Abstract
The potential of recombinant human interleukin-12 (IL-12) to enhance the capacity of human monocytes (MNC) to elicit an oxidative burst and damage hyphae of Aspergillus fumigatus was investigated. Incubation of peripheral blood mononuclear cells (PBMC) from healthy adults with 10 to 100 ng of IL-12/ml at 37°C for 2 to 3 days enhanced the production of superoxide anion (O2−) in response to phorbol myristate acetate (PMA) (P = 0.04) and unopsonized A. fumigatus hyphae (P = 0.03) and further enhanced hyphal damage (P = 0.009). Anti-gamma interferon (anti-IFN-γ) blocked secretion of IFN-γ by IL-12-treated PBMC but did not inhibit IL-12-induced O2− production by these cells in response to PMA. In addition, IL-12-treated elutriated MNC secreted no IFN-γ or tumor necrosis factor alpha but exhibited enhanced O2− production compared to controls (P = 0.013). These findings demonstrate that IL-12 augments oxidative antifungal activities of MNC via an IFN-γ-independent route, suggesting a novel pathway of IL-12 action in antifungal defense.
Invasive aspergillosis is a serious opportunistic fungal infection in immunocompromised hosts, including those with AIDS, patients undergoing antineoplastic chemotherapy, and those who have undergone organ transplantation (6). Despite recent advances in antifungal chemotherapy, invasive aspergillosis remains an important cause of morbidity and mortality in these patients. Aspergillus fumigatus is the most frequent species isolated.
Circulating phagocytes, including monocytes (MNC), possess a critical role in host defense against Aspergillus spp., damaging hyphae of the organism (8, 18). Oxidative mechanisms are predominantly involved in this process. On the other hand, lymphocytes also respond to Aspergillus spp. by producing cytokines, such as gamma interferon (IFN-γ), upon stimulation by the organism (10). However, the role of T helper 1-related immunoregulatory cytokines in host defense against A. fumigatus is incompletely understood (4).
Human interleukin-12 (IL-12) is a heterodimeric T helper 1-type cytokine composed of 40- and 35-kDa subunits which are produced by MNC, B lymphocytes, and other antigen-presenting cells in response to bacteria, fungi, and intracellular parasites (11, 21, 23). IL-12 was originally identified as a human Epstein-Barr virus-transformed B-cell line product (9) that stimulates T and NK lymphocytes to secrete IFN-γ (24). This pluripotent cytokine has multiple effects on T and NK lymphocytes as well as other cells of the immune system. These effects include increased secretion of cytokines, particularly IFN-γ (3, 5); enhancement of cytotoxic activity (20); and increased mitogen-induced cell proliferation (13).
IL-12 has been shown to have beneficial effects in treatment of murine candidiasis (16, 17). However, these beneficial effects have been thought to be mediated through its capacity to induce secretion of IFN-γ by T lymphocytes. We hypothesized that IL-12 might also mediate a direct effect on MNC in host defenses against invasive aspergillosis. To our knowledge, no previous studies have examined whether IL-12 has such an effect. We therefore investigated whether IL-12 directly regulates human mononuclear phagocytic host defenses against A. fumigatus, as evidenced by oxidative bursts (O2− production) of MNC, upon stimulation with phorbol myristate acetate (PMA) and unopsonized hyphae of A. fumigatus, as well as by monocyte-mediated damage of A. fumigatus hyphae.
(This study was presented in part at the 8th European Congress on Chemotherapy, Microbiology, and Infectious Diseases, Lausanne, Switzerland, 25 to 28 May 1997 [15a].)
MATERIALS AND METHODS
Preparation of effector cells.
MNC were studied as two preparations.
(i) Mixed PBMC.
Mixed peripheral blood mononuclear cells (PBMC) were isolated by Ficoll centrifugation of buffy coats from anticoagulated venous blood obtained from healthy adult volunteers at the Transfusion Medicine Department of Hippokration Hospital, Thessaloniki, Greece, as previously described (15). They were washed twice with Ca2+- and Mg2+-free Hanks buffered salt solution (HBSS) and resuspended in complete medium (CM) (RPMI 1640 supplemented with 10% fetal calf serum [Gibco], 100 U of penicillin/ml, and 100 μg of streptomycin/ml). The percentage of MNC was estimated by modified May-Grunwald-Giemsa staining. The viability of cells was greater than 95%, and approximately 25 to 45% of them were MNC. The concentration of MNC was adjusted to 5 × 106 per ml. Generally, these cell populations were approximately 30 to 40% CD14+ and nonspecific esterase positive. PBMC were used initially to detect indirect effects of IL-12 on MNC function through increased production of lymphokines.
(ii) EHM, >95% CD14+ and nonspecific esterase positive.
Elutriated human MNC (EHM) were isolated from blood of healthy adult donors by a two-step procedure consisting of automated leukapheresis and counterflow centrifugal elutriation at the Transfusion Medicine Department of Warren Grant Magnuson Clinical Center, National Institutes of Health, Bethesda, Md. (1). They were washed and resuspended in CM.
Organism.
Strain 4215 of A. fumigatus, isolated from a cancer patient with invasive pulmonary aspergillosis at the National Institutes of Health, was used in these studies. This strain was preserved on potato dextrose agar slants frozen at −70°C. Conidia were harvested by scraping the surfaces of the slants, suspended in phosphate-buffered saline, (PBS), filtered through sterile gauze, washed with PBS, and kept in PBS at 4°C as previously described (15). One day before performance of the experiments, 1-ml aliquots of a suspension with a density of 105 conidia per ml in yeast nitrogen base supplemented with 2% glucose were plated in the wells of 24-well plates (Costar). The plates were incubated at 30°C for 16 h, by which time more than 95% of the conidia had germinated to hyphae of approximately 150 to 200 μm in length. Plates with unopsonized hyphae were either used immediately or stored at 4°C for no longer than 1 to 2 h.
Reagents and treatment of effector cells with IL-12.
Recombinant human IL-12 with a specific activity of 5 × 106 U/mg was a kind gift of The Genetics Institute, Cambridge, Mass. Endotoxin in the preparation was undetected according to the manufacturer’s assay. IL-12 was diluted in Ca2+- and Mg2+-free HBSS to a concentration of 10 μg/ml, and this stock was kept at −35°C. Anti-IFN-γ antibody, a kind gift from Genentech, South San Francisco, Calif., was supplied at a concentration of 1 mg/ml. Enzyme-linked immunosorbent assay (ELISA) kits for measurements of the concentrations of IFN-γ and tumor necrosis factor alpha (TNF-α) in the supernatants of monocyte cultures were purchased from R&D Systems (Minneapolis, Min.).
PBMC or EHM, at a concentration of 2 × 106 per ml, were incubated with various concentrations of IL-12 in CM in tissue culture flasks at 37°C in air supplemented with 5% CO2 for 2 to 3 days. At the end of the incubation, supernatants were aspirated and frozen for measurements of TNF-α and IFN-γ whereas the cells were scraped, off the flasks, washed in Ca2+- and Mg2+-free HBSS, and then resuspended in the same. The cells were counted by trypan blue staining and were used for the functional assays as described below.
Superoxide anion production.
Production of O2− in response to PMA and to unopsonized hyphae of A. fumigatus was assessed spectrophotometrically by determining superoxide dismutase-inhibitable reduction of cytochrome c (15). One million MNC, which had been incubated with CM only or with IL-12-containing CM in each well of 12-well plates (Costar), were mixed with 50 μM cytochrome c (Sigma). As a stimulus, PMA at 0.1 μg/ml (Sigma) or unopsonized hyphae of A. fumigatus at an effector-to-target cell (E/T) ratio of 1:1 were added to the MNC in 1 ml of HBSS, and the cell suspension was then incubated on a shaker at 37°C for 15 min. O2− production was then assessed as the difference in absorbance at 550 nm, as measured on a Gilford model 260 spectrophotometer (Ciba-Corning Diagnostics Corp., Oberlin, Ohio) or a MicroElisa Strip Reader model 301 photometer (Bio-Tek Instruments, Winooski, Vt.), between test and control cells. The number of nanomoles of O2− produced by 106 MNC in 15 min was calculated by using an extinction coefficient of 29.5.
Hyphal damage.
A colorimetric (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (12, 15) was employed to study Aspergillus hyphal damage caused by MNC. Briefly, the supernatants were aspirated and MNC that had been incubated with or without IL-12 were added to the wells at final E/T ratios of 5:1 and 20:1 for EHM and 2:1, 20:1, and 40:1 for PBMC. After 2 h at 37°C in an atmosphere containing 5% CO2, supernatants were aspirated, MNC were lysed by adding 300 μl of 0.5% sodium deoxycholate, and hyphae were washed three times with sterile water. Subsequently, 1 ml of RPMI 1640 containing 0.5 mg of MTT/ml was added to each well, and the plates were further incubated at 37°C in an atmosphere with 5% CO2 for 3 h. The wells were then aspirated dry, 200 μl of isopropanol was used to extract the dye from each well, volumes of 150 μl were transferred into the wells of a 96-well plate, and the color was measured on a Titertek Multiscan microplate spectrophotometer (Flow Laboratories, McLean, Va.) at 570 nm, using as a reference the wavelength 690 nm. A well containing only isopropanol was used as a blank. Control wells containing hyphae and buffer only but not effector cells were included in each experiment. Antifungal activity (hyphal damage) was calculated with the following formula: percentage of hyphal damage = (1 − X/C) × 100, where X is the optical density of test wells at 2 h and C is the optical density of control wells containing hyphae only. Each set of conditions was tested in duplicate or quadruplicate, and the results were averaged.
ELISA cytokine measurements.
Concentrations of IFN-γ and TNF-α in culture supernatants of PBMC and EHM were measured by ELISA (R&D Systems). Samples were run in duplicate wells, and the results were read in an automated ELISA reader (Titertek Multiscan microplate spectrophotometer; Flow Laboratories). ΔSOFT II 3.67 F for Macintosh and Excel 4.0 software were used to analyze the data. Control wells containing defined concentrations of cytokines were used to construct a standard curve for each ELISA plate. The lower limits of detection were 3 pg/ml for IFN-γ and 4.4 pg/ml for TNF-α.
Statistical analysis.
Each experiment was performed with the cells of one donor and by the use of duplicate or quadruplicate wells for each set of conditions; the average value of these replicate wells was taken as the value for this particular donor and experiment. The averages of the replicate wells of each experiment were then used in the data analysis to calculate the mean ± standard error of mean (SEM) for all of the experiments performed under the same set of conditions. The statistical program Instat Statistics 2.03 for Macintosh was used. Trends of change were evaluated by analysis of variance with Dunnett’s correction for multiple comparisons. Differences between baseline levels and levels for individual cytokine concentrations in the supernatants were assessed by Student’s t test or Mann-Whitney U test when appropriate. A P value of <0.05 indicated statistical significance. All P values reported are two sided.
RESULTS
Enhancement of superoxide anion concentration in response to PMA.
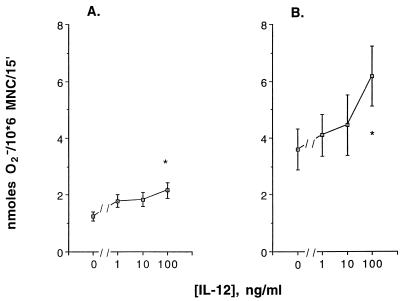
Incubation of PBMC with 100 ng of IL-12/ml at 37°C for 2 to 3 days enhanced O2− production in response to PMA from a mean ± SEM of 1.25 ± 0.16 (control value) to 2.16 ± 0.27 nmol/106 MNC/15 min (P = 0.04) (Fig. 1A). Similarly, IL-12 at 100 ng/ml enhanced O2− production by EHM in response to PMA (P = 0.013) (Fig. 1B). A concentration of 500 ng/ml did not further enhance O2− production by EHM (data not shown).
FIG. 1.
Effect of incubation of human PBMC (A) or EHM (B) with IL-12 for 2 to 3 days on superoxide anion production by these cells in response to PMA at 0.1 μg/ml. (A) The vertical bars indicate SEMs derived from 20 experiments. ∗, the difference in superoxide anion production between the value for PBMC treated with IL-12 at 100 ng/ml and control value is significant (P = 0.04). (B) The vertical bars indicate SEMs derived from eight to nine experiments. ∗, the difference between the value for EHM treated with IL-12 at 100 ng/ml and the control value is significant (P = 0.013).
Enhancement of antifungal activities against A. fumigatus.
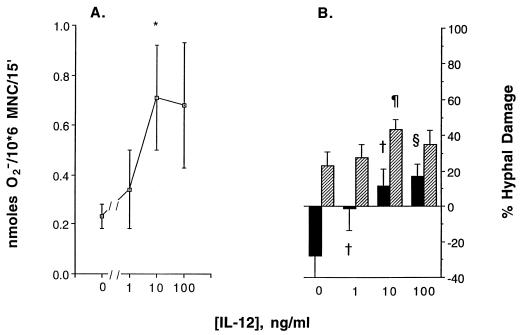
Incubation of PBMC with IL-12 at 10 ng/ml significantly enhanced O2− production in response to A. fumigatus hyphae (P = 0.03) (Fig. 2A). Figure 2B demonstrates that at a low E/T ratio (2:1), IL-12 at concentrations of 10 and 100 ng/ml significantly enhanced hyphal damage caused by PBMC. At a high E/T ratio, neither a 10- nor a 100-ng/ml concentration showed a significant enhancement; however, PBMC incubated with IL-12 at 10 ng/ml exhibited a trend toward enhanced damage of unopsonized hyphae, from 22.9% ± 7.8% (for controls) to 43.1% ± 5.7% (P = 0.063). In addition, incubation of EHM with IL-12 at 100 and 500 ng/ml somewhat increased hyphal damage at an E/T ratio of 5:1 (from 7.7% ± 8.8% to 11.1% ± 8.5% and 12.0% ± 9.3%, respectively), but these differences also were not significant.
FIG. 2.
Effect of incubation of PBMC with IL-12 at 1 to 100 ng/ml for 2 to 3 days on superoxide anion production in response to unopsonized hyphae of A. fumigatus (A) and on the percentage of A. fumigatus hyphae damaged by human PBMC (B), as measured by the MTT assay, at E/T ratios of 2:1 (closed columns) and 40:1 (hatched columns). (A) The vertical bars indicate SEMs derived from five experiments. ∗, the difference in superoxide anion production by PBMC treated with 10 ng/ml IL-12 and the control (0 ng/ml) is significant (P = 0.031). (B) The vertical bars indicate SEMs derived from six experiments. † and §, the differences from control PBMC (0 ng/ml) were significant (P < 0.05 and P < 0.01, respectively); ¶, the corresponding concentration was slightly different from that of control PBMC (P = 0.063).
Secretion of IFN-γ and TNF-α and effects of anti-IFN-γ.
Concentrations of IFN-γ measured in the supernatants of IL-12-treated PBMC were higher than those in supernatants of untreated control PBMC, confirming the findings of previous studies (3, 5, 24) (Table 1). However, the enhanced O2− production by IL-12-treated PBMC was not directly related to this increased IFN-γ production. Anti-IFN-γ blocked IFN-γ secreted into the medium by IL-12-treated PBMC but did not inhibit the IL-12-induced increase in O2− production by PBMC in response to PMA. Moreover, while no IFN-γ was secreted by IL-12-treated EHM, O2− production by EHM was significantly enhanced after incubation with IL-12. On the other hand, secretion of TNF-α was increased by IL-12 treatment of PBMC but not of EHM, suggesting that secretion of TNF-α is also not required for enhancement of O2− production by EHM (Table 1).
TABLE 1.
Superoxide production in response to PMA and concentrations of IFN-γ and TNF-α in the supernatants of PBMC and EHM cultured with medium only (controls), IL-12 of 100 ng/ml, anti-IFN-γ, or both IL-12 at 100 ng/ml and anti-IFN-γ for 2 to 3 days
| Cells and culture supplement(s) | Mean concn ± SEM fora:
|
||
|---|---|---|---|
| IFN-γ (pg/ml) | TNF-α (pg/ml) | O2− (nmol/106 MNC/15 min) | |
| PBMC | |||
| None (control) | 83 ± 42 | 71 ± 26 | 1.3 ± 0.2 |
| IL-12 | 1,000 ± 172* | 167 ± 23† | 2.2 ± 0.3§ |
| Anti-IFN-γ | 16 ± 13 | 89 ± 32 | 1.4 ± 0.2 |
| IL-12 + anti-IFN-γ | 11 ± 5 | 134 ± 62‡ | 2.1 ± 0.3¶ |
| EHM | |||
| None (control) | 5 ± 1 | 6 ± 1 | 3.6 ± 0.7 |
| IL-12 | 3 ± 0 | 4 ± 0 | 6.2 ± 1.1** |
The values for IFN-γ and TNF-α concentrations are means ± SEMs of duplicate readings of four supernatants. The values for O2− production by PBMC and EHM are means ± SEMs derived from 20 or 21 and 8 or 9 experiments, respectively. *, P = 0.03 in comparison to the value for control PBMC; †, P = 0.045 in comparison to the value for control PBMC; §, P = 0.04 in comparison to the value for control PBMC; ‡ P = 0.55 in comparison to the value for anti-IFN-γ-treated PBMC; ¶, P = 0.03 in comparison to the value for anti-IFN-γ-treated PBMC; **, P = 0.013 in comparison to the value for control EHM.
DISCUSSION
In this study, we demonstrated that IL-12 augments oxidative antifungal activities of MNC as evidenced by oxidative bursts and damage to A. fumigatus hyphae. The augmentation of oxidative bursts appears to occur via an IFN-γ-independent mechanism of activation.
These findings, to our knowledge, are novel, with no previous study having reported the direct effects of IL-12 on oxidative bursts and antifungal activity of MNC. These effects of IL-12 on MNC may depend in part on the marker of activation (superoxide anion) and the organism (A. fumigatus). A previous study did not show a change in nitric oxide production by murine macrophages when these cells were treated with IL-12 (22). In addition, IL-12 treatment of human MNC-derived macrophages did not result in augmented inhibition of growth of Mycobacterium avium (2).
IL-12 exerts its known effects on T and NK lymphocytes through binding to specific IL-12 receptors (19). Although receptors for IL-12 on the surface of MNC have not been reported (7, 19), this augmentation of oxidative bursts and antifungal activity appears to occur as a direct effect of IL-12 on MNC. Whether this effect is mediated through an IL-12 receptor or another cytokine receptor remains to be elucidated. We have previously shown that TNF-α enhances both O2− production and hyphal damage (14). However, only negligible amounts of TNF-α were secreted following IL-12 treatment of MNC.
The above-described findings suggest a new mechanism by which IL-12 exerts its beneficial effects on the host response to Aspergillus spp. and may have important implications with regard to our further understanding of immunoregulation of host defenses by this cytokine. An IFN-γ-independent pathway appears to underlie the upregulatory impact of IL-12 on antifungal phagocytic defenses against A. fumigatus and needs to be elucidated at the molecular level.
ACKNOWLEDGMENTS
We are grateful to Anna Manitsa, Head, and the staff of the Transfusion Medicine Department of Hippokration Hospital as well as to the staff of the Transfusion Medicine Department of Warren Grant Magnuson Clinical Center, National Institutes of Health, for providing leukocytes from healthy donors.
REFERENCES
- 1.Abrahamsen T G, Carter C S, Read E J, Rubin M, Goetzman H G, Lizzio E F, Lee Y L, Hanson M, Pizzo P A, Hoffman T. Stimulatory effect of counterflow centrifugal elutriation in large-scale separation of peripheral blood monocytes can be reversed by storing the cells at 37°C. J Clin Apheresis. 1991;6:48–53. doi: 10.1002/jca.2920060110. [DOI] [PubMed] [Google Scholar]
- 2.Bermudez L E, Wu M, Young L S. Interleukin-12-stimulated natural killer cells can activate human macrophages to inhibit growth of Mycobacterium avium. Infect Immun. 1995;63:4099–4104. doi: 10.1128/iai.63.10.4099-4104.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carson W E, Ross M E, Baiocchi R A, Marien M J, Boiani N, Grabstein K, Caligiuri M A. Endogenous production of interleukin 15 by activated human monocytes is critical for optimal production of interferon-gamma by natural killer cells in vitro. J Clin Investig. 1995;96:2578–2582. doi: 10.1172/JCI118321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cenci E, Perito S, Enssle K-H, Mosci P, Latgé J-P, Romani L, Bistoni F. Th1 and Th2 cytokines in mice with invasive aspergillosis. Infect Immun. 1997;65:564–570. doi: 10.1128/iai.65.2.564-570.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.D’Andrea A, Aste-Amezaga M, Valiante N M, Ma X, Kubin M, Trinchieri G. Interleukin 10 (IL-10) inhibits human lymphocyte interferon γ production by suppressing natural killer cell stimulatory factor/IL-12 synthesis in accessory cells. J Exp Med. 1993;178:1041–1048. doi: 10.1084/jem.178.3.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Denning D W. Invasive aspergillosis. Clin Infect Dis. 1998;26:781–803. doi: 10.1086/513943. [DOI] [PubMed] [Google Scholar]
- 7.Desai B B, Quinn P M, Wolitzky A G, Mongini P K, Chizzonite R, Gately M K. IL-12 receptor. II. Distribution and regulation of receptor expression. J Immunol. 1992;148:3125–3132. [PubMed] [Google Scholar]
- 8.Diamond R D, Huber E, Haudenschild C C. Mechanisms of destruction of Aspergillus fumigatus hyphae mediated by human monocytes. J Infect Dis. 1983;147:474–483. doi: 10.1093/infdis/147.3.474. [DOI] [PubMed] [Google Scholar]
- 9.German T, Rude E. Interleukin-12. Int Arch Allergy Immunol. 1995;108:103–112. doi: 10.1159/000237126. [DOI] [PubMed] [Google Scholar]
- 10.Grazziutti M L, Savary C A, Ford A, Anaissie E J, Cowart R E, Rex J H. Aspergillus fumigatus conidia induce a Th1-type cytokine response. J Infect Dis. 1997;176:1579–1583. doi: 10.1086/514157. [DOI] [PubMed] [Google Scholar]
- 11.Hendrzak J A, Brunda M J. Interleukin-12. Biologic activity, therapeutic utility, and role in disease. Lab Investig. 1995;72:619–637. [PubMed] [Google Scholar]
- 12.Levitz S M, Diamond R D. A rapid colorimetric assay of fungal viability with the tetrazolium salt MTT. J Infect Dis. 1985;152:938–945. doi: 10.1093/infdis/152.5.938. [DOI] [PubMed] [Google Scholar]
- 13.Maruo S, Toyo-oka K, Oh-hora M, Tai X G, Iwata H, Takenaka H, Yamada S, Ono S, Hamaoka T, Kobayashi M, Wysocka M, Trinchieri G, Fujiwara H. IL-12 produced by antigen-presenting cells induces IL-2-independent proliferation of T helper cell clones. J Immunol. 1996;156:1748–1755. [PubMed] [Google Scholar]
- 14.Roilides E, Dimitriadou-Georgiadou A, Sein T, Kadiltsoglou I, Walsh T J. Tumor necrosis factor alpha enhances antifungal activities of polymorphonuclear and mononuclear phagocytes against Aspergillus fumigatus. Infect Immun. 1998;66:5999–6003. doi: 10.1128/iai.66.12.5999-6003.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roilides E, Dimitriadou A, Kadiltsoglou I, Sein T, Karpouzas J, Pizzo P A, Walsh T J. IL-10 exerts suppressive and enhancing effects on antifungal activity of mononuclear phagocytes against Aspergillus fumigatus. J Immunol. 1997;158:322–329. [PubMed] [Google Scholar]
- 15a.Roilides E, Tsaparidou S, Kadiltsoglou I, Sein T, Walsh T J. Effects of interleukin-IL)-12 on antifungal activity of mononuclear phagocytes against Aspergillus fumigatus. Clin Microbiol Infect. 1997;3(Suppl 2):197–198. [Google Scholar]
- 16.Romani L, Mencacci A, Tonnetti L, Spaccapelo R, Cenci E, Puccetti P, Wolf S F, Bistoni F. Interleukin-12 is both required and prognostic in vitro for T helper type 1 differentiation in murine candidiasis. J Immunol. 1994;152:5167–5175. [PubMed] [Google Scholar]
- 17.Romani L, Mencacci A, Tonnetti L, Spaccapelo R, Cenci E, Wolf S, Puccetti P, Bistoni F. Interleukin-12 but not interferon-gamma production correlates with induction of T helper type 1 phenotype in murine candidiasis. Eur J Immunol. 1994;24:909–915. doi: 10.1002/eji.1830240419. [DOI] [PubMed] [Google Scholar]
- 18.Schaffner A, Douglas H, Braude A. Selective protection against conidia by mononuclear and against mycelia by polymorphonuclear phagocytes in resistance to Aspergillus: observations on these two lines of defense in vivo and in vitro with human and mouse phagocytes. J Clin Investig. 1982;69:617–631. doi: 10.1172/JCI110489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stern A S, Gubler U, Presky D H, Magram J. Structural and functional aspects of the IL-12 receptor complex. Chem Immunol. 1997;68:23–37. doi: 10.1159/000058692. [DOI] [PubMed] [Google Scholar]
- 20.Trinchieri G. Interleukin-12: a cytokine produced by antigen-presenting cells with immunoregulatory functions in the generation of T-helper cells type 1 and cytotoxic lymphocytes. Blood. 1994;84:4008–4027. [PubMed] [Google Scholar]
- 21.Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol. 1995;13:251–276. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- 22.Wigginton J M, Kuhns D B, Back T C, Brunda M J, Wiltrout R H, Cox G W. Interleukin 12 primes macrophages for nitric oxide production in vivo and restores depressed nitric oxide production by macrophages from tumor-bearing mice: implications for the antitumor activity of interleukin 12 and/or interleukin 2. Cancer Res. 1996;56:1131–1136. [PubMed] [Google Scholar]
- 23.Wolf S F, Sieburth D, Sypek J. Interleukin-12: a key modulator of immune function. Stem Cells. 1994;12:154–168. doi: 10.1002/stem.5530120203. [DOI] [PubMed] [Google Scholar]
- 24.Ye J, Ortaldo J R, Conlon K, Winkler-Pickett R, Young H A. Cellular and molecular mechanisms of IFN-γ production induced by IL-2 and IL-12 in a human NK cell line. J Leukoc Biol. 1995;58:225–233. doi: 10.1002/jlb.58.2.225. [DOI] [PubMed] [Google Scholar]