Abstract
Purpose
The study aimed to determine the feasibility of remotely delivered exercise (tele-exercise) for older, rural cancer survivors and to explore the effects of tele-exercise on physical function, physical activity, and patient-reported outcomes.
Methods
Participants were rural cancer survivors age 60 years (79% female; mean age 70.4 ± 5.7) randomly assigned to the remotely delivered EnhanceFitness (tele-EF) exercise program, inclusive of aerobic, strength, and balance training and led by American Council on Exercise certified instructors for 1 h, 3 days/week for 16 weeks (n = 20) or to a waitlist control group (n = 19). We assessed feasibility, physical function, accelerometer-measured physical activity, and patient-reported outcomes at baseline and post intervention.
Results
Among those screened as eligible, 44 (64%) consented to participate with 39 randomized after completing baseline measures. Attrition was equivalent between groups (n = 1, each) with 95% completing the study. The median class attendance rate was 86.9% (interquartile range: 79–94%). Compared to controls, tele-EF participants had statistically significant improvement in the five-time sit-to-stand test (− 3.4 vs. − 1.1 s, p = 0.03, effect size = 0.44), mean daily light physical activity (+ 38.5 vs 0.5 min, p = 0.03, effect size = 0.72) and step counts (+ 1977 vs. 33, p = 0.01, effect size = 0.96). There were no changes in self-efficacy for exercise, fatigue, or sleep disturbance between groups.
Conclusions
Findings indicate that tele-EF is feasible in older, rural cancer survivors and results in positive changes in physical function and physical activity.
Implications for Cancer Survivors
Tele-EF addresses common barriers to exercise for older, rural cancer survivors, including limited accessible opportunities for professional instruction and supervision.
Supplementary information
The online version contains supplementary material available at 10.1007/s11764-022-01292-y.
Keywords: Tele-exercise, Older adults, EnhanceFitness, Cancer, Physical function
Introduction
The number of cancer survivors is rising and projected to increase to 20 million by 2026, with the majority being age 65 years and older [1]. Despite improvements in cancer diagnosis and treatment, cancer survivors report five comorbidities on average, with even more severe health conditions among those with obesity and inadequate physical activity [2]. The burden of chronic disease is even greater in rural residents, who have worse health outcomes and increased mortality risk compared to those living in urban areas [3, 4].
Participation in regular exercise confers benefits such as improvements in physical functioning, quality of life, and cancer-related fatigue among cancer survivors [5, 6]. Moderate-to-vigorous intensity physical activity (MVPA) reduces risk for many chronic diseases, in addition to mortality risk in cancer survivors [7]. Evidence-based guidelines strongly recommend multicomponent exercise for people living with and beyond cancer based on robust evidence for the benefits [8, 9]. However, access to exercise programs that address the unique needs of cancer survivors is lacking [10], particularly in rural communities where cancer survivors have significantly lower levels of physical activity compared to those in urban areas [3, 11].
Previous work has identified unique barriers to exercise for cancer survivors. Known preferences for rural survivors include professional guidance, home-based programs, and consideration of the rural environment [12, 13]. The majority of past interventions targeting rural cancer survivors were created for breast cancer survivors, and did not address preferences for supervised exercise programming [11]. While the COVID-19 pandemic resulted in overall lower rates of physical activity participation among older adults [14], it also led to increased use and familiarity of technology in older adults [15]. The advances and acceptance of interactive videoconferencing technology provides a potential option for rural older adults to participate in remotely delivered group-based exercise programs from home.
EnhanceFitness (EF), an evidence-based, group exercise program for older adults, was adapted for remote delivery in 2020 [16, 17]. The development of remotely delivered EnhanceFitness (tele-EF), inclusive of aerobic, strength, balance, and flexibility exercise led by certified instructors, provides a unique and timely opportunity to engage hard-to-reach cancer survivors. Previous studies on the in-person version of EF have shown effect sizes in the range of 0.4–0.6 for physical function [18, 19], which, if replicated in a remote version, could provide evidence for tele-EF as a comparable option. Therefore, we sought to determine the feasibility of tele-EF for older, rural cancer survivors and to explore the preliminary effects of tele-exercise on physical function, physical activity, and patient-reported outcomes.
Methods
This was a randomized, waitlist-controlled pilot trial with 1:1 group assignment. The trial was conducted in accordance with the Consolidated Standards of Reporting Trials (CONSORT) for randomized pilot and feasibility trials [20]. Recruitment occurred between March and December 2021, using online forums, support groups, and patient navigator referrals. Recruitment was ongoing; however, the majority of people responded and enrolled during three targeted recruitment waves. Randomization to the tele-EF intervention or waitlist control group took place after baseline measures were completed using QMinim Online Minimization, stratifying on age (60–74 vs.75 +) and sex [21].
Participants
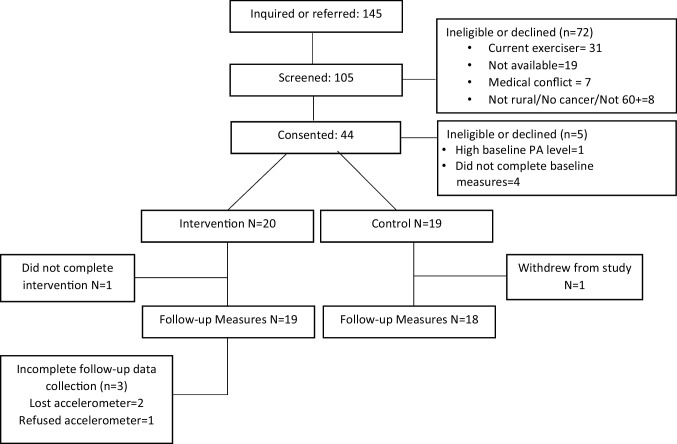
Eligible participants were age 60 or older, self-reported cancer survivors, as defined by Stage I–III cancer history with completion of adjuvant chemotherapy, radiation therapy, or surgery for their cancer diagnosis, and residing in a rural community, as indicated by zip code in RUCA areas designated as 4–10 [22]. Participants were not excluded on cancer type. Other criteria included minimal participation in exercise, confirmed with baseline accelerometer measures (< 150 min/week MVPA consistent with “insufficient physical activity” per Physical Activity Guidelines for Americans [23]). Figure 1 displays participant flow from recruitment to study completion. All procedures were approved by the University of Vermont Institutional Review Board. The trial was registered at clinicaltrials.gov prior to the start of data collection (NCT04806139).
Fig. 1.
CONSORT diagram
Procedures
All procedures were completed remotely. The principal investigator or a trained research assistant screened for eligibility over the phone and obtained informed consent. Participants completed the initial questionnaire through the REDCap electronic data portal (Vanderbilt University, Nashville, TN) [24] or were mailed a paper copy, depending on preference. Physical activity and sedentary behavior were assessed with a thigh worn activPAL accelerometer (PAL Technologies Ltd, Glasgow, UK) over a 7-day period. The accelerometer was mailed to participants with detailed written instructions for use. A link to a demonstration video was provided by email. Physical performance tests were completed on Zoom (Zoom, San Jose, CA). All measures were repeated after 16 weeks (post-intervention).
Intervention
Intervention participants were assigned to tele-EF after baseline measurements were complete. Prior to starting the class, all participants were screened using the Screening Cancer Survivors for Unsupervised Moderate-to-Vigorous Intensity Exercise [25] and the Screening for Physical Activity Readiness Questionnaire (PAR-Q) [26]. As a result of the screening, three participants were required to obtain medical clearance. Additionally, prior to starting the class, all participants had a one-on-one technology orientation meeting with a research assistant. During these sessions, participants were guided on navigating Zoom, and room and equipment set-up for the exercise sessions. One participant was provided with a tablet to participate in the exercise classes, one participant was provided with an external camera, and one participant was provided with a fish-eye lens to allow for full body viewing during the exercise classes. All participants were provided with two five-pound adjustable cuff weights to use during the class.
The tele-EF classes followed the procedures as prescribed in the Remote Class Delivery Guidance provided by Sound Generations [17]. An assistant joined each class to monitor for safety and address technical challenges. Participants were invited to join the Zoom session 10 min prior to the start of the exercise as an opportunity for social interaction and to ask questions. The 1-h classes were delivered by an EF-certified instructor 3 days a week (Monday, Wednesday, and Friday) for 16 weeks and followed the prescribed format of a 5–8-min warm-up, 20 min of moderate intensity aerobic exercise, and a 3–5-min cool down. The remainder of the class was allocated to 20 min of progressive strengthening exercise using weights, 4–8 static and dynamic balance exercises, and 5–10 min of flexibility exercise. Instructors that have certification by the American Council on Exercise and undergo 12 h of training with an EF Master Trainer that includes audiovisual materials, live demonstration, and teach backs that demonstrate understanding of program protocols and how to modify exercises including seated options, as necessary. Per standard EF procedure, instructors demonstrate two levels for each exercise with Level 1 being a less strenuous, often seated, version, and Level 2 being the more strenuous option. Participants are encouraged to choose either level for any exercise depending on functional ability and symptoms.
Waitlist control group
Participants allocated to the waitlist control group completed all study measurements at baseline and again after 16 weeks. Once the measures were complete, control group participants were invited to enroll in tele-EF classes.
Measures
Feasibility
We assessed feasibility through recruitment and retention in the study. These metrics included identification of referral sources, measurement completion, and retention in each arm. Attendance was recorded for each session along with reasons for missing class, including technology challenges that limited participation. Based on previous trials in cancer survivors [27, 28], we defined feasibility as enrollment of at least 50% of eligible participants with an 80% retention rate. Feasibility for attendance was set at 75% of the sessions based on the minimum recommendation for cancer survivors [29, 30].
Demographic and health characteristics
Self-report questionnaires were used to collect age, sex, race, education, marital status, employment status, health history, and cancer history.
Patient-reported outcomes
Physical function was assessed with the Patient Reported Outcome Measurement System (PROMIS)-Physical Function short form. This measure has 10 items and been validated for use in multiple populations including cancer survivors and people with multiple chronic conditions [31]. Six additional domains of health-related quality of life (fatigue, sleep disturbance, depression, anxiety, satisfaction in social roles, and pain interference) were assessed with the PROMIS-29. The raw scores for each of the seven PROMIS domains (range: 0–100) are transformed into T scores with a mean of 50 and a standard deviation of 10 for the US general population. Higher scores indicate higher levels of the concept being measured.
Self-efficacy for exercise
Participants completed the 15-item “Self-Efficacy to Regulate Exercise Survey” developed by Garcia and King [32], which has demonstrated high internal consistency (Cronbach’s alpha = 0.90) and test–retest correlation of 0.67 (p < 0.001). A mean self-efficacy score is obtained by calculating the mean of the 15 responses (range: 0–100).
Physical performance
Three tests of physical performance were conducted over the Zoom videoconference platform. A trained research assistant, blinded to group allocation, conducted the assessments at baseline and follow-up. Procedures for assessor training are described in the Online Resource. Participants were provided written instructions on how to prepare the testing area (e.g., chair with no arms or wheels, near a table or sturdy surface, video camera placed to allow full view of body). The tests included the five-time-sit-to stand, 4-stage balance test, and 30-s-sit-to-stand [33]. Recent work has validated remote assessment of these measures [34, 35].
Physical activity and sedentary behavior were assessed using the activPAL4 accelerometer which has been previously validated for PA, cadence, and sedentary behavior in older adults and acceptability in cancer survivors [36–38]. Participants were mailed the activPAL4, encased in a nitrile sleeve, and Tegaderm™ dressings. Participants wore the device 24 h/day for 7 days. A paper log was used to document any periods where the device was removed and for bed/wake times each day. Participants with allergies to the Tegaderm were provided paper tape and instructions to remove the device for bathing. After the accelerometers were returned, the data was downloaded and processed using the PAL software suite. Additional processing of the minute-epoch files was conducted with a macro-enabled Excel program. Derived metrics included mean daily MVPA based on the step cadence of ≥ 100 steps/min [39, 40] and light intensity physical activity (LPA) based on a cadence of 1–99 steps/min sedentary time and total step counts. The activPAL4 measurements were conducted at baseline and again at the end of the 4-month intervention period. Methods for identifying peak 30-min intensity and cadence outcomes are reported in the Online Material.
Sample size and data analysis
A goal of this pilot trial was to estimate the intervention effect size and its precision to help inform power calculations for a larger scale efficacy trial. We aimed to retain 15 participants in each group to provide for nominal estimation (using a 95% confidence interval) of the mean changes in dependent variables [41]. With a sample size of 15 participants in each group, we had 80% power to detect a treatment effect on patient-reported physical function of 1.06 SD at the 2-sided 0.05 level of statistical significance. Additional participants were recruited to allow for up to 25% attrition and to ensure an optimal class size for tele-EF.
Feasibility was assessed with descriptive statistics to examine recruitment, measurement completion, exercise class attendance, and retention in each arm. Baseline characteristics were compared between groups using percentages or means with standard deviations (SD). Within-group differences for outcomes were assessed with paired-t-tests or chi-square tests. We used linear mixed models to assess group by time interactions in baseline and follow-up measures. Due to the small sample size and stratification at randomization on age and sex, the models did not include additional variables for adjustment. Effect sizes (Cohen’s d) for between-group differences were calculated using the pooled baseline standard deviation. [42]. Significance testing was set at 0.05 a priori. Data analysis was carried out using STATA 17 (Stata Corp., College Station, TX).
Results
Baseline characteristics for the study population as a whole and by group allocation are presented in Table 1. Participants were predominantly female (79%) with a mean age of 70.4 years (SD: 5.7, range 61–84). Two-thirds of participants (67%) were classified as obese (BMI ≥ 30 kg/m2). The majority were breast cancer survivors (42%). More than a third (37%) were employed full- or part-time. Nineteen participants (49%) were not able to maintain a single leg stand for at least 10 s. Twenty participants (72%) needed 12 or more seconds to complete the five-time sit-to-stand test, indicating increased fall risk [43]. There were no statistically significant differences between groups on baseline measures.
Table 1.
Baseline characteristics of study participants
| Total mean (SD) or n (%) N = 39 |
Tele-EF mean (SD) or n (%) N = 20 |
Control mean (SD) or n (%) N = 19 |
P | |
|---|---|---|---|---|
| Age (in years) | 70.4 (5.7) | 70.1 (5.3) | 70.6 (6.2) | 0.78 |
| Sex | ||||
| Female | 31 (79%) | 16 (80%) | 15 (78%) | 0.87 |
| Race and ethnicity | ||||
| White/Non-Hispanic | 36 (97%) | 19 (95%) | 19 (100%) | 0.35 |
| American Indian | 1 (1.2%) | 1 (5%) | 0 | |
| Educational status | ||||
| College/University degree | 33 (85%) | 17 (85%) | 16 (84%) | 0.89 |
| Marital status | ||||
| Married or living as married | 22 (56%) | 12 (60%) | 10 (53%) | |
| Divorced/Widowed | 9 (23%) | 5 (25%) | 4 (21%) | 0.68 |
| Single/Never married | 8 (21%) | 3 (15%) | 5 (26%) | |
| Employment status | ||||
| Full time | 6 (15%) | 1 (5%) | 5 (26%) | 0.17 |
| Part time | 8 (21%) | 5 (25%) | 3 (16%) | |
| BMI | 30.5 (7.1) | 31.6 (7.5) | 29.3 ± 6.7 | 0.33 |
| Cancer type | ||||
| Breast | 16 (42%) | 9 (45%) | 7 (37%) | 0.59 |
| Prostate | 4 (11%) | 3 (15%) | 1 (5%) | |
| Cervical/Endometrial | 6 (16%) | 3 (15%) | 3 (16%) | |
| Other | 13 (33%) | 5 (25%) | 8 (42%) | |
| Patient-report physical function (0–100) | 46.1 (7.0) | 45.2 (6.5) | 46.9 (7.6) | 0.43 |
| Five-time sit-to-stand (s) | 14.3 (4.4) | 14.6 (5.2) | 13.9 (3.4) | 0.64 |
| Single leg stand for 10 s (Y/N) | 21 (54%) | 10 (50%) | 11 (58%) | 0.62 |
| Fatigue (0–100) | 52.5 (8.6) | 54.3 (7.0) | 50.5 (9.8) | 0.17 |
| Sleep disturbance (0–100) | 48.9 (7.6) | 49.8 (7.3) | 48.0 (7.9) | 0.47 |
| Mean daily MVPA (min) | 4.0 (5.6) | 3.0 (5.3) | 5.2 (5.8) | 0.24 |
| Mean daily step counts | 5598.5 (2256.5) | 5082.3 (1947.0) | 6172.2 (2486.7) | 0.14 |
Feasibility
One-hundred forty-five people responded to recruitment postings or were referred; of these 117 (81%) were screened for eligibility (see Fig. 1). Twenty-eight people (19%) did not respond to contact attempts after they were provided with inclusion criteria and study details. Among those screened, 46 (39%) were ineligible to participate based on inclusion criteria (e.g., no history of cancer, not living in a rural area, currently engaged in regular exercise). Among those screened as eligible, 44 recruits (64%) consented to participate, higher than our expectation of 50%. After consent, four did not complete the baseline measures and one participant was deemed ineligible due to high physical activity levels as measured by the accelerometer. Thirty-nine participants completed baseline measures and were randomized to the intervention (n = 20) or waitlist control group (n = 19). One intervention group participant withdrew at 9 weeks due to medical complications unrelated to the study and one control group participant withdrew prior to the follow-up measures. Therefore, follow-up measures at 16 weeks were completed by 19 participants in the intervention group (95%) and 18 participants in the control group (95%) resulting in a 5% attrition between baseline and follow-up measures. Three participants in the intervention group did not complete the follow-up physical activity assessment (activPAL) due to losing the device (n = 2) or refusal to wear it (n = 1).
The median class attendance rate was 86.9% (interquartile range: 79–94%). Participants missed classes primarily because of medical appointments or illness (32%), vacation or family engagements (22%), and caregiving responsibilities (15%). There were no serious adverse events associated with the intervention. Reported non-serious adverse events included a neck and a knee strain (exercises modified, no missed classes), a quadriceps strain, and a painful shoulder symptom re-occurrence (exercises modified, single missed class). One participant modified some of the standing exercises due to an episode of piriformis syndrome that did not resolve until the exercise location was changed to a room without a slanted floor.
Physical function outcomes
Baseline and follow-up outcomes for patient-reported physical function and physical performance measures are presented in Table 2. Participants in the intervention group had a statistically significant improvement in the PROMIS physical function measure (+ 2.4 points, p = 0.04), the five-time sit-to-stand test (− 3.4 s, p = < 0.001), and the 30-s sit-to-stand test (+ 1.9 reps, p = 0.002) after participating in tele-EF. In contrast, the control group did not demonstrate significant change on the same measures. Group by time interaction for mean change in the five-time sit-to-stand test was significantly different for the intervention group compared to the control group (p = 0.03; d = 0.44). There was no significant difference between or within groups for change in the single leg stand balance test.
Table 2.
Physical function and physical performance outcomes
| Tele-EF group Mean (SD) or n (%) N = 19 |
Control group Mean (SD) or n (%) N = 18 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 16 weeks | Change | P | Baseline | 16 weeks | Change | p | Group × time p-value |
Effect size | |
| PROMIS Physical function (0-100) | 45.5 (1.5) | 47.9 (2.0) | 2.4 | 0.04 | 47.1 (1.8) | 47.2 (1.7) | 0.1 | 0.94 | 0.22 | 0.33 |
| Five-time sit-to-stand (s) | 14.8 (5.3) | 11.4 (3.5) | -3.4 | < 0.001 | 13.8 (3.5) | 12.7 (3.1) | -1.1 | 0.08 | 0.03 | 0.44 |
| 30-s sit-to-stand (reps) | 10.9 (3.6) | 12.8 (3.5) | 1.9 | 0.002 | 11.4 (2.2) | 12.3 (3.1) | 0.2 | 0.19 | 0.21 | 0.33 |
| Single-leg stand for 10 s (Y/N) | 10 (53%) | 11 (58%) | + 5.4% | 0.60 | 10 (56%) | 11 (61%) | + 5.4% | 0.60 | 0.79 | 0.07 |
Bolded p-values indicate significance < 0.05
Physical activity outcomes
There were significant improvements in the intervention group for time spent in MVPA and LPA, step counts, and a reduction in time spent being sedentary (Table 3). Notably, time spent in LPA increased by 38.5 min (p = 0.01), mean daily sedentary time decreased by 56 min (p = 0.01), and mean daily step counts increased by 1977 steps (p = 0.003) after the intervention. There were no significant changes in the control group for the same outcomes. The between-group comparison showed significant differences in the physical activity outcomes for LPA (p = 0.03, d = 0.72), sedentary time (p = 0.001, d = 0.81), and total step count (p = 0.01, d = 0.96). Mean daily MVPA increased significantly in the intervention group by 3.2 min (p = 0.02). Several measures of cadence outcomes improved significantly among those in the intervention group but not in the control group (see Online Resource 2).
Table 3.
Changes in accelerometer-derived physical activity and sedentary time
| Tele-EF group Mean (SD) | Control group Mean (SD) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Physical activity | Baseline | 16 weeks | Change | p | Baseline | 16 weeks | Change | P | Group × time p-value | Effect size |
| MVPA mean (min) | 3.2 (5.7) | 6.5 (6.9) | 3.2 (5.0) | 0.02 | 4.9 (5.9) | 5.7 (7.6) | 0.8 (6.9) | 0.65 | 0.32 | 0.53 |
| Light intensity (min) | 237.8 (54.0) | 276.3 (52.4) | 38.5 (53.8) | 0.01 | 244.2 (81.3) | 244.7 (106.3) | 0.5 (66.4) | 0.98 | 0.03 | 0.72 |
| Sedentary (min) | 592.8 (108.9) | 536.5 (91.7) | − 56.3 (79.6) | 0.01 | 591.7 (119.4) | 610.1 (105.2) | 18.4 (65.3) | 0.26 | 0.001 | 0.81 |
| Total step | 5201.9 (1980.4) | 7178.8 (2799.7) | 1976.9 (2247.0) | 0.003 | 6071.7 (2525.2) | 6104.8 (3136.0) | 33.2 (1929.5) | 0.94 | 0.01 | 0.96 |
Bolded p-values indicate significance < 0.05
Self-efficacy and other patient-reported outcomes
There were no significant changes in self-efficacy between baseline and follow-up for either group. Changes in patient-reported outcomes are presented in Online Resource 3. Of the six non-physical function PROMIS domains, there were no changes except for a significant increase in depression in the control group.
Discussion
We investigated the feasibility of remote exercise for older, rural cancer survivors and to examine the effects of remote exercise on physical function, and secondary outcomes of PA and patient-reported outcomes. The study was conducted remotely with no in-person recruitment or interaction. Despite this constraint, we exceeded our feasibility standards for recruitment and retention of older cancer survivors living in rural communities. Attrition was lower than expected (5% vs 20%) with one drop-out from the exercise class due to unrelated medical issues and one drop-out from the control group.
The median class attendance rate of 87% was higher than the 64–75% reported in previous studies of in-person exercise classes for older adults [44, 45]. Attendance also exceeded the minimum recommendation of 75% attendance for cancer survivors [29, 30]. Both retention and class attendance provide evidence that similar to in-person exercise classes, EnhanceFitness classes offered 1 h, 3 days per week over 16 weeks which are feasible through remote delivery. In contrast to unsupervised work-out videos and fitness apps, videoconferencing provides opportunity for live interaction between participants and the exercise instructor. Real-time instruction of group-based classes helps motivate older adults to exercise by establishing relationships, fostering social support, and receiving corrective and supportive feedback from instructors [46, 47].
Consistent with other pilot studies of remote exercise in older adults, participants in tele-EF demonstrated improved physical function [46, 48] with moderate effect sizes. This provides evidence that the frequency and intensity of tele-EF was sufficient to improve self-reported and objectively measured physical function. Collectively, these outcomes represent functional improvements for this population at higher risk for falls and functional limitations. In particular, the improvement in the five-time sit-to-stand test suggests the intervention group had a reduction in fall risk. Given the higher fall rates in older cancer survivors [49], and designation of EF as an evidence-based falls prevention program [50], a future trial assessing efficacy of tele-EF on fall reduction in older cancer survivors is warranted.
After completing tele-EF, participants had an average daily increase of 39 min of LPA activity and close to an hour reduction in average daily time spent being sedentary. Further, there was a corresponding 1977 step increase in mean daily step counts, which is an increase of almost one mile per day. In older adults, increases in light activity are associated with physical health and well-being [51]. Prior studies have estimated that replacing 30 min/day of sedentary time with equal amounts of light physical activity is associated with improved physical and cardiometabolic health, and reduced mortality risk [52]. Although the tele-EF intervention did not include explicit behavior change components, the multi-component exercise program resulted in positive lifestyle changes with potential for significant health benefits.
The PA outcomes were assessed once the tele-EF program ended. Therefore, it is not surprising that the mean daily increase in MVPA of 3.2 min, though statistically significant, was not substantial. The challenge of maintenance of exercise behavior change post intervention is well documented [53]. Unlike many previous exercise interventions for cancer survivors that did not include a maintenance option, tele-EF is already disseminated and available in the community through organizations such as the YMCA and senior centers. However, one limitation of the current dissemination model is potentially higher costs for tele-EF. Traditional EF classes incur a relatively fixed fee in securing a supply of dumbbells and cuff weights for use among participants whereas tele-EF requires individuals to have their own set of weights. A future efficacy trial will need to examine the additional costs of weights and technology needed for participation compared to the transportation cost savings. Although it was beyond the scope of the current study, future implementation studies can evaluate uptake of tele-EF for exercise maintenance among older rural cancer survivors. This should include examination of continued participation rates in tele-EF and maintenance of improvements in those who continue participation versus those who do not, with evaluation of outcomes at least 6 months after the 16-week intervention ends.
Fatigue and sleep interference, common post-treatment symptoms among cancer survivors, improved, but not significantly. Similarly, we did not see improvement in self-efficacy for exercise or other patient-reported outcomes, suggesting that access to supervised exercise, without additional intervention components, is not sufficient to see a change in those outcomes. As we did not limit recruitment to people immediately post cancer treatment, it is possible that these outcomes had already stabilized for the participants. Additionally, we recruited a heterogeneous group with respect to cancer type, which may have impacted the severity of these patient-reported outcomes. Longer follow-up, more precise dose assessments, or additional measures may help to elucidate the lack of change in these outcomes.
As a pilot study, we acknowledge several limitations. The population recruited for the study was predominantly white, female, and college educated. It will be critical to include a more diverse population in fully powered trials to establish the efficacy of tele-EF for rural, older cancer survivors. This will require partnerships with community-based organizations and primary care and oncology practices who serve rural and more diverse communities, tailored communication, recruitment of trainers from the same communities, and multiple options for class timing [54, 55]. Additionally, the sample size was not sufficient to identify changes in many of the patient-reported secondary outcomes. Physical activity measurement with accelerometers is not infallible; limitations of accelerometry include inability to capture upper extremity movement, potential for misclassification of intensity, and restrictions on the types of activity that are measured. A challenge of conducting exercise feasibility studies is the tendency for people who already exercise, or are interested in exercise, to be the first respondents to recruitment notices. Key to future implementation is the need for strategies to reach and recruit older rural cancer survivors who may not place a high value on exercise to participate in tele-exercise, particularly in situations where access is limited.
This study builds on previous work that established that older adults are capable of using technology to participate in tele-exercise, particularly when they have support and it is a valued activity [16, 46]. Our findings demonstrate that tele-EF is feasible for older, rural cancer survivors and warrants further study given the limited access to community-based exercise options for cancer survivors [10], particularly in rural communities. Based on the positive changes and moderate to large effect sizes in physical function and physical activity, future implementation-effectiveness studies with larger and more diverse populations are needed to establish evidence for tele-EF as an effective exercise program for aging cancer survivors.
Supplementary information
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank Paige Dennison, Rebecca Reynolds, Moira Mulligan, Rachel Adams, and Elise Hoffman for their assistance with this research.
Author contribution
Nancy Gell, Kim Dittus, and Kushang V. Patel contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Nancy Gell, Jacqueline Caefer, Anita Martin, and Myeongjin Bae. The first draft of the manuscript was written by Nancy Gell, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by a grant from the University of Vermont Cancer Center.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Ethical approval
All procedures performed in the study involving human participants were in accordance with the ethical standards of the 1964 Helsinki declaration. Approval was granted by the University of Vermont Institutional Research Committee (March 12, 2021/Study #1375).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66:271–289. doi: 10.3322/caac.21349. [DOI] [PubMed] [Google Scholar]
- 2.Leach CR, Weaver KE, Aziz NM, Alfano CM, Bellizzi KM, Kent EE, et al. The complex health profile of long-term cancer survivors: prevalence and predictors of comorbid conditions. J Cancer Surviv. 2015;9:239–251. doi: 10.1007/s11764-014-0403-1. [DOI] [PubMed] [Google Scholar]
- 3.Weaver KE, Palmer N, Lu L, Case LD, Geiger AM. Rural–urban differences in health behaviors and implications for health status among US cancer survivors. Cancer Causes Control. 2013;24:1481–1490. doi: 10.1007/s10552-013-0225-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson TJ, Saman DM, Lipsky MS, Lutfiyya MN. A cross-sectional study on health differences between rural and non-rural U.S. counties using the County Health Rankings. BMC Health Serv Res. 2015;15:441. doi: 10.1186/s12913-015-1053-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li T, Wei S, Shi Y, Pang S, Qin Q, Yin J, et al. The dose–response effect of physical activity on cancer mortality: findings from 71 prospective cohort studies. Br J Sports Med. 2015;50(6):339–45. doi: 10.1136/bjsports-2015-094927. [DOI] [PubMed] [Google Scholar]
- 6.McNeely ML. Effects of exercise on breast cancer patients and survivors: a systematic review and meta-analysis. Can Med Assoc J. 2006;175:34–41. doi: 10.1503/cmaj.051073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buffart LM, Galvão DA, Brug J, Chinapaw MJM, Newton RU. Evidence-based physical activity guidelines for cancer survivors: Current guidelines, knowledge gaps and future research directions. Cancer Treat Rev. 2014;40:327–340. doi: 10.1016/j.ctrv.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 8.Patel AV, Friedenreich CM, Moore SC, Hayes SC, Silver JK, Campbell KL, et al. American College of Sports Medicine Roundtable Report on Physical Activity, Sedentary Behavior, and Cancer Prevention and Control. Med Sci Sports Exerc. 2019;51:2391–2402. doi: 10.1249/MSS.0000000000002117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campbell KL, Winters-Stone K, Wiskemann J, May AM, Schwartz AL, Courneya KS, et al. Exercise guidelines for cancer survivors: consensus statement from International Multidisciplinary Roundtable. Med Sci Sports Exerc. 2019;51:2375–2390. doi: 10.1249/MSS.0000000000002116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmitz KH, Stout NL, Maitlin-Shepard M, Campbell A, Schwartz AL, Grimmett C, et al. Moving Through Cancer: Setting the agenda to make exercise standard in oncology practice. Cancer. 2021;127:476–484. doi: 10.1002/cncr.33245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith-Turchyn J, Gravesande J, Sabiston CM. Exercise interventions for survivors of cancer living in rural or remote settings: a scoping review. Rehabil Oncol. 2020;38:61–80. doi: 10.1097/01.REO.0000000000000208. [DOI] [Google Scholar]
- 12.Rogers LQ, Markwell SJ, Verhulst S, McAuley E, Courneya KS. Rural breast cancer survivors: exercise preferences and their determinants. Psychooncology. 2009;18:412–421. doi: 10.1002/pon.1497. [DOI] [PubMed] [Google Scholar]
- 13.Rogers LQ, Markwell SJ, Courneya KS, McAuley E, Verhulst S. Exercise preference patterns, resources, and environment among rural breast cancer survivors. J Rural Health. 2009;25:388–391. doi: 10.1111/j.1748-0361.2009.00249.x. [DOI] [PubMed] [Google Scholar]
- 14.Hoffman GJ, Malani PN, Solway E, Kirch M, Singer DC, Kullgren JT. Changes in activity levels, physical functioning, and fall risk during the COVID-19 pandemic. J Am Geriatr Soc. 2022;70:49–59. doi: 10.1111/jgs.17477. [DOI] [PubMed] [Google Scholar]
- 15.Choi NG, DiNitto DM, Marti CN, Choi BY. Telehealth use among older adults during COVID-19: associations with sociodemographic and health characteristics, Technology Device Ownership, and Technology Learning. J Appl Gerontol. 2022;41:600–609. doi: 10.1177/07334648211047347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gell N, Hoffman E, Patel K. Technology support challenges and recommendations for adapting an evidence-based exercise program for remote delivery to older adults: exploratory mixed methods study. JMIR Aging. 2021;4:e27645. doi: 10.2196/27645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Enhance®Fitness | Project Enhance n.d. https://projectenhance.org/enhancefitness/ (accessed October 26, 2020).
- 18.Belza B, Shumway-Cook A, Phelan EA, Williams B, Snyder SJ, LoGerfo JP. The effects of a community-based exercise program on function and health in older adults: the EnhanceFitness program. J Appl Gerontol. 2006;25:291–306. doi: 10.1177/0733464806290934. [DOI] [Google Scholar]
- 19.Moore-Harrison TL, Johnson MA, Quinn ME, Cress ME. An evidence-based exercise program implemented in congregate-meal sites. J Phys Act Health. 2009;6:247–251. doi: 10.1123/jpah.6.2.247. [DOI] [PubMed] [Google Scholar]
- 20.Eldridge SM, Chan CL, Campbell MJ, Bond CM, Hopewell S, Thabane L, CONSORT, , et al. statement: extension to randomised pilot and feasibility trials. BMJ. 2010;2016:355. doi: 10.1136/bmj.i5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saghaei M, Saghaei S. Implementation of an open-source customizable minimization program for allocation of patients to parallel groups in clinical trials. J Biomed Sci Eng. 2011;4:734. doi: 10.4236/jbise.2011.411090. [DOI] [Google Scholar]
- 22.USDA ERS - Rural-Urban Commuting Area Codes n.d. https://www.ers.usda.gov/data-products/rural-urban-commuting-area-codes/ (accessed August 9, 2022).
- 23.Physical Activity Guidelines Advisory Committee Physical Activity Guidelines Advisory Committee Scientific Report. Wash DC US Dep Health Hum Serv. 2018;2018:779. [Google Scholar]
- 24.Harris P, Taylor R, Thielke R, Payne J, Gonzalez N, Conde J. Research Electronic Data Capture (REDCap) — a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown JC, Ko EM, Schmitz KH. Development of a risk-screening tool for cancer survivors to participate in unsupervised moderate- to vigorous-intensity exercise: results from a survey study. PM&R. 2015;7:113–122. doi: 10.1016/j.pmrj.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thomas S, Reading J, Shephard RJ. Revision of the Physical Activity Readiness Questionnaire (PAR-Q) Can J Sport Sci. 1992;17:338–345. [PubMed] [Google Scholar]
- 27.Mishra SI, Scherer RW, Geigle PM, Berlanstein DR, Topaloglu O, Gotay CC, et al. Exercise interventions on health-related quality of life for cancer survivors. Cochrane Database Syst Rev. 2012 doi: 10.1002/14651858.CD007566.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singh B, Spence RR, Steele ML, Sandler CX, Peake JM, Hayes SC. A systematic review and meta-analysis of the safety, feasibility, and effect of exercise in women with stage II+ breast cancer. Arch Phys Med Rehabil. 2018;99:2621–2636. doi: 10.1016/j.apmr.2018.03.026. [DOI] [PubMed] [Google Scholar]
- 29.Turner RR, Steed L, Quirk H, Greasley RU, Saxton JM, Taylor SJ, et al. Interventions for promoting habitual exercise in people living with and beyond cancer. Cochrane Database Syst Rev. 2018;9(9):CD010192. doi: 10.1002/14651858.CD010192.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bourke L, Homer KE, Thaha MA, Steed L, Rosario DJ, Robb KA, et al. Interventions to improve exercise behaviour in sedentary people living with and beyond cancer: a systematic review. Br J Cancer. 2014;110:831–841. doi: 10.1038/bjc.2013.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jensen RE, Moinpour CM, Potosky AL, Lobo T, Hahn EA, Hays RD, et al. Responsiveness of 8 patient-reported outcomes measurement information system (PROMIS) measures in a large, community-based cancer study cohort. Cancer. 2017;123:327–335. doi: 10.1002/cncr.30354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garcia AW, King AC. Predicting long-term adherence to aerobic exercise: a comparison of two models. J Sport Exerc Psychol. 1991;13:394–410. doi: 10.1123/jsep.13.4.394. [DOI] [Google Scholar]
- 33.Overcash JA, Rivera HR. Physical performance evaluation of older cancer patients: a preliminary study. Crit Rev Oncol Hematol. 2008;68:233–241. doi: 10.1016/j.critrevonc.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 34.Blair CK, Harding E, Herman C, Boyce T, Demark-Wahnefried W, Davis S, et al. Remote assessment of functional mobility and strength in older cancer survivors: protocol for a validity and reliability study. JMIR Res Protoc. 2020;9:e20834 . doi: 10.2196/20834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guidarelli C, Lipps C, Stoyles S, Dieckmann NF, Winters-Stone KM. Remote administration of physical performance tests among persons with and without a cancer history: establishing reliability and agreement with in-person assessment. J Geriatr Oncol. 2022;13:691–697. doi: 10.1016/j.jgo.2022.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lyden K, Keadle SK, Staudenmayer J, Freedson PS. The activPAL™ accurately classifies activity intensity categories in healthy adults. Med Sci Sports Exerc. 2017;49:1022–1028. doi: 10.1249/MSS.0000000000001177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grant PM, Dall PM, Mitchell SL, Granat MH. Activity-monitor accuracy in measuring step number and cadence in community-dwelling older adults. J Aging Phys Act. 2008;16:201–214. doi: 10.1123/japa.16.2.201. [DOI] [PubMed] [Google Scholar]
- 38.Maddocks M, Byrne A, Johnson CD, Wilson RH, Fearon KCH, Wilcock A. Physical activity level as an outcome measure for use in cancer cachexia trials: a feasibility study. Support Care Cancer. 2010;18:1539–1544. doi: 10.1007/s00520-009-0776-2. [DOI] [PubMed] [Google Scholar]
- 39.O’Brien MW, Kivell MJ, Wojcik WR, D’Entremont G, Kimmerly DS, Fowles JR. Step rate thresholds associated with moderate and vigorous physical activity in adults. Int J Environ Res Public Health. 2018;15:2454. doi: 10.3390/ijerph15112454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tudor-Locke C, Mora-Gonzalez J, Ducharme SW, Aguiar EJ, Schuna JM, Barreira TV, et al. Walking cadence (steps/min) and intensity in 61–85-year-old adults: the CADENCE-Adults study. Int J Behav Nutr Phys Act. 2021;18:129. doi: 10.1186/s12966-021-01199-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lancaster GA, Dodd S, Williamson PR. Design and analysis of pilot studies: recommendations for good practice. J Eval Clin Pract. 2004;10:307–312. doi: 10.1111/j.2002.384.doc.x. [DOI] [PubMed] [Google Scholar]
- 42.Morris SB. Estimating effect sizes from pretest-posttest-control group designs. Organ Res Methods. 2008;11:364–386. doi: 10.1177/1094428106291059. [DOI] [Google Scholar]
- 43.Tiedemann A, Shimada H, Sherrington C, Murray S, Lord S. The comparative ability of eight functional mobility tests for predicting falls in community-dwelling older people. Age Ageing. 2008;37:430–435. doi: 10.1093/ageing/afn100. [DOI] [PubMed] [Google Scholar]
- 44.Ory MG, Lee S, Zollinger A, Bhurtyal K, Jiang L, Smith ML. Translation of fit & strong! For middle-aged and older adults: examining implementation and effectiveness of a lay-led model in central Texas. Front Public Health. 2014;2:187. doi: 10.3389/fpubh.2014.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tomioka M, Braun KL, Wu YY, Holt K, Keele P, Tsuhako L, et al. Twelve-month retention in and impact of Enhance®Fitness on older adults in Hawai‘i. J Aging Res. 2019;2019:e9836181 . doi: 10.1155/2019/9836181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Patel KV, Hoffman EV, Phelan EA, Gell NM. Remotely delivered exercise to rural older adults with knee osteoarthritis: a pilot study. ACR Open Rheumatol. 2022;4(8):735–744. doi: 10.1002/acr2.11452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bethancourt HJ, Rosenberg DE, Beatty T, Arterburn DE. Barriers to and facilitators of physical activity program use among older adults. Clin Med Res. 2014;12:10–20. doi: 10.3121/cmr.2013.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Buckinx F, Aubertin-Leheudre M, Daoust R, Hegg S, Martel D, Martel-Thibault M, et al. Feasibility and acceptability of remote physical exercise programs to prevent mobility loss in pre-disabled older adults during isolation periods such as the COVID-19 pandemic. J Nutr Health Aging. 2021;25:1106–1111. doi: 10.1007/s12603-021-1688-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bird M-L, Cheney MJ, Williams AD. Accidental fall rates in community-dwelling adults compared to cancer survivors during and post-treatment: a systematic review with meta-analysis. Oncol Nurs Forum. 2016;43:E64–72. doi: 10.1188/16.ONF.E64-E72. [DOI] [PubMed] [Google Scholar]
- 50.Evidence-Based Falls Prevention Programs for Older Adults. @NCOAging n.d. https://www.ncoa.org/article/evidence-based-falls-prevention-programs (accessed August 9, 2022).
- 51.Buman MP, Hekler EB, Haskell WL, Pruitt L, Conway TL, Cain KL, et al. Objective light-intensity physical activity associations with rated health in older adults. Am J Epidemiol. 2010;172:1155–1165. doi: 10.1093/aje/kwq249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.del Pozo-Cruz J, García-Hermoso A, Alfonso-Rosa RM, Alvarez-Barbosa F, Owen N, Chastin S, et al. Replacing sedentary time: meta-analysis of objective-assessment studies. Am J Prev Med. 2018;55:395–402. doi: 10.1016/j.amepre.2018.04.042. [DOI] [PubMed] [Google Scholar]
- 53.Jankowski CM, Ory MG, Friedman DB, Dwyer A, Birken SA, Risendal B. Searching for maintenance in exercise interventions for cancer survivors. J Cancer Surviv. 2014;8:697–706. doi: 10.1007/s11764-014-0386-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.O’Regan A, Bengoechea EG, Clifford AM, Casey M, Gallagher S, Glynn L, et al. How to improve recruitment, sustainability and scalability in physical activity programmes for adults aged 50 years and older: a qualitative study of key stakeholder perspectives. PLoS ONE. 2020;15:e0240974 . doi: 10.1371/journal.pone.0240974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marquez DX, Aguiñaga S, Castillo A, Hughes SL, Der Ananian C, Whitt-Glover MC. ¡Ojo! What to expect in recruiting and retaining older Latinos in physical activity programs. Transl Behav Med. 2019;10:1566–1572. doi: 10.1093/tbm/ibz127. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.