author Present Address
‡ Synnovation Therapeutics, 200 Powder Mill Road, E500, Wilmington, Delaware, 19803, United States
This research was funded by Incyte Corporation.
The authors declare the following competing financial interest(s): All authors are current or former employees of Incyte Corporation.
Supplementary Material
References
- Asghar U. W.; Witkiewicz A. K.; Turner N. C.; Knudsen E. S. The History and Future of Targeting Cyclic-dependent Kinases. Nat. Rev. Drug Discovery 2015, 14 (2), 130–146. 10.1038/nrd4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Martinez C.; Gelbert L. M.; Lallena M. J.; de Dios A. Cyclin Dependent Kinase (CDK) Inhibitors as Anticancer Drugs. Bioorg. Med. Chem. Lett. 2015, 25 (17), 3420–3435. 10.1016/j.bmcl.2015.05.100. [DOI] [PubMed] [Google Scholar]
- Siemeister G.; Lücking U.; Wengner A. M.; Lienau P.; Steinke W.; Schatz C.; Mumberg D.; Ziegelbauer K. BAY 1000394, a Novel Cyclin-Dependent Kinase Inhibitor, with Potent Antitumor Activity in Mono- and in Combination Treatment upon Oral Application. Mol. Cancer Ther. 2012, 11 (10), 2265–2273. 10.1158/1535-7163.MCT-12-0286. [DOI] [PubMed] [Google Scholar]
- Brasca M. G.; Amboldi N.; Ballinari D.; Cameron A.; Casale E.; Cervi G.; Colombo M.; Colotta F.; Croci V.; D’Alessio R.; Fiorentini F.; Isacchi A.; Mercurio C.; Moretti W.; Panzeri A.; Pastori W.; Pevarello P.; Quartieri F.; Roletto F.; Traquandi G.; Vianello P.; Vulpetti A.; Ciomei M. Identification of N,1,4,4-tetramethyl-8-{[4-(4-methylpiperazin-1-yl)phenyl]amino}-4,5-dihydro-1H-py razolo[4,3-h]quinazoline-3-carboxamide (PHA-848125), a potent, orally available cyclin dependent kinase inhibitor. J. Med. Chem. 2009, 52 (16), 5152–5163. 10.1021/jm9006559. [DOI] [PubMed] [Google Scholar]
- Jackson R. C.; Barnett A. L.; McClue S. J.; Green S. R. Seliciclib, a cell-cycle modulator that acts through the inhibition of cyclin-dependent kinases. Expert Opin. on Drug Discovery 2008, 3 (1), 131–143. 10.1517/17460441.3.1.131. [DOI] [PubMed] [Google Scholar]
- Parry D.; Guzi T.; Shanahan F.; Davis N.; Prabhavalkar D.; Wiswell D.; Seghezzi W.; Paruch K.; Dwyer M. P.; Doll R.; Nomeir A.; Windsor W.; Fischmann T.; Wang Y.; Oft M.; Chen T.; Kirschmeier P.; Lees E. M. Dinaciclib (SCH 727965), a Novel and Potent Cyclin-Dependent Kinase Inhibitor. Mol. Cancer Ther. 2010, 9 (8), 2344–2353. 10.1158/1535-7163.MCT-10-0324. [DOI] [PubMed] [Google Scholar]
- Santamaría D.; Barrière C.; Cerqueira A.; Hunt S.; Tardy C.; Newton K.; Cáceres J. F.; Dubus P.; Malumbres M.; Barbacid M. Cdk1 is sufficient to drive the mammalian cell cycle. Nature 2007, 448 (7155), 811–815. 10.1038/nature06046. [DOI] [PubMed] [Google Scholar]
- Laderian B.; Fojo T. CDK4/6 Inhibition as a Therapeutic Strategy in Breast Cancer: Palbociclib, Ribociclib, and Abemaciclib. Semin. Oncol. 2017, 44 (6), 395–403. 10.1053/j.seminoncol.2018.03.006. [DOI] [PubMed] [Google Scholar]
- Herrera-Abreu M. T.; Palafox M.; Asghar U.; Rivas M. A.; Cutts R. J.; Garcia-Murillas I.; Pearson A.; Guzman M.; Rodriguez O.; Grueso J.; Bellet M.; Cortes J.; Elliott R.; Pancholi S.; Lord C. J.; Baselga J.; Dowsett M.; Martin L.-A.; Turner N. C.; Serra V. Early Adaptation and Acquired Resistance to CDK4/6 Inhibition in Estrogen Receptor-Positive Breast Cancer. Cancer Res. 2016, 76 (8), 2301–2313. 10.1158/0008-5472.CAN-15-0728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Leary B.; Cutts R. J.; Liu Y.; Hrebien S.; Huang X.; Fenwick K.; André F.; Loibl S.; Loi S.; Garcia-Murillas I.; Cristofanilli M.; Huang Bartlett C.; Turner N. C. The Genetic Landscape and Clonal Evolution of Breast Cancer Resistance to Palbociclib plus Fulvestrant in the PALOMA-3 Trial. Cancer Discovery 2018, 8 (11), 1390–1403. 10.1158/2159-8290.CD-18-0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etemadmoghadam D.; Weir B. A.; Au-Yeung G.; Alsop K.; Mitchell G.; George J.; Davis S.; D’Andrea A. D.; Simpson K.; Hahn W. C.; Bowtell D. D. L. Synthetic lethality between CCNE1 amplification and loss of BRCA1. Proc. Natl. Acad. Sci., USA 2013, 110 (48), 19489–19494. 10.1073/pnas.1314302110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaltriti M.; Eichhorn P. J.; Cortés J.; Prudkin L.; Aura C.; Jiménez J.; Chandarlapaty S.; Serra V.; Prat A.; Ibrahim Y. H.; Guzmán M.; Gili M.; Rodríguez O.; Rodríguez S.; Pérez J.; Green S. R.; Mai S.; Rosen N.; Hudis C.; Baselga J. Cyclin E amplification/overexpression is a mechanism of trastuzumab resistance in HER2 breast cancer patients. Proc. Natl. Acad. Sci. USA 2011, 108 (9), 3761–3766. 10.1073/pnas.1014835108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etemadmoghadam D.; Au-Yeung G.; Wall M.; Mitchell C.; Kansara M.; Loehrer E.; Batzios C.; George J.; Ftouni S.; Weir B. A.; Carter S.; Gresshoff I.; Mileshkin L.; Rischin D.; Hahn W. C.; Waring P. M.; Getz G.; Cullinane C.; Campbell L. J.; Bowtell D. D. Resistance to CDK2 Inhibitors Is Associated with Selection of Polyploid Cells in CCNE1-Amplified Ovarian Cancer. Clin. Cancer Res. 2013, 19 (21), 5960–5971. 10.1158/1078-0432.CCR-13-1337. [DOI] [PubMed] [Google Scholar]
- McDonald E. R. 3rd; de Weck A.; Schlabach M. R.; Billy E.; Mavrakis K. J.; Hoffman G. R.; Belur D.; Castelletti D.; Frias E.; Gampa K.; Golji J.; Kao I.; Li L.; Megel P.; Perkins T. A.; Ramadan N.; Ruddy D. A.; Silver S. J.; Sovath S.; Stump M.; Weber O.; Widmer R.; Yu J.; Yu K.; Yue Y.; Abramowski D.; Ackley E.; Barrett R.; Berger J.; Bernard J. L.; Billig R.; Brachmann S. M.; Buxton F.; Caothien R.; Caushi J. X.; Chung F. S.; Cortes-Cros M.; deBeaumont R. S.; Delaunay C.; Desplat A.; Duong W.; Dwoske D. A.; Eldridge R. S.; Farsidjani A.; Feng F.; Feng J.; Flemming D.; Forrester W.; Galli G. G.; Gao Z.; Gauter F.; Gibaja V.; Haas K.; Hattenberger M.; Hood T.; Hurov K. E.; Jagani Z.; Jenal M.; Johnson J. A.; Jones M. D.; Kapoor A.; Korn J.; Liu J.; Liu Q.; Liu S.; Liu Y.; Loo A. T.; Macchi K. J.; Martin T.; McAllister G.; Meyer A.; Molle S.; Pagliarini R. A.; Phadke T.; Repko B.; Schouwey T.; Shanahan F.; Shen Q.; Stamm C.; Stephan C.; Stucke V. M.; Tiedt R.; Varadarajan M.; Venkatesan K.; Vitari A. C.; Wallroth M.; Weiler J.; Zhang J.; Mickanin C.; Myer V. E.; Porter J. A.; Lai A.; Bitter H.; Lees E.; Keen N.; Kauffmann A.; Stegmeier F.; Hofmann F.; Schmelzle T.; Sellers W. R. Project DRIVE: A Compendium of Cancer Dependencies and Synthetic Lethal Relationships Uncovered by Large-Scale, Deep RNAi Screening. Cell 2017, 170 (3), 577–592. 10.1016/j.cell.2017.07.005. [DOI] [PubMed] [Google Scholar]
- Tadesse S.; Caldon E. C.; Tilley W.; Wang S. Cyclin-Dependent Kinase 2 Inhibitors in Cancer Therapy: An Update. J. Med. Chem. 2019, 62 (9), 4233–4251. 10.1021/acs.jmedchem.8b01469. [DOI] [PubMed] [Google Scholar]
- Jorda R.; Hendrychova D.; Voller J.; Reznickova E.; Gucky T.; Krystof V. How Selective Are Pharmacological Inhibitors of Cell-Cycle-Regulating Cyclin-Dependent Kinases?. J. Med. Chem. 2018, 61 (20), 9105–9120. 10.1021/acs.jmedchem.8b00049. [DOI] [PubMed] [Google Scholar]
- Freeman-Cook K. D.; Hoffman R. L.; Behenna D. C.; Boras B.; Carelli J.; Diehl W.; Ferre R. A.; He Y.-A.; Hui A.; Huang B.; Huser N.; Jones R.; Kephart S. E.; Lapek J.; McTigue M.; Miller N.; Murray B. W.; Nagata A.; Nguyen L.; Niessen S.; Ninkovic S.; O’Doherty I.; Ornelas M. A.; Solowiej J.; Sutton S. C.; Tran K.; Tseng E.; Visswanathan R.; Xu M.; Zehnder L.; Zhang Q.; Zhang C.; Dann S. Discovery of PF-06873600, a CDK2/4/6 Inhibitor for the Treatment of Cancer. J. Med. Chem. 2021, 64 (13), 9056–9077. 10.1021/acs.jmedchem.1c00159. [DOI] [PubMed] [Google Scholar]
- Hoffman R. L.The discovery of PF-07104091: A CDK2 selective inhibitor for the treatment of cyclic E amplified cancers. Presented at AACR Annual Meeting 2021, Virtual Meeting, 2021.
- Coxon C. R.; Anscombe E.; Harnor S. J.; Martin M. P.; Carbain B.; Golding B. T.; Hardcastle I. R.; Harlow L. K.; Korolchuk S.; Matheson C. J.; Newell D. R.; Noble M. E.; Sivaprakasam M.; Tudhope S. J.; Turner D. M.; Wang L. Z.; Wedge S. R.; Wong C.; Griffin R. J.; Endicott J. A.; Cano C.. Cyclin-Dependent Kinase (CDK) Inhibitors: Structure-Activity Relationships and Insights into the CDK-2 Selectivity of 6-Substituted 2-Arylaminopurines. J. Med. Chem., 2017, 60, (5), , 1746−1767. Although the CDK family selectvity reported for compounds in this paper is quite high, in our hands, the enzyme activity did not translate into a cellular setting. 10.1021/acs.jmedchem.6b01254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardcastle I. R.; Arris C. E.; Bentley J.; Boyle F. T.; Chen Y.; Curtin N. J.; Endicott J. A.; Gibson A. E.; Golding B. T.; Griffin R. J.; Jewsbury P.; Menyerol J.; Mesguiche V.; Newell D. R.; Noble M. E.; Pratt D. J.; Wang L. Z.; Whitfield H. J. N2-substituted O6-cyclohexylmethylguanine derivatives: potent inhibitors of cyclin-dependent kinases 1 and 2. J. Med. Chem. 2004, 47 (15), 3710–3722. 10.1021/jm0311442. [DOI] [PubMed] [Google Scholar]
- McInnes C.; Wang S.; Anderson S.; O’Boyle J.; Jackson W.; Kontopidis G.; Meades C.; Mezna M.; Thomas M.; Wood G.; Lane D. P.; Fischer P. M. Structural determinants of CDK4 inhibition and design of selective ATP competitive inhibitors. Chem. Biol. 2004, 11 (4), 525–534. 10.1016/j.chembiol.2004.03.022. [DOI] [PubMed] [Google Scholar]
- Lucking U.; Jautelat R.; Kruger M.; Brumby T.; Lienau P.; Schafer M.; Briem H.; Schulze J.; Hillisch A.; Reichel A.; Wengner A. M.; Siemeister G. The lab oddity prevails: discovery of pan-CDK inhibitor (R)-S-cyclopropyl-S-(4-{[4-{[(1R,2R)-2-hydroxy-1-methylpropyl]oxy}-5-(trifluorome thyl)pyrimidin-2-yl]amino}phenyl)sulfoximide (BAY 1000394) for the treatment of cancer. ChemMedChem. 2013, 8 (7), 1067–1085. 10.1002/cmdc.201300096. [DOI] [PubMed] [Google Scholar]
- Traquandi G.; Ciomei M.; Ballinari D.; Casale E.; Colombo N.; Croci V.; Fiorentini F.; Isacchi A.; Longo A.; Mercurio C.; Panzeri A.; Pastori W.; Pevarello P.; Volpi D.; Roussel P.; Vulpetti A.; Brasca M. G. Identification of potent pyrazolo[4,3-h]quinazoline-3-carboxamides as multi-cyclin-dependent kinase inhibitors. J. Med. Chem. 2010, 53 (5), 2171–2187. 10.1021/jm901710h. [DOI] [PubMed] [Google Scholar]
- A reviewer pointed out that this represents two changes and the relative importance of two methyl groups on hWB is unclear. Unfortunately, a direct comparison is not available in this case, but numerous examples across the project highlighted that methylation of the sulfonamide was sufficient to restore hWB potency.
- Smith D. L.; Keasling H. H.; Forist A. A. The Metabolism of N-Alkyl-4-bromobenzenesulfonamides in the Mouse. Correlation with Anticonvulsant Activity. J. Med. Chem. 1965, 8 (4), 520–524. 10.1021/jm00328a025. [DOI] [PubMed] [Google Scholar]
- Fauber B. P.; René O.; Deng Y.; DeVoss J.; Eidenschenk C.; Everett C.; Ganguli A.; Gobbi A.; Hawkins J.; Johnson A. R.; La H.; Lesch J.; Lockey P.; Norman M.; Ouyang W.; Summerhill S.; Wong H. Discovery of 1-{4-[3-Fluoro-4-((3S,6R)-3-methyl-1,1-dioxo-6-phenyl-[1,2]thiazinan-2-ylmethyl)-phenyl]-piperazin-1-yl}-ethanone (GNE-3500): a Potent, Selective, and Orally Bioavailable Retinoic Acid Receptor-Related Orphan Receptor C (RORc or RORγ) Inverse Agonist. J. Med. Chem. 2015, 58 (13), 5308–5322. 10.1021/acs.jmedchem.5b00597. [DOI] [PubMed] [Google Scholar]
- Stepan A. F.; Karki K.; McDonald W. S.; Dorff P. H.; Dutra J. K.; DiRico K. J.; Won A.; Subramanyam C.; Efremov I. V.; O’Donnell C. J.; Nolan C. E.; Becker S. L.; Pustilnik L. R.; Sneed B.; Sun H.; Lu Y.; Robshaw A. E.; Riddell D.; O’Sullivan T. J.; Sibley E.; Capetta S.; Atchison K.; Hallgren A. J.; Miller E.; Wood A.; Obach R. S. Metabolism-Directed Design of Oxetane-Containing Arylsulfonamide Derivatives as γ-Secretase Inhibitors. J. Med. Chem. 2011, 54 (22), 7772–7783. 10.1021/jm200893p. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Zhu J.; Liu J.; Chen X.; Mihalic J.; Deignan J.; Yu M.; Sun D.; Kayser F.; McGee L. R.; Lo M.-C.; Chen A.; Zhou J.; Ye Q.; Huang X.; Long A. M.; Yakowec P.; Oliner J. D.; Olson S. H.; Medina J. C. Optimization beyond AMG 232: Discovery and SAR of sulfonamides on a piperidinone scaffold as potent inhibitors of the MDM2-p53 protein-protein interaction. Bioorg. Med. Chem. Lett. 2014, 24 (16), 3782–3785. 10.1016/j.bmcl.2014.06.073. [DOI] [PubMed] [Google Scholar]
- Shao P. P.; Ye F.; Chakravarty P. K.; Herrington J. B.; Dai G.; Bugianesi R. M.; Haedo R. J.; Swensen A. M.; Warren V. A.; Smith M. M.; Garcia M. L.; McManus O. B.; Lyons K. A.; Li X.; Green M.; Jochnowitz N.; McGowan E.; Mistry S.; Sun S.-Y.; Abbadie C.; Kaczorowski G. J.; Duffy J. L. Improved Cav2.2 Channel Inhibitors through a gem-Dimethylsulfone Bioisostere Replacement of a Labile Sulfonamide. ACS Med. Chem. Lett. 2013, 4 (11), 1064–1068. 10.1021/ml4002612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbeson S. L.; Tung R. D.. Deuterium in Drug Discovery and Development. In Annual Reports in Medicinal Chemistry; Macor J. E., Ed.; Academic Press: 2011; Vol. 46, Chapter 24, pp 403–417. [Google Scholar]
- Chen H.; Volgraf M.; Do S.; Kolesnikov A.; Shore D. G.; Verma V. A.; Villemure E.; Wang L.; Chen Y.; Hu B.; Lu A.-J.; Wu G.; Xu X.; Yuen P.-w.; Zhang Y.; Erickson S. D.; Dahl M.; Brotherton-Pleiss C.; Tay S.; Ly J. Q.; Murray L. J.; Chen J.; Amm D.; Lange W.; Hackos D. H.; Reese R. M.; Shields S. D.; Lyssikatos J. P.; Safina B. S.; Estrada A. A. Discovery of a Potent (4R,5S)-4-Fluoro-5-methylproline Sulfonamide Transient Receptor Potential Ankyrin 1 Antagonist and Its Methylene Phosphate Prodrug Guided by Molecular Modeling. J. Med. Chem. 2018, 61 (8), 3641–3659. 10.1021/acs.jmedchem.8b00117. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.