Abstract
Background
A suboptimal response to the 2-dose COVID-19 vaccine series in the immunocompromised population prompted recommendations for a 3rd primary dose. We aimed to determine the humoral and cellular immune response to the 3rd COVID-19 vaccine in immunocompromised children.
Methods
Prospective cohort study of immunocompromised participants, 5–21 years old, who received 2 prior doses of an mRNA COVID-19 vaccine. Humoral and CD4/CD8 T-cell responses were measured to SARS-CoV-2 spike antigens prior to receiving the 3rd vaccine dose and 3–4 weeks after the 3rd dose was given.
Results
Of the 37 participants, approximately half were solid organ transplant recipients. The majority (86.5%) had a detectable humoral response after the 2nd and 3rd vaccine doses, with a significant increase in antibody levels after the 3rd dose. Positive T-cell responses increased from being present in 86.5% to 100% of the cohort after the 3rd dose.
Conclusions
Most immunocompromised children mount a humoral and cellular immune response to the 2-dose COVID-19 vaccine series, which is significantly augmented after receiving the 3rd vaccine dose. This supports the utility of the 3rd vaccine dose and the rationale for ongoing emphasis for vaccination against COVID-19 in this population.
Impact
Most immunocompromised children mount a humoral and cellular immune response to the 2-dose COVID-19 vaccine series, which is significantly augmented after receiving the 3rd vaccine dose.
This is the first prospective cohort study to analyze both the humoral and T-cell immune response to the 3rd COVID-19 primary vaccine dose in children who are immunocompromised.
The results of this study support the utility of the 3rd vaccine dose and the rationale for ongoing emphasis for vaccination against COVID-19 in the immunosuppressed pediatric population.
Introduction
Vaccines against SARS-CoV-2 have been instrumental in decreasing COVID-19 related morbidity and mortality.1,2 Although vaccine efficacy is high in immunocompetent adults, those who are immunocompromised have demonstrated a suboptimal response to the primary 2-dose COVID-19 vaccine series with higher hospitalization rates and greater severity of illness.3–6 A recent meta-analysis found that the 2-dose COVID-19 vaccine efficacy was 70.4% in immunocompromised adults, with only 63% developing anti-SARS-CoV2 spike protein IgG antibodies compared to 99.1% in the healthy population.7 Studies evaluating a 3rd dose of the COVID-19 vaccine in immunocompromised adults showed a significant increase in seroconversion which prompted the FDA to recommend a 3rd primary dose for the immunocompromised population.8–11 Subsequently, multiple booster vaccine doses have now been recommended as well.
Notwithstanding the previous recommendation, there is limited evidence regarding the degree and duration of vaccine-induced immunogenicity in immunocompromised children. Recent pediatric-specific studies have revealed a suboptimal antibody response to the 2 and 3-dose COVID-19 vaccine series in adolescent kidney transplant recipients which is comparable to observations in immunocompromised adults.12,13 In contrast, a cohort of children with inflammatory bowel disease did not have a suppressed antibody response to the 2-dose series, despite receiving immunosuppressive medications.14 While the humoral response is more commonly studied, less is known regarding the T-cell response to the COVID-19 vaccine in immunocompromised individuals, particularly in children.9,15 In turn, we aimed to evaluate both the humoral and T-cell immune response to the 3rd COVID-19 primary vaccine dose in a population of immunocompromised children and adolescents.
Methods
This was a prospective cohort study with immunocompromised participants 5–21 years of age. Participants were characterized as being immunocompromised based upon the following criteria published by the Center for Disease Control and Prevention (CDC):
“Received a solid organ transplant (heart, liver, kidney) and receiving immunosuppressive therapy, OR
Receiving active cancer treatment (tumor or blood cancers), OR
Received a stem cell transplant within the last 2 years, OR
Those with a moderate/severe primary immunodeficiency, OR
Receiving chronic immunosuppression defined as active treatment with high-dose corticosteroids (i.e., ≥20 mg prednisone or equivalent per day), alkylating agents, antimetabolites, transplant-related immunosuppressive drugs, cancer chemotherapeutic agents classified as severely immunosuppressive, tumor necrosis factor (TNF) blockers, and other biologic agents that are immunosuppressive or immunomodulatory”16
Inclusion criteria required participants to have previously received the initial 2 vaccine doses of either the BNT162b2 (Comirnaty®) or mRNA-1273 (Spikevax®) vaccine, as per the age-related recommendations from the CDC, and have plans to receive the 3rd primary vaccine dose. Participants were excluded if any of their immunosuppressive medication dosages were increased by >25% of their maintenance dose in the 2 weeks prior to receiving the 3rd vaccine dose because of the influence that enhanced immunosuppression might have on vaccine response. Primary physicians and coordinators within each participating division approached eligible participants during clinic visits for recruitment. Interested participants were then contacted by study team members for consent.
The study consisted of 2 visits with an online survey and lab assays for humoral and cellular immune response at each visit. The 1st study visit occurred immediately prior to receiving the 3rd dose of the COVID-19 vaccine, which participants were instructed to receive through any of the available vaccine clinics at Children’s Mercy Kansas City or within the community. The 2nd study visit occurred 3–4 weeks post-3rd dose vaccination. Humoral response was measured by quantitative SARS-CoV-2 immunoglobulin G (IgG) antibody binding levels to 4 viral proteins and an ACE-receptor blocking assay measured the neutralizing antibody level (≥ 30% indicated a positive response). Cellular response was measured by SARS-CoV-2 antigen-specific T-cell (CD4/CD8) assays and reported as “detected” or “not detected”. Online surveys were obtained via REDCap electronic data capture tools hosted at Children’s Mercy Kansas City.17,18 Surveys included initial demographic information with subsequent surveys assessing medication changes and COVID-19 breakthrough illnesses.
Humoral immune response
SARS-CoV-2 viral antigen multiplexed binding assay
Antibody levels to SARS-CoV-2 spike subunit proteins (spike subunit 1 (S1), spike subunit 2 (S2), receptor-binding domain (RBD)) and nucleocapsid (NP) antigens were measured with a bead-based multiplex assay and Luminex xMAP technology, using reagent kits (Millipore, #HC19SERG1-85K) with secondary antibodies specific for IgG. Each kit provided SARS-CoV-2 antigen conjugated beads (S1, S2, RBD, NP) along with 4 positive control beads and a negative control bead set. The positive control beads were coated with different concentrations of IgG while the negative control beads determined nonspecific binding. The 4 positive control beads and 1 negative control bead were mixed and incubated with each plasma sample which was diluted to 1:100 with assay buffer. With each assay plate, at least two sample wells with only buffer and no plasma were included to determine assay background. Finally, PE-anti-human IgG conjugate detection antibodies were utilized to determine antibody isotype responses to each of the SARS-CoV-2 antigens. Using the positive control beads, the inter-assay (plate-to-plate) coefficient of variation (CV) was determined to be 5.16% for each assay. To acquire and analyze data, the Luminex analyzer (MAGPIX) and Luminex xPONENT acquisition software were utilized. Samples were run in duplicate, and after acquisition, net mean fluorescence intensity (MFI) was determined (MFI with background well MFI subtracted). Positive control beads were utilized to ensure positive detection of the well and to identify any inter- and/or intra-assay technical variation. The level of nonspecific binding was detected by using the negative control samples’ MFI.
SARS-CoV-2 viral neutralizing antibody assays
To detect viral neutralizing antibodies, the SARS-CoV-2 Surrogate Virus Neutralization Test kit was used (Genscript, #L00847) according to the standard protocol.19–21 Samples were run in duplicate with blocking values averaged. This kit detected antibodies that block the interaction between the receptor binding domain of the viral spike glycoprotein with the Angiotensin Converting Enzyme 2 (ACE2) cell surface receptor and has been approved by the FDA for emergency use. Plasma samples, along with positive (anti-RBD antibody) and negative (buffer only) controls, were incubated with a Horseradish Peroxidase (HRP) conjugated recombinant SARS-CoV-2 RBD (original strain 2019-nCoV) or SARS-CoV-2 RBD (Omicron) fragment. The mixture was then added to a capture plate coated with the human ACE2 protein. The unbound HRP-RBD then binds to the plate. After washing, 3,3’,5,5’-Tetramethylbenzidine (TMB) solution was added to develop the HRP signal and was read at 450 nm in a microtiter plate reader. The absorbance of the sample was inversely dependent on the titer of the anti-SARS-CoV-2 neutralizing antibodies. A cutoff of ≥30% was considered positive for SARS-CoV-2 neutralizing antibody. Plasma samples were diluted 1:10 for all samples.
Cellular immune response
Patient whole blood specimens were sent to a commercial reference laboratory (Eurofins-Viracor Laboratories) for the SARS-CoV-2 inSIGHT™ T-cell immunity panel testing. Aliquots of the samples were left unstimulated to measure background or were stimulated with the SARS-CoV-2 peptide mix for the spike (S) protein, the nucleocapsid peptide (NP) mix (JPT Peptide Technologies) or with Staphylococcus aureus enterotoxin B (SEB) as a positive control. Samples were stimulated overnight in a 37 °C 5% CO2 incubator in the presence of CD28/49d and Brefeldin A (BD Biosystems). After the overnight incubation, erythrocytes were lysed, and the samples were fixed and permeabilized for intracellular cytokine staining. Fluorochrome labeled antibodies used for identifying specific T-cell populations and functional activation were CD3-PerCPCy5.5, CD4-Pacific Blue, CD8-APC-Cy7, CD69-PE-Cy7, IFNγ-FITC, IL-2-PE and TNFα-APC (BD Biosciences). Samples were acquired on the Cytek Aurora® flow cytometer and the data were analyzed using FCS Express™. Activated T-cells expressed CD69 and at least one of the cytokines. The fold-increase over the background (unstimulated) responses for each population were calculated and a 3-fold increase or higher in both a single cytokine (IFNγ, IL-2 or TNFα) expressing T-cell population and a polyfunctional (defined as 2 or more individual cytokines detected in individual cells) expressing T-cell population from either S or NP stimulated aliquots were reported as immunity detected. This T-cell assay was validated using samples from healthy volunteers who were vaccinated against SARS-CoV-2 or had been infected and were recovered. The sensitivity of the assay from these samples was determined to be 90% and the specificity was 78% when the cutoff for positivity was 3-fold above the background in the responding cell populations.
Statistical analysis
McNemar’s test was used to compare the frequency of detected vs. non-detected between time points. The Wilcoxon signed-rank test was used when comparing continuous outcomes between time points. Data was analyzed using R software (version 4.1.2; R Core Team: Vienna Austria).
Results
Study population
Thirty-seven participants completed the 2 study visits assessing the immune response prior to and 3–4 weeks after receiving the 3rd COVID-19 vaccine dose. Of these participants, 17 had a solid organ transplant and 20 were receiving immunosuppressive medication for other chronic illnesses (Table 1). The mean age was 15.4 years, 62.2% male, with most participants prescribed a calcineurin inhibitor, prednisone, mycophenolate mofetil, and/or a tumor necrosis factor alpha (TNFα) blocking agent. Six participants self-reported COVID-19 illnesses prior to receiving the 3rd COVID-19 vaccine dose (Table 1).
Table 1.
Demographics.
| Age (mean, range) | 15.4 years (5–21 years) |
| Sex (N, % male) | 23 (62.2%) |
| Race (N, %) | |
| White | 27 (73%) |
| Black/African American | 3 (8.1%) |
| Asian | 2 (5.4%) |
| American Indian/Alaska Native | 1 (2.7%) |
| Multiracial | 2 (5.4%) |
| Unknown | 2 (5.4%) |
| Ethnicity (N, %) | |
| Hispanic/Latino | 3 (8.1%) |
| Non-Hispanic/Latino | 28 (75.7%) |
| Unknown/Other | 6 (16.2%) |
| Medical History (N, %) | |
| Kidney transplant | 12 (32.4%) |
| Liver transplant | 5 (13.5%) |
| Stem cell transplant | 1 (2.7%) |
| Rheumatologic disease | 13 (35.1%) |
| Gastrointestinal disease | 6 (16.2%) |
| Immunosuppressive medications (N) | |
| Tacrolimus, Mycophenolate mofetil, Prednisone | 11 |
| Methotrexate, Adalimumab/Infliximab | 6 |
| Adalimumab or Infliximab | 6 |
| Tacrolimus | 3 |
| Methotrexate | 2 |
| Sirolimus | 2 |
| Hydrocortisone/Stem cell transplant | 1 |
| Sirolimus, Mycophenolate mofetil, Prednisone | 1 |
| Rituximab | 1 |
| Anakinra, Infliximab | 1 |
| Cellcept, Prednisone | 1 |
| Tocilizumab | 1 |
| Abatacept | 1 |
| Self-reported COVID-19 Illness Prior to 3rd Vaccine (N, %) | |
| Yes | 6 (16.2%) |
| No | 29 (78.4%) |
| Unknown | 2 (5.4%) |
| Months between 2nd and 3rd COVID vaccine (mean, range) | 4.38 months (1–9 months) |
Humoral immune response
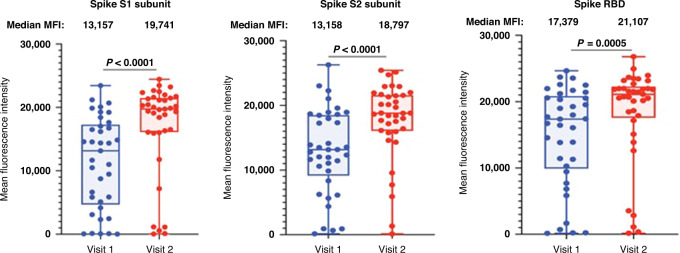
Levels of binding antibodies to 3 SARS-CoV-2 spike protein subunits (S1, S2 and receptor-binding domain (RBD)) were detected in most participants after the 2-dose vaccine series with median MFI levels of 13,157 (95% CI: 6639–16,188), 13,158 (95% CI: 11,434–17,259), and 17,379 (95% CI: 13,838–19,540) for S1, S2, and RBD subunits, respectively (Fig. 1). These levels were significantly boosted after the 3rd vaccine dose for all three spike subunit proteins to median MFI levels of 19,741 (95% CI: 18,426–21,108), 18,797 (95% CI: 17,532–20,542), and 21,107 (95% CI 20,097–21,913) for S1, S2, and RBD subunits, respectively (p ≤ 0.0005) (Fig. 1). Five participants had low levels (<2000 MFI) for all spike protein subunits, which remained low even after the 3rd vaccine dose, suggesting a limited humoral immune response to the vaccine (Fig. 1).
Fig. 1. Antibody binding levels to SARS-CoV-2 spike proteins.
Multiplex bead-based antibody binding assay that measures the IgG antibody response to SARS-CoV-2 spike subunit 1 (S1), spike subunit 2 (S2) and receptor-binding domain (RBD). Median Fluorescent Intensity (MFI) is shown and background well subtraction has been used to remove nonspecific signal. Each dot represents an individual (n = 37) after the two-dose vaccine series (Visit 1) and after the 3rd vaccine dose (Visit 2). P values were determined using Wilcoxon matched pairs signed rank test. Group median values are displayed above the graph.
The spike protein is the only SARS-CoV-2 viral component in the COVID-19 vaccine. Thus, antibody responses to other viral proteins, such as the nucleocapsid protein (NP), likely indicate prior infection with SARS-CoV2. As expected, most participants had low NP antibody levels before and after receiving the 3rd vaccine. Five of the six participants who had a self-reported COVID-19 illness prior to receiving the 3rd vaccine dose had elevated NP antibodies (>5000 MFI). Three of those participants had elevated NP antibodies after the 2-dose vaccine series (visit 1) and two additional participants developed elevated NP antibodies after receiving the 3rd vaccine dose (visit 2) (Fig. 2).
Fig. 2. Antibody binding levels to SARS-CoV-2 nucleocapsid proteins.
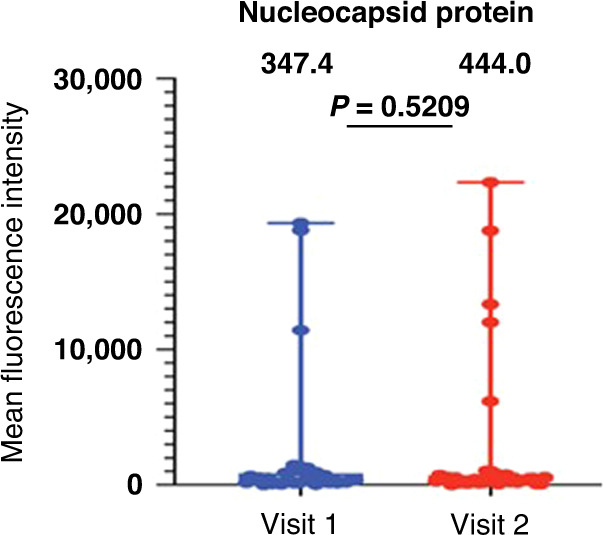
A multiplex bead-based antibody binding assay measured the IgG antibody response to SARS-CoV-2 nucleocapsid protein. Median Fluorescent Intensity (MFI) is shown and background well subtraction has been used to remove nonspecific signal. Each dot represents an individual (n = 37) after the two-dose vaccine series (Visit 1) and after the 3rd vaccine dose (Visit 2). P values were determined using Wilcoxon matched pairs signed rank test. Group median values are displayed above the graph.
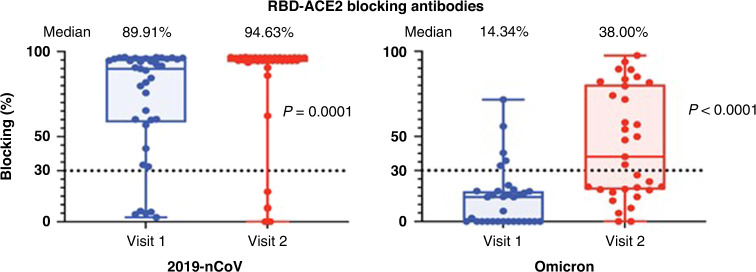
As a surrogate to neutralizing antibody detection, the levels of antibodies that could block the human host receptor angiotensin converting enzyme 2 (ACE2) and viral RBD binding were measured. Blocking antibodies were measured against the original vaccine-matched strain (2019-nCoV) and the Omicron variant that emerged in the fall of 2021. Detectable blocking antibodies were found in 86.5% of participants before and after the 3rd vaccine dose against the 2019-nCoV strain. The five participants that had low levels of binding antibodies to the spike protein also had undetectable blocking antibodies before and after the 3rd vaccine dose (Fig. 3). The magnitude of blocking antibodies increased from a median of 89.91% (95% CI: 65.25–94.45%) after the 2-dose vaccine series to a median of 94.63% (95%CI: 94.51–96.37%) following the 3rd vaccine dose (p = 0.0001). On further evaluation of the 5 participants with no antibody response following the 3rd vaccine dose, 4 of these children were kidney transplant recipients and were prescribed mycophenolate mofetil, tacrolimus, and prednisone and 1 child had received rituximab for a rheumatologic disease 3 months prior to receiving the 3rd vaccine dose.
Fig. 3. Surrogate neutralizing antibodies against SARS-CoV-2 vaccine-matched and Omicron variant.
A neutralization antibody proxy assay determined the level of antibodies that block binding of the spike protein receptor-binding domain to the human host receptor angiotensin-converting enzyme 2 (ACE2). The assay threshold for positivity was 30% indicating the presence of neutralizing antibodies. RBD from the original 2019-nCoV or RBD from the SARS-CoV-2 Omicron variant were utilized. Plasma samples (n = 37) were obtained after 2-doses of the vaccine (Visit 1) and after the 3rd vaccine dose (Visit 2). The median percentage of surrogate neutralizing antibodies is displayed above the graph.
In contrast to the response to the vaccine-matched strain (2019-nCoV), only 15.2% of participants had surrogate neutralizing antibodies (median 14.34%, 95% CI: 0.00–16.82%) to the Omicron variant after 2 doses of the vaccine. After the 3rd vaccine dose, Omicron surrogate neutralizing antibodies were detected in 54.6% of participants (median 38%, 95% CI: 19.98–71.72%), but the magnitude remained lower than the response to the original vaccine-strain (p < 0.0001) (Fig. 3).
Cellular immune response
Most participants (86.5%) had a positive cellular response after the 2-dose vaccine series, which increased to 100% after the 3rd vaccine dose (p = 0.063). When characterized into CD4 and CD8 response, the CD4 response was identical to the overall cellular response. In contrast, 20 participants had a negative CD8 response after the 2-dose vaccine series with only 5 additional participants converting to a positive response after the 3rd vaccine dose; 11 participants had a positive response at both time points (Fig. 4). Of the 5 participants with initial negative cellular response, 3 were kidney transplant recipients, 2 had rheumatologic diseases, and 1 had received a stem-cell transplant. Only 1 of the 5 also had a negative humoral response.
Fig. 4. Cellular immune response to the 3rd COVID-19 vaccine dose.
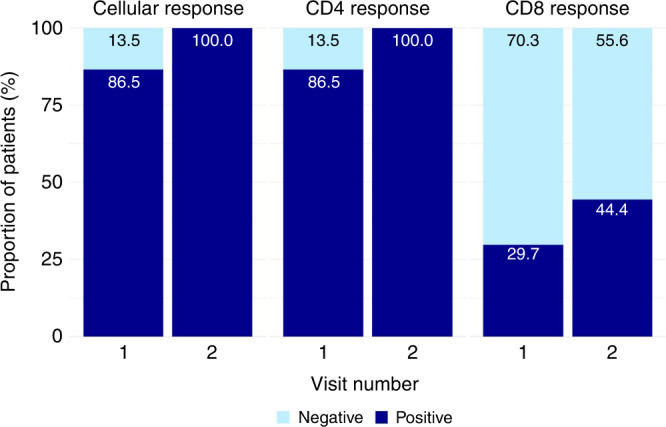
Cellular immune response was measured via the SARS-CoV-2 inSIGHT™ T-cell immunity panel. The overall response (detected/not detected) was further categorized into CD4 and CD8 response. Samples (n = 37) were obtained after 2-doses of the vaccine (Visit 1) and after the 3rd vaccine dose (Visit 2). The proportion of participants with positive/negative results are displayed within the graph.
Discussion
Children who are immunocompromised have been shown to mount an inadequate immune response compared with immunocompetent children, both in terms of their capacity to respond to active infections as well as their response to vaccinations. Medications that are frequently prescribed for these children cause a variable degree of immunosuppression. It is well documented that medications such as B-cell depletion therapies, high dose glucocorticoids, and mycophenolate mofetil significantly reduce the immune response to vaccinations.5,6,22 This has led to concerns regarding protection against SARS-CoV-2 despite vaccination in the immunocompromised population. Strategies such as a decreased dose of immunosuppressive medications or transition to fewer immunosuppressive medications in anticipation of vaccine administration have been suggested by some to augment vaccine responsiveness to SARS-CoV-2 in this population, with the associated risk for transplant rejection or suboptimal control of the underlying disorder.5,6,22 While inhibition of the humoral immune response to vaccination is well documented in the immunocompromised population, less is known regarding the effect of vaccination on the cellular immune response.
In this cohort, most immunocompromised children did mount a humoral (86.5%) and cellular (86.5%) immune response to the 2-dose COVID-19 vaccine series. Not surprisingly, this response remained lower than what has been demonstrated for healthy children who received the BNT162b2 2-dose vaccine series in whom a humoral response was detected in 90.7%, 100%, and 95% of participants for age groups 5–11 years, 12–15 years, and ≥16 years, respectively.23,24 While the humoral response to the original strain of COVID-19 was present in the majority of participants in our immunocompromised cohort, only a minority of participants (15.2%) mounted a response to the Omicron variant following receipt of the initial 2 vaccine doses. Whereas a response to the Omicron variant did improve and was found to be present in 54.6% of participants after the 3rd COVID-19 vaccine dose, the frequency and strength of the response remains suboptimal compared to the response to the original strain. This is consistent with other adult and pediatric studies, which also found a reduced effectiveness of current COVID-19 vaccines against the Omicron variant and emphasizes the importance of booster vaccine doses and continued attention to recommended public health care measures in hopes of providing adequate protection against this variant.25–27
Impressively, all participants had a detectable cellular response after receiving the 3rd vaccine dose, including the 5 participants who did not mount a humoral response. This is consistent with a few studies that have shown detectable cellular immune responses despite diminished vaccine induced humoral immune responses in immunocompromised individuals.12,22,28 This highlights the multifaceted complexity of immunity, specifically that protection against COVID-19 is likely dependent on both humoral and cellular mechanisms. When the cellular immune response was categorized as a CD4 and CD8 response, the CD4 response was more prominent, although both improved following the 3rd COVID-19 vaccine dose. Painter et al also noted a robust initial CD4 response to COVID-19 vaccination which supports the development of memory B cells and antibody formation. The CD8 response occurred more slowly and improved with subsequent COVID-19 vaccine doses.29
There are several strengths to our study including its prospective, pediatric-specific design in a particularly vulnerable patient population, and the assessment of both humoral and T-cell immune responses to the 3rd COVID-19 vaccine. The humoral response assessment included analysis of several spike proteins along with assessment of neutralizing antibodies to the original strain, as well as to the Omicron variant. The study limitations include a small sample size from a single site, a short follow up period, and limited racial and ethnic diversity within the study cohort. The small sample size and the variety of immunosuppressive medication regimens also precluded further meaningful subgroup analysis regarding the effects of the underlying diagnosis and types of immunosuppressive medications on the response to the 3rd COVID vaccine dose. Nevertheless, the study does provide the opportunity for collection of additional longitudinal data from a more diverse population of immunocompromised children from multiple divisions within our institution to evaluate the duration of the humoral and T-cell immune responses and their association with subsequent COVID-19 infections.
Conclusion
Most immunocompromised children mount a humoral and cellular immune response to the 2-dose COVID-19 vaccine series, a response that is significantly augmented after receiving the 3rd vaccine dose. This supports the need for ongoing emphasis on the benefits of vaccination against COVID-19 in this population. Subsequent analyses regarding the duration of vaccine induced immunogenicity and the frequency and severity of breakthrough infections will help inform the long-term vaccine strategy.
Acknowledgements
Funding was provided by Eurofins-Viracor and an internal grant from Dr. Bradley Warady’s Endowed Chair Fund.
Author contributions
All authors listed have met authorship criteria as outlined in the publishing instructions.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Ethics approval
This study was approved by the institutional review board (IRB) at Children’s Mercy Kansas City. Verbal consent was obtained from one guardian and assent was obtained from the participant.
Consent to participate
Consent was obtained by one parent and assent was obtained by the participant.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Andrews N, et al. Duration of protection against mild and severe disease by Covid-19 vaccines. N. Engl. J. Med. 2022;386:340–350. doi: 10.1056/NEJMoa2115481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnson AG, et al. COVID-19 incidence and death rates among unvaccinated and fully vaccinated adults with and without booster doses during periods of delta and omicron variant emergence - 25 U.S. jurisdictions, April 4-December 25, 2021. MMWR Morb. Mortal. Wkly Rep. 2022;71:132–138. doi: 10.15585/mmwr.mm7104e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zheng C, et al. Real-world effectiveness of COVID-19 vaccines: a literature review and meta-analysis. Int J. Infect. Dis. 2022;114:252–260. doi: 10.1016/j.ijid.2021.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Embi PJ, et al. Effectiveness of 2-dose vaccination with mRNA COVID-19 vaccines against COVID-19-associated hospitalizations among immunocompromised adults - nine states, January–September 2021. MMWR Morb. Mortal. Wkly Rep. 2021;70:1553–1559. doi: 10.15585/mmwr.mm7044e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luxi N, et al. COVID-19 vaccination in pregnancy, paediatrics, immunocompromised patients, and persons with history of allergy or prior SARS-CoV-2 infection: overview of current recommendations and pre- and post-marketing evidence for vaccine efficacy and safety. Drug Saf. 2021;44:1247–1269. doi: 10.1007/s40264-021-01131-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deepak P, et al. Effect of immunosuppression on the immunogenicity of mRNA vaccines to SARS-CoV-2: a prospective cohort study. Ann. Intern Med. 2021;174:1572–1585. doi: 10.7326/M21-1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marra, A. R. et al. Short-term effectiveness of COVID-19 vaccines in immunocompromised patients: A systematic literature review and meta-analysis [published online ahead of print, 2022 Jan 1]. J Infect. S0163–S4453 00658-7. 10.1016/j.jinf.2021.12.035 (2022). [DOI] [PMC free article] [PubMed]
- 8.Mbaeyi S, et al. The advisory committee on immunization practices’ interim recommendations for additional primary and booster doses of COVID-19 vaccines - United States, 2021. MMWR Morb. Mortal. Wkly Rep. 2021;70:1545–1552. doi: 10.15585/mmwr.mm7044e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Espi M, et al. A prospective observational study for justification, safety, and efficacy of a third dose of mRNA vaccine in patients receiving maintenance hemodialysis. Kidney Int. 2022;101:390–402. doi: 10.1016/j.kint.2021.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bachelet T, et al. Humoral response after SARS-CoV-2 mRNA vaccines in dialysis patients: Integrating anti-SARS-CoV-2 Spike-Protein-RBD antibody monitoring to manage dialysis centers in pandemic times. PLoS One. 2021;16:e0257646. doi: 10.1371/journal.pone.0257646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shroff RT, et al. Immune responses to two and three doses of the BNT162b2 mRNA vaccine in adults with solid tumors. Nat. Med. 2021;27:2002–2011. doi: 10.1038/s41591-021-01542-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crane C, Phebus E, Ingulli E. Immunologic response of mRNA SARS-CoV-2 vaccination in adolescent kidney transplant recipients. Pediatr. Nephrol. 2022;37:449–453. doi: 10.1007/s00467-021-05256-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kermond, R. F. et al. Immunologic response to SARS-CoV-2 mRNA vaccination in pediatric kidney transplant recipients [published online ahead of print, 2022 Jul 14]. Pediatr Nephrol. 1–8. 10.1007/s00467-022-05679-y (2022). [DOI] [PMC free article] [PubMed]
- 14.Spencer EA, Klang E, Dolinger M, Pittman N, Dubinsky MC. Seroconversion following SARS-CoV-2 infection or vaccination in pediatric IBD patients. Inflamm. Bowel Dis. 2021;27:1862–1864. doi: 10.1093/ibd/izab194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amodio D, et al. Humoral and cellular response following vaccination with the BNT162b2 mRNA COVID-19 vaccine in patients affected by primary immunodeficiencies. Front Immunol. 2021;12:727850. doi: 10.3389/fimmu.2021.727850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention. (n.d.). Covid-19 vaccines for people who are moderately or severely immunocompromised. Centers for Disease Control and Prevention. Retrieved April 24, 2022, from https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/immuno.html
- 17.Harris PA, et al. Research electronic data capture (REDCap) – A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inf. 2009;42(Apr):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harris, P. A. et al. REDCap Consortium, The REDCap consortium: Building an international community of software partners, J Biomed Inform. (2019). 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed]
- 19.Bradley T, et al. Antibody responses after a single dose of SARS-CoV-2 mRNA vaccine. N. Engl. J. Med. 2021;384:1959–1961. doi: 10.1056/NEJMc2102051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fraley E, et al. Humoral immune responses during SARS-CoV-2 mRNA vaccine administration in seropositive and seronegative individuals. BMC Med. 2021;19:169. doi: 10.1186/s12916-021-02055-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fraley, E. et al. The Impact of Prior Infection and Age on Antibody Persistence After Severe Acute Respiratory Syndrome Coronavirus 2 Messenger RNA Vaccine. Clin Infect Dis. 2022;75:e902–e904. [DOI] [PMC free article] [PubMed]
- 22.Friedman MA, Curtis JR, Winthrop KL. Impact of disease-modifying antirheumatic drugs on vaccine immunogenicity in patients with inflammatory rheumatic and musculoskeletal diseases. Ann. Rheum. Dis. 2021;80:1255–1265. doi: 10.1136/annrheumdis-2021-221244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frenck RW, Jr, et al. Safety, immunogenicity, and efficacy of the BNT162b2 Covid-19 vaccine in adolescents. N. Engl. J. Med. 2021;385:239–250. doi: 10.1056/NEJMoa2107456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walter EB, et al. Evaluation of the BNT162b2 Covid-19 vaccine in children 5 to 11 years of age. N. Engl. J. Med. 2022;386:35–46. doi: 10.1056/NEJMoa2116298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andrews N, et al. Covid-19 vaccine effectiveness against the omicron (B.1.1.529) variant. N. Engl. J. Med. 2022;386:1532–1546. doi: 10.1056/NEJMoa2119451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoffmann M, et al. The Omicron variant is highly resistant against antibody-mediated neutralization: Implications for control of the COVID-19 pandemic. Cell. 2022;185:447–456.e11. doi: 10.1016/j.cell.2021.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Price, A. M. et al. BNT162b2 Protection against the Omicron Variant in Children and Adolescents [published online ahead of print, 2022 Mar 30]. N Engl J Med. NEJMoa2202826. 10.1056/NEJMoa2202826 (2022). [DOI] [PMC free article] [PubMed]
- 28.Ma AL, et al. Antibody responses to 2 doses of mRNA COVID-19 vaccine in pediatric patients with kidney diseases. Kidney Int. 2022;101:1069–1072. doi: 10.1016/j.kint.2022.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Painter MM, et al. Rapid induction of antigen-specific CD4+ T cells is associated with coordinated humoral and cellular immunity to SARS-CoV-2 mRNA vaccination. Immunity. 2021;54:2133–2142.e3. doi: 10.1016/j.immuni.2021.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.