Abstract
Purpose
Dementia and cardio-metabolic diseases share many risk factors. Management of these risk factors could contribute to successful aging, including the prevention of cardio-metabolic disease and dementia. The increasing use of smartphones offers an opportunity for remote preventive interventions. We provided a systematic review of telephone and smartphone-based interventions targeting the prevention of cognitive decline, dementia cardio-metabolic diseases or their risk factors among adults aged over 50 years.
Patients and Methods
We searched Pubmed and the International Clinical Trials Registry Platform for experimental studies. We used the Cochrane risk-of-bias tool (Version 2) for randomized trials or TREND (Transparent Reporting of Evaluations with Nonrandomized Designs) checklists to assess study quality for completed studies.
Results
We analyzed 21 completed (3 for cognition, 18 for cardio-metabolic outcomes) and 50 ongoing studies (23 for cognition, 27 for cardio-metabolic outcomes). Smartphone interventions were used in 26 studies (3 completed, 23 ongoing). Other interventions involved telephone vocal support and text messaging. Few studies were at low risk of bias. There were heterogeneous cognitive and cardio-metabolic outcomes. The highest quality studies found no significant effects on cognition, and inconsistent results for HbA1c, blood pressure or physical activity. The lower quality-studies found effects on global cognition, working memory, memory and language and inconsistent results for clinical, biological or behavioral cardio-metabolic outcomes.
Conclusion and Implications
Despite the large number of commercially available mobile health applications, the magnitude of the scientific evidence base remains very limited. Based on published studies, the added value of telephone and smartphone tools for the prevention of cardio-metabolic diseases, cognitive decline or dementia is currently uncertain, but, there are several ongoing studies expected to be completed in the coming years.
Keywords: aging, telephone, smartphone, cognition, dementia, cardio, vascular outcomes
Introduction
Promoting successful aging through the prevention of non-communicable diseases, such as dementia, cardio-metabolic diseases (cardiovascular (eg stroke, angina) or metabolic (eg dyslipidemia, obesity) diseases or mental health problems among older adults has become one of the main World Health Organization (WHO) priorities.1 Indeed, non-communicable diseases represent 71% of all deaths worldwide with a higher burden among middle-aged and older adults.2 Most cardio-vascular diseases could be prevented by modifying their risk factors; for example such as improving physical activity, stopping smoking, having a healthy diet, controlling weight or blood pressure, or decreasing cholesterol, lipids or blood sugar could also have an effect on cardio-vascular disease incidence.3 Moreover, these risk factors are also known to be associated with dementia incidence.4,5 Therefore, managing these risk factors could contribute to both cognitive and cardio-metabolic disease prevention. Even though some studies suggest that many older adults have only basic health literacy,6 others suggest that middle-aged and older adults may be attentive to these factors, know the notion of risk for them7 and could be prone to initiate healthy behavior changes with the help of promotional interventions.
Information and communication technologies could help to promote healthier behaviors, particularly through the use of mobile technology tools such as smartphones. Mobile and smartphone accessibility has rapidly increased worldwide, first, in high income countries and now also in low or middle-income countries.8 The number of mobile phone was reached 7 billion in 2021 worldwide, of whom 6.3 billion were smartphone users.9 The number of mobile applications is also increasing worldwide, with, for example, 47,478 iOS applications classified as “health care” applications available in the first quarter of 2020.10 Adults over 65 years are increasingly using smartphones, but there are evident disparities, with the oldest individuals, those with lowest household income and lowest levels of education less often owning a smartphone11 and more often using simple message and weather applications rather than more interactive ones, compared to younger individuals.12,13 These disparities are also evident in the telemedicine field, although some studies have shown that telemedicine is feasible among older populations.14–18
Telephone and smartphone interventions may be interesting tools for prevention in middle-aged and older adults given their common usage, inexpensive cost, and accessibility. Moreover, mobile-phones are portable, and well-integrated in daily life. Finally, mobile phones are a potentially powerful tool for monitoring and communicating with patients (and thus providing interventions) in a continuous way. In a clinical trial setting, they may improve recruitment rates by reducing participant burden, for example by reducing/removing travel to study visits.19 They could therefore be a powerful method for encouraging behavior changes and consequently having an impact on long-term disease prevention. Mobile phones also enable “big data” approaches through the collection of medical (and other) data in a non-decontextualized (ecological) environment via text messages,20 mobile applications and smartphone sensors (accelerometer, location, etc.). Telephones could also facilitate cognitive assessment21 and improve early detection of cognitive and functional decline22 or other health issues. However, clinical trials using such tools in older populations could be particularly affected by selection bias, given the disparities in use in older populations.
Despite the many promising advantages, it is necessary to evaluate the evidence concerning the effectiveness of such strategies.
Thus, the main aim of this review was to assess the effectiveness of telephone and smartphone-based interventions to prevent cognitive decline, dementia or cardiometabolic diseases, which share many risk factors, in older adults.
When data were available, we described strategies measuring intervention adherence, factors associated with adherence and the implementation of such strategies.
Materials and Methods
Searches were run in PubMed (including MEDLINE) and the WHO International Clinical Trials Registry Platform (ICTRP), which includes various registries from around the world (eg International Standard Randomised Controlled Trials Number (ISRCTN), Clinicaltrials.gov, Australian New-Zealand Clinical Trials Registry (ANZCTR), Brazilian Clinical Trials Registry), until 17 January 2022, using the search equations outlined in the Appendix.
Eligibility Criteria (See Appendix Box A)
Studies that met the following criteria were included:
Inclusion of individuals aged 50 and over living independently at home
Randomized clinical trial (RCT), quasi-experimental or pilot study
Assessment of efficacy (as a primary or secondary outcome) of mobile-phone or other telephone interventions targeting the prevention of cognitive decline, dementia or cardio-metabolic diseases or their risk factors (overweight, physical activity, sedentarity, sugar or lipid profile, diet food and nutrition)
Articles written in English
We excluded studies focusing on other interfaces, such as tablet, computer or personal digital assistants, because we wanted to exclusively focus on telephone-interventions since they are more portable and more integrated into everyday routine. We included all kinds of telephone interventions, ie telephone calls, short messages system (SMS), applications or telephone-accessible platforms. For cognitive outcomes, as we focused on studies concerning the prevention of dementia or cognitive impairment, we excluded studies involving participants with serious diseases likely to affect cognitive function (dementia, depression, schizophrenia, Parkinson’s disease), but we did not exclude studies of participants with mild cognitive impairment or subjective cognitive decline. For cardio-metabolic outcomes, we excluded participants with congenital cardio-vascular disease. We also excluded studies of advanced disease (cancer, palliative care, malnutrition).
Data Extraction
Each study’s eligibility was assessed by two authors (LA and CG). Study selection was firstly based on title and abstract, and the full-text was read where necessary. Publications which possibly met inclusion criteria were then assessed by both investigators independently. In cases of discordances regarding the eligibility of an article, there was discussion between the two investigators until consensus was reached.
The reference lists of the eligible articles were also checked in order to identify other studies of potential interest that were not identified in the literature search, as well as the reference lists of selected reports and papers in our own files.
Data were extracted by one author from each study regarding setting, participant characteristics, intervention description and outcomes.
Quality Assessment
We assessed the quality of completed studies using the items of the RoB2 (Version 2 of the Cochrane risk-of-bias tool for randomized trials).23 Quality is defined into 3 levels: low risk of bias, some concerns and high risk of bias. Due to a lack of existing scales, we used the TREND (Transparent Reporting of Evaluations with Nonrandomized Designs) statement items to evaluate the quality of non-randomized controlled studies.24 We divided scores into 3 groups. Poor quality was defined by a score of ≤9 criteria, good quality by a score of >18 criteria, and fair quality by a score between 10 to 18.
Results
Telephone and Smartphone Interventions for the Prevention of Cognitive Decline or Dementia
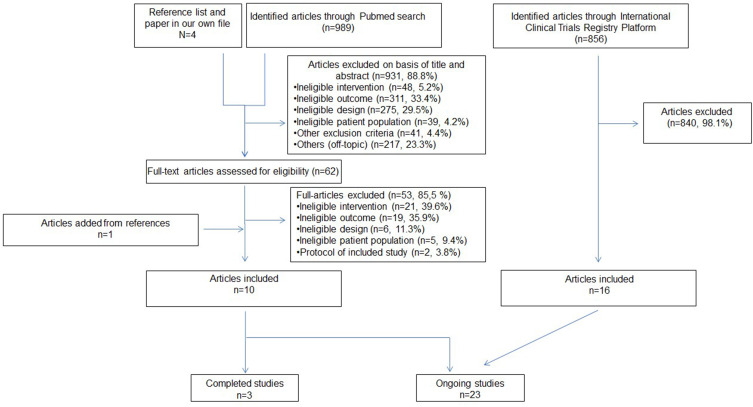
The Pubmed searches yielded 989 papers, of which 10 were eligible for our review (Figure 1). Of the 10 included articles, 3 described completed studies, 7 detailed protocols of ongoing studies.
Figure 1.
Study selection flow-chart for the cognitive outcome articles.
In the ICTRP, 16 ongoing studies were included of the 856 identified (Figure 1).
In total, we included 3 completed (Table 1) and 23 ongoing studies (Appendix Table A).
Table 1.
Characteristics of Completed Studies Evaluating the Effect of Telephone and Smartphone Interventions on Cognitive Outcomes
| First Author Year Country | Quality Assessment | Design | N | Length of Intervention Follow-Up (if Different) | Cognitive Outcomes | Phone Intervention | Population | Results | |
|---|---|---|---|---|---|---|---|---|---|
| Age | Cognitive Criteria | ||||||||
| Kumar et al25 2018 USA | Fair | Single arm pre-post pilot study | 82 | 24 weeks 52 weeks |
Primary: outcome: change in RBANS total score and sub-dimensions | Multidomain lifestyle intervention (main intervention) on computer supported by health telephone and email/text support: physical, nutritional, cognitive training and social engagement (difficulties, lifestyle behavior) |
60–75 | • At risk: subjective cognitive decline and worries about it • Free of dementia, mental illness and neurologic conditions |
Compared to pre-intervention scores, there were significant differences at 24 weeks for immediate memory (p<0.001), and at 52 weeks for total RBANS scores (p<0.001), language (p<0.001), delayed memory (p<0.001) and immediate memory Compared to 24 week scores, there were significant effects at 52 weeks for total RBANS scores (p<0.001), immediate memory (p<0.001), language (p<0.001), attention (p<0.01) and delayed memory (p<0.01) There were no differences for visuo-spatial and attention scores. |
| Oh et al26 2018 South-Korea | High risk of bias | RCT | 53 | 8 weeks | Primary outcome: K-MMSE, Korean, K-WAIS-IV, Memory Diagnostic System, Korean language version of the SCWT |
Weekly call phone or text messages (progress and participation) • Intervention: SMART application brain training • Active control: Fit Brain training application • Control: no intervention |
50–69 | •At risk: subjective memory complaints • K-MMSE ≥ 24 • No cognitive impairment |
Working memory on the Memory Diagnostic Memory (p < 0.001) and auditory-verbal working memory (p < 0.001) increased significantly between pre and post intervention in the SMART group. There was an interaction with time on auditory-verbal working memory test There were no other effects on outcomes in other groups |
| Sakakibara et al27 2021 Canada | Low risk of bias | Single-blinded RCT | 126 | 6 months 12months | Tertiary outcomea: MoCA | Intervention: telehealth self-management: telephone lifestyle coaching sessions to improve controlling of cardiovascular risk factors with phone call. Self-monitoring kit and self-management manual •Telephone memory training program: memory coaching, memory training manual |
≥50 | • Vascular stroke survivors (modified Rankin Scale between 1 to 4) • Free of cognitive impairment • MoCA ≥23 • No clinically important neurological conditions • No severe aphasia or dysarthria |
Stroke coach intervention did not change MOCA score at 12 months compared to memory training group (p=0.430) Adherence: •Intervention: 98% completed all coaching sessions and 96% all check-in sessions. Mean of 188.7 days (sd=30.2) •Control: 96% completed all sessions and 85% completed all check-in sessions. Mean of 188.0 days (sd= 52.5 days) |
Notes: aPrimary outcome not relevant to our work.
Abbreviations: RCT, randomized clinical trial; ≥, superior or equal to; <, inferior to; =, equal to; %, percentage; sd, standard deviation; p, p value; K-MMSE, Korean-Mini Mental State Examination; SMART, Smartphone-based brain Anti-aging and memory Reinforcement Training; WAIS-IV, Wechsler Adult Intelligence Scale-IV; SCWT, Stroop Color and Word Test; RBANS, Repeatable Battery for the Assessment of Neuropsychological Status; N, number of subjects; MoCA, Montreal Cognitive Assessment.
Quality Assessment
Based on the RoB2 and TREND Checklist scoring systems, only one study was at low risk of bias.27 The other two studies had higher risks of bias. For example, Oh et al26 did not detail the allocation sequence generation and did not describe who was blinded. For the remaining study,25 details concerning location, date of inclusion and setting details of the intervention as well as side effects were missing.
Description of Completed Studies (Table 1)
Sakakibara et al27 evaluated in a low-risk of bias randomized clinical trial, the effect of telephone lifestyle coaching sessions (aiming to improve control of cardio-metabolic risk factors) among 126 Canadian stroke survivors adults aged 50 years and over without cognitive impairment. Participants received stroke risk factors manual, a kit to monitor risk factors and 7 coaching telephone sessions of 30–45 minutes to coach, motivate and help them to change lifestyle and 5 additional follow-up calls over 6 months. Control group received a memory training, an agenda to make reminder notes and 7 memory coaching telephone sessions of 30–45 minutes and 5 additional follow-up calls over 6 months. At 12 months, there was no change in MoCA (Montreal Cognitive Assessment), studied as tertiary outcome in intervention group compared with a memory training control group.
Oh and al26 evaluated, in an 8-week randomized trial, the effect of a smartphone-based brain training application (Smartphone-based brain Anti-aging and memory Reinforcement Training (SMART)) among 53 South-Korean adults with subjective memory complaints and a Mini Mental State Examination (MMSE) score greater or equal to 24 (mean baseline scores: 28.06 (2.04) in the SMART group, 28.68 (1.06) in the fit Brain group and 28.25 (1.57) in the wait list group). This application, which targeted attention and working memory, was compared with another commercialized cognitive training application and a control group (no intervention). Participants used applications 5 times a week for 15 to 20 minutes. Weekly telephone calls and text messaging assessed progress and conscientious participation. The study found significant improvement between pre and post intervention on working memory for SMART group only, but not for the other tests. It should be noted that this was a poor-quality study, with several limitations, including.
The last study,25 evaluated in an uncontrolled single arm study, a computer-based multidomain lifestyle intervention with telephone and email or text health support for a single group of 82 American aged 60–75 year-old with subjective cognitive decline. Participants received personalized coaching sessions, focusing on nutrition, physical activity, cognitive training and social engagement. The authors found significant improvement at 52 weeks compared to baseline scores for the primary outcomes on the total Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) scores (mean improvement=5.8, p<0.001), memory (immediate recall mean improvement=7.8, p<0.001, delayed recall mean improvement=5.4, p<0.001), language (mean improvement=5.7, p<0.001). Immediate recall significantly decreased at 24 weeks, but no other significant differences were found.
Description of Ongoing Studies
Australia is the country most frequently enrolling participants in the ongoing studies (7 studies). Six studies are being conducted in Asia, 4 in European countries, and 3 in North/South America (Canada, Brazil and USA). The remaining three are enrolling participants in multiple locations (Australia, Europe, Asia for one study, China and United Kingdom for the second, and Germany, the Netherlands and Norway in the third). (Appendix Table A and Figure 1)
The interventions are due to last between 4 weeks and 36 months, with a median of 12 months of follow-up. Moreover, the number of participants varies highly, from 9 for the smallest study to 3498 in the largest.
Ten of the studies are enrolling participants considered to be at risk of cognitive decline, due to the presence of memory complaints, mild cognitive impairment or stroke comorbidity (without cognitive impairment), and the others are including participants with no specific risk factors.
The interventions tested in the ongoing trials can be divided into 2 categories:
The first, and most frequent, is mobile-phone applications or mobile health interventions, which are being used by 14 studies28–31 (CTRI/2018/01/011090, NCT03058146, DRKS00010595, NCT04692974, NCT04184037, ACTRN12619001634167, DRKS00020943, JPRN-UMIN000041926, ACTRN12620001037998, JPRN-UMIN000042123). Two of these studies (CTRI/2018/01/011090, JPRN-UMIN000042123) are using mobile phone-based cognitive training, one a physical exercise program (ACTRN12620001037998), another a meditation application (NCT04184037) while the others are using mobile phone interventions for training, monitoring, tracking, supporting and/or promoting healthy behaviors28–31 (NCT03058146, DRKS00010595, NCT04692974, ACTRN12619001634167, DRKS00020943, JPRN-UMIN000041926).
The second type of intervention is telephone support and coaching which is being evaluated in 7 studies32–34 (ACTRN12617000082303, NCT01012947, ACTRN12621000977875, ACTRN12620000978965). In these studies, telephone calls or messages are used to provide advice or encouragement, improve risk factor control, or increase adherence, or are used as reminders to carry out the intervention. For instance, Cox et al32 are evaluating the effect of mentor telephone counselling. Another study (ACTRN12618000513213) is evaluating the effect of standardized reminder or reinforcement messages.
The effects of telephone interventions on cognitive function are being evaluated as a primary outcome in 12 studies, as a secondary outcome in 9 studies, using neuropsychological scores or changes on a single test (n=6) or on a battery of neuropsychological tests (n=16).
Finally, two study are evaluating the impact of a smartphone intervention on a dementia incidence, as a primary or secondary outcome (Eggink et al,31 JPRN-UMIN000041926) and 2 trials on dementia risk score (Eggink et al,31 ACTRN12621000977875)).
Implementation results
Few studies reported implementation results. In a 3-month pilot phase, the portability, usability and acceptability of a physical, cognitive, psychological and social mobile platform intervention were evaluated in a limited sample of 20 participants. Protocols, questionnaires and platform technical aspects were found to be good.29 This study29 concluded that the intervention platform was suitable (no additional details were provided), and another31 improved functionality of application and logistic issues before beginning the main trial. Adherence, duration, frequency of use or feedback will be recorded in several trials.28–30,32 For instance, Summer et al29 are recording duration and frequency of mobile application use and time spent on cognitive training, and another study30 is recording the number of logins and days using the mobile application.
Telephone and Smartphone-Based Interventions for Cardio-Metabolic Outcomes and Risk Factors
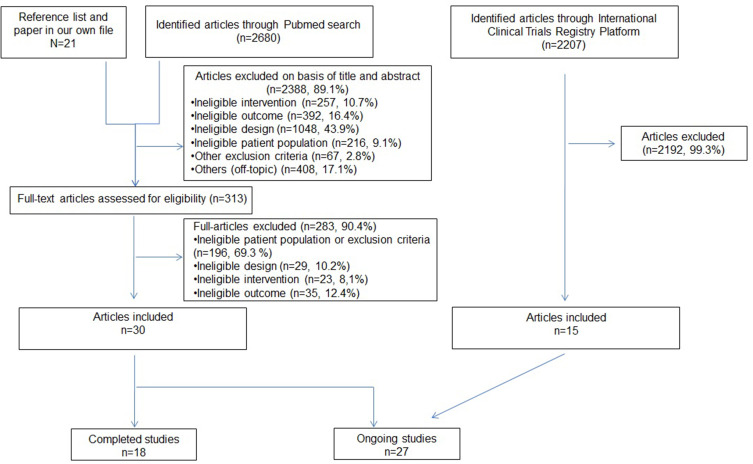
Of the 2680 articles identified in Pubmed, 30 (1.1%), published from 2007 onwards, were included (Figure 2). In the ICTRP, we identified 2207 records, and 15 studies were included. In total, 18 completed and 27 ongoing studies were analyzed.
Figure 2.
Study selection flow-chart for the cardio-metabolic outcome articles.
Quality Assessment
Based on the Rob2 and TREND checklists, the 18 completed studies,27,35–51 4 were of good quality,27,37,44,46 and the rest were fair or poor of quality. Among the lowest quality studies, missing information included randomization allocation,35,39 or sample size calculations, for example. In one study, we did not find details concerning blinding of participants39 or if assessor was blinded during assessment of outcomes.40,42
Description of Completed Studies (Tables 2–4)
Table 2.
Characteristics of Completed Studies Evaluating the Effect of Smartphone Interventions on Cardio-Vascular Outcomes
| First Author Year Country | Quality Assessment | Design | N | Length of Intervention Follow-Up (if Different) | Cardio-Metabolic Outcomes and Risk Factors | Phone Intervention | Population | Results | |
|---|---|---|---|---|---|---|---|---|---|
| Age | Cardio-Metabolic Outcomes and Risk Factors Criteria | ||||||||
| Lim and al38 2011 South Korea | Some concerns | RCT | 154 | 6 months | Primary outcome: proportion of patients with HbA1c< 7% without hypoglycemia Secondary outcome: BMI change, body weight change, fasting blood samples, frequency of self-glucose monitoring, fasting and postprandial glucose, HbA1c, compliance to measure blood glucose level |
•U-healthcare group: wired telephone-connected glucometer plus adapted glucose medication SMS on mobile phone •Self-monitored blood glucose: 8 times a week • Control: routine care |
≥ 60 | •At risk: • Type 2 diabetes • HbA1c: 6.5–10.5% • No severe diabetes complications |
Primary outcome: Proportion of HbA1c without hypoglycemia was lower at 6 months among U-healthcare group (30.6%) compared to self-monitored blood glucose (23.4%) (p=0.027) and control (14.0%) groups (p=0.019) HbA1c was lower in U-healthcare group compared to self-monitored blood glucose (p<0.05) at 6 months and to control (p<0.05) at 3 and 6 months Compared to pre intervention score, intervention decreased weight, BMI, fasting glucose and LDL cholesterol and increased self-monitoring glucose concentration among U-healthcare group, Self-monitoring glucose increased in U-healthcare group compared to control (p<0.01) and increased among self-monitored blood glucose group compared to control (p<0.001) No other significant differences Study completion rate: between 92.2 to 96.1% Target frequency of glucose testing (≥8 times/week): 81.2% for u-healthcare, 68.5%, for self-monitored and 31.2% for control group |
| Poppe et al42 2019 Belgium | Some concerns | RCT | 63 | 6 weeks 6 months |
Primary outcome: Change in self-reported PA (International PA Questionnaire), objective total, light and self-reported sedentary behavior via the Longitudinal Aging Study Amsterdam, physical activity Sedentary time and break via accelerometer |
Phone was optional • Intervention: five motivation website sessions to increase physical (arm 1) or decrease sedentary behavior activity (arm 2) + mobile application (optional) • Control: access to the intervention after the study |
≥50 | • At risk: type 2 diabetes | Compared to control group, participants focusing to improve PA improve self-reported total PA No other significant effects were found Compared to control group, participants aiming to decrease sedentary did not improve cardio-metabolic outcome 5 (8%) used the optional mobile app |
| Zheng et al49 2018 USA | Poor | Pre-post test pilot study | 9 | 8 weeks | Secondary outcomea: weight, steps and calorie intake change | • Intervention: biweekly self-regulation theory-based weight loss intervention and self-monitoring (1) iPhone Plus, (2) the Lose It! app for self-monitoring of dietary intake, (3) Fitbit for self-monitoring of physical activity, (4) Bluetooth-enabled scale for daily weight, and (5) Bluetooth-enabled blood glucose monitor for testing blood glucose levels |
≥65 | • BMI between 27–40 • Diagnosed type 2 diabetes • Prescribed insulin or oral medications • No severe complications of diabetes or current use of weight loss medication • No participation in diabetes education in the previous 12 months • Able to walk 2 blocks •No severe hypertension |
Compared to pre intervention, following the intervention, there was a significant percentage of weight loss (p=0.0004), decreased calorie intake and increased steps (p=0.02) Percentage of days of using intervention components: Lose It!=92.7, Fitbit= 93.7, glucometer 76.4 |
Notes: aPrimary outcome not relevant to our review.
Abbreviations: N, number of subjects; RCT, randomized-controlled trial; HbA1c, glycosylated haemoglobin; ≥, superior or equal to; <, inferior to; =, equal to; %, percentage; kg, kilogram; BMI, Body Mass Index; p, p value; SMS, Short Message System; PA, Physical Activity.
Table 3.
Characteristics of Completed Studies Evaluating the Effect of Telephone Call Interventions on Cardio-Vascular Outcomes
| First Author Year Country | Quality Assessment | Design | N | Length of Intervention Follow-Up (if Different) | Cardio-Metabolic Outcomes and Risk Factors | Phone Intervention | Population | Results | |
|---|---|---|---|---|---|---|---|---|---|
| Age | Cardio-Metabolic Outcomes and Risk Factors Criteria | ||||||||
| Chapman et al37 2018 China | Low risk of bias | RCT | 753 | 18 months | Primary outcome: HbA1c Secondary outcome: weight, BMI, systolic and diastolic BP, waist and hip circumference, fasting blood samples, diabetes self-care activities, diabetes management self-efficacy |
• Intervention: face-to-face and telephone health coaching + usual care • Control: usual care |
≥50 | • At risk: type 2 diabetes | No effect of the intervention on change from baseline to 18 months on HbA1c (mean change=−0.07, p=0.769) or on secondary outcomes, compared to the control group The participant attrition: 13.4% for intervention and 21.5% for control |
| Gillham et al40 2010 UK | Some concerns | RCT | 52 | 3 months | Secondary outcomea: change in self-reported •smoking status •exercise behavior •fruit and vegetable consumption |
• Intervention: risk factor information and healthy behavior + telephone support and follow-up discuss progress • Control: usual care |
Mean age= 68.3 | • At risk: first minor stroke or transient ischemic attack | Compared to control group • exercise frequency score between pre and post intervention increased in the intervention group (p=0.007) • change in fruit and vegetable consumption score between pre and post intervention increased in the intervention group (p=0.033) • There was no change concerning smoking status |
| Hartman et al50 2021 Netherland | Fair | Pre-post intervention | 15 | 16 weeks | PA and sedentary behavior (accelerometer), fasting glucose levels, insulin, total cholesterol, HDL cholesterol, LDL cholesterol, triglycerides, BP | Information to promote physical activity and wearing an activity monitor + weekly telephone/online coaching | ≥55 | At risk: • >40 hours /week of self-reported sedentary behavior • One or more cardiovascular risk factors: BMI >28, high BP |
Compared to pre intervention scores, participants significantly decreased sedentary time (p< 0.01) and increased standing time (p=0.03), walking time (p<0.01) and step count (p<0.01). There were no significant differences for other outcomes |
| Aunger et al43 2020 UK | Some concerns | RC feasability study | 35 | <18 weeks | Secondary outcomea: SPPB, sitting time, standing time, sit-to-stand transitions, quantity of sedentary bouts >30 minutes, older Adults’ Sedentary Time, International PA Questionnaire Short Form, body weight, BMI, Short Form Mini Nutritional Assessment, LDL, HDL, triglyceride | • Intervention: Sedentary behavior reduction program + bi-weekly supportive phone calls • Control: usual care |
≥60 | – | No formal statistical analysis was performed in this feasibility study which was not powered to detect differences Participant uptake rate was 14.2%, and retention rate 85.7% For the entries: overall mean average weekly self-reported goal adherence =3.9 /5 Overall mean self-reported adherence= 4.2/5 |
| Kolt et al45 2007 New-Zealand | Some concern | RCT | 186 | 3 months 12 months |
Primary outcome PA: Auckland Heart Study Physical | • Intervention: combination of telephone-based motivational interviewing and cognitive-behavioral techniques • Control: no intervention |
≥65 | • Sedentary adults (< 30 minutes of physical activity on five or more days per week) • No walking contraindication | At 12 months, moderate or vigorous leisure physical activity per week increased in intervention compared with control (significant interaction with time) No other significant effects were found at 12 months |
| Perri et al35 2008 USA | Some concerns | RT | 234 | 12 last months 18 months |
Primary outcome: change in body weight, BMI Secondary outcome: changes in BP, lipid profile, glycemic control, number of self- monitoring records, attrition, attendance and telephone calls completion | •Intervention Arm 1: 26 biweekly face-to-face group counseling sessions Arm 2: 26 biweekly telephone counseling sessions •Control: 26 biweekly newsletters mail with weight management advice |
50–75 | • At risk: • Women with BMI > 30 and a body weight<159.1 kg • Free of uncontrolled hypertension and diabetes • No manifestation of cardio-vascular disease. • No weight loss medication taken in the last 6 months • No weight loss >4,5 kg taken in the last 6 months |
Primary outcome: compared to control group, telephone counseling group regained less weight (1.2 ±0.7kg vs 3.7±0.7kg, p=0.02) and had smaller increase in BMI (0.45± 0.27 vs 1.42± 0.26, p=0.03) There were no effects for telephone counseling on secondary outcomes compared to control group Completed face to face sessions: 13.8 (±8.6) Completed telephone sessions: 21.1 (±5.7) Adherence higher in the telephone (p=0.006) and face-to-face (p=0.003) arms compared with control group |
| Sakakibara et al27 2021 Canada | Low risk of bias | RCT | 126 | 6 months 12 months | Secondary outcomesa: •HBP, cholesterol, glucose •Diet (fiber and fat intake) • BMI •Daily walking physical activity •Protocol adherence |
• Stroke Coach: telehealth self-management: telephone lifestyle coaching sessions to improve controlling of cardiovascular risk factors with phone call. Self-monitoring kit and self-management manual • Memory training program: memory coaching, memory training manual |
≥50 | • At risk: vascular stroke survivors (modified Rankin Scale between 1–4) • Free of cognitive impairment • MoCA ≥23 • No clinically important neurological conditions • No severe aphasia or dysarthria |
Stroke coach intervention improved HbA1c control (p=0.034) compared to Memory training group. There were no interactions between group or time. No-other significant results were found for secondary outcomes for Stroke coach intervention compared to control Adherence: •Intervention: 98% completed all coaching sessions and 96% all check-in sessions. Mean of 188.7 days (sd=30.2) •Control: 96% completed all sessions and 85% completed all check-in sessions. Mean of completed program: 188.0 days (sd= 52.5 days) |
| Vita et al36 2015 Australia | Fair | Uni-center quasi experimental study | 1238 | 12 months | Change goal: ≥ 210 minutes of MVPA per week, body weight, BMI, waist circumference, progressive resistance training, PA, saturated food, total fat, fibers, total energy, fasting blood samples |
One behavioral individual, 3 face to face group sessions and 3 follow-up calls with coaching call (professional) | 50–65 | •At risk of diabetes (AUSDRISK tool ≥ 15) • No undiagnosed diabetes, use of blood glucose lowering or weight loss medications |
Between pre- and post-intervention, there was a significantl decrease in weight (p<0.02), BMI (p<0.01), waist circumference (p<0.0001), total cholesterol (p<0.000), LDL cholesterol (p<0.01), triglycerides (p<0.01), saturated fat (p<0.0001), total fat (p<0.0001), total energy (p<0.00001), ≤30% of total energy (p<0.001), ≤ 10% of total energy intake from saturated fat (p<0.001), ≥15 g/1000 kcal of fibre (p<0.001) and a significant increase in physical activity (p<0.05), resistance training (p<0.0001) grams of fibre (p<0.0001), Non-significant improvement was found for other outcomes Adherence: 75 to 77% of participants were present at each telephone follow-up. 62% completed all the 3 sessions. |
| Venditti et al44 2021 USA | Low risk of bias | RCT | 322 | 12 months 24 months |
Primary outcome: change in body weight Secondary outcome: change in waist circumference, fasting lipid profile, fasting glucose, systolic and diastolic BP, nutrition, physical function performance, PA (minutes/per week), achieved ≥3 days of moderate intensity PA per week |
• Intervention: comprehensive evidence-based lifestyle intervention + 8 conference calls for social support and problem • Control: comprehensive evidence-based lifestyle intervention + 4 additional newsletters |
65–80 | • BMI ≥ 27 and at least one cardio-metabolic risk factors 1) large waist circumference 2) HBP or hypertension drug 3) elevated lipids or medication for lipids or triglycerides 4) pre-diabetes or a score of 15 on the American Diabetes Association risk test • No diabetes |
Compared to the control group, the intervention significantly improved weight and weight percentage loss (p=0.01) and BMI (p=0.02) at 12, but not 24, months, and improved HDL at 24 (p=0.01), but not 12, months. No significant results for other outcomes Attendance at the 8 phone group sessions> 85% |
Notes: aPrimary outcome was not relevant for our review.
Abbreviations: UK, United Kingdom; USA, United State of America; RCT, randomized-controlled trial; HbA1c, glycosylated haemoglobin; RT, randomized trial; kg, kilogram; MVPA, moderate to vigorous physical activity; ±, plus or minus; ≥, superior or equal to; ≤, inferior or equal; =, equal to; >, superior to; <, inferior to; +, plus; %, percentage; sd, standard deviation; BMI, Body Mass Index; p, p value; PA, Physical activity; HDL, High Density lipoprotein; LDL, Low Density lipoprotein; SPPB, Short Physical Performance Battery; BP, Blood Pressure; HBP, high Blood Pressure; MoCA, Montreal Cognitive Assessment; AUSDRISK, Australian Type 2 Diabetes Risk; vs, versus; N, number of subjects.
Table 4.
Characteristics of Completed Studies Evaluating the Effect of Text Messaging or Text Messaging with Telephone Calls Interventions on Cardio-Vascular Outcomes
| First Author Year Country | Quality Assessment | Design | N | Length of Intervention Follow-Up (if different) | Cardio-Metabolic Outcomes and Risk Factors | Phone Intervention | Population | Results | |
|---|---|---|---|---|---|---|---|---|---|
| Age | Cardio-Metabolic Outcomes and Risk Factors Criteria | ||||||||
| Bennell et al46 2020 Australia | Low risk of bias | RCT | 110 | 24 weeks | Primary outcome: adherence to home exercise: Self-reported number of exercise sessions, Exercise Adherence Rating Scale Section B and number of days home exercises completed in the past week Secondary outcome: PA scale for the elderly, adherence to home exercise program three times per week |
Both groups had completed a previous RCT of two different exercise programs • Intervention: SMS aiming to support and facilitate adherence to the home exercise program and identify and address the barriers (< 3 sessions in the previous week) • Control: no SMS support |
≥50 | • Obesity (BMI≥30) | Significant differences between intervention and control groups for: • Adherence to home exercise (mean difference 3.1, p=0.01) • Number of days home exercises completed in the past week (mean difference 0.6, p=0.01) No other significant effects on secondary outcomes Completed outcomes at week 24 86% SMS group, 94% for control |
| Jones et al41 2016 USA | Fair | Pre/post pilot study | 40 | 12 weeks | Secondary outcomea: change in • BP • Lipid profile • HbA1c • BMI • Waist circumference •Adherence to cardio-vascular disease risk reducing behaviors |
Intervention: received more than 250 informational + motivational messages designed to reduce cardio-vascular disease risk and cancer risk factors. Reply was possible. |
≥50 | At risk for cancer or cardio-vascular disease (≥2 criteria) • diagnosis of HBP or taking medications or found to be hypertensive by the research team • diagnosis of hyperlipidemia or taking medication, or any lipid abnormality found on screening • diagnosis of type II diabetes or HbA1c >7%• overweight or obese • waist circumference > 40 inches in men and >35 inches in women • sedentary lifestyle |
There were significant improvements from pre to post intervention scores on total cholesterol (p<0.001), LDL cholesterol (p=0.015), waist circumference (p=0.002), systolic BP (p=0.009), diastolic BP (p=0.02), adherence behaviors (p<0.001) There were no effects on HDL cholesterol, triglycerides, HbA1c and BMI increased (p=0.03) |
| Kim et al39 2013 USA | High risk of bias | RCT | 36 | 6 weeks | Change in • Step count • Leisure Time Exercise Questionnaire |
• Intervention: pedometers and walking manual and simple + motivational text messages • Control: pedometers and walking manual |
60–85 | No medical problems that restricted them from walking | There was an improvement in the number of steps per day and on the Leisure Time Exercise Questionnaire score from pre to post intervention in the text messages group (p=0.001) There was no improvement for control |
| Wallis et al48 2017 Australia | Some concerns | RCT | 46 | 12 weeks | Secondary outcomea: PA: daily number of steps, daily minutes spent walking, weekly minutes spent walking at moderate intensity cadence, weekly minutes spent walking at moderate intensity cadence and at least 10 min of continuous bouts, daily hours spent sitting or lying, systolic and diastolic BP, BMI, waist circumference, fasting lipid profile, fasting glucose level 40 meters fast paced walk test, 30-second chair stand | • Intervention: walking sessions and progress monitor with phone call or send weekly SMS reminders • Control: usual care |
≥50 | • Cardiovascular risk profile (at least 2 total risk factors) | ITT analysis: intervention improved the 40-meters walking test and the proportion of participants with systolic BP<140mmHg compared to control PP analysis: intervention improved the 40-meters walking test, and in systolic blood pressure, steps per days, time walking and decreased waist circumference, and BP compared to control. No other associations 70% of the intervention group completed 9 out the 12 sessions of weekly dose (70 minutes ± 10 minutes) |
| Wong et al47 2021 Singapore | Some concern | RCT | 580 | 6 months | Primary outcome: lipid profile, blood glucose, levels of physical activity behavior: self-reported PA Secondary outcome: weight, BMI, percentage of body fat, dietary behaviors, waist and hip circumference, Waist-Hip Ratio, diastolic and systolic BP |
• Intervention: multi-component intervention with nutritionist and program ambassadors telephone calls and healthy text messages • Control: fall prevention booklet |
≥50 | • < 150 minutes of moderate intensity physical activity per week • No medical condition that prohibit involvement in physical activity | Compared to control, intervention improved moderate PA (p<0.001), vigorous PA (p<0.001), total physical activity (p=0.004), intake of fruit (p=0.001), sugar beverages (p=0.019), vegetable (p=0.019), salt and salty sauce (p=0.042), and decreased systolic BP (p=0.020), diastolic BP (p=0.001), % of body fat (p<0.001) No other significant effects were found. Attrition rate 16% for intervention 14% for control |
| Zacharia et al51 2020 Australia | Poor | Pilot study | 17 | 2 weeks | Change in the 14-point Mediterranean Diet Score | The AusMed diet program: 1 education materials, AusMed diet program and individual goal setting + twice weekly supported text messages |
≥55 | – | Compared to pre intervention score, mean Mediterranean Diet score increased at 2 weeks (p < 0.001), Extra Virgin Olive Oil eating vegetable, legume, fish (p = 0.009) and sofrito also significantly increased. Eating pastries or red meat significantly decreased. No significant associations were found for other intakes. Text messages were found of be appropriate and were beneficial in achieving their goals. |
Note: aPrimary outcome was not relevant for our review.
Abbreviations: RCT, randomized Control Trial; USA, United State of America; PA, Physical Activity; SMS, Short Message System; HbA1c, glycosylated haemoglobin; BP, Blood Pressure; HBP, High Blood Pressure; LDL, Low Density Lipoprotein; HDL, High Density Lipoprotein; ITT, Intention To Treat; PP, Per Protocol; <, inferior to; =, equal to; ≥, superior or equal to; >, superior to; +, plus; ±, plus or minus; %, percentage; mmHg, millimeters of mercury; BMI, Body Mass Index; p, p value; N, number of subjects.
Five of the studies included participants from the USA, while the others included participants from the Australia (n=4), UK (n=2), China (n=1), Belgium (n=1), Netherland (n=1), Canada (n=1), South Korea (n=1), Singapore (n=1) and New-Zealand (n=1).
Participants at risk of cardio-metabolic diseases were included in 15 studies, defined by the presence of stroke comorbidities,27,40 type 2 diabetes,36–38,41,42,49 obesity,35,44,46 hypertriglyceridemia and/or high blood pressure41,48 or sedentarism.45,47,50 Interventions lasted between 2 weeks and 24 months.
Telephone vocal support to motivate participants to reach a goal, increase intervention adherence or resolve problems was the most frequent type of telephone intervention used, representing 9 studies’ interventions.27,35–37,40,43–45,50
Automated motivational or reminder text messaging for adherence was used for 5 studies.39,41,46,48,51 A wired telephone-connected glucometer associated with short message service and telephone technical support was evaluated in one study,38 while another study49 studied a self-monitoring dietary intake application for weight loss. The final study42 evaluated a website with motivational sessions to increase physical activity or decrease sedentarism, associated with an optional mobile application with behavior monitoring, goal and notification reminders.
The effect of these interventions on cardio-metabolic outcomes or risk factors was evaluated as a primary outcome in eight studies using various measures, but none used hard clinical outcomes such as cardio-vascular mortality. The outcomes used include hemoglobin A1c (HbA1c),37,38,41 body weight,35,37,38,41,43,44,47 lipid and glycemic profile,35–38,41,43 blood pressure,27,35,37,41,44,48,50 waist and hip circumference,36,37,41,44,47,48 smoking status,40 fruit, vegetable or other consumption27,36,40,43,47,49,51 and physical activity (eg step count) outcomes.27,36,39,40,42–50
The effect of telephone-support or text messaging on these outcomes was discordant. For example, high blood pressure improved in a 12 week single arm study including 40 adults aged 50 years and over at risk of cardiometabolic diseases or cancer with informational and motivational text messaging in one study41 but no effect was found in a 18 months trial evaluating the effect of bi-weekly telephone counselling sessions in obese women aged 50 to 75 years.35 Moreover, interventions involving telephone support seemed to be associated with self-reported dietary changes with improvement in fruit and vegetable consumption after 3 months of risk factor information and healthy behavior with telephone support among 52 stroke or transient ischemic attack adults.40 Vita et al36 found also healthier diet with more fiber intake and lower saturated fat intake, but a high quality study27 find no effect of coaching sessions on fiber and fat intake change after 12 months of follow-up. Intervention effects on HbA1c were discordant in the highest quality studies: an 18-month telephone health coaching intervention37 was not associated with improvement in mean HbA1c compared to the control group, contrary to an 12-month telephone lifestyle counselling intervention which improved HbA1c control compared to a memory training program group.27 Results were also discordant among the highest quality studies of the effect of telephone and smartphone interventions on blood pressure27,37,44 or physical activity,25,44,46 although there were various differences in study population, type of telephone intervention and duration.
Description of Ongoing Studies
Eight studies are being conducted in Australia, four in the US, two in Thailand, one each in Spain, Malaysia, Slovenia, Poland, China, Iran, India, Finland, Singapore and Japan, and three in several locations (one in European countries, one in UK and China and the last one in Europe, Asia and Australia).
Participants are at risk of cardio-metabolic diseases in the majority of studies, defined by stroke comorbidities, type 2 diabetes, overweight or obesity, insufficient physical activity or smoking status (Appendix Table B).
Interventions are lasting between 6 weeks to 48 months and studies are including 30 to 2400 participants.
Mobile-phone applications are being used in 13 studies28–31,52,53 (NCT01307137, NCT04819256, TCTR20211021006, ACTRN12621001136897, ACTRN12621000236897, TCTR20190902004, ISRCTN31471852). For instance, participants with diabetes are using smartphones to record meals and self-monitor weight (NCT04819256). Another application is using an interactive coach supported mobile application31 among participants at risk of dementia.
Telephone vocal support is the second method used in the ongoing cardio-metabolic studies (n=12).
Finally, 2 studies are using both telephone vocal coaching and health or reminder text message interventions (ACTRN12617001022358, JPRN-UMIN000024416).
18 studies are evaluating cardio-metabolic outcomes as primary outcomes using various cardio-metabolic outcomes and risk factors, with physical activity and HbA1c being the most commonly used. Smoking status, lipid or glycemic profile, dietary outcomes or waist and hip circumference, blood pressure, BMI are also being used, but, as in the completed studies, no hard clinical outcomes.
Implementation Results
For all completed studies, adherence was high with more than 70% of participants completing the studies. Measures of adherence, including the number of sessions completed27,36,43,44,46,48,49 or the number of participants who completed the study visit,38 were described in eleven studies.27,35–38,43,44,46–49 Vital et al36 found that 71% of the initial population attended the last follow-up visit with 75 to 77% of participants receiving all follow-up telephone calls. Lim et al38 reported higher adherence (ie 92.2 to 96.1%). Text messaging frequency (twice weekly) was also found to be appropriate.51 Moreover a second study46 evaluated the effect of text messages to support engagement with a home exercises program and found 86% of participants completed the study (94% for control group). Another study49 found that a dietary intake monitoring application was used for 92.7% of the 8-week follow-up period.
When reported, the main reasons for participants withdrawing from studies were being too busy or family/personal issues.38 In the study by Perri et al,35 participants completed a mean of 21.1±5.7 out of 26 telephone sessions. The participants who dropped-out seemed to have higher BMI, lower income, were younger and less often had private insurance, compared to those who did not drop out.
Discussion
We found few high-quality completed studies evaluating the effectiveness of telephone or smartphone-based interventions on cognitive or cardio-metabolic outcomes among middle-age and older adults. Indeed, 21 completed studies were identified, of which only four had a good quality rating, and some of them included both cognitive and cardio-metabolic outcomes. A further 50 other studies are still ongoing. Based on these studies, no conclusions about the efficacy of telephone or smartphone-based interventions on cognition can be made because of a lack of high quality-data, and cardio-metabolic results varied depending on outcomes, interventions or the study population.
There were fewer completed trials evaluating the effects of telephone and notably smartphone-based interventions on cognitive or cardio-metabolic outcomes than we expected, given the large number of smartphone applications currently available. Indeed, many health applications have been developed without any scientific evaluation of their impact. Finally, the use of new technology tools differs in different age groups, and implementation data are still needed for older age groups.
There may be several reasons for the low number of studies identified. First, the mobile phone market is relatively recent, compared to other interfaces (ie computers). Second, mobile phone use initially grew mostly among young adults, although 62% of adults aged 70 and over owned smartphones in 201954 compared to 18% of adults aged 65 years and over in 201311 in the US. However, older adults are still the less likely to be smartphone users compared to younger adults.55 Furthermore, mobile and smartphones were designed for younger populations, and may not always be practical for older adults. Ergonomics should be adapted for age-related features, such as larger buttons or telephone contrast.56 This could help to increase participation and reduce attrition for smartphone interventions in older age groups. Moreover, smartphone applications should also be specifically designed for older populations. For instance, the frequency of daily smartphone access depends on age, and older individuals tend to favor weather and personal information manager applications over communication applications, such as instant messaging, compared to younger individuals.13
Concerning the interventions we reviewed, some used telephone support, but we do not know if they concerned landline and/or mobile phones. Furthermore, many of the studies included in the review were multicomponent interventions and the results relate to the efficacy of the intervention as a whole, and not just the telephone component.
Older adults are usually less often included in clinical trials,57,58 and more particularly in clinical trials using new technologies.59 Furthermore, the individuals included in the study populations might be higher-educated and healthier than the general population, since they firstly accepted to take part in a clinical trial,60 and also because telephone and smartphone ownership depends on socio-economic status.11 This selection of the population may have limited the effects of the interventions. Furthermore, the studies included in our review might be too small to detect intervention effects, since only 3 studies31,36 (JPRN-UMIN000041926) include more than 1000 participants.
High quality study results concerning short and long-term effect on cognition, cardio-metabolic diseases and their common risk factors in middle aged and older adults are therefore still needed. Given the relatively wide-scale availability of commercial applications, we expected that cognitive healthcare applications would have been well-evaluated in clinical trials. With only three completed studies,25–27 we could not draw any conclusions about the effectiveness of telephone-based interventions on cognition. Telephone call support was the main type of telephone intervention identified and none of the completed studies evaluated the effects of telephone or smartphone-based interventions on dementia incidence. Only 2 ongoing trials are evaluating dementia incidence.31 (JPRN-UMIN000041926) However, a meta-analysis61 found a small to moderate effect on cognition for web-based lifestyle intervention compared to control. Additionally, study-follow-up (maximum 24 months) might be too short to expect significant changes in this outcome.
We found discordant results for cardio-metabolic effects of telephone or smartphone-based interventions between studies, and the highest-quality studies27,37,44,46 found discrepant effects on HbA1c, physical activity and blood pressure. Previous literature reviews have also found discordant results. Indeed, Widmer et al62 reviewed the effect of any digital health intervention on cardio-metabolic outcomes and found that results depended on whether the interventions were used for primary or secondary prevention. For example, beneficial effects of digital interventions were found on systolic blood pressure for participants having cardio-metabolic risk factors (primary prevention) but not for those who had cardio-metabolic diseases (secondary prevention). However, high blood pressure decreased with telephone support after a myocardial infarction in a meta-analysis.63 We did not find different effects of telephone and smartphone interventions based on primary or secondary prevention in older adults. Moreover, mobile technology (smartphone and wearable sensor) seemed to be associated with better management of cardio-metabolic diseases or risk factors among community-dwelling adults64 and a systematic review and meta-analysis based on web-based prevention of cardio-vascular outcomes found 57 studies with significant effects on blood pressure, HbA1c and weight.59 Nevertheless, these reviews covered any kind of digital health intervention, and these studies evaluated young as well as older adults.
Our review shows that telephone and smartphone-based interventions suitable for older adults are still in the development phase, since there are relatively few eligible completed studies. However, they could be promising in older age groups since studies have showed that their use is feasible and a major application promoting health aging is currently being implemented in clinical practice.65
Our review has some limitations. First, our search equation included the term “prevention” which may have limited the number of relevant articles identified but we considered that this was in important term, since our aim was to evaluate telephone and smartphone for the prevention of cognitive and cardio-metabolic disease and their risk factors. However, we also scrutinized reference lists from the studies we identified, but this only led to the identification of one further study, which suggests that our original search was exhaustive. Secondly, the majority of included articles had poor or fair quality scores, and few results have so far been published in older populations, thus limiting the evidence base on which to draw conclusions. Moreover, for some studies (notably the ongoing studies identified in clinical trial registries), data were not always very detailed which limited the exhaustiveness of our descriptions of interventions or outcomes.
Research on telephone and smartphone interventions in older populations is in its early stages. However, some recommendations can be made to improve future randomized trials in this field. Digital tools (eg emails, social media advertisements) are effective for recruiting older participants66 even those with mild cognitive impairment.67 However, it is indispensable to take into account limitations, barriers and needs of the target population to better adapt devices and interventions.68,69 One of the main barriers to older people using mobile technology and devices should be as simple as possible and if applicable, easy to wear.68 Motivational behavior change techniques should be used to improve engagement with interventions69 and therefore efficacy. Moreover, to improve engagement and digital literacy, a session to explain how to use study device with trained-staff is recommended. Reminders (by telephone calls or text messaging) could also improve engagement as well as using supervised interventions.70 Implementation evaluation is also indispensable, in order to provide better recommendations. Finally, it is important to evaluate multiple outcomes associated with similar risk factors as was done in some of studies we identified,27,29–34 since interventions could have simultaneous benefits on multiple age-related disease outcomes by improving these factors.
Conclusions
Few studies on telephone and smartphone for the prevention of dementia and cardio-metabolic diseases have been specifically performed in middle-aged and older adults, and few are at low of risk of bias. Most completed studies reported no statistically significant effects of their interventions. Many studies are ongoing and can be classified as pilot or Phase 2 studies. Overall, in spite of the wealth of mobile health applications available and given a lack of research data and evaluation at the current time, we cannot demonstrate the supplementary value of such technologies compared to usual intervention strategies. Nonetheless, this is a growing area of research which will help to develop patient-centered approaches to prevention, and several new studies are underway, meaning that efficacy and implementation results for middle-aged or older adults should be available in the near future.
Acknowledgments
This review was performed as part of the Prodemos Project. The research leading to these results has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement no 779238 and the National Key R&D Programme of China (2017YFE0118800). The members of the PRODEMOS group are: Edo Richard, Pim van Gool, Eric Moll van Charante, Marieke Hoevenaar-Blom, Esmé Eggink, Melanie Hafdi, Patrick Witvliet (Academic Medical Center, University of Amsterdam, Amsterdam, The Netherlands); Carol Brayne, Linda Barnes, Rachael Brooks (University of Cambridge, Cambridge, UK); Wei Wang, Wenzhi Wang, Youxin Wang, Manshu Song (Capital Medical University, Beijing, China; Edith Cowan University (ECU), Perth Australia); Anders Wimo, Ron Handels (Karolinska Institutet, Stockholm, Sweden); Sandrine Andrieu, Nicola Coley (INSERM UMR1295, Toulouse France); Jean Georges, Cindy Birck (Alzheimer Europe, Luxembourg); Bram van de Groep, Rick Mast (Vital Health Software, Ede, the Netherlands). The present work was also performed in the context of the Inspire Program, a research platform supported by grants from the Region Occitanie/Pyrénées-Méditerranée (Reference number: 1901175) and the European Regional Development Fund (ERDF) (Project number: MP0022856).
Disclosure
Dr Sandrine Andrieu reports grants from CHU, Université Paul Sabatier, UMR1295, Toulouse, during the conduct of the study; personal fees from Roche, outside the submitted work. The authors declare no other conflicts of interest relevant to the current work.
References
- 1.World Health Organization. The Global strategy and action plan on ageing and health. WHO. Available from: http://www.who.int/ageing/global-strategy/en/. Accessed November 20, 2017. [Google Scholar]
- 2.Non communicable diseases. Available from: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases. Accessed February 14, 2022.
- 3.Budreviciute A, Damiati S, Sabir DK, et al. Management and prevention strategies for Non-communicable Diseases (NCDs) and their risk factors. Front Public Health. 2020;8:574111. doi: 10.3389/fpubh.2020.574111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Norton S, Matthews FE, Barnes DE, Yaffe K, Brayne C. Potential for primary prevention of Alzheimer’s disease: an analysis of population-based data. Lancet Neurol. 2014;13(8):788–794. doi: 10.1016/S1474-4422(14)70136-X [DOI] [PubMed] [Google Scholar]
- 5.Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396(10248):413–446. doi: 10.1016/S0140-6736(20)30367-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kutner M, Greenberg E, Jin Y, Paulsen C. The Health Literacy of America’s Adults: results From the 2003 National Assessment of Adult Literacy (NCES 2006–483). Washington, DC: U.S. Department of Education, National Center for Education Statistics; 2006: 76. [Google Scholar]
- 7.Kaba Z, Khamisa N, Tshuma N. Age-group differences in risk perceptions of non-communicable diseases among adults in Diepsloot township, Johannesburg, South Africa: a cross-sectional study based on the health belief model. S Afr Med J. 2017;107(9):797–804. doi: 10.7196/SAMJ.2017.v107i9.12168 [DOI] [PubMed] [Google Scholar]
- 8.Bastawrous A, Hennig B, Livingstone I. mHealth possibilities in a changing world. Distribution of global cell phone subscriptions. J Mob Technol Med. 2013;2(1S):22–25. doi: 10.7309/jmtm.2.1.4 [DOI] [Google Scholar]
- 9.How many people have smartphones worldwide; February, 2022. Available from: https://www.bankmycell.com/blog/how-many-phones-are-in-The-world. Accessed February 3, 2022.
- 10.Healthcare apps available apple app store 2020 | Statista. Available from: https://www.statista.com/statistics/779910/health-apps-available-ios-worldwide/. Accessed August 30, 2020.
- 11.Technology use among seniors; May 17, 2017. Available from: http://www.pewinternet.org/2017/05/17/technology-use-among-seniors/. Accessed October 4, 2018.
- 12.Technology use among seniors. Pew Research Center: Internet, Science & Tech; May 17, 2017. Available from: https://www.pewresearch.org/internet/2017/05/17/technology-use-among-seniors/. Accessed February 17, 2022. [Google Scholar]
- 13.Rosales A, Fernández-Ardèvol M. Beyond WhatsApp: older people and smartphones. Romanian J Commun Public Relat. 2016;18(1):27–47. doi: 10.21018/rjcpr.2016.1.200 [DOI] [Google Scholar]
- 14.Şahin E, Yavuz Veizi BG, Naharci MI. Telemedicine interventions for older adults: a systematic review. J Telemed Telecare. 2021;1357633X211058340. doi: 10.1177/1357633X211058340 [DOI] [PubMed] [Google Scholar]
- 15.Goldberg EM, Jiménez FN, Chen K, et al. Telehealth was beneficial during COVID-19 for older Americans: a qualitative study with physicians. J Am Geriatr Soc. 2021;69(11):3034–3043. doi: 10.1111/jgs.17370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greenwald P, Stern ME, Clark S, Sharma R. Older adults and technology: in telehealth, they may not be who you think they are. Int J Emerg Med. 2018;11(1):2. doi: 10.1186/s12245-017-0162-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Castilla D, Botella C, Miralles N, et al. Teaching digital literacy skills to the elderly using a social network with linear navigation: a case study in a rural area. Int J Hum Comput Stud. 2018;118:24–37. doi: 10.1016/j.ijhcs.2018.05.009 [DOI] [Google Scholar]
- 18.Lam K, Lu AD, Shi Y, Covinsky KE. Assessing telemedicine unreadiness among older adults in the United States during the COVID-19 pandemic. JAMA Intern Med. 2020;180(10):1389–1391. doi: 10.1001/jamainternmed.2020.2671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steinhubl SR, Wolff-Hughes DL, Nilsen W, Iturriaga E, Califf RM. Digital clinical trials: creating a vision for the future. NPJ Digit Med. 2019;2(1):126. doi: 10.1038/s41746-019-0203-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Piau A, Crissey R, Brechemier D, Balardy L, Nourhashemi F. A smartphone Chatbot application to optimize monitoring of older patients with cancer. Int J Med Inform. 2019;128:18–23. doi: 10.1016/j.ijmedinf.2019.05.013 [DOI] [PubMed] [Google Scholar]
- 21.Diaz-Asper C, Chandler C, Turner RS, Reynolds B, Elvevåg B. Acceptability of collecting speech samples from the elderly via the telephone. Digit Health. 2021;7:20552076211002104. doi: 10.1177/20552076211002103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.König A, Sacco G, Bensadoun G, et al. The role of information and communication technologies in clinical trials with patients with Alzheimer’s disease and related disorders. Front Aging Neurosci. 2015;7:110. doi: 10.3389/fnagi.2015.00110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 24.Vlahov D. Transparent Reporting of Evaluations with Nonrandomized Designs (TREND). J Urban Health. 2004;81(2):163–164. doi: 10.1093/jurban/jth099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumar S, Tran JL, Moseson H, et al. The impact of the virtual cognitive health program on the cognition and mental health of older adults: pre-post 12-month pilot study. JMIR Aging. 2018;1(2):2. doi: 10.2196/12031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oh SJ, Seo S, Lee JH, Song MJ, Shin MS. Effects of smartphone-based memory training for older adults with subjective memory complaints: a randomized controlled trial. Aging Ment Health. 2018;22(4):526–534. doi: 10.1080/13607863.2016.1274373 [DOI] [PubMed] [Google Scholar]
- 27.Sakakibara BM, Lear SA, Barr SI, et al. Telehealth coaching to improve self-management for secondary prevention after stroke: a randomized controlled trial of Stroke Coach. Int J Stroke. 2021:17474930211017700. doi: 10.1177/17474930211017699. [DOI] [PubMed] [Google Scholar]
- 28.Taraldsen K, Mikolaizak AS, Maier AB, et al. Protocol for the PreventIT feasibility randomised controlled trial of a lifestyle-integrated exercise intervention in young older adults. BMJ Open. 2019;9(3):3. doi: 10.1136/bmjopen-2018-023526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Summers MJ, Rainero I, Vercelli AE, et al. The My Active and Healthy Aging (My-AHA) ICT platform to detect and prevent frailty in older adults: randomized control trial design and protocol. Alzheimers Dement. 2018;4(1):252–262. doi: 10.1016/j.trci.2018.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Recio-Rodríguez JI, Lugones-Sanchez C, Agudo-Conde C, et al. Combined use of smartphone and smartband technology in the improvement of lifestyles in the adult population over 65 years: study protocol for a randomized clinical trial (EVIDENT-Age study). BMC Geriatr. 2019;19(1). doi: 10.1186/s12877-019-1037-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eggink E, Hafdi M, Hoevenaar-Blom MP, et al. Prevention of dementia using mobile phone applications (PRODEMOS): protocol for an international randomised controlled trial. BMJ Open. 2021;11(6):e049762. doi: 10.1136/bmjopen-2021-049762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cox KL, Cyarto EV, Etherton-Beer C, et al. A randomized controlled trial of physical activity with individual goal-setting and volunteer mentors to overcome sedentary lifestyle in older adults at risk of cognitive decline: the INDIGO trial protocol. BMC Geriatr. 2017;17(1):215. doi: 10.1186/s12877-017-0617-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Edgren J, Karinkanta S, Rantanen T, et al. Counselling for physical activity, life-space mobility and falls prevention in old age (COSMOS): protocol of a randomised controlled trial. BMJ Open. 2019;9(9):9. doi: 10.1136/bmjopen-2019-029682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ponvel P, Shahar S, Singh DKA, et al. Multidomain intervention for reversal of cognitive frailty, towards a personalized approach (AGELESS Trial): study design. J Alzheimers Dis. 2021;82(2):673–687. doi: 10.3233/JAD-201607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perri MG, Limacher MC, Durning PE, et al. Extended-care programs for weight management in rural communities: the treatment of obesity in underserved rural settings (TOURS) randomized trial. Arch Intern Med. 2008;168(21):2347–2354. doi: 10.1001/archinte.168.21.2347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vita P, Cardona-Morrell M, Bauman A, et al. Type 2 diabetes prevention in the community: 12-Month outcomes from the Sydney Diabetes Prevention Program. Diabetes Res Clin Pract. 2016;112:13–19. doi: 10.1016/j.diabres.2015.11.010 [DOI] [PubMed] [Google Scholar]
- 37.Chapman A, Browning CJ, Enticott JC, et al. Effect of a health coach intervention for the management of individuals with type 2 diabetes mellitus in china: a pragmatic cluster randomized controlled trial. Front Public Health. 2018;6:252. doi: 10.3389/fpubh.2018.00252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lim S, Kang SM, Shin H, et al. Improved glycemic control without hypoglycemia in elderly diabetic patients using the ubiquitous healthcare service, a new medical information system. Diabetes Care. 2011;34(2):308–313. doi: 10.2337/dc10-1447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim BH, Glanz K. Text messaging to motivate walking in older African Americans: a randomized controlled trial. Am J Prev Med. 2013;44(1):71–75. doi: 10.1016/j.amepre.2012.09.050 [DOI] [PubMed] [Google Scholar]
- 40.Gillham S, Endacott R. Impact of enhanced secondary prevention on health behaviour in patients following minor stroke and transient ischaemic attack: a randomized controlled trial. Clin Rehabil. 2010;24(9):822–830. doi: 10.1177/0269215510367970 [DOI] [PubMed] [Google Scholar]
- 41.Jones AR, Moser DK, Hatcher J. Using text messages to promote health in African-Americans: #HeartHealthyandCancerFree. Ethn Health. 2018;23(3):307–320. doi: 10.1080/13557858.2016.1263289 [DOI] [PubMed] [Google Scholar]
- 42.Poppe L, De Bourdeaudhuij I, Verloigne M, et al. Efficacy of a self-regulation-based electronic and mobile health intervention targeting an active lifestyle in adults having type 2 diabetes and in adults aged 50 years or older: two randomized controlled trials. J Med Internet Res. 2019;21(8):e13363. doi: 10.2196/13363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aunger JA, Greaves CJ, Davis ET, Asamane EA, Whittaker AC, Greig CA. A novel behavioural INTErvention to REduce Sitting Time in older adults undergoing orthopaedic surgery (INTEREST): results of a randomised-controlled feasibility study. Aging Clin Exp Res. 2020;32(12):2565–2585. doi: 10.1007/s40520-020-01475-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Venditti EM, Marcus MD, Miller RG, Arena VC, Greenspan SL, Rockette-Wagner B. Group lifestyle phone maintenance for weight, health, and physical function in adults aged 65–80 years: a randomized clinical trial. J Gerontol a Biol Sci Med Sci. 2021;76(2):352–360. doi: 10.1093/gerona/glaa229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kolt GS, Schofield GM, Kerse N, Garrett N, Oliver M. Effect of telephone counseling on physical activity for low-active older people in primary care: a randomized, controlled trial. J Am Geriatr Soc. 2007;55(7):986–992. doi: 10.1111/j.1532-5415.2007.01203.x [DOI] [PubMed] [Google Scholar]
- 46.Bennell K, Nelligan RK, Schwartz S, et al. Behavior change text messages for home exercise adherence in knee osteoarthritis: randomized trial. J Med Internet Res. 2020;22(9):e21749. doi: 10.2196/21749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wong EYS, James AP, Lee AH, Jancey J. Effectiveness of a Singaporean community-based physical activity and nutrition intervention: a cluster randomized controlled trial. Asia Pac J Public Health. 2021;33(2–3):196–204. doi: 10.1177/1010539520977311 [DOI] [PubMed] [Google Scholar]
- 48.Wallis JA, Webster KE, Levinger P, Singh PJ, Fong C, Taylor NF. A walking program for people with severe knee osteoarthritis did not reduce pain but may have benefits for cardiovascular health: a Phase II randomised controlled trial. Osteoarthritis Cartilage. 2017;25(12):1969–1979. doi: 10.1016/j.joca.2016.12.017 [DOI] [PubMed] [Google Scholar]
- 49.Zheng Y, Weinger K, Gregas M, et al. Acceptability of a self-regulation theory-based mhealth behavior intervention for older adults with type 2 diabetes and obesity. Diabetes. 2018;67(Supplement_1):826–P. doi: 10.2337/db18-826-P [DOI] [Google Scholar]
- 50.Hartman YW, Tillmans LCM, Benschop DL, et al. Long-term and acute benefits of reduced sitting on vascular flow and function. Med Sci Sports Exerc. 2021;53(2):341–350. doi: 10.1249/MSS.0000000000002462 [DOI] [PubMed] [Google Scholar]
- 51.Zacharia K, Patterson AJ, English C, MacDonald-Wicks L. Feasibility of the AusMed diet program: translating the Mediterranean diet for older Australians. Nutrients. 2020;12(4):1044. doi: 10.3390/nu12041044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fanning J, Opina MT, Leng I, Lyles MF, Nicklas BJ, Rejeski WJ. Empowered with Movement to Prevent Obesity & Weight Regain (EMPOWER): design and methods. Contemp Clin Trials. 2018;72:35–42. doi: 10.1016/j.cct.2018.07.010 [DOI] [PubMed] [Google Scholar]
- 53.Wong AKC, Wong FKY, Chang KKP. A proactive mobile health application program for promoting self-care health management among older adults in the community: study protocol of a three-arm randomized controlled trial. Gerontology. 2020;66(5):506–513. doi: 10.1159/000509129 [DOI] [PubMed] [Google Scholar]
- 54.2020 Tech and the 50+ Survey - AARP. Available from: https://www.aarp.org/research/topics/technology/info-2019/2020-technology-trends-older-americans.html. Accessed August 30, 2020.
- 55.NW 1615 L. St, Washington S 800, Inquiries D 20036 U 419 4300 | m 419 4349 | f 419 4372 | m. Demographics of Mobile Device Ownership and Adoption in the United States | Pew Research Center. Available from: https://www.pewinternet.org/fact-sheet/mobile/. Accessed May 9, 2019.
- 56.Farage MA, Miller KW, Ajayi F, Hutchins D. Design principles to accommodate older adults. Glob J Health Sci. 2012;4(2):2–25. doi: 10.5539/gjhs.v4n2p2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Beers E, Moerkerken DC, Leufkens HGM, Egberts TCG, Jansen PAF. Participation of older people in preauthorization trials of recently approved medicines. J Am Geriatr Soc. 2014;62(10):1883–1890. doi: 10.1111/jgs.13067 [DOI] [PubMed] [Google Scholar]
- 58.Nguyen QD, Peters E, Wassef A, Desmarais P, Rémillard-Labrosse D, Tremblay-Gravel M. Evolution of age and female representation in the most-cited randomized controlled trials of cardiology of the last 20 years. Circ Cardiovasc Qual Outcomes. 2018;11(6):e004713. doi: 10.1161/CIRCOUTCOMES.118.004713 [DOI] [PubMed] [Google Scholar]
- 59.Beishuizen CRL, Stephan BCM, van Gool WA, et al. Web-based interventions targeting cardiovascular risk factors in middle-aged and older people: a systematic review and meta-analysis. J Med Internet Res. 2016;18(3):e55. doi: 10.2196/jmir.5218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mosenifar Z. Population issues in clinical trials. Proc Am Thorac Soc. 2007;4(2):185–187;discussion 187–188. doi: 10.1513/pats.200701-009GC [DOI] [PubMed] [Google Scholar]
- 61.Wesselman LM, Hooghiemstra AM, Schoonmade LJ, de Wit MC, van der Flier WM, Sikkes SA. Web-based multidomain lifestyle programs for brain health: comprehensive overview and meta-analysis. JMIR Ment Health. 2019;6(4):4. doi: 10.2196/12104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Widmer RJ, Collins NM, Collins CS, West CP, Lerman LO, Lerman A. Digital health interventions for the prevention of cardiovascular disease: a systematic review and meta-analysis. Mayo Clin Proc. 2015;90(4):469–480. doi: 10.1016/j.mayocp.2014.12.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kotb A, Hsieh S, Wells GA. The Effect of telephone support interventions on Coronary Artery Disease (CAD) patient outcomes during cardiac rehabilitation: a systematic review and meta-analysis. PLoS One. 2014;9(5):5. doi: 10.1371/journal.pone.0096581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Burke LE, Ma J, Azar KMJ, et al. Current science on consumer use of mobile health for cardiovascular disease prevention: a scientific statement from the American Heart Association. Circulation. 2015;132(12):1157–1213. doi: 10.1161/CIR.0000000000000232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tavassoli N, Piau A, Berbon C, et al. Framework Implementation of the INSPIRE ICOPE-CARE program in collaboration with the World Health Organization (WHO) in the Occitania region. J Frailty Aging. 2020:1–7. doi: 10.14283/JFA.2020.26. [DOI] [PubMed] [Google Scholar]
- 66.Miller EG, Nowson CA, Dunstan DW, et al. Recruitment of older adults with type 2 diabetes into a community-based exercise and nutrition randomised controlled trial. Trials. 2016;17(1):467. doi: 10.1186/s13063-016-1589-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shadyab AH, LaCroix AZ, Feldman HH, et al. Recruitment of a multi-site randomized controlled trial of aerobic exercise for older adults with amnestic mild cognitive impairment: the EXERT trial. Alzheimers Dement. 2021;17(11):1808–1817. doi: 10.1002/alz.12401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kruse CS, Mileski M, Moreno J. Mobile health solutions for the aging population: a systematic narrative analysis. J Telemed Telecare. 2017;23(4):439–451. doi: 10.1177/1357633X16649790 [DOI] [PubMed] [Google Scholar]
- 69.Pach D, Rogge AA, Wang J, Witt CM. Five lessons learned from randomized controlled trials on mobile health interventions: consensus procedure on practical recommendations for sustainable research. JMIR Mhealth Uhealth. 2021;9(2):e20630. doi: 10.2196/20630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Coley N, Ngandu T, Lehtisalo J, et al. Adherence to multidomain interventions for dementia prevention: data from the FINGER and MAPT trials. Alzheimers Dement. 2019;15(6):729–741. doi: 10.1016/j.jalz.2019.03.005 [DOI] [PubMed] [Google Scholar]