Abstract
Insulin is a peptide secreted by the pancreas and plays an important role in the regulation of glucose metabolism in peripheral tissues. Although the role of insulin in the periphery is well understood, less is known about its multifactorial role in the brain. However, emerging evidence from human and animal studies indicate that insulin influences cerebral bioenergetics, enhances synaptic viability and dendritic spine formation, and increases turnover of neurotransmitters, such as dopamine. Insulin also has a role in proteostasis, influencing clearance of the amyloid β peptide and phosphorylation of tau, which are hallmarks of Alzheimer’s disease. Insulin also modulates vascular function through effects on vasoreactivity, lipid metabolism, and inflammation. Through these multiple pathways, insulin dysregulation could contribute to neurodegeneration. Thus, new approaches to restore cerebral insulin function that could offer therapeutic benefit to adults with Alzheimer’s disease, vascular cognitive impairment, or related disorders are being investigated.
Introduction
Alzheimer’s disease is the leading cause of dementia and its prevalence is expected to increase in response to an ageing population.1 There are no disease-modifying treatments for Alzheimer’s disease; current approaches target symptoms without addressing the underlying pathology, and their clinical benefit are limited in scope and duration.2 Drugs that reduce the aggregation or production of amyloid β (Aβ), the hallmark pathology of Alzheimer’s disease, have been extensively studied but yielded no treatment effect.3 These disappointing results highlight the need for a better understanding of Alzheimer’s disease and related disorders, including the mechanisms underlying the aggregation of Aβ and tau and other contributory factors (eg, synaptic loss and neuroinflammation), thereby enabling identification of alternative therapeutic options. One such area of research focuses on impaired brain metabolism and, in particular, the role of insulin. Insulin has long been implicated in Alzheimer’s disease, with studies suggesting that adults with Alzheimer’s disease show dysregulated insulin functioning in peripheral tissues.4 One of the most well known effects of impaired peripheral insulin regulation is the presence of hyperglycaemia, which can negatively affect brain function through a variety of mechanisms, such as glucose neurotoxicity, vascular injury, and accumulation of advanced glycation end products.5 A discussion of these complex effects is outside the scope of this Review. This Review focusses on the evidence regarding the role of dysregulated brain insulin in Alzheimer’s disease and related disorders and discusses the implications of this work for the identification of mechanistic pathways and novel therapeutic approaches. Specifically, we discuss aspects of insulin dysregulation that can occur independently or concomitantly with hyperglycaemia and affect key features of Alzheimer’s disease pathology, including Aβ and tau aggregation and synaptic loss, as well as associated pathologies, such as bioenergetic deficiencies, vascular dysfunction, inflammation, and dyslipidaemia. This Review evaluates research on brain insulin resistance as a risk factor for Alzheimer’s disease and related disorders, with a particular focus on Alzheimer’s disease and the relationship of vascular factors to Alzheimer’s disease pathology and symptoms. First, we discuss methods for assessing insulin resistance in the brain and peripheral tissues. Second, we review the results of clinical trials on improving brain insulin function and discuss their implications for development of effective therapies. We conclude with a discussion of potential avenues for future research.
Definition and assessment of brain insulin resistance
Insulin is a peptide hormone secreted principally by pancreatic β-cells and is well known for regulating glucose metabolism in peripheral tissues. Insulin also has a multifaceted role in the CNS.6 In normal physiology, insulin readily crosses the blood–brain barrier (BBB) via a receptor-mediated transport process, and the rate of transport can be modulated by several conditions, such as obesity and inflammation. Insulin can also access brain regions that are unprotected by the BBB, such as the hypothalamus.7 There is debate as to whether insulin is produced within the CNS, although rodent studies have documented insulin mRNA in brain and release of insulin from GABAergic interneurons and choroid plexus epithelial cells.8,9
Insulin resistance is defined as a failure of target tissues to mount a normal response to insulin.10 In the peripheral tissue, insulin resistance is commonly assessed with the Homoeostatic Model Assessment for Insulin Resistance (HOMA-IR).11,12 The gold standard assessment is provided by the hyperinsulinemic-euglycaemic (HI-EG) clamp, 13 during which insulin is infused intravenously at a constant rate while dextrose is infused variably to maintain euglycaemia. Higher rates of dextrose infusion indicate greater insulin sensitivity. The HI-EG clamp increases concentrations of insulin in the CNS,14 and has been paired with techniques such as MRI, EEG, and magnetoencephalography (MEG) to examine the effects of insulin on the brain and the relationship of insulin sensitivity in the brain and the periphery.15 One limitation of the HI-EG clamp is that BBB transport of insulin might be reduced as a result of chronic hyperinsulinaemia or other factors associated with insulin resistance in the periphery;7 thus some uncertainty exists about the amount of insulin reaching the CNS during the clamp.
An alternative approach uses intranasal administration to bypass the BBB and deliver insulin directly to the CNS, with minimal increase of insulin in the periphery.16,17 Typically, the effects of intranasal insulin are compared with placebo during controlled stimulation with measures, such as food images and memory tests, in healthy adults compared with those who have a relevant condition (eg, obesity or cognitive impairment). Failure of intranasal insulin to invoke a neuroimaging response (measured with neuroimaging methods such as MRI or PET) or a neurophysiological response in healthy adults is interpreted as evidence of brain insulin resistance, as summarised elsewhere.15,18 Studies using intranasal insulin paired with controlled stimulation have shown that insulin resistance in the peripherical tissues of people with related disorders (eg, obesity and type 2 diabetes) is associated with a reduced response to intranasal insulin in the CNS, as measured by functional MRI, EEG, or MEG.15,18 Moreover, improving peripheral metabolism in patients with obesity and diabetes through dietary restrictions has been shown to restore CNS insulin sensitivity, and treating CNS insulin sensitivity improves peripheral metabolism.18 Thus, there is a close (and often reciprocal) relationship between the function of insulin in peripheral tissues and the brain. Whether insulin resistance in the periphery and CNS can exist independently is not clear yet. Similarly, there is no accepted criterion for classifying a neuroimaging response (measured by MRI or PET) or neurophysiological response as indicative of brain insulin resistance.
On a molecular level, insulin insensitivity in the periphery or CNS can reflect a wide range of underlying changes, which include reduced insulin receptor concentrations, reduced binding affinity of insulin, and disruption of the insulin signalling cascade. Insulin signals through two main pathways: the Ras-Mitogen Activated Protein kinase (MAPK) and the PI3K-Akt pathways.19 The activation of an insulin receptor leads to the recruitment of insulin receptor substrate proteins. These proteins can be phosphorylated at various tyrosine sites to stimulate activation of the PI3K-Akt pathway, while phosphorylation on serine residues inhibits the pathway. The ratio of serine-phosphorylated insulin receptor substrate to total phosphorylated insulin receptor substrate can be used as a marker of insulin resistance in either the brain or peripheral tissues, with a greater ratio indicating increased insulin resistance. This ratio, together with ex vivo stimulation of brain tissue with insulin, has been successfully applied to show brain insulin resistance in patients with Alzheimer’s disease.20,21
One method that holds great promise for providing a reliable and accessible marker of brain insulin resistance is derived from neuronal-enriched extracellular vesicles in plasma, in which phosphorylation changes in insulin signalling (that signify neuronal insulin resistance) can be measured.22 Neuronal-enriched extracellular vesicle markers of insulin resistance are increased in people with type 2 diabetes and those with Alzheimer’s disease.23
Insulin resistance in neurodegenerative diseases
Several studies have investigated the relationship between insulin resistance in the periphery and brain and Alzheimer’s disease and related disorders. We review studies of Alzheimer’s disease, the most common neurodegenerative disease, and then turn to vascular cognitive impairment, which can occur independently or together with Alzheimer’s disease.
Alzheimer’s disease
Two reviews have extensively discussed the link between insulin metabolism and Alzheimer’s disease.6,12 One study showed that adults with Alzheimer’s disease had reduced peripheral insulin sensitivity and hyperinsulinaemia in both fasting states and in response to an oral glucose tolerance test.24 Given that chronic peripheral hyperinsulinaemia downregulates BBB insulin receptors and decreases the amount of insulin transported into the brain,25 peripheral hyperinsulinaemia might result in lower brain insulin concentrations in patients with Alzheimer’s disease.26 Additionally, reduced or mislocalised (ie, not located on the membrane surface) insulin receptors and decreased receptor affinity for insulin in the brain has been reported in patients with Alzheimer’s disease compared with controls.27
Insulin affects Alzheimer’s disease pathology directly via its interactions with the Aβ peptide.12 Insulin protects against Aβ synaptotoxicity and modulates clearance through its effects on lipid metabolism and proteases, such as the insulin degrading enzyme.12 A study showed that insulin resistance in the periphery in patients with Alzheimer’s disease was positively correlated with brain Aβ deposition in the frontal and temporal areas.28 Peripheral insulin resistance might precede Aβ accumulation as shown by a study in which midlife HOMA-IR predicted Aβ aggregation, as assessed by amyloid PET 15 years after HOMA-IR was measured.29 Peripheral insulin resistance was also associated with increased accumulation of Aβ over the course of 2 years in cognitively healthy adults who were Aβ positive compared with those who were Aβ negative.30,31
Although these studies have reported associations between midlife insulin resistance (as assessed by HOMA-IR) and amyloid deposition or future onset of Alzheimer’s disease, the association of amyloid β and type 2 diabetes—a condition strongly associated with insulin-resistance—is more complex. Although there is evidence for a association between type 2 diabetes and Alzheimer’s disease,32 several neuropathological studies failed to show an association between type 2 diabetes and Alzheimer’s disease pathology.33-35 It has been suggested that type 2 diabetes and Alzheimer’s disease could share pathogenic mechanisms that similarly affect cognition that are downstream from amyloid β in Alzheimer’s disease, such as increased inflammation and oxidative stress, dyslipidaemia, impaired mitochondrial and synaptic function, and impaired brain insulin signalling.33 Medications used to treat diabetes might also affect the severity of Alzheimer’s disease pathology, such as amyloid plaques and neurofibrillary tangles. This possibility has been addressed in two neuropathological studies in which patients’ diabetes treatment status was carefully documented. Brains from adults with Alzheimer’s disease and with untreated diabetes had amyloid pathology similar to adults with Alzheimer’s disease without diabetes, whereas adults who were clinically diagnosed with Alzheimer’s disease, who had diabetes and were treated with insulin and oral medications, had reduced amyloid pathology compared with adults who did not have Alzheimer’s disease or diabetes.36,37
With regard to tau, although neuropathological studies do not indicate increased neurofibrillary tangle deposition in people with type 2 diabetes,33 one meta-analysis reported increased CSF tau concentration in people with type 2 diabetes compared with adults without diabetes and who were cognitively healthy.38 Colocalisation of tangles and insulin resistance markers have also been shown in a neuropathological study of Alzheimer’s disease.12 Adults diagnosed with both Alzheimer’s disease and type 2 diabetes had more extensive neocortical tau deposition assessed with 11C-PBB3 PET, a pattern observed more commonly in people who have diabetes who received insulin therapy and who reported more frequent episodes of hypoglycaemia.39 In cognitively healthy adults, increased peripheral insulin resistance was correlated with worse cognitive scores and higher concentrations of both CSF phosphorylated and total tau.40 Conversely, tau can also affect insulin signalling. In preclinical studies, tau knock out mice had reduced brain insulin sensitivity, defects in synaptic plasticity, and impairments in short-term memory.41 This interaction between tau and insulin might conceivably lead to a vicious cycle, resulting in neurodegeneration and cognitive impairment. Such a possibility is consistent with reports that peripheral insulin resistance, as measured by the HOMA-IR, was negatively correlated with grey matter volume in the medial prefrontal cortex and temporal and parietal regions in adults with Alzheimer’s disease.42 Furthermore, neuronal-enriched extracellular vesicle markers of brain insulin resistance also correlated with grey matter atrophy,43,44 predicted development of future clinical Alzheimer’s disease, and measures of tau and cognition.31,45
Synaptic compromise is thought to be present at the earliest stages (ie, at the initiation of the Alzheimer’s pathological cascade) of Alzheimer’s disease. Insulin has well documented effects on synapse formation and maintenance, with corresponding effects on long-term potentiation and depression.12 Brain insulin resistance impairs synaptic integrity, and Aβ and tau can also interfere with the actions of insulin at the synapse.46 In animal models, intranasal insulin enhanced synaptic function.47,48 A lower cerebral metabolic rate of glucose use (assessed with 18F-fluorodeoxyglucose PET [FDG-PET]) could be an early marker of synaptic or neuronal injury or neurodegeneration, and was shown in adults with Alzheimer’s disease and in middle-aged adults at risk of Alzheimer’s disease.49 Peripheral insulin resistance (assessed by HOMA-IR) has been associated with lower glucose metabolism using FDG-PET in regions that are susceptible to Alzheimer’s disease (eg, posterior cingulate) in adults who are middle aged and cognitively healthy with pre-diabetes or newly diagnosed, untreated type 2 diabetes compared with adults without diabetes who are cognitively healthy.50
Vascular cognitive impairment
Vascular cognitive impairment is the second leading cause of dementia,51 often termed vascular dementia. The heterogeneous causes and classification of vascular cognitive impairment and vascular dementia have been extensively reviewed elsewhere.52 Vascular cognitive impairment can result from widespread small vessel dysfunction, which adversely affects cerebral perfusion, cerebrovascular reactivity, and BBB and white matter integrity.53,54 Although vascular cognitive impairment and its associated pathophysiology are most commonly attributed to hypertension, the degree to which peripheral and brain insulin resistance contributes to the underlying pathology requires further investigation, particularly given the multifactorial role of insulin in vascular function. Insulin acts as a vasoactive hormone that modulates both cerebral and peripheral blood flow,55 binding to receptors on endothelial cells, where it increases nitric oxide that acts to dilate blood vessels. However, insulin can alternatively constrict blood vessels by stimulating production of endothelin-1 via the MAPK pathway.56 Through these effects, insulin acts as vasodilator when at normal concentrations and a vasoconstrictor at high concentrations. As a result, insulin resistance-associated chronic hyperinsulinaemia promotes vasoconstriction,56 resulting in higher blood pressure and reduced cerebral perfusion, a pattern that might be observed years before the onset of cognitive symptoms characteristic for vascular cognitive impairment. For example, in adults who were middle aged and cognitively healthy, higher HOMA-IR correlated with lower cerebral blood flow throughout the brain in both macro-vessels and micro-vessels, as assessed with arterial spin labelling MRI.57 Similarly, the HI-EG clamp, which acutely raises CNS insulin, increased the functional MRI blood oxygen level-dependent signal during a working memory task compared with saline infusion, and the degree of activation was reduced for adults with greater peripheral insulin resistance at baseline, compared with adults with lower baseline peripheral resistance.58
Type 2 diabetes is commonly associated with hypertension and vascular injury, including structural lesions (eg, white matter hyperintensities and lacunar infarcts) and impaired neurovascular coupling. Two studies suggested that directly manipulating brain insulin acutely with intranasal insulin administration improves cerebrovascular function and cognition in patients with type 2 diabetes compared with placebo.59,60 An ongoing trial of intranasal insulin in people with type 2 diabetes that is examining its effects on brain volume, cerebrovascular function, and cognition could provide additional insight into the therapeutic value of this approach (NCT02415556).61
Therapeutic approaches to modulate brain insulin resistance
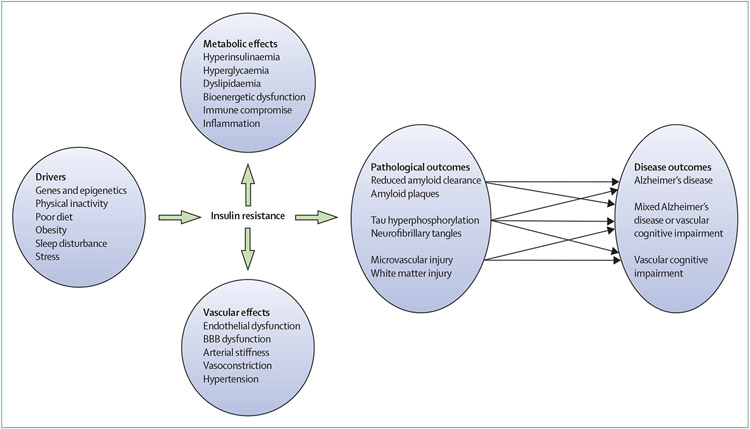
Evidence shows that insulin is crucial for brain health and that peripheral and brain insulin dysregulation might contribute to the development of Alzheimer’s disease and cerebrovascular pathology (figure). This research suggests a novel area for therapeutic investigation in which increasing the availability of insulin in the CNS or increasing sensitivity to insulin could prevent or delay Alzheimer’s disease and related disorders. We first discuss intranasal insulin, a strategy that directly targets the brain, followed by a discussion of other approaches that enhance both central and peripheral insulin sensitivity.
Figure: Association between insulin resistance and Alzheimer’s disease and related disorders.
BBB=blood-brain barrier.
Intranasal insulin
Peripheral insulin administration used for the treatment of diabetes might induce hypoglycaemia in individuals who do not have diabetes, and might also be ineffective because of impaired BBB insulin transport. Thus, investigations have focused on intranasal insulin administration, in which insulin travels via bulk flow to the brain along olfactory nerve channels and trigeminal perivascular channels, bypassing the BBB. Studies in cognitively healthy adults showed that intranasal insulin affects CNS measures, such as functional MRI, EEG, or MEG.18 In rodent models of Alzheimer’s disease, intranasal insulin improved Alzheimer’s disease pathology and preserved short-term and long-term memory.62 Short-term (ie, acute or 21 day) administration of intranasal insulin improved episodic memory in participants who were cognitively healthy without diabetes compared with placebo.63,64 Two studies in patients with mild cognitive impairment or Alzheimer’s disease showed that intranasal administration of insulin (acutely and for 21 days) improved episodic memory; an effect that was moderated by the APOE genotype.65,66 A randomised controlled trial66 of 60 adults with mild cognitive impairment or Alzheimer’s disease compared 21 days administration of a placebo or a low dose (20 IU) or a high dose (40 IU) of long-acting insulin detemir and showed that only the high dose group had improved memory. However, in a subsequent trial, the improvement with long-acting insulin was attenuated after a 120 day treatment period, although beneficial effects continued to be observed for the arm that received regular insulin.67
In one study,68 104 participants received either 20 IU or 40 IU of insulin or placebo for 120 days. Both doses improved performance on the Alzheimer’s Disease Assessment Scale-Cognitive subscale (ADAS-Cog12), a measure of global cognition. Preserved functional abilities and higher cerebral glucose use, as assessed by FDG-PET, were also observed for both doses. Post-hoc analyses suggested that the effects of intranasal insulin might be modulated by APOE genotype and sex, with men responding more favourably to higher doses than women.69 Although animal and in vitro studies showed that APOE-ε4 can modulate the brain insulin response by trapping the insulin receptor within the neuronal endosomal compartment,70 or by interfering with the BBB transport of insulin,71 human studies have not yet examined mechanisms that might underlie APOE-related response differences to intranasal insulin.71
The promising results of these studies prompted the first multi-site phase 2 and 3 placebo-controlled trial72 of 289 participants with mild cognitive impairment or Alzheimer’s disease to determine the feasibility, safety, and efficacy of 40 IU of intranasal insulin per day for 12 months, followed by a 6 month open label extension. The primary outcome was the change in ADAS-Cog12 score from baseline to month 12, and secondary clinical outcomes included Alzheimer’s disease CSF biomarkers. The study was initiated using a device (ie, device 1) that had been used in previous studies in Alzheimer’s disease.66-68 However, modifications of this device for this trial, which were intended to facilitate use, led to malfunctions in some devices. After the first 49 participants, a decision was made to switch to a newly available device (device 2) for the remaining 240 participants. This device had not been used previously in studies of Alzheimer’s disease but had shown good reliability in delivering insulin to the olfactory cleft73 (a key portal for nose-to-brain delivery) in nasal cavity models (a standard model for testing intranasal delivery devices targeting the CNS).74 Compliance rates were acceptable for both devices, ranging from 73–86% for device 1 and 90–92% for device 2. The device 2 intention-to-treat cohort was designated as the primary cohort, and the device 1 intention-to-treat cohort was designated as the secondary cohort.
Primary analyses with the device 2 cohort showed no effects of insulin compared with placebo for the ADAS-Cog12, or for other clinical or CSF outcomes at 12 months or at the 18 month open label timepoint. By contrast, secondary analyses with the device 1 cohort showed better ADAS-Cog12 performance for the insulin group compared with placebo at 12 months. In open label analyses, ADAS-Cog12 performance of the insulin group in the device 1 cohort was also better at 18 months compared with those who received placebo. Although individual biomarkers did not differ between arms for the device 1 group, the ratios of aβ1–42 to aβ1–40 and aβ1–42 to tau were increased compared with the placebo group.
One question raised by these discrepant results was whether both devices were able to successfully deliver insulin to the CNS. Device 1 had been used in previous studies in which intranasal insulin was associated with benefits in cognition and cerebral glucose metabolism measured with FDG PET,65,68 whereas device 2 has not yet been tested in Alzheimer’s disease trials; however, it had successfully delivered insulin to the olfactory cleft in nasal cavity models.73 The conflicting results of this study suggest that the indirectly measured access using nasal models is an insufficient standard and that the access of insulin to the CNS needs to be directly shown. This could be done by administering insulin or other therapeutic models with an intranasal-delivery device, then measuring acute increases in CSF, or alternatively by administering labelled insulin and measuring brain distribution with PET.
Although the results of secondary analyses from this trial must be interpreted with caution, there was better cognitive performance associated with insulin treatment for the device 1 cohort, with the groups approaching a 6 point difference in ADAS-Cog12 scores at 18 months. This effect was considered clinically significant, particularly given that participants were allowed to be on background therapy (eg, cholinesterase inhibitors or memantine). Further support for clinical significance is provided by the observation that participants treated with insulin in the device 1 cohort also showed improved CSF Aβ42 to tau and Aβ42 to 40 ratios. Compared with individual CSF biomarkers, these ratios were better able to predict progression from mild cognitive impairment in research cohorts and reliably predict brain Aβ deposition (assessed with PET).75,76 Taken together, these results suggest that the specific delivery device is an important consideration in the intranasal administration of therapeutic agents. Further research is warranted to assess the therapeutic benefit of intranasal insulin for patients with mild cognitive impairment and Alzheimer’s disease.
Insulin sensitisers
An alternative strategy to the increasing insulin concentrations is instead to use interventions that make tissues more responsive to lower insulin concentrations. Several insulin sensitising compounds have been tested to restore CNS insulin sensitivity, although evidence of their effects in the human brain is scarce. Metformin is possibly the most well studied class of drugs, and is widely used to treat type 2 diabetes. In mouse models of Alzheimer’s disease, metformin improved memory and decreased concentrations of Aβ, hyperphosphorylated tau, and activated microglia.77,78 These benefits were accompanied by improved brain insulin signalling. In a randomised placebo-controlled trial, 80 participants without diabetes who had mild cognitive impairment received metformin or placebo daily for 12 months.79 Although promising results were obtained for memory assessed with the selective reminding test for the metformin group compared with the placebo group, no differences were observed for the ADAS-Cog12, CSF Aβ42, and cerebral glucose metabolism, as measured by FDG PET. These results motivated a phase 2 trial for patients with mild cognitive impairment and Alzheimer’s disease that is currently underway (NCT04098666).
Other insulin sensitisers, such as PPAR agonists, have been studied with mixed results. In a pilot study of 30 patients with mild cognitive impairment and Alzheimer’s disease, improved memory and stabilised plasma Aβ42 concentrations were observed after 6 months of treatment with rosiglitazone compared with placebo.80 However, a phase 2 trial showed cognitive improvement (as measured by the ADAS-Cog12) after 24 weeks of treatment with rosiglitazone or placebo in only APOE-ε4 non-carriers,81 and a phase 3 trial showed no effects on cognition regardless of APOE genotype.82 A phase 3 placebo-controlled trial of pioglitazone to delay the onset of mild cognitive impairment which, according to ClinicalTrials.gov, enrolled 3500 cognitively healthy adults based on their APOE and TOMM40 genotype, was terminated early because of lack of efficacy (NCT01931566), although no results have been published yet. In summary, there is weak evidence that insulin sensitisers can enhance brain insulin sensitivity or can act as effective therapeutic agents in Alzheimer’s disease.
GLP-1 receptor agonists
Insulinotropic hormones, such as GLP-1 receptor agonists, stimulate insulin secretion and can regulate glucostasis. GLP-1 receptor agonists are found in the brain, where they mediate GLP-1 actions that increase cell proliferation and growth, and protect against excitotoxic cell death and apoptosis.83-85 GLP-1 receptor agonists are used to treat type 2 diabetes by increasing peripheral insulin sensitivity.86 Liraglutide, for example, has been suggested as a potential therapeutic option for Alzheimer’s disease.87 In mouse models, liraglutide treatment preserved memory and increased hippocampal neuronal density.88,89 Similar effects have been reported in a primate model in which liraglutide protected against insulin receptor loss and synaptic dysfunction, while also reducing hyperphosphorylated tau.89 Subcutaneous daily administration of liraglutide in 18 patients with Alzheimer’s disease for 26 weeks prevented the decline of brain glucose metabolism compared with placebo but had no effect on cognition or Aβ burden, although the study was underpowered to detect such changes.90 An ongoing phase 2, randomised, placebo-controlled clinical trial of 12 months of daily liraglutide treatment in patients with Alzheimer’s disease is examining treatment effects on FDG-PET (primary outcome), cognitive performance, and other imaging biomarkers (NCT01843075).
Non-pharmacological approaches
Lifestyle interventions targeting dietary patterns and physical activity are well known effective modulators of peripheral insulin resistance and, since 2015, have been proposed as a possible preventative or therapeutic strategy for Alzheimer’s disease. Adults consuming diets that are high in simple carbohydrates and saturated fats have an increased risk of developing Alzheimer’s disease compared with those consuming diets that are high in lean proteins and poly-unsaturated fats.91 Although several studies have shown that diets that are high in fat impair brain insulin signalling in animal models,92,93 only a few human studies have directly examined the effects of diet on brain insulin. In a randomised controlled trial, cognitively healthy adults and adults with mild cognitive impairment consumed a diet that was high in saturated fat and high in simple carbohydrates (ie, a high fat and high glycaemic diet) or a eucaloric diet that was low in saturated fat and low in sugars (ie, a low saturated fat and low glycaemic diet) for 4 weeks. The high fat and high glycaemic diet lowered CSF insulin concentrations, moving healthy adults in a direction as commonly observed in those with Alzheimer’s disease, whereas the low fat and low glycaemic diet increased insulin concentrations in people with mild cognitive impairment to levels similar to those observed in cognitively healthy people.94 Diet-induced changes in CSF insulin also correlated with CSF Aβ42 changes.94 In another study, dietary restriction in adults who were obese or who had diabetes improved the brain’s response to food stimuli, as assessed with functional MRI following intranasal insulin administration.18 Although the mechanisms through which these effects are mediated are probably multifactorial, diet modulates many risk factors associated with Alzheimer’s disease, such as brain and peripheral insulin resistance, inflammation, obesity, diabetes, and vascular disease.55
Three systematic reviews that examined dietary interventions that increased consumption of polyunsaturated fatty acids, nuts, and plant-based foods while reduced saturated fats, animal-derived proteins, and refined sugars showed that higher adherence to this dietary pattern was correlated with greater peripheral insulin sensitivity and a lower risk of age-related cognitive decline and Alzheimer’s disease.91,95,96 The extent to which protective effects of diet are mediated though enhancement of brain insulin function is an important question for future research.
Exercise is arguably the most powerful modulator of peripheral insulin resistance and has also become an active field of research in the prevention of Alzheimer’s disease and cognitive decline. A systematic review concluded that physical activity reduces the risk of Alzheimer’s disease.97 Many insulin resistance-related risk factors for Alzheimer’s disease, such as hypertension and metabolic disease, can be prevented or treated through exercise. Although exercise improved brain insulin sensitivity in rodent studies, resulting in enhanced mitochondrial function, reduced oxidative stress, and reduced tau hyperphosphorylation and aggregation in neurons,98,99 no human studies have yet examined the effects of exercise on brain insulin sensitivity. Thus, whether the effects of exercise on peripheral insulin resistance can be also observed in the human brain warrants further investigation, potentially providing insight into the relationship between exercise and risk of Alzheimer’s disease and related disorders.
Future directions
The field of precision Alzheimer’s disease therapeutics, in which genetic, environmental, and lifestyle data are integrated to design targeted strategies for treatment or prevention, has lagged behind other fields such as cancer. This lag is partly because there is no clear consensus on which therapeutic approaches could be effective. With the advent of new therapeutic options and the increased availability of large genetic datasets with deep phenotyped individuals, targeted approaches are likely to become more feasible. Such datasets could reveal genes at the intersection of insulin resistance and Alzheimer’s disease, enabling the identification of individuals for whom interventions aimed at ameliorating insulin resistance are most likely to be effective.
Sex could also be an important consideration. Women are at a higher risk of developing both peripheral insulin resistance and Alzheimer’s disease,100,101 and could also respond differently to metabolic treatments. For example, women show different response patterns to intranasal insulin compared with men.102 Biomarkers of peripheral insulin resistance or brain insulin resistance could also be used to guide personalised approaches to therapy, given that neuronal-enriched extracellular vesicles predicted a positive response to intranasal insulin.103
Finally, lifestyle and environmental factors can further modulate risk and therapeutic benefit. Examples include diet and exercise, early life stress or deprivation, and air quality, all of which have been related to metabolic health and greater risk of Alzheimer’s disease.104
Conclusion
Insulin plays a crucial role in brain health, and insulin dysregulation can contribute to conditions of pathological brain ageing, such as Alzheimer’s disease and vascular cognitive impairment, through a myriad of mechanisms. Research is underway to investigate the mechanisms underlying the neuroprotective effects of insulin to create effective strategies for prevention and treatment. The multifactorial nature of insulin’s role in Alzheimer’s disease and related disorders will require further research. It is likely that different aspects of insulin function, such as its effects on synaptic health, inflammation, energy metabolism, and vascular function, are perturbed for subgroups of affected individuals, or that for other subgroups, pathways independent of insulin are important to disease pathogenesis. New methods from systems network analyses could help to identify these patterns. This knowledge will facilitate future development of targeted prevention and treatment strategies for Alzheimer’s disease and related conditions, and thereby promote the preservation of brain health and cognitive vitality throughout life.
Search strategy and selection criteria.
We searched PubMed from January 1, 2015, to May 31, 2020, and references from relevant articles available in English, using the search terms “insulin”, “insulin resistance”, “brain”, “diabetes”, “vascular” “Alzheimer’s Disease”, “vascular cognitive impairment”, “vascular dementia”, “intranasal”, “insulin sensitiser”, “angiotensin receptor blocker”, “GLP-1 agonist”, “lifestyle”, “diet”, “exercise”, “therapeutic”, “beta secretase enzyme”, “PET”, “MRI”, “amyloid beta”, “tau”, “grey matter volume”, and “neurovascular unit”. The final reference list was generated on the basis of relevance to the topics covered in this Review.
Footnotes
Declaration of interests
We declare no competing interests.
Contributor Information
Derek Kellar, Department of Physiology and Pharmacology, Wake Forest School of Medicine, NC, USA.
Suzanne Craft, Internal Medicine-Geriatrics, Wake Forest School of Medicine, NC, USA.
References
- 1.Alzheimer’s Association. 2019 Alzheimer’s disease facts and figures. https://www.alz.org/media/documents/alzheimers-facts-and-figures-2019-r.pdf (assessed July 21, 2020). [Google Scholar]
- 2.Graham WV, Bonito-Oliva A, Sakmar TP. Update on Alzheimer’s disease therapy and prevention strategies. Annu Rev Med 2017; 68: 413–30. [DOI] [PubMed] [Google Scholar]
- 3.Rygiel K Novel strategies for Alzheimer’s disease treatment: an overview of anti-amyloid beta monoclonal antibodies. Indian J Pharmacol 2016; 48: 629–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoyer S, Möller D, Plaschke K. Desensitization of brain insulin receptor. Effect on glucose/energy and related metabolism. J Neural Transm Suppl 1994; 44: 259–68. [DOI] [PubMed] [Google Scholar]
- 5.Hamed SA. Brain injury with diabetes mellitus: evidence, mechanisms and treatment implications. Expert Rev Clin Pharmacol 2017; 10: 409–28. [DOI] [PubMed] [Google Scholar]
- 6.Neth BJ, Craft S. Insulin resistance and Alzheimer’s disease: bioenergetic linkages. Front Aging Neurosci 2017; 9: 345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rhea EM, Banks WA. Role of the blood—brain barrier in central nervous system insulin resistance. Front Neurosci 2019; 13: 521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mazucanti CH, Liu QR, Lang D, et al. Release of insulin produced by the choroid plexis is regulated by serotonergic signaling. JCI Insight 2019; 4: e131682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nemoto T, Toyoshima-Aoyama F, Yanagita T, et al. New insights concerning insulin synthesis and its secretion in rat hippocampus and cerebral cortex: amyloid- β 1-42-induced reduction of proinsulin level via glycogen synthase kinase-3β. Cell Signal 2014; 26: 253–59. [DOI] [PubMed] [Google Scholar]
- 10.Reaven GM. The insulin resistance syndrome: definition and dietary approaches to treatment. Annu Rev Nutr 2005; 25: 391–406. [DOI] [PubMed] [Google Scholar]
- 11.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985; 28: 412–19. [DOI] [PubMed] [Google Scholar]
- 12.Arnold SE, Arvanitakis Z, Macauley-Rambach SL, et al. Brain insulin resistance in type 2 diabetes and Alzheimer disease: concepts and conundrums. Nat Rev Neurol 2018; 14: 168–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 1979; 237: E214–23. [DOI] [PubMed] [Google Scholar]
- 14.Fishel MA, Watson GS, Montine TJ, et al. Hyperinsulinemia provokes synchronous increases in central inflammation and beta-amyloid in normal adults. Arch Neurol 2005; 62: 1539–44. [DOI] [PubMed] [Google Scholar]
- 15.Heni M, Kullmann S, Preissl H, Fritsche A, Häring HU. Impaired insulin action in the human brain: causes and metabolic consequences. Nat Rev Endocrinol 2015; 11: 701–11. [DOI] [PubMed] [Google Scholar]
- 16.Renner DB, Svitak AL, Gallus NJ, Ericson ME, Frey WH 2nd, Hanson LR. Intranasal delivery of insulin via the olfactory nerve pathway. J Pharm Pharmacol 2012; 64: 1709–14. [DOI] [PubMed] [Google Scholar]
- 17.Lochhead JJ, Kellohen KL, Ronaldson PT, Davis TP. Distribution of insulin in trigeminal nerve and brain after intranasal administration. Sci Rep 2019; 9: 2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kullmann S, Heni M, Hallschmid M, Fritsche A, Preissl H, Häring HU. Brain insulin resistance at the crossroads of metabolic and cognitive disorders in humans. Physiol Rev 2016; 96: 1169–209. [DOI] [PubMed] [Google Scholar]
- 19.Kim B, Feldman EL. Insulin resistance in the nervous system. Trends Endocrinol Metab 2012; 23: 133–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Talbot K, Wang HY, Kazi H, et al. Demonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J Clin Invest 2012; 122: 1316–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yarchoan M, Toledo JB, Lee EB, et al. Abnormal serine phosphorylation of insulin receptor substrate 1 is associated with tau pathology in Alzheimer’s disease and tauopathies. Acta Neuropathol 2014; 128: 679–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kapogiannis D, Boxer A, Schwartz JB, et al. Dysfunctionally phosphorylated type 1 insulin receptor substrate in neural-derived blood exosomes of preclinical Alzheimer’s disease. FASEB J 2015; 29: 589–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diehl T, Mullins R, Kapogiannis D. Insulin resistance in Alzheimer’s disease. Transl Res 2017; 183: 26–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Craft S, Newcomer J, Kanne S, et al. Memory improvement following induced hyperinsulinemia in Alzheimer’s disease. Neurobiol Aging 1996; 17: 123–30. [DOI] [PubMed] [Google Scholar]
- 25.Verdile G, Keane KN, Cruzat VF, et al. Inflammation and oxidative stress: the molecular connectivity between insulin resistance, obesity, and Alzheimer’s disease. Mediators Inflamm 2015; 2015: 105828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sartorius T, Peter A, Heni M, et al. The brain response to peripheral insulin declines with age: a contribution of the blood—brain barrier? PLoS One 2015; 10: e0126804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Steen E, Terry BM, Rivera EJ, et al. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer’s disease—is this type 3 diabetes? J Alzheimers Dis 2005; 7: 63–80. [DOI] [PubMed] [Google Scholar]
- 28.Willette AA, Johnson SC, Birdsill AC, et al. Insulin resistance predicts brain amyloid deposition in late middle-aged adults. Alzheimers Dement 2015; 11: 504–10.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ekblad LL, Johansson J, Helin S, et al. Midlife insulin resistance, APOE genotype, and late-life brain amyloid accumulation. Neurology 2018; 90: e1150–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gomez G, Beason-Held LL, Bilgel M, et al. Metabolic syndrome and amyloid accumulation in the aging brain. J Alzheimers Dis 2018; 65: 629–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kapogiannis D, Mustapic M, Shardell MD, et al. Association of extracellular vesicle biomarkers with Alzheimer disease in the Baltimore longitudinal study of aging. JAMA Neurol 2019; 76: 1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mittal K, Katare DP. Shared links between type 2 diabetes mellitus and Alzheimer’s disease: a review. Diabetes Metab Syndr 2016; 10 (suppl 1): S144–49. [DOI] [PubMed] [Google Scholar]
- 33.Chornenkyy Y, Wang WX, Wei A, Nelson PT. Alzheimer’s disease and type 2 diabetes mellitus are distinct diseases with potential overlapping metabolic dysfunction upstream of observed cognitive decline. Brain Pathol 2019; 29: 3–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dos Santos Matioli MNP, Suemoto CK, Rodriguez RD, et al. Diabetes is not associated with Alzheimer’s disease neuropathology. J Alzheimers Dis 2017; 60: 1035–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pruzin JJ, Nelson PT, Abner EL, Arvanitakis Z. Review: relationship of type 2 diabetes to human brain pathology. Neuropathol Appl Neurobiol 2018; 44: 347–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beeri MS, Schmeidler J, Silverman JM, et al. Insulin in combination with other diabetes medication is associated with less Alzheimer neuropathology. Neurology 2008; 71: 750–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sonnen JA, Larson EB, Brickell K, et al. Different patterns of cerebral injury in dementia with or without diabetes. Arch Neurol 2009; 66: 315–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu Y, Jiang X, Liu S, Li M. Changes in cerebrospinal fluid tau and β-amyloid levels in diabetic and prediabetic patients: a meta-analysis. Front Aging Neurosci 2018; 10: 271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takenoshita N, Shimizu S, Kanetaka H, et al. Classification of clinically diagnosed Alzheimer’s disease associated with diabetes based on amyloid and tau PET results. J Alzheimers Dis 2019; 71: 261–71. [DOI] [PubMed] [Google Scholar]
- 40.Laws SM, Gaskin S, Woodfield A, et al. Insulin resistance is associated with reductions in specific cognitive domains and increases in CSF tau in cognitively normal adults. Sci Rep 2017; 7: 9766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Biundo F, Del Prete D, Zhang H, Arancio O, D’Adamio L. A role for tau in learning, memory and synaptic plasticity. Sci Rep 2018; 8: 3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morris JK, Vidoni ED, Perea RD, et al. Insulin resistance and gray matter volume in neurodegenerative disease. Neuroscience 2014; 270: 139–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abner EL, Jicha GA, Shaw LM, Trojanowski JQ, Goetzl EJ. Plasma neuronal exosomal levels of Alzheimer’s disease biomarkers in normal aging. Ann Clin Transl Neurol 2016; 3: 399–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mullins RJ, Mustapic M, Goetzl EJ, Kapogiannis D. Exosomal biomarkers of brain insulin resistance associated with regional atrophy in Alzheimer’s disease. Hum Brain Mapp 2017; 38: 1933–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fiandaca MS, Kapogiannis D, Mapstone M, et al. Identification of preclinical Alzheimer’s disease by a profile of pathogenic proteins in neurally derived blood exosomes: a case-control study. Alzheimers Dement 2015; 11: 600–07.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Forner S, Baglietto-Vargas D, Martini AC, Trujillo-Estrada L, LaFerla FM. Synaptic impairment in Alzheimer’s disease: a dysregulated symphony. Trends Neurosci 2017; 40: 347–57. [DOI] [PubMed] [Google Scholar]
- 47.Gabbouj S, Ryhänen S, Marttinen M, et al. Altered insulin signaling in Alzheimer’s disease brain—special emphasis on PI3K-Akt pathway. Front Neurosci 2019; 13: 629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guo Z, Chen Y, Mao YF, et al. Long-term treatment with intranasal insulin ameliorates cognitive impairment, tau hyperphosphorylation, and microglial activation in a streptozotocin-induced Alzheimer’s rat model. Sci Rep 2017; 7: 45971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mosconi L, McHugh PF. FDG- and amyloid-PET in Alzheimer’s disease: is the whole greater than the sum of the parts? Q J Nucl Med Mol Imaging 2011; 55: 250–64. [PMC free article] [PubMed] [Google Scholar]
- 50.Baker LD, Cross DJ, Minoshima S, Belongia D, Watson GS, Craft S. Insulin resistance and Alzheimer-like reductions in regional cerebral glucose metabolism for cognitively normal adults with prediabetes or early type 2 diabetes. Arch Neurol 2011; 68: 51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dichgans M, Leys D. Vascular cognitive impairment. Circ Res 2017; 120: 573–91. [DOI] [PubMed] [Google Scholar]
- 52.Kalaria RN. The pathology and pathophysiology of vascular dementia. Neuropharmacology 2018; 134: 226–39. [DOI] [PubMed] [Google Scholar]
- 53.Smith EE, Beaudin AE. New insights into cerebral small vessel disease and vascular cognitive impairment from MRI. Curr Opin Neurol 2018; 31: 36–43. [DOI] [PubMed] [Google Scholar]
- 54.Frantellizzi V, Pani A, Ricci M, Locuratolo N, Fattapposta F, De Vincentis G. Neuroimaging in vascular cognitive impairment and dementia: a systematic review. J Alzheimers Dis 2020; 73: 1279–94. [DOI] [PubMed] [Google Scholar]
- 55.Hughes TM, Craft S. The role of insulin in the vascular contributions to age-related dementia. Biochim Biophys Acta 2016; 1862: 983–91. [DOI] [PubMed] [Google Scholar]
- 56.Muniyappa R, Yavuz S. Metabolic actions of angiotensin II and insulin: a microvascular endothelial balancing act. Mol Cell Endocrinol 2013; 378: 59–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hoscheidt SM, Starks EJ, Oh JM, et al. Insulin resistance is associated with increased levels of cerebrospinal fluid biomarkers of Alzheimer’s disease and reduced memory function in at-risk healthy middle-aged adults. J Alzheimers Dis 2016; 52: 1373–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Williams VJ, Trombetta BA, Jafri RZ, et al. Task-related fMRI BOLD response to hyperinsulinemia in healthy older adults. JCI Insight 2019; 5: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang H, Hao Y, Manor B, et al. Intranasal insulin enhanced resting-state functional connectivity of hippocampal regions in type 2 diabetes. Diabetes 2015; 64: 1025–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Novak V, Milberg W, Hao Y, et al. Enhancement of vasoreactivity and cognition by intranasal insulin in type 2 diabetes. Diabetes Care 2014; 37: 751–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Galindo-Mendez B, Trevino JA, McGlinchey R, et al. Memory advancement by intranasal insulin in type 2 diabetes (MemAID) randomized controlled clinical trial: design, methods and rationale. Contemp Clin Trials 2020; 89: 105934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Barone E, Tramutola A, Triani F, et al. Biliverdin reductase-a mediates the beneficial effects of intranasal insulin in Alzheimer disease. Mol Neurobiol 2019; 56: 2922–43. [DOI] [PubMed] [Google Scholar]
- 63.Benedict C, Hallschmid M, Hatke A, et al. Intranasal insulin improves memory in humans. Psychoneuroendocrinology 2004; 29: 1326–34. [DOI] [PubMed] [Google Scholar]
- 64.Benedict C, Hallschmid M, Schmitz K, et al. Intranasal insulin improves memory in humans: superiority of insulin aspart. Neuropsychopharmacology 2007; 32: 239–43. [DOI] [PubMed] [Google Scholar]
- 65.Reger MA, Watson GS, Green PS, et al. Intranasal insulin improves cognition and modulates beta-amyloid in early AD. Neurology 2008; 70: 440–48. [DOI] [PubMed] [Google Scholar]
- 66.Claxton A, Baker LD, Hanson A, et al. Long acting intranasal insulin detemir improves cognition for adults with mild cognitive impairment or early-stage Alzheimer’s disease dementia. J Alzheimers Dis 2015; 45: 1269–70. [DOI] [PubMed] [Google Scholar]
- 67.Craft S, Claxton A, Baker LD, et al. Effects of regular and long-acting insulin on cognition and Alzheimer’s disease biomarkers: a pilot clinical trial. J Alzheimers Dis 2017; 57: 1325–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Craft S, Baker LD, Montine TJ, et al. Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: a pilot clinical trial. Arch Neurol 2012; 69: 29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Claxton A, Baker LD, Wilkinson CW, et al. Sex and ApoE genotype differences in treatment response to two doses of intranasal insulin in adults with mild cognitive impairment or Alzheimer’s disease. J Alzheimers Dis 2013; 35: 789–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhao N, Liu C-C, Van Ingelgom AJ, et al. Apolipoprotein E4 impairs neuronal insulin signaling by trapping insulin receptor in the endosomes. Neuron 2017; 96: 115–29.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rhea EM, Raber J, Banks WA. ApoE and cerebral insulin: Trafficking, receptors, and resistance. Neurobiol Dis 2020; 137: 104755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Craft S, Raman R, Chow TW, et al. Safety, efficacy, and feasibility of intranasal insulin for the treatment of mild cognitive impairment and Alzheimer disease dementia: a randomized clinical trial. JAMA Neurol 2020; published online June 22. DOI: 10.1001/jamaneurol.2020.1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Impel NeuroPharma Investigator Brochure. Version 11. WA USA: Impel Neuropharma, 2014. [Google Scholar]
- 74.Erdő F, Bors LA, Farkas D, Bajza Á, Gizurarson S. Evaluation of intranasal delivery route of drug administration for brain targeting. Brain Res Bull 2018; 143: 155–70. [DOI] [PubMed] [Google Scholar]
- 75.Dumurgier J, Hanseeuw BJ, Hatling FB, et al. Alzheimer’s disease biomarkers and future decline in cognitive normal older adults. J Alzheimers Dis 2017; 60: 1451–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Janelidze S, Zetterberg H, Mattsson N, et al. CSF A (β42/A (β40 and A (β42/A β38 ratios: better diagnostic markers of Alzheimer disease. Ann Clin Transl Neurol 2016; 3: 154–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ou Z, Kong X, Sun X, et al. Metformin treatment prevents amyloid plaque deposition and memory impairment in APP/PS1 mice. Brain Behav Immun 2018; 69: 351–63. [DOI] [PubMed] [Google Scholar]
- 78.Farr SA, Roesler E, Niehoff ML, Roby DA, McKee A, Morley JE. Metformin improves learning and memory in the SAMP8 mouse model of Alzheimer’s Disease. J Alzheimers Dis 2019; 68: 1699–710. [DOI] [PubMed] [Google Scholar]
- 79.Luchsinger JA, Perez T, Chang H, et al. Metformin in amnestic mild cognitive impairment: results of a pilot randomized placebo controlled clinical trial. J Alzheimers Dis 2016; 51: 501–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Watson GS, Cholerton BA, Reger MA, et al. Preserved cognition in patients with early Alzheimer disease and amnestic mild cognitive impairment during treatment with rosiglitazone: a preliminary study. Am J Geriatr Psychiatry 2005; 13: 950–58. [DOI] [PubMed] [Google Scholar]
- 81.Risner ME, Saunders AM, Altman JF, et al. Efficacy of rosiglitazone in a genetically defined population with mild-to-moderate Alzheimer’s disease. Pharmacogenomics J 2006; 6: 246–54. [DOI] [PubMed] [Google Scholar]
- 82.Harrington C, Sawchak S, Chiang C, et al. Rosiglitazone does not improve cognition or global function when used as adjunctive therapy to AChE inhibitors in mild-to-moderate Alzheimer’s disease: two phase 3 studies. Curr Alzheimer Res 2011; 8: 592–606. [DOI] [PubMed] [Google Scholar]
- 83.Hamilton A, Hölscher C. Receptors for the incretin glucagon-like peptide-1 are expressed on neurons in the central nervous system. Neuroreport 2009; 20: 1161–66. [DOI] [PubMed] [Google Scholar]
- 84.Perfetti R, Zhou J, Doyle ME, Egan JM. Glucagon-like peptide-1 induces cell proliferation and pancreatic-duodenum homeobox-1 expression and increases endocrine cell mass in the pancreas of old, glucose-intolerant rats. Endocrinology 2000; 141: 4600–05. [DOI] [PubMed] [Google Scholar]
- 85.Perry T, Lahiri DK, Sambamurti K, et al. Glucagon-like peptide-1 decreases endogenous amyloid-beta peptide (Abeta) levels and protects hippocampal neurons from death induced by Abeta and iron. J Neurosci Res 2003; 72: 603–12. [DOI] [PubMed] [Google Scholar]
- 86.Anderson J The pharmacokinetic properties of glucagon-like peptide-1 receptor agonists and their mode and mechanism of action in patients with type 2 diabetes. J Fam Pract 2018; 67 (suppl): S8–13. [PubMed] [Google Scholar]
- 87.Freiherr J, Hallschmid M, Frey WH 2nd , et al. Intranasal insulin as a treatment for Alzheimer’s disease: a review of basic research and clinical evidence. CNS Drugs 2013; 27: 505–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hansen HH, Fabricius K, Barkholt P, et al. The GLP-1 receptor agonist liraglutide improves memory function and increases hippocampal CA1 neuronal numbers in a senescence-accelerated mouse model of Alzheimer’s disease. J Alzheimers Dis 2015; 46: 877–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Batista AF, Forny-Germano L, Clarke JR, et al. The diabetes drug liraglutide reverses cognitive impairment in mice and attenuates insulin receptor and synaptic pathology in a non-human primate model of Alzheimer’s disease. J Pathol 2018; 245: 85–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gejl M, Gjedde A, Egefjord L, et al. In Alzheimer’s Disease, 6-month treatment with GLP-1 analog prevents decline of brain glucose metabolism: randomized, placebo-controlled, double-blind clinical trial. Front Aging Neurosci 2016; 8: 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Solfrizzi V, Custodero C, Lozupone M, et al. Relationships of dietary patterns, foods, and micro- and macronutrients with Alzheimer’s disease and late-life cognitive disorders: a systematic Review. J Alzheimers Dis 2017; 59: 815–49. [DOI] [PubMed] [Google Scholar]
- 92.Clegg DJ, Gotoh K, Kemp C, et al. Consumption of a high-fat diet induces central insulin resistance independent of adiposity. Physiol Behav 2011; 103: 10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Carvalheira JBC, Ribeiro EB, Araújo EP, et al. Selective impairment of insulin signalling in the hypothalamus of obese Zucker rats. Diabetologia 2003; 46: 1629–40. [DOI] [PubMed] [Google Scholar]
- 94.Bayer-Carter JL, Green PS, Montine TJ, et al. Diet intervention and cerebrospinal fluid biomarkers in amnestic mild cognitive impairment. Arch Neurol 2011; 68: 743–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.van den Brink AC, Brouwer-Brolsma EM, Berendsen AAM, van de Rest O. The Mediterranean, Dietary Approaches to Stop Hypertension (DASH), and Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) diets are associated with less cognitive decline and a lower risk of Alzheimer’s disease-a review. Adv Nutr 2019; 10: 1040–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pistollato F, Iglesias RC, Ruiz R, et al. Nutritional patterns associated with the maintenance of neurocognitive functions and the risk of dementia and Alzheimer’s disease: a focus on human studies. Pharmacol Res 2018; 131: 32–43. [DOI] [PubMed] [Google Scholar]
- 97.Stephen R, Hongisto K, Solomon A, Lönnroos E. Physical activity and Alzheimer’s disease: a systematic review. J Gerontol A Biol Sci Med Sci 2017; 72: 733–39. [DOI] [PubMed] [Google Scholar]
- 98.Ruegsegger GN, Vanderboom PM, Dasari S, et al. Exercise and metformin counteract altered mitochondrial function in the insulin-resistant brain. JCI Insight 2019; 4: 130681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jeong JH, Koo JH, Cho JY, Kang EB. Neuroprotective effect of treadmill exercise against blunted brain insulin signaling, NADPH oxidase, and tau hyperphosphorylation in rats fed a high-fat diet. Brain Res Bull 2018; 142: 374–83. [DOI] [PubMed] [Google Scholar]
- 100.Pike CJ. Sex and the development of Alzheimer’s disease. J Neurosci Res 2017; 95: 671–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mittendorfer B Insulin resistance: sex matters. Curr Opin Clin Nutr Metab Care 2005; 8: 367–72. [DOI] [PubMed] [Google Scholar]
- 102.Benedict C, Kern W, Schultes B, Born J, Hallschmid M. Differential sensitivity of men and women to anorexigenic and memory-improving effects of intranasal insulin. J Clin Endocrinol Metab 2008; 93: 1339–44. [DOI] [PubMed] [Google Scholar]
- 103.Mustapic M, Tran J, Craft S, Kapogiannis D. Extracellular vesicle biomarkers track cognitive changes following intranasal insulin in Alzheimer’s disease. J Alzheimers Dis 2019; 69: 489–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Finch CE, Kulminski AM. The Alzheimer’s disease exposome. Alzheimers Dement 2019; 15: 1123–32. [DOI] [PMC free article] [PubMed] [Google Scholar]