Abstract
Background
HIV testing is a crucial starting point for prevention, early diagnosis, and treatment of HIV. Sub-Saharan Africa has the highest global HIV/AIDS prevalence and mortality, yet HIV testing remains sub-optimal. Thus, this study aimed to identify the prevalence of HIV testing and associated factors among young adolescents aged 10 to 14 years in Eswatini, a country with the highest HIV prevalence in the world.
Methods
Data were obtained from Swaziland HIV Incidence Measurement Survey between 2016 and 2017 (SHIMS 2), an internationally supported national survey aimed at combating HIV/AIDS. A total of 739 young adolescents aged 10 to 14 years were selected for the final analysis after deleting cases with missing values for the key variables. The effects of demographic characteristics, HIV knowledge, HIV risk perception, belief about HIV testing, perceived service accessibility, and parent-child sexual and reproductive health communication on lifetime HIV testing as an outcome variable, were explored using multivariable logistic regression.
Results
Only 52.0% of young adolescents reported “ever tested” for HIV in their lifetime. Age (OR = 0.81, 95% CI = 0.73–0.90), residence (OR = 0.56, 95% CI = 0.43–0.74), and perceived service accessibility (OR = 3.10, (95% CI = 1.47–6.56) were identified as important factors associated with receiving HIV testing among young adolescents.
Conclusions
A low rate of HIV testing was identified among young adolescents in Eswatini compared to the intended global goal of HIV testing coverage. Our findings suggested the importance of young adolescent-friendly educational and environmental interventions needed to improve the prevalence of HIV testing by reducing misperceptions about the risk of HIV and alleviating environmental constraints to access to HIV services.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12887-022-03698-0.
Keywords: Adolescent; HIV testing; Perception, Prevalence, Eswatini
Background
HIV testing is a crucial starting point for prevention, early diagnosis, and treatment of HIV to increase survival rate, improve quality of life, and preclude further transmission [1, 2]. In order to improve motivation toward maximizing HIV testing coverage, which is an optimal measure to achieve an HIV-free generation, the Joint United Nations Programme on HIV/AIDS (UNAIDS) outlined a global target named 90–90-90 in 2014. The 90–90-90 target aimed to ensure that 90% of the people living with HIV (PLHIV) should be diagnosed and know their HIV status, 90% of people living with HIV should be initiated on antiretroviral therapy (ART), and 90% of those started on ART should have their viral load suppressed by 2020 [3]. The 90–90-90 target was further accelerated to 95–95-95 by 2030, following the remarkable progress made by the UNAIDS and other global stakeholders in achieving the 90–90-90 targets in the general population of individuals aged 15–49 years in a couple of countries globally including the Sub-Saharan Africa by 2020 [4–6]. In 2020, Eswatini was the first country in Africa to achieve the 95–95-95 target [6, 7]. However, considering the disparities in accessing HIV prevention and treatment interventions [8], it is not clear whether this success can be attributed to young adolescents.
Sub-Saharan Africa is a region with the highest prevalence rate of HIV among adolescents and young adults [9]. Considering the high prevalence of HIV and the anticipated increased risk of transmission in a high HIV-burdened country like Eswatini, the HIV management guidelines of Eswatini suggested that young adolescents should be tested for HIV at least once a year depending on risk assessment [10]. However, the latest report by United Nations Children’s Fund (UNICEF) showed that approximately 88% of all HIV-infected adolescents aged 10 to 19 years live in Sub-Saharan Africa, yet only 17 to 25% of this age group had tested for HIV in the past year in 2020 [11]. Similarly, another review study with data from 18 countries in Sub-Saharan Africa also reported the low rate of HIV testing among adolescents aged 15 to 19 years, showing 2–24% in male adolescents and 4–44% in female adolescents across countries [12]. According to a recent analysis of data from PHIA surveys conducted in seven countries, including Eswatini, about 39% of children and young adolescents (1–14 years) living with HIV were undiagnosed, meaning they were unaware of their HIV status [13]. Low rates of HIV testing can lead to a reduction of early treatment, resulting in insufficient support and increasing mortality in adolescents living with HIV [8, 14].
Considering that HIV-related mortality has ranked as a top killer in Sub-Sahara African adolescents between the ages of 10 and 19 years [15], these young adolescents should be prioritized early in the development life cycle before being exposed to environmental and societal factors with potentially damaging consequences for sexual and reproductive health [16]. However, sufficient attention has not been paid to young adolescents. This gap is probably related to limited age-specific data to identify potential factors for facilitating the availability, accessibility, and utilization of HIV testing services [17]. A systematic review on HIV testing among children and adolescents in the Sub-Saharan region highlighted that data from existing literature had not been adequately stratified by age [17]. For this reason, strategies used in HIV testing and treatment that were developed for older adolescents and young adults were mostly repeated with little consideration for the needs of young adolescents [17–20]. As a result, HIV-related mortality varied with age [14]. Only a 10% reduction was reported among adolescents aged 10–19 years while 74 and 64% reductions were found in children aged 0–9 years and other age groups, respectively [21].
According to existing literature, HIV testing uptake among adolescents and young people was associated with several factors, including age, gender, residence, HIV knowledge, and risk perception, stigma, HIV-related belief, accessibility to HIV testing services, and prior experience to participate in sexual reproductive health (SRH) communication [18, 22–27]. Despite the valuable information from previous studies regarding the low rate of HIV testing and associated factors, further investigations are needed to determine the relevance of what is given from different age groups as factors that can play the same role in explaining the HIV testing uptake in young adolescents. Young adolescence was highlighted as a critical target population for the prevention and treatment of HIV to achieve the global target of combating HIV by 2030 [28]. Therefore, this study aimed to identify the prevalence of HIV testing uptake and examine the factors associated with HIV testing among young adolescents aged 10 to 14 years in Eswatini.
Methods
Research design and sample
This study is a secondary data analysis obtained from the Swaziland HIV Incidence Measurement Survey (SHIMS 2). This nationally representative survey was conducted between September 2016 and March 2017 to gather demographic and HIV-related data as part of the multicounty Population-based HIV Impact Assessment (PHIA) project. To obtain participants for SHIMS 2, a two-stage, stratified cluster sample design was used. The sample frame for this survey included all households in Eswatini, estimated at 212,195 homes, including 2064 enumeration areas (E.A.) based on the country’s 2007 census of population and housing. In the first sampling stage, the sample was stratified by urban or rural status within four regions (Hhohho, Manzini, Lubombo, and Shiselweni) of Eswatini, and a total of 287 EAs were selected with probability proportionate to the number of households. The second stage involved a random selection of homes from each stratum using the equal probability method, which rendered an average of 20 households selected from each cluster. Children were eligible to participate if they resided in a selected household and slept in the house the night before the interview. Households were eligible for selection if 1) they were known households during the time of listing, 2) occupied at the time of the interview, and 3) vacant dwelling units that could potentially be occupied at the time of the interview. Before data collection, consent from parents or guardians of children 0 to 14 years was sought to approach a minor, and assent was sought from children 10 to 14 years whose parents or guardians had consented to their participation.
The original data set contained 7796 children aged 0 to 14 years [29]. For the purposes of this study, we selected data from those who were adolescents aged 10 to 14 years and had provided information about HIV testing and key variables including age, gender, residence, educational level, HIV knowledge, HIV risk perception, HIV test-related belief, HIV testing service accessibility, and parent-child SRH communication. After deleting respondents younger than 10 years, a sample of 2, 545 respondents remained. The additional exclusion was made when individuals had missing values on the outcome variable and other independent variables selected for purpose of this study. Finally, a sample size of 739 was included in the analysis, and weights were attached to account for the respondents with missing or incomplete values for the demographic variables of each respondent. A sample flow chart is presented in Fig. 1. below.
Fig. 1.
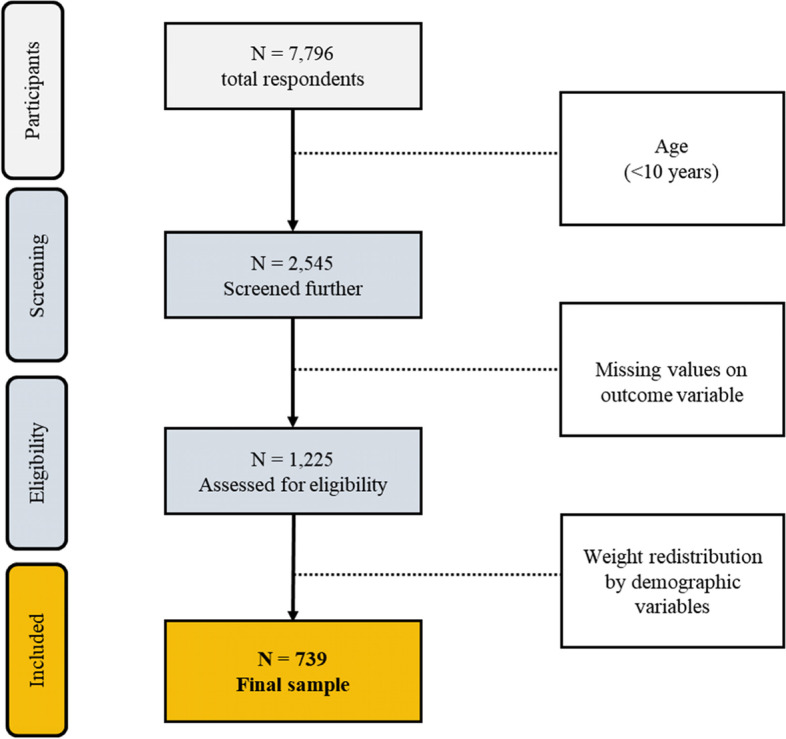
A flow of sample selection
Measures
HIV testing was selected as an outcome variable in this study. Independent variables included HIV knowledge, HIV risk perception, HIV test-related belief, HIV testing service accessibility, and parent-child SRH communication. Sociodemographic factors including age, gender, residence, and educational level were evaluated to define the sample characteristics.
HIV testing was assessed using one question on individuals’ past experience of getting an HIV test: “Have you ever tested for HIV?” This question was answered with “yes” or “no.”
HIV knowledge was measured as a categorical variable by asking 8 questions to define comprehensive knowledge about HIV. Participants were asked to respond “yes” or “no” to each question. The score of HIV knowledge ranged from 0 to 8, with higher scores interpreted as more accurate knowledge [29, 30]. If participants had 0 or 1 correct answer to the questions, they were classified into the group with a low level of HIV knowledge. If participants had 2 to 5 correct answers, they were considered the group with middle-level HIV knowledge. If participants answered 6 or more questions correctly, they were categorized as a high knowledge level group.
HIV risk perception was measured by using one question to assess the perceived risk of HIV infection: “How likely do you think it is for you to get HIV?”. The participants answered the question by selecting one of four options, including “likely,” “somewhat likely,” “unlikely,” and “already know my HIV status”. Individuals who answered with “already know my status” were excluded from the analysis of HIV risk perception because, according to the SHIMS 2 codebook, these were individuals who had already tested positive for HIV. To simplify the presentation and interpretation of the results, this variable was converted into a categorical variable by splitting the scale using the median as a cut-off point to re-define the high and low groups in this study [30–32]. Participants with 1 or less scores were classified into the group with lower HIV risk perception, and those who scored 3 and above were classified into the high HIV risk perception group.
HIV test-related belief was measured as a categorical variable by asking one question on individuals’ beliefs about HIV testing: “Should everyone get tested for HIV?” This question was answered with “yes” or “no” [29].
Parent-child SRH communication was measured as a categorical variable by asking participants 3 questions to define young adolescents’ openness to discuss sex-related topics with their parents or guardians. These questions included, “Have you ever discussed HIV with your parent or guardian?”, “Have you ever talked to parent/guardian about sex?” and “If you have a problem, can you freely discuss it with your parent or guardian?” They were asked to respond “yes” or “no” to these questions. The selection of these three items and classifying them as “parent-child SRH communication” single variable was based on previous studies which demonstrated a close relationship between these items [33, 34]. A sum score including all three items was computed. If participants had a score of less than 2, then that group was classified as “low”, meaning they could not freely talk about SRH related issues with parents or caregivers. If participants had a score of 2 and above, they were classified as the “high”, meaning they could freely talk about sexual and reproductive health issues with their parents/caregivers.
Perceived service accessibility was measured as a categorical variable to assess individuals’ perceived ease or difficulty to access HIV testing services: “If you wanted to, could you get an HIV test”? Participants were asked to respond “yes” or “no” [29]. An additional questionnaire file shows this in more detail (see Additional file 1).
Statistical analyses
All data analyses were conducted in SPSS version 26. Descriptive statistics, including frequency, mean and standard deviations, were used to describe the sample characteristics. Chi-square tests were conducted to examine the differences in HIV testing by HIV knowledge, HIV risk perception, HIV test-related belief, parent-child SRH communication, and perceived service accessibility and sociodemographic factors including age, gender, and residence. Multivariable logistic regression model was developed to determine the factors associated with HIV testing. In this model, eight independent variables were selected based on theoretical and statistical considerations [22–25] as possible predictors of HIV testing; a) age as a continuous variable; b) gender, c) residence, d) HIV-test related belief, e) perceived service accessibility, and f) HIV risk perception as categorical variables; g) HIV knowledge and h) parent-child SRH communication as continuous variables. All analyses in this study were performed using sample weights and complex sample approaches to account for the survey design used by SHIMS 2 [35].
Results
Sociodemographic characteristics
Sociodemographic characteristics of 739 respondents are presented in Table 1. All participants were young adolescents whose ages ranged from 10 to 14 years, with an average age of 12.14 years. More than half of them were females, resided in urban areas, and were in primary school at the time of data collection. Interestingly, those who reported having ever tested for HIV were only 52.00%, with a higher HIV testing rate observed in females than males.
Table 1.
Weighted sample distribution by sociodemographic characteristics (N = 739)
| Variables | n (%) | M (SE) |
|---|---|---|
| Age (Years) | 12.14 (0.04) | |
| Gender | ||
| Male | 342 (46.30) | |
| Female | 397 (53.70) | |
| Residence | ||
| Rural | 277 (37.50) | |
| Urban | 462 (62.50) | |
| Level of education | ||
| Primary School | 618 (83.60) | |
| High School | 116 (15.80) | |
| HIV testing | ||
| Ever tested | 384 (52.00) | |
| Never tested | 355 (48.20) | |
Differences between HIV testing and independent variables
As presented in Table 2, HIV testing in a lifetime differed significantly depending on the residence, level of education, service accessibility, and parent-child SRH communication. Individuals residing in rural areas had fewer chances of getting an HIV test than those in urban areas (χ 2 = 11.98, p < .001). Individuals in the lower grades were less likely to have ever tested for HIV than those in higher grades (χ 2 = 10.84, p < .001). Individuals with perceived ease of accessibility to testing services were more likely to have ever tested for HIV test than those with difficulty accessing HIV testing services (χ 2 = 14.80, p < .001). Young adolescents with experience of having parent-child SRH communication with their parents or guardian were more likely to report having ever tested for HIV than those who did not (χ 2 = 5.72, p = .02). HIV testing did not significantly differ by gender, HIV knowledge, and HIV test-related belief in this analysis.
Table 2.
Weighted differences between HIV testing and independent variables among participants
| Variables | HIV testing | χ2 | |
|---|---|---|---|
| Yes | No | ||
| Gender | |||
| Male | 186 (48.3) | 157 (44.1) | 1.22 |
| Female | 199 (51.7) | 198 (55.9) | |
| Residence | |||
| Rural | 168 (43.6) | 109 (30.8) | 11.98*** |
| Urban | 217 (56.4) | 246 (69.2) | |
| Level of education | |||
| Primary School | 333 (86.6) | 285 (80.3) | 10.84** |
| High School | 47 (12.2) | 70 (19.7) | |
| HIV Knowledge | |||
| Low | 17 (4.4) | 23 (6.6) | 2.99 |
| Middle | 132 (34.5) | 134 (37.9) | |
| High | 234 (61.1) | 197 (55.5) | |
| HIV Risk Perception | |||
| Low | 336 (87.4) | 300 (84.5) | 1.26 |
| High | 48 (12.6) | 55 (15.5) | |
| HIV test-related belief | |||
| No | 12 (3.1) | 13 (3.7) | .20 |
| Yes | 372 (96.9) | 342 (96.3) | |
| Service accessibility | |||
| No | 12 (3.1) | 36 (10.3) | 14.80*** |
| Yes | 372 (96.9) | 318 (89.7) | |
| Parent-child SRH communication | |||
| No | 238 (62.1) | 251 (70.7) | 5.72* |
| Yes | 146 (37.9) | 104 (29.3) | |
Abbreviation: SRH sexual and reproductive health
Note: *p < .05, **p < .001
Multivariable logistic regression analysis
The results of multivariable logistic regression analysis are presented in Table 3. Getting tested for HIV was significantly associated with age, residence, and perceived service accessibility. One unit increase in age was associated with 0.81 times (95% CI = 0.73–0.90) higher odds of getting an HIV test. Individuals living in urban areas had 5.6% higher odds of getting tested (OR = 0.56, 95% CI = 0.43–0.74) than those in rural areas. The OR for adolescents who could access HIV testing was 3.10 (95% CI = 1.47–6.56) compared to those who could not. Parent-child SRH communication was not significantly associated with HIV testing uptake. Similarly, HIV knowledge, HIV risk perception, and HIV test-related belief were not significant factors of HIV testing for young adolescents.
Table 3.
Multivariable logistic regression model
| Variables | B (SE) | OR (95% CI) |
|---|---|---|
| Age | 0.21 (.05) | 0.81 (0.73–0.90)** |
| Gender (female) | 0.24 (.16) | 0.79 (0.57–1.09) |
| Residence (urban) | 0.58 (.14) | 0.56 (0.43–0.74)** |
| Sum of HIV knowledge | 0.87 (.89) | 2.38 (0.40–14.00) |
| HIV Risk perception (high) | 0.19 (.29) | 1.21 (0.68–2.15) |
| HIV test-related belief (yes) | 0.03 (.43) | 1.03 (0.43–2.44) |
| Perceived service accessibility (yes) | 1.13(.38) | 3.10 (1.47–6.56)* |
| Parent-child SRH communication | 0.30 (.31) | 1.47 (0.80–2.70) |
Abbreviations: SRH sexual and reproductive health, OR Odds Ratio, CI Confidence Interval
Note: * p < .05, ** p < .001
Discussion
Eswatini made remarkable progress in HIV testing coverage among the general population of individuals aged 15 to 49 years and was applauded for being among the first countries to reach the global target of the first 90% along with other countries like Botswana and Namibia in Africa in 2018 [5]. In 2020, Eswatini was the first country in Africa to reach the 95–95-95 global target [7]. However, it was difficult to say that the same progress was obtained in the age group of young adolescents. This study found that the lifetime HIV testing rate among young adolescents aged 10 to 14 years in Eswatini was 52% compared to 98% in the general population [7], indicating a huge gap between the rate in this study and the goal proposed by the global targets. This age-related hidden gap may be of serious concern and highlights the need for greater participation of marginalized young adolescents who may be missed out on HIV response programs as some may be unknowingly infected hence delaying enrolment into care [16].
HIV testing uptake was significantly associated with greater access to HIV testing services in this study. As anticipated perceived accessibility to HIV testing services was significantly associated with HIV testing uptake among young adolescents. Respondents who reported difficult accessibility to HIV testing services were less likely to have received an HIV test in a lifetime. This finding is consistent with previous studies showing that service accessibility issues were associated with lower participation in HIV testing among adolescents [24, 28]. To bridge the gap in receiving HIV tests by dealing with barriers to accessibility among young adolescents, introducing adolescent-friendly health services (AFHS) within the communities in which young adolescents reside was recommended [36, 37]. The AFHS was defined as an evidence-based and specialized approach to addressing health system barriers and catering to developmental needs by providing a more accessible, acceptable, equitable, appropriate, and effective social environment for young people [38]. A recently published systematic review study supported that AFHS in health facilities and clinical settings positively influenced health service utilization for HIV testing and other sexually transmitted diseases or performing preventive behaviors in Sub-Saharan Arica [39]. However, there is a lack of understanding and limited knowledge in the literature regarding implementing appropriate strategies for delivering AFHS to help young adolescents receive HIV testing in resource-constrained out-of-facility settings [39]. Considering the effectiveness of AFHS in meeting adolescents’ sexual and reproductive health needs, future research is needed to investigate how AFHS can be applied over an extended period in marginalized communities to achieve the desired goals, especially in ending HIV.
HIV risk perception is vital in determining health behavior practices such as routine HIV testing as prevention. However, HIV testing uptake was not significantly associated with higher risk perception for HIV infection in this study. More than 80% of young adolescents reported lower HIV risk perception regardless of HIV test status. Although young adolescents who reported lower HIV risk perception were more likely to get an HIV test than those with higher HIV risk perception, the difference did not reach the significance level. Interestingly, this age group tends to believe that they are at low risk of contracting HIV, and their misperception about HIV transmission may increase the likelihood of engaging in risky sexual behaviors and their vulnerability to HIV infection [36]. Similarly, a couple of other studies conducted among African adolescents showed that adolescents and young people were likely to have incorrect HIV risk perception such that even those at high risk of HIV acquisition had lower HIV risk perception [27, 36, 37]. Therefore, future research is required to understand better the drivers of HIV risk perception among young adolescents.
There is a growing literature showing that young adolescents who freely communicated about sex-related topics and HIV with their parents or caregivers were likely to practice safer sex behaviors, including HIV prevention [40]. Contrary to these findings, the association between parent-child SRH communication and HIV testing uptake was not significant in this study. This unexpected inconsistency in the effect of parent-child SRH communication on health behavior practices and HIV prevention may be associated with the characteristics of communications and the individuals involved in SRH communication. First, a meta-analysis study suggested that the frequency, content, or how pleasant the conversations were likely to influence the effectiveness of the parent-child SRH communication among adolescents [40, 41]. Our data did not provide detailed information about the nature of the communication according to the above-mentioned factors, except for the knowledge that some adolescents engaged in SRH communication with parents or caregivers. Second, significant differences in the effect of communication on healthy behaviors such as HIV prevention were found between the gender of the adolescents and parents or caregivers, with more substantial effects observed in girls than in boys. Parent-child SRH communication between mothers and daughters was more significantly associated with positive outcomes compared to father-son communication [40, 42, 43]. It is necessary to consider that the interaction between the nature of SRH communication and the gender of the individuals involved may have different effects on communication outcomes [33]. Therefore, robust and well-designed future research on the determinants of parent-child SRH communication’s positive effects on healthy behavior practices and HIV prevention among young adolescents is recommended.
Strengths and limitations
The strength of this study is that it is the first-ever study to provide information about the prevalence of HIV testing and associated factors among young adolescents in Eswatini and probably in the low- and middle-income countries highly burdened by HIV. The findings of this study can inform policymakers to plan timely and effective interventions that will accelerate remarkable progress of HIV testing coverage among young adolescents. However, this study also had some limitations. First, this study is that it only focused on individual factors that influence HIV testing among young adolescents. However, to achieve maximum HIV testing coverage, there is a need to apply ecological approaches of integrating other factors, including environmental, community, organizational, and societal level factors, which were not addressed in this research. Hence, future research must consider examining the influence of such factors on HIV testing uptake among young adolescents. Second, this analysis did not present a comprehensive model for factors related to HIV testing uptake due to the lack of data that could be explained by sociocultural factors of Eswatini, a high HIV-burdened country, such as parent’s HIV status, information from parents/guardians regarding previous children’s HIV testing, and the lack of information about the barriers of HIV testing among young adolescents. Future research needs to consider the application of a tested and validated conceptual model when examining the factors associated with HIV testing among young adolescents and across different age groups.
Conclusion
This study shows that HIV testing among young adolescents in Eswatini is significantly lower than the global targets. Our findings highlighted some important factors associated with HIV testing among young adolescents, including increasing age, urban residence, and higher awareness of the ease of access to testing services. International organizations, national policymakers, and healthcare professionals need to carefully consider these factors and other differentiated approaches that cater to differences in personal characteristics and external resources when developing appropriate strategies to reduce possible gaps in receiving HIV tests. Additionally, primary research on the barriers to HIV testing is recommended to expand understanding and planning of programs to alleviate barriers and further research to provide more data about the prevalence of HIV testing and gender-specific associated factors among young adolescents. Age-and-gender specific, practical, and culturally appropriate strategies should be considered when developing interventions to enhance the uptake of HIV testing among young adolescents.
Supplementary Information
Acknowledgments
We would like to thank the children and parents who participated in SHIMS 2 for their time and insights. The SHIMS 2 national survey was funded by the Centers for Disease Control and Prevention, USA. Also, acknowledge the support and hard work from all the authors of this study, and extend our gratitude to Brain Korea 21 Plus Program and Samsung Dream Foundation for their continued support.
Abbreviations
- AFHS
Adolescent-Friendly Health Services
- AIDS
Acquired Immunodeficiency Syndrome
- ART
Anti-Retroviral Therapy
- CI
Confidence interval
- EA
Enumeration Areas
- HIV
Human Immunodeficiency Virus
- OR
Odds Ratio
- PHIA
Population-based HIV Impact Assessment
- PLHIV
People Living with HIV
- SHIMS
Swaziland HIV Incidence Measurement Survey
- SRH
Sexual Reproductive Health
- UNAIDS
Joint United Nations Programme on HIV/AIDS
- UNICEF
United Nations Children’s Fund
Authors’ contributions
Mi Sook Jung: involved in planning, supervising the findings of this work, and was involved in compiling the final draft. Nondumiso S. Dlamini: came up with the idea and worked on statistical analyses and writing the manuscript. Xirong Cui: contributed to the writing of the manuscript. Kyeongin Cha: contributed to data analysis and presentation of findings. All authors discussed the results and contributed to the final manuscript. The author(s) read and approved the final manuscript.
Funding
The authors did not receive any specific grant from the public, commercial, or not-for-profit funding agencies for this article’s analysis, authorship, and publication. This work was supported by research fund of Chungnam National University (2022–0710-01).
Availability of data and materials
The datasets used and analyzed in the current study are available upon request from the corresponding author.
Declarations
Ethics approval and consent to participate
All methods were performed in compliance with relevant guidelines and regulations, and the study was conducted in accordance with the Declaration of Helsinki. SHIMS-2 is a part of a multicounty Population-based HIV Impact Assessment (PHIA) project approved by the Eswatini National Health Research Review Board, Westat Institutional Review Board (IRB), the Center of Disease Control, and the Columbia University Medical Center Institutional Review Board. This study is a secondary data analysis, therefore, the need for informed consent was waived by Chungnam National University ethics committee, reference number: 202106-BR-102-01.
Consent for publication
This study is a secondary data analysis, so consent for publication was not required. However, permission to use the SHIMS 2 data for this research was sought and approved by the project manager.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Oginni AB, Adebajo SB, Ahonsi BA. Trends and determinants of comprehensive knowledge of HIV among adolescents and young adults in Nigeria: 2003-2013. Afr J Reprod Health. 2017;21(1):26–34. doi: 10.29063/ajrh2017/v21i2.4. [DOI] [PubMed] [Google Scholar]
- 2.Kurth AE, Lally MA, Choko AT, Inwani IW, Fortenberry JD. HIV testing and linkage to services for youth. J Int AIDS Soc. 2015;18:19433. doi: 10.7448/IAS.18.2.19433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.UNAIDS . Understanding Fast-Track: accelerating action to end the AIDS epidemic by 2030. 2015. [Google Scholar]
- 4.UNAIDS . UNAIDS Data. 2020. [Google Scholar]
- 5.Marsh K, Eaton JW, Mahy M, Sabin K, Autenrieth CS, Wanyeki I, et al. Global, regional and country-level 90–90–90 estimates for 2018: assessing progress towards the 2020 target. AIDS (London, England) 2019;33(Suppl 3):S213. doi: 10.1097/QAD.0000000000002355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.UNAIDS . Understanding Fast-Track: accelerating action to end the AIDS epidemic by 2030. 2015. [Google Scholar]
- 7.The Global Fund. Results Report 2020 2020 [Available from: https://www.theglobalfund.org/media/10103/corporate_2020resultsreport_report_en.pdf. Accessed 23 Aug 2022.
- 8.Idele P, Gillespie A, Porth T, Suzuki C, Mahy M, Kasedde S, et al. Epidemiology of HIV and AIDS among adolescents: current status, inequities, and data gaps. JAIDS J Acquired Immune Deficiency Syndr. 2014;66:S144–S153. doi: 10.1097/QAI.0000000000000176. [DOI] [PubMed] [Google Scholar]
- 9.UNAIDS. Global HIV & AIDS statistics — 2020 fact sheet. Geneva. Available from: https://www.unaids.org/en/resources/fact-sheet. Accessed 21 Apr 2020
- 10.Ministry of Health Kingdom of Swaziland. Swaziland Integrated HIV Management Guidelines 2018. [Available from: http://swaziaidsprogram.org/wp-content/uploads/2021/07/2018-Integrated-HIV-Management-Guidelines_final-1.pdf. Accessed 22 Aug 2022.
- 11.UNICEF. Monitoring the situation of children and women: Adolescent HIV prevention. 2021. Retrieved April 27, 2022, Available from: https://data.unicef.org/topic/hivaids/adolescents-young-people/. Accessed 27 Apr 2022.
- 12.Sam-Agudu NA, Folayan MO, Ezeanolue EE. Seeking wider access to HIV testing for adolescents in sub-Saharan Africa. Pediatr Res. 2016;79(6):838–845. doi: 10.1038/pr.2016.28. [DOI] [PubMed] [Google Scholar]
- 13.Teasdale CA, Zimba R, Abrams EJ, Sachathep K, Ndagije F, Nuwagaba-Biribonwoha H, et al. Estimates of the prevalence of undiagnosed HIV among children living with HIV in Eswatini, Lesotho, Malawi, Namibia, Tanzania, Zambia, and Zimbabwe from 2015 to 2017: an analysis of data from the cross-sectional Population-based HIV Impact Assessment surveys. Lancet HIV. 2022;9(2):e91–101. doi: 10.1016/S2352-3018(21)00291-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.UNICEF . Children and AIDS: Statistical update. 2017. [Google Scholar]
- 15.UNAIDS. Global AIDS Update 2020: Seizing the moment. Geneva, UNAIDS; 2020. Available from: https://www.unaids.org/en/resources/documents/2020/global-aids-report. Accessed 23 May 2022
- 16.Jerene D, Abebe W, Taye K, Ruff A, Hallstrom I. Adolescents living with HIV are at higher risk of death and loss to follow up from care: analysis of cohort data from eight health facilities in Ethiopia. PLoS One. 2019;14(10):e0223655. doi: 10.1371/journal.pone.0223655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Govindasamy D, Ferrand RA, Wilmore SM, Ford N, Ahmed S, Afnan-Holmes H, et al. Uptake and yield of HIV testing and counselling among children and adolescents in sub-Saharan Africa: a systematic review. J Int AIDS Soc. 2015;18(1):20182. doi: 10.7448/IAS.18.1.20182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alhasawi A, Grover SB, Sadek A, Ashoor I, Alkhabbaz I, Almasri S. Assessing HIV/AIDS knowledge, awareness, and attitudes among senior high school students in Kuwait. Med Princ Pract. 2019;28(5):470–476. doi: 10.1159/000500307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ong’wen P, Samba BO, Moghadassi M, Okoko N, Bukusi EA, Cohen CR, et al. Chain Peer Referral Approach for HIV Testing Among Adolescents in Kisumu County, Kenya. AIDS Behav. 2020;24(2):484–490. doi: 10.1007/s10461-019-02560-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Asaolu IO, Gunn JK, Center KE, Koss MP, Iwelunmor JI, Ehiri JE. Predictors of HIV testing among youth in sub-Saharan Africa: a cross-sectional study. PLoS One. 2016;11(10):e0164052. doi: 10.1371/journal.pone.0164052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.UNICEF. Global and regional trends 2021 [Available from: https://data.unicef.org/topic/hivaids/global-regional-trends/. Accessed 22 Aug 2022.
- 22.Ssebunya RN, Wanyenze RK, Namale L, Lukolyo H, Kisitu GP, Nahirya-Ntege P, et al. Prevalence and correlates of HIV testing among adolescents 10–19 years in a post-conflict pastoralist community of Karamoja region, Uganda. BMC Public Health. 2018;18(1):1–8. doi: 10.1186/s12889-018-5544-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Djibuti M, Zurashvili T, Kasrashvili T, Berg CJ. Factors associated with HIV counseling and testing behavior among undergraduates of universities and vocational technical training schools in Tbilisi, Georgia. BMC Public Health. 2015;15(1):1–9. doi: 10.1186/s12889-015-1760-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Agbemenu K, Hannan M, Kitutu J, Terry MA, Doswell W. "Sex will make your fingers grow thin and then you die": The interplay of culture, myths, and taboos on African immigrant mothers' perceptions of reproductive health education with their daughters aged 10–14 years. J Immigr Minor Health. 2018;20(3):697–704. doi: 10.1007/s10903-017-0675-4. [DOI] [PubMed] [Google Scholar]
- 25.Yumo HA, Kuaban C, Ajeh RA, Nji AM, Nash D, Kathryn A, et al. Active case finding: comparison of the acceptability, feasibility and effectiveness of targeted versus blanket provider-initiated-testing and counseling of HIV among children and adolescents in Cameroon. BMC Pediatr. 2018;18(1):1–9. doi: 10.1186/s12887-018-1276-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Madiba S, Mokgatle M. Fear of stigma, beliefs, and knowledge about HIV are barriers to early access to HIV testing and disclosure for perinatally infected children and adolescents in rural communities in South Africa. S Afr Fam Pract. 2017;59(5):175–181. doi: 10.1080/20786190.2017.1329489. [DOI] [Google Scholar]
- 27.Tsegay G, Edris M, Meseret S. Assessment of voluntary counseling and testing service utilization and associated factors among Debre Markos University Students, North West Ethiopia: a cross-sectional survey in 2011. BMC Public Health. 2013;13(1):1–7. doi: 10.1186/1471-2458-13-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.UNICEF. Collecting and reporting of sex- and age-disaggregated data on adolescents at the sub-national level. New York; 2016. Available from: hhttps://childrenaids.org/collecting-reporrting-sex-agedisaggregated-data. Accessed 20 Aug 2021
- 29.Swaziland HIV Incidence Measurement Survey 2. Government of the Kingdom of Eswatini. Final report. 2019 Mbabane, Eswatini. (2016-2017). Available from: https://phia.icap.columbia.edu/wp-content/uploads/2019/05/SHIMS2_Final-Report_05.03.2019_forWEB.pdf. Accessed 20 Apr 2020.
- 30.Rucker DD, McShane BB, Preacher KJ. A researcher's guide to regression, discretization, and median splits of continuous variables. J Consum Psychol. 2015;25(4):666–678. doi: 10.1016/j.jcps.2015.04.004. [DOI] [Google Scholar]
- 31.MacCallum RC, Zhang S, Preacher KJ, Rucker DD. On the practice of dichotomization of quantitative variables. Psychol Methods. 2002;7(1):19. doi: 10.1037/1082-989X.7.1.19. [DOI] [PubMed] [Google Scholar]
- 32.Badru T, Mwaisaka J, Khamofu H, Agbakwuru C, Adedokun O, Pandey SR, et al. HIV comprehensive knowledge and prevalence among young adolescents in Nigeria: evidence from Akwa Ibom AIDS indicator survey, 2017. BMC Public Health. 2020;20(1):1–0. doi: 10.1186/s12889-019-7890-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bastien S, Kajula LJ, Muhwezi WW. A review of studies of parent-child communication about sexuality and HIV/AIDS in sub-Saharan Africa. Reprod Health. 2011;8(1):1–7. doi: 10.1186/1742-4755-8-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Balaji AB, Oraka E, Fasula AM, Jayne PE, Carry MG, Raiford JL. Association between parent–adolescent communication about sex-related topics and HIV testing, United States. 2006–2013. AIDS Care. 2017;29(3):344–349. doi: 10.1080/09540121.2016.1238443. [DOI] [PubMed] [Google Scholar]
- 35.Langkamp DL, Lehman A, Lemeshow S. Techniques for handling missing data in secondary analyses of large surveys. Acad Pediatr. 2010;10(3):205–210. doi: 10.1016/j.acap.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Onyechi KC, Eseadi C, Okere AU, Otu MS. Effects of rational-emotive health education program on HIV risk perceptions among in-school adolescents in Nigeria. Medicine. 2016;95(29):e3967. [DOI] [PMC free article] [PubMed]
- 37.Price JT, Rosenberg NE, Vansia D, Phanga T, Bhushan NL, Maseko B, et al. Predictors of HIV, HIV risk perception, and HIV worry among adolescent girls and young women in Lilongwe, Malawi. J Acquired Immune Deficiency Syndr (1999) 2018;77(1):53. doi: 10.1097/QAI.0000000000001567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Armstrong A, Baggaley R, Ferguson J, Van der Kwaak A, Wolmarans L. The voices, values and preference of adolescents on HIV testing and counselling. Geneva: World Health Organization (WHO); 2013. [Google Scholar]
- 39.Kidman R, Waidler J, Palermo T. Uptake of HIV testing among adolescents and associated adolescent-friendly services. BMC Health Serv Res. 2020;20(1):1–0. doi: 10.1186/s12913-020-05731-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.WHO . Making health services adolescent friendly: Developing national quality standards for adolescent friendly health services. Geneva: World Health Organization; 2012. [Google Scholar]
- 41.Widman L, Choukas-Bradley S, Noar SM, Nesi J, Garrett K. Parent-adolescent sexual communication and adolescent safer sex behavior: A meta-analysis. JAMA Pediatr. 2016;170(1):52–61. doi: 10.1001/jamapediatrics.2015.2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Obiezu-Umeh C, Nwaozuru U, Shato T, Mason S, Carter V, Manu S, David A, Iwelunmor J. Implementation strategies to enhance youth-friendly sexual and reproductive health services in sub-Saharan Africa: a systematic review. In CUGH 2021 Virtual Conference 2021. CUGH. [DOI] [PMC free article] [PubMed]
- 43.Widman L, Choukas-Bradley S, Helms SW, Golin CE, Prinstein MJ. Sexual communication between early adolescents and their dating partners, parents, and best friends. J Sex Res. 2014;51(7):731–741. doi: 10.1080/00224499.2013.843148. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and analyzed in the current study are available upon request from the corresponding author.


