Abstract
Background
CDK4/6 inhibitors combined with endocrine therapy are standard first- or second-line treatment for patients with HR-positive and HER2-negative advanced breast cancer, however, there is currently no optimal recommendation for therapeutic strategies after progression on CDK4/6i. The aim of this study is to analyze the efficacy and safety of HDAC inhibitor Tucidinostat combined with endocrine therapy in patients after prior CDK4/6 inhibitor progression.
Methods
The pathological and clinical data of 44 HR-positive and HER2-negative breast cancer patients treated with tucidinostat after progression on CDK4/6i at the Breast Oncology Department of the Fifth Medical Center of the PLA General Hospital from July 2019 to October 2021 were retrospectively analyzed. Observation indexes included progression-free survival (PFS), overall survival (OS), clinical benefit rate (CBR), objective response rate (ORR) and adverse events. At the same time, we attempted to identify potential genomic predictors using available next-generation sequencing (NGS).
Results
A total of 44 patients were enrolled in this study. Median follow-up was 10 months (1–26 months) by the data cutoff date (February 2022). The CBR was 6.8% (3/44), the median PFS was 2.0 months (95% CI 1.9–2.1), and the median OS was 14 months (95% CI 6.3–21.7). The mPFS was 4.1 months (95%CI: 0–8.2) in patients with 1 metastatic site, and the mPFS was 4.5 months (95%CI: 4.2–4.8) in patients who received sequential tucidinostat after CDK4/6i failure. Multivariate analysis showed that patients with 1 metastatic site or sequential tucidinostat treatment after failure of CDK4/6i were more likely to benefit from tucidinostat combined with endocrine therapy. Preliminary data showed PIK3CA mutation may be associated with resistance of tucidinostat therapy. No grade 4 adverse events and no treatment-related deaths were recorded in the study. Dose reductions because of adverse events occurred in 4 (9.1%) patients.
Conclusions
This study preliminarily shows that tucidinostat combined with endocrine therapy may be an optional sequential strategy for patients with HR+/HER2-advanced breast cancer that has progressed on CDK4/6 inhibitor, especially for these with lower tumor burden and fewer prior palliative treatment.
Keywords: Tucidinostat, Hormone receptor-positive, Metastatic breast cancer, Endocrine therapy, CDK4/6 inhibitor
Highlights
-
•
CDK4/6 inhibitor became standard treatment for HR-positive MBC patients.
-
•
There is no optimal treatment strategy after progression on CDK4/6i.
-
•
Tcucidinostat was the only HDAC inhibitor approved in solid tumors worldwide.
-
•
The mPFS of Tcucidinostat was 4.5 months when used sequentially after prior CDK4/6i.
-
•
PIK3CA mutation may be associated with resistance of tucidinostat.
1. Introduction
Breast cancer (BC) is the most common malignancy among women worldwide, of which more than 70% are hormone receptor(HR)-positive, HER2-negative diseases. Although the survival rate of patients with breast cancer has improved significantly, advanced breast cancer remains incurable [1,2]. Endocrine therapy is the standard of care for HR-positive patients. The advent of cyclin-dependent kinase (CDK) 4/6 inhibitor has changed the treatment pattern of patients with HR-positive HER2-negative advanced breast cancer, which in combination with endocrine therapy has become the standard of care in either the first-line setting or second-line setting. Nevertheless, despite significant improvement in progression-free survival (PFS) and overall survival (OS), primary or acquired resistance to CDK4/6 inhibitor eventually occurs. 15–20% of patients on first-line therapy and 30–40% of patients on second-line therapy may exhibit primary resistance, while almost all patients will develop acquired resistance [[3], [4], [5], [6], [7], [8], [9], [10], [11], [12]]. There is currently no optimal recommendation for therapeutic strategies after progression on CDK4/6 inhibitor. Alternative strategies include chemotherapy and endocrine therapy in combination with other pathway inhibitors such as alpelisib, an inhibitor for patients with phosphoinositide-4, 5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA) mutations, and everolimus, a mammalian target of rapamycin (mTOR) pathway inhibitor, etc [13].
Tucidinostat, a histone deacetylase (HDAC) inhibitor, induces cell cycle arrest, differentiation, and death in cancer cells by modifying the status of acetylation on histone and non-histone proteins and changes the tumor microenvironment to exert anti-tumor effects [14]. Based on the results of the ACE study which demonstrated a significant improvement in PFS with the combination of tucidinostat plus exemestane (EXE) (median, 9.6 months) compared with EXE alone (median, 3.8 months), tucidinostat in combination with aromatase inhibitors has been approved in China as the first HDAC inhibitor approved in solid tumors worldwide for the treatment of patients with advanced, HR-positive, HER2-negative breast cancer that progressed after previous endocrine therapy [15]. The patients enrolled in ACE study did not include those with prior CDK4/6i exposure because this trial was conducted in the period before the abovementioned CDK4/6i available in China. Currently, there is no clinical data on the efficacy of tucidinostat in combination with endocrine in HR + HER2-metastatic breast cancer (MBC) patients with progression on prior CDK4/6 inhibitor-based treatments. The main objective of this study is to analyze the efficacy and safety of tucidinostat combined with endocrine therapy in patients whose disease was refractory to CDK4/6i combinations.
2. Methods
2.1. Study design and participants
This study was a single-institution, retrospective cohort study. Electronic medical records were reviewed to identify MBC patients who had received tucidinostat therapy between July 2019 and October 2021. All consecutive patients were then reviewed for inclusion and exclusion criteria.
Inclusion criteria: (1) age ≥18 years; (2) pathologically diagnosed breast cancer with unresectable locally advanced or metastatic disease; (3) HR positive, which was defined as ER or PR positive cells detected by immunohistochemistry (IHC) Ratio >1%; (4) HER2 negative, which was defined as IHC 0–1+ or FISH/CISH non-amplified; (5) progression on prior CDK4/6i which was received for at least 4 weeks; (6) at least one efficacy evaluation after tucidinostat therapy.
Exclusion criteria: (1) received tucidinostat prior to receiving CDK4/6i; (2) received >1 line of CDK4/6i regimen prior to receiving tucidinostat; (3) discontinued the prior CDK4/6i because of toxicity or other reasons except for disease progression; (5) termination of treatment due to toxicity, progression, or death observed ≤14 days of tucidinostat initiation; (6) incomplete medical records.
Medical records of eligible patients were analyzed respectively for relevant clinical metrics, including baseline patient characteristics, treatment history, special interest adverse events due to tucidinostat, response evaluation, death or most recent clinic visit date (if applicable). This study was approved by the Ethics Committee of the Fifth Medical Center of the Chinese People's Liberation Army General Hospital and informed consent was obtained from all patients. This study was registered with ClinicalTrials.gov (ClinicalTrials.gov identifier: NCT 05276713).
Endocrine sensitivity was defined as relapsed after 24 months of adjuvant endocrine therapy or had a clinical benefit from prior endocrine therapy in the context of advanced disease. Benefit from CDK4/6i was defined as gained complete response or partial response or stable disease lasting ≥24 weeks from CDK4/6i treatment.
2.2. Procedures
The recommended initial dose of tucidinostat was 30 mg, twice a week orally (either, Monday and Thursday, Tuesday and Friday, or Wednesday and Saturday), and dose reduction, permanent discontinuation, or delay was performed according to patients' tolerance. Adverse events were handled in time according to relevant guidelines. The endocrine drugs combined with tucidinostat were given based on previous endocrine treatments and at the discretion of the treating physicians. After excluding the endocrine drugs that have previously proven as ineffective, those administered during the present study included: selective estrogen receptor modulators (SERMs), including tamoxifen (20 mg/day, orally) and toremifene (60 mg/day, orally); aromatase inhibitors (AIs), including letrozole (2.5 mg/day, orally), anastrozole (1 mg/day, orally), exemestane (25 mg/day, orally); fulvestrant (500 mg 1/28 day, intramuscular injection), and medroxyprogesterone (1000 mg/day, orally).
2.3. Efficacy evaluation criteria
The efficacy was routinely evaluated every two months, and adverse events were recorded. Imaging assessment was conducted immediately when clinical symptoms suggested disease progression. Treatment continued until disease progression, unacceptable toxicity or death, with last follow-up time in February 2022.
Tumor response was assessed according to the RECIST version 1.1 evaluation criteria, and adverse events were recorded and graded according to the National Cancer Institutes Common Terminology Criteria for Adverse Events (version 4.0).
2.4. Outcomes
Observation indexes included PFS, OS, clinical benefit rate (CBR), and objective response rate (ORR). PFS was defined as the time from the start of treatment to either the first documented disease progression or death from any cause. OS was defined as the time from the start of treatment to death from any cause. CBR was defined as the proportion of patients with a best overall response of complete response or partial response or an overall lesion response of stable disease lasting ≥24 weeks. ORR was defined as the proportion of patients with a complete response or a partial response.
2.5. Statistical analysis
Statistical analyses were performed using SPSS V.19.0. Measurement data were expressed as medians (ranges); count data were expressed as adoption rates or composition ratios. Chi-square test or Fisher's exact test was used to analyze group differences. Kaplan-Meier curves and log-rank tests were used for analyzing PFS and OS. Cox regression models were used to calculate the hazard ratios (HR) and 95% confidence interval (CI). Log-rank tests were used for univariate analyses. Cox multivariate analyses were performed based on the results of univariate analyses. P values and CI were both bilaterally tested. P value < 0.05 was considered as indicating statistical significance.
3. Results
3.1. Patients’ characteristics
A total of 44 patients were enrolled in this study, and their baseline characteristics are shown in Table 1. Median age of the patients was 53 years (range: 30–73 years), and 52.3% of the patients were postmenopausal. All enrolled patients had previously received palliative endocrine therapy, with 95.5% of patients receiving palliative chemotherapy and 4.5% receiving adjuvant chemotherapy only. 77.3% of patients had visceral metastases, and 59.1% had ≥3 metastatic sites. 15.9% of patients received tucidinostat with endocrine therapy sequentially (immediately after disease progression on the initial CDK4/6i), 84.1% of patients received tucidinostat in combination with endocrine therapy nonsequentially, and the median number of treatment lines between tucidinostat and CDK4/6i was 1 (range: 0–7 lines). The median number of palliative therapy lines was 6 (range: 2–16), the median number of previous palliative endocrine therapy lines was 2 (range: 1–5), and the median number of palliative chemotherapy lines was 2 (range: 0–7). Only one patient (2.3%) received tucidinostat as 2nd line therapy, six patients (13.6%) received tucidinostat as 3rd line therapy, and the remaining 84.1% received tucidinostat as 4th and later line therapy.
Table 1.
Patient characteristics at baseline.
| Characteristics | Number of cases (%) |
|---|---|
| Age (years) | |
| Median (range) | 53 (30–73) |
| Hormone receptor status | |
| ER+/PR+ | 42 (95.5%) |
| ER+/PR- | 2 (4.5%) |
| Menopausal status | |
| Postmenopausal | 23 (52.3%) |
| Premenopausal | 21 (47.7%) |
| Disease-free survival (months) | |
| Median (range) | 28.5 (0–144) |
| De novo | 13 (29.5%) |
| ≤24 | 8 (18.2%) |
| >24 | 23 (52.3%) |
| Number of metastatic sites | |
| 1 | 6 (13.6%) |
| 2 | 12 (27.3%) |
| ≥3 | 26 (59.1%) |
| Metastatic site | |
| Bone | 28 (63.6%) |
| Viscera | 34 (77.3%) |
| Liver | 25 (56.8%) |
| Lung | 24 (54.5%) |
| Brain | 4 (9.1%) |
| Lines of previous therapy of CDK4/6i | |
| 1 | 9 (20.5%) |
| 2 | 7 (15.9%) |
| ≥3 | 28 (63.6%) |
| Benefits of CDK4/6 Inhibitor | |
| Benefits | 27 (61.4%) |
| No benefits | 17 (38.6%) |
| Number of therapy between CDK4/6i and tucidinostat | |
| Median (range) | 1 (0–7) |
| 0 | 7 (15.9%) |
| 1 | 22 (50.0%) |
| 2 | 6 (13.6%) |
| ≥3 | 9 (20.5%) |
| Previous endocrine therapy in the metastatic setting | |
| Tamoxifen or toremifene | 8 (18.2%) |
| Letrozole or anastrozole | 29 (65.9%) |
| Exemestane | 17 (38.6%) |
| Fulvestrant | 34 (77.3%) |
| Everolimus | 5 (11.4%) |
| Sensitivity to prior hormonal therapy | |
| Yes | 39 (88.6%) |
| No | 5 (11.4%) |
| Endocrine partner of Tucidinostat | |
| Tamoxifen or toremifene | 5 (11.4%) |
| Letrozole or anastrozole | 8 (18.2%) |
| Exemestane | 19 (43.2%) |
| Fulvestrant | 10 (22.7%) |
| Medroxyprogesterone | 2 (4.5%) |
| Number of previous chemotherapy for MBC | |
| Median (range) | 2 (0–7) |
| 0 | 2 (4.5%) |
| 1 | 10 (22.7%) |
| 2 | 13 (29.5%) |
| ≥3 | 19 (43.2%) |
| Previous chemotherapy | |
| (Neo)adjuvant only | 2 (4.5%) |
| Metastatic ± (neo)adjuvant | 42 (95.5%) |
| Number of previous endocrine therapy for MBC | |
| Median (range) | 2 (1–5) |
| 1 | 11 (25.0%) |
| 2 | 17 (38.6%) |
| ≥3 | 16 (36.4%) |
| Number of previous therapy for MBC | |
| Median (range) | 5 (1–15) |
| 1 | 1 (2.3%) |
| 2 | 6 (13.6%) |
| ≥3 | 37 (84.1%) |
3.2. Efficacy
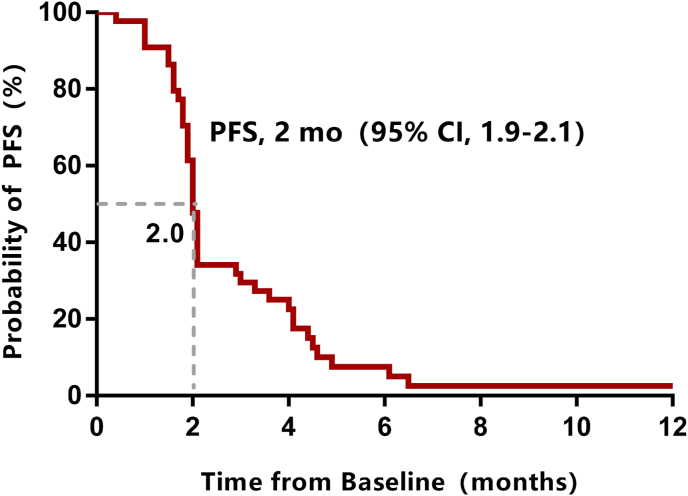
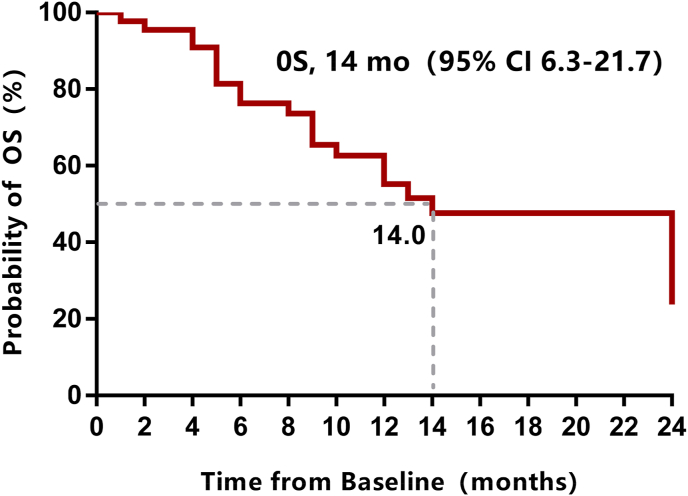
Median follow-up was 10 months (1–26 months) by the data cutoff date (February 2022). Treatment was ongoing only in one patient (2.3%), 42 patients (95.5%) had discontinued treatment due to disease progression, and one patient (2.3%) discontinued treatment due to tucidinostat-related adverse events. At the time of data cutoff, 20 patients (45.5%) died. No patients achieved complete or partial response, and three patients experienced stable disease for more than six months. The CBR was 6.8%, the median PFS was 2.0 months (95%CI: 1.9–2.1, Fig. 1), and the median OS was 14 months (95%CI: 6.3–21.7, Fig. 2). The mPFS of 4.1 months (95%CI: 0–8.2) in patients with 1 metastatic site was significantly longer than the mPFS of 2.0 months (95%CI: 1.9–2.1) in patients with ≥2 metastatic sites. Median PFS was 4.5 months (95%CI: 4.2–4.8) in patients who received sequential tucidinostat after CDK4/6i progression, which was significantly longer than that of those who received nonsequential tucidinostat after CDK4/6i (2.0 months, 95%CI: 1.9–2.1) (Fig. 3). The patients who received tucidinostat as 2nd or 3rd line therapy exhibited significantly longer PFS than that of those who received tucidinostat as 4th and later line therapy (4.5 months, 95%CI: 2.7–6.3 vs. 2.0 months, 95%CI: 1.9–2.1).Multivariate analysis showed that patients with 1 metastatic site and sequential tucidinostat treatment after progression on CDK4/6i were more likely to benefit from tucidinostat combined with endocrine therapy, as shown in Table 2 and Table 3.
Fig. 1.
PFS for patients receiving tucidinostat therapy after prior CDK4/6 inhibitor progression.
Fig. 2.
OS for patients receiving tucidinostat therapy after prior CDK4/6 inhibitor progression.
Fig. 3.
PFS for patients with sequential or nonsequential use of tucidinostat after CDK4/6 inhibitor.
Table 2.
Univariate analysis of factors affecting PFS.
| Factors | PFS (months) | HR | 95% CI | p |
|---|---|---|---|---|
| Age | ||||
| <50 (n = 21) | 2 | 0.75 | 0.39–1.42 | 0.372 |
| ≥50 (n = 23) | 2.1 | |||
| Endocrine partner | ||||
| Anti-estrogens (n = 5) | 2 | 0.94 | 0.69–1.28 | 0.679 |
| Letrozole or anastrozole (n = 8) | 2.1 | |||
| Exemestane (n = 19) | 1.9 | |||
| Fulvestrant (n-10) | 2 | |||
| Medroxyprogesterone (n = 2) | 2 | |||
| Liver metastasis | ||||
| No (n = 19) | 2 | 1.11 | 0.60–2.06 | 0.732 |
| Yes (n = 25) | 2 | |||
| Lung metastasis | ||||
| No (n = 20) | 2 | 1.03 | 0.56–1.91 | 0.916 |
| Yes (n = 24) | 2 | |||
| Visceral metastasis | ||||
| No (n = 10) | 1.9 | 0.81 | 0.38–1.71 | 0.583 |
| Yes (n = 34) | 2 | |||
| Number of metastatic sites | ||||
| 1 (n = 6) | 4.1 | 2.79 | 1.09–7.92 | 0.035 |
| ≥2 (n = 38) | 2 | |||
| Chemotherapy lines for MBC | ||||
| ≤2 (n = 25) | 2.1 | 1.99 | 1.03–3.84 | 0.039 |
| ≥3 (n = 19) | 2 | |||
| Menopausal status | ||||
| Premenopausal (n = 21) | 2 | 0.99 | 0.54–1.83 | 0.981 |
| Postmenopausal (n = 23) | 2 | |||
| Endocrine sensitivity | ||||
| No (n = 5) | 1.8 | 0.39 | 0.15–1.04 | 0.061 |
| Yes (n = 39) | 2.1 | |||
| Benefits of CDK4/6i | ||||
| No benefits (n = 17) | 2 | 0.66 | 0.35–1.23 | 0.187 |
| Benefits (n = 27) | 2.1 | |||
| DFS | ||||
| ≤24 months (n = 21) | 2 | 0.78 | 0.42–1.44 | 0.426 |
| >24 months (n = 23) | 2.1 | |||
| Number of therapy between tucidinostat and CDK4/6i | ||||
| 0 (n = 7) | 4.5 | 3.96 | 1.48–10.61 | 0.006 |
| ≥1 (n = 37) | 2 | |||
| Number of tucidinostat treatment | ||||
| ≤3 (n = 7) | 4.5 | 2.55 | 1.08–6.62 | 0.037 |
| ≥3 (n = 37) | 2 | |||
Table 3.
Multivariate analysis of factors affecting PFS.
| Factors | PFS (months) | HR | 95% CI | p |
|---|---|---|---|---|
| Visceral metastasis | ||||
| No (n = 10) | 1.9 | 0.75 | 0.34–1.62 | 0.461 |
| Yes (n = 34) | 2 | |||
| Number of metastatic sites | ||||
| 1 (n = 6) | 4.1 | 3.11 | 1.09–8.90 | 0.034 |
| ≥2 (n = 38) | 2 | |||
| Chemotherapy lines for MBC | ||||
| ≤2 (n = 25) | 2.1 | 1.67 | 0.82–3.40 | 0.155 |
| ≥3 (n = 19) | 2 | |||
| Endocrine sensitivity | ||||
| Not sensitive (n = 5) | 1.8 | 0.48 | 0.18–1.29 | 0.147 |
| Sensitive (n = 39) | 2.1 | |||
| Number of therapy between tucidinostat and CDK4/6i | ||||
| 0 (n = 7) | 4.5 | 4.27 | 1.59–11.48 | 0.004 |
| ≥1 (n = 37) | 2 | |||
| Number of tucidinostat treatment | ||||
| ≤3 (n = 7) | 4.5 | 1.97 | 0.72–5.42 | 0.19 |
| ≥3 (n = 37) | 2 | |||
The population enrolled in this trial is truly heavily pretreated, the median number of previous palliative endocrine therapy lines was 2 (range: 1–5). Though we avoided applying the same endocrine drug which was previously ineffective, 26 patients (59.0%) rechallenged with the same class agent in combination with tucidinostat, all of which switch between steroid AI and non-steroid AI. Median PFS in patients who rechallenged with the same class endocrine agent was 2.1months (95%CI: 1.9–2.3), which was similar with that in patients who changed another class endocrine drug(2.0months, 95%CI: 1.9–2.1, p = 0.711).
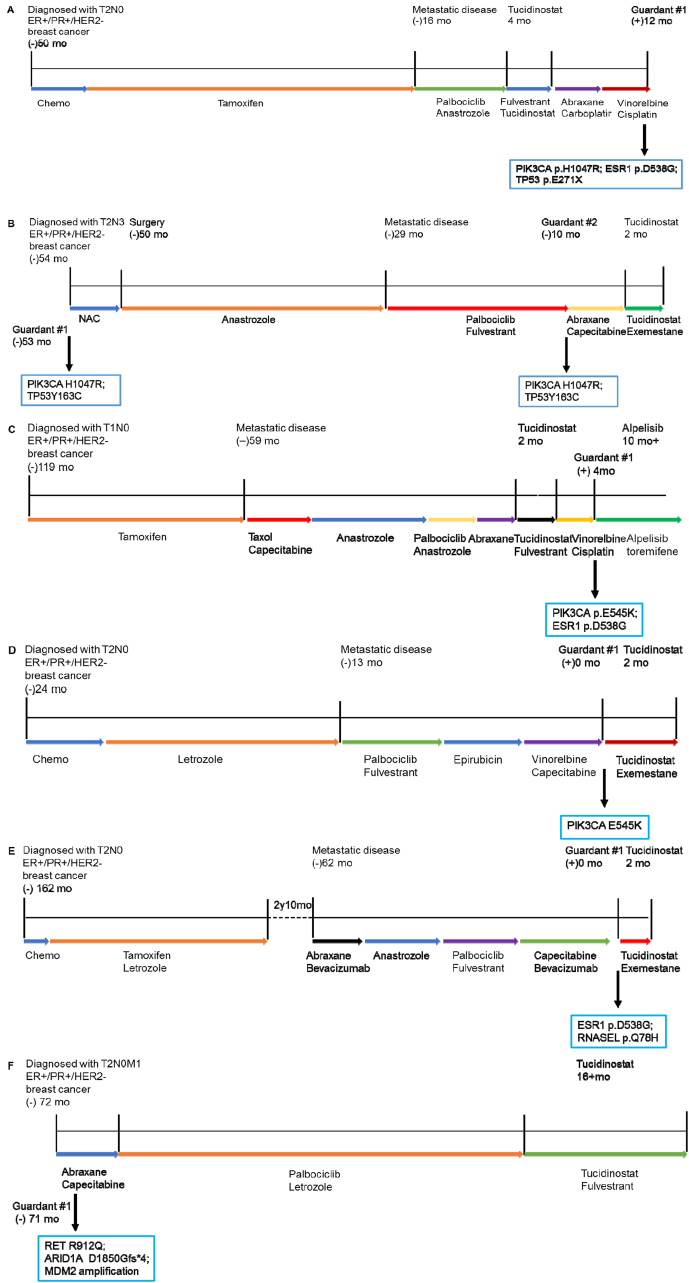
We attempted to analyze available next-generation sequencing (NGS) results to explore factors predicting the efficacy of tucidinostat combination therapy after progression on CDK4/6 inhibitor. 6 patients had NGS results, 2 of which was tested by FoundationOne CDx while the other 4 was tested by domestic medical laboratory which had completed the technical platform verification according to relevant technical guidelines. Alterations in PIK3CA were identified via next-generation sequencing of tumor tissue and blood samples in 4 out 5 patients who didn't obtain clinical benefit from tucidinostat (Fig. 4A-E). No target mutations associated with breast cancer therapy were detected in one patient who benefited from tucidinostat treatment (Fig. 4F).
Fig. 4.
Clinical vignettes with next-generation sequencing provide insight into potential genomic predictors for efficacy of tucidinostat combination therapy after progression on CDK4/6 inhibitor. Treatment histories are provided along with results from next-generation sequencing in representative patients. Patients who didn’‘t obtain clinical benefit from tucidinostat harbored alterations in (A, B, C, D) PIK3CA, and (E) ESR1.
3.3. Adverse events
Adverse events in all patients are shown in Table 4. No grade 4 adverse events and no treatment-related deaths were recorded in the study. Dose reductions because of adverse events occurred in 4 (9.1%) patients, including one case for grade 2 thrombocytopenia, three cases for grade 2 nausea, and two cases with concurrent grade 2 diarrhea.
Table 4.
Adverse events.
| Adverse events | All grades | Grade 3 or worse |
|---|---|---|
| Nausea | 11 (25.0) | 0 (0) |
| Fatigue | 12 (27.3) | 0 (0) |
| Weight loss | 3 (6.8) | 0 (0) |
| Diarrhea | 4 (9.1) | 0 (0) |
| Urinary infection | 2 (4.5) | 0 (0) |
| Anemia | 16 (36.4) | 2 (4.5) |
| Leukopenia | 20 (45.5) | 2 (4.5) |
| Neutropenia | 21 (47.7) | 4 (9.1) |
| Thrombocytopenia | 15 (34.1) | 1 (2.3) |
| Hyperglycemia | 14 (31.8) | 0 (0) |
| Increased alanine aminotransferase | 9 (20.5) | 1 (2.3) |
| Increased aspartate aminotransferase | 11 (25.0) | 2 (4.5) |
| Increased transpeptidase | 17 (38.6) | 4 (9.1) |
| Hypoproteinemia | 18 (40.9) | 0 (0) |
| Hypokalemia | 11 (25.0) | 1 (2.3) |
| Hypocalcemia | 10 (22.7) | 0 (0) |
| Hypophosphatemia | 8 (18.2) | 0 (0) |
| Hypertriglyceridemia | 5 (11.4) | 0 (0) |
4. Discussion
Endocrine therapy has always been the standard care for patients with HR-positive and HER2-negative advanced breast cancer, the advent of inhibitors such as mTOR, PIK3CA, HDAC, and CDK4/6 has prompted it enters the era of targeted therapy. Current guidelines recommend CDK4/6 inhibitor combined with endocrine therapy as the first- or second-line treatment for patients without visceral crisis [13,16]. With the widespread use of CDK4/6i in front-line therapy in clinical practice, clinicians inevitably have to face the issue of their resistance. There is currently no optimal recommendation for therapeutic strategies after progression on CDK4/6 inhibitor. Key registration clinical trials of mTOR, PIK3CA, and HDAC inhibitor were all conducted before the approval of CDK4/6i, however, the gene expression profiles and tumor microenvironment of patients who progressed on CDK4/6i must differ from those of CDK4/6i-naive [17]; therefore, it is necessary to explore their efficacy in the era after CDK4/6 inhibitor.
This study retrospectively analyzed the efficacy and safety of tucidinostat combined with endocrine therapy in patients with HR + HER2- MBC who have progressed on prior CDK4/6 inhibitor therapy for the first time. A total of 44 patients were enrolled in this study, and the mPFS was 2.0 months (95% CI: 1.9–2.1) which was shorter than the 7.4 months in the ACE study. Possible reasons for this phenomenon were larger tumor burden, more refractory disease, and progression on previous CDK4/6 inhibitor in the patients enrolled in this study [15]. The incidence of adverse events in this study was lower than that in the ACE study, which was due to incomplete records because of retrospective study and physicians'improved safety awareness and preventive management measures of tucidinostat. The combination of tucidinostat and endocrine therapy was generally well tolerated with no new safety events.
At present, there is no prospective clinical trial to confirm which treatment strategy is the best after progression on CDK4/6 inhibitor. After the failure of CDK4/6i, the mPFS of chemotherapy was 4.2–5.5 months and the mPFS of endocrine therapy combined with everolimus was 4–5 months, which was negatively affected by treatment line [8,[18], [19], [20]]. MAINTAIN trial showed the mPFS of ribociclib combined with endocrine was 5.29 months beyond previous CDK4/6i [21]. Elacestrant, a new oral selective estrogen receptor degrader, exhibited 2.79 months mPFS after previous CDK4/6i in EMERAL trial [22].For patients with PIK3CA mutations Alpelisib plus fulvestrant was a treatment options with 7.3 months mPFS after CDK4/6i with an AI as immediate prior therapy [23,24].
The CDK4/6i was firstly approved by China Food and Drug Administration in 2018 and was not covered by medical insurance until 2022, so the patients enrolled in our study were heavily pretreated who accepted muti-line treatments including endocrine therapy and chemotherapy. Only 20.5% patients received CDK4/6i as first line treatment. According to guideline and consensus, other CDK4/6i, mTOR inhibitor everolimus and PIK3CA inhibitor combined with endocrine drug followed by CDK4/6i failure are options.Unfortunately, except Palbociclib, other CDK4/6i and PIK3CA inhibitor haven't been approved in China. mTOR inhibitor everolimus hasn't been approved in breast cancer. HDACi tucidinostat was approved for breast cancer in 2019 in China, but it hasn't been covered by medical insurance until today. So in our clinical practice, most patients accepted chemotherapy after CDK4/6i failure. Tucidinostat was the first HDAC inhibitor approved for breast cancer. Based on the HDAC mechanism, patients after CDK 4/6i failure maybe benefit from tucidinostat treatment. So we collected our real-world data to show effect of tucidinostat for MBC patients who failure in CDK4/6i. We hope we can understand the tucidinostat efficacy and possible influence factors on efficacy from this study. In the future, we are going to carry out new clinical trail on HDACi and hope to give new treatment strategy for all over the word MBC patients. In our this study, mPFS of tucidinostat combined with endocrine therapy was 4.5 months when used sequentially after progression on CDK4/6i. In fact the patients were all heavily pretreated patients.
We have to admit only 7(16%) patients received tucidinostat-based treatment after progression on CDK4/6i, owing to tucidinostat has not yet covered by medical insurance even though it got approvement for MBC by cFDA in November 2019.26(59%) patients received subsequent monochemotherapy, while the remaining 11(25%) patients received combination chemotherapy or chemotherapy combined with anti-vascular endothelial growth factor receptor therapy. The mPFS of patients who received subsequent monochemotherapy was 5.0 months(95%CI: 3.9–6.1, Appendix Fig S1, online only).We also reviewed and analyzed other 48 patients who received monochemotherapy beyond CDK4/6i sequentially during the period of this study, the mPFS was 5.0 months(95%CI: 4.0–6.0, Appendix Fig S2, online only). The baseline characteristics of patients who received tucidinostat-based or monochemotherapy were basically balanced, similar mPFS were also obtained in both groups(Appendix Fig S3, online only).In all enrolled populations, the mPFS of patients with low tumor burden (1 metastatic site) and few prior palliative treatment (≤2 lines) exhibited longer mPFS(4.1 months and 4.5 months, respectively), which were comparable to those in the above-mentioned studies.
HDAC inhibitors are an emerging targeted inhibitor, and there are many HDAC subtypes, thus there is no clear predictor of efficacy till now. Preclinical data showed that factors such as loss of JUN DNA, activation of PIK3CA-AKT-mTOR pathway, p21 upregulation may lead to HDAC inhibitor resistance. A total of 6 patients in this article had the results of next-generation sequencing, alterations in PIK3CA were identified in 4 out 5 patients who didn’t obtain clinical benefit from tucidinostat, and no target mutations associated with breast cancer therapy was detected in one patient who benefited from tucidinostat treatment. Combined with preclinical research data, it is preliminarily speculated that patients with PIK3CA mutations who are candidates for PIK3CA inhibitor therapy may not benefit from HDAC inhibitor therapy [[25], [26], [27]]. However, more research is needed to explore and confirm this phenomenon in the future.
There are several limitations in this study, including retrospective design, relatively small sample size at a single center, patient selection bias, large differences between patients' previous treatment and short follow-up period. In addition, the visit time and compliance may be different in different patients in real-world clinical practice, which may lead to varying cycles of treatment response assessment and thus make an impact on the determination of PFS. However, our study provides crucial data on the efficacy of tucidinostat combined with endocrine therapy in patients with HR+/HER2-advanced breast cancer that has progressed on CDK4/6 inhibitor.
5. Conclusions
Our study preliminarily shows that tucidinostat combined with endocrine therapy may be an optional sequential strategy for patients with HR+/HER2-advanced breast cancer that has progressed on CDK4/6 inhibitor, especially for these with lower tumor burden and fewer prior palliative treatment. Additional studies with larger sample sizes ideally in prospective trials are required to confirm these findings and molecular predictors of tucidinostat.
Availability of data and materials
The datasets generated during and/or analyzed during the current study are not publicly available due to privacy of participants but are available from the corresponding author on reasonable request.
Author contributions
Tao Wang and Zefei Jiang conceived and designed the study. Jinmei Zhou, Xuexue Wu, Huiqiang Zhang, Xiaobo Wang, Yang Yuan, and Shaohua Zhang collected the data. Jinmei Zhou and Xuexue Wu performed the statistical analyses. Jinmei Zhou wrote the manuscript. Tao Wang and Zefei Jiang reviewed and revised the manuscript. All authors read and approved the final manuscript
Funding
This study was supported by National Natural Science Foundation of China (Grant No. 81572597) and Beijing Municipal Natural Science Foundation (Grant No. 7192198).
Ethics approval and consent to participate
This study was approved by the Ethics Committee of the Fifth Medical Center of the Chinese People's Liberation Army General Hospital and informed consent was obtained from all patients. Informed consent for data collection was obtained in accordance with the Declaration of Helsinki. This study was registered with ClinicalTrials.gov (ClinicalTrials.gov identifier: NCT 05276713).
Consent for publication
Written consent was obtained for the publication of this study from the patients.
Declaration of competing interest
All authors have no conflicts of interest.
Acknowledgements
The authors thank all of the patients and their families, as well as all technicians, physicians, and nurses who participated in this study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.breast.2022.10.018.
Contributor Information
Zefei Jiang, Email: jiangzefei@csco.org.cn.
Tao Wang, Email: wangtao733073@163.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Cao W., Chen H.D., Yu Y.W., Li N., Chen W.Q. Changing profiles of cancer burden worldwide and in China: a secondary analysis of the global cancer statistics 2020. Chin Med J. 2021;134(7):783–791. doi: 10.1097/CM9.0000000000001474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cardoso F., Paluch-Shimon S., Senkus E., et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5) Ann Oncol. 2020;31(12):1623–1649. doi: 10.1016/j.annonc.2020.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cristofanilli M., Turner N.C., Bondarenko I., et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016;17(4):425–439. doi: 10.1016/S1470-2045(15)00613-0. [DOI] [PubMed] [Google Scholar]
- 4.Finn R.S., Martin M., Rugo H.S., et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med. 2016;375(20):1925–1936. doi: 10.1056/NEJMoa1607303. [DOI] [PubMed] [Google Scholar]
- 5.Hortobagyi G.N., Stemmer S.M., Burris H.A., et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med. 2016;375(18):1738–1748. doi: 10.1056/NEJMoa1609709. [DOI] [PubMed] [Google Scholar]
- 6.Goetz M.P., Toi M., Campone M., et al. MONARCH 3: abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol. 2017;35(32):3638–3646. doi: 10.1200/JCO.2017.75.6155. [DOI] [PubMed] [Google Scholar]
- 7.Sledge G.W., Jr., Toi M., Neven P., et al. MONARCH 2: abemaciclib in combination with fulvestrant in women with HR+/HER2- advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol. 2017;35(25):2875–2884. doi: 10.1200/JCO.2017.73.7585. [DOI] [PubMed] [Google Scholar]
- 8.Turner N.C., Slamon D.J., Ro J., et al. Overall survival with palbociclib and fulvestrant in advanced breast cancer. N Engl J Med. 2018;379(20):1926–1936. doi: 10.1056/NEJMoa1810527. [DOI] [PubMed] [Google Scholar]
- 9.Im S.A., Lu Y.S., Bardia A., et al. Overall survival with ribociclib plus endocrine therapy in breast cancer. N Engl J Med. 2019;381(4):307–316. doi: 10.1056/NEJMoa1903765. [DOI] [PubMed] [Google Scholar]
- 10.Finn R.S., Boer K., Bondarenko I., et al. Overall survival results from the randomized phase 2 study of palbociclib in combination with letrozole versus letrozole alone for first-line treatment of ER+/HER2- advanced breast cancer (PALOMA-1, TRIO-18) Breast Cancer Res Treat. 2020;183(2):419–428. doi: 10.1007/s10549-020-05755-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sledge G.W., Jr., Toi M., Neven P., et al. The effect of abemaciclib plus fulvestrant on overall survival in hormone receptor-positive, ERBB2-negative breast cancer that progressed on endocrine therapy-MONARCH 2: a randomized clinical trial. JAMA Oncol. 2020;6(1):116–124. doi: 10.1001/jamaoncol.2019.4782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Slamon D.J., Neven P., Chia S., et al. Ribociclib plus fulvestrant for postmenopausal women with hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer in the phase III randomized MONALEESA-3 trial: updated overall survival. Ann Oncol. 2021;32(8):1015–1024. doi: 10.1016/j.annonc.2021.05.353. [DOI] [PubMed] [Google Scholar]
- 13.Gennari A., André F., Barrios C.H., et al. ESMO Clinical Practice Guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann Oncol. 2021;32(12):1475–1495. doi: 10.1016/j.annonc.2021.09.019. [DOI] [PubMed] [Google Scholar]
- 14.Falkenberg K.J., Johnstone R.W. Histone deacetylases and their inhibitors in cancer, neurological diseases and immune disorders. Nat Rev Drug Discov. 2014;13(9):673–691. doi: 10.1038/nrd4360. [DOI] [PubMed] [Google Scholar]
- 15.Jiang Z., Li W., Hu X., et al. Tucidinostat plus exemestane for postmenopausal patients with advanced, hormone receptor-positive breast cancer (ACE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20(6):806–815. doi: 10.1016/S1470-2045(19)30164-0. [DOI] [PubMed] [Google Scholar]
- 16.Li Jianbin, Jiang Zefei. Chinese society of clinical Oncology breast cancer guideline version 2021: updates and interpretations. Natl Med J China (Peking) 2021;101(24):1835–1838. doi: 10.3760/cma.j.cn112137-20210421-00954. [DOI] [PubMed] [Google Scholar]
- 17.Fassl A., Geng Y., Sicinski P. CDK4 and CDK6 kinases: from basic science to cancer therapy. Science. 2022;375(6577) doi: 10.1126/science.abc1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Y., Li W., Gong C., et al. A multicenter analysis of treatment patterns and clinical outcomes of subsequent therapies after progression on palbociclib in HR+/HER2- metastatic breast cancer. Ther Adv Med Oncol. 2021;13 doi: 10.1177/17588359211022890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xi J., Oza A., Thomas S., et al. Retrospective analysis of treatment patterns and effectiveness of palbociclib and subsequent regimens in metastatic breast cancer. J Natl Compr Cancer Netw. 2019;17(2):141–147. doi: 10.6004/jnccn.2018.7094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rozenblit M., Mun S., Soulos P., Adelson K., Pusztai L., Mougalian S. Patterns of treatment with everolimus exemestane in hormone receptor-positive HER2-negative metastatic breast cancer in the era of targeted therapy. Breast Cancer Res. 2021;23(1):14. doi: 10.1186/s13058-021-01394-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kevin Kalinsky, Melissa Kate Accordino, Codruta Chiuzan, et al. A randomized, phase II trial of fulvestrant or exemestane with or without ribociclib after progression on anti-estrogen therapy plus cyclin-dependent kinase 4/6 inhibition (CDK 4/6i) in patients (pts) with unresectable or hormone receptor–positive (HR+), HER2-negative metastatic breast cancer (MBC): MAINTAIN trial. J Clin Oncol 40, 2022 (suppl 17; abstr LBA1004).
- 22.Bardia A., Neven P., Montero A.J., et al. Poster presented at: 39th annual miami breast cancer conference. March 3-6, 2022. Elacestrant, an oral selective estrogen receptor degrader (SERD), vs investigator's choice of endocrine monotherapy for ER+/HER2- advanced/metastatic breast cancer (mBC) following progression on prior endocrine and CDK4/6 inhibitor therapy: results of EMERALD phase 3 trial. [Miami Beach, FL] [Google Scholar]
- 23.Rugo H.S., Lerebours F., Ciruelos E., et al. Alpelisib plus fulvestrant in PIK3CA-mutated, hormone receptor-positive advanced breast cancer after a CDK4/6 inhibitor (BYLieve): one cohort of a phase 2, multicentre, open-label, non-comparative study. Lancet Oncol. 2021;22(4):489–498. doi: 10.1016/S1470-2045(21)00034-6. [DOI] [PubMed] [Google Scholar]
- 24.Turner S., Chia S., Kanakamedala H., et al. Effectiveness of alpelisib + fulvestrant compared with real-world standard treatment among patients with HR+, HER2-, PIK3CA-mutated breast cancer. Oncol. 2021;26(7):e1133–e1142. doi: 10.1002/onco.13804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tanioka M., Mott K.R., Hollern D.P., Fan C., Darr D.B., Perou C.M. Identification of Jun loss promotes resistance to histone deacetylase inhibitor entinostat through Myc signaling in luminal breast cancer. Genome Med. 2018;10(1):86. doi: 10.1186/s13073-018-0597-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mrakovcic M., Fröhlich L.F. Molecular determinants of cancer therapy resistance to HDAC inhibitor-induced autophagy. Cancers. 2019;12(1) doi: 10.3390/cancers12010109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chakraborty A.R., Robey R.W., Luchenko V.L., et al. MAPK pathway activation leads to Bim loss and histone deacetylase inhibitor resistance: rationale to combine romidepsin with an MEK inhibitor. Blood. 2013;121(20):4115–4125. doi: 10.1182/blood-2012-08-449140. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available due to privacy of participants but are available from the corresponding author on reasonable request.