Abstract
Metallic Li is one of the most promising anodes for high‐energy secondary batteries. However, the enormous volume changes and severe dendrite formation during the Li plating/stripping process hinder the practical application of Li metal anodes (LMAs). We have developed a sulfate‐assisted strategy to synthesize a self‐standing host composed of N,S‐doped porous carbon nanobelts embedded with MoS2 nanosheets (MoS2@NSPCB) for use in LMAs. In situ measurements and theoretical calculations reveal that the uniformly distributed MoS2 derivatives within the carbon nanobelts serve as stable lithiophilic sites which effectively homogenize Li nucleation and suppress dendrite formation. In addition, the hierarchical porosity and 3D nanobelt networks ensure fast Li‐ion diffusion and accommodate the volume change of Li deposits during the plating/stripping process. As a result, a Li–Li symmetric cell using the MoS2@NSPCB host operates steadily over 1500 h with an ultralow voltage hysteresis (≈24.2 mV) at 3 mA cm−2/3 mAh cm−2. When paired with a LiFePO4 cathode, the current collector‐free LMA endows the full cell with a high energy density of 460 Wh kg−1 and good cycling performance (with a capacity retention of ≈70% even after 1600 cycles at 10 C).
Keywords: carbon nanobelts, heteroatom doping, Li metal anode, lithiophilic site, self‐standing host
N,S‐doped porous carbon nanobelts embedded with MoS2 nanosheets were prepared by a simple sulfate‐assistant method, showing superior performance as a self‐standing host for dendrite‐free lithium metal anodes in half cells and full cells. The modification mechanism of the host was detailedly investigated and revealed by in situ measurements combined with theoretical calculations.
1. Introduction
The energy densities of Li‐ion batteries based on graphite anodes cannot meet the ever‐increasing energy demands of electric vehicles and power stations.[ 1 , 2 ] Metallic Li is considered an ideal anode material for high‐energy rechargeable batteries because of its ultrahigh theoretical specific capacity (3860 mAh g−1) and ultralow electrochemical potential (−3.04 V vs standard hydrogen electode).[ 3 ] Unfortunately, the practical application of Li metal anodes (LMAs) involves two major challenges—undesirable dendrite formation and enormous volume change—which continuously decompose the electrolyte by destroying/rebuilding the solid electrolyte interface (SEI), and can even cause a short circuit leading to potential safety problems.[ 4 , 5 , 6 , 7 ]
Many strategies have been proposed to improve LMAs, including designing liquid/solid electrolytes, constructing artificial SEIs, and introducing 3D hosts.[ 8 , 9 , 10 , 11 ] Prestoring Li in 3D hosts can efficiently regulate the electric fields governing Li‐ion transport resulting in uniform current distributions for Li nucleation and growth.[ 12 , 13 , 14 , 15 ] Carbon‐based frameworks are ideal hosts for high‐performance LMAs because of their excellent electrical conductivity and high electrochemical stability.[ 16 ] However, the lithiophobic nature of carbon materials results in high barriers for Li nucleation, resulting in an inability to regulate subsequent Li growth.[ 17 ] Although a variety of metal and non‐metal atoms and their compounds have been introduced into a carbon matrix in an attempt to change the surface chemistry of the carbon‐based hosts,[ 18 , 19 , 20 ] some issues remain unresolved: i) complicated preparation procedures are usually required to ensure the efficient introduction of lithiophilic sites; ii) how to balance the conflicting requirements of high structural stability and high surface activity of lithiophilic sites; iii) hosts having both abundant lithiophilic sites and good mechanical properties are difficult to fabricate; iv) mechanisms of host modification in LMAs are not well understood.
We report the use of a simple sulfate‐assisted method to fabricate a new self‐standing host consisting of N,S‐doped carbon nanobelts with hierarchical porosity impregnated with MoS2 nanosheets (denoted as MoS2@NSPCB). The MoS2@NSPCB host has abundant metal/non‐metal lithiophilic sites facilitating uniform Li nucleation. Additionally, the hierarchical porosity and N,S‐doped carbon support endow the lithiophilic sites with good structural stability as well as high surface chemical activity. Moreover, the 3D conductive nanobelt networks ensure fast Li‐ion diffusion, preventing the formation of dendrites in the LMA during the plating/stripping process. LMAs based on the MoS2@NSPCB host show excellent performance in half cells. Furthermore, the current collector‐free LMAs endow full cells with high energy density, long cycling and fast‐charge/discharge performance.
2. Results and Discussion
Figure 1a shows a schematic illustration of the synthesis for the MoS2@NSPCB host. An aqueous solution of gelatin containing Na2SO4 and ammonium molybdate ((NH4)6Mo7O24·4H2O) was used to electrospin films, which were then pyrolyzed and washed with deionized water. In contrast to previously reported gelatin‐derived carbon films, the MoS2@NSPCB film shows superb flexibility as a self‐standing host (Figure 1b). Two other gelatin‐derived carbon samples were prepared using the same procedure, one without Na2SO4 and the other without ammonium molybdate. As shown in Figure S1 (Supporting Information), the former (denoted as MoO x @NCF) is also a self‐standing film, while the latter (denoted as NSPCB) is easily broken into small pieces, indicating that the introduction of ammonium molybdate improves the mechanical strength of the final samples due to the coordination between Mo centers and functional groups in gelatin.[ 21 , 22 ]
Figure 1.
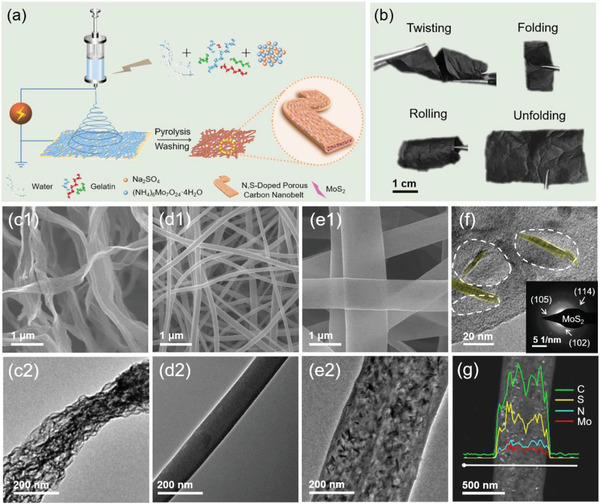
a) Schematic illustration of the synthesis of MoS2@NSPCB films. b) Digital photos showing the flexibility of the MoS2@NSPCB film in various states, including twisting, rolling, and folding. SEM images of (c1) NSPCB, (d1) MoOx@NCF, and (e1) MoS2@NSPCB. TEM images of (c2) NSPCB, (d2) MoOx@NCF, and (e2) MoS2@NSPCB. f) High‐resolution TEM image (inset: selected area electron diffraction pattern) and g) EDX linear scanning analysis of MoS2@NSPCB.
X‐ray diffraction (XRD) and Raman spectroscopy confirm the presence of gelatin‐derived carbons in all three samples (Figure S2, Supporting Information). Characteristic peaks of MoS2 are observed in the XRD pattern and Raman spectrum of MoS2@NSPCB, while no characteristic peaks of Mo compounds are shown in those of MoO x @NCF. Due to the similar weight content of noncarbon materials for MoO x @NCF and MoS2@NSPCB (≈40%, Figure S3, Supporting Information), the metallic compound within MoOx@NCF is considered to have an amorphous structure. X‐ray photoelectron spectroscopy (XPS, Figure S4, Supporting Information) confirms that MoO x and MoS2 are present in MoO x @NCF and MoS2@NSPCB, respectively.[ 23 ] This suggests that Na2SO4 acts as a sulfurizing reagent and reacts with molybdate ions to form MoS2. N species are observed in the XPS spectra of all the samples and additional S species are observed in the XPS spectra of NSPCB and MoS2@NSPCB, suggesting that Na2SO4 also plays a role as a dopant by introducing S heteroatoms into the carbon framework.
The addition of Na2SO4 not only affects the chemical composition but also regulates the structural morphology of the samples. Scanning electron microscopy (SEM) results (Figure 1c1–e1) show that NSPCB and MoS2@NSPCB have ultrathin nanobelt structures, while MoO x @NCF has a nanofiber structure. This suggests that Na2SO4 can influence the rheological or other properties of the solution and favor the formation of a nanobelt structure during the electrospinning process (Figure S5, Supporting Information). Transmission electron microscopy (TEM) images show that obvious pores exist in NSPCB and MoS2@NSPCB, while no pores can be observed in MoOx@NCF (Figure 1c2–e2). N2 adsorption/desorption measurements verify the hierarchical porosities of NSPCB and MoS2@NSPCB and the non‐porous structure of MoO x @NCF, suggesting that Na2SO4 also acts as a template for nanopore formation (Figure S6, Supporting Information). Well‐dispersed nanosheets are observed in MoS2@NSPCB (Figure 1f; and Figure S7, Supporting Information), which are confirmed to be MoS2 by selected area electron diffraction (Figure 1f, inset).[ 24 , 25 ] It should be noted that these MoS2 nanosheets are embedded within the porous carbon nanobelts since they are unobservable in the SEM image. Energy dispersive X‐ray (EDX) results of MoS2@NSPCB are displayed in Figure 1g; and Figure S7d (Supporting Information). The linear scanning curves exhibit a mesa‐like shape, characteristic of a nanobelt structure. The high dispersion of C, N, and S in the elemental mapping images indicate that N/S atoms are doped in the carbon nanobelts, consistent with the XPS results.
The self‐standing films of MoS2@NSPCB and MoOx@NCF were used as hosts for LMAs. Li|Cu, Li|MoO x @NCF, and Li|MoS2@NSPCB half‐cells were assembled in order to compare the Li plating/stripping behaviors. As shown in Figure 2a, the MoS2@NSPCB host displays the smallest overpotential for Li nucleation and plating in the initial cycle, corresponding to the lowest nucleation barrier and fastest Li‐ion migration.[ 15 ] Moreover, the Li|MoS2@NSPCB cell performs the highest Coulombic efficiency of all the cells with a good cycling performance over 350 cycles at 0.5 mA cm−2 for 0.5 mAh cm−2 (Figure 2b). An average Coulombic efficiency of ≈99% was obtained at 1 mA cm−2 for 1 mAh cm−2 (Figure S8, Supporting Information), indicative of the high reversibility of Li plating/stripping in the MoS2@NSPCB host. Symmetric cells were assembled to evaluate the galvanostatic cyclic performance and the interfacial stability of the LMAs with and without the hosts. At a current of 1 mA cm−2 for 1 mAh cm−2, a Li|Li symmetric cell using the MoS2@NSPCB host shows a stable cycling life over 2000 h with an ultralow voltage hysteresis of ≈22.7 mV (Figure 2c). In contrast, Li|Li symmetric cells with the MoOx@NCF host and Cu foil display high voltage hysteresis of ≈73.0 and ≈53.0 mV, respectively (Figure S9, Supporting Information). A short‐circuit eventually occurred after ≈65 h, causing a sudden drop in the voltage hysteresis.
Figure 2.
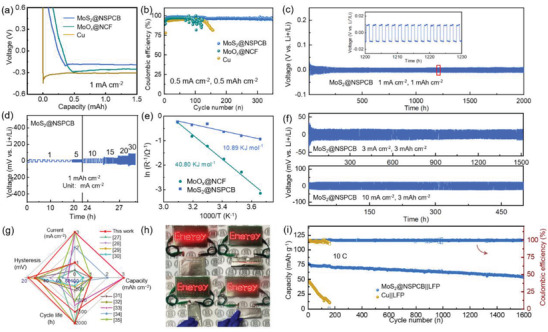
a) The voltage−capacity curves in the initial cycle and b) Coulombic efficiency (the initial cycle is not shown) of Li|Cu, Li|MoO x @NCF, and Li|MoS2@NSPCB half cells. c,d) Galvanostatic cycling performance of Li|Li symmetric cells using MoS2@NSPCB hosts at different current densities. e) Activation energy for Li‐ion diffusion with the MoS2@NSPCB and MoO x @NCF hosts. f) Galvanostatic cycling performance of Li|Li symmetric cells using MoS2@NSPCB hosts with plating/stripping capacity of 3 mAh cm−2. g) Comparison of the electrochemical performance of Li|Li symmetric cells using the MoS2@NSPCB host with the cells using other hosts. h) Digital photos of a MoS2@NSPCB‐Li|LiFePO4 pouch cell powering LED lights in different states. i) Cycling performance of the MoS2@NSPCB‐Li|LiFePO4 and Cu‐Li|LiFePO4 full cells.
The MoS2@NSPCB host also endows the Li|Li cell with an excellent rate performance (Figure 2d), with stable working even at 30 mA cm−2 with a low voltage overpotential below 100 mV. Electrochemical impedance spectroscopy (EIS) was employed to investigate the kinetics of charge transfer and Li‐ion diffusion (Figure S10, Supporting Information). The Li|MoS2@NSPCB cell shows a much smaller charge‐transfer resistance (5.5 Ω) than the Li|MoO x @NCF cell (27.2 Ω) at 30 °C, demonstrating the enhanced charge transport at the electrode/electrolyte interface. The activation energies for Li‐ion diffusion within the different hosts were calculated based on the Arrhenius plots (Figure 2e).[ 26 ] The Li|MoS2@NSPCB cell has a much smaller activation energy of 10.89 kJ mol−1 than the Li|MoOx@NCF cell (40.80 kJ mol−1), suggesting that the continuous structure of the hierarchical porous nanobelts in the MoS2@NSPCB host allows faster Li‐ion diffusion. By virtue of the high porosity and controllable thickness, the MoS2@NSPCB host enables the Li|Li symmetric cells to show good performance, even at a plating/stripping capacity of 3 mAh cm−2 (Figure 2f). Long‐term stabilities of over 1500 and 500 h were obtained at 3 and 10 mA cm−2, respectively. The half‐cell performance of the Li|Li symmetric cells using the MoS2@NSPCB host is superior to similar cells using other hosts reported in the recent literature (Figure 2g; and Table S2, Supporting Information).[ 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 ]
Full cells were assembled using LiFePO4 (LFP) as the cathode in order to evaluate the practical performance of the MoS2@NSPCB host‐modified LMA (MoS2@NSPCB‐Li). The N/P ratio and electrolyte amount were fixed at 1:1 and ≈3.7 µL mg−1, respectively. The galvanostatic charge–discharge (GCD) profiles (Figure S11, Supporting Information) indicate that MoS2@NSPCB‐Li||LFP exhibits a smaller voltage polarization than Cu‐Li||LFP (≈63.0 mV vs ≈90.0 mV at 1 C), highlighting the faster kinetics of the MoS2@NSPCB host. A high reversible capacity of ≈80 mAh g−1 is maintained even at 10 C, showing good rate capability. The MoS2@NSPCB‐Li||LFP full cell can deliver a high energy of ≈460 Wh kg−1 based on the total weight of LFP and MoS2@NSPCB‐Li. Even after considering the weight of the current collectors, the MoS2@NSPCB‐Li||LFP full cell can deliver an energy density of 348 Wh kg−1, much higher than that of the Cu‐Li||LFP full cell (246 Wh kg−1). The MoS2@NSPCB‐Li||LFP pouch cell can easily power 68 LEDs and showed good bending performance by virtue of the self‐standing properties of the MoS2@NSPCB host (Figure 2h). Moreover, the MoS2@NSPCB‐Li||LFP full cell shows a remarkable cycling performance with a capacity retention of ≈70% even after 1600 cycles at 10 C (Figure 2i).
The mechanism responsible for the modification of the MoS2@NSPCB host in LMAs was investigated using in situ Raman and XRD measurements on half cells. As shown in Figure 3a,b, MoS2 is gradually lithiated, initially giving Li x MoS2, which is finally transformed into Li2S and Mo during the discharge process (MoS2 + 4Li+ + 4e−→ Mo + 2Li2S). The characteristic peaks of Li2S and Mo are observed at low potential during the discharge process and the subsequent charge process, confirming their good stability as the active sites for regulating Li plating/stripping (Figure S14, Supporting Information). In addition, the high degree of recovery of the D and G bands in the Raman spectra suggests good reversibility of the change in the N,S‐doped carbon framework. TEM images and the corresponding selected area electron diffraction results of the cycled MoS2@NSPCB host further verify that the MoS2 derivatives are uniformly anchored on the nanobelts with good structural integrity.
Figure 3.
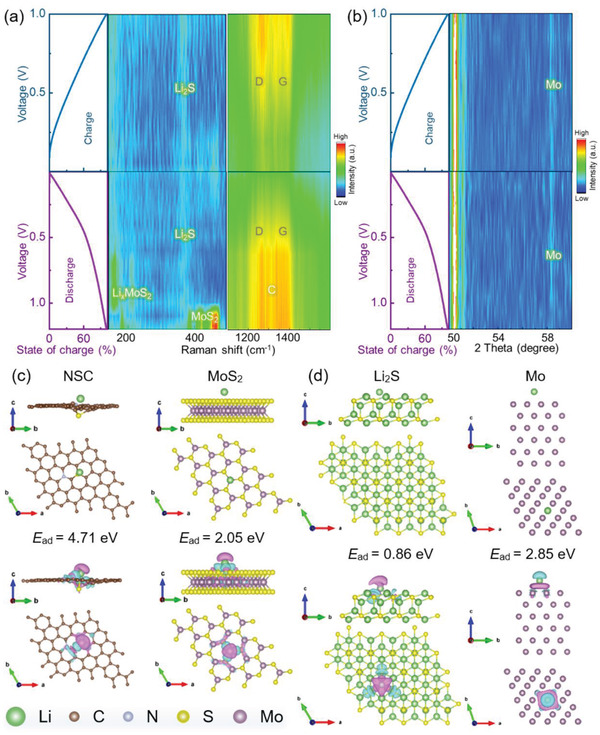
a) Galvanostatic charge–discharge (GCD) plots of the MoS2@NSPCB host at 0.1 A g−1 and the corresponding in situ Raman spectra. b) GCD plots of the MoS2@NSPCB host at 0.1 A g−1 and the corresponding in situ XRD patterns. Top and side views of different geometries, real‐space charge densities, and E ad values of Li atoms on c) NSC, MoS2, d) Li2S, Mo. The purple and blue colors, respectively, represent decreases and increases in the charge densities.
DFT calculations were used to probe the lithiophilicity and stability of the active sites within the MoS2 @NSPCB host. Models of Li atoms adsorbed on N,S‐doped carbon (NSC), MoS2, Li2S, and Mo were constructed, and the optimized results are displayed in Figure 3c,d. The calculated adsorption energies (E ad) of NSC and MoS2 are 4.71 and 2.05 eV, respectively, indicating that the N/S heteroatoms and MoS2 are good lithiophilic sites that can adsorb Li ions on the interface between the electrode and electrolyte in the initial cycle. As compared with N/S heteroatoms, Li2S and Mo show a lower E ad of 0.86 and 2.85 eV, respectively. The moderate Li adsorption of these materials is beneficial to both Li plating and stripping because of the low nucleation/diffusion barriers of Li and good chemical stability to Li.[ 36 ] Although the N,S doping is not favorable for Li diffusion and striping, it enables good carbon support which firmly anchors the ideal lithiophilic sites (MoS2 derivatives). The structural stability of the lithiophilic sites was further revealed by comparing the E ad of active sites on a pure carbon support (C) and NSC (Figure S15, Supporting Information). The N,S‐doped carbon has a higher E ad than the undoped carbon, confirming that the lithiophilic sites in the MoS2@NSPCB host have both high surface activity and good structural stability, thus efficiently guiding uniform Li nucleation and facilitating highly reversible Li plating/stripping.[ 37 ]
In situ optical microscopy was further performed to investigate the inhibitory effects of the hosts on Li dendrites. As shown in Figure 4a, Li dendrites formed quickly on the Cu surface, resulting in a highly porous structure with a large volume change. Although Li dendrites were not observed on the MoO x @NCF host in the initial plating process, obvious volumetric expansion and surface Li dendrite formation were eventually observed due to the limited space for Li growth (Figure 4b). In contrast, a smooth surface without any observable dendrites was observed for the MoS2@NSPCB host throughout the entire deposition process (Figure 4c). This shows that the self‐standing MoS2@NSPCB host with its abundant lithiophilic sites and 3D porous structure favors homogeneous Li plating and suppresses the formation of Li dendrites.
Figure 4.
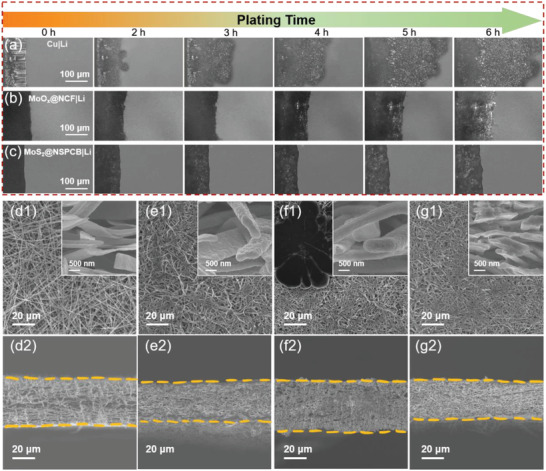
In situ optical microscopy of Li plating behavior on a) Cu foil, b) MoOx@NCF, and (c) MoS2@NSPCB at 1 mA cm–2 for 6 h. Ex situ surface and cross‐section SEM images of the MoS2@NSPCB hosts: (d1, d2) before Li plating, (e1, e2) after Li plating with a capacity of 3 mAh cm–2 at 1 mA cm–2, (f1, f2) after Li plating with a capacity of 6 mAh cm–2 at 1 mA cm–2, and (g1, g2) after Li stripping with a capacity of 6 mAh cm–2 at 1 mA cm–2.
Ex situ SEM images for different Li plating/stripping states clearly show the morphological evolution of the MoS2@NSPCB host. As shown in Figure 4d, the host has an initial thickness of ≈40 µm with an ultrathin nanobelt structure; After a deposition capacity of 3 mAh cm−2, the host thickness of MoS2@NSPCB decreases due to the pressure within the cells, while the compactness of the host increases significantly (Figure 4e, 2). In addition, the thickness of the nanobelt increases from ≈100 to ≈300 nm, showing the uniform Li plating (Figure 4e, 1, inset). At a higher deposition capacity of 6 mAh cm−2, the host thickness increases slightly, and some Li deposits with an island‐like structure are observed on the host surface (Figure 4f). The nanobelt still exhibits a smooth surface with a thickness of 500–600 nm (Figure 4f, inset). After Li stripping with a capacity of 6 mAh cm−2, the thickness of the host and nanobelt decreases to ≈30 and ≈100 nm, respectively (Figure 4g). The good structural stability and high Li‐plating/stripping reversibility of the MoS2@NSPCB host endow the LMAs with excellent cycling performance.
3. Conclusions
We have fabricated a self‐standing MoS2@NSPCB host for LMAs using an efficient sulfate‐assisted method. LMAs using the MoS2@NSPCB host remain dendrite‐free with good half‐cell and full‐cell performance. A detailed physicochemical study has shown that the N,S‐doped carbon nanobelts firmly anchor the MoS2‐derived lithiophilic sites, which homogenize the Li nucleation and suppress the formation of Li dendrites. In addition, the presence of a conductive 3D structure with hierarchical porosity accelerates Li‐ion diffusion and accommodates the volume changes of the Li deposits. This work has not only proposed a new self‐standing host for LMAs but also suggested new ideas for the modification of other metallic anodes.
4. Experimental Section
Synthesis of MoS2@NSPCB
An aqueous gelatin solution (15 wt%, 9 mL) was mixed with Na2SO4 (570 mg) and (NH4)6Mo7O24·4H2O (700 mg) by stirring at 60 °C for 1 h. The solution was used to electrospin films (ET2535X, Ucalery). The gelatin films obtained after electrospinning for 6 h were stabilized by heating at 270 °C for 1 h in air, followed by pyrolysis at 700 °C for 1 h under an argon atmosphere (heating rate: 2.5 °C min−1). The pyrolyzed samples were washed by water and dried overnight at 60 °C.
Synthesis of NSPCB and MoO x @NCF
The preparation procedures were similar to that for MoS2@NSPCB. NSPCB and MoO x @NCF were prepared without adding (NH4)6Mo7O24·4H2O and Na2SO4, respectively.
Conflict of Interest
The authors declare no conflict of interest.
Supporting information
Supporting Information
Acknowledgements
B.L. and W.C. contributed equally to this work. This work was supported by National Natural Science Foundation of China (Nos. 52130206, U20A20337, 22178016, 52003025, and 52002012), the Fundamental Research Funds for the Central Universities (No. buctrc202024, JD2211), and the high‐performance computing platform of BUCT.
Li B., Cao W., Wang S., Cao Z., Shi Y., Niu J., Wang F., N,S‐Doped Porous Carbon Nanobelts Embedded with MoS2 Nanosheets as a Self‐Standing Host for Dendrite‐Free Li Metal Anodes. Adv. Sci. 2022, 9, 2204232. 10.1002/advs.202204232
Contributor Information
Jin Niu, Email: niujin@mail.buct.edu.cn.
Feng Wang, Email: wangf@mail.buct.edu.cn.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. Fu C., Lin S., Zhao C., Wang J., Wang L., Bao J. L., Wang Y., Liu T., Energy Storage Mater. 2022, 45, 1109. [Google Scholar]
- 2. Wang C., Liu M., Thijs M., Ooms F. G. B., Ganapathy S., Wagemaker M., Nat. Commun. 2021, 12, 6536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hobold G. M., Lopez J., Guo R., Minafra N., Banerjee A., Shirley Y. M., Shao‐Horn Y., Gallant B. M., Nat. Energy 2021, 6, 951. [Google Scholar]
- 4. Cao J., Xie Y., Yang Y., Wang X., Li W., Zhang Q., Ma S., Cheng S., Lu B., Adv. Sci. 2022, 9, 2104689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wu Q. P., Zheng Y. J., Guan X., Xu J., Cao F. H., Li C. L., Adv. Funct. Mater. 2021, 31, 2101034. [Google Scholar]
- 6. Yang Q. F., Cui M. N., Hu J. L., Chu F. L., Zheng Y. J., Liu J. J., Li C. L., ACS Nano 2020, 14, 1866. [DOI] [PubMed] [Google Scholar]
- 7. Meng J. W., Li C. L., Energy Storage Mater. 2021, 37, 466. [Google Scholar]
- 8. Guo Z., Pang Y., Xia S., Xu F., Yang J., Sun L., Zheng S., Adv. Sci. 2021, 8, 2100899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kim S., Kim J. S., Miara L., Wang Y., Jung S. K., Park S. Y., Song Z., Kim H., Badding M., Chang J., Roev V., Yoon G., Kim R., Kim J. H., Yoon K., Im D., Kang K., Nat. Commun. 2022, 13, 1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Huang M., Yao Z., Yang Q., Li C., Angew. Chem., Int. Ed. 2021, 60, 14040. [DOI] [PubMed] [Google Scholar]
- 11. Auffarth S., Dafinger W., Mehler J., Ardizzon V., Preuster P., Wasserscheid P., Thiele S., Kerres J., J. Mater. Chem. A 2022, 10, 17208. [Google Scholar]
- 12. Fang Y., Zhang S. L., Wu Z. P., Luan D., Lou X. W., Sci. Adv. 2021, 7, eabg3626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lin H., Zhang Z., Wang Y., Zhang X. L., Tie Z., Jin Z., Adv. Funct. Mater. 2021, 31, 2102735. [Google Scholar]
- 14. Mei Y., Zhou J., Hao Y., Hu X., Lin J., Huang Y., Li L., Feng C., Wu F., Chen R., Adv. Funct. Mater. 2021, 31, 2106676. [Google Scholar]
- 15. Chen C., Guan J., Li N. W., Lu Y., Luan D., Zhang C. H., Cheng G., Yu L., Lou X. W., Adv. Mater. 2021, 33, 2100608. [DOI] [PubMed] [Google Scholar]
- 16. Kwon H., Lee J. H., Roh Y., Baek J., Shin D. J., Yoon J. K., Ha H. J., Kim J. Y., Kim H. T., Nat. Commun. 2021, 12, 5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang T. S., Liu X., Wang Y., Fan L. Z., Adv. Funct. Mater. 2021, 31, 2001973. [Google Scholar]
- 18. Deng Y., Gao J., Wang M., Luo C., Zhou C., Wu M., Energy Storage Mater. 2022, 48, 114. [Google Scholar]
- 19. Zhou T., Shen J., Wang Z., Liu J., Hu R., Ouyang L., Feng Y., Liu H., Yu Y., Zhu M., Adv. Funct. Mater. 2020, 30, 1909159. [Google Scholar]
- 20. Li K., Hu Z., Ma J., Chen S., Mu D., Zhang J., Adv. Mater. 2019, 31, 1902399. [DOI] [PubMed] [Google Scholar]
- 21. Liu Y., Wang S., Sun X., Zhang J., uz Zaman F., Hou L., Yuan C., Energy Environ. Mater. 2022, 10.1002/eem2.12263 [DOI] [Google Scholar]
- 22. Zhang B., Qin L., Fang Y., Chai Y., Xie X., Lu B., Liang S., Zhou J., Sci. Bull. 2022, 67, 955. [DOI] [PubMed] [Google Scholar]
- 23. Lee S. M., Kim J., Moon J., Jung K. N., Kim J. H., Park G. J., Choi J. H., Rhee D. Y., Kim J. S., Lee J. W., Park M. S., Nat. Commun. 2021, 12, 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nguyen D. C., Luyen Doan T. L., Prabhakaran S., Tran D. T., Kim D. H., Lee J. H., Kim N. H., Nano Energy 2021, 82, 105750. [Google Scholar]
- 25. Shan A., Teng X., Zhang Y., Zhang P., Xu Y., Liu C., Li H., Ye H., Wang R., Nano Energy 2022, 94, 106913. [Google Scholar]
- 26. Wang R., Yu J., Tang J., Meng R., Nazar L. F., Huang L., Liang X., Energy Storage Mater. 2020, 32, 178. [Google Scholar]
- 27. Qian Y., Zhu Z., Li Y., Pan Z., Wang L., Tian J., Zhou H., Lin N., Qian Y., Energy Storage Mater. 2022, 47, 620. [Google Scholar]
- 28. Wu J., Rao Z., Liu X., Shen Y., Fang C., Yuan L., Li Z., Zhang W., Xie X., Huang Y., Adv. Mater. 2021, 33, 2007428. [DOI] [PubMed] [Google Scholar]
- 29. Qian J., Wang S., Li Y., Zhang M., Wang F., Zhao Y., Sun Q., Li L., Wu F., Chen R., Adv. Funct. Mater. 2021, 31, 2006950. [Google Scholar]
- 30. Wei C., Fei H., Tian Y., An Y., Guo H., Feng J., Qian Y., Energy Storage Mater. 2020, 26, 223. [Google Scholar]
- 31. Gao P., Wu H., Zhang X., Jia H., Kim J. M., Engelhard M. H., Niu C., Xu Z., Zhang J. G., Xu W., Angew. Chem., Int. Ed. 2021, 60, 16506. [DOI] [PubMed] [Google Scholar]
- 32. Fang Y., Zeng Y., Jin Q., Lu X. F., Luan D., Zhang X., Lou X. W., Angew. Chem., Int. Ed. 2021, 60, 8515. [DOI] [PubMed] [Google Scholar]
- 33. He X., Jin S., Miao L., Cai Y., Hou Y., Li H., Zhang K., Yan Z., Chen J., Angew. Chem., Int. Ed. 2020, 59, 16705. [DOI] [PubMed] [Google Scholar]
- 34. Yang T., Li L., Zhao T., Ye Y., Ye Z., Xu S., Wu F., Chen R., Adv. Energy Mater. 2021, 11, 2102454. [Google Scholar]
- 35. Huang Z., Li Z., Zhu M., Wang G., Yu F., Wu M., Xu G., Dou S. X., Liu H. K., Wu C., Nano Lett. 2021, 21, 10453. [DOI] [PubMed] [Google Scholar]
- 36. Yu L., Su Q., Li B., Huang L., Du G., Ding S., Zhao W., Zhang M., Xu B., Chem. Eng. J. 2022, 429, 132479. [Google Scholar]
- 37. Li S. Q., Zhang L., Liu T. T., Zhang Y. W., Guo C., Wang Y., Du F. H., Adv. Mater. 2022, 34, 2201801. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


