Abstract
Objective
This study aimed to explore the prognostic value of the lymphocyte (LYM)-to-white blood cell (WBC) ratio (LWR) in patients with decompensated liver cirrhosis (DLC).
Methods
This study was conducted by recruiting 214 patients with DLC with different aetiologies (development cohort). Receiver operating characteristic (ROC) curve analyses were used to assess the predictive accuracy of the LWR, and Youden’s index was used to determine the optimal cut-off values of the LWR based on the ROC curve. Next, patients were divided into high- and low-LWR groups according to the cut-off values. Multivariate logistic analyses were performed to determine the independent predictors for the 1-, 3- and 6-month mortality. Restricted cubic spline (RCS) was used to determine and visualize the association between LWR and the risk of death. We verified the predictive ability of LWR in the validation cohort of 139 patients.
Results
In the development cohort, there were 16 (7.5%), 22 (10.3%) and 30 patients (14.0%) who died at 1, 3 and 6 months, respectively. The LWR was significantly lower in non-survivors than in survivors and was an independent predictor of poor outcomes. The ROC analyses with the Delong test showed that the LWR had comparable predictive power with the Model for End-Stage Liver Disease (MELD) score, neutrophil-to-LYM ratio (NLR) and Chronic Liver Failure consortium score for acute decompensated (CLIF-C ADs). RCS showed a non-linear relationship between the LWR and the risk of death at 1 and 3 months, whereas a linear relationship was observed between the LWR and the risk of death at 6 months. We verified that the decreased LWR was an independent predictor of adverse outcomes at 3-, and 6-month follow-up endpoints in the validation cohort.
Conclusions
Our findings indicate that a lower LWR is an independent factor for unfavourable outcomes and may serve as a potential novel prognostic predictor in patients with DLC.
KEY MESSAGES
This study is the first report on the prognostic value of the lymphocyte (LYM)-to-white blood cell (WBC) ratio (LWR) in patients with decompensated liver cirrhosis (DLC).
Decreased LWR is an independent factor for adverse outcomes in patients with DLC.
Keywords: Decompensated liver cirrhosis, lymphocyte-to-white blood cell ratio, prognosis
Introduction
Liver cirrhosis is prevalent worldwide and associated with high morbidity and mortality [1]. In China, the annual progression rate from compensated liver cirrhosis to decompensated liver cirrhosis (DLC) is approximately 3% [2,3], and a 5-year mortality rate of 85% is estimated in patients with DLC [4–6]. Although liver transplantation can significantly improve the survival rates, it is not widely used owing to insufficient sources of donor livers, high costs and serious post-transplantation complications [7]. This necessitates the accurate and early detection of high-risk patients, which will aid the timely decision of a treatment strategy to ameliorate the prognosis of patients with DLC.
A growing body of evidence has shown that haematological inflammation indicators, such as the neutrophil-to-lymphocyte (LYM) ratio (NLR), platelet-to-white blood cell (WBC) ratio, monocyte-to-LYM ratio and C-reactive protein-to-albumin ratio are reliable prognostic indicators in various diseases, such as cerebral haemorrhage, ischemic stroke, major cardiac events, renal failure, acute respiratory distress syndrome, cancer, acute-on-chronic liver failure and infectious pathologies [8–18]. In recent years, inflammation-based markers have received increasing attention in clinical practice and are used in the prognosis of liver cirrhosis [19–24]. Moreover, it has been reported that the LYM-to-WBC ratio (LWR) is a predictor of prognosis in patients suffering from cancer, COVID-19, acute heart failure and infective endocarditis [25–30]. However, the role of LWR in the prognosis of patients with DLC remains unclear. Therefore, the present investigation aims to explore the prognostic value of the LWR in patients with DLC.
Materials and methods
Patients
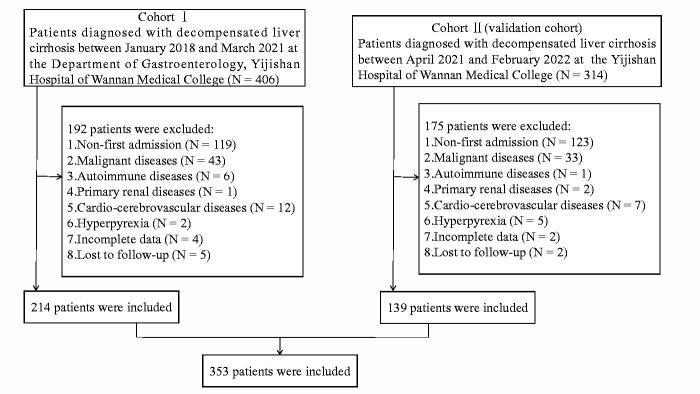
Patients with DLC referred to the Department of Gastroenterology, Yijishan Hospital of Wannan Medical College between January 2018 and March 2021 were included in this study as the development cohort (cohort I). We recruited patients with DLC as the validation cohort (cohort II) in the Yijishan Hospital of Wannan Medical College between April 2021 and February 2022. Liver decompensation was defined by the clinical, laboratory and imaging data and endoscopic and histological findings [31]. The exclusion criteria were as follows: (1) non-first admission, (2) malignant diseases, (3) autoimmune diseases, (4) primary kidney disease, (5) cardio-cerebrovascular diseases, (6) hyperpyrexia, (8) loss to follow-up and (9) incomplete data. Survival was assessed at 1, 3 and 6 months based on medical records and/or by direct telephonic conversations with patients or patient families. Since the study was retrospective in nature, the requirement for informed consent from the patients was waived. The study was approved by the Institutional Review Board of the Yijishan Hospital of Wannan Medical College (2022–26).
Data collection
Demographic data, laboratory variables and clinical information of the patients, including gender, age, routine blood test reports, hepatic function test reports, coagulation parameters, creatinine (Cr), cause of liver cirrhosis and modes of decompensation, were comprehensively collected on admission. The LWR was calculated as the LYM count (×109/L) divided by the WBC count (×109/L) [25]. In addition, the NLR, Model for End-Stage Liver Disease (MELD) score, and Chronic Liver Failure consortium score for acute decompensated (CLIF-C ADs) were calculated as previously described [13,32,33].
Statistical analysis
Continuous variables were presented as the means ± standard deviation or medians (25th–75th percentiles). Categorical variables were expressed as a frequency. Continuous variables were compared using an independent sample t-test or the Mann–Whitney U test, whereas categorical variables were compared using the chi-square test or Fisher’s exact test [34]. To evaluate the correlation between the LWR and MELD scores, Spearman’s correlation test was used [35]. The predictive accuracy of the LWR was evaluated by analysing the area under the receiver operating characteristic (ROC) curve, whereas Youden’s index was used to determine the optimal cut-off values of the LWR based on the ROC curve [36]. The Delong test was used to compare the value of the area under the ROC curve [37]. Patients with DLC were categorized into high- and low-LWR groups according to the cut-off values. Multivariate logistic regression analysis was used to identify the independent predictors for mortality. Besides, restricted cubic spline (RCS) was used to evaluate the non-linear relationship between the LWR and risk of death based on multivariate analysis [38]. To achieve an optimum fit and avoid overfitting in the main splines, the number of knots between three and five was selected according to the minimum value for the Akaike information criterion [39]. The statistical analyses were performed using R version 4.0.2 (The R Foundation for Statistical Computing, Vienna, Austria), SPSS version 25.0 (SPSS Inc., Chicago, IL) and Medcalc version 15.2 (MedCalc Inc., Ostend, Belgium). All statistical tests were two-sided, and p values < 0.05 were considered to be statistically significant.
Results
Baseline characteristics
In this study, 353 eligible patients with DLC were recruited (Figure 1). In the cohort I, the average age of patients was 61.4 ± 12.9 years, and approximately 60% of the patients were male. The main aetiology of liver cirrhosis was chronic infection with hepatitis B virus. The causes of decompensation events included ascites (84.6%), variceal bleeding (32.2%), hepatic encephalopathy (4.2%) and hepatorenal syndrome (2.3%). The mortality rates of all patients at 1, 3 and 6 months were 7.5%, 10.3% and 14.0%, respectively. In addition, the baseline characteristics of patients in the cohort II are shown in Supplementary Table 1.
Figure 1.
Flow chart of the patient selection process.
Baseline characteristics of patients between the survival and the Non-Survival groups
The clinical-laboratory characteristics of the patients with DLC in the cohort I are shown in Table 1. The patients with DLC were divided into survival and non-survival groups according to their outcomes at 1, 3 and 6 months. During the stages of follow-up, the WBC, platelet, NLR, CLIF-C ADs and MELD score of the non-survivor were significantly higher than the survivor (p < 0.05). However, the LWR and albumin (ALB) of the non-survivor were significantly lower than the survivor (p< .05). Besides, the prothrombin time (PT) of the non-survivor was significantly higher than the survivor at 1 month (p < 0.05), but no significant differences were observed at 3 or 6 months (p > 0.05). Similarly, no significant differences in gender, age, LYM, total bilirubin, alanine aminotransferase, aspartate aminotransferase, γ-glutamyl transpeptidase (GGT) or Cr were observed at any time point during the follow-up (p > 0.05). In addition, the clinical-laboratory characteristics of the patients between the survival and the non-survival groups in the cohort II are listed in Supplementary Table 1.
Table 1.
Clinical and laboratory characteristics of the decompensated liver cirrhosis patients in cohort I at the 1-, 3- and 6-month follow-ups.
| Variables | All patients (n = 214) | 1 month |
3 months |
6 months |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Survivors (n = 198) | Non-survivors (n = 16) | p Value | Survivors (n = 192) | Non-survivors (n = 22) | p Value | Survivors (n = 184) | Non-survivors (n = 30) | p Value | ||
| Gender (n, %) | 0.429 | 0.161 | 0.054 | |||||||
| Male | 127 (59.3%) | 119 (60.1%) | 8 (50.0%) | 117 (60.9%) | 10 (45.5%) | 114 (62.0%) | 13 (43.3%) | |||
| Female | 87 (40.7%) | 79 (39.9%) | 8 (50.0%) | 75 (39.1%) | 12 (54.5%) | 70 (38.0%) | 17 (56.7%) | |||
| Age (years) | 61.4 ± 12.9 | 61.4 ± 13.0 | 61.8 ± 11.5 | 0.906 | 61.1 ± 13.0 | 64.6 ± 11.7 | 0.228 | 60.8 ± 12.7 | 65.1 ± 13.7 | 0.094 |
| WBC (109/L) | 3.80 (2.60–5.40) | 3.60 (2.50–5.10) | 5.65 (4.33–8.13) | <0.001 | 3.60 (2.50–5.10) | 5.05 (3.85–6.40) | 0.004 | 3.60 (2.50–5.10) | 4.45 (3.55–6.63) | 0.005 |
| LYM (109/L) | 0.80 (0.60–1.20) | 0.80 (0.60–1.20) | 0.85 (0.50–1.30) | 0.965 | 0.80(0.60–1.20) | 0.85 (0.50–1.23) | 0.893 | 0.80 (0.60–1.20) | 0.80 (0.50–1.23) | 0.940 |
| HGB (g/L) | 96.29 ± 25.35 | 96.42 ± 24.54 | 94.81 ± 34.78 | 0.858 | 96.35 ± 24.70 | 95.86 ± 31.12 | 0.932 | 96.25 ± 25.09 | 96.60 ± 27.32 | 0.944 |
| PLT (109/L) | 61.50 (44.00–98.50) | 58.50 (43.75–95.00) | 94.50 (63.00–124.50) | 0.013 | 58.00 (43.25–94.00) | 92.50 (62.75–152.25) | 0.007 | 57.50 (43.25–90.75) | 98.50 (62.75–130.75) | 0.004 |
| ALB (g/L) | 29.61 ± 6.17 | 29.92 ± 6.14 | 25.76 ± 5.39 | 0.009 | 30.02 ± 6.18 | 26.04 ± 4.89 | 0.004 | 30.23 ± 6.13 | 25.81 ± 5.02 | <0.001 |
| TBIL (umol/L) | 24.78 (16.64–37.51) | 24.40 (16.62–36.28) | 38.90 (21.23–83.36) | 0.050 | 24.43 (16.81–36.59) | 34.07 (16.50–56.15) | 0.170 | 24.47 (17.35–36.75) | 27.06 (15.27–51.50) | 0.669 |
| ALT (U/L) | 25.00 (16.00–41.00) | 24.43 (16.62–36.28) | 21.50 (11.00–44.50) | 0.477 | 25.00 (17.00–41.00) | 23.50 (13.25–41.50) | 0.465 | 25.00 (17.00–41.75) | 23.00 (14.75–34.25) | 0.241 |
| AST (U/L) | 34.00 (23.00–58.00) | 25.00 (17.00–41.00) | 47.00 (22.25–62.25) | 0.620 | 33.00 (22.25–58.00) | 43.00 (26.75–59.75) | 0.422 | 34.00 (22.25–58.00) | 32.50 (26.00–55.00) | 0.994 |
| GGT (U/L) | 54.50 (23.00–145.25) | 33.00 (23.00–58.00) | 46.50 (18.25–208.75) | 0.906 | 55.50 (23.50–136.25) | 46.50 (18.75–200.25) | 0.815 | 55.50 (25.00–132.50) | 47.00 (18.75–214.75) | 0.757 |
| Cr (umol/L) | 67.25 (55.98–85.30) | 55.50 (24.50–138.50) | 65.95 (43.10–93.93) | 0.510 | 67.25 (55.93–84.35) | 69.65 (54.20–120.35) | 0.383 | 67.25 (56.05–84.35) | 68.10 (54.38–104.65) | 0.532 |
| PT (S) | 14.55 (13.30–16.23) | 14.40 (13.28–16.13) | 16.10 (14.93–19.45) | 0.022 | 14.40 (13.23–16.18) | 15.65 (14.35–17.20) | 0.050 | 14.40 (13.30–16.10) | 15.65 (13.08–19.15) | 0.080 |
| LWR | 0.23 (0.17–0.31) | 0.25 (0.18–0.32) | 0.14 (0.10–0.22 | 0.001 | 0.25 (0.18–0.32) | 0.15 (0.12–0.23) | 0.002 | 0.25 (0.18–0.32) | 0.17 (0.13–0.23) | 0.001 |
| NLR | 2.72 (1.78–4.21) | 2.56 (1.73–4.00) | 5.27 (2.93–8.26) | 0.001 | 2.55 (1.72–4.00) | 4.90 (2.83–6.63) | 0.002 | 2.50 (1.71–4.00) | 4.17 (2.72–6.24) | 0.002 |
| CLIF-C ADS | 41.72 (37.11–48.06) | 41.28 (36.77–47.25) | 51.34 (45.50–54.61) | <0.001 | 41.24 (36.77–46.32) | 51.09 (45.06–55.24) | <0.001 | 41.20 (36.71–46.65) | 49.73 (43.98–54.51) | <0.001 |
| MELD score | 11.22 (9.14–13.62) | 11.17 (9.05–13.21) | 14.08 (10.10–18.81) | 0.031 | 11.16 (9.02–13.21) | 14.08 (10.61–17.88) | 0.005 | 11.16 (9.03-12.95) | 13.59 (9.99-17.31) | 0.017 |
| Aetiology (n, %) | ||||||||||
| HBV | 134 (62.6%) | 123 (62.1%) | 11 (68.8%) | 119 (62.0%) | 15 (68.2%) | 112 (60.9%) | 22 (73.3%) | |||
| HCV | 7 (3.3%) | 7 (3.5%) | 0 | 7 (3.6%) | 0 | 7 (3.8%) | 0 | |||
| Alcoholism | 5 (2.3%) | 5 (2.5%) | 0 | 5 (2.6%) | 0 | 5 (2.7%) | 0 | |||
| Others | 68 (31.8%) | 63 (31.8%) | 5 (31.3%) | 61 (31.8%) | 7 (31.8%) | 60 (32.6%) | 8 (26.7%) | |||
| Modes of decompensation (n, %) | ||||||||||
| Ascites | 181 (84.6%) | 168(84.8%) | 13 (81.3%) | 162 (84.4%) | 19 (86.4%) | 155 (84.2%) | 26 (86.7%) | |||
| Variceal bleeding |
69 (32.2%) | 64 (32.3%) | 5(31.3%) | 64 (33.3%) | 5 (22.7%) | 63 (34.2%) | 6 (20.0%) | |||
| HE | 9 (4.2%) | 5 (2.5%) | 4 (25.0%) | 5 (2.6%) | 4 (18.2%) | 5 (2.7%) | 4 (13.3%) | |||
| HRS | 5 (2.3%) | 3 (1.5%) | 2 (12.5%) | 1 (0.5%) | 4 (18.2%) | 0 | 5 (16.7%) | |||
Data are expressed as number, mean ± standard deviation, median (25th- 75th percentiles), or frequency (percentage (%)).
WBC: white blood cell; LYM: lymphocyte; HGB: haemoglobin; PLT: platelet; ALB: albumin; TBIL: total bilirubin; ALT: alanine aminotransferase; AST: aspartate aminotransferase; GGT: γ-glutamyl transpeptidase; PT: prothrombin time; Cr: creatinine; LWR: lymphocyte-to-white blood cell ratio; NLR: neutrophil-to-lymphocyte ratio; CLIF-C ADs: Chronic Liver Failure-consortium score for acute decompensated; MELD: Model for End-Stage Liver Disease; HBV: hepatitis B virus; HCV: hepatitis C virus; HE: hepatic encephalopathy; HRS: hepatorenal syndrome.
Baseline characteristics of patients with different LWR levels
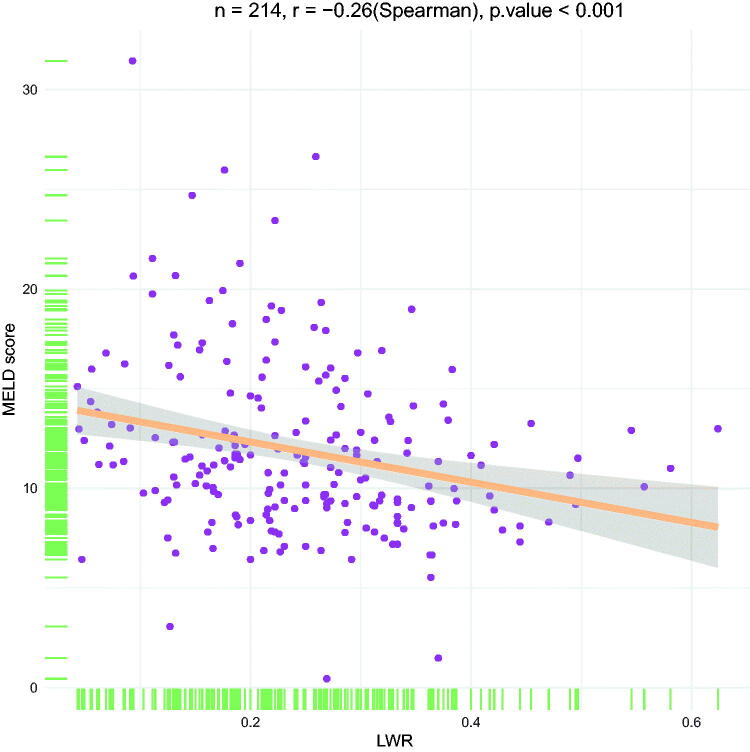
The LWR cut-off values for 1, 3 and 6 months are 0.163, 0.163 and 0.264, respectively. The clinical-laboratory characteristics of the patients with DLC in the high- and low-LWR groups in the cohort I are listed in Table 2. A decreased LWR was associated with higher WBC, higher PT, higher MELD score, higher NLR, higher CLIF-C ADs, lower LYM, lower ALB, lower GGT and higher mortality. Moreover, Spearman’s correlation test showed a significant negative correlation between the LWR and the MELD score (r= −0.26, p < 0.001) (Figure 2). In addition, the baseline characteristics of patients in the high- and low-LWR groups in the cohort II are shown in Supplementary Table 2.
Table 2.
Comparison of Clinical and laboratory characteristics between low and high LWR groups in patients with decompensated liver cirrhosis in cohort I.
| Variables | 1 month |
3 months |
6 months |
||||||
|---|---|---|---|---|---|---|---|---|---|
| LWR < 0.163 (n = 49) | LWR > 0.163 (n = 165) | p Value | LWR < 0.163 (n = 49) | LWR > 0.163 (n = 165) | p Value | LWR < 0.264 (n = 124) | LWR > 0.264 (n = 90) | p Value | |
| Gender (n, %) | 0.194 | 0.194 | 0.127 | ||||||
| Male | 33 (67.3%) | 94 (57.0%) | 33 (67.3%) | 94 (57.0%) | 79 (63.7%) | 48 (53.3%) | |||
| Female | 16 (32.7%) | 71 (43.0%) | 16 (32.7%) | 71 (43.0%) | 45 (36.3%) | 42 (46.7%) | |||
| Age (years) | 59.2 ± 12.5 | 62.1 ± 13.0 | 0.153 | 59.2 ± 12.5 | 62.1 ± 13.0 | 0.153 | 61.1 ± 13.4 | 62.0 ± 12.3 | 0.615 |
| WBC (109/L) | 5.30 (3.50–7.15) | 3.50 (2.45–4.70) | <0.001 | 5.30 (3.50–7.15) | 3.50 (2.45–4.70) | <0.001 | 4.25 (2.90–6.08) | 3.20 (2.20–4.43) | <0.001 |
| LYM (109/L) | 0.50 (0.40–0.80) | 0.90 (0.70–1.40) | <0.001 | 0.50 (0.40–0.80) | 0.90 (0.70–1.40) | <0.001 | 0.70 (0.43–1.00) | 1.05 (0.80–1.50) | <0.001 |
| HGB (g/L) | 94.80 ± 27.37 | 96.75 ± 24.79 | 0.637 | 94.80 ± 27.37 | 96.75 ± 24.79 | 0.637 | 93.36 ± 26.51 | 100.35 ± 23.20 | 0.046 |
| PLT (109/L) | 59.00 (31.50–97.00) | 62.00 (46.50–101.00) | 0.300 | 59.00 (31.50–97.00) | 62.00 (46.50–101.00) | 0.300 | 61.50 (41.00–104.75) | 61.00 (48.00–90.00) | 0.793 |
| ALB (g/L) | 28.28 ± 5.73 | 30.00 ± 6.26 | 0.086 | 28.28 ± 5.73 | 30.00 ± 6.26 | 0.086 | 28.90 ± 6.17 | 30.58 ± 6.07 | 0.048 |
| TBIL (umol/L) | 30.92 (15.65–51.28) | 23.76 (16.97–36.75) | 0.241 | 30.92 (15.65–51.28) | 23.76 (16.97–36.75) | 0.241 | 25.99 (16.50–40.95) | 23.67 (17.53–36.08) | 0.490 |
| ALT (U/L) | 24.00 (15.00–36.50) | 25.00 (17.00–41.50) | 0.504 | 24.00 (15.00–36.50) | 25.00 (17.00–41.50) | 0.504 | 24.00 (14.00–39.75) | 25.50 (20.00–46.25) | 0.144 |
| AST (U/L) | 29.00 (21.50–51.00) | 36.00 (23.50–59.00) | 0.077 | 29.00 (21.50–51.00) | 36.00 (23.50–59.00) | 0.077 | 30.00 (22.00–54.00) | 39.50 (26.50–67.25) | 0.015 |
| GGT (U/L) | 31.00 (18.00–76.50) | 64.00 (27.00–155.50) | 0.003 | 31.00 (18.00–76.50) | 64.00 (27.00–155.50) | 0.003 | 37.00 (18.00–131.50) | 78.50 (37.75–156.75) | 0.001 |
| Cr (umol/L) | 67.30 (60.20–91.85) | 67.20 (54.45–84.80) | 0.281 | 67.30 (60.20–91.85) | 67.20 (54.45–84.80) | 0.281 | 70.00 (58.63–92.28) | 63.50 (52.68–79.38) | 0.011 |
| PT (s) | 15.70 (14.35–16.95) | 14.20 (13.10–16.10) | 0.003 | 15.70 (14.35–16.95) | 14.20 (13.10–16.10) | 0.003 | 15.00 (13.43–16.58) | 14.20 (12.98–16.03) | 0.021 |
| LWR | 0.13 (0.08–0.14) | 0.27 (0.22–0.33) | <0.001 | 0.13 (0.08–0.14) | 0.27 (0.22–0.33) | <0.001 | 0.18 (0.13–0.22) | 0.33 (0.29–0.38) | <0.001 |
| NLR | 6.33 (5.23–10.44) | 2.20 (1.63–3.15) | <0.001 | 6.33 (5.23–10.44) | 2.20 (1.63–3.15) | <0.001 | 4.00 (3.08–6.00) | 1.70 (1.33–2.00) | <0.001 |
| CLIF-C ADs | 45.26 (40.34–54.12) | 41.05 (36.56–46.14) | <0.001 | 45.26 (40.34–54.12) | 41.05 (36.56–46.14) | <0.001 | 44.02 (38.76–50.75) | 39.57 (33.92–42.68) | <0.001 |
| MELD score | 12.39 (10.40–16.51) | 10.78 (8.79–12.88) | 0.001 | 12.39 (10.40–16.51) | 10.78 (8.79–12.88) | 0.001 | 11.61 (9.74–15.52) | 9.84 (8.29–12.48) | 0.001 |
| Mortality (n, %) | 11 (22.4%) | 5 (3.0%) | <0.001 | 13 (26.5%) | 9 (5.5%) | <0.001 | 26 (21.0%) | 4 (4.4%) | 0.001 |
Data are expressed as number, mean ± standard deviation, median (25th–75th percentiles), or frequency (percentage (%)).
WBC: white blood cell; LYM: lymphocyte; HGB: haemoglobin; PLT: platelet; ALB: albumin; TBIL: total bilirubin; ALT: alanine aminotransferase; AST: aspartate aminotransferase; GGT: γ-glutamyl transpeptidase; PT: prothrombin time; Cr: creatinine; LWR: lymphocyte-to-white blood cell ratio; NLR: neutrophil-to-lymphocyte ratio; CLIF-C ADs: Chronic Liver Failure-consortium score for acute decompensated; MELD: Model for End-stage Liver Disease
Figure 2.
Scatter plot illustrating the correlation between the LWR and the MELD score in the development cohort.
Low LWR as an independent risk factor for death in patients with DLC
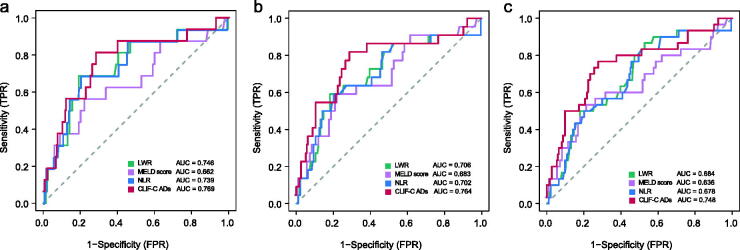
We demonstrated that decreased LWR was an independent factor of unfavourable outcomes in patients with DLC in the cohort I after adjusting the effects of covariates on 1-, 3- and 6- months mortality, respectively (Table 3). As shown in Figure 3, the LWR had comparable predictive power with the NLR, MELD score and CLIF-C ADs (all Delong test p value > 0.05).
Table 3.
Indicators associated with 1-month, 3-month, and 6-month mortality in multivariate analyses in patients with decompensated liver cirrhosis in cohort I& II.
| Cohort I |
Cohort II (validation cohort) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variables | 1 month OR (95% CI) | p | 3 months OR (95% CI) | p | 6 months OR (95% CI) | p | 1 month OR (95% CI) | p | 3 months OR (95% CI) | p | 6 months OR (95% CI) | p |
| PLT (109/L) | 1.008 (1.003–1.014) | 0.004 | 1.006 (1.001–1.012) | 0.013 | 1.005 (1.000–1.010) | 0.026 | 1.009 (0.995–1.023) | 0.197 | 1.006 (0.995–1.018) | 0.285 | 1.003 (0.992–1.014) | 0.595 |
| ALB (g/L) | 0.959 (0.844–1.082) | 0.504 | 0.943 (0.850–1.040) | 0.251 | 0.913 (0.836–0.992) | 0.037 | 0.851 (0.688–1.053) | 0.137 | 0.901 (0.774–1.049) | 0.179 | 0.877 (0.776–0.991) | 0.036 |
| TBIL (umol/L) | 0.997 (0.977–1.015) | 0.766 | 0.998 (0.972–1.024) | 0.866 | ||||||||
| AST (U/L) | 1.001 (0.997–1.006) | 0.531 | 1.002 (0.998–1.006) | 0.395 | 0.943 (0.888–1.001) | 0.054 | 0.988 (0.972–1.005) | 0.165 | ||||
| PT (s) | 1.161 (0.900–1.507) | 0.251 | 0.999 (0.795–1.249) | 0.996 | 1.032 (0.853–1.249) | 0.743 | 0.970 (0.782–1.203) | 0.782 | 0.942 (0.771–1.150) | 0.554 | 1.148 (0.924–1.425) | 0.213 |
| MELD score | 1.087 (0.887–1.307) | 0.395 | 1.125 (0.970–1.300) | 0.114 | 1.070 (0.942–1.212) | 0.284 | 1.297 (1.002–1.680) | 0.049 | 1.238 (1.032–1.487) | 0.022 | 1.05 (0.918–1.214) | 0.445 |
| LWR | 0.002 | 0.002 | 0.014 | 0.095 | 0.007 | 0.026 | ||||||
| Low | Ref | Ref | Ref | Ref | Ref | Ref | ||||||
| High | 0.139 (0.037–0.456) | 0.211 (0.075-0.574) | 0.234 (0.064–0.679) | 0.245 (0.047–1.276) | 0.169 (0.047–0.613) | 0.271 (0.085–0.858) | ||||||
PLT: platelet; ALB: albumin; TBIL: total bilirubin; AST: aspartate aminotransferase; PT: prothrombin time; MELD: Model for End-stage Liver Disease; LWR: lymphocyte-to-white blood cell ratio; OR: odds ratio; CI: confidence interval.
Age, sex, haemoglobin, PLT, ALB, TBIL, AST, PT, LWR, MELD score, alanine aminotransferase, γ-glutamyl transpeptidase and creatinine were included in the univariate logistic regression analysis. Variables that did not have a significant effect on mortality in the univariate logistic regression analysis were not included in the multivariate logistic regression analysis.
Figure 3.
Receiver operating curves of the NLR, MELD score, CLIF-C ADs and the LWR for prediction of mortality at (a) 1, (b) 3 and (c) 6 months in patients with decompensated liver cirrhosis in the development cohort.
Nonlinear relationship between the LWR and risk of death
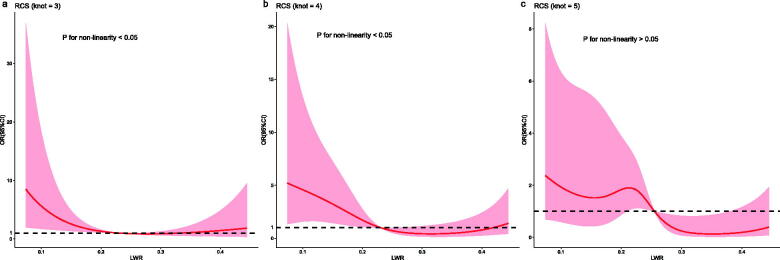
RCS was used to flexibly model and visualize the relationship between the LWR and the risk of death in patients with DLC based on multivariate analysis. The odds ratio (OR) curves showed an overall declining tendency with an increase in LWR at the 1- and 3-month follow-up endpoints (Figure 4). A nonlinear relationship was observed between the LWR and the risk of death 1 and 3 months (p for non-linearity < 0.05), Moreover, the risk of death decreased with an increase in LWR and was relatively flat when the LWR approached 0.23. However, a linear correlation was observed between the LWR and the 6-month risk of death (p for non-linearity > 0.05), indicating that the risk of death decreased with an increase in LWR.
Figure 4.
Associations of the LWR with the risk of death at (a) 1, (b) 3 and (c) 6 months in patients with decompensated liver cirrhosis using restricted cubic spline in the development cohort.
Validation of the predictive accuracy of the LWR
In the cohort II, we demonstrated that the LWR was an independent predictor of 3- and 6-month mortality in patients with DLC; but not for 1-month follow-up endpoint (OR, 0.245; 95% CI, 0.047–1.276) (Table 3). Furthermore, the ROC analysis showed that the LWR had comparable predictive ability with the NLR, MELD score and CLIF-C ADs, no matter in the cohort II or all patients (All Delong test p value > 0.05) (Supplementary Figure 1). The exact p values for Delong test for the comparisons of the area under the ROC curve of these biomarkers are listed in Supplementary Table 3.
Discussion
In this research, we investigated the prognostic value of the LWR in patients with DLC. Our results indicated that the reduction in LWR was an independent predictor of adverse outcomes at 3- and 6-month follow-up endpoints.
The MELD score has been extensively used to predict the prognosis of liver disease patients. The MELD score helps to prioritize patients for liver transplants and is determined by three complex parameters (Cr, total bilirubin and international normalized ratio) that are not convenient for use in clinical application [40]. Conversely, LWR uses two routine indicators of blood, which make it more convenient and easier to calculate than the MELD score. Moreover, the LWR is a ratio and is not influenced by the test location or method, which makes it an objective predictive indicator with no additional cost or resource requirement [41]. Our results showed a significant negative correlation between the LWR and MELD scores, and the LWR has comparable predictive ability with the MELD score in patients with DLC.
Systemic inflammation is a well-recognized feature of DLC, and several studies have shown the important role of the inflammatory response in the pathogenesis of advanced cirrhosis and their correlation with unfavourable outcomes [42–45]. Zhang et al. reported that elevated WBC reflected the severity of systemic inflammation following liver injury, which may affect the prognosis of patients with DLC [24]. It has also been reported that LYMs play a critical role in the body’s immune response, immune surveillance and immune defence functions [25]. Lower LYM counts may be associated with a poor nutritional status and impaired immune response in patients with liver disease and are a predictor of prognosis on the liver transplant waiting list [46,47]. Besides, LWR can be regarded as a combined marker of immune status and inflammatory. Decreased levels of LWR may reflect a suppressed immune response and/or an enhanced inflammatory response. Several studies have evaluated the association between the LWR and the prognosis of various diseases [25–30]. Zhang et al. reported that a lower LWR is an independent risk factor for mortality in patients with infective endocarditis [29]. Further, Ding et al. reported that a decreased LWR was associated with poor overall survival, disease-free survival, and metastasis-free survival and was also an independent factor of decreased metastasis-free survival in patients with oral squamous cell carcinoma [30]. Pitre et al. reported that a lower LWR was correlated with a poor prognosis for patients with COVID-19 [25]. Similar to these findings, our findings indicated that the LWR can be serving as an independent indicator for predicting the prognosis of patients with DLC.
Several predictive scoring systems have been developed to assess the prognosis of patients with advanced liver disease, such as the MELD score, sequential organ failure assessment and CLIF-C ADs [32,33,48–50]. However, most predictive scoring systems do not consider the systemic inflammatory response, which is significantly associated with the progression of cirrhosis and related complications, except CLIF-C ADs [42–45]. In previous studies, CLIF-C ADs have been shown to be an independent predictor of mortality in patients with DLC [33,51,52]. Based on the results of our study, we found that LWR had comparable predictive power to CLIF-C ADs at all follow-up endpoints, while CLIF-C ADs had better predictive power than the MELD score in the development cohort and all patients at 6-month follow-up endpoints (Supplementary Table 3). The results were similar to the findings of the above studies. Previous studies have identified various inflammatory indicators associated with prognosis in patients with liver cirrhosis, such as neutrophil- LYM ratio, monocyte-LYM ratio and platelet-WBC ratio [19–24], and these indicators may be complementary to the widely used prognostic scoring systems, as discussed earlier. Our results showed that the LWR may be used as a potential indicator to predict prognosis in patients with DLC.
There are several limitations to our study. First, it is a retrospective and single-centre study, and thus, selection biases were inevitable. Second, the study did not evaluate pro-inflammatory cytokines, such as TNF-α and IL-6, which may help in understanding the mechanism. Therefore, further prospective studies at multiple centres are required to validate the predictive value of the LWR for patients with DLC.
Conclusions
In summary, the LWR can serve as a potential prognostic indicator in the early identification of high-risk patients with DLC. The index can be conveniently calculated, is easily accessible, and has comparable predictive ability with the MELD score. Thus, our findings may help clinicians improve the disease predictability as well as prevent and manage the condition.
Supplementary Material
Funding Statement
The authors reported there is no funding associated with the work featured in this article.
Author contributions
Wei Wang designed the research. Yanan Xie, Chiyi He and Wei Wang analysed the data. Yanan Xie drafted the paper and interpreted the data. Wei Wang revised the paper critically for intellectual content. All authors read and approved the final manuscript to be published. All authors agree to be accountable for all aspects of the work.
Disclosure statement
We declare that there is no conflict of interest regarding the publication of this article.
Data availability statement
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
References
- 1.Sepanlou SG, Safiri S, Bisignano C, et al. The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet Gastroenterol Hepatol. 2020;5(3):245–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xiao J, Wang F, Wong NK, et al. Global liver disease burdens and research trends: analysis from a chinese perspective. J Hepatol. 2019;71(1):212–221. [DOI] [PubMed] [Google Scholar]
- 3.Lee HM, Banini BA.. Updates on chronic HBV: current challenges and future goals. Curr Treat Options Gastroenterol. 2019;17(2):271–291. [DOI] [PubMed] [Google Scholar]
- 4.D'Amico G. The clinical course of cirrhosis. Population based studies and the need of personalized medicine. J Hepatol. 2014;60(2):241–242. [DOI] [PubMed] [Google Scholar]
- 5.Angeli P, Bernardi M, Villanueva C, et al. EASL clinical practice guidelines for the management of patients with decompensated cirrhosis. Journal of Hepatology. 2018;69(2):406–460. [DOI] [PubMed] [Google Scholar]
- 6.Tsochatzis EA, Bosch J, Burroughs AK.. Liver cirrhosis. Lancet. 2014;383(9930):1749–1761. [DOI] [PubMed] [Google Scholar]
- 7.Cardenas A, Gines P.. Management of patients with cirrhosis awaiting liver transplantation. Gut. 2011;60(3):412–421. [DOI] [PubMed] [Google Scholar]
- 8.Kinoshita A, Onoda H, Imai N, et al. The C-Reactive protein/albumin ratio, a novel Inflammation-Based prognostic score, predicts outcomes in patients with hepatocellular carcinoma. Ann Surg Oncol. 2015;22(3):803–810. [DOI] [PubMed] [Google Scholar]
- 9.Park JS, Seo KW, Choi BJ, et al. Importance of prognostic value of neutrophil to lymphocyte ratio in patients with ST-elevation myocardial infarction. Medicine (Baltimore). 2018;97(48):e13471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamamoto T, Kawada K, Obama K.. Inflammation-related biomarkers for the prediction of prognosis in colorectal cancer patients. Int J Mol Sci. 2021;22(15):8002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jie Y, Gong J, Xiao C, et al. Low platelet to white blood cell ratio indicates poor prognosis for acute-on-chronic liver failure. Biomed Res Int. 2018;2018:7394904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang L, Gao C, Li F, et al. Monocyte-to-lymphocyte ratio is associated with 28-day mortality in patients with acute respiratory distress syndrome: a retrospective study. J Intensive Care. 2021;9(1):49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lattanzi S, Cagnetti C, Rinaldi C, et al. Neutrophil-to-lymphocyte ratio improves outcome prediction of acute intracerebral hemorrhage. J Neurol Sci. 2018;387:98–102. [DOI] [PubMed] [Google Scholar]
- 14.Garbens A, Wallis CJD, Bjarnason G, et al. Platelet to white blood cell ratio predicts 30-day postoperative infectious complications in patients undergoing radical nephrectomy for renal malignancy. Can Urol Assoc J. 2017;11(11):E414–E420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen Z, Huang Y, Li S, et al. Platelet-to-White blood cell ratio: a prognostic predictor for 90-Day outcomes in ischemic stroke patients with intravenous thrombolysis. J Stroke Cerebrovasc Dis. 2016;25(10):2430–2438. [DOI] [PubMed] [Google Scholar]
- 16.Song H, Jeong MJ, Cha J, et al. Preoperative neutrophil-to-lymphocyte, platelet-to-lymphocyte and monocyte-to-lymphocyte ratio as a prognostic factor in non-endometrioid endometrial cancer. Int J Med Sci. 2021;18(16):3712–3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chiriac S, Stanciu C, Singeap AM, et al. Prognostic value of neutrophil-to-lymphocyte ratio in cirrhotic patients with acute-on-chronic liver failure. Turk J Gastroenterol. 2020;31(12):868–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reddan DN, Klassen PS, Szczech LA, et al. White blood cells as a novel mortality predictor in haemodialysis patients. Nephrol Dial Transplant. 2003;18(6):1167–1173. [DOI] [PubMed] [Google Scholar]
- 19.Huang SS, Xie DM, Cai YJ, et al. C-reactive protein-to-albumin ratio is a predictor of hepatitis B virus related decompensated cirrhosis: time-dependent receiver operating characteristics and decision curve analysis. Eur J Gastroenterol Hepatol. 2017;29(4):472–480. [DOI] [PubMed] [Google Scholar]
- 20.Wu J, Mao W, Li X.. Mean platelet volume/lymphocyte ratio as a prognostic indicator for HBV-Related decompensated cirrhosis. Gastroenterol Res Pract. 2020;2020:4107219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han Z, He X, Peng S.. Neutrophil count to albumin ratio as a prognostic indicator for HBV‐associated decompensated cirrhosis. J Clin Lab Anal. 2021;35(4):e23730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang H, Sun Q, Mao W, et al. Neutrophil-to-Lymphocyte ratio predicts early mortality in patients with HBV-Related decompensated cirrhosis. Gastroenterol Res Pract. 2016;2016:4394650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cai YJ, Dong JJ, Dong JZ, et al. A nomogram for predicting prognostic value of inflammatory response biomarkers in decompensated cirrhotic patients without acute-on-chronic liver failure. Aliment Pharmacol Ther. 2017;45(11):1413–1426. [DOI] [PubMed] [Google Scholar]
- 24.Zhang J, Qiu Y, He X, et al. Platelet‐to‐white blood cell ratio: a novel and promising prognostic marker for HBV‐associated decompensated cirrhosis. J Clin Lab Anal. 2020;34(12):e23556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pitre T, Jones A, Su J, et al. Inflammatory biomarkers as independent prognosticators of 28-day mortality for COVID-19 patients admitted to general medicine or ICU wards: a retrospective cohort study. Intern Emerg Med. 2021;16(6):1573–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao W, Wang P, Jia H, et al. Lymphocyte count or percentage: which can better predict the prognosis of advanced cancer patients following palliative care? BMC Cancer. 2017;17(1):514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Formiga F, Chivite D, Salvatori M, et al. Lymphocyte-to-white blood cells ratio in older patients experiencing a first acute heart failure hospitalization. Eur Geriatr Med. 2018;9(3):365–370. [DOI] [PubMed] [Google Scholar]
- 28.Tang C, Cheng X, Yu S, et al. Platelet-to-lymphocyte ratio and lymphocyte-to-white blood cell ratio predict the efficacy of neoadjuvant chemotherapy and the prognosis of locally advanced gastric cancer patients treated with the oxaliplatin and capecitabine regimen. Onco Targets Ther. 2018;11:7061–7075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang M, Ge Q, Qiao T, et al. Prognostic value of lymphocyte-to-White blood cell ratio for in-Hospital mortality in infective endocarditis patients. Int J Clin Pract. 2022;2022:8667054–8667056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ding M, Song Y, Jing J, et al. The ratio of preoperative serum biomarkers predicts prognosis in patients with oral squamous cell carcinoma. Front Oncol. 2021;11:719513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ginès P, Krag A, Abraldes JG, et al. Liver cirrhosis. Lancet. 2021;398(10308):1359–1376. [DOI] [PubMed] [Google Scholar]
- 32.Kamath PS, Kim WR, Advanced Liver Disease Study Group. Advanced liver disease study G. The Model for End-Stage Liver Disease (MELD). Hepatology. 2007;45(3):797–805. [DOI] [PubMed] [Google Scholar]
- 33.Jalan R, Pavesi M, Saliba F, et al. The CLIF consortium acute decompensation score (CLIF-C ADs) for prognosis of hospitalised cirrhotic patients without acute-on-chronic liver failure. J Hepatol. 2015;62(4):831–840. [DOI] [PubMed] [Google Scholar]
- 34.Du Prel JB, Rohrig B, Hommel G, et al. Choosing statistical tests: part 12 of a series on evaluation of scientific publications. Dtsch Arztebl Int. 2010;107(19):343–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spearman C. The proof and measurement of association between two things. Int J Epidemiol. 2010;39(5):1137–1150. [DOI] [PubMed] [Google Scholar]
- 36.Walter SD. The partial area under the summary ROC curve. Stat Med. 2005;24(13):2025–2040. [DOI] [PubMed] [Google Scholar]
- 37.DeLong ER, DeLong DM, Clarke-Pearson DL.. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837–845. [PubMed] [Google Scholar]
- 38.Harrell FE, Jr., Lee KL, Pollock BG.. Regression models in clinical studies: determining relationships between predictors and response. J Natl Cancer Inst. 1988;80(15):1198–1202. [DOI] [PubMed] [Google Scholar]
- 39.Johannesen CDL, Langsted A, Mortensen MB, et al. Association between low density lipoprotein and all cause and cause specific mortality in Denmark: prospective cohort study. BMJ. 2020;371:m4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Freeman RB, Jr., Wiesner RH, Harper A, et al. The new liver allocation system: moving toward evidence-based transplantation policy. Liver Transpl. 2002;8(9):851–858. [DOI] [PubMed] [Google Scholar]
- 41.Raghav K, Hwang H, Jacome AA, et al. Development and validation of a novel nomogram for individualized prediction of survival in cancer of unknown primary. Clin Cancer Res. 2021;27(12):3414–3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bajaj JS, Heuman DM, Hylemon PB, et al. Altered profile of human gut microbiome is associated with cirrhosis and its complications. J Hepatol. 2014;60(5):940–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arroyo V, Angeli P, Moreau R, et al. The systemic inflammation hypothesis: towards a new paradigm of acute decompensation and multiorgan failure in cirrhosis. J Hepatol. 2021;74(3):670–685. [DOI] [PubMed] [Google Scholar]
- 44.Cazzaniga M, Dionigi E, Gobbo G, et al. The systemic inflammatory response syndrome in cirrhotic patients: relationship with their in-hospital outcome. J Hepatol. 2009;51(3):475–482. [DOI] [PubMed] [Google Scholar]
- 45.Abdel-Khalek EE, El-Fakhry A, Helaly M, et al. Systemic inflammatory response syndrome in patients with liver cirrhosis. Arab J Gastroenterol. 2011;12(4):173–177. [DOI] [PubMed] [Google Scholar]
- 46.Leithead JA, Rajoriya N, Gunson BK, et al. Neutrophil-to-lymphocyte ratio predicts mortality in patients listed for liver transplantation. Liver Int. 2015;35(2):502–509. [DOI] [PubMed] [Google Scholar]
- 47.Fernandez-Ruiz M, Lopez-Medrano F, Romo EM, et al. Pretransplant lymphocyte count predicts the incidence of infection during the first two years after liver transplantation. Liver Transpl. 2009;15(10):1209–1216. [DOI] [PubMed] [Google Scholar]
- 48.Poca M, Alvarado-Tapias E, Concepcion M, et al. Predictive model of mortality in patients with spontaneous bacterial peritonitis. Aliment Pharmacol Ther. 2016;44(6):629–637. [DOI] [PubMed] [Google Scholar]
- 49.Fleming KM, Aithal GP, Card TR, et al. The rate of decompensation and clinical progression of disease in people with cirrhosis: a cohort study. Aliment Pharmacol Ther. 2010;32(11–12):1343–1350. [DOI] [PubMed] [Google Scholar]
- 50.Cholongitas E, Senzolo M, Patch D, et al. Risk factors, sequential organ failure assessment and Model for End-Stage Liver Disease scores for predicting short term mortality in cirrhotic patients admitted to intensive care unit. Aliment Pharmacol Ther. 2006;23(7):883–893. [DOI] [PubMed] [Google Scholar]
- 51.Baldin C, Piedade J, Guimaraes L, et al. CLIF-C AD score predicts development of acute decompensations and survival in hospitalized cirrhotic patients. Dig Dis Sci. 2021;66(12):4525–4535. [DOI] [PubMed] [Google Scholar]
- 52.Shi Y, Shu Z, Sun W, et al. Risk stratification of decompensated cirrhosis patients by chronic liver failure consortium scores: classification and regression tree analysis. Hepatol Res. 2017;47(4):328–337. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, upon reasonable request.